Abstract
Objectives:
To investigate the association between subjective memory complaints (SMCs) and long-term risk of cognitive impairment in aging because most previous studies have followed individuals for only a few years.
Methods:
Participants were 1,107 cognitively normal, community-dwelling older women (aged 65 years and older at baseline) in a prospective study of aging. SMCs were assessed shortly after baseline and repeatedly over time with the yes/no question, “Do you feel you have more problems with memory than most?” Cognitive status 18 years later (normal or impaired with mild cognitive impairment or dementia) was determined by an expert panel. Using logistic regression, we investigated the association between SMCs over time and risk of cognitive impairment, adjusting for demographics, baseline cognition, and characteristics that differed between those with and without SMCs.
Results:
At baseline, 8.0% of participants (n = 89) endorsed SMCs. Baseline SMCs were associated with increased risk of cognitive impairment 18 years later (adjusted odds ratio [OR] = 1.7, 95% confidence interval 1.1–2.8). Results were unchanged after excluding participants with depression. The association between SMCs and cognitive impairment was greatest at the last SMC assessment time point (18 years before diagnosis: adjusted OR = 1.7 [1.1–2.9]; 14 years before diagnosis: adjusted OR = 1.6 [0.9–2.7]; 10 years before diagnosis: adjusted OR = 1.9 [1.1–3.1]; 4 years before diagnosis: adjusted OR = 3.0 [1.8–5.0]).
Conclusions:
SMCs are associated with cognitive impairment nearly 2 decades later among older women. SMCs may be a very early symptom of an insidious neurodegenerative disease process, such as Alzheimer disease.
Alzheimer disease (AD) and other neurodegenerative disorders manifest with an insidious course, making it challenging to detect their subtle beginnings in an individual patient. Indeed, the neuropathologic changes of AD may occur many years before an individual begins to exhibit cognitive impairment.1 Subjective cognitive complaints among cognitively normal older adults may be an early symptom of AD or other neurodegenerative processes.2 Some studies of cognitively normal older adults have found that individuals with subjective memory complaints (SMCs) have greater amyloid burden,3,4 while others have found no such associations5,6 or have argued that SMCs merely reflect symptoms of depression or anxiety.5 A recent meta-analysis7 of longitudinal studies supports an overall association between SMCs and the development of mild cognitive impairment (MCI) and dementia, but also revealed that previous studies have followed individuals for an average of only 4 to 5 years.7 This is a key limitation in the field given that neurodegenerative processes such as AD can take several years to decades to unfold.8
Additional research is needed to clarify the long-term predictive utility of an older adult's subjective report of memory problems and the extent to which SMCs may reflect an early symptom of a neurodegenerative disorder. Our objective was to examine whether SMCs are associated with long-term risk of cognitive impairment (MCI or dementia) 18 years later among older women. We also explored the association between SMCs assessed across time and risk of cognitive impairment, in order to evaluate whether the sensitivity of SMCs as a potential early indicator of future cognitive impairment may change over time.
METHODS
Population.
Participants were community-dwelling older women (aged 65 years and older) in the prospective cohort Study of Osteoporotic Fractures (SOF), recruited by study centers in Baltimore, MD, Minneapolis, MN, Portland, OR, and the Monongahela Valley near Pittsburgh, PA. A total of 9,704 Caucasian women were enrolled between 1986 and 1988, and 662 African American women were enrolled later, between 1997 and 1998. Women with a history of hip fracture or bilateral hip replacement or who were unable to walk without assistance were excluded. Further details regarding SOF recruitment methods and study design have been published previously.9
As part of the SOF–Women, Cognitive Impairment Study of Exceptional Aging ancillary study, year-20 (2006–2008) clinical cognitive diagnosis of normal cognition, MCI, or dementia was evaluated by an expert panel among 1,338 women from the Caucasian cohort. For our primary analyses, we included 1,107 women who were cognitively normal at baseline (as defined by a baseline modified Mini-Mental State Examination [mMMSE] score no more than 1.5 SD below the mean compared with age- and education-matched peers in the entire Caucasian cohort), who completed SMC assessment shortly after baseline (year 2), and whose year-20 cognitive diagnosis was determined. Compared with the remaining women in the SOF Caucasian cohort, these 1,107 women were younger and more highly educated, had fewer comorbidities and endorsed fewer depressive symptoms (all p < 0.001), and had higher body mass index (p = 0.03). Included women were less likely to endorse SMCs at baseline and at all subsequent SMC assessments (all p < 0.001).
Standard protocol approvals, registrations, and patient consents.
All participants gave written informed consent to participate in SOF. The study was approved by institutional review boards at each study site and at the University of California, San Francisco (coordinating center).
Measures.
Subjective memory complaints.
SMCs were assessed shortly after baseline at year 2 (henceforth referred to as baseline SMCs) and again in years 6, 10, and 16 using the following yes/no question from the 15-item Geriatric Depression Scale (GDS)10: “Do you feel you have more problems with memory than most?”
Cognition and cognitive diagnosis.
Global cognitive functioning was assessed using a 26-item mMMSE11 at baseline and repeatedly over time. At year 20, a larger cognitive battery was given that included the following: (1) the mMMSE,9 which is an expanded measure of global cognition; (2) the California Verbal Learning Test–II Short Form,12 a measure of learning and memory; (3) Digit Span–Forward and Backward,13 a measure of auditory attention/working memory; (4) Trail Making Test, Part B,14 a measure of executive functioning; and (5) Verbal Associative Fluency (letter and category),15 a measure of language. Year-20 clinical cognitive diagnosis of normal cognition, MCI, or dementia was determined using a previously described process.16 First, the following screening criteria were applied as indicators of possible cognitive impairment: (1) mMMSE score <8817; (2) California Verbal Learning Test delayed recall score <412; (3) Informant Questionnaire on Cognitive Decline in the Elderly score ≥3.618; (4) self-reported dementia diagnosis; or (5) living in a nursing home. Women who did not meet any of these screening criteria were considered cognitively normal. Data from women who met any of the screening criteria were then further examined by a team of clinicians, who reviewed individuals' cognitive test results from year 20 and all prior cognitive assessment data, functional status, medical history, medications, and depressive symptoms and then diagnosed individuals as having MCI (based on modified Peterson criteria),19 dementia (based on DSM-IV criteria), or as being cognitively normal.
Other variables.
Participants completed self-report questionnaires assessing basic demographics and educational history at baseline. Information about comorbidities was collected repeatedly over time, based on participants' self-report of physician diagnoses of hypertension, diabetes, stroke, and myocardial infarction. Body mass index was calculated based on participants' height and weight (kg/m2). Depressive symptoms were assessed with the 15-item GDS.10 Because our primary variable of interest (SMCs) was taken from this inventory, we utilized a GDS score out of 14 that excluded the memory item, and we applied a prorated cutoff (6 of 14) to define depression in keeping with traditional cutpoint.10
Statistical analysis.
We first compared basic demographic and other baseline characteristics between individuals with and without baseline SMCs using t tests, χ2, or Fisher exact tests as appropriate. We then conducted logistic regression models to investigate whether baseline SMCs predicted clinically significant cognitive impairment (MCI/dementia vs normal cognition) 18 years later. We conducted this analysis using an unadjusted model as well as a model adjusting for demographics (age, education), baseline mMMSE score, and participant characteristics that significantly differed between groups (p < 0.05). We also explored whether the association between SMCs and risk of cognitive impairment changed over time. Similar to our primary analyses, we used logistic regression models to investigate the association between SMCs at each time point (years 2, 6, 10, 16) and later diagnosis of cognitive impairment, unadjusted and adjusted for the same factors as above measured at each respective time point. These models were conducted among participants who had complete SMC data across all time points and who were cognitively normal at the examined time point as determined using the same age- and education-based mMMSE cutoffs as defined above (year 2: n = 1,025; year 6: n = 990; year 10: n = 977; year 16: n = 943).
RESULTS
At baseline, 8.0% of participants (n = 89) endorsed SMCs. Demographics and other participant characteristics are compared between those with and without baseline SMCs in table 1. Compared to those without baseline SMCs, women with SMCs had lower education (p = 0.01), greater myocardial infarction history (p = 0.01), and higher depressive symptoms (p < 0.001).
Table 1.
Baseline characteristics by presence of SMCs among 1,107 older women
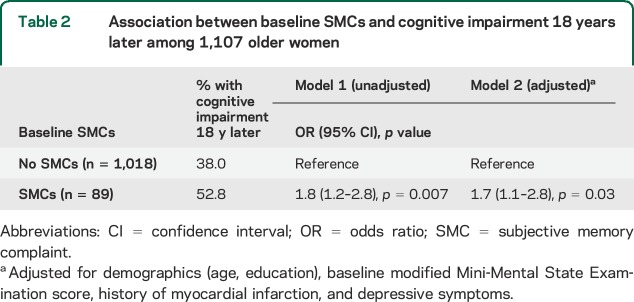
Women with baseline SMCs were more likely to be diagnosed with cognitive impairment at year 20 compared to women without baseline SMCs (52.8% vs 38.0%; χ2 = 7.5, p = 0.01). As shown in table 2, baseline SMCs were significantly associated with increased risk of cognitive impairment 18 years later even after adjustment for demographics, baseline mMMSE score, history of myocardial infarction, and depressive symptoms (adjusted odds ratio [OR] = 1.7, 95% confidence interval [CI] 1.1–2.8, p = 0.03). In a sensitivity analysis, we excluded 26 participants with baseline depression, and the effect of SMCs was unchanged (fully adjusted OR = 1.7, 95% CI 1.01–2.8, p = 0.046).
Table 2.
Association between baseline SMCs and cognitive impairment 18 years later among 1,107 older women
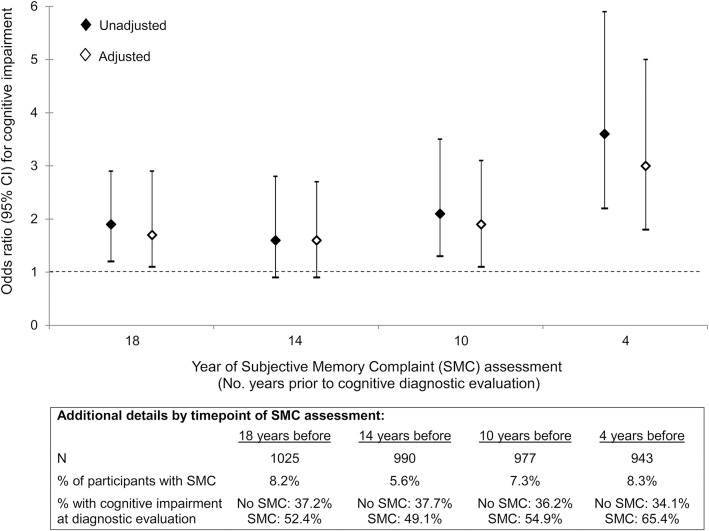
The figure displays the association between SMCs assessed at varying time points before the diagnostic evaluation and risk of cognitive impairment. Although SMCs measured 14 years before the diagnostic evaluation were not significantly associated with cognitive impairment (adjusted OR = 1.6, 95% CI 0.9–2.7, p = 0.12), the adjusted OR was in the same direction and of similar magnitude as the significant association between SMCs measured 18 years before the diagnostic evaluation (adjusted OR = 1.7, 95% CI 1.1–2.9, p = 0.03) and SMCs measured 10 years before the diagnostic evaluation (adjusted OR = 1.9, 95% CI 1.1–3.1, p = 0.01). The association between SMCs and cognitive impairment was greatest at the last time point, i.e., SMCs measured 4 years before the diagnostic evaluation (adjusted OR = 3.0, 95% CI 1.8–5.0, p < 0.001).
Figure. SMCs at varying time points before the diagnostic evaluation and risk of cognitive impairment.
Results of logistic regression analyses examining the association between SMCs assessed at varying time points before the diagnostic evaluation and risk of cognitive impairment. Adjusted models include adjustment for demographics (age, education) as well as mMMSE score, history of myocardial infarction, and depressive symptoms as measured at each SMC assessment time point. At each time point, individuals with an impaired mMMSE score at that time point were excluded (using −1.5 SD age- and education-based cutoffs). CI = confidence interval; mMMSE = modified Mini-Mental State Examination; SMC = subjective memory complaint.
In sensitivity analyses among individuals with complete SMC data who remained cognitively normal at year 16 (n = 943), we explored the independent effects of SMCs at particular time points and the effects of individuals' pattern of SMC endorsement over time. For the former, we included all 4 SMC assessment time points as predictors in a logistic regression model, adjusted for the same covariates as above. There was a significant overall effect of endorsing SMCs at some point on likelihood of cognitive impairment (p < 0.001) and significant heterogeneity of effects among the SMC time points (p = 0.02). Pairwise comparisons revealed that the last SMC time point (4 years before the cognitive diagnostic evaluation) was most strongly associated with cognitive impairment compared with other SMC time points (the last SMC time point was significantly different from every other time point [all p < 0.05]; there were no other significant pairwise differences [all p > 0.05]). To explore individuals' pattern of SMC endorsement over time, we first compared risk of cognitive impairment between individuals who endorsed SMCs at 0, 1, vs ≥2 out of the 4 SMC assessment visits. Compared with those who never endorsed SMCs, individuals who endorsed SMCs at one or more visits were more likely to be diagnosed with cognitive impairment (SMCs at only 1 visit [10.7% of participants; n = 101]: adjusted OR = 2.1, 95% CI 1.3–3.2, p = 0.001; SMCs at ≥2 visits [7.3% of participants; n = 69]: adjusted OR = 2.0, 95% CI 1.2–3.4, p = 0.01). We then classified individuals as endorsing patterns of (1) persistent SMCs—those who endorsed SMCs at baseline and ≥1 additional visit (n = 47), (2) incident SMCs—those who denied SMCs at baseline but endorsed SMCs at ≥1 later visit (n = 91), (3) transient SMCs—those who endorsed SMCs at baseline but denied SMCs at all other visits (n = 32), vs (4) those who never endorsed SMCs (n = 773). Compared with individuals who never endorsed SMCs, individuals with incident SMCs (adjusted OR = 2.4, 95% CI 1.5–3.9, p < 0.001) and persistent SMCs (adjusted OR = 1.9, 95% CI 1.01–3.5, p = 0.046) were more likely to develop cognitive impairment. The effect of transient SMCs was not statistically significant (adjusted OR = 1.4, 95% CI 0.7–3.0, p = 0.36).
DISCUSSION
We investigated the long-term association between SMCs and cognitive outcomes among older women and found that SMCs were significantly associated with cognitive impairment nearly 2 decades later. We also examined the association between SMCs assessed repeatedly over time and subsequent diagnosis of cognitive impairment and found that the strongest association was present when SMCs were assessed just a few years in advance of participants' clinical cognitive diagnostic evaluation. While women with SMCs 18 years before the diagnostic evaluation had 1.7 times greater odds of receiving an impaired diagnosis, women with SMCs 4 years before the diagnostic evaluation had 3 times greater odds of receiving an impaired diagnosis. As further evidence of the significance of SMCs, we found that even women who endorsed SMCs at only one point in time were at increased risk of cognitive impairment and that patterns of persistent SMCs and incident SMCs over time were both significantly associated with risk of cognitive impairment. Our findings provide further evidence that SMCs in aging warrant close attention as a possible early warning sign of future cognitive problems, even several years in advance. Indeed, a recent study quantified that older adults with SMCs who went on to develop MCI progressed to this diagnosis an average of 9.2 years after they first endorsed SMCs,20 documenting a prolonged prodromal period between SMCs and the development of cognitive impairment.
Our results add further support to the possibility that SMCs may be an early symptom of an underlying neurodegenerative process, such as AD. That the association between SMCs and risk of cognitive impairment was present very early (i.e., for SMCs endorsed nearly 20 years before our cognitive diagnostic evaluation) and was strongest for SMCs closest in time to the diagnostic evaluation, this timing pattern suggests that SMCs may signal an insidious disease process as it first emerges and continues to unfold. This would be consistent with the long, prodromal period of AD, during which amyloid deposition, thought to be the beginnings of the AD neuropathology cascade, is present many years before the clinical manifestation of objective cognitive impairment.21 Moreover, neuroimaging studies corroborate this possibility in finding that older adults with SMCs have greater amyloid burden3,4 and patterns of atrophy similar to older adults with amnestic MCI and AD.22,23 Nevertheless, SMCs may not be specific to AD but could also precede objective cognitive impairment in the context of the gradual accumulation of other neuropathology in aging, such as cerebrovascular disease.24,25 Despite the above possibilities and our findings, it should be noted that the associations we observed (particularly for early years of SMC assessment) are somewhat modest. There may be other reasons for an older adult to endorse SMCs that do not necessarily lead to the development of MCI or dementia.
Strengths of the present study include our ability to investigate the predictive utility of SMCs over an extended follow-up period of 18 years and to examine SMCs assessed repeatedly over time. In addition, our study benefits from the completion of a comprehensive clinical cognitive diagnostic evaluation to determine participants' cognitive status (normal, MCI, dementia). Nevertheless, there are some limitations in the assessment of cognitive status in our study. Because the clinical cognitive diagnostic evaluation was only conducted at the final time point, we cannot be certain exactly when individuals first met diagnostic criteria for MCI/dementia. Our utilization of global cognitive screen (mMMSE) scores to exclude individuals with cognitive impairment at baseline and at subsequent SMC assessment time points reduces the likelihood that significant cognitive impairment was present at those time points. However, it is possible that more subtle cognitive deficits were not detected by these screens. An additional limitation is that we cannot be certain of the extent to which survival bias may have influenced our results. Women included in the present study were generally healthier than those who were not, were less likely to endorse SMCs, and were required to have completed a 20th year of study participation. While some survival bias in our study is therefore likely, it is perhaps even more compelling that we found long-term associations between SMCs and cognitive impairment among this relatively healthier cohort, as survival bias would most likely bias our findings toward an underestimate of risk. Particularly as included women were less likely to have SMCs than those not included, some women with SMCs may have actually been lost to follow-up before year 20 related to the development of cognitive impairment. Further limitations include that our findings cannot be generalized to men or to other racial/ethnic groups.
It is important to consider that we investigated SMCs among community-dwelling older women participating in a population-based study. SMCs endorsed by women in the general population may reflect a different phenomenon than what occurs when older adults are concerned enough about their cognition to present to a clinic for SMCs. Moreover, because we assessed SMCs using one yes/no question, we likely did not capture many complexities inherent in asking an older adult to evaluate his or her own cognitive functioning. As highlighted by the 2014 Subjective Cognitive Decline Initiative Workgroup,2 valuable data may be gleaned from a more comprehensive assessment that asks individuals to appraise their functioning in multiple cognitive domains both in comparison to their peers and relative to their own prior ability levels. However, it appears notable that, despite the simplicity of our SMC measure and despite studying women from the general population rather than clinic patients, we still found an association between SMCs and risk of cognitive impairment. Therefore, our results support the idea that clinical providers, even in primary care settings, should consider incorporating assessment of SMCs into their routine checkups of older patients, as endorsement of SMCs may be informative even when the patient's primary reason for presenting to clinic is not their cognition. Our results also raise the possibility that even a relatively brief SMC assessment could be a valuable screening tool, perhaps in the form of a questionnaire administered in the waiting room to help minimize provider burden. Moreover, as we found associations between SMCs and risk of cognitive impairment among individuals with unimpaired scores on a global cognitive screen, we recommend that providers should not be entirely reassured by a “normal” cognitive screen performance but instead take care to monitor individuals with SMCs over time for possible cognitive decline.
SMCs among cognitively normal older women appear to be an early indicator of risk of cognitive impairment and may be a subtle signal of an underlying neurodegenerative disease process such as AD that is still in its earliest stages. Early detection of AD and other dementias is likely needed to enable potential interventions to be applied as early in the disease course as possible. Our results suggest that dementia prevention research trials should target older women with SMCs as a high-risk group, in order to attempt to intervene among those who may be showing the earliest symptoms of neurodegeneration.
ACKNOWLEDGMENT
The authors thank the following personnel associated with the Study of Osteoporotic Fractures (SOF) Research Group. Investigators in the SOF Research Group: San Francisco Coordinating Center (California Pacific Medical Center Research Institute and University of California San Francisco): S.R. Cummings (principal investigator), D.C. Bauer (coinvestigator), D.M. Black (coinvestigator), W. Browner (coinvestigator), P.M. Cawthon (coinvestigator), N. Lane (coinvestigator), M.C. Nevitt (coinvestigator), C. McCulloch (coinvestigator), A. Schwartz (coinvestigator), K.L. Stone (coinvestigator), G. Tranah (coinvestigator), K. Yaffe (coinvestigator), R. Benard, T. Blackwell, L. Concepcion, D. Evans, S. Ewing, C. Fox, R. Fullman, S.L. Harrison, M. Jaime-Chavez, D. Kriesel, W. Liu, L. Lui, L. Palermo, N. Parimi, K. Peters, M. Rahorst, C. Schambach, and J. Ziarno. University of Maryland: M.C. Hochberg (principal investigator), R. Nichols (clinic coordinator), and S. Link. University of Minnesota: K.E. Ensrud (principal investigator), S. Diem (coinvestigator), M. Homan (coinvestigator), P. Van Coevering (program coordinator), S. Fillhouer (clinic director), N. Nelson (clinic coordinator), K. Moen (assistant program coordinator), K. Jacobson, M. Forseth, R. Andrews, S. Luthi, K. Atchison, and L. Penland-Miller. University of Pittsburgh: J.A. Cauley (principal investigator), L.H. Kuller (co–principal investigator), J.M. Zmuda (coinvestigator), L. Harper (project director), L. Buck (clinic coordinator), M. Danielson (project administrator), D. Cusick, A. Flaugh, M. Gorecki, and C. Newman. The Kaiser Permanente Center for Health Research, Portland, OR: T. Hillier (principal investigator), K. Vesco (coinvestigator), K. Pedula (coinvestigator), J. Van Marter (project director), M. Summer (clinic coordinator), A. MacFarlane, J. Rizzo, K. Snider, and J. Wallace.
GLOSSARY
- AD
Alzheimer disease
- CI
confidence interval
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition)
- GDS
Geriatric Depression Scale
- MCI
mild cognitive impairment
- mMMSE
modified Mini-Mental State Examination
- OR
odds ratio
- SMC
subjective memory complaint
- SOF
Study of Osteoporotic Fractures
AUTHOR CONTRIBUTIONS
Dr. Kaup designed and conceptualized the study, conducted data analysis, interpreted the data, and drafted and revised the manuscript. Dr. Nettiksimmons assisted with data analysis and data interpretation and reviewed and revised the manuscript. Dr. LeBlanc assisted with data interpretation and reviewed and revised the manuscript. Dr. Yaffe designed and conceptualized the study, interpreted the data, reviewed and revised the manuscript, and supervised the study. All authors have approved the manuscript.
STUDY FUNDING
The Study of Osteoporotic Fractures (SOF) is supported by NIH funding. The National Institute on Aging (NIA) provides support under the following grants: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, and R01 AG027576. The research described here was also supported in part by NIA grant K24AG031155 (awarded to Kristine Yaffe, MD); by Career Development Award 1IK2RX001629 (awarded to Allison Kaup, PhD) from the US Department of Veterans Affairs, Rehabilitation Research and Development Service; and the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Medical Research Service of the San Francisco Veterans Affairs Medical Center, and the Department of Veterans Affairs Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC). The contents of this manuscript do not represent the views of the US Department of Veterans Affairs or the United States Government.
DISCLOSURE
A. Kaup and J. Nettiksimmons report no disclosures relevant to the manuscript. E. LeBlanc's institution has received funding from Amgen, Bristol-Myers Squibb, and AstraZeneca for research grants unrelated to the current study. K. Yaffe is a consultant for Novartis and Pfizer. She serves on DSMBs for Takeda, Inc., and an NIA-sponsored study and also serves on the Beeson Scientific Advisory Board. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol 2009;68:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement 2014;10:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amariglio RE, Becker JA, Carmasin J, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 2012;50:2880–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: a Pittsburgh compound B positron emission tomography study in normal elderly individuals. Arch Neurol 2012;69:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckley R, Saling M, Ames D, et al. Factors affecting subjective memory complaints in the AIBL Aging Study: biomarkers, memory, affect, and age. Int Psychogeriatr 2013;25:1307–1315. [DOI] [PubMed] [Google Scholar]
- 6.Hollands S, Lim YY, Buckley R, et al. Amyloid-β related memory decline is not associated with subjective or informant rated cognitive impairment in healthy adults. J Alzheimers Dis 2015;43:677–686. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell A, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta‐analysis. Acta Psychiatr Scand 2014;130:439–451. [DOI] [PubMed] [Google Scholar]
- 8.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association Workgroups on Diagnostic Guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teng EL, Chui HC. The modified Mini-Mental State Examination (3MS). Can J Psychiatry 1987;41:114–121. [PubMed] [Google Scholar]
- 10.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press; 1986:165–173. [Google Scholar]
- 11.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry 1983;40:812. [DOI] [PubMed] [Google Scholar]
- 12.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test–Second Edition (CVLT-II). San Antonio: Psychological Corporation; 2000. [Google Scholar]
- 13.Strauss E, Sherman E, Spreen O. General cognitive functioning, neuropsychological batteries, and assessment of premorbid intelligence. In: A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 3rd ed New York: Oxford University Press; 2006:283–285. [Google Scholar]
- 14.Reitan RM. Trail Making Test: Manual for Administration and Scoring. Tucson: Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- 15.Strauss E, Sherman E, Spreen O. Executive functions. In: A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 3rd ed New York: Oxford University Press; 2006:499–503. [Google Scholar]
- 16.Tranah GJ, Blackwell T, Stone KL, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol 2011;70:722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espeland MA, Rapp SR, Robertson J, et al. Benchmarks for designing two-stage studies using modified Mini-Mental State Examinations: experience from the Women's Health Initiative Memory Study. Clin Trials 2006;3:99–106. [DOI] [PubMed] [Google Scholar]
- 18.Jorm A, Jacomb P. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med 1989;19:1015–1022. [DOI] [PubMed] [Google Scholar]
- 19.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58:1985–1992. [DOI] [PubMed] [Google Scholar]
- 20.Kryscio RJ, Abner EL, Cooper GE, et al. Self-reported memory complaints implications from a longitudinal cohort with autopsies. Neurology 2014;83:1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jack CR, Knopman DS, Jagust WJ, et al. Update on hypothetical model of Alzheimer's disease biomarkers. Lancet Neurol 2013;12:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peter J, Scheef L, Abdulkadir A, et al. Gray matter atrophy pattern in elderly with subjective memory impairment. Alzheimers Dement 2014;10:99–108. [DOI] [PubMed] [Google Scholar]
- 23.Saykin A, Wishart H, Rabin L, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 2006;67:834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sajjad A, Mirza SS, Portegies ML, et al. Subjective memory complaints and the risk of stroke. Stroke 2015;46:170–175. [DOI] [PubMed] [Google Scholar]
- 25.van Norden AG, van Uden IW, de Laat KF, et al. Cerebral microbleeds are related to subjective cognitive failures: the RUN DMC Study. Neurobiol Aging 2013;34:2225–2230. [DOI] [PubMed] [Google Scholar]