Abstract
Background and Aims
Endothelial cell-selective adhesion molecule (ESAM) is selectively expressed on vascular endothelium and is postulated to play a role in atherogenesis. We investigated the association of serum soluble ESAM (sESAM) levels with subsequent cardiovascular outcomes in patients with stable ischemic heart disease.
Methods
We measured sESAM levels in 981 patients with stable coronary disease enrolled between September 2000 and December 2002 in a prospective cohort study. Poisson regression models were used to define the relationship between baseline sESAM levels and cardiovascular outcomes, including myocardial infarction, heart failure hospitalization, and mortality.
Results
There were 293 occurrences of the composite endpoint over a median follow-up of 8.9 years. After adjusting for demographic and clinical risk factors, participants in the highest sESAM quartile (compared to the lower three sESAM quartiles) had a higher rate of the composite endpoint (incident rate ratio (IRR) 1.52 (95% CI 1.16–1.99) as well as of its individual components: myocardial infarction (IRR1.64 (1.06–2.55)), heart failure hospitalizations (IRR 1.96 (1.32–2.81)), and death (IRR 1.5 (1.2–1.89)). These associations were no longer significant after adjustment for estimated glomerular filtration rate.
Conclusions
sESAM levels associate with myocardial infarction, heart failure, and death after adjustment for demographic and clinical risk factors, but not after adjustment for kidney function. sESAM may be involved in the pathogenesis of concurrent kidney and cardiovascular disease.
Keywords: atherosclerosis, endothelium, kidney disease, cardiovascular mortality
INTRODUCTION
Endothelial cell-selective adhesion molecule (ESAM) is a recently discovered member of the immunoglobulin superfamily of cellular adhesion molecules (CAM) that is highly expressed in vascular and glomerular endothelial cells1, 2 as well as in platelets.3 Studies in vitro have suggested that ESAM facilitates monocyte and neutrophil migration to sites of endovascular injury by regulating endothelial tight junctions, endothelial permeability and angiogenesis.1, 4, 5 As leukocyte diapedesis is a key step in the formation of atherosclerotic plaques,5, 6 these functions of ESAM suggest that it might play a pivotal role in the genesis of atherosclerosis.5, 7
Murine models of atherosclerosis have provided support for this hypothesis. In apolipoprotein E deficient mice, genetic inactivation of ESAM resulted in markedly smaller atherosclerotic lesions, accompanied by a reduction in arterial macrophages and in the density of vasa vasorum.5 A recent cross-sectional, observational study of healthy middle-aged subjects from the Dallas Heart Study demonstrated that higher soluble ESAM (sESAM) levels were associated with more atherosclerosis measured by its surrogates – greater aortic wall thickness, higher coronary artery calcium scores, and reduced aortic compliance.7 A longitudinal study demonstrated that sESAM was associated with increased risk of kidney function decline.8 However, no study to date has correlated sESAM levels with cardiovascular outcomes. As endothelial function has also been implicated in heart failure,9, 10 we sought to investigate associations of sESAM with cardiovascular outcomes including myocardial infarction, heart failure, and mortality in a well-characterized cohort of subjects with stable ischemic heart disease. We hypothesized that higher levels of sESAM would be independently associated with increased rates of cardiovascular events.
MATERIALS AND METHODS
Participants
We evaluated subjects from The Heart and Soul Study, a prospective cohort study designed to investigate the effects of psychosocial factors on health outcomes in patients with stable ischemic heart disease (IHD). Methods have been previously described.11 In brief, patients were eligible if they had at least 1 of the following: history of myocardial infarction, angiographic evidence of ≥50% stenosis in ≥1 coronary vessels, evidence of exercise-induced ischemia by treadmill ECG or stress nuclear perfusion imaging, or a history of coronary revascularization. Patients were excluded if they were unable to walk one block, had an acute coronary syndrome within the previous six months, or were likely to move out of the area within three years.
Between September 2000 and December 2002, 1024 subjects were recruited from 12 outpatient clinics in the San Francisco Bay Area, including 549 (54%) with a history of myocardial infarction, 237 (23%) with a history of revascularization but not myocardial infarction, and 238 (23%) with a diagnosis of coronary disease that was documented by their physician, based on a positive angiogram or treadmill test in over 98% of cases. All participants completed a full-day study including medical history, extensive questionnaires, and an exercise treadmill test with baseline and stress echocardiograms. 12-hour fasting serum samples were obtained in the morning prior to stress test and frozen at −80° C. Of 1024 participants, 39 were excluded from this analysis because serum was not available to perform the ESAM assay, and 4 were lost to follow-up, yielding 981 participants for this analysis. Institutional Review Boards at each site approved this study protocol. All participants provided written informed consent.
Measurement of sESAM
Serum ESAM levels were determined by a multiplexed bead-based immunoassay on microtiter plates. The ESAM antibody (Alere, San Diego, CA) was conjugated to modified paramagnetic Luminex beads (Radix Biosolutions); antigens were biotinylated. Fluorescent signals were generated using Streptavidin-R-Phycoerythrin (SA-RPE: Prozyme PJ31S) and read on a Luminex LX200 reader. An 8-point calibration curve was made gravimetrically by spiking each antigen into a calibration matrix. The antigen concentrations were calculated using a standard curve determined by fitting a five parameter logistic function to the signals obtained for the 8-point calibration curves (Alere, San Diego, CA). Each sample was assayed in duplicate, and sESAM level was calculated as the average of two measurements. The limit of detection for this assay is 0.4 ng/mL. In our study, the intra-assay coefficients of variation were 5% at low concentrations (31.0 ng/mL) and 11% at high concentrations (79.5ng/mL) of sESAM. The inter-assay coefficients of variation for this assay were 6% at low concentrations (31.0ng/mL) and 12% at high concentrations (79.5ng/mL) of sESAM. There was no significant cross reactivity with other antibodies.
Primary and Secondary Outcomes
Annual telephone interviews were conducted with participants or their proxy to inquire about interval hospitalizations or deaths. For any reported event, medical records, electrocardiograms, death certificates, autopsy, and coroner’s reports were obtained. Each event was adjudicated by two independent and blinded reviewers. In the event of disagreement, the adjudicators conferred, reconsidered their classification, and requested consultation from a third blinded adjudicator.
The primary outcome was a composite of myocardial infarction, hospital admission for congestive heart failure, and death from any cause. We also evaluated a combined coronary heart disease outcome, comprising myocardial infarction, stroke, revascularization, and cardiovascular death. Each individual outcome from the composite primary outcome constituted a secondary endpoint. Myocardial infarction was defined using standard diagnostic criteria.12 Heart failure was defined as hospitalization for incident heart failure or exacerbation. Stroke was defined as a new neurological deficit not known to be secondary to brain trauma, tumor, infection, or other cause. Deaths were verified by death certificates. Methods have been described in greater detail previously.13
Patient characteristics
Demographic characteristics, medical history, and smoking status were assessed by self-report questionnaire. We measured weight and height and calculated the body mass index (BMI) (kg/m2). Participants were asked to bring their medication bottles to study appointments, and research personnel recorded all current medications. Medications were categorized using Epocrates Rx (San Mateo, CA).
Laboratory tests
Low-density lipoprotein (LDL) cholesterol, triglycerides, glycosylated hemoglobin, and high sensitivity C-reactive protein (CRP)14 were determined from 12-h fasting serum samples. Levels of the amino terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP) were determined using Roche Diagnostics Elecsys NT-proBNP electrochemiluminescence immunoassay (ElecsysproBNP, Roche Diagnostics, Indianapolis, IN)13. Serum cystatin C was measured from frozen samples collected at the baseline study visit with the use of a BNII nephelometer (Dade Behring, Inc, Deerfield, Ill) with a particle-enhanced immunonephelometric assay (N Latex Cystatin C, Dade Behring, Inc)15. Estimated glomerular filtration rate (eGFR) was calculated from levels of creatinine and Cystatin C using the combined creatinine-cystatin C equation.16 For comparison, we also used the creatinine-based Modification of Diet in Renal Disease (MDRD) equation to calculate eGFR.17 Leptin18, Monocyte Chemotactic Protein-119, Tumor Necrosis Factor-alpha20, and Interleukin-621 levels were measured using immunoassays as previously described.
Participants underwent complete resting two-dimensional echocardiograms with all standard views using an Acuson Sequoia ultrasound system (Siemens Medical Solutions, Mountain View, CA) with a 3.5-MHz transducer and Doppler ultrasound examination. Standard two-dimensional parasternal short-axis and apical two- and four-chamber views were obtained during held inspiration and were used to calculate the left ventricular ejection fraction.22 After resting echocardiography, participants underwent symptom-limited exercise stress testing according to a standard Bruce protocol (those unable to complete the standard protocol were converted to a manual protocol) with continuous 12-lead electrocardiogram monitoring. Exercise capacity was estimated as the total metabolic equivalents (METs) achieved at peak exercise.
Statistical analysis
Participants were divided into quartiles of sESAM levels. As sESAM levels were normally distributed in the population studied, baseline participant characteristics across quartiles were compared using analysis of variance (ANOVA) for continuous variables and χ2 test for dichotomous variables. We compared rates of the primary composite outcome and its components as well as a combined coronary heart disease outcome between quartile IV versus quartiles I–III in multivariable Poisson regression models. We adjusted for baseline demographics (age, sex, race), clinical risk factors (smoking status, hypertension, beta-blocker use, LDL, hemoglobin A1c), and eGFR. Participants were classified as achieving the primary composite endpoint if they reached any of the individual events. In addition to the quartile analysis, we also constructed Poisson regression models treating sESAM as a continuous variable. Analyses were performed using Statistical Analysis Software (version 9.2; SAS Institute Inc., Cary, NC) and STATA (version 12.0; Statacorp LP, College Station, TX).
RESULTS
Baseline characteristics
Among 981 participants enrolled in this study the median follow-up period was 8.9 years (IQR 3.76 years). There were 293 composite events constituting the primary outcome (116 myocardial infarctions, 359 deaths and 164 hospitalizations for heart failure). The mean level of sESAM was 57.5 +/− 18.0 ng/mL. When separated into quartiles by sESAM levels, there were significant differences between groups in age, race, gender, smoking status, LDL-cholesterol and glycosylated hemoglobin (Table 1). Increasing quartiles of sESAM were also associated with lower estimated glomerular filtration rate (eGFR) and lower treadmill exercise capacity (Table 1). Levels of sESAM were significantly correlated with other serum biomarkers of disease, with Spearman correlation coefficients of 0.33 for NT-pro-BNP, 0.61 for cystatin C, −0.6120 for eGFR, 0.2 for TNF-α, and 0.2 for IL-6 (Table 2) (p<0.0001 for all).
TABLE 1.
Baseline clinical characteristics of 981 participants by quartile of sESAM
| Quartile of sESAM | |||||
|---|---|---|---|---|---|
| I (N = 246) 0.4–45.3 ng/mL |
II (N = 246) 45.4–54.6 ng/mL |
III (N = 244) 54.6–66.8 ng/mL |
IV (N = 245) 66.8–130.0 ng/mL |
p-value | |
| Demographic factors | |||||
| Age (years) | 62.1 (10.4) | 65.6 (10) | 67.6 (10.6) | 71.6 (10.7) | 0.0001 |
| White race (%) | 44.3 | 61.4 | 67.1 | 68.3 | <0.0001 |
| Male sex (%) | 73% | 81% | 85% | 86% | 0.001 |
| Body mass index (kg/m2) | 28.7 (5.8) | 28.5 (4.7) | 28.5 (5.9) | 27.9 (5) | 0.5804 |
| Never smoker | 27% | 29% | 32% | 33% | <0.0001 |
| Clinical History | |||||
| Hypertension | 70.7% | 64.1% | 72.4% | 75.7% | 0.062 |
| Diabetes mellitus | 23.2% | 23.3% | 30.1% | 28.6% | 0.181 |
| Myocardial infarction | 50.6% | 54.1% | 51% | 59% | 0.218 |
| Heart failure | 14.6% | 15.6% | 18.4% | 22% | 0.132 |
| COPD/Asthma | 18.3% | 14.3% | 14.7% | 16.3% | 0.610 |
| Current medication use | |||||
| Aspirin | 76.1% | 78.5% | 81.7% | 73.2% | 0.138 |
| Statin | 60.3% | 68.7% | 65% | 62.6% | 0.248 |
| ACE inhibitor or ARB | 45.8% | 49.6% | 56.5% | 53.3% | 0.095 |
| Beta blocker | 53.4% | 61.8% | 53.3% | 61.4% | 0.077 |
| Metabolic markers | |||||
| HDL cholesterol (mg/dl) | 46.6 (13.9) | 45.3 (12.8) | 44.7 (14.5) | 45.7 (14.5) | 0.1877 |
| LDL cholesterol (mg/dl) | 109.6 (34.8) | 104.9 (31.7) | 102.9 (34) | 100.2 (34.4) | 0.0130 |
| Triglycerides (mg/dl) | 137.7 (122) | 143.7 (139.6) | 141.1 (140.1) | 142.3 (112.5) | 0.7029 |
| eGFR (ml/min/1.73m2) | 85.4 (10.1) | 76.6 (16.7) | 69.1 (18) | 51.4 (19.3) | 0.0001 |
| Glycosylated hemoglobin (%) | 5.8 (1.1) | 5.8 (0.9) | 6.1 (1.3) | 6.2 (1.3) | 0.0001 |
| Cardiac function | |||||
| LV ejection fraction (%) | 62.5 (9.1) | 61.8 (8.9) | 61.9 (10.8) | 61 (9.6) | 0.0973 |
| Treadmill exercise capacity (METs) | 7.8 (3.3) | 7.9 (3.4) | 7.3 (3.2) | 6.2 (3.3) | 0.0001 |
Values are expressed as mean +/− standard deviation or number (% within quartile). P value is by χ2 for categorical variables and ANOVA for continuous variables.
Abbreviations: COPD: Chronic Obstructive Pulmonary Disease; ACE: angiotensin converting enzyme; ARB: angiotensin receptor blocker; HDL: high-density lipoprotein; LDL: low-density lipoprotein; eGFR: estimated glomerular filtration rate; LV: left ventricular; METs: Metabolic Equivalents.
TABLE 2.
Correlation matrix for biomarkers in 981 participants
| ESAM | hsCRP | NT-proBNP | Leptin | MCP-1 | cystatin C | eGFR | TNF-alpha | IL-6 | |
|---|---|---|---|---|---|---|---|---|---|
| ESAM | 1 | ||||||||
| hsCRP | 0.0674 | 1 | |||||||
| NTproBNP | 0.3303 | 0.1271 | 1 | ||||||
| Leptin | 0.0799 | 0.0066 | 0.0011 | 1 | |||||
| MCP-1 | 0.0127 | −0.0286 | −0.0476 | 0.1130 | 1 | ||||
| cystatin C | 0.6072 | 0.1523 | 0.4795 | 0.0392 | −0.0276 | 1 | |||
| eGFR | −0.6120 | −0.1110 | −0.3444 | −0.0540 | −0.0083 | −0.7646 | 1 | ||
| TNF-alpha | 0.1989 | 0.0624 | 0.1457 | −0.0034 | 0.079 | 0.2746 | −0.2733 | 1 | |
| IL-6 | 0.2047 | 0.5221 | 0.2668 | 0.0268 | −0.0091 | 0.2815 | −0.2885 | 0.1589 | 1 |
Values represent the Spearman correlation coefficient.
Abbreviations: hsCRP: high sensitivity C-Reactive Protein; NT-proBNP: amino terminal fragment of the prohormone of brain-type natriuretic peptide; MCP-1: Monocyte Chemotactic Protein 1 ; eGFR: estimated glomerular filtration rate; IL-6: Interleukin 6.
Associations of sESAM with cardiovascular endpoints
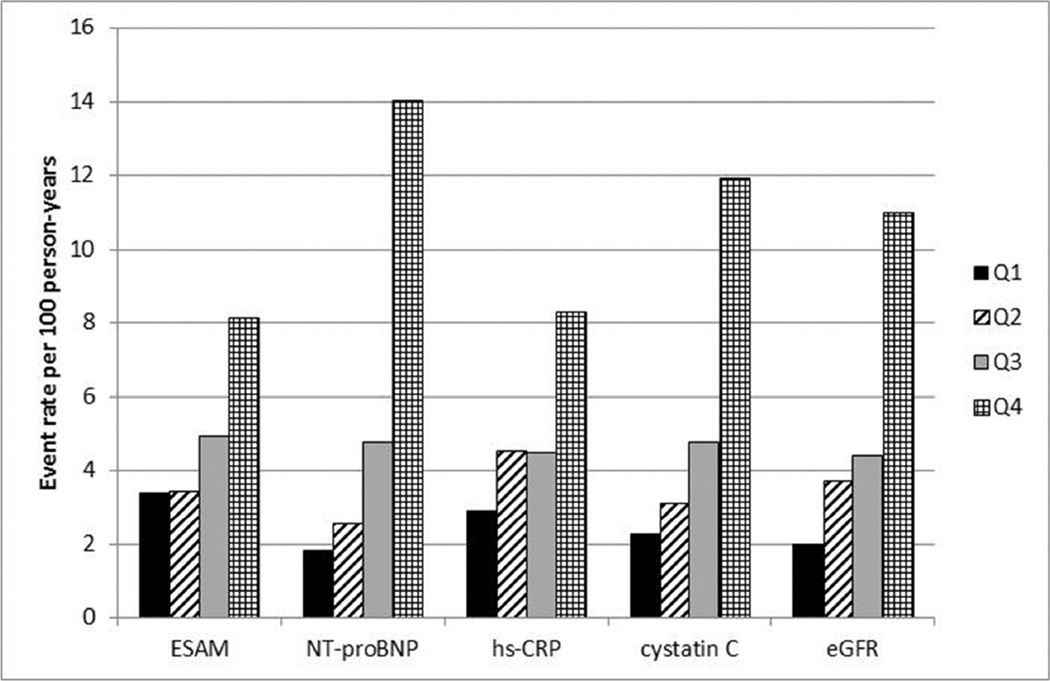
In the highest quartile of sESAM levels, 106 participants achieved the primary endpoint compared to 55 in the lowest quartile (Table 3) with significant differences in event rates across the 4 groups (p<0.0001). There were also significant differences between groups for individual outcomes (myocardial infarction, heart failure hospitalizations, and death) within the composite endpoint (Table 3). The association between sESAM and the primary endpoint was similar in magnitude to that reported previously from this cohort for NT-proBNP, hsCRP, and cystatin C13–15 (Figure 1).
TABLE 3.
Cardiovascular outcomes by quartile of sESAM
| Quartile of sESAM Number of events (incidence rate per 100 person-years) |
p-value (trend) |
||||
|---|---|---|---|---|---|
| I (n=246) 0.4–45.3 ng/mL |
II (n=246) 45.4–54.6 ng/mL |
III (n=244) 54.6–66.8 ng/mL |
IV (n=245) 66.8–130.0 ng/mL |
||
| Myocardial Infarction | 17 (1%) | 24 (1.37%) | 34 (2.1%) | 41 (2.84%) | 0.0001 |
| Heart Failure | 25 (1.49%) | 31 (1.79%) | 36 (2.17%) | 72 (5.24%) | <0.0001 |
| Stroke | 14 (0.83%) | 3 (0.16%) | 14 (0.82%) | 12 (0.79%) | 0.2749 |
| Combined Coronary Heart Disease | 41 (2.5%) | 41 (2.4%) | 61 (3.9%) | 72 (5.1%) | 0.0001 |
| All-cause mortality | 68 (3.9%) | 66 (3.47%) | 92 (5.29%) | 133 (8.63%) | <0.0001 |
| Composite primary outcome | 55 (3.41%)) | 56 (3.4%) | 76 (4.84%) | 106 (8.11%) | <0.0001 |
Values expressed as number with events (% of patients in quartile).
p values for trend across quartiles.
Combined Coronary Heart Disease includes MI, stroke, revascularization, and cardiovascular death
Composite primary outcome includes MI, HF, or all-cause death
Figure 1.
Rate of composite endpoint by quartiles of biomarkers measured in the Heart and Soul Study
Rate of primary composite endpoint (myocardial infarction, heart failure, or death) by quartiles of sESAM, NT-proBNP, cystatin C, and hs-CRP. Abbreviations: NT-proBNP: amino terminal fragment of the prohormone of brain-type natriuretic peptide; hsCRP: high sensitivity C-Reactive Protein. eGFR: estimated glomerular filtration rate. Please note quartiles of eGFR are shown form best eGFR to worst eGFR, to follow same pattern as other predictors.
Poisson models were used to further characterize the relationship between sESAM and outcomes. In univariate analysis, participants in the highest quartile of sESAM levels had a higher likelihood of achieving the composite endpoint than participants in the lower three quartiles (IRR 2.11, 95% CI 1.64–2.71, p<0.001). Similar associations were found in the individual components of the composite endpoint: myocardial infarction (IRR 2, 95% CI 1.34–2.98, p=0.0006), heart failure hospitalizations (HR 2.92, 95% CI 2.12–4.02, p<0.0001), and death (IRR 2.06 (95% CI 1.68–2.52), p<0.0001).
To examine the influence of baseline characteristics on differences in outcomes between the highest and lower quartiles of sESAM, multivariate Poisson regression models were applied for the outcomes of myocardial infarction, heart failure hospitalizations, death, and the composite outcome of myocardial infarction, heart failure, and death (Table 4). After adjusting for demographic factors (age, race, gender) and for clinical factors (smoking status, hypertension, use of beta-blockers, LDL-cholesterol level and level of glycosylated hemoglobin), incident rate ratios for the composite primary endpoint (1.52, 95% CI 1.16–1.99, p=0.0027) as well as for its components of myocardial infarction (IRR 1.64, 95% CI 1.06–2.55, p=0.0266), heart failure hospitalizations (IRR 1.96, 95% CI 1.36–2.81, p=0.0003), and death (IRR 1.5, 95% CI 1.2–1.89, p=0.0004), remained robust. Results were similar for the combined coronary heart disease outcome (IRR 1.4, 95% CI 1.02–1.92, p=0.0384). After adjusting for eGFR, the association between sESAM quartile and the composite primary endpoint was attenuated (Table 4). Results were similar when the creatinine-derived MDRD equation was used to determine eGFR (adjusted IRR 1.09 (95% CI 0.8–1.48), p=0.5773). As a continuous variable, each SD increase in sESAM (18.0 ng/mL) was associated with a 46% increase in the rate of the composite endpoint (IRR 1.46, 95% CI 1.3–1.65, p< 0.0001), and the association persisted after adjustment for demographics and clinical factors (IRR 1.27, 95% CI 1.11–1.46). This association was no longer apparent after adjusting for eGFR (IRR 1.01, 95% CI 0.85–1.2) (Supplementary Table 1).
TABLE 4.
Association of sESAM quartiles with cardiovascular outcomes.
| IRR (95% CI) Quartile IV versus Quartiles I–III of sESAM | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myocardial Infarction |
p-value | Heart Failure |
p-value | Stroke | p-value | Combined CHD |
p-value | All-cause mortality |
p-value | Composite primary outcome |
p-value | |
| Unadj. | 2 (1.34–2.98) |
0.0006 | 2.92 (2.12–4.02) |
<0.0001 | 1.63 (0.79–3.35) |
0.1855 | 1.79 (1.33– 2.39) |
0.0001 | 2.06 (1.68–2.52) |
<0.0001 | 2.11 (1.64–2.71) |
<0.0001 |
| Model 1 |
1.76 (1.29–4.67) |
0.0062 | 2.33 (1.64–3.31) |
<0.0001 | 1.46 (0.68–3.14) |
0.3372 | 1.5 (1.09– 2.05) |
0.0119 | 1.61 (1.3–1.99) |
<0.0001 | 1.71 (1.31–2.25) |
0.0001 |
| Model 2 |
1.64 (1.06–2.55) |
0.0266 | 1.96 (1.36–2.81) |
0.0003 | 1.15 (0.5–2.63) |
0.7378 | 1.4 (1.02– 1.92) |
0.0384 | 1.5 (1.2–1.89) |
0.0004 | 1.52 (1.16–1.99) |
0.0027 |
| Model 3 |
1.19 (0.72–1.97) |
0.4969 | 1.05 (0.69–1.61) |
0.8149 | 0.67 (0.29–1.57) |
0.3575 | 0.93 (0.52– 1.36) |
0.4841 | 1.07 (0.84–1.36) |
0.5676 | 0.86 (0.63–1.15) |
0.3152 |
| Model 3b |
1.38 (0.86–2.21) |
0.1849 | 1.51 (1.02–2.24) |
0.0397 | 0.8 (0.33–1.91) |
0.6088 | 1.04 (0.83–1.22) |
0.9275 | 1.19 (0.92–1.24) |
0.1845 | 1.14 (0.85–1.54) |
0.3721 |
Unadj. = unadjusted model
Model 1 adjusts for demographics (age, sex, race)
Model 2 adjusts for Model 1 + Clinical risk factors (smoking status, hypertension, beta-blocker use, low density lipoprotein-cholesterol, hemoglobin A1c)
Model 3 adjusts for Model 2 + estimated glomerular filtration rate (ml/min) by combined equation
Model 3b adjusts for Model 2 + cystatin C
Combined Coronary Heart Disease includes MI, stroke, revascularization, and cardiovascular death
Composite primary outcome includes MI, HF, or all-cause death
Additional Analyses
There was no significant interaction between sESAM levels and age (p=0.63), eGFR (0.71), or smoking status (p=0.68) with regard to the composite endpoint. There was evidence of an interaction with regard to gender (p=0.05) and race (p=0.03). When stratified by gender, the unadjusted incident rate ratio was 1.82 (1.39–2.39, p<0.0001) for men and 4.69 (95% CI 2.34–9.39, p<0.0001) for women. When stratified by race, the unadjusted IRR for blacks was 2.45 (95% CI 1.35–4.46, p=0.0032) and for whites was 1.81 (1.32–1.49, p=0.0003). On the other hand, there were no significant interactions between eGFR measured by cystatin C and age, gender, or race (p for interaction 0.99, 0.87, and 0.13, respectively).
To evaluate the effect of mortality as the driver of the composite endpoint of MI, heart failure hospitalizations, and death, we performed sensitivity analyses adjusting for troponin, NT-proBNP, and left ventricular ejection fraction. Adjustment for NT-proBNP attenuated the associations of sESAM and the composite outcome, but adjustment for NT-proBNP or left ventricular ejection fraction did not attenuate the association of sESAM and heart failure (Supplementary Table 2).
DISCUSSION
In this study, we examined associations between levels of sESAM and cardiovascular outcomes in patients with stable ischemic heart disease in order to study possible biological mechanisms in a clinical population. Higher levels of sESAM were associated with increased rates of the composite primary endpoint (myocardial infarctions, heart failure hospitalizations, and death) as well as with its individual components, independent of demographic and clinical factors. The observed relationship between sESAM levels and cardiovascular events was rendered non-significant after adjustment for eGFR.
A link between ESAM and cardiovascular diseases has been considered in non-human models almost since its discovery.2, 4, 23 Its abundant expression on the surface of vascular endothelium along with its key role in trans-endothelial migration and vascular angiogenesis provided significant support for its potential importance in atherosclerosis generation7. In addition, vascular inflammation and angiogenesis increase the likelihood of plaque rupture;6 we therefore hypothesized that ESAM might promote acute coronary syndromes. Our analysis supports this hypothesis, as we found a significant, robust relationship between sESAM levels and cardiovascular events even after adjusting for demographic and clinical factors in patients with known ischemic heart disease. Interestingly, we also identified an association between sESAM levels and exacerbations of heart failure hospitalizations, which has not been reported previously or explained mechanistically. Adjustment for NT-proBNP attenuated the associations of sESAM and the composite outcome, but adjustment for NT-proBNP did not attenuate the association of sESAM and heart failure, suggesting a possible novel pathway of endothelial disruption in heart failure.
Associations between sESAM and cardiovascular outcomes in our analysis were attenuated after adjustment for eGFR. To our knowledge, our study is the first to explicitly recognize this. Rohatgi et al in the Dallas Heart Study cohort found a significant correlation between sESAM and aortic wall thickness, coronary calcium and aortic stiffness7, all measures of atherosclerosis that are used as surrogates for clinical outcomes. In their study, ESAM was strongly correlated with cystatin C, but multivariable models were not adjusted for cystatin C or other measures of GFR7.
The mechanisms underpinning the correlation between cystatin C and sESAM remain speculative. Levels of cystatin C reflect glomerular filtration rates. It is possible that sESAM accumulates with declining glomerular filtration rates, and as impaired glomerular filtration predicts cardiovascular events,24, 25 the relationship of sESAM to cardiovascular events might simply reflect a passive relationship of sESAM to renal function. However, previously reported hazard ratios for cardiovascular events25 in the range of eGFR present in this cohort are appreciably lower than the hazard ratio associated with escalating sESAM levels in our analysis, even after adjustment for eGFR. This may suggest an additive role of sESAM because it reflects an element of cardiovascular risk imposed by CKD that was not captured by eGFR in this cohort.
In an earlier study in this cohort, we found that sESAM was significantly associated with albuminuria and faster kidney function decline.8 With respect to CKD, ESAM is expressed abundantly on glomerular endothelial cells.1, 2 It regulates vascular permeability1, 4, 5 and is independently associated with chronic kidney disease26. Compelling evidence for the potential role of ESAM in the pathogenesis of renal disease comes from experimental studies in animal models by Hara et al,27 who demonstrated a loss of regulated albumin exchange across the glomerulus in ESAM knockout mice. This reported change in structure and function of glomerular tight junctions with the loss of ESAM points to its pivotal role in normal kidney homeostasis. It is therefore plausible that ESAM might represent one pathophysiological link shared by CKD (loss of glomerular homeostasis) and CVD (loss of vascular homeostasis). A statistical adjustment for ESAM-mediated renal disease might then be expected to weaken any association between sESAM and cardiovascular outcomes.
The study has some potential weaknesses. ESAM was measured as a soluble protein in serum at a single time point only. We and others7 have assumed that sESAM accurately reflects its expression on vascular and glomerular endothelium. Our results therefore should be interpreted in the context of this assumption. As we do not have measurements of other soluble cellular adhesion molecules such as intracellular cellular adhesion molecule 1 (ICAM-1) and vascular cellular adhesion molecule 1 (VCAM-1), we cannot compare the incremental value of sESAM to these markers. In addition, due to the observational nature of the study, we cannot exclude residual confounding from variables not adequately accounted for in our models. Finally, our study did not evaluate sESAM biomarker performance metrics, as it was intended as an exploratory study of biology and pathophysiology of heart disease rather than as a new risk prediction model.
Our study also has important strengths. It is the first report of an association between levels of soluble ESAM and cardiovascular outcomes in human subjects with stable CHD. In a large cohort of well-characterized subjects and a relatively long clinical follow-up, we found that sESAM levels are strongly associated with cardiovascular outcomes (myocardial infarction, heart failure hospitalizations, or death) after adjusting for demographic factors and clinical co-morbidities. Our findings are consistent with and extend the Dallas Heart Study report of an association between sESAM and surrogate measures of atherosclerosis burden. Further investigations will be required to better understand the biologic mechanisms underlying our observations in this study, including the link between cardiovascular and renal diseases.
Supplementary Material
HIGHLIGHTS.
Endothelial cell-selective adhesion molecule (ESAM) is selectively expressed on vascular endothelium and is postulated to play a role in atherogenesis
We found that in the Heart and Soul Study, soluble ESAM levels associate with myocardial infarction, heart failure, and death after adjustment for demographic and clinical risk factors. These associations are completely attenuated after adjustment for kidney function.
sESAM may be a marker for cardiovascular risk among individuals with chronic kidney disease.
Acknowledgments
The authors would like to thank the participants in the Heart and Soul study and their families.
Sources of Funding
This work was supported by Meyeon Park’s NIH/NIDDK K23 DK099238. The Heart and Soul Study was funded by the Department of Veteran Affairs (Epidemiology Merit Review Program), Washington, DC; grant R01 HL-079235 from the National Heart, Lung, and Blood Institute, Bethesda, MD; the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), Princeton, NJ; the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), New York, NY; and the Ischemia Research and Education Foundation, South San Francisco, CA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All authors report no conflicts of interest.
References
- 1.Hirata K, Ishida T, Penta K, Rezaee M, Yang E, Wohlgemuth J, et al. Cloning of an immunoglobulin family adhesion molecule selectively expressed by endothelial cells. J Biol Chem. 2001;276(19):16223–16231. doi: 10.1074/jbc.M100630200. [DOI] [PubMed] [Google Scholar]
- 2.Nasdala I, Wolburg-Buchholz K, Wolburg H, Kuhn A, Ebnet K, Brachtendorf G, et al. A transmembrane tight junction protein selectively expressed on endothelial cells and platelets. J Biol Chem. 2002;277(18):16294–16303. doi: 10.1074/jbc.M111999200. [DOI] [PubMed] [Google Scholar]
- 3.Stalker TJ, Wu J, Morgans A, Traxler EA, Wang L, Chatterjee MS, et al. Endothelial cell specific adhesion molecule (ESAM) localizes to platelet-platelet contacts and regulates thrombus formation in vivo. J Thromb Haemost. 2009;7(11):1886–1896. doi: 10.1111/j.1538-7836.2009.03606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wegmann F, Petri B, Khandoga AG, Moser C, Khandoga A, Volkery S, et al. ESAM supports neutrophil extravasation, activation of Rho, and VEGF-induced vascular permeability. J Exp Med. 2006;203(7):1671–1677. doi: 10.1084/jem.20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue M, Ishida T, Yasuda T, Toh R, Hara T, Cangara HM, et al. Endothelial cell-selective adhesion molecule modulates atherosclerosis through plaque angiogenesis and monocyte-endothelial interaction. Microvasc Res. 2010;80(2):179–187. doi: 10.1016/j.mvr.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Lippi G, Franchini M, Targher G. Arterial thrombus formation in cardiovascular disease. Nat Rev Cardiol. 2011;8(9):502–512. doi: 10.1038/nrcardio.2011.91. [DOI] [PubMed] [Google Scholar]
- 7.Rohatgi A, Owens AW, Khera A, Ayers CR, Banks K, Das SR, et al. Differential associations between soluble cellular adhesion molecules and atherosclerosis in the Dallas Heart Study: a distinct role for soluble endothelial cell-selective adhesion molecule. Arteriosclerosis, thrombosis, and vascular biology. 2009;29(10):1684–1690. doi: 10.1161/ATVBAHA.109.190553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park M, Vittinghoff E, Ganz P, Peralta CA, Whooley M, Shlipak MG. Role of soluble endothelial cell-selective adhesion molecule biomarker in albuminuria and kidney function changes in patients with coronary artery disease: the Heart and Soul Study. Arterioscler Thromb Vasc Biol. 2014;34(1):231–236. doi: 10.1161/ATVBAHA.113.301806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillege HL, Fidler V, Diercks GFH, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, et al. Urinary Albumin Excretion Predicts Cardiovascular and Noncardiovascular Mortality in General Population. Circulation. 2002;106(14):1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 10.Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, et al. Incremental Prognostic Significance of Peripheral Endothelial Dysfunction in Patients With Heart Failure With Normal Left Ventricular Ejection Fraction. Journal of the American College of Cardiology. 2012;60(18):1778–1786. doi: 10.1016/j.jacc.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 11.Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. Jama. 2008;300(20):2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 13.Bibbins-Domingo K, Gupta R, Na B, Wu AH, Schiller NB, Whooley MA. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. Jama. 2007;297(2):169–176. doi: 10.1001/jama.297.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beattie MS, Shlipak MG, Liu H, Browner WS, Schiller NB, Whooley MA. C-reactive protein and ischemia in users and nonusers of beta-blockers and statins: data from the Heart and Soul Study. Circulation. 2003;107(2):245–250. doi: 10.1161/01.cir.0000044387.23578.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115(2):173–179. doi: 10.1161/CIRCULATIONAHA.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. New England Journal of Medicine. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Ku IA, Farzaneh-Far R, Vittinghoff E, Zhang MH, Na B, Whooley MA. Association of low leptin with cardiovascular events and mortality in patients with stable coronary artery disease: the Heart and Soul Study. Atherosclerosis. 2011;217(2):503–508. doi: 10.1016/j.atherosclerosis.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcus GM, Whooley MA, Glidden DV, Pawlikowska L, Zaroff JG, Olgin JE. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the Heart and Soul Study. American heart journal. 2008;155(2):303–309. doi: 10.1016/j.ahj.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsui JI, Whooley MA, Monto A, Seal K, Tien PC, Shlipak M. Association of hepatitis C virus seropositivity with inflammatory markers and heart failure in persons with coronary heart disease: data from the Heart and Soul study. Journal of cardiac failure. 2009;15(5):451–456. doi: 10.1016/j.cardfail.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farzaneh-Far R, Harris WS, Garg S, Na B, Whooley MA. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis. 2009;205(2):538–543. doi: 10.1016/j.atherosclerosis.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 1989;2(5):358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 23.Maynard DM, Heijnen HF, Horne MK, White JG, Gahl WA. Proteomic analysis of platelet alpha-granules using mass spectrometry. J Thromb Haemost. 2007;5(9):1945–1955. doi: 10.1111/j.1538-7836.2007.02690.x. [DOI] [PubMed] [Google Scholar]
- 24.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. The New England journal of medicine. 2005;352(20):2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 25.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 26.Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS One. 2010;5(12):e15004. doi: 10.1371/journal.pone.0015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hara T, Ishida T, Cangara HM, Hirata K. Endothelial cell-selective adhesion molecule regulates albuminuria in diabetic nephropathy. Microvasc Res. 2009;77(3):348–355. doi: 10.1016/j.mvr.2009.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.