Abstract
BACKGROUND & AIMS
Dual oxidase 2 (DUOX2), a hydrogen-peroxide generator at the apical membrane of gastrointestinal epithelia, is upregulated in patients with inflammatory bowel disease (IBD) before the onset of inflammation, but little is known about its effects. We investigated the role of DUOX2 in maintaining mucosal immune homeostasis in mice.
METHODS
We analyzed the regulation of DUOX2 in intestinal tissues of germ-free vs conventional mice, mice given antibiotics or colonized with only segmented filamentous bacteria, mice associated with human microbiota, and mice with deficiencies in interleukin (IL)23 and 22 signaling. We performed 16S rRNA gene quantitative PCR of intestinal mucosa and mesenteric lymph nodes of Duoxa−/− mice that lack functional DUOX enzymes. Genes differentially expressed in Duoxa−/− mice compared to co-housed wild type littermates were correlated with gene expression changes in early-stage IBD using gene set enrichment analysis.
RESULTS
Colonization of mice with segmented filamentous bacteria upregulated intestinal expression of DUOX2. DUOX2 regulated redox-signaling within mucosa-associated microbes and restricted bacterial access to lymphatic tissues of the mice, thereby reducing microbiota-induced immune responses. Induction of Duox2 transcription by microbial colonization did not require the mucosal cytokines IL17 or IL22, although IL22 increased expression of Duox2. Dysbiotic, but not healthy human microbiota, activated a DUOX2 response in recipient germ-free mice that corresponded to abnormal colonization of the mucosa with distinct populations of microbes. In Duoxa−/− mice, abnormalities in ileal mucosal gene expression at homeostasis recapitulated those in patients with mucosal dysbiosis.
CONCLUSIONS
DUOX2 regulates interactions between the intestinal microbiota and the mucosa to maintain immune homeostasis in mice. Mucosal dysbiosis leads to increased expression of DUOX2, which might be a marker of perturbed mucosal homeostasis in patients with early-stage IBD.
Keywords: intestine, gastroenterology, microbial dysbiosis, inflammatory bowel disease
A single layer of epithelial cells constitutes a physical and immune barrier between the gut-associated lymphatic tissue (GALT) and >1013 commensal microbes in the intestinal lumen. The function of this barrier plays a central role in maintaining normal mucosal homeostasis and protecting against potentially life-threatening pathogens by creating spatial separation and adequate innate defense response against luminal microbes 1.
Conceivably the most primordial form of innate defense response, present in all metazoans, is the release of reactive oxygen species (ROS) generated by membrane integral enzymes. In animals, prime candidates for such ROS-based defense system at barrier epithelia are the dual oxidases (DUOX), epithelial-specific NADPH oxidases that generate extracellular H2O2. Of the two DUOX isoenzymes, DUOX1 is highly expressed in bronchial epithelium and urothelium, whereas in the gut the expression is dominated by DUOX2 2,3. Expression of a DUOX2 homolog in the gut epithelium is evolutionary conserved. Indeed, studies in invertebrates indicate an important role of DUOX in innate host defense against luminal bacteria 4,5. We recently provided evidence in mice that DUOX2-generated H2O2 restricts Helicobacter felis colonization within the gastric mucus layer, providing a paradigm for the nonredundant function of DUOX in mammalian innate defense 6. A critical function of DUOX2 in the epithelial defense response against the abundant intestinal microbiota would be consistent with the robust upregulation of DUOX2 in conditions frequently associated with dysbiosis, even in the absence of manifest inflammation. For instance, DUOX2 is markedly upregulated in the intestine of patients with irritable bowel syndrome 7, in the normal appearing proximal small intestine after ileal pouch-anal anastomosis 8, and in noninflamed ilea of patients with colon-only Crohn’s disease (cCD) 9. However, whether DUOX2 induction is a consequence of microbial dysbiosis and plays a role in maintaining immune homeostasis is currently unclear.
In the present study we provide evidence that an epithelial-attaching commensal, segmented filamentous bacterium (SFB), is sufficient to induce DUOX2. DUOX activity modulates redox-signaling in mucosa-associated microbes and restricts the access of bacteria to the GALT system, thereby dampening microbiota-induced mucosal immune responses. Dysbiotic microbiota from patients transplanted into germ-free recipient mice leads to differential mucosal colonization and Duox2 induction compared to microbiota from healthy donors. In addition, a loss of DUOX activity alone is sufficient to cause ileal gene expression abnormalities reminiscent of that associated with mucosal dysbiosis in cCD. These findings implicate DUOX2 as a critical modulator in mutualistic host-microbiota interactions that are fundamental in maintaining gut immune homeostasis.
Materials and Methods
Animals
Duoxa−/− and gender-matched wild type (wt) littermates in 129S6 genetic background were cohoused (3–5 animals/cage) in microisolator cages under SPF conditions 6. Food and water were supplied ad libitum, with the latter including a supplemental dose of L-thyroxine to maintain euthyroidism of Duoxa−/− mice 10. Il22−/−, Il23r−/−, and RORgt−/− mice (all in B6 background) have been described previously 11–13. C57BL6 mice with distinct resident microbiota were purchased from Taconic Farms and Jackson Laboratory, respectively. For all studies, mice were used at 9–12 weeks of age. Studies were approved by the University of Michigan Institutional Animal Care and Use Committee (PRO-00004497 and PRO-00002436).
Detailed methods for tissue collection, mono-association of mice with SFB, human-flora associated mouse model, enteric Salmonella Typhimurium infection, dextran sodium sulfate challenge, intestinal permeability assay, microarray-based gene expression profiling, histology and morphometric analysis, 16S rRNA in situ hybridization and immunostaining, DNA and RNA extraction, realtime reverse transcription PCR (rt-qPCR), Western blotting and ileal enteroid culture are available in the Supplementary materials and methods.
Statistics
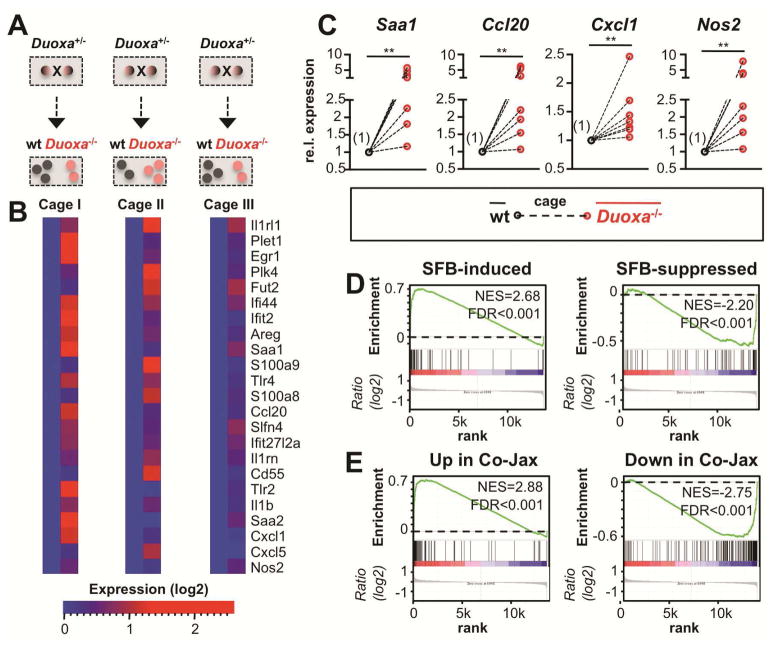
Log-transformed expression data from unpaired groups were analyzed using Welch’s t-test with multiple comparisons adjustment or with one-way ANOVA and Bonferroni post-hoc tests. The Wilcoxon matched-pairs signed-rank test was used to test for differences between genotype groups in mixed housing experiments. Each cage was analyzed as a pair of the means obtained in Duoxa−/− and cohoused wt littermates (n=2–3 mice per genotype and cage). Data were analyzed using GraphPad Prism 6.0 (San Diego, CA).
Results
Intestinal expression of DUOX2 in response to microbial colonization
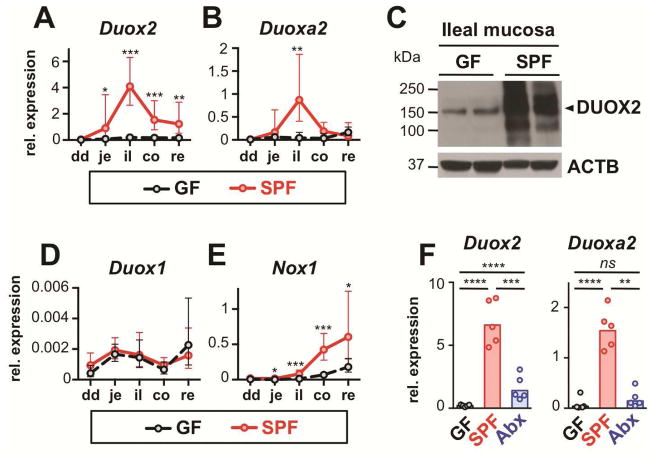
We initially analyzed longitudinal DUOX2 expression profiles in the gut of mice kept in our specific pathogen-free (SPF) or germfree (GF) environment. Both DUOX2 subunit genes (i.e., Duox2 and Duoxa2; Supplementary Figure S1A) were robustly induced by the microbiota as reported previously 14, but expression did not directly correlate with luminal bacterial density as Duox2 mRNA induction peaked in the terminal ileum (Figures 1A and B). Compared to GF mice, DUOX2 protein expression was also robustly induced in the ilea of SPF mice (Figure 1C; Supplementary Figure S2). Expression of other epithelial NADPH oxidases was exceedingly low (Duox1, Figure 1D) or predominant in the colorectum (Nox1, Figure 1E). Duox2 expression was maintained in response to continuous stimulation by the microbiota, since levels of Duox2 and Duoxa2 mRNA were significantly diminished following a single dose of antibiotics by oral gavage (Figure 1F).
Figure 1.
Intestinal DUOX2 expression depends on microbial colonization. Relative Duox2 (A) and Duoxa2 (B) mRNA expression in GF (n=5) and SPF (n=7) mice. Data represent geometric means±95% CI. dd, duodenum; je, jejunum; il, ileum; co, colon; re, rectum. (C) Immunoblot of DUOX2 protein. ACTB, β-actin loading control. (D, E) Relative mRNA expression of Duox1 and Nox1. Data represent geometric means±95% CI. (F) Effect of acute enteral antibiotic treatment on ileal Duox2 and Duoxa2 expression. Mice were analyzed 24 hours following oral gavage with streptomycin (20 mg; Abx); SPF, sham treated control. Bars indicate geometric means. ****, P<.0001; ***, P<.001; **, P<.01; *, P<.05.
Induction of DUOX2 by colonization with a mucosa-adherent commensal
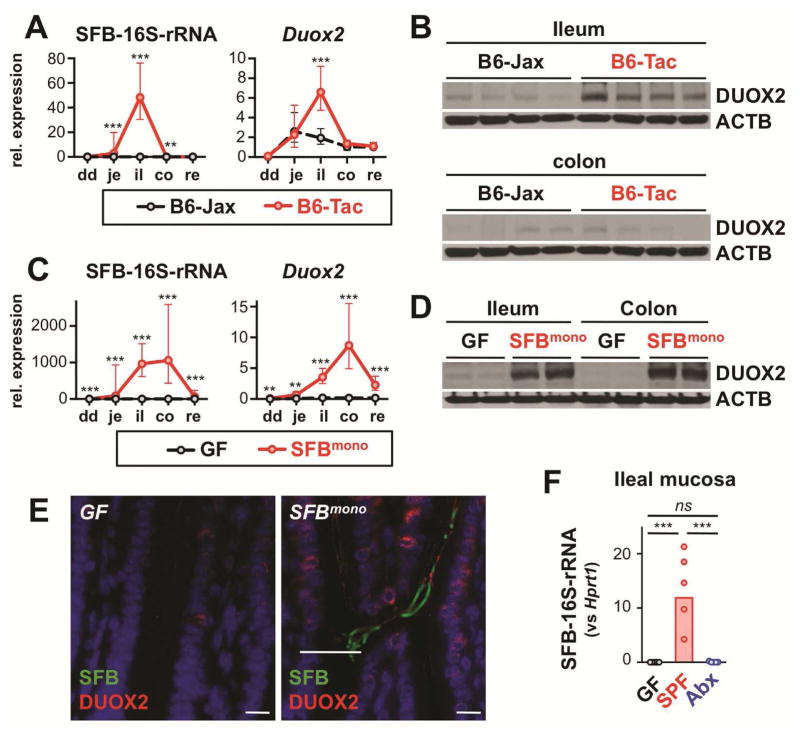
Because the murine ileum is typically colonized by SFB that, unusual for commensal bacteria, grow firmly attached to the intestinal epithelium in a symbiotic relationship, we hypothesized that SFB contributed to the ileum-dominant expression of DUOX2. We compared mice maintained in either an SFBneg SPF environment (Jackson Laboratories; B6-Jax) with those housed in SFBpos SPF environment (Taconic Farms (B6-Tac)15 or our own SFBpos SPF animal facilities). Quantitative PCR of SFB-specific 16S rRNA confirmed that mucosa-associated SFB were essentially restricted to the ileum in SFBpos animals but absent in SFBneg B6-Jax mice (Figure 2A). SPF mice from these different environments had indistinguishable Duox2 expression profiles except that B6-Jax mice lacked the peak ileal expression (Figure 2A and B; Supplementary Figure S2). To corroborate that SFB was sufficient to trigger DUOX2 induction, GF mice were gavaged with cecal content from SFB-monocolonized (SFBmono) mice. After one week, a high level of mucosa-associated SFB was detected in both the ileum and colon (Figure 2C). SFB colonization was accompanied by robust induction of Duox2 mRNA (Figure 2C) and protein (Figure 2D and E; Supplementary Figure S2) confirming that mono-association with SFB was sufficient for Duox2 induction. Twenty-four hours following treatment with streptomycin, SFB-specific 16S rRNA in ileal mucosal samples (as surrogate for viable SFB) was reduced to less than 0.1% (Figure 2F), in agreement with the suppression of Duox2 and Duoxa2 mRNA (Figure 1F). Overall, these results indicate that epithelial-attaching SFB strongly triggers DUOX2 expression.
Figure 2.
Mucosa-adherent SFB are a dominant inducer of ileal DUOX2 expression. (A) Mucosa-adherent SFB (16S-rRNA) and Duox2 mRNA level in SFBneg (B6-Jax; n=5) and SFBpos (B6-Tac; n=5) mice. Data represent geometric means±95% CI. dd, duodenum; je, jejunum; il, ileum; co, colon; re, rectum. (B) DUOX2 protein expression in ilea of B6-Jax and B6-Tac mice. Each lane represents an individual mouse. ACTB, β-actin loading control. (C, D) Mucosa-associated SFB and expression of Duox2 mRNA and protein in mice mono-associated for one week with SFB (SFBmono; n=5) or sham-treated GF controls (n=5). Data represent geometric means±95% CI. (E) Detection of DUOX2 protein by indirect immunofluorescence (red) and SFB by in situ hybridization (green) in the terminal ileum of GF and SFBmono mice. Scale bars, 10 μm. (F) Ileal mucosa-adherent SFB (16S-rRNA) in mice treated with oral streptomycin (Abx) or sham treated controls (SPF). ***, P<.001; **, P<.01; *, P<.05.
Microbial induction of DUOX2 does not require a functional IL-22 pathway
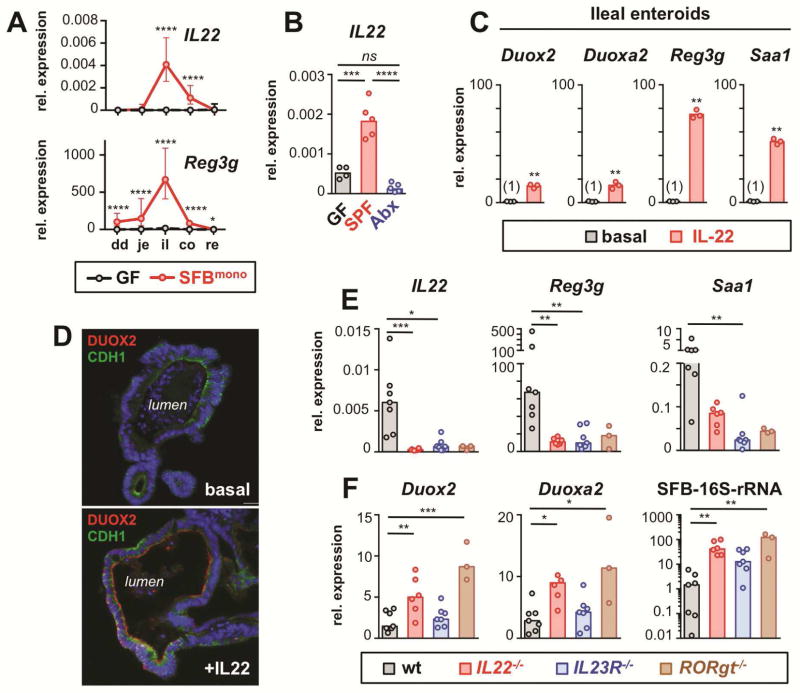
SFB is known to be a particular powerful inducer of mucosal innate lymphoid cells (ILC) to produce interleukin-22 (IL-22), which is a master regulator of epithelial defense responses 15. In fact, IL-22 was strongly stimulated in the ileum of SFBmono mice (Figure 3A; Supplementary Figure S3A) and its expression was acutely abrogated in mice treated with enteral streptomycin (Figure 3B), reminiscent of the sharp decline in mucosal SFB colonization and Duox2 expression (Figures 1F and 2F). The anti-microbial lectin Reg3g, a known IL-22 target gene, was also strongly upregulated in the ileum of SFBmono mice (Figures 3A).
Figure 3.
IL-22 can augment DUOX2 expression but is not essential for DUOX2 induction by microbial colonization. (A) Expression of IL22 and Reg3g along the intestinal tract of GF and SFBmono mice (n=5 per group). Data represent geometric means±95% CI. Note lower colonic expression of IL22 and Reg3g despite high level of mucosal SFB and DUOX2 (Fig 2C). (B) Acute microbial regulation of ileal IL22 expression. SPF (SFBpos) mice were analyzed 24 hours following oral gavage with streptomycin (Abx) or sham treatment (SPF). (C) Ileal enteroids were cultured with or without IL-22 (50 ng/ml for 18 h) and gene expression of selected genes determined by RT-qPCR. (D) Immunofluorescence of DUOX2 protein in IL-22-stimulated ileal enteroids. Cdh1, E-cadherin. (E) Ileal expression of IL22 and IL-22 target genes in wt and IL-22 deficient mice (IL22−/−, IL23R−/−, RORgt−/−). (F) Expression of DUOX2 subunit genes and mucosal SFB-colonization. Bars in panels E and F indicate median values. ****, P<.0001; ***, P<.001; **, P<.01; *, P<.05.
To directly test whether IL-22 can activate Duox2 expression, ileal enteroids were stimulated with recombinant IL-22. Both Duox2 and Duoxa2 were induced about 15-fold within 18 hours (Figure 3C) and DUOX protein became clearly detectable at the luminal surface of enteroids (Figure 3D). In contrast, the proinflammatory cytokine TNFα was unable to induce the Duox2 expression pathway in enteroids (Supplementary Figure S3B). To investigate whether IL-22 is essential to maintain Duox2 expression in vivo, we examined whether disrupted IL-22-signaling in mice abrogates Duox2 expression. As expected, IL22−/−, IL23R−/− and RORgt−/− (deficient in both TH17 and ILC development) mice all lacked ileal IL22 expression compared to wt controls sharing the same environment (Figure 3E). Accordingly, the former were also deficient in expression of the IL-22 target genes Reg3g and Saa1. In stark contrast, Duox2 and Duoxa2 were both upregulated in mice lacking IL-22-mediated defense (Figure 3F), indicating that IL-22 is not essential for high ileal DUOX2 expression. Interestingly, induction of Duox2 in these IL-22 deficient models was associated with higher level of mucosa-adherent SFB (Figure 3F), further corroborating the concept that increased commensal-epithelial interaction can trigger Duox2 expression independently of IL-22. Exposure of ileal enteroids to sterile microbial extracts prepared from either SFBneg or SFBpos mice induced epithelial toll-like receptor downstream targets, but completely failed to induce Duox2 (Supplementary Figure S4), suggesting that active bacteria, rather than soluble bacterial products, are critical for the Duox2 response.
DUOX supports luminal containment of bacteria
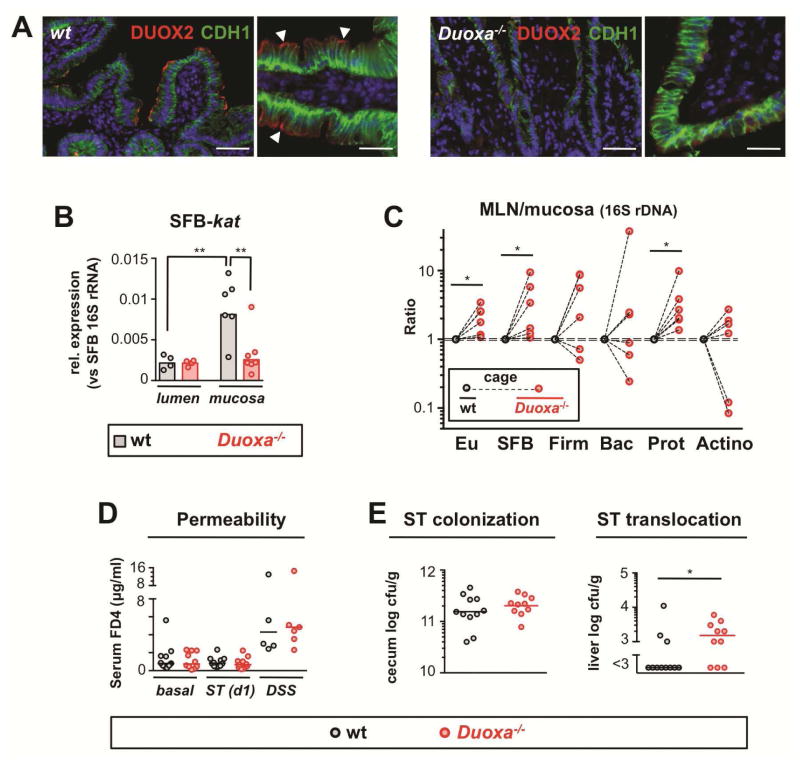
To investigate further the role of the DUOX response, we utilized mice lacking functional DUOX enzymes (Duoxa−/−) 6 (Figure 4A; Supplementary Figure S1B). A ROS-inducible SFB catalase gene (SFB-kat) (Supplementary Figure S5) was measured as a marker of H2O2 exposure. We found that kat expression of mucosal, but not luminal, SFB was markedly higher in wt compared to Duoxa−/− mice (Figure 4B), confirming DUOX deficiency status of Duoxa−/− mice and the ability of DUOX to modulate redox-sensitive pathways in mucosa-adherent SFB. The expression of several other tested SFB genes was not under control of DUOX activity indicating specificity of the SFB-kat response (Supplementary Figure S6A). In contrast to SFB-kat, expression of epithelial anti-oxidative enzymes was not significantly affected by DUOX status (Supplementary Figure S6B). Thus, DUOX activity does not lead to global changes in epithelial redox status, at least under homeostatic conditions. Duoxa−/− mice did not manifest signs of spontaneous intestinal inflammation (such as, body weight loss or cellular infiltration) (Supplementary Figure S7) and SFB colonization levels did not differ between Duoxa−/− mice and cohoused wt littermates nor did the level of mucosa-associated ileal microbiota surveyed using 16S qPCR assays specific for the major bacterial phyla (Supplementary Figure S8).
Figure 4.
DUOX2 restricts transepithelial uptake of bacteria in the small intestine. (A) Detection of DUOX2 protein (red) at the epithelial brush border in the terminal ileum of wt but not Duoxa−/− littermates; the basolateral cell marker E-cadherin (CDH1) is shown in green; scale bars represent 50 μm (left panels) and 20 μm (right panels), respectively. (B) SFB-specific catalase (kat) expression in mucosa-adherent and luminal SFB. (C) Graph depicts the ratio of 16S rDNA in MLN vs ileal mucosa indicative of transepithelial bacterial flux. Dashed lines connect mean bacterial DNA level of Duoxa−/− mice and cohoused littermate controls. Eu, eubacteria; Firm, Firmicutes; Bac, Bacteroidetes; Prot, Gammaproteobacteria; Actino, Actinobacteria. (D) In vivo intestinal permeability for 4 kDa dextran in Duoxa−/− and wt mice at baseline, following acute enteral Salmonella infection (ST), and after treatment with dextran sulfate sodium (DSS) to induce unspecific epithelial injury. (E) Acute systemic dissemination of enteral Salmonella Typhimurium in Duoxa−/− and wt animals 24 h post enteral infection.
Although considered a strict anaerobe unable to survive within host tissues, SFB or remnants thereof are translocated across the epithelial barrier 16, potentially involving epithelial endocytosis 17,18. We therefore investigated whether induction of DUOX2 by commensal bacteria regulates their transepithelial passage, an integral part of small intestinal immune surveillance. As proxy for transepithelial flux of bacterial DNA, we compared the relative concentration of bacterial DNA in mesenteric lymph nodes (MLN) and corresponding mucosal samples. MLN-to-mucosa ratios for γ–Proteobacteria and SFB were significantly elevated in Duoxa−/− mice compared to littermate controls sharing the same environment (Figure 4C; Supplementary Figure S9). Paracellular permeability to dextran (4 kDa) was not altered in Duoxa−/− mice (Figure 4D) excluding an unspecific defect in the intestinal epithelial barrier.
The ability of DUOX2 to restrict translocation of indigenous bacteria appeared to be congruent with in vitro studies, where DUOX2 conferred cell-autonomous protection against pathogens, such as, Salmonella Typhimurium 19, Listeria monocytogenes 20, and Campylobacter jejuni 21. To assess whether DUOX interfered with pathogen translocation, mice were orally challenged with Salmonella Typhimurium, which preferentially targets the small intestinal epithelium 22. Following pretreatment with streptomycin, acute infection induced ileal Duoxa2 expression to a level observed in mice with an SFB-containing microbiota (Supplementary Figure S11A). Salmonella colonization was not different in Duoxa−/− mice and wt controls (Figure 4E). The epithelial chemokines Cxcl1 and Ccl20 are robustly induced by acute Salmonella infection in vitro and in vivo, a response attributed to stimulation of toll-like 5 receptors 23,24. Both chemokines showed exacerbated induction in the ileum of infected Duoxa−/− mice compared to infected controls, compatible with increased activation of basolateral receptors (Supplementary Figures S11B and C). That Duoxa−/− mice were less able to contain luminal Salmonella was further indicated by increased early systemic dissemination of the pathogen (Figure 4E; Supplementary Figure S12). Unspecific intestinal permeability in infected mice was indistinguishable between Duoxa−/− and wt mice (Figure 4D). Overall, these studies indicate that DUOX reduces early systemic dissemination of a bona fide epithelial-invasive pathogen providing further evidence as to its role in maintaining the innate epithelial barrier in the small intestine.
Loss of DUOX2 augments SFB-induced changes in ileal gene expression
To further examine whether loss of DUOX activity perturbs immune homeostasis, we analyzed genome-wide expression profiles in the ileal mucosa of Duoxa−/− and cohoused wt littermates (Figure 5A). We identified genes with consistent induction in Duoxa−/− mice (Figure 5B). Functional pathway analysis revealed an enrichment of annotations related to innate defense and inflammatory responses (Supplementary Figure S13). Since DUOX2 was strongly induced by SFB (Figure 2C) and restricted their mucosal passage (Figure 4C), we hypothesized that loss of DUOX activity would exaggerate the effects of SFB on host gene expression. We used gene set enrichment analysis (GSEA) to test whether SFB-regulated genes 15 show concordant expression differences between Duoxa−/− and wt littermate controls. Genes strongly upregulated (or downregulated) by monocolonization of GF mice with SFB (or by cohousing of B6-Jax with B6-Tac animals, respectively) were significantly enriched (P<.001; 1000 permutations) among the genes upregulated (or downregulated) in Duoxa−/− (Figures 5D and 5E). There was also a trend towards higher expression of IL-17/22 cytokines in Duoxa−/− mice (Supplementary Figure S14). Results of these studies imply that SFB not only induces DUOX2 expression, but that normal DUOX activity may dampen other SFB-induced innate mucosal responses under homeostatic conditions.
Figure 5.
Loss of DUOX activity disturbs mucosal homeostasis in the terminal ileum. (A) Experimental setup for gene expression profiling. (B) Expression heat maps depicting selected genes upregulated in the ileum of Duoxa−/− animals. (C) Ileal gene expression analyzed by RT-qPCR. Mean expression in Duoxa−/− animals is plotted relative to the mean in cohoused wt littermates (set to 1) in cage-wise comparisons (n=7). **, P<.01. (D, E) Genes controlled by SFB-monocolonization or by cohousing of B6-Jax (SFBneg) with B6-Tac (SFBpos) mice 15 were tested for correlation with genes affected by loss of DUOX activity using GSEA (see Supplementary Methods). Within each plot, genes are sorted for their relative ileal expression in Duoxa−/− mice (left side: up in Duoxa−/−; right side: down in Duoxa−/−). Genes upregulated (downregulated) by introduction of SFB are significantly correlated with those upregulated (downregulated) in mice with loss of DUOX activity. Core enriched genes are listed in Supplementary Tables S16-S19. FDR, false discovery rate q-value; NES, normalized enrichment score.
Dysbiotic microbiota from IBD patients confers Duox2 induction in human-microbiota associated mice
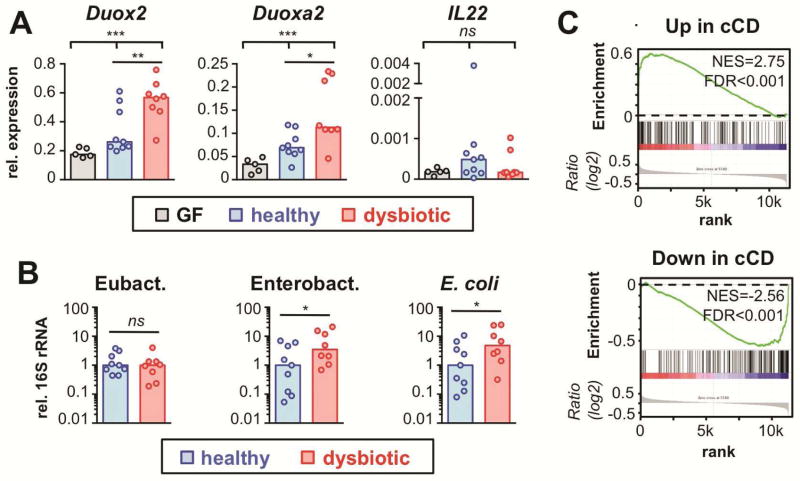
Our findings above indicate that murine DUOX2 functions as an epithelial first-line defense system affecting normal mucosal immune homeostasis. In humans, DUOX2 and DUOXA2 have been identified among the top upregulated genes in the ilea of IBD patients 9 including those without ileal inflammation, suggesting a role in abnormal immune homeostasis preceding the manifestation of histological lesions. In these patients, dysbiotic changes of the mucosal microbiota have been described, although a causative relationship to DUOX2 upregulation has not been established. To explore whether abnormal microbiota is sufficient to explain overexpression of DUOX2 in the pre-clinical stage of IBD, we analyzed Duox2 expression in GF mice following association with human microbiota from IBD patients or healthy donors. As observed previously 25, association of mice with normal human microbiota has little or no effect on the expression of host defense genes highly responsive to mouse microbiota (Figure 6A). However, significantly higher induction of the DUOX2 system was observed among mice inoculated with dysbiotic fecal samples from patients with active colitis. Although intestinal samples from mice colonized with healthy or dysbiotic human microbiota did not differ in the overall mucosa-adherent eubacterial load, there was higher mucosal association with members of the family of Enterobacteriaceae (Figure 6B). While the specific microbial species responsible for DUOX2 induction are unknown and likely donor specific, it is tempting to speculate that, in the context of dysbiotic microbiota, indigenous pathobionts gain increased access to the intestinal epithelium triggering a DUOX2 defense response.
Figure 6.
DUOX2 is induced by constituents of the microbiota from IBD patients and acts as a compensatory mucosal defense pathway. (A) Gene expression in colonic mucosa of mice colonized for two weeks with fecal material from healthy donors or patients with ulcerative colitis-associated dysbiosis. Bars indicate median values. Kruskal-Wallis test; ***, P<.001; **, P<.01; *, P<.05. (B) 16S rRNA level in mucosal samples corresponding to (A). *, P<.05. (C) Genes dysregulated in the non-affected ileum of patients with colon-only CD (cCD vs healthy controls) 9 were tested for correlation with genes affected by loss of DUOX2 activity using GSEA. Genes upregulated (downregulated) in non-inflamed tissue from CD patients are significantly enriched among those upregulated (downregulated) in mice with loss of DUOX activity. Leading edge gene subsets are depicted in Tables S20 and S21. FDR, false discovery rate q-value; NES, normalized enrichment score.
The gene expression changes in DUOX defective mice resemble mucosal dysbiosis associated changes in non-inflamed ilea of IBD patients
Since a DUOX defect exaggerated the ileal immune response to epithelial-attaching SFB, we hypothesized that a defect in DUOX would trigger similar pathogenetic changes as those observed in non-inflamed ilea affected by mucosal dysbiosis. Cross-species GSEA revealed that genes with strongest upregulation in non-inflamed ilea of patients with cCD 9 were significantly enriched among genes upregulated in Duoxa−/− animals, whereas genes with suppressed expression in cCD patients showed concordant downregulation in Duoxa−/− (Figure 6C). Hence, a loss of DUOX activity in mice leads to subtle gene expression changes that recapitulated the abnormalities found in non-inflamed ilea of patients with mucosal dysbiosis.
Discussion
Our current study establishes mammalian DUOX2 as a critical modulator of mutualistic host-microbiota interactions that are fundamental in maintaining gut immune homeostasis. While the induction of murine Duox2 by the normal microbiota has been reported recently 14, our results provide a link between an epithelial-attaching commensal and DUOX2 activation. Induction does not depend on mucosal IL-23/22 cytokine circuitry, although the latter can boost expression of DUOX2. DUOX activity modulates redox-signaling in mucosa-associated commensals and restricts the translocation of bacterial material into the GALT system, thereby dampening microbiota-induced mucosal immune responses. Ultimately, our study provides a functional link between DUOX2 induction in early stages of IBD and the associated changes in mucosal microbiota. Not only is DUOX2 induced by constituents found in the dysbiotic flora of IBD patients, but lack of the DUOX defense system is sufficient to cause ileal gene expression changes reminiscent of those in an early pathogenetic stage of IBD associated with mucosal dysbiosis.
There are several lines of evidence that support the concept that direct commensal epithelial contact is a primary trigger for DUOX2 expression. We showed that monocolonization with SFB was sufficient for high-level DUOX2 induction corresponding to the mucosal abundance of SFB along the intestinal tract. This finding was in contrast to a recent report showing that other indigenous bacteria that, unlike SFB, are normally kept at distance from the epithelium, did not stimulate Duox2 expression 14. Consistent with the concept that DUOX2 induction is triggered by access of bacteria to the epithelium, we found that microbial extracts prepared from either SFBneg or SFBpos microbiota were not sufficient to induce Duox2 in ileal enteroids (Supplementary Figure S4). Arguing against a role of soluble factors is also the fact that microbiota-induced Duox2 expression at homeostasis does not depend on toll-like receptor signaling since it was not abolished in Myd88−/−Trif−/− mice 14. The regulation of DUOX2 by epithelial contact with constituents of the microbiota is consistent with a role of mammalian DUOX2 as sentinel response against potential threats to the epithelial barrier. In SFBneg SPF mice, induction of DUOX2 was still higher in the small intestine compared to the colon, despite the latter having higher bacterial load (bacteria per ml luminal content: ileum ~108; colon ~1011). This observation is likely related to the distinct physical properties of the colonic mucus layer, which is less penetrable for bacteria-sized particles 26. To directly examine SFB-induced cell-autonomous signaling and its effects on Duox2 expression will require the coculture of SFB with highly differentiated intestinal epithelial cells in vitro. Given the recent progress in the in vitro culture of SFB 27 and enteroid-derived monolayers 28, such studies should become feasible in the future.
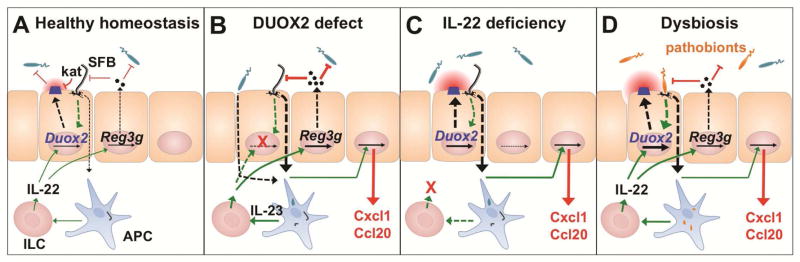
Epithelial innate defense responses are coordinated by IL-22, which stimulates expression of secreted antimicrobial effectors 29 (Figure 7). IL-22 is produced in TH17 cells and ILC found in the intestinal lamina propria, Peyer’s patches and MLN 30, whereas IL-22 receptor is almost exclusively detected in epithelial cells. A master regulator for activation of the IL-22 pathway is IL-23 produced by lamina propria macrophages upon contact with bacteria or sensing of bacterial products 31. We showed that IL-22 robustly induced Duox2 in enteroids indicating that activation of the IL-23/22 pathway can amplify DUOX2-mediated defense. However, in contrast to expression of Reg3g or Saa1, expression of Duox2 was upregulated rather than diminished in IL-22 deficient animals. In this scenario, a lack of antimicrobial effectors allowed increased mucosal access of indigenous bacteria (e.g., SFB) inducing Duox2 expression (Figure 7C). In terms of Duox2 expression, increased bacterial-epithelial contact in IL-22 deficient mice apparently overrides the lack of IL-22 mediated stimulation. We thus hypothesize that the first line defense provided by DUOX2 restricts activation of the IL-22 pathway under homeostatic conditions, the latter being strongly activated following bacterial sensing by lamina propria macrophages (Figure 7A). In fact, even in an SPF environment (SFBpos), Duoxa−/− displayed a trend towards higher ileal expression of IL-17/22 cytokines (Figure 7B; Supplementary Figure S14).
Figure 7.
Model for the integration of the DUOX2 system into the intestinal epithelial defense response. (A) Epithelial contacting microbes (e.g., SFB) induce DUOX2 by an IL-22-independent pathway at homeostasis. DUOX2 activity triggers an anti-oxidative response (kat) in mucosa-adherent SFB but does not prevent their colonization. (B) Lack of DUOX2 activity leads to increased uptake of bacterial material (e.g., SFB, Proteobacteria). Expression of compensatory host defense systems resulting in a proinflammatory milieu. (C) Lack of IL-22 dependent mucosal defense leads to mucosal dysbiosis and compensatory induction of DUOX2. (D) In IBD-associated mucosal dysbiosis, increased epithelial contact with and uptake of indigenous pathobionts triggers a compensatory DUOX2 response before the onset of clinical inflammation. Sensing of bacterial factors by antigen-presenting cells (APC) activates the IL-23/IL-22 cascade for secretion of antimicrobial effectors and further enhancement of DUOX2 expression.
We found that loss of DUOX activity increased mucosal penetration of a microbiota subset at homeostasis despite normal paracellular permeability. One potential mechanism is the suppression of bacterial virulence within an oxidative microenvironment. For instance, in vitro exposure of Campylobacter jejuni to DUOX2-generated H2O2 suppresses polysaccharide capsule synthesis and thereby invasion of epithelial cells 21. We showed here that DUOX was sufficient to regulate expression of a redox-sensitive response element (putative perR-kat operon) in mucosa-associated SFB. Such DUOX-dependent change in the redox-status of juxtaepithelial bacteria was also evident in our prior study of gastric Helicobacter felis infection 6. Hence, DUOX-generated H2O2 can modulate protein function within mucosa-associated bacteria, potentially suppressing virulence behavior of indigenous pathobionts. In addition, DUOX may also support epithelial-cell intrinsic defense mechanisms that contain endocytosed bacteria, a concept compatible with the protective effect of DUOX2 in epithelial invasion studies 19,20. Endocytosed bacteria are targeted for lysosomal degradation via selective macroautophagy (xenophagy). Such epithelial xenophagy delays early Salmonella dissemination to extraintestinal sites 32,33 and may be facilitated by oxidative inactivation of the delipidation enzyme ATG4B 34. It is thus an attractive hypothesis that in intestinal epithelium, DUOX-generated H2O2 supports the targeting of autophagy activity to endocytosed bacteria. This concept would also be consistent with the previous finding that DUOX2 co-localizes at the bacterial entry site with the bacterial pattern recognition receptor NOD2 20, which can directly recruit the autophagocytic protein ATG16L1 35.
Although low level of transepithelial uptake of bacteria is part of the normal immune surveillance, exaggerated translocation of bacteria into Peyer’s patches is a feature found in CD patients, despite normal permeability to protein and absent inflammation 36,37. The genes most specifically associated with CD are crucial for epithelial containment of intracellular commensals 32 and invasive pathogens, such as, Salmonella in animal models 32,33,38. The mild phenotype observed in these models is not unlike the phenotype of Duoxa−/− mice (Figure 7B). The robust upregulation of DUOX2 in non-inflamed ilea of colon-only CD patients 9 could therefore provide a compensatory response limiting bacterial translocation. In fact, we showed that a defect in the DUOX response alone is sufficient to cause ileal gene expression profiles reminiscent of those found in noninflamed ilea of cCD patients, suggesting that a functional defect in the DUOX2 system could be a novel susceptibility event in CD. Here we also showed that dysbiotic microbiota from IBD patients, but not microbiota from healthy donors, was able to trigger the DUOX2 system when transferred into GF mice. Mice reconstituted with a dysbiotic microbiota showed a higher abundance of mucosa-associated Enterobacteriaceae. In this context, it is interesting that adherent, invasive E. coli have been frequently isolated from mucosal samples of CD patients 39.
Since unbalanced production of ROS can exacerbate inflammation 40, DUOX2 upregulation in IBD is often deemed to be deleterious. Here we demonstrate that loss of DUOX activity not only increases mucosal bacterial uptake, but caused an ileal gene expression profile reminiscent of early CD-associated changes, indicating a distinct paradigm for the role of DUOX2 in early CD pathogenesis. Accordingly, the upregulation of DUOX2 in patients with quiescent ileal disease can be interpreted as a sentinel response against increased commensal-epithelial interaction reflecting a defective host defense mechanism that normally keeps commensals at bay. Thus, increased ileal DUOX2 level may prove to be an important marker of perturbed mucosal homeostasis early in the pathogenesis of IBD.
Supplementary Material
Acknowledgments
We thank X. De Deken and F. Miot for providing DUOX antibodies. This study was supported by the National Institutes of Health grants RO1DK087708-01 (J.Y.K.) and RO1DK055732-15 (J.L.M.), a JSPS Postdoctoral Fellow for Research Abroad (S. K. and H. N.-K.), the Crohn’s and Colitis Foundation of America (N.K.) and the Michigan Gastrointestinal Peptide Research Center NIDDK 5P30DK034933 (H.G., N. K.).
Abbreviations
- CD
Crohn’s disease
- cCD
colon-only Crohn’s disease
- GALT
gut-associated lymphatic tissue
- GF
germ-free
- GSEA
gene set enrichment analysis
- IBD
inflammatory bowel disease
- ILC
innate lymphoid cells
- MLN
mesenteric lymph node
- SFB
segmented filamentous bacterium
- SPF
specific pathogen-free
Footnotes
Author contributions
HG: designed study, acquired and analyzed data, prepared manuscript; JG, HNK, SK, MZ, KE, ABS: developed experimental tools; NK: supplied critical materials and reviewed manuscript; MEZ: performed microscopy; JLM and CO: contributed funding and reviewed manuscript. JYK: designed study, revised and edited manuscript, obtained funding.
Disclosures: The authors have nothing to disclose.
Accession number: NCBI GEO series GSE60933.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goto Y, Ivanov II. Intestinal epithelial cells as mediators of the commensal-host immune crosstalk. Immunol Cell Biol. 2013;91:204–14. doi: 10.1038/icb.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geiszt M, Witta J, Baffi J, et al. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–4. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 3.El Hassani RA, Benfares N, Caillou B, et al. Dual oxidase2 is expressed all along the digestive tract. American journal of physiology Gastrointestinal and liver physiology. 2005;288:G933–42. doi: 10.1152/ajpgi.00198.2004. [DOI] [PubMed] [Google Scholar]
- 4.Ha EM, Oh CT, Bae YS, et al. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–50. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- 5.Chavez V, Mohri-Shiomi A, Garsin DA. Ce-Duox1/BLI-3 generates reactive oxygen species as a protective innate immune mechanism in Caenorhabditis elegans. Infection and immunity. 2009;77:4983–9. doi: 10.1128/IAI.00627-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grasberger H, El-Zaatari M, Dang DT, et al. Dual oxidases control release of hydrogen peroxide by the gastric epithelium to prevent Helicobacter felis infection and inflammation in mice. Gastroenterology. 2013;145:1045–54. doi: 10.1053/j.gastro.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aerssens J, Camilleri M, Talloen W, et al. Alterations in mucosal immunity identified in the colon of patients with irritable bowel syndrome. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2008;6:194–205. doi: 10.1016/j.cgh.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanai H, Ben-Shachar S, Baram L, et al. Gene expression alterations in ulcerative colitis patients after restorative proctocolectomy extend to the small bowel proximal to the pouch. Gut. 2014;64:756–64. doi: 10.1136/gutjnl-2014-307387. [DOI] [PubMed] [Google Scholar]
- 9.Haberman Y, Tickle TL, Dexheimer PJ, et al. Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J Clin Invest. 2014;124:3617–33. doi: 10.1172/JCI75436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grasberger H, De Deken X, Mayo OB, et al. Mice deficient in dual oxidase maturation factors are severely hypothyroid. Mol Endocrinol. 2012;26:481–92. doi: 10.1210/me.2011-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y, Valdez PA, Danilenko DM, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–9. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 12.Cox JH, Kljavin NM, Ota N, et al. Opposing consequences of IL-23 signaling mediated by innate and adaptive cells in chemically induced colitis in mice. Mucosal Immunol. 2012;5:99–109. doi: 10.1038/mi.2011.54. [DOI] [PubMed] [Google Scholar]
- 13.Eberl G, Marmon S, Sunshine MJ, et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 14.Sommer F, Backhed F. The gut microbiota engages different signaling pathways to induce Duox2 expression in the ileum and colon epithelium. Mucosal Immunol. 2014 doi: 10.1038/mi.2014.74. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obata T, Goto Y, Kunisawa J, et al. Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc Natl Acad Sci U S A. 2010;107:7419–24. doi: 10.1073/pnas.1001061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamauchi KE, Snel J. Transmission electron microscopic demonstration of phagocytosis and intracellular processing of segmented filamentous bacteria by intestinal epithelial cells of the chick ileum. Infect Immun. 2000;68:6496–504. doi: 10.1128/iai.68.11.6496-6504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caselli M, Holton J, Boldrini P, et al. Morphology of segmented filamentous bacteria and their patterns of contact with the follicle-associated epithelium of the mouse terminal ileum: implications for the relationship with the immune system. Gut Microbes. 2010;1:367–72. doi: 10.4161/gmic.1.6.14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botteaux A, Hoste C, Dumont JE, et al. Potential role of Noxes in the protection of mucosae: H2O2 as a bacterial repellent. Microbes Infect. 2009;11:537–44. doi: 10.1016/j.micinf.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Lipinski S, Till A, Sina C, et al. DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. J Cell Sci. 2009;122:3522–30. doi: 10.1242/jcs.050690. [DOI] [PubMed] [Google Scholar]
- 21.Corcionivoschi N, Alvarez LA, Sharp TH, et al. Mucosal reactive oxygen species decrease virulence by disrupting Campylobacter jejuni phosphotyrosine signaling. Cell host & microbe. 2012;12:47–59. doi: 10.1016/j.chom.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008;6:53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 23.Sierro F, Dubois B, Coste A, et al. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc Natl Acad Sci U S A. 2001;98:13722–7. doi: 10.1073/pnas.241308598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vijay-Kumar M, Carvalho FA, Aitken JD, et al. TLR5 or NLRC4 is necessary and sufficient for promotion of humoral immunity by flagellin. Eur J Immunol. 2010;40:3528–34. doi: 10.1002/eji.201040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–89. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Johansson ME, Phillipson M, Petersson J, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–9. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnupf P, Gaboriau-Routhiau V, Gros M, et al. Growth and host interaction of mouse segmented filamentous bacteria in vitro. Nature. 2015 doi: 10.1038/nature14027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moon C, VanDussen KL, Miyoshi H, et al. Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunol. 2014;7:818–28. doi: 10.1038/mi.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 31.Kinnebrew MA, Buffie CG, Diehl GE, et al. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–87. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjamin JL, Sumpter R, Jr, Levine B, et al. Intestinal epithelial autophagy is essential for host defense against invasive bacteria. Cell Host Microbe. 2013;13:723–34. doi: 10.1016/j.chom.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conway KL, Kuballa P, Song JH, et al. Atg16l1 is required for autophagy in intestinal epithelial cells and protection of mice from Salmonella infection. Gastroenterology. 2013;145:1347–57. doi: 10.1053/j.gastro.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scherz-Shouval R, Shvets E, Fass E, et al. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–60. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Travassos LH, Carneiro LA, Ramjeet M, et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11:55–62. doi: 10.1038/ni.1823. [DOI] [PubMed] [Google Scholar]
- 36.Keita AV, Salim SY, Jiang T, et al. Increased uptake of non-pathogenic E. coli via the follicle-associated epithelium in longstanding ileal Crohn’s disease. J Pathol. 2008;215:135–44. doi: 10.1002/path.2337. [DOI] [PubMed] [Google Scholar]
- 37.Salim SY, Silva MA, Keita AV, et al. CD83+CCR7- dendritic cells accumulate in the subepithelial dome and internalize translocated Escherichia coli HB101 in the Peyer’s patches of ileal Crohn’s disease. Am J Pathol. 2009;174:82–90. doi: 10.2353/ajpath.2009.080273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lapaquette P, Glasser AL, Huett A, et al. Crohn’s disease-associated adherent-invasive E. coli are selectively favoured by impaired autophagy to replicate intracellularly. Cell Microbiol. 2010;12:99–113. doi: 10.1111/j.1462-5822.2009.01381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darfeuille-Michaud A, Boudeau J, Bulois P, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology. 2004;127:412–21. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 40.Biasi F, Leonarduzzi G, Oteiza PI, et al. Inflammatory bowel disease: mechanisms, redox considerations, and therapeutic targets. Antioxid Redox Signal. 2013;19:1711–47. doi: 10.1089/ars.2012.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.