Abstract
The phenotypic and genetic similarities between Xiphophorus and human melanoma render Xiphophorus a useful animal model for studying the genetic basis of melanoma etiology. In the Xiphophorus model, melanoma has been shown to be inducible by ultraviolet light (UVB) exposure among interspecies hybrids, but not in parental line fish similarly treated. This leads to questions of what genes are responsive to UVB exposure in the skin of the interspecies hybrids, as well as how parental alleles in hybrids may be differentially regulated and the potential roles they may play in induced melanomagenesis. To address these questions, we produced X. maculatus Jp 163 B × X. couchianus (Sp-Couch) F1 hybrid fish, exposed both hybrid and parental fish to UVB, and performed gene expression profiling of the skin using RNA-Seq methodology. We characterized a group of unique UVB-responsive genes in Sp-Couch hybrid including dct, pmela, tyr, tyrp1a, slc2a11b, rab38a, rab27, tspan10, slc45a2, oca2, slc24a5, ptn and mitfa. These genes are associated with melanin production and melanocyte proliferation. They were also up-regulated in Sp-Couch hybrid, indicating their UVB response is hybridization initiated. In the hybrid, several melanin production and pigmentation related genes, including slc45a2, tspan10, dct, slc2a11b and ptn showed either X. couchianus or X. maculatus allele specific expression. The finding that these genes exhibit allele specific expression regulatory mechanisms in Sp-Couch hybrids, but do not exhibit a corresponding UVB response in either one of the parental fishes, may suggest UVB targets and imply mechanisms regarding the susceptibility of Sp-Couch to induced melanomagenesis.
Keywords: Ultraviolet light, UVB, RNA-Seq, Differential gene expression, melanoma, Allele specific gene expression, hybrid, pigment
Introduction
The most studied Xiphophorus melanoma model is the one originally introduced by Myron Gordon and Kurt Kosswig in the late 1920’s (Gordon, 1927; Häussler, 1928; Kosswig, 1928). This model, often referred to as the Gordon-Kosswig melanoma model, employs X. maculatus and X. hellerii interspecies hybrids to produce melanoma in 25% of backcross progeny (Gordon, 1927; Häussler, 1928; Kosswig, 1928). The Gordon-Kosswig model led to the discovery of the xmrk oncogene, which is a mutant copy of the fish orthologue of the human Epidermal Growth Factor Receptor (EGFR; Weis and Schartl, 1998). Recently, the X. maculatus derived xmrk oncogene, driven by the pigment cell specific mitf promoter, has been used to produce a transgenic medaka (Japanese rice fish, Oryzias latipes) that develop aggressive melanoma within days of hatching with 100% penetrance (Schartl et al., 2010; Schartl et al., 2012). These transgenic Medaka models serve as experimentally tractable melanoma models and are currently being developed for small molecule screening regimen.
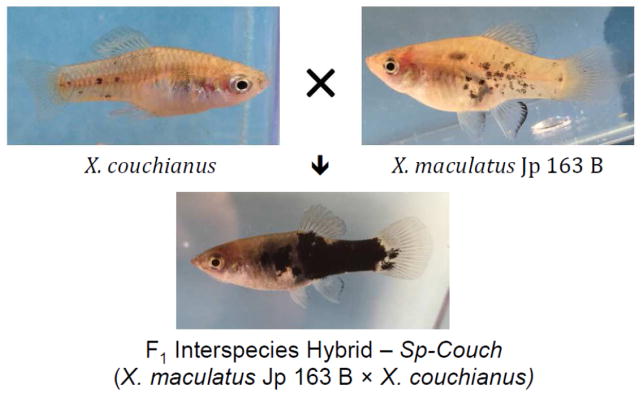
In contrast to the Gordon-Kosswig melanoma model, certain hybrids between other Xiphophorus species do not develop malignant melanoma spontaneously but have been shown to produce backcross progeny that are susceptible to melanoma induction after exposure to ultraviolet light (UV; Setlow et al., 1989; Setlow et al., 1993; Nairn et al., 1996a,b; Mitchell et. al, 2010) or treatment with known mutagens such as N-methyl-N-nitrosourera (MNU; Kaziains et al, 2001; Rahn et al., 2009). For example, a particularly well-utilized model of UVB inducible melanoma involves the backcross [X. maculatus JP 163 B × X. couchianus] × X. couchianus (termed the Sp-Couch hybrid) (Setlow et al., 1993). The Sp-Couch backcross occurs between two parental species that are quite different in regard to pigment patterns. The X. maculatus Jp 163 B carries both xmrk and the Spot Side (termed Sp) pigment pattern consisting of black macromelanophore spots distributed on the flanks of the animals (Figure 1), while X. couchianus lacks macromelanophore spots (for reviews see: Patton et al., 2011; Walter and Kazianis et al., 2001). Upon interspecies crossing, the X. maculatus JP 163 B × X. couchianus F1 progeny show a greatly expanded macromelanophore (black) pigmentation (Figure 1) that may become even further enhanced in select backcross hybrids. If Sp-Couch backcross progeny are exposed to UVB at six days post birth, about 30 to 40% of the heavily pigmented interspecies hybrids may develop melanoma from 6–9 months later (Nairn et al., 1996b; Mitchell et al., 2010). Association between sunlight exposure and melanoma has been well established in humans (Armstrong and Kricker, 2001) and this Xiphophorus UVB inducible melanoma model has been employed in attempts to define the specific wavelengths that may be responsible for sunlight induced melanomagenesis (Setlow et al., 1993; Wood et al, 2006; Mitchell et al., 2010). However, there remains controversy on the mechanism of melanoma induction in this model system. Evidence has been reported showing both direct DNA damage by UVB (Mitchell et al., 2004; Mitchell et al., 2010), and indirect cellular damage from oxidative radical production by photosensitizers (e.g., melanin) may be involved in triggering melanoma induction (Setlow et al., 1993; Wood et al., 2006).
Figure 1.
X. maculatus, X. couchianus and Sp-Couch pigmentation pattern
UV radiation is composed of UVA (320–400nM), UVB (290–320nM) and UVC (200–290nM; Luther et al., 2000). Although UVC is highly mutagenic, it is completely absorbed by atmospheric ozone and does not reach the earth surface (Luther et al., 2000). Both UVA and UVB are genotoxic. While UVB is considered a direct cause of mutation due to DNA damage, UVA may produce genotoxicity through indirect mechanisms involving production of reactive oxygen species (ROS; Ananthaswamy and Pierceall, 1990; Kanofsky and Sima, 1991; de Laat et al., 1996; de Gruijl and van der Leun, 2002).
In most studies to date the biology and molecular genetic capabilities of the organism being used to model tumor induction have not been as deeply addressed as have the photochemical properties of the light source being applied to induce tumors. However, contemporary RNA-Seq methods allow one to perform global assessment of alterations in the molecular genetic profiles of cells, tissues and organs after exposure to varied light sources. Accordingly, we have initiated RNA-Seq studies of skin from parental Xiphophorus strains utilized in crosses that produce melanoma susceptible experimental genotypes after exposure to various light types and sources (Walter et al., 2014; Yang et al., 2014; and other papers this journal edition). Here we document novel results defining UVB regulated expression signatures and showing patterns of allele specific gene expression in the skin of Sp-Couch F1 interspecies hybrids after exposure to UVB light.
Materials and Methods
Animal model
The X. maculatus Jp 163 B, X. couchianus and Sp-Couch hybrids used in this study were supplied by the Xiphophorus Genetic Stock Center (Figure 1. For contact information see: http://www.xiphophorus.txstate.edu/). All fish used in this study were 9-month-old sibling males. X. maculatus Jp 163 B used in this study were in the 101st generation of brother-sister inbreeding (pedigree JP163B 101.c); X. couchianus used in this study were from the 77th inbred generation (Xc.77b). The parents of the Sp-Couch F1 hybrids were from the 100th generation X. maculatus (female; pedigree JP163B 100.c) and 76th inbred generation of X. couchianus (male; pedigree Xc 76d).
UVB exposure
Two fish were used for each experiment and control condition. UVB exposures were as detailed previously (Yang et al., 2014). Briefly, 9-month-old adult male fish were kept in dark for 12 hours prior to UVB exposure. Immediately before exposure to UVB the fish were transferred to UV-transparent cuvettes containing 95 ml of water. The cuvettes were placed between two banks of two (4 lights total) unfiltered narrow spectrum UVB lamps (Philips UVB TL 20W/01 RS SLV) emitting principally 311 nm light that were mounted horizontally inside of a wooden exposure box. Flounce was determined to be 12.2 J/m2/s on each side of the chamber as measured by an IL-1400A Radiometer/photometer coupled to a SEL 240/UVB detector containing a 280 nm Sharp Cutoff Filter (International Light, Newburyport, MA). Fish were then exposed in duplicate to either 0 kJ/m2 or 8 kJ/m2 of UVB by varying the amount of time in the exposure box. After exposure, the fish were returned to dark for 6 hours, to allow time for modulation of gene expression, prior to being sacrificed for dissection and RNA isolation.
RNA isolation and RNA sequencing
Total RNA was isolated as previously detailed (Walter et al., 2014; Yang et al.. 2014) using TriReagent (Sigma Inc., St. Louis, MO, USA). Skin from each individual fish was homogenized in Tri-reagent followed by addition of 200 μl chloroform and the samples vigorously shaken and subjected to centrifugation at 12,000 g for 15min at 4 °c. Total RNA was further purified using RNeasy mini RNA isolation kit (Qiagen, Valencia, CA, USA). Residual DNA was eliminated by performing on column DNase digestion at 37°C for 30 min. RNA integrity was assessed by gel electrophoresis (2% agarose in TAE running buffer) and total RNA concentration was determined using a spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). RNA quality was verified on an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA) to confirm that RIN scores were above 8 prior to sequencing.
RNA sequencing was performed upon libraries constructed using the Illumina TrueSeq library preparation system that employs a polyA selection. RNA libraries were sequenced as 100bp pair-end fragments using Illumina Hi-Seq 2000 system (Illumina, Inc., San Diego, CA, USA). 70–80 million raw reads were generated for each fish (Table 1). Raw reads were filtered and trimmed based on quality scores by using a custom filtration algorithm that removed low-scoring sections of each read and preserved the longest remaining fragment (Garcia et al., 2012). All raw reads were subsequently truncated by similarity to library adaptor sequences using a custom Perl script (Table 1).
Table 1.
RNA-Seq result statistics
| X. couchianus | X. couchianus | X. maculatus | X. maculatus | SP-Couch | SP-Couch | |
|---|---|---|---|---|---|---|
| control | UVB | control | UVB | control | UVB | |
| raw read counts (in million) | 79.3 | 67.7 | 81.8 | 77.5 | 81.6 | 72.0 |
| filtered read counts (in million) | 39.6 | 36.3 | 38.6 | 44.1 | 38.5 | 37.9 |
| raw total length (in billion bp) | 7.9 | 6.8 | 8.2 | 7.7 | 8.2 | 7.2 |
| filtered total length ((in billion bp) | 3.4 | 3.0 | 3.3 | 3.7 | 3.3 | 3.2 |
| Transcriptome Coverage | 73X | 66X | 71X | 81X | 71X | 69X |
Differentially expressed genes (DEGs) analysis
The trimmed and filtered reads were mapped to X. maculatus genome (Xmac_JpA.ens70_genome.fa) from Ensemble using Tophat2 (Kim et al., 2013). Mapped reads were quantified as raw counts in each file by the Subreads package function “featureCounts” and then are converted to Count Per Million (cpm) for each sample (Liao et al., 2013; Liao et al., 2014). Genes with cpm values of no more than 4 in all samples were discarded as low counts transcripts. Differentially expressed genes were analyzed using fisher exact test by R-Bioconductor package edgeR (Robinson et al., 2010). For a gene to be a Differentially Expressed Genes (DEGs), it has to alter at least 2 fold with False Discovery Rate (FDR) less than 0.05 (Log2FC ≥ 1 or Log2FC ≤ −1, FDR < 0.05). Since the transcriptome available on Ensembl does not contain xmrk sequence, we aligned short read to xmrk sequence independently and used the library size to normalize the xmrk read counts in different samples. Xmrk differential expression was also tested by edgeR (Robinson et al., 2010). For data visualization, the normalized gene expression value in the format of cpm was plotted by R package gplots.
Allele specific expressed (ASE) gene analysis
Herein, we defined ASE genes in the Sp-Couch hybrid skin as those genes that exhibit parental allele expression ratios that deviate from 1:1. We used single nucleotide polymorphisms (SNP) and other sequence differences between the two parental alleles to identify transcripts as being derived from different parental alleles. To compare the gene expression from each parental allele, an artificial transcriptome was constructed. X. maculatus and X. couchianus cDNAs were aligned to each other using Blastn (-evalue 1e-6, -best_hit_score_edge 0.1, -best_hit_overhang 0.1, -num_alignments 1, -max_hsps 1, Shen et al., 2013). Reciprocal-best-hit sequences were kept and gene IDs carried over using a customized Perl script to construct the transcriptome containing both parental allele sequences. Short reads were mapped to the artificial transcriptomes using Bowtie2 (Langmead et al., 2009). Customized Perl script was used to retrieve and quantify the short reads that only aligned to one of the parental alleles. Quantified allele expression values were normalized to the total reads number of each allele and differential expression between two alleles was tested using edgeR (Robinson et al., 2010). Relative Log2Fold Change (Log2FC) was used to convert to allele frequency:
A gene is determined as X. maculatus allele specific If the X. maculatus allele frequency is over 60%, or X. couchianus allele specific if the X. maculatus allele frequency is below 40%.
Gene Ontology analysis
Xiphophorus Ensembl gene IDs were converted to human homolog gene Ensembl gene IDs and gene symbol using R-Bioconductor package BiomaRt. Gene Ontology (GO) term of each gene was retrieved from human genome database (org.Hs.eg.db). All genes that have a GO term were collected as background genes. Over-represented GO terms were tested using hypergeomatric test by R-Bioconductor package GOstat (ontology = Biological Process, p value cutoff = 0.001; Beissbarth and Speed, 2004).
Quantitative real time PCR
Allele specific expression was verified for selected genes by qualitative RT-PCR analysis as described before (Shen et al., 2013, Walter et al., 2014). QRT-PCR was performed by SYBR Green-based detection with an Applied Biosystems 7500Fast system (Applied Bioscience, Carlsbad, CA, USA). Initially, each set of designed primers was tested for allele specificity in a 20 μL reaction consisting of 1 μL of cDNA, 0.5 μM of each primer, and 10 μL SYBR Green ready mix. Each reaction was subjected to 40 cycles each at 95 °C for 20 s, 95 °C for 15 s, and 60 °C for 30 s, before being subjected to melting curve analysis. The 18S gene was selected for normalization of all samples. The mean CT values from triplicate runs were used to calculate relative expression levels. The allele specific primers were then used to determine efficiency following the same procedure outlined above but using a series of dilutions of cDNA to establish a standard curve (100 ng, 10 ng, 1 ng, and 0.1 ng, respectively). Only primer sets with an efficiency percentage of ±10% of one another were utilized. Once the primer efficiency and allele specificity were established, the primers were used to test relative expression of each allele in X. maculatus, X. couchianus, Sp-Couch under control condition and UVB exposed Sp-Couch hybrid. Primer sequences and real time PCR results are presented in Supplementary Table 4.
Results and Discussion
Unique UVB-responsive genes in the Sp-Couch hybrid
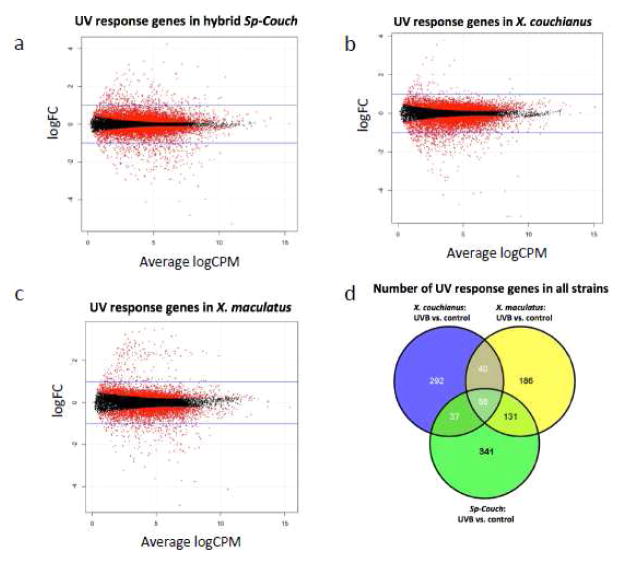
To characterize how skin from X. maculatus and X. couchianus interspecies hybrids (i.e., Sp-Couch) respond to UVB exposure, Sp-Couch hybrids were exposed to 8 kJ/m2 UVB and the skin mRNA was used for gene expression profiling analysis. Compared to unexposed controls of Sp-Couch skin samples, we identified 564 genes altered (298 down-regulated; 266 up-regulated) in response to 8 kJ/m2 UVB (Figure 2a).
Figure 2. Differentially expressed genes in response to UVB in the Sp-Couch hybrid, X. couchianus and X. maculatus.
Sp-Couch hybrid, X. couchianus and X. maculatus were exposed to UVB and differentially expressed genes were analyzed in skin. (a) 564 genes differentially expressed in Sp-Couch hybrid; (b) 425 genes differentially expressed in X. couchianus; (c) 413 differentially expressed genes are identified in X. maculatus. (d) Venn diagram shows 56 UVB-responsive genes common in all three fish types (both parental species and the F1 hybrid). The Sp-Couch hybrid shares more UVB-responsive genes with X. maculatus, than to X. couchianus..
We sought to assess whether these UVB-responsive genes in the Sp-Couch hybrid skin exhibit a conserved response in parental fish or are uniquely altered in the hybrid fish. To characterize the gene expression of each parental fish strain in response to UVB exposure X. maculatus and X. couchianus were also exposed to 8 kJ/m2 UVB and the skin mRNA was used for gene expression profiling analysis. Compared to unexposed controls, 425 DEGs were identified in X. couchianus (356 down-regulated; 69 up-regulated), while 413 genes were differentially expressed in X. maculatus (217 down-regulated; 196 up-regulated), (Figure 2b,c Supplement Table 1). Although Sp-Couch shares UVB-responsive genes with each parental fish, there are 341 unique UVB-response genes in the hybrid genetic background, indicating there is extended UVB response in the hybrid compared to either parental fish strains (Figure 2d).
Functional enrichment analysis of UVB-responsive genes for all 3 different fish types (i.e., parental and the hybrid) showed that melanin and pigment related categories are only present in UVB exposed Sp-Couch (Supplement Figure 2a,b). The genes that belong to these functional categories include canonical melanocyte pathway genes mc1r, mitf1, tyr, tyrp1a and dct. Mc1r, mitf1, tyr, tyrp1a and dct are all consistently up-regulated, indicating that UVB exposure positively induces the melanocyte-signaling pathway only in the Sp-Couch hybrids. Melanin, the product of these activities of the encoded genes, acts as a photosensitizer and could induce pre-mutagenic DNA lesions that may account for the susceptibility of melanoma in Sp-Couch hybrid.
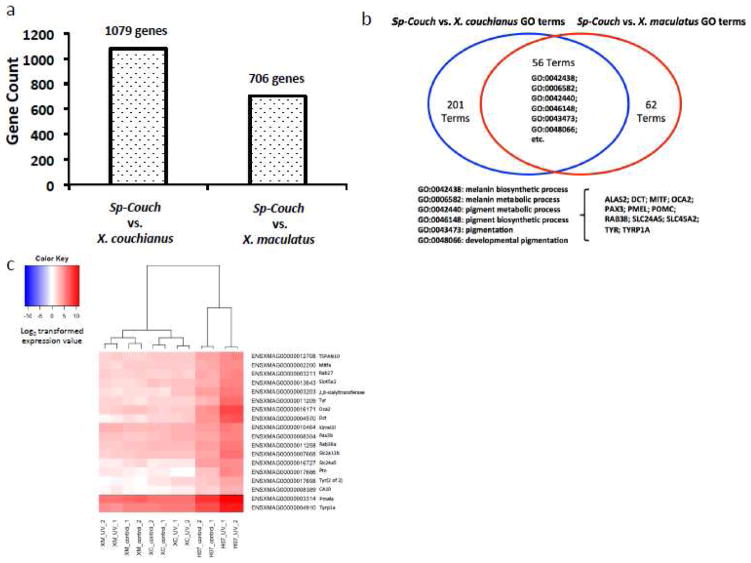
Hybridization initiated UVB-responsive genes in the Sp-Couch hybrid
Since there are 341 genes that responded to UVB exposure only in the hybrid genetic background, we hypothesized the hybridization event disrupted these genes to initiate their UVB response. We compared the gene expression profile of unexposed Sp-Couch hybrid control fish to the parental gene expression profiles, also from unexposed fish to assess gene expression that is altered by hybridization. We distinguished 1,079 differentially expressed genes between Sp-Couch hybrid and X. couchianus, and 706 differentially expressed genes between Sp-Couch hybrid and X. maculatus (Figure 3a, Supplement Table 2). Functional analysis showed that both sets of differentially expressed genes have melanin synthesis and pigmentation related functions (Figure 3b). Since increased mRNA transcription of specific genes serves to represents activation of a pathways as a cellular response, we focused on the up-regulated genes in Sp-Couch hybrid. By comparing the genes that were up-regulated by hybridization to the 341 Sp-couch unique UVB-responsive genes, we found 18 genes were up-regulated in Sp-Couch hybrid compared to both X. maculatus and X. couchianus (Figure 3c). These genes showed a pattern of up-regulation due to hybridization and also further up-regulation effected by UVB exposure. Among these 18 genes, 4 genes are related to melanin synthesis (mitfa, tyr, tyrp1a, dct), 3 genes are related to melanosome transport (rab27, rab38a, pmela), 4 genes are related to pigmentation (slc2a11b, slc24a5, oca2, pax3b), and 4 genes are melanoma/melanocyte marker (tspan10, slc45a2, ptn, 2,8-sialyltranferase) (Kobayashi et al., 1994; Kuroda et al., 2005; Kang et al., 2007; Zhang et al., 2013; Haltaufderhyde and Oancea 2014; Kimura et al., 2014; Liu et al., 2014; Morice-Picard et al., 2014; Choi et al., 2015; Eaton et al., 2015; Lindsay et al., 2015). The remaining two genes (CA10 and kirrel3l) are not well studied and their functions poorly charactrized.
Figure 3. Differentially expressed genes between hybrid and parental fish strains.
(a) The Sp-Couch hybrid has different gene expression compared to either X. couchianus or X. maculatus parent. Statistical analysis showed 1079 differentially expressed genes between Sp-Couch hybrid and X. couchianus and 706 differentially expressed genes between Sp-Couch hybrid and X. maculatus. (b) Functional enrichment of differentially expressed genes between Sp-Couch hybrid and each of the parental fish species is analyzed using Gene Ontology (GO). Of the 257 enriched functions between the Sp-Couch hybrid and X. couchianus, and 118 enriched functions between the Sp-Couch hybrid and X. maculatus, 56 functions overlapped. A significant part of the overlapping functions are related to melanin synthesis and pigmentation. (c) 18 genes are identified as hybridization-initiated and UVB-responsive genes. This group of genes does not show a response to UVB in any parental strain. The hybridization event served to up-regulate the expression of these genes while UVB exposure further stimulated the their expression levels.
It is documented that mitf can be transactivated by the Xiphophorus Melanoma Receptor Kinase gene (xmrk), a mutant ortholog of the human EGFR (Delfgaauw et al., 2003; Wellbrock et al., 2002). The xmrk gene in the platyfish X. maculatus is closely linked to the Sp (or Sd, see Schartl et al., 2010) pigmentary locus on the X chromosome. The loss of one copy of the R/Diff locus, which is thought to play a role in macromelanophore differentiation in Sp-Couch, leads to heavy pigmentation of Sp-Couch hybrids (Walter and Kazianis, 2001; Patton et. al, 2010). This is consistent with the up-regulation of mitf genes, and mitf target genes, in Sp-Couch hybrid. However, it is interesting how mitfa might become further up-regulated in Sp-Couch hybrids after UVB exposure, as well as the effects of UVB exposure on mitf target genes, such as dct, pmela, rab38a, tyr, tryp1a. It is noteworthy to mention that mitf levels are correlated with aggressive stage melanoma malignancy in the transgenic medaka-Xmrk melanoma model as well as human melanoma (Koludrovic et al., 2013; Schartl et al., 2010; Schartl et al., 2012). This may suggest that UVB serves to further up-regulate mitf and overwhelm normal cellular mechanisms (i.e., a single R/Diff gene copy) to keep xmrk in check and thereby initiating melanomagenesis.
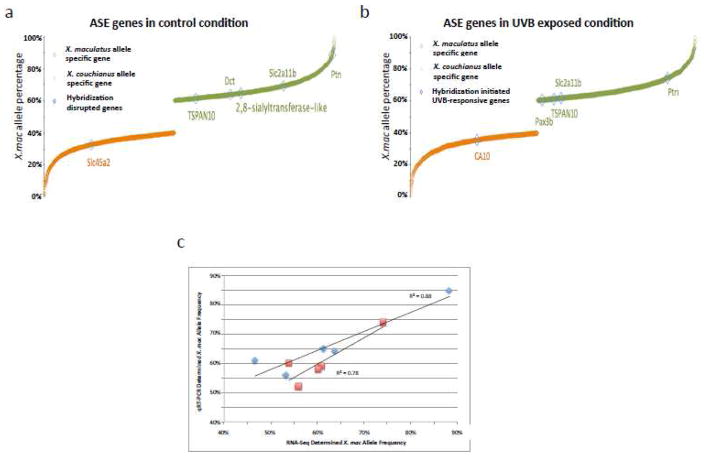
Sp-Couch hybrids show ASE of pigment related genes
We considered if certain parental alleles would be more favored in response to UVB exposure, and sought to identify genes showing allele specific expression (ASE) responses in the hybrid genetic background. To find ASE genes in the Sp-Couch hybrid, we identified sequence variations between the two parental alleles and used them to mark parental alleles in the hybrid genetic background and calculated the ratio of reads emanating from each parental allele. We identified 1,255 X. couchianus allele specific genes in Sp-Couch hybrid without UVB exposure, and 1,533 X. couchianus allele specific genes after UVB exposure. We also identified 1,271 X. maculatus allele specific genes in Sp-Couch hybrid without UVB exposure, and 1,566 X. maculatus allele specific genes in Sp-Couch hybrid after UVB exposure (Supplement Table 3). QRT-PCR assay confirmed the allele specificity of selected genes observed by RNA-Seq (Figure 4c). Eight of the hybridization-initiated UVB-responsive genes were identified to be ASE genes (Figure 4). As mentioned before, 7 of these 8 genes are pigment metabolism and melanoma related. Ptn, slc2a11b, dct, 2,8-sialytransferase-like, tspan10 are X. maculatus ASE genes in unexposed control skin; ptn, slc2a11b, tspan10 and pax3b were detected to be X. maculatus ASE genes after UVB exposure. While slc45a2 and CA10 are X. couchianus ASE genes in unexposed or the UVB exposed condition, respectively (Figure 4a,b). The dominance of the X. maculatus allele in the expression of these genes infers genetic interactions in the hybrid genetic background produces X. mauclatus bias in allele activation for pigmentation related genes and in the hybrid response to UVB.
Figure 4. Allele Specific Gene Expression.
(a) Allele specific expressed genes in Sp-Couch hybrid where 6 hybridization-responsive genes showed allele specificity. (b) Allele specific genes in Sp-Couch hybrid after UVB exposure, 5 hybridization-initiated UVB responsive genes showed allele specificity. (c) Allele specificity of 5 genes (ptn, tspan10 and dct showed strong allele specificity, pomcb and tyr showed no allele specificity) from Sp-Couch hybrid in control and UVB-exposed conditions were tested using qRT-PCR. Blue represents unexposed control conditions and red represents the UVB-exposed condition.
Conclusions
By using UVB exposed parental and interspecies hybrids between X. maculatus and X. couchianus, we found unique gene expression patterns in the hybrid related to both the hybridization event and to UVB induced gene expression. Melanin synthesis and transport related genes were highly expressed in Sp-Couch hybrid and further up-regulated after UVB exposure and their expression is contributed more by X. maculatus alleles. The expression pattern of these genes suggests they may play a role in UVB induced hybrid melanomagenesis.
Supplementary Material
Acknowledgments
The author would like to thank Markita Savage and the other employees of the Xiphophorus Genetic Stock Center, Texas State University, for maintaining the pedigreed fish lines, performing interspecies crosses, and caring for the hybrid animals used in this study. The author would also like to thank Dr. Yingjia Shen, Dr. Tzuni Garcia and Dr. Wesley Warren for sequencing and assembling the genome and transcriptome of X. couchianus. This work was supported by the National Institutes of Health, Division of Comparative Medicine, R24 OD-011120 and R24 OD-011199, and R24 OD-018555.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yuan Lu, Email: y_l54@txstate.edu.
Mikki Bowswell, Email: mboswell@txstate.edu.
William Bowswell, Email: wb1016@txstate.edu.
Kuan Yang, Email: yangkuan81@gmail.com.
Manfred Schartl, Email: phch1@biozentrum.uni-wuerzburg.de.
References
- Ananthaswamy HN, Pierceall WE. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochemistry and photobiology. 1990;52:1119–1136. doi: 10.1111/j.1751-1097.1990.tb08452.x. [DOI] [PubMed] [Google Scholar]
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. Journal of photochemistry and photobiology. B, Biology. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- Beissbarth T, Speed TP. GOstat: find statistically overrepresented Gene Ontologies within a group of genes. Bioinformatics. 2004;20:1464–1465. doi: 10.1093/bioinformatics/bth088. [DOI] [PubMed] [Google Scholar]
- Choi WJ, Kim M, Park JY, Park TJ, Kang HY. Pleiotrophin inhibits melanogenesis via Erk1/2-MITF signaling in normal human melanocytes. Pigment cell & melanoma research. 2015;28:51–60. doi: 10.1111/pcmr.12309. [DOI] [PubMed] [Google Scholar]
- De Gruijl FR, van der Leun JC. Physical variables in experimental photocarcinogenesis and quantitative relationships between stages of tumor development. Frontiers in bioscience: a journal and virtual library. 2002;7:d1525–1530. doi: 10.2741/A732. [DOI] [PubMed] [Google Scholar]
- De Laat A, van Tilburg M, van der Leun JC, van Vloten WA, de Gruijl FR. Cell cycle kinetics following UVA irradiation in comparison to UVB and UVC irradiation. Photochemistry and photobiology. 1996;63:492–497. doi: 10.1111/j.1751-1097.1996.tb03075.x. [DOI] [PubMed] [Google Scholar]
- Delfgaauw J, Duschl J, Wellbrock C, Froschauer C, Schartl M, Altschmied J. MITF-M plays an essential role in transcriptional activation and signal transduction in Xiphophorus melanoma. Gene. 2003;320:117–126. doi: 10.1016/s0378-1119(03)00817-5. [DOI] [PubMed] [Google Scholar]
- Eaton K, Edwards M, Krithika S, Cook G, Norton H, Parra EJ. Association study confirms the role of two OCA2 polymorphisms in normal skin pigmentation variation in East Asian populations. American journal of human biology: the official journal of the Human Biology Council. 2015 doi: 10.1002/ajhb.22678. [DOI] [PubMed] [Google Scholar]
- Garcia TI, Shen Y, Catchen J, Amores A, Schartl M, Postlethwait J, Walter RB. Effects of short read quality and quantity on a de novo vertebrate transcriptome assembly. Comparative biochemistry and physiology. Toxicology & pharmacology: CBP. 2012;155:95–101. doi: 10.1016/j.cbpc.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon M. The Genetics of a Viviparous Top-Minnow Platypoecilus; the Inheritance of Two Kinds of Melanophores. Genetics. 1927;12:253–283. doi: 10.1093/genetics/12.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltaufderhyde KD, Oancea E. Genome-wide transcriptome analysis of human epidermal melanocytes. Genomics. 2014;104:482–489. doi: 10.1016/j.ygeno.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussler G. Über Melanombildungen bei Bastarden von Xiphophorus Helleri und Platypoecilus Maculatus var. Rubra. Journal of Molecular Medicine. 1928;7:1561–1562. [Google Scholar]
- Kang NY, Kim CH, Kim KS, Ko JH, Lee JH, Jeong YK, Lee YC. Expression of the human CMP-NeuAc:GM3 alpha2,8-sialyltransferase (GD3 synthase) gene through the NF-kappaB activation in human melanoma SK-MEL-2 cells. Biochimica et biophysica acta. 2007;1769:622–630. doi: 10.1016/j.bbaexp.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kanofsky JR, Sima P. Singlet oxygen production from the reactions of ozone with biological molecules. The Journal of biological chemistry. 1991;266:9039–9042. [PubMed] [Google Scholar]
- Kazianis S, Gimenez-Conti I, Trono D, Pedroza A, Chovanec LB, Morizot DC, Nairn RS, Walter RB. Genetic analysis of neoplasia induced by N-nitroso-N-methylurea in Xiphophorus hybrid fish. Marine biotechnology. 2001;3:S37–43. doi: 10.1007/s10126001-0025-2. [DOI] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome biology. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Nagao Y, Hashimoto H, Yamamoto-Shiraishi Y, Yamamoto S, Yabe T, Takada S, Kinoshita M, Kuroiwa A, Naruse K. Leucophores are similar to xanthophores in their specification and differentiation processes in medaka. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:7343–7348. doi: 10.1073/pnas.1311254111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Urabe K, Orlow SJ, Higashi K, Imokawa G, Kwon BS, Potterf B, Hearing VJ. The Pmel 17/silver locus protein. Characterization and investigation of its melanogenic function. The Journal of biological chemistry. 1994;269:29198–29205. [PubMed] [Google Scholar]
- Koludrovic D, Davidson I. MITF, the Janus transcription factor of melanoma. Future oncology. 2013;9:235–244. doi: 10.2217/fon.12.177. [DOI] [PubMed] [Google Scholar]
- Kosswig K. Über Bastarde der Teleostier Platypoecilus und Xiphophorus. Z Indukt Abstamm Vererbungsl. 1928;44:150–158. [Google Scholar]
- Kuroda TS, Itoh T, Fukuda M. Functional analysis of slac2-a/melanophilin as a linker protein between Rab27A and myosin Va in melanosome transport. Methods in enzymology. 2005;403:419–431. doi: 10.1016/S0076-6879(05)03037-5. [DOI] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome biology. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic acids research. 2013;41:e108. doi: 10.1093/nar/gkt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- Lindsay AJ, Miserey-Lenkei S, Goud B. Analysis of the interactions between rab GTPases and class v myosins. Methods in molecular biology. 2015;1298:73–83. doi: 10.1007/978-1-4939-2569-8_6. [DOI] [PubMed] [Google Scholar]
- Liu D, Zhao ZG, Jiao ZL, Li HJ. Identifying differential expression genes and single nucleotide variations using RNA-seq in metastatic melanoma. Genetics and molecular research: GMR. 2014;13:8153–8162. doi: 10.4238/2014.October.7.10. [DOI] [PubMed] [Google Scholar]
- Luther U, Dichmann S, Schlobe A, Czech W, Norgauer J. UV light and skin cancer. Medizinische Monatsschrift fur Pharmazeuten. 2000;23:261–266. [PubMed] [Google Scholar]
- Mitchell DL, Fernandez AA, Nairn RS, Garcia R, Paniker L, Trono D, Thames HD, Gimenez-Conti I. Ultraviolet A does not induce melanomas in a Xiphophorus hybrid fish model. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9329–9334. doi: 10.1073/pnas.1000324107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DL, Nairn RS, Johnston DA, Byrom M, Kazianis S, Walter RB. Decreased levels of (6-4) photoproduct excision repair in hybrid fish of the genus Xiphophorus. Photochemistry and photobiology. 2004;79:447–452. doi: 10.1562/ca-03-14.1. [DOI] [PubMed] [Google Scholar]
- Morice-Picard F, Lasseaux E, Francois S, Simon D, Rooryck C, Bieth E, Colin E, Bonneau D, Journel H, Walraedt S, Leroy BP, Meire F, Lacombe D, Arveiler B. SLC24A5 mutations are associated with non-syndromic oculocutaneous albinism. The Journal of investigative dermatology. 2014;134:568–571. doi: 10.1038/jid.2013.360. [DOI] [PubMed] [Google Scholar]
- Nairn RS, Kazianis S, McEntire BB, Della Coletta L, Walter RB, Morizot DC. A CDKN2-like polymorphism in Xiphophorus LG V is associated with UV-B-induced melanoma formation in platyfish-swordtail hybrids. Proceedings of the National Academy of Sciences of the United States of America. 1996a;93:13042–13047. doi: 10.1073/pnas.93.23.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn RS, Morizot DC, Kazianis S, Woodhead AD, Setlow RB. Nonmammalian models for sunlight carcinogenesis: genetic analysis of melanoma formation in Xiphophorus hybrid fish. Photochem Photobiol. 1996b;64:440–448. doi: 10.1111/j.1751-1097.1996.tb03089.x. [DOI] [PubMed] [Google Scholar]
- Patton EE, Mathers ME, Schartl M. Generating and analyzing fish models of melanoma. Methods in cell biology. 2011;105:339–366. doi: 10.1016/B978-0-12-381320-6.00014-X. [DOI] [PubMed] [Google Scholar]
- Patton EE, Mitchell DL, Nairn RS. Genetic and environmental melanoma models in fish. Pigment Cell Melanoma Res. 2010;23:314–337. doi: 10.1111/j.1755-148X.2010.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn JJ, Trono D, Gimenez-Conti I, Butler AP, Nairn RS. Etiology of MNU-induced melanomas in Xiphophorus hybrids. Comparative biochemistry and physiology. Toxicology & pharmacology: CBP. 2009;149:129–133. doi: 10.1016/j.cbpc.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartl M, Kneitz S, Wilde B, Wagner T, Henkel CV, Spaink HP, Meierjohann S. Conserved expression signatures between medaka and human pigment cell tumors. PloS one. 2012;7:e37880. doi: 10.1371/journal.pone.0037880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartl M, Wilde B, Laisney JA, Taniguchi Y, Takeda S, Meierjohann S. A mutated EGFR is sufficient to induce malignant melanoma with genetic background-dependent histopathologies. The Journal of investigative dermatology. 2010;130:249–258. doi: 10.1038/jid.2009.213. [DOI] [PubMed] [Google Scholar]
- Setlow RB, Grist E, Thompson K, Woodhead AD. Wavelengths effective in induction of malignant melanoma. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:6666–6670. doi: 10.1073/pnas.90.14.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow RB, Woodhead AD, Grist E. Animal model for ultraviolet radiation-induced melanoma: platyfish-swordtail hybrid. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:8922–8926. doi: 10.1073/pnas.86.22.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Carcia T, Pabuwal V, Boswell M, Pasqiali A, Beldorth I, Warren Wes, Schartl M, Cresko WA, Walter RB. Comparative Biochemistry and Physiology. Part D, Genomics & proteomics. 2013;8:11–16. doi: 10.1016/j.cbd.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielkind U. Genetic control of cell differentiation in platyfish-swordtail melanomas. The Journal of experimental zoology. 1976;196:197–204. doi: 10.1002/jez.1401960207. [DOI] [PubMed] [Google Scholar]
- Walter DJ, Boswell M, Volk de Garcia SM, Walter SM, Breitenfeldt EW, Boswell W, Walter RB. Characterization and differential expression of CPD and 6-4 DNA photolyases in Xiphophorus species and interspecies hybrids. Comparative biochemistry and physiology. Toxicology & pharmacology: CBP. 2014;163:77–85. doi: 10.1016/j.cbpc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter RB, Kazianis S. Xiphophorus interspecies hybrids as genetic models of induced neoplasia. ILAR J. 2001;42:299–321. doi: 10.1093/ilar.42.4.299. [DOI] [PubMed] [Google Scholar]
- Weis S, Schartl M. The macromelanophore locus and the melanoma oncogene Xmrk are separate genetic entities in the genome of Xiphophorus. Genetics. 1998;149:1909–1920. doi: 10.1093/genetics/149.4.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellbrock C, Weisser C, Geissinger E, Troppmair J, Schartl M. Activation of p59(Fyn) leads to melanocyte dedifferentiation by influencing MKP-1-regulated mitogen-activated protein kinase signaling. The Journal of biological chemistry. 2002;277:6443–6454. doi: 10.1074/jbc.M110684200. [DOI] [PubMed] [Google Scholar]
- Wood SR, Berwick M, Ley RD, Walter RB, Setlow RB, Timmins GS. UV causation of melanoma in Xiphophorus is dominated by melanin photosensitized oxidant production. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4111–4115. doi: 10.1073/pnas.0511248103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolcock BW, Schmidt BM, Kallman KD, Vielkind JR. Differences in transcription and promoters of Xmrk-1 and Xmrk-2 genes suggest a role for Xmrk-2 in pigment pattern development in the platyfish, Xiphophorus maculatus. Cell growth & differentiation: the molecular biology journal of the American Association for Cancer Research. 1994;5:575–583. [PubMed] [Google Scholar]
- Yang K, Boswell M, Walter DJ, Downs KP, Gaston-Pravia K, Garcia T, Shen Y, Mitchell DL, Walter RB. UVB-induced gene expression in the skin of Xiphophorus maculatus Jp 163 B. Comparative biochemistry and physiology. Toxicology & pharmacology: CBP. 2014;163:86–94. doi: 10.1016/j.cbpc.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Tao K, Huang W, Tian Y, Liu X. Elevated expression of pleiotrophin in human hypertrophic scars. Journal of molecular histology. 2013;44:91–96. doi: 10.1007/s10735-012-9453-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.