Abstract
Increasing evidence has highlighted the critical role of early life environment in shaping the future health outcomes of an individual. Moreover, recent studies have revealed that early life perturbations can affect the health of subsequent generations. Hypothesized mechanisms of multi- and transgenerational inheritance of abnormal developmental phenotypes include epigenetic misregulation in germ cells. In this review, we will focus on the available data demonstrating the ability of endocrine disrupting chemicals (EDCs), including bisphenol A (BPA), phthalates, and parabens, to alter epigenetic marks in rodents and humans. These epigenetic marks include DNA methylation, histone post-translational modifications, and non-coding RNAs. We also review the current evidence for multi- and transgenerational inheritance of abnormal developmental changes in the offspring following EDC exposure. Based on published results, we conclude that EDC exposure can alter the mouse and human epigenome, with variable tissue susceptibilities. Although increasing data suggest that exposure to EDCs is linked to transgenerational inheritance of reproductive, metabolic, or neurological phenotypes, more studies are needed to validate these observations and to elucidate further whether these developmental changes are directly associated with the relevant epigenetic alterations.
Keywords: Endocrine disrupting chemicals, bisphenol A, phthalates, parabens, epigenetics, transgenerational inheritance
1. Introduction
The Developmental Origins of Health and Disease (DOHaD) hypothesis suggests that early life experiences can influence health outcomes later in life [1]. David Barker and colleagues were among the first to demonstrate this phenomenon over 25 years ago, correlating low birth weight with an increased risk of cardiovascular and metabolic diseases during adulthood [2]. Various environmental factors can disrupt proper developmental trajectories, and endocrine disrupting chemicals (EDCs) have received considerable attention due to their ubiquity in the environment and the increased incidence of endocrine-related disorders in humans, including pregnancy complications, genital malformations (i.e. cryptorchidism and hypospadias in male infants), and cancer (i.e. breast, ovarian, prostate, testicular) [3]. EDCs are natural or synthetic compounds capable of interfering with the biosynthesis, storage, release, transport, and/or receptor binding of endogenous hormones, ultimately interfering with the proper functions of these hormones [4]. About 800 commercial chemicals are suspected to interfere with the endocrine system, but only a small fraction of these has been tested for potential adverse effects [3]. Although the precise mechanisms responsible for exposure-induced phenotypes are unknown, epigenetic mechanisms have been proposed to mediate developmental reprogramming and subsequent disease susceptibility that occurs later in life.
The fetus and neonate represent particularly vulnerable populations to EDC exposures. Early development requires precise timing of hormone action to promote proper growth of tissues and organs, and EDCs can interfere with the endogenous activities of these hormones. In addition, the enzymes involved in xenobiotic biotransformation and the processes required to eliminate these compounds are not fully developed in the fetus or neonate [5,6]. Therefore, a toxic compound can persist and accumulate, reaching levels sufficient to cause adverse effects on target organs among these populations. Finally, large-scale epigenetic reprogramming events occur at two critical time points during early development to establish totipotency in the zygote and to specify the germ cell lineage [7]. EDCs could prevent the proper erasure, reestablishment, or maintenance of epigenetic marks during these periods of development, alter the cellular epigenome, and subsequently enhance postnatal disease susceptibility. If germline epigenetic marks are disrupted, this could result in the transmission of adverse phenotypes across multiple generations.
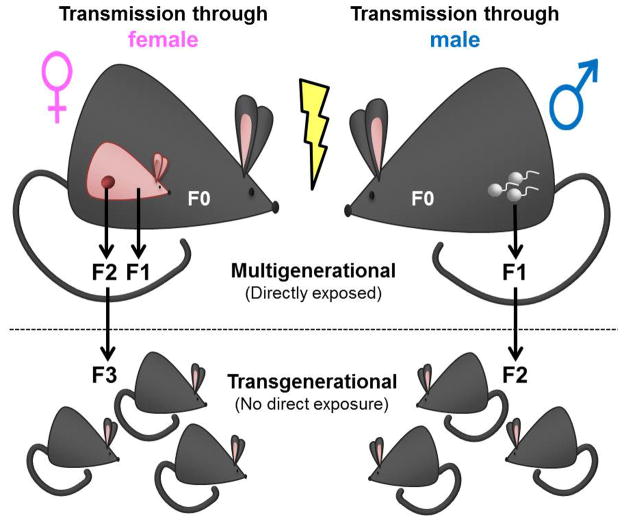
A growing research interest within the DOHaD field is the multi- and transgenerational inheritance of an abnormal phenotype. These two phenomena differ depending on whether the affected generation had direct exposure to the original stimulus. If a pregnant mother (designated as the filial [F] 0) is exposed to an adverse stimulus, her child (designated the F1) may be affected as a consequence of direct exposure to the same stimulus in utero (Figure 1). Moreover, because the germ cells of the F1 offspring are developing throughout gestation, the grandchildren (designated F2) are also directly exposed. Effects seen in the F2 generation would be considered multigenerational. In contrast, effects observed in the F3 generation that had no direct exposure to the original stimulus would be transgenerational. An important note regarding transmission of an abnormal phenotype through exposure from the mother is the presence of maternal effects (e.g., behavior or metabolic milieu), which may confound the associated epigenetic change and observed phenotype [8]. When an exposure occurs through the F0 father, transgenerational effects are observed in the F2 generation, as the only other generation directly exposed to the original stimulus is the future F1 offspring, which is exposed as a germ cell (Figure 1).
Figure 1. Multigenerational vs. transgenerational effects transmitted through the F0 female vs. F0 male.
Exposure of a pregnant F0 dam directly exposes both the F1 (exposed as developing fetus) and F2 (exposed as developing germ cells of F1) generations. The first generation to experience no direct exposure to the original stimulus (lightning bolt) from maternal exposure is the F3 generation. Paternal F0 exposure directly exposes the F1 generation only (exposed as germ cells). Effects observed in the F2 generation are, therefore, considered transgenerational.
To elicit a transgenerational phenotype, EDCs must affect the germ cell directly or indirectly by altering the function of its supporting cells. If epigenetic profiles are disrupted in the developing sperm or oocyte, the phenotypic consequences of aberrant erasure, establishment, and maintenance of epigenetic marks could be transmitted to future generations. Recent technological advances provide abundant tools to study epigenetic changes in low cell number populations such as germ cells, including single-cell technologies and modifications on chromatin immunoprecipitation (ChIP)-based methods that allow for analyses of limited starting material [9,10].
In this review, we will present possible mechanisms of transgenerational epigenetic inheritance and discuss the mechanisms of action (with an emphasis on epigenetic regulation) of three ubiquitous EDCs: bisphenol A (BPA), phthalates, and parabens. Our discussion will focus on the effects of in utero and neonatal (i.e., perinatal) exposures in rodents and identify parallels between these studies and human epidemiological findings.
2. Mechanisms of transgenerational epigenetic inheritance
How an EDC reaches an organism, or its route of delivery, greatly impacts its bioavailability. The primary routes of exposure to xenobiotic compounds in humans include oral (via ingestion), dermal, and inhalational. In animal models, oral exposure can be mimicked through dietary supplementation in the feed or manual administration by oral gavage. Of note, oral gavage is not an ideal route of delivery, as gavage has been shown to induce stress in animals and affect offspring health [11].
Prior to entering the bloodstream, EDCs that are ingested will undergo first-pass metabolism in the liver. Not all of the ingested EDCs, however, will be completely metabolized, subsequently increasing the bioavailability of the parent compound. For most EDCs, the parent (i.e. unmetabolized) compounds are the most active form, also known as the ultimate toxicant [12]. Upon entry into the bloodstream, the ultimate toxicant is now capable of reaching, and acting on, its target cell(s). If the target cell is a germ cell, epigenetic alterations that occur, and their associated phenotypes, can persist across generations. Because the detoxification machinery is still developing in the fetus and neonate, these populations are particularly susceptible to EDCs, and their germ cells represent targets of EDC-induced multi- or transgenerational effects.
EDC-induced phenotypic consequences greatly depend on the window of exposure. While we have already established that the fetus and neonate represent particularly susceptible populations, the critical time points within early development that are associated with increased disease risk later in life remain to be determined. For a transgenerational effect to occur, one critical window is germ cell development, as the inheritance of an aberrant phenotype must initially occur through the germline and subsequently propagate into developing somatic tissues. EDC exposures that disrupt epigenetic reprogramming in germ cells that do not get corrected during developmental reprogramming in subsequent generations provide a mechanism for the origin and maintenance of transgenerational effects. Therefore, assessing changes in the activity of epigenetic regulatory enzymes involved in the erasure, establishment, and maintenance of epigenetic marks will be critical to understanding the mechanistic basis of transgenerational epigenetic inheritance.
Studies have described three major epigenetic regulatory mechanisms that mediate the phenotypes associated with EDC exposure. DNA methylation is an epigenetic regulatory mechanism critical for proper development, with central roles in genome stability, X chromosome inactivation, and genomic imprinting [13]. DNA methyltransferase (DNMT) enzymes catalyze the addition of a methyl group to the cytosine base within a CpG dinucleotide context, and methylation in gene promoters is typically associated with transcriptional repression [13]. Additionally, other modifications to DNA have been described, including the conversion of methylcytosine to hydroxymethyl-, carboxyl-, and/or formylcytosine as part of a multi-step DNA demethylation process [13]. These modifications are catalyzed by the ten-eleven translocase (TET) enzymes [14]; the function of each intermediate modification, however, remains to be determined. Next, histone post-translational modifications (PTMs) can modify the chromatin landscape by altering the charge between nucleosomes and DNA, subsequently changing the accessibility of DNA to transcription factors [15]. Numerous histone writers and erasers responsible for the addition or removal of histone PTMs, respectively, have been identified, and compromised activity of these enzymes is associated with various diseases [16]. Finally, non-coding RNAs (ncRNAs) have emerged as potential regulators of transgenerational epigenetic inheritance. Long, ncRNAs may act as scaffolds to chaperone chromatin-modifying enzymes to target loci, while short, ncRNAs may prevent protein biogenesis through mRNA degradation or translation inhibition [17]. Of note, these epigenetic regulatory mechanisms do not work in isolation, but work together in a complex regulatory network [18]. Perturbations in any of the aforementioned epigenetic regulatory mechanisms are associated with increased disease risk [19], and epigenetic misregulation that occurs in germ cells provides a mechanism for transgenerational epigenetic inheritance of abnormal phenotypes.
The link between EDC exposure and altered epigenetic regulation is an ongoing topic of investigation. Ligand-activated transcription factors such as estrogen receptors (ERs) have been postulated to mediate some of the underlying molecular changes [20] (Figure 2). Because of their ability to mimic endogenous hormones, EDCs are able to bind to ERs and other hormone receptors that are expressed in target cells [21]. EDCs exert their effects through ligand-activated receptors using both genomic (i.e. nuclear) and non-genomic (i.e. signal transduction) mechanisms [22,23]. Disrupting binding of the endogenous ligand to its receptor and inappropriately activating or repressing the downstream effects of the ligand-receptor complex results in a variety of diseases including cancer, infertility, obesity, and diabetes [23,24].
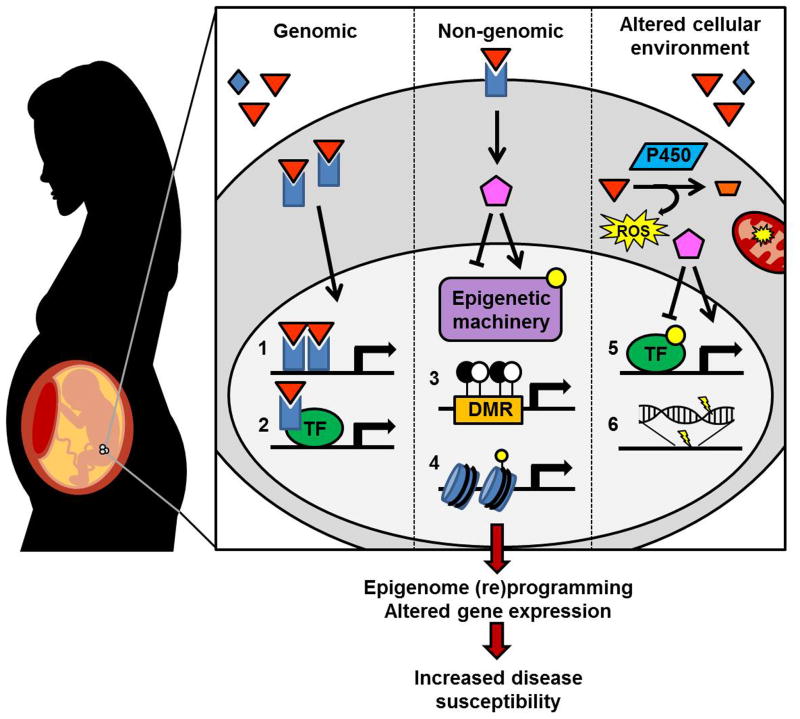
Figure 2. Mechanisms of EDC exposure-induced fetal reprogramming.
Upon exposure and delivery of an EDC (orange triangle) to the target cell, gene regulation can be disrupted via genomic and non-genomic actions of ligand-activated transcription factors (TFs) as well as altered cellular environments. Genomic actions include ligand-activated heterodimers binding hormone response elements (1) and interactions of ligand-activated TFs with other TF complexes (2) to activate or repress downstream target genes (2). Non-genomic actions include the induction of signal transduction cascades (pink pentagon) that may alter (e.g. post-translationally modify, depicted as yellow dot) epigenetic machinery involved in DNA methylation (3) or histone PTMs (4), affecting chromatin accessibility and TF binding. Finally, EDC exposure may alter the cellular environment, generating reactive oxygen species (ROS) as a consequence of EDC metabolism or altered mitochondrial function and subsequently activating a variety of signaling cascades affecting protein function (5) or directly damaging the DNA itself (6). Not only can EDCs mimic endogenous hormones, but they can also competitively inhibit and block the action of endogenous hormones (blue diamond, not shown). Ultimately, expression of coding and/or non-coding genes is altered and susceptibility to disease is enhanced. DMR: differentially methylated region; P450: cytochrome P450 enzyme.
Interestingly, ligand-activated transcription factors and epigenetic machinery appear to be regulated bidirectionally. Phosphorylation at serine (S) 10 on histone (H) 3 (H3S10) and subsequent gene transcription, for example, depends on aryl hydrocarbon receptor (AhR) signaling [25]. Additionally, non-genomic signal transduction cascades initiated by ligand binding can post-translationally modify epigenetic regulatory enzymes, enhancing or attenuating their activity [23]. Conversely, ligand-activated transcription factors themselves are subject to epigenetic regulation [26,27]. Epigenetic regulatory machinery and ligand-activated transcription factors can also act cooperatively, as epigenetic machinery can serve as a coactivator or corepressor of gene transcription [22]. One such example is the lysine (K) methyltransferase MLL2, which was identified as a coactivator for ERα [28].
Finally, EDCs may elicit their adverse effects independent of ligand-activated transcription factors. Instead, EDC exposure could alter the extra- and/or intracellular environment (Figure 2). The availability of plasma proteins that normally bind to, and prevent, EDCs from reaching their target cell can be altered following EDC exposure [29]. Additionally, the detoxification process that follows xenobiotic exposure can result in the formation of electrophiles, free radicals, nucleophiles, and redox-active reactants that can accumulate and damage the cell [30]. Although the mechanisms of EDC action are complex, they are crucial to our understanding of how adverse phenotypes manifest and to the development of intervention and/or prevention strategies.
3. BPA, phthalates, and parabens: modes and mechanisms of action
We will address physiological changes, epigenetic mechanisms of action, and transgenerational effects of BPA, phthalates, and parabens. Other EDCs will be briefly discussed at the end of the section.
3.1 BPA
BPA is an EDC found in epoxy resins and polycarbonate plastics. It is one of the most abundant chemicals manufactured globally, and the majority of the US population has detectable urinary levels of BPA [31]. A major route of human exposure is through ingestion of food and beverages containing BPA [32]. As an EDC, BPA can act as an estrogen mimic in vitro and in vivo and can bind to ERα and ERβ, although with weak binding affinities [33].
Three widely investigated BPA-induced endpoints in humans and rodents are reproductive, metabolic, and behavioral phenotypes [33]. Reproductive tissues such as the uterus and testes are targeted by BPA following perinatal exposure and result in pregnancy complications and impaired steroidogenesis, respectively [24,34]. BPA likely targets the liver and/or pancreas as well, as associations between perinatal BPA exposure and altered body composition have been observed in humans [35,36] and rodents [37]. Finally, neurological disorders associated with BPA exposure such as increased aggression and anxiety in children [38] and rodents [39] suggest that the brain is another target tissue of BPA. Additional behavioral changes reported in rodent studies include depression-like phenotypes [40] and hyperactivity [41].
Recent studies have confirmed that BPA exposure alters epigenetic regulation in rodents. Dolinoy and colleagues were among the first to demonstrate that fetal BPA exposure is capable of inducing changes in DNA methylation at the Agouti locus in mice, which correlated with shifts in offspring coat color [42]. A more recent study did not support the ability of BPA to alter offspring coat color; DNA methylation analysis, however, was not investigated in this study [43]. The disparity between the two studies may originate from animal age, parity, and/or environmental background [43]. Anderson et al. (2012) demonstrated that maternal exposure to BPA at varying doses differentially affected offspring coat color distributions, and this did not always correlate with changes in DNA methylation at the Agouti locus. This suggests that changes in DNA methylation are associated with, but not necessarily causing, changes in coat color patterning in the agouti mouse model. Because the Agouti locus is metastable and has limited developmental relevance to humans, this study served as a proof-of-principle that BPA was capable of inducing epigenetic changes. Later studies reported altered DNA methylation at genes critical for development following perinatal BPA exposure [44–46]. For example, imprinted genes play a critical role in fetal growth and development, and in utero BPA exposure was shown to disrupt DNA methylation in imprinted gene regulatory regions [45,47,48]. DNA methylation in regulatory regions of various non-imprinted genes was also affected, including altered methylation of ER-encoding Esr genes [44,49–52]. Tissue-specific changes in DNA methylation at other regions of the genome, such as repetitive elements (e.g. LINE-1), have also been demonstrated following BPA exposure [53]. Furthermore, improper silencing of repetitive elements and the adjacent genes has been suggested to be a mode of transgenerational epigenetic inheritance [54]. The altered DNA methylation profiles observed in these studies may be due to compromised expression or activity of DNMTs [47,50,55]. In addition to DNA methylation, studies have linked BPA exposure and altered histone PTMs in reproductive tissues [56,57]. Wong et al (2015) reported significantly upregulated mRNA expression of a major androgen binding protein Scgb2a1 that was linked to altered acetylation of H3K9 in the rat prostate [58]. Disruption of ncRNA expression has also been reported upon BPA exposure in human placental cells and mouse Sertoli cells [59,60]. Although these observations demonstrate strong evidence for perinatal BPA exposure altering epigenetic regulation, whether these epigenetic changes are mediated by ligand-activated transcription factors and/or directly drive the observed physiological changes remains to be determined.
Susiarjo et al. (2015) recently investigated a causal role for epigenetic misregulation in the manifestation of a multigenerational metabolic phenotype upon perinatal BPA exposure [61]. Altered DNA methylation was observed in F1 and F2 male mice at a differentially methylated region (DMR) that regulates expression of the imprinted Igf2 gene, a critical fetal growth-promoting factor with roles in metabolic homeostasis [61]. Using a genetically engineered mouse model of Igf2 overexpression, the metabolic changes observed in the BPA-exposed mice could be phenocopied, suggesting that misregulation of Igf2 is at least partially responsible for the observed metabolic phenotype in F1 and F2 male mice.
Two rodent studies have reported transgenerational effects of BPA. Wolstenholme et al. (2013) reported that exposure in the F0 pregnant mouse altered behavior up to the F4 generation, and the transgenerational phenotype was accompanied by altered expression of the Avp and Oxt genes, which encode for neuropeptides involved in social recognition [62]. No epigenetic analyses, however, were conducted in these studies. Manikkam et al. (2013) studied transgenerational outcomes in rats following exposure to a combination of BPA and phthalates and reported altered reproductive parameters through the F3 generation [63]. An obesity phenotype was also observed in the F3, but not F1 generation. Altered DNA methylation was detected in F3 sperm at the Gdnf and Ntf3 genes, which are indirectly associated with obesity [63]. However, DNA methylation changes at these obesity-associated loci were not assessed in F1 or F2 generation sperm, so it is uncertain whether these genes, or the epigenetic regulatory changes associated with them, are driving the observed obesity phenotype in F3 rats.
In these in utero exposure models, one potential mechanism for the observed transgenerational phenotype is altered epigenetic reprogramming in fetal germ cells of the F1 generation that persist into the later F2 and F3 generations. Although limited transgenerational assessments have been conducted following BPA exposure, studies examining the effects of BPA on germ cells may provide a clue as to whether these aberrant epigenetic effects can be passed across multiple generations. BPA was shown to alter DNA methylation in primordial germ cells [48] and cultured oocytes [47], suggesting that the germ cell epigenome is a target of BPA and changes in this cell population may result in adverse health effects in subsequent generations.
3.2 Phthalates
Phthalates are a family of phthalic acid diesters typically used as plasticizers to soften and increase the flexibility of polyvinyl chloride plastic products [64]. Because these compounds are not covalently bound to the products they are used in, they can leach out and contaminate food processed or stored in plastic products [65]. As EDCs, phthalates are best studied for their anti-androgenic activity [66].
Similar to BPA, investigations assessing the role of phthalate exposure have mostly focused on reproductive, metabolic, and neurological outcomes. Prenatal exposure to phthalates has been associated with testicular dysgenesis syndrome (TDS) in rodents and humans. TDS encompasses a group of reproductive disorders with possible underlying environmental causes, including genital malformations, impaired hormone production, and a predisposition to hyperplastic growth [67]. Other than germ cell cancer, perinatal exposure to EDCs in animal models has recapitulated all TDS-associated reproductive problems in humans [67]. In humans and rodents, reduced anogenital distance has been associated with phthalate exposure and serves as a predictor of poor semen quality [68,69]. Phthalate exposure is, therefore, a potential risk factor for male infertility. Additionally, because exposure to phthalates has been positively correlated with altered metabolic parameters in humans [70] and rodents [71,72], these compounds have been considered obesogenic. Finally, links between phthalate exposure and adverse neurodevelopmental outcomes have been described, including increased aggression, inattention, and delinquent behavior in children [38]. Studies in rodents have also reported behavioral changes including enhanced anxiolytic and depressed symptoms as well as altered social interactions [55,73,74].
Most studies assessing epigenetic modifications following phthalate exposures have focused on DNA methylation. Human epidemiological studies correlated higher urinary levels of phthalate metabolites with altered imprinted gene and LINE-1 methylation in placental tissue [75,76]. Rodent studies have reported altered global and locus-specific DNA methylation following di(2-ethylhexyl)phthalate (DEHP) exposure [77,78]. Additionally, DNMT and methyl-CpG binding proteins were affected in various tissue types upon DEHP exposure in rodents [73,79].
Recent in vitro studies have provided additional insight into potential mechanisms of phthalate-induced epigenetic changes. Treatment of MCF-7 human breast cancer cells with benzyl butyl phthalate (BBP) or dibutyl phthalate (DBP) resulted in DNA demethylation at the ERα promoter [80], providing a link between ligand-activated transcription factors and epigenetic changes. Interestingly, phthalates have also been shown to induce proliferation in an ER-independent manner. When ER-negative MDA-MB-231 breast cancer cells were treated with BBP or DBP, cell proliferation increased through AhR stimulation [81]. In addition to DNA methylation changes, expression and/or activity levels of several histone modifying enzymes were disrupted upon phthalate exposure in vitro [81–84]. These cell culture experiments have begun to elucidate how phthalates may affect epigenetic regulation in the cell and provide a good starting point for in vivo mechanistic studies.
Studies describing the transgenerational epigenetic effects of phthalates are limited. Following in utero exposure to DEHP, sperm count and mobility were reduced in F1 through F4 mice [85]. No epigenetic mechanisms, however, were assayed in this study. Because altered DNA methylation in oocytes of offspring prenatally exposed to DEHP were reported in an independent study, this suggests aberrant phenotypes could be transmitted across multiple generations [86]. Future studies will need to confirm the transgenerational transmission of an aberrant phenotype that results from phthalate-induced epigenetic misregulation in germ cells.
3.3 Parabens
Parabens are a family of esters of p-hydroxybenzoic acid and were first introduced in the mid-1920s as a preservative in pharmaceutical products [87]. Today, the use of parabens has expanded to cosmetics, food commodities, and industrial products, resulting in widespread exposure through multiple routes [87]. Parabens have been best studied as an ER agonist in vitro and in vivo, and their estrogenicity depends on the length of their alkyl side chains [87].
Human epidemiological studies reporting adverse effects of paraben exposure are limited. A National Health and Nutrition Examination Survey study suggested paraben exposure altered thyroid hormone levels [88], which are critical for growth, development, and metabolism [89]. Animal studies have demonstrated adverse outcomes on reproductive and neurological endpoints, reiterating the notion of tissue-specific EDC susceptibility [87]. Although fewer studies have assessed the ability of parabens to induce metabolic alterations, perinatal paraben exposure was linked to lower birth weight followed by compensatory postnatal growth in rats [90]. Because this type of postnatal growth pattern is associated with an increased susceptibility to obesity, parabens may be another obesogenic compound contributing to metabolic disease in humans [91].
A major shortcoming of the current reported paraben literature is the frequent use of supraphysiological doses to study exposure-related effects in rodent models. While some studies have reported adverse phenotypes at these high doses, not all studies have been replicable across investigators, and other studies have presented negative data [87]. Any conclusions about paraben exposures on human health at this time, therefore, are premature. A hallmark feature of EDCs is their ability to elicit low-dose effects. Therefore, more studies utilizing physiologically relevant doses of parabens will help improve the risk assessment of these compounds on human health and disease.
Studies have not yet assessed the ability of parabens to elicit multi- or transgenerational phenotypes, but evidence suggests paraben exposure can perturb epigenetic regulation in human and rodent tissues. Higher paraben levels have been associated with reduced methylation at DMR2 of IGF2 in human placental tissue [75]. In the rat, postnatal exposure to butylparaben was shown to alter DNA methylation in sperm from F0 males, but no breeding was conducted to investigate transmission of an altered phenotype [92]. In an in vitro study, Pugazhendhi et al. (2007) observed upregulation of MLL2 upon paraben treatment in MCF-7 cells [93]. While the adverse effects of paraben exposure have been less thoroughly studied relative to BPA and phthalates, the reported epigenetic changes warrant further investigation of the potential health consequences associated with paraben exposure and its underlying mechanisms of action.
3.4 Other Compounds
As mentioned before, more than 800 compounds are suspected to interfere with the endocrine system, but few have been well studied. Diethylstilbestrol (DES), a synthetic non-steroidal estrogen, was administered to women beginning in the 1940s to prevent miscarriages. In the early 1970s, the Food and Drug Administration withdrew the use of DES in pregnant women, because daughters of DES-exposed mothers presented with an increased incidence of a rare vaginal cancer [94]. Various epidemiological studies have demonstrated adverse reproductive effects and increased cancer frequencies in both daughters and sons exposed to DES in utero [95]. Interestingly, multigenerational reproductive effects have also been observed in both female and male grandchildren of DES-treated women [95]. At the molecular level, studies have reported various epigenetic modifications in both human and rodent tissues exposed to DES [95], and these effects appear to be mediated, at least in part, by ERα (Table 1) [96–98]. Studies to determine transgenerational transmission of DES-induced phenotypes in humans are ongoing, as the grandchildren of DES-exposed mothers are beginning to approach reproductive age.
Table 1.
Molecular and gross phenotypic changes associated with early life EDC exposure
| EDC | Exposure | Changes in epigenetic and/or ligand-activated transcription factor regulation; transgenerational effects (when applicable) | Ref. |
|---|---|---|---|
| DES | Perinatal | Increased Ezh2 expression (mRNA and protein) and increased H3K37me3 in F1 mouse mammary gland | [56] |
| In utero | Hypermethylation of Hoxa10 gene, increased expression of Dnmt1 and Dnmt3b genes in F1 mouse uterus | [96] | |
| Postnatal | Increased phospho-EZH2 via ER-activated PI3K/Akt pathway, reduced H3K27me3 in F1 rat uterus; ERα knockout mouse failed activate PI3K/Akt signaling cascade | [110] | |
| Vinclozolin | In utero | Increased spermatogenic cell apoptosis, reduced epididymal sperm counts and sperm mobility in F1–F4 rats, altered DNA methylation in F2 and F3 epididymal sperm | [99] |
| In utero | Altered DNA methylation at imprinted genes in F1–F3 mouse sperm | [100] | |
| In utero | Reduced AR expression, increased NF-κB activation, increased Dnmt3a and Dnmt3b expression in F1 rat prostate; reduced Dnmt1 and Dnmt3l but increased Dnmt3b in F1 rat testis | [111] | |
| In utero | Altered methylation at 24 DMRs in primordial germ cells and 14 DMRs in prospermatogonia of F3 rat embryos; no assessment of Dnmt levels | [112] | |
| Methoxychlor | In utero | Altered methylation at imprinted genes in F1 and F2 mouse sperm; not significant in F3 | [113] |
| In utero | 37 DMRs in F3 rat sperm; DMR associated genes involved in PI3K signaling system and steroid hormone biosynthesis | [102] | |
| Perinatal | Hypermethylation in Esr2 promoter, reduced Esr2 expression, increased Dnmt3b expression in F1 rat ovaries | [114] | |
| DDT | Postnatal | Decreased AR expression in testis and upregulation of PXR in F0 male mouse liver | [115] |
| TCDD | In utero | Altered methylation and expression at imprinted genes in muscle and liver; increased expression of lncRNA Airn in F1 mouse sperm | [116] |
| Perinatal | AR expression reduced in F1 rat ventral prostate | [117] | |
| In utero | 50 DMRs in promoter regions of F3 rat sperm, including Jmjd8 and Hdac3 genes | [118] | |
| Tributyltin | In utero | Increase in obesity-related parameters (i.e. increased white adipose tissue, hepatic lipid accumulation) in F1–F3 mice | [119] |
Perinatal refers to exposure in utero and after birth
In addition to DES, various pesticides (e.g., vinclozolin, dichlorodiphenyltrichloroethane [DDT], and methoxychlor) and dioxins (2,3,7,8-tetrachlorodibenzo-p-dioxin [TCDD]) have been shown to disrupt epigenetic regulation and/or expression of ligand-activated transcription factors in a multi- and transgenerational manner (Table 1). One of the first EDCs to demonstrate exposure-induced transgenerational effects was vinclozolin, an anti-androgenic fungicide [99]. Maternal exposure to vinclozolin during the time of gonadal sex determination resulted in various adverse reproductive outcomes in F1 male rats, with altered DNA methylation patterns being reported in the germline [99,100]. Whether or not these epigenetic changes were causally responsible for the observed decrease in sperm cell number and viability, as well as the increased incidence of male infertility, remains uncertain.
In a very recent publication by Iqbal et al. (2015), F0 pregnant mice were exposed to vinclozolin, BPA, or DEHP from embryonic day (E) 8.5-E12.5 or E12.5-E16.5. These stages coincide with global erasure of DNA methylation in primordial germ cells (PGCs) and sex-specific remethylation of DNA in prospermatogonia, respectively. The authors observed minimal changes in DNA methylation and allele-specific expression of imprinted genes in F1 male mice. When the authors assessed whether any of the minor changes in the F1 generation would persist into the somatic and germ cells of F2 and F3 mice, no persistent aberration of DNA methylation or allele-specific expression of imprinted genes was observed. Based on these findings, the authors concluded that the adverse effects of EDCs are corrected for during developmental reprogramming, therefore preventing transgenerational inheritance. Brieno-Enriquez et al (2015) and Stouder and Paolini-Giacobino (2011) exposed gestating mothers to similar doses of vinclozolin for greater periods, which included the exposure window in the Iqbal et al. (2015) study, that did result in transgenerational epigenetic and physiological changes in offspring [100,101]. Therefore, the window of exposure appears to be a critical factor in the manifestation of multi- and transgenerational phenotypes. The extent to which these epigenetic changes drive the observed phenotypes across generations remains to be determined.
4. Considerations of sex
When assessing multi- or transgenerational inheritance of a phenotype, it is important to examine transmission through the maternal and/or paternal lineage (i.e. through the oocyte and/or sperm). Studies have shown differences in parental germline transmission of a phenotype following EDC exposure, suggesting that female and male germ cells have different susceptibilities to environmental perturbations [99,102].
It has also become increasingly clear that phenotypes observed in EDC-exposed offspring are often sex-dependent [38,72]. Males and females may also adapt to stimuli differently in utero, suggestive of dimorphic fetal reprogramming and subsequent postnatal disease susceptibility [103]. Interestingly, one common developmental effect of EDCs is the neutralization of sexually dimorphic phenotypes [104]. There are a couple of possible explanations for these observations. First, basal levels of gonadal hormones in males and females differ, and environmental exposures that affect these hormones can differentially affect males and females [105]. For example, estrogen has been suggested to have a neuroprotective effect [106]. Alternatively, genetic effects based on differences in the complementary sex chromosome in females and males (i.e. XX and XY chromosome complements, respectively) could disproportionately affect one sex over the other. Genes disrupted on the Y chromosome would specifically affect males. Genes that escape X chromosome inactivation are expressed at greater levels in females compared to males. If these genes are affected, males, again, may be more susceptible to an adverse phenotype, as a change in expression of an X inactivation escape gene will have a proportionally greater effect on males. Notably, some X-linked genes that escape X-inactivation have epigenetic regulatory roles, including the Kdm family of histone demethylases [107]. Mouse models known as the Four Core Genotypes (FCG) mice have been utilized to distinguish whether a sexually dimorphic phenotype is due to differing gonadal hormone secretion or a genetic effect [105]. The sex-specific effects of EDC-induced phenotypes are not only intriguing, but provide additional insight into the mechanism by which these EDCs elicit their actions.
5. Concluding remarks
Our understanding of how EDCs act, and whether their effects can be transmitted across multiple generations, continues to grow. Although causal relationships between EDC exposure and disease endpoints have not been fully established, there is strong evidence to demonstrate that the ubiquitous presence of EDCs in the environment should not go unnoticed. Because EDC exposures elicit a myriad of phenotypes, it is unlikely that EDCs act through any single mechanism. In fact, EDCs elicit non-monotonic dose responses, and the varied responses at different doses may be due to different mechanisms of action.
Many EDCs require further investigation, and the effect of EDC exposure on the more vulnerable fetal and neonatal populations is ongoing. EDC-induced transgenerational epigenetic inheritance is a relatively young field, and continued work in this area will help answer several key remaining questions. First, what tissues (more specifically, cell types) are targeted by EDCs? Second, what genes are susceptible to EDC-induced epigenetic misregulation? Finally, is epigenetic misregulation causing the observed physiological changes in the F1 offspring, and is it the driving force for transgenerational inheritance of abnormal phenotypes? Incorporating genetically engineered mouse models deficient for epigenetic regulatory enzymes into exposure-based studies will help to address the necessity of specific epigenetic factors in mediating the observed phenotypes.
Importantly, many studies have only assessed single compound exposures and subsequent disease endpoints. These studies are a crucial starting point to understand the mechanism of action of each EDC. Studies assessing multi-compound exposures are also critical, as humans are rarely, if ever, exposed to any EDCs in isolation. Hotchkiss et al. (2010) demonstrated cumulative adverse effects in offspring exposed in utero to procymidone, a pesticide with androgen receptor (AR)-antagonizing activity, in combination with the phthalate DBP, an inhibitor of fetal testosterone synthesis [108]. Interventions such as diet/nutrition and/or exercise at the time of exposure or after may ameliorate the effects of EDC exposure and are worth investigating.
The concept of transgenerational inheritance greatly alters the public health perception and risk of EDCs. Not only does the health of the directly exposed generation need be considered, but the transmission of adverse health outcomes and disease susceptibility to future generations must also be taken into account. The precautionary principle suggests that preventive, anticipatory measures should be taken in the face of activities that may pose a risk to human health, even when cause-and-effect relationships have not been fully established [109]. Employing the precautionary principle in the context of EDCs may help reduce the incidence of various diseases with currently unknown etiology.
Acknowledgments
The authors have been supported by T32ES019851 (FX), K99ES022244 (MS), March of Dimes (MSB), and NIEHS ES023284 (MSB). Due to space limitations, we frequently referred to review articles and apologize to those researchers whose original work was not directly cited.
Abbreviations
- AhR
aryl hydrocarbon receptor
- BPA
bisphenol A
- DDT
dichlorodiphenyltrichloroethane
- DMR
differentially methylated region
- DES
diethylstilbestrol
- DOHaD
developmental origins of health and disease
- DNMT
DNA methyltransferase
- EDC
endocrine disrupting chemical
- ER
estrogen receptor
- F[#]
filial generation
- ncRNA
non-coding RNA
- PTM
post-translational modification
- ROS
reactive oxygen species
- TDS
testicular dysgenesis syndrome
- TET
ten-eleven translocase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dover GJ. The Barker hypothesis: how pediatricans will diagnose and prevent common adult-onset diseases. Trans Am Clin Climatol Assoc. 2009;120:199–207. [PMC free article] [PubMed] [Google Scholar]
- 2.Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–80. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 3.Bergman Å, Heindel JJ, Jobling S, Kidd KA, Zoeller RT, Jobling SKKA. State of the science of endocrine disrupting chemicals 2012: an assessment of the state of the science of endocrine disruptors prepared by a group of experts for the United Nations Environment Programme and World Health Organization. World Health Organization; 2013. [Google Scholar]
- 4.Yoon K, Kwack SJ, Kim HS, Lee B-M. Estrogenic endocrine-disrupting chemicals: molecular mechanisms of actions on putative human diseases. J Toxicol Environ Health B Crit Rev. 2014;17:127–74. doi: 10.1080/10937404.2014.882194. [DOI] [PubMed] [Google Scholar]
- 5.de Wildt SN, Kearns GL, Leeder JS, van den Anker JN. Glucuronidation in humans. Pharmacogenetic and developmental aspects. Clin Pharmacokinet. 1999;36:439–52. doi: 10.2165/00003088-199936060-00005. [DOI] [PubMed] [Google Scholar]
- 6.Choudhary D, Jansson I, Schenkman JB, Sarfarazi M, Stoilov I. Comparative expression profiling of 40 mouse cytochrome P450 genes in embryonic and adult tissues. Arch Biochem Biophys. 2003;414:91–100. doi: 10.1016/s0003-9861(03)00174-7. [DOI] [PubMed] [Google Scholar]
- 7.Saitou M, Kagiwada S, Kurimoto K. Epigenetic reprogramming in mouse pre-implantation development and primordial germ cells. Development. 2012;139:15–31. doi: 10.1242/dev.050849. [DOI] [PubMed] [Google Scholar]
- 8.Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. Cell. 2014;157:95–109. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spaethling JM, Eberwine JH. Single-cell transcriptomics for drug target discovery. Curr Opin Pharmacol. 2013;13:786–90. doi: 10.1016/j.coph.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Brind’Amour J, Liu S, Hudson M, Chen C, Karimi MM, Lorincz MC. An ultra-low-input native ChIP-seq protocol for genome-wide profiling of rare cell populations. Nat Commun. 2015;6:6033. doi: 10.1038/ncomms7033. [DOI] [PubMed] [Google Scholar]
- 11.Jensen Peña C, Monk C, Champagne FA. Epigenetic effects of prenatal stress on 11β-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS ONE. 2012;7:e39791. doi: 10.1371/journal.pone.0039791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aubert N, Ameller T, Legrand J-J. Systemic exposure to parabens: pharmacokinetics, tissue distribution, excretion balance and plasma metabolites of [14C]-methyl-, propyl- and butylparaben in rats after oral, topical or subcutaneous administration. Food Chem Toxicol. 2012;50:445–54. doi: 10.1016/j.fct.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 13.Schübeler D. Function and information content of DNA methylation. Nature. 2015;517:321–6. doi: 10.1038/nature14192. [DOI] [PubMed] [Google Scholar]
- 14.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–5. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–95. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov. 2014;13:673–91. doi: 10.1038/nrd4360. [DOI] [PubMed] [Google Scholar]
- 17.Esteller M. Non-coding RNAs in human disease. Nature Reviews Genetics. 2011;12:861–74. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 18.Robertson KD. DNA methylation and chromatin - unraveling the tangled web. Oncogene. 2002;21:5361–79. doi: 10.1038/sj.onc.1205609. [DOI] [PubMed] [Google Scholar]
- 19.Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28:1057–68. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- 20.Greathouse KL, Bredfeldt T, Everitt JI, Lin K, Berry T, Kannan K, et al. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Mol Cancer Res. 2012;10:546–57. doi: 10.1158/1541-7786.MCR-11-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swedenborg E, Rüegg J, Mäkelä S, Pongratz I. Endocrine disruptive chemicals: mechanisms of action and involvement in metabolic disorders. J Mol Endocrinol. 2009;43:1–10. doi: 10.1677/JME-08-0132. [DOI] [PubMed] [Google Scholar]
- 22.Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–42. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 23.Wong RLY, Walker CL. Molecular Pathways: Environmental Estrogens Activate Nongenomic Signaling to Developmentally Reprogram the Epigenome. Clinical Cancer Research. 2013;19:3732–7. doi: 10.1158/1078-0432.CCR-13-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, et al. Bisphenol a and reproductive health: update of experimental and human evidence, 2007–2013. Environ Health Perspect. 2014;122:775–86. doi: 10.1289/ehp.1307728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurita H, Schnekenburger M, Ovesen JL, Xia Y, Puga A. The Ah receptor recruits IKKα to its target binding motifs to phosphorylate serine-10 in histone H3 required for transcriptional activation. Toxicol Sci. 2014;139:121–32. doi: 10.1093/toxsci/kfu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X, Phillips DL, Ferguson AT, Nelson WG, Herman JG, Davidson NE. Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res. 2001;61:7025–9. [PubMed] [Google Scholar]
- 27.Ottaviano YL, Issa JP, Parl FF, Smith HS, Baylin SB, Davidson NE. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res. 1994;54:2552–5. [PubMed] [Google Scholar]
- 28.Mo R, Rao SM, Zhu Y-J. Identification of the MLL2 complex as a coactivator for estrogen receptor alpha. J Biol Chem. 2006;281:15714–20. doi: 10.1074/jbc.M513245200. [DOI] [PubMed] [Google Scholar]
- 29.Kerdivel G, Habauzit D, Pakdel F. Assessment and molecular actions of endocrine-disrupting chemicals that interfere with estrogen receptor pathways. Int J Endocrinol. 2013;2013:501851–14. doi: 10.1155/2013/501851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minamiyama Y, Ichikawa H, Takemura S, Kusunoki H, Naito Y, Yoshikawa T. Generation of reactive oxygen species in sperms of rats as an earlier marker for evaluating the toxicity of endocrine-disrupting chemicals. Free Radic Res. 2010;44:1398–406. doi: 10.3109/10715762.2010.510523. [DOI] [PubMed] [Google Scholar]
- 31.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–5. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saal vom FS, Welshons WV. Evidence that bisphenol A (BPA) can be accurately measured without contamination in human serum and urine, and that BPA causes numerous hazards from multiple routes of exposure. Molecular and Cellular Endocrinology. 2014;398:101–13. doi: 10.1016/j.mce.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee D-H, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocrine Reviews. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nanjappa MK, Simon L, Akingbemi BT. The industrial chemical bisphenol A (BPA) interferes with proliferative activity and development of steroidogenic capacity in rat Leydig cells. Biol Reprod. 2012;86:135-1-12. doi: 10.1095/biolreprod.111.095349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. Jama. 2012;308:1113–21. doi: 10.1001/2012.jama.11461. [DOI] [PubMed] [Google Scholar]
- 36.Ashley-Martin J, Dodds L, Arbuckle TE, Ettinger AS, Shapiro GD, Fisher M, et al. A birth cohort study to investigate the association between prenatal phthalate and bisphenol A exposures and fetal markers of metabolic dysfunction. Environ Health. 2014;13:84. doi: 10.1186/1476-069X-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001;109:675–80. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenfeld CS. Bisphenol A and phthalate endocrine disruption of parental and social behaviors. Front Neurosci. 2015;9:57. doi: 10.3389/fnins.2015.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan BC, Vandenbergh JG. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm Behav. 2006;50:85–93. doi: 10.1016/j.yhbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Xu X-H, Zhang J, Wang Y-M, Ye Y-P, Luo Q-Q. Perinatal exposure to bisphenol-A impairs learning-memory by concomitant down-regulation of N-methyl-D-aspartate receptors of hippocampus in male offspring mice. Horm Behav. 2010;58:326–33. doi: 10.1016/j.yhbeh.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Ishido M, Masuo Y, Kunimoto M, Oka S, Morita M. Bisphenol A causes hyperactivity in the rat concomitantly with impairment of tyrosine hydroxylase immunoreactivity. J Neurosci Res. 2004;76:423–33. doi: 10.1002/jnr.20050. [DOI] [PubMed] [Google Scholar]
- 42.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USa. 2007;104:13056–61. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenfeld CS, Sieli PT, Warzak DA, Ellersieck MR, Pennington KA, Roberts RM. Maternal exposure to bisphenol A and genistein has minimal effect on A(vy)/a offspring coat color but favors birth of agouti over nonagouti mice. Proc Natl Acad Sci USa. 2013;110:537–42. doi: 10.1073/pnas.1220230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prins GS, Tang W-Y, Belmonte J, Ho S-M. Perinatal exposure to oestradiol and bisphenol A alters the prostate epigenome and increases susceptibility to carcinogenesis. Basic Clin Pharmacol Toxicol. 2008;102:134–8. doi: 10.1111/j.1742-7843.2007.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Susiarjo M, Sasson I, Mesaros C, Bartolomei MS. Bisphenol a exposure disrupts genomic imprinting in the mouse. PLoS Genet. 2013;9:e1003401. doi: 10.1371/journal.pgen.1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bromer JG, Zhou Y, Taylor MB, Doherty L, Taylor HS. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. Faseb J. 2010;24:2273–80. doi: 10.1096/fj.09-140533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chao H-H, Zhang X-F, Chen B, Pan B, Zhang L-J, Li L, et al. Bisphenol A exposure modifies methylation of imprinted genes in mouse oocytes via the estrogen receptor signaling pathway. Histochem Cell Biol. 2012;137:249–59. doi: 10.1007/s00418-011-0894-z. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H-Q, Zhang X-F, Zhang L-J, Chao H-H, Pan B, Feng Y-M, et al. Fetal exposure to bisphenol A affects the primordial follicle formation by inhibiting the meiotic progression of oocytes. Mol Biol Rep. 2012;39:5651–7. doi: 10.1007/s11033-011-1372-3. [DOI] [PubMed] [Google Scholar]
- 49.Doshi T, Mehta SS, Dighe V, Balasinor N, Vanage G. Hypermethylation of estrogen receptor promoter region in adult testis of rats exposed neonatally to bisphenol A. Toxicology. 2011;289:74–82. doi: 10.1016/j.tox.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 50.Wolstenholme JT, Taylor JA, Shetty SRJ, Edwards M, Connelly JJ, Rissman EF. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS ONE. 2011;6:e25448. doi: 10.1371/journal.pone.0025448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho S-M, Tang W-Y, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–32. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma Y, Xia W, Wang DQ, Wan YJ, Xu B, Chen X, et al. Hepatic DNA methylation modifications in early development of rats resulting from perinatal BPA exposure contribute to insulin resistance in adulthood. Diabetologia. 2013;56:2059–67. doi: 10.1007/s00125-013-2944-7. [DOI] [PubMed] [Google Scholar]
- 53.Nahar MS, Liao C, Kannan K, Harris C, Dolinoy DC. In utero bisphenol A concentration, metabolism, and global DNA methylation across matched placenta, kidney, and liver in the human fetus. Chemosphere. 2015;124:54–60. doi: 10.1016/j.chemosphere.2014.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nature Reviews Genetics. 2012;13:153–62. doi: 10.1038/nrg3188. [DOI] [PubMed] [Google Scholar]
- 55.Xu X, Yang Y, Wang R, Wang Y, Ruan Q, Lu Y. Perinatal exposure to di-(2-ethylhexyl) phthalate affects anxiety- and depression-like behaviors in mice. Chemosphere. 2015;124:22–31. doi: 10.1016/j.chemosphere.2014.10.056. [DOI] [PubMed] [Google Scholar]
- 56.Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm Cancer. 2010;1:146–55. doi: 10.1007/s12672-010-0015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dhimolea E, Wadia PR, Murray TJ, Settles ML, Treitman JD, Sonnenschein C, et al. Prenatal Exposure to BPA Alters the Epigenome of the Rat Mammary Gland and Increases the Propensity to Neoplastic Development. PLoS ONE. 2014;9:e99800. doi: 10.1371/journal.pone.0099800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong RLY, Wang Q, Treviño LS, Bosland MC, Chen J, Medvedovic M, et al. Identification of secretaglobin Scgb2a1 as a target for developmental reprogramming by BPA in the rat prostate. Epigenetics. 2015;10:127–34. doi: 10.1080/15592294.2015.1009768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho H, Kim SJ, Park H-W, Oh M-J, Yu SY, Lee SY, et al. A relationship between miRNA and gene expression in the mouse Sertoli cell line after exposure to bisphenol A. BioChip J. 2010;4:75–81. [Google Scholar]
- 60.Avissar-Whiting M, Veiga KR, Uhl KM, Maccani MA, Gagne LA, Moen EL, et al. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod Toxicol. 2010;29:401–6. doi: 10.1016/j.reprotox.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Susiarjo M, Xin F, Bansal A, Stefaniak M, Li C, Simmons RA, et al. Bisphenol A exposure disrupts metabolic health across multiple generations in the mouse. Endocrinology. 2015:en20142027. doi: 10.1210/en.2014-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolstenholme JT, Goldsby JA, Rissman EF. Transgenerational effects of prenatal bisphenol A on social recognition. Horm Behav. 2013;64:833–9. doi: 10.1016/j.yhbeh.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS ONE. 2013;8:e55387. doi: 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koch HM, Preuss R, Angerer J. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure-- an update and latest results. Int J Androl. 2006;29:155–65. doi: 10.1111/j.1365-2605.2005.00607.x. –discussion181–5. [DOI] [PubMed] [Google Scholar]
- 65.Bosnir J, Puntarić D, Skes I, Klarić M, Simić S, Zorić I. Migration of phthalates from plastic products to model solutions. Coll Antropol. 2003;27(Suppl 1):23. [PubMed] [Google Scholar]
- 66.Fisher JS. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–15. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- 67.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–8. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 68.Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–61. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mendiola J, Stahlhut RW, Jørgensen N, Liu F, Swan SH. Shorter anogenital distance predicts poorer semen quality in young men in Rochester, New York. Environ Health Perspect. 2011;119:958–63. doi: 10.1289/ehp.1103421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect. 2007;115:876–82. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rajesh P, Balasubramanian K. Phthalate exposure in utero causes epigenetic changes and impairs insulin signalling. J Endocrinol. 2014;223:47–66. doi: 10.1530/JOE-14-0111. [DOI] [PubMed] [Google Scholar]
- 72.Hao C, Cheng X, Xia H, Ma X. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci Rep. 2012;32:619–29. doi: 10.1042/BSR20120042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Betz A, Jayatilaka S, Joshi J, Ramanan S, Debartolo D, Pylypiw H, et al. Chronic exposure to benzyl butyl phthalate (BBP) alters social interaction and fear conditioning in male adult rats: alterations in amygdalar MeCP2, ERK1/2 and ERα. Neuro Endocrinol Lett. 2013;34:347–58. [PubMed] [Google Scholar]
- 74.Wang D-C, Chen T-J, Lin M-L, Jhong Y-C, Chen S-C. Exercise prevents the increased anxiety-like behavior in lactational di-(2-ethylhexyl) phthalate-exposed female rats in late adolescence by improving the regulation of hypothalamus-pituitary-adrenal axis. Horm Behav. 2014;66:674–84. doi: 10.1016/j.yhbeh.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 75.LaRocca J, Binder AM, McElrath TF, Michels KB. The impact of first trimester phthalate and phenol exposure on IGF2/H19 genomic imprinting and birth outcomes. Environ Res. 2014;133:396–406. doi: 10.1016/j.envres.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao Y, Shi H-J, Xie C-M, Chen J, Laue H, Zhang Y-H. Prenatal phthalate exposure, infant growth, and global DNA methylation of human placenta. Environ Mol Mutagen. 2014;56 doi: 10.1002/em.21916. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 77.Martinez-Arguelles DB, Papadopoulos V. Identification of hot spots of DNA methylation in the adult male adrenal in response to in utero exposure to the ubiquitous endocrine disruptor plasticizer di-(2-ethylhexyl) phthalate. Endocrinology. 2015;156:124–33. doi: 10.1210/en.2014-1436. [DOI] [PubMed] [Google Scholar]
- 78.Kostka G, Urbanek-Olejnik K, Wiadrowska B. Di-butyl phthalate-induced hypomethylation of the c-myc gene in rat liver. Toxicol Ind Health. 2010;26:407–16. doi: 10.1177/0748233710369124. [DOI] [PubMed] [Google Scholar]
- 79.Wu S, Zhu J, Li Y, Lin T, Gan L, Yuan X, et al. Dynamic effect of di-2-(ethylhexyl) phthalate on testicular toxicity: epigenetic changes and their impact on gene expression. Int J Toxicol. 2010;29:193–200. doi: 10.1177/1091581809355488. [DOI] [PubMed] [Google Scholar]
- 80.Kang SC, Lee B-M. DNA methylation of estrogen receptor alpha gene by phthalates. J Toxicol Environ Health Part A. 2005;68:1995–2003. doi: 10.1080/15287390491008913. [DOI] [PubMed] [Google Scholar]
- 81.Hsieh T-H, Tsai C-F, Hsu C-Y, Kuo P-L, Lee J-N, Chai C-Y, et al. Phthalates induce proliferation and invasiveness of estrogen receptor-negative breast cancer through the AhR/HDAC6/c-Myc signaling pathway. Faseb J. 2012;26:778–87. doi: 10.1096/fj.11-191742. [DOI] [PubMed] [Google Scholar]
- 82.Hsieh T-H, Tsai C-F, Hsu C-Y, Kuo P-L, Lee J-N, Chai C-Y, et al. Phthalates stimulate the epithelial to mesenchymal transition through an HDAC6-dependent mechanism in human breast epithelial stem cells. Toxicol Sci. 2012;128:365–76. doi: 10.1093/toxsci/kfs163. [DOI] [PubMed] [Google Scholar]
- 83.Guida N, Laudati G, Galgani M, Santopaolo M, Montuori P, Triassi M, et al. Histone deacetylase 4 promotes ubiquitin-dependent proteasomal degradation of Sp3 in SH-SY5Y cells treated with di(2-ethylhexyl)phthalate (DEHP), determining neuronal death. Toxicol Appl Pharmacol. 2014;280:190–8. doi: 10.1016/j.taap.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 84.Kuo C-H, Hsieh C-C, Kuo H-F, Huang M-Y, Yang S-N, Chen L-C, et al. Phthalates suppress type I interferon in human plasmacytoid dendritic cells via epigenetic regulation. Allergy. 2013;68:870–9. doi: 10.1111/all.12162. [DOI] [PubMed] [Google Scholar]
- 85.Doyle TJ, Bowman JL, Windell VL, McLean DJ, Kim KH. Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol Reprod. 2013;88:112–2. doi: 10.1095/biolreprod.112.106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li L, Zhang T, Qin X-S, Ge W, Ma H-G, Sun L-L, et al. Exposure to diethylhexyl phthalate (DEHP) results in a heritable modification of imprint genes DNA methylation in mouse oocytes. Mol Biol Rep. 2014;41:1227–35. doi: 10.1007/s11033-013-2967-7. [DOI] [PubMed] [Google Scholar]
- 87.Błędzka D, Gromadzińska J, Wąsowicz W. Parabens. From environmental studies to human health. Environ Int. 2014;67:27–42. doi: 10.1016/j.envint.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 88.Koeppe ES, Ferguson KK, Colacino JA, Meeker JD. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007–2008. Sci Total Environ. 2013;445–446:299–305. doi: 10.1016/j.scitotenv.2012.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nandi-Munshi D, Taplin CE. Thyroid-Related Neurological Disorders and Complications in Children. Pediatr Neurol. 2015;52:373–82. doi: 10.1016/j.pediatrneurol.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 90.Zhang L, Dong L, Ding S, Qiao P, Wang C, Zhang M, et al. Effects of n-butylparaben on steroidogenesis and spermatogenesis through changed E2 levels in male rat offspring. Environ Toxicol Pharmacol. 2014;37:705–17. doi: 10.1016/j.etap.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 91.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 92.Park CJ, Nah WH, Lee JE, Oh YS, Gye MC. Butyl paraben-induced changes in DNA methylation in rat epididymal spermatozoa. Andrologia. 2012;44(Suppl 1):187–93. doi: 10.1111/j.1439-0272.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 93.Pugazhendhi D, Sadler AJ, Darbre PD. Comparison of the global gene expression profiles produced by methylparaben, n-butylparaben and 17beta-oestradiol in MCF7 human breast cancer cells. J Appl Toxicol. 2007;27:67–77. doi: 10.1002/jat.1200. [DOI] [PubMed] [Google Scholar]
- 94.Herbst AL, Ulfelder H, Poskanzer DC, Longo LD. Adenocarcinoma of the vagina. Vol. 181. Association of maternal stilbestrol therapy with tumor appearance in young women; 1971. 1999. [DOI] [PubMed] [Google Scholar]
- 95.Hilakivi-Clarke L. Maternal exposure to diethylstilbestrol during pregnancy and increased breast cancer risk in daughters. Breast Cancer Res. 2014;16:208. doi: 10.1186/bcr3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bromer JG, Wu J, Zhou Y, Taylor HS. Hypermethylation of homeobox A10 by in utero diethylstilbestrol exposure: an epigenetic mechanism for altered developmental programming. Endocrinology. 2009;150:3376–82. doi: 10.1210/en.2009-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Couse JF, Dixon D, Yates M, Moore AB, Ma L, Maas R, et al. Estrogen receptor-alpha knockout mice exhibit resistance to the developmental effects of neonatal diethylstilbestrol exposure on the female reproductive tract. Developmental Biology. 2001;238:224–38. doi: 10.1006/dbio.2001.0413. [DOI] [PubMed] [Google Scholar]
- 98.Li Y, Hamilton KJ, Lai AY, Burns KA, Li L, Wade PA, et al. Diethylstilbestrol (DES)-stimulated hormonal toxicity is mediated by ERα alteration of target gene methylation patterns and epigenetic modifiers (DNMT3A, MBD2, and HDAC2) in the mouse seminal vesicle. Environ Health Perspect. 2014;122:262–8. doi: 10.1289/ehp.1307351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–9. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stouder C, Paoloni-Giacobino A. Transgenerational effects of the endocrine disruptor vinclozolin on the methylation pattern of imprinted genes in the mouse sperm. Reproduction. 2010;139:373–9. doi: 10.1530/REP-09-0340. [DOI] [PubMed] [Google Scholar]
- 101.Brieño-Enríquez MA, García-López J, Cárdenas DB, Guibert S, Cleroux E, Děd L, et al. Exposure to Endocrine Disruptor Induces Transgenerational Epigenetic Deregulation of MicroRNAs in Primordial Germ Cells. PLoS ONE. 2015;10:e0124296. doi: 10.1371/journal.pone.0124296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Manikkam M, Haque MM, Guerrero-Bosagna C, Nilsson EE, Skinner MK. Pesticide methoxychlor promotes the epigenetic transgenerational inheritance of adult-onset disease through the female germline. PLoS ONE. 2014;9:e102091. doi: 10.1371/journal.pone.0102091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clifton VL. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(Suppl):S33–9. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 104.Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147:3681–91. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- 105.Arnold AP. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J Neuroendocrinol. 2009;21:377–86. doi: 10.1111/j.1365-2826.2009.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alonso A, Gonzalez C. Neuroprotective role of estrogens: relationship with insulin/IGF-1 signaling. Front Biosci (Elite Ed) 2012;4:607–19. doi: 10.2741/403. [DOI] [PubMed] [Google Scholar]
- 107.Berletch JB, Yang F, Disteche CM. Escape from X inactivation in mice and humans. Genome Biology. 2010;11:213. doi: 10.1186/gb-2010-11-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hotchkiss AK, Rider CV, Furr J, Howdeshell KL, Blystone CR, Wilson VS, et al. In utero exposure to an AR antagonist plus an inhibitor of fetal testosterone synthesis induces cumulative effects on F1 male rats. Reprod Toxicol. 2010;30:261–70. doi: 10.1016/j.reprotox.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Friis R. Essentials of Environmental Health. Jones & Bartlett Publishers; 2011. [Google Scholar]
- 110.Bredfeldt TG, Greathouse KL, Safe SH, Hung M-C, Bedford MT, Walker CL. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol Endocrinol. 2010;24:993–1006. doi: 10.1210/me.2009-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cowin PA, Gold E, Aleksova J, O’Bryan MK, Foster PMD, Scott HS, et al. Vinclozolin exposure in utero induces postpubertal prostatitis and reduces sperm production via a reversible hormone-regulated mechanism. Endocrinology. 2010;151:783–92. doi: 10.1210/en.2009-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Skinner MK, Guerrero-Bosagna C, Haque M, Nilsson E, Bhandari R, McCarrey JR. Environmentally induced transgenerational epigenetic reprogramming of primordial germ cells and the subsequent germ line. PLoS ONE. 2013;8:e66318. doi: 10.1371/journal.pone.0066318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stouder C, Paoloni-Giacobino A. Specific transgenerational imprinting effects of the endocrine disruptor methoxychlor on male gametes. Reproduction. 2011;141:207–16. doi: 10.1530/REP-10-0400. [DOI] [PubMed] [Google Scholar]
- 114.Zama AM, Uzumcu M. Fetal and neonatal exposure to the endocrine disruptor methoxychlor causes epigenetic alterations in adult ovarian genes. Endocrinology. 2009;150:4681–91. doi: 10.1210/en.2009-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chaturvedi NK, Kumar S, Negi S, Tyagi RK. Endocrine disruptors provoke differential modulatory responses on androgen receptor and pregnane and xenobiotic receptor: potential implications in metabolic disorders. Mol Cell Biochem. 2010;345:291–308. doi: 10.1007/s11010-010-0583-6. [DOI] [PubMed] [Google Scholar]
- 116.Somm E, Stouder C, Paoloni-Giacobino A. Effect of developmental dioxin exposure on methylation and expression of specific imprinted genes in mice. Reprod Toxicol. 2013;35:150–5. doi: 10.1016/j.reprotox.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 117.Ohsako S, Miyabara Y, Nishimura N, Kurosawa S, Sakaue M, Ishimura R, et al. Maternal exposure to a low dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) suppressed the development of reproductive organs of male rats: dose-dependent increase of mRNA levels of 5alpha-reductase type 2 in contrast to decrease of androgen receptor in the pubertal ventral prostate. Toxicol Sci. 2001;60:132–43. doi: 10.1093/toxsci/60.1.132. [DOI] [PubMed] [Google Scholar]
- 118.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations. PLoS ONE. 2012;7:e46249. doi: 10.1371/journal.pone.0046249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chamorro-García R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environ Health Perspect. 2013;121:359–66. doi: 10.1289/ehp.1205701. [DOI] [PMC free article] [PubMed] [Google Scholar]