Abstract
Arylalkylamine N-acyltransferase like 2 (AANATL2) catalyzes the formation of N-acylarylalkylamides from the corresponding acyl-CoA and arylalkylamine. The N-acylation of biogenic amines in Drosophila melanogaster is a critical step for the inactivation of neurotransmitters, cuticle sclerotization, and melatonin biosynthesis. In addition, D. melanogaster has been used as a model system to evaluate the biosynthesis of fatty acid amides: a family of potent cell signaling lipids. We have previously showed that AANATL2 catalyzes the formation of N-acylarylakylamides, including long-chain N-acylserotonins and N-acyldopamines. Herein, we define the kinetic mechanism for AANATL2 as an ordered sequential mechanism with acetyl-CoA binding first followed by tyramine to generate the ternary complex prior to catalysis. Bell shaped kcat,app – acetyl-CoA and (kcat/Km)app – acetyl-CoA pH-rate profiles identified two apparent pKa,app values of ~7.4 and ~8.9 that are critical to catalysis, suggesting the AANATL2-catalyzed formation of N-acetyltyramine occurs through an acid/base chemical mechanism. Site-directed mutagenesis of a conserved glutamate that corresponds to the catalytic base for other D. melanogaster AANATL enzymes did not produce a substantial depression in the kcat,app value nor did it abolish the pKa,app value attributed to the general base in catalysis (pKa ~7.4). These data suggest that AANATL2 catalyzes the formation of N-acylarylalkylamides using either different catalytic residues or a different chemical mechanism relative to other D. melanogaster AANATL enzymes. In addition, we constructed other site-directed mutants of AANATL2 to help define the role of targeted amino acids in substrate binding and/or enzyme catalysis.
Keywords: AANATL2, chemical mechanism, structure-function relationships, N-acyldopamines, N-acylserotonins
Graphical abstract
1. Introduction
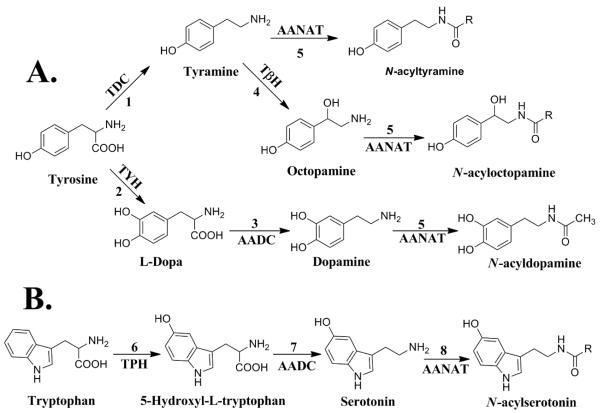
The biogenic arylalkylamines identified in Drosophila melanogaster consist of serotonin, dopamine, octopamine, and tyramine (Blenau and Baumann, 2011). Outlined in Scheme 1 is the metabolic flow of tyrosine to N-acyltryramine, N-acyloctopamine, and N-acyldopamine (Pathway A) and tryptophan to N-acylserotonin (Pathway B). Each biogenic amine and their respective N-acylated product are derived from the cognate amino acid. Two general reactions found in each pathway shown in Scheme 1 are amino acid decarboxylation and N-acylation. L-Amino acid decarboxylase is the decarboxylase involved in these pathways and is a relatively well understood (Han et al., 2010). The arylalkylamine N-acyltransferases (AANATs) catalyzing the acyl-CoA-dependent N-acylation reactions shown in Scheme 1 are likely members of the GCN5-related N-acetyltransferase (GNAT) superfamily of enzymes (Dyda et al., 2000; Vetting et al., 2005). However, the role played by the AANATs in vivo is not fully appreciated. Much of the focus on the AANATs has centered on their function in amine N-acetylation because the N-acetyl derivatives of most biogenic amines are known metabolites and N-acetylation is important in neurotransmitter inactivation, cuticle sclerotization, and melatonin biosynthesis (Andersen, 2010; Brunet, 1980; Finocchiaro et al., 1988; Wright, 1987).
Scheme 1.
Biosynthetic pathway for the biogenic arylalkylamines and their N-acylated products in D. melanogaster. (A) Tyrosine biosynthesis. Reaction 1, tyrosine decarboxylase (TDC); 2, tyrosine hydroxylase (TYH); 3, aromatic amino acid decarboxylase (AADC); 4, tyramine β-hydroxylase (TβH); 5, arylalkylamine N-acetyltransferase (AANAT). (B) Tryptophan biosynthesis. Reaction 6, tryptophan decarboxlyase (TPH); 7, aromatic amino acid decarboxylase (AADC); 8, arylalkylamine N-acetyltransferase (AANAT).
AANATs represent a fascinating family of enzymes from an evolutionary perspective. The AANAT family, all belonging to the GNAT superfamily of acyltransferases, is comprised of the insect AANATs, (iAANATs), the non-vertebrate AANATs (NV-AANATs), and the vertebrate AANATs (VTAANATs) (Coon and Klein, 2006; Falcón et al., 2014; Hiragaki et al., 2015; Pavlicek et al., 2010). The division of the AANAT family of enzymes into iAANATs, NV-AANATs, and VT-AANATs is based on sequence and phylogenetic analyses. The VT-AANATs function primarily in the melatonin metabolism and changes in VT-AANAT activity correlate to the rhythmic production of melatonin in the pineal gland (Klein, 2006). Hence, VT-AANAT has been called the “timezyme” and the evolution of the timezyme seems intimately tied to pineal gland and vertebrate photodetection (Falcón et al., 2014; Klein, 2007). The NV-AANATs function broadly in arylalkylamine detoxification and also in DNA biology resulting from polyamine acetylation (Ganguly et al., 2001; Pavlicek et al, 2010; Wittich and Walter, 1990). In fact, arylalkylamine acetylation is thought to have played a role in primitive photodetection by protecting retinaldehyde, leading to the proposal that VT-AANAT evolved from NV-AANAT as the pineal gland evolved from a primitive photosensor (Falcón et al., 2014). By virtue of their ability to acetylate melatonin, dopamine, and other biogenic amines, iAANATs are important in sclerotization, photoperiodism, amine detoxification, and cell signaling (Dempsey et al., 2014b; Hiragaki et al, 2015). The expression of an array of iaaNATs in a specific insect, as many as seven in Drosophila (Amherd et al, 2000; Dempsey et al., 2014a) and 13 in Aedes (Mehere et al., 2011) as examples, further complicates our understanding of the iaaNATs. The issue of multiple AANATs is important in organisms other than insects as amphioxus express seven NB-AANATs (Pavlicek et al., 2010) and teleost fish express three VT-AANATs (Cazaméa-Catalan et al., 2014; Coon and Klein, 2006). One important step in better understanding the various AANATs is a careful and thorough characterization of these enzymes individually, particularly in consideration of their potential involvement in fatty acid amide biosynthesis.
We have long thought that an AANAT or AANAT-like (AANATL) enzyme would be involved in biosynthesis of long-chain N-fatty acid amides (Merkler et al., 1995), a diverse family of cell signaling lipids (Farrell and Merkler, 2008; Fezza, et al., 2014). The identification of glycine N-acyltransferase-like enzymes that catalyze the formation of N-fatty acylglycines (Waluk et al., 2010) and our discovery that AANATL2 will catalyze the formation of N-fatty acylserotonins and N-fatty acyldopamines support our hypothesis. D. melanogaster is an excellent model organism for the study of the fatty acid amides, the AANAT-like enzymes, and their putative role in fatty acid amide metabolism. This organism is known to produce fatty acid amides (Jeffries et al., 2014; Tortoriello et al., 2013); therefore, one of our goals is to clone, over-express, and characterize all of these putative D. melanogaster N-acyltransferases and, ultimately, define how these enzymes contribute to fatty acid amide metabolism the flies. Our earlier work strongly suggests that AANATL2 is involved in the generation of N-fatty acylserotonins and N-fatty acyldopamines in D. melanogaster in vivo (Dempsey et al., 2014b). In addition, AANATL2 will accept a broad set of short-chain acyl-CoA thioesters as substrates, providing convenient tools for the study of its kinetic and chemical mechanisms.
Herein, we report the characterization of D. melanogaster AANATL2 that has been over-expressed in E. coli. We define the amine substrate specificity of the enzyme, have solved the kinetic mechanism by using double reciprocal analysis and dead-end inhibition, and propose a chemical mechanism for catalysis based on pH-rate studies and the results of site-directed mutagenesis experiments of targeted amino acid residues.
2. Material and methods
2.1 Materials
Antarctic Phosphatase, T4 DNA ligase, NdeI, and XhoI were purchased from New England Biolabs; XL10 E.coli cells, the pET-28a(+) vector, and BL21 (DE3) E.coli cells were purchased from Novagen; kanamycin monosulfate and IPTG were purchased from Gold Biotechnology; acetyl-CoA and oleoyl-CoA were purchased from Sigma-Aldrich; PCR primers were purchased from Eurofins MWG Operon; PfuUltra High-Fidelity DNA polymerase was purchased from Agilent; and ProBond™ nickel-chelating resin was purchased from Invitrogen. Wild-type AANATL2 was expressed and purified using the same procedures as published by Dempsey et al. (Dempsey et al., 2014b). All other reagents were of the highest quality available from either Sigma-Aldrich or Fisher Scientific.
2.2 Cloning, expression, and purification of AANATL2 site-directed mutants
All the AANATL2 mutants were generated by using the overlap extension method (Ho et al., 1989). PCR primers (Table S1) were generated for each mutant by using the Agilent QuickChange Primer Design tool. The following PCR conditions were used with PfuUltra High-Fidelity DNA polymerase: initial denaturing step of 95°C for 2 min, then 30 cycles of (95°C for 30 s; 60°C for 30 s; 72°C for 1 min), and a final extension step of 72°C for 10 min. The AANATL2 mutant PCR product was then inserted into a pET-28a(+) vector using NdeI and XhoI restriction sites. The AANATL2-mutant-pET-28a product was transformed into E. coli XL10 competent cells, plated on Luria Broth (LB) agar plates supplemented with 40 μg/mL kanamycin, and incubated overnight at 37°C. The following day, a single colony from the LB agar plate containing the E. coli XL10 cells with the AANATL2-mutant-pET-28a vector was used to inoculate an overnight culture in LB media, supplemented with 40 μg/mL kanamycin at 37°C. The next day, the AANATL2-mutant-pET-28a plasmid was purified using Promega Wizard® Plus SV Minipreps DNA purification kit and sequenced by Eurofins MWG operon. Following confirmation of the AANATL2 mutant sequence, the AANATL2-mutant-pET-28a plasmid was then transformed into BL21 (DE3) E.coli cells. Each AANATL2 mutant protein was expressed and purified using the same procedures that were defined for the wild-type enzyme (Dempsey et al.).11 After the purification of each enzyme, the AANATL2 mutants and wild-type enzyme were dialyzed into 20 mM Tris pH 7.4, 200 mM NaCl for 16 hr at 4°C.
2.3 Enzyme assay
Ellman’s reagent (DTNB) was used to determine the kinetic constants for different amino donor substrates and acetyl-CoA with AANATL2 by measuring the rate of coenzyme A release at 412 nm (molar extinction coefficient = 13,600 M−1 cm−1) (Ellman, 1959) at 22°C and measuring the initial velocities with a Cary 300 Bio UV-Visible spectrophotometer. The assay solutions consisted of 300 mM Tris pH 8.0, 150 μM DTNB, varying concentration of one substrate while holding the other substrate at a fixed saturating concentration. The resulting data was fit to equation 1, in which vo is the initial velocity, Vmax is the maximal velocity, [S] is the substrate concentration, and Km is the Michaelis constant. Each assay was performed in triplicate and the uncertainty for the kcat,app and (kcat/Km)app values were calculated by using equation 2 (Morrison and Uhr, 1966), for which σ is the standard error.
| Equation 1 |
| Equation 2 |
2.4 Kinetic mechanism
Initial velocity patterns for acetyl-CoA and tyramine were used to distinguish between the sequential or ping-pong kinetic mechanisms. The initial velocities were measured for acetyl-CoA by holding the tyramine concentration constant (1 μM, 2 μM, 10 μM, and 25 μM) and varying the concentration of acetyl-CoA. The initial velocities for tyramine were measured by holding the acetyl-CoA concentration constant (1 μM, 2 μM, 10 μM, and 25 μM) and varying the concentration of tyramine. The resulting data were fit to equation 3 for a Bi Bi sequential kinetic mechanism and plotted as a double reciprocal plot using IGOR Pro 6.34A. In equation 3, Kia is the dissociation constant for substrate A (acetyl-CoA), Ka is the Km (Michaelis constant) for substrate A (acetyl-CoA), and Kb is the Km (Michaelis constant) for substrate B (tyramine).
| Equation 3 |
Following the double reciprocal analysis, dead-end inhibitor analysis was used to differentiate between an ordered or random sequential kinetic mechanism. Oleoyl-CoA and tyrosol were used as dead-end inhibitors as these are analogs for acetyl-CoA and tyramine, respectively. Initial velocities for the inhibition experiments were measured for acetyl-CoA and tyramine by varying the concentration of one substrate, holding the other substrate at a fixed concentration equal to the respective Km,app (acetyl-CoA = 7.2 μM; tyramine = 3.9 μM), at different concentrations of the inhibitor. Inhibition patterns were determined by fitting the resulting data, using SigmaPlot 12.0, to equations 4 – 6; for competitive, noncompetitive, and uncompetitive inhibition, respectively. The terms in equations 4 – 6 represent the following: vo is the initial velocity, Vmax is the maximal velocity, [S] is the substrate concentration, Km is the Michaelis constant, [I] is the inhibitor concentration, and Ki is the inhibition constant. Each assay was performed in triplicate.
| Equation 4 |
| Equation 5 |
| Equation 6 |
2.5 Rate versus pH dependence
The pH dependence on the kinetic constants for the wild-type and E29A mutant enzymes were determined for acetyl-CoA. The kinetic constants were determined at pH values ranging from 6.5 – 9.5 at intervals of every 0.5 pH units. The following buffers were used for the pH dependence study: MES (pH 6.5 – 7.0), Tris (pH 7.0 – 9.0), and AMeP (pH 9.0 – 9.5). The kinetic constants from the pH dependence experiments were fit using IGOR Pro 6.34A, to equation 7 [log (kcat/Km – tyramine)] and equation 8 [log (kcat)], where c is the pH-independent plateau. Each assay was performed in duplicate.
| Equation 7 |
| Equation 8 |
3. Results and discussion
3.1 Structure-activity relationships: amine substrates AANATL2
We have previously published a limited set of data regarding the substrate specificity of AANATL2 because our goal, in that study, was to correlate the identification of long-chain N-acylserotonins in D. melanogaster to substrates for the enzyme. We found that long-chain acyl-CoAs would only serve as AANATL2 substrates when the amine substrate was serotonin or dopamine. However, all the known biogenic arylalkylamines in D. melanogaster (serotonin, dopamine, octopamine, and tyramine) are AANATL2 substrates when acetyl-CoA is the acyl-CoA donor (Dempsey et al., 2014b). In the work we report here, we further evaluate the structure-activity relationships for other amino donor substrates with acetyl-CoA serving as the corresponding acyl-CoA substrate (Table 1). We focused on five major structural features for amino donor substrates consisting of: (a) modification of the of the indole ring for tryptamines, (b) modification of the phenyl ring for phenethylamines, (c) modification of the two methylene spacer between the phenyl ring and the primary amine in phenethylamine analogs, (d) alternations in the space length between the phenyl ring and the primary amine in phenethylamine analogs, and (e) other non-arylalkylamine amino donors.
Table 1.
| Substrate | Structure | Km, app (μM) |
kcat, app (s−1) |
(kcat/Km)app (M−1s−1) |
|---|---|---|---|---|
| 3-(Trifluoromethyl)phenethylamine |
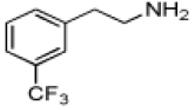
|
10 ± 2 | 4.5 ± 0.2 | (4.4 ± 0.7) × 105 |
| 4-Methoxyphenethylamine |
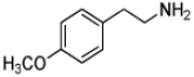
|
10 ± 2 | 3.7 ± 0.1 | (3.7 ± 0.6) × 105 |
| 3-Methoxyphenethylamine |

|
13 ± 2 | 4.4 ± 0.2 | (3.5 ± 0.6) × 105 |
| 5-Benzyloxytryptamine |
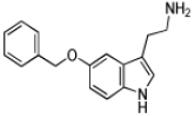
|
8 ± 1 | 2.8 ± 0.1 | (3.3 ± 0.4) × 105 |
| Serotoninc |
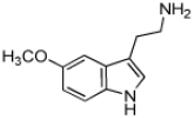
|
7 ± 1 | 2.4 ± 0.1 | (3.3 ± 0.1) × 105 |
| β-Methylphenethylamine |
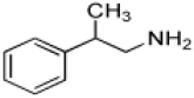
|
9 ± 1 | 2.9 ± 0.1 | (3.2 ± 0.4) × 105 |
| Tryptamine |
|
14 ± 3 | 4.3 ± 0.3 | (3.1 ± 0.6) × 105 |
| Tyramine |
|
5 ± 1 | 1.5 ± 0.03 | (2.9 ± 0.3) × 105 |
| 3,4-Methylenedioxyphenethylamine |
|
27 ± 5 | 6.3 ± 0.4 | (2.3 ± 0.4) × 105 |
| Phenethylamine |

|
35 ± 10 | 7.3 ± 1 | (2.1 ± 0.7) × 105 |
| Norepinephrine |
|
27 ± 3 | 5.6 ± 0.3 | (2.1 ± 0.3) × 105 |
| Dopaminec |
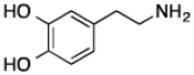
|
42 ± 10 | 7 ± 1 | (1.6 ± 0.2) × 105 |
| 4-Phenylbutylamine |
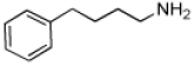
|
9.5 ± 0.4 | 1.0 ± 0.02 | (1.1 ± 0.05) × 105 |
| 5-Methoxytyrptamine |
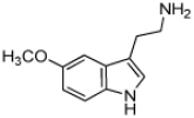
|
25 ± 2 | 2.6 ± 0.1 | (1.0 ± 0.1) × 105 |
| Octopaminec |
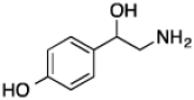
|
78 ± 4 | 2.3 ± 0.04 | (3.0 ± 0.05) × 104 |
| Benzylamine |
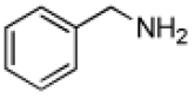
|
210 ± 4 | 0.092 ± 0.002 |
430 ± 10 |
| Histamine |
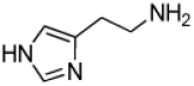
|
3500 ± 200 | 0.90 ± 0.02 |
260 ± 5 |
| Ethanolamine |

|
(1.1 ± 0.1) × 105 |
0.82 ± 0.03 |
7 ± 1 |
Reaction condition – 300 mM Tris pH 8.0, 150 μM DTNB, 50 μM acetyl-CoA, and varying concentration of amine substrate.
Kinetic constants are reported as ± standard error (n = 3).
Data is published in Dempsey et al., 2014b
Tryptamine (2-(1H-Indol-3-yl)ethanamine), the decarboxylated analog of tryptophan, possesses an indole ring. With the exception of 5-methoxytryptamine, the (kcat/Km)app values the set of tryptamine analogs we evaluated as AANATL2 substrates were the same within experimental error (Table 1). The relatively small decrease in the (kcat/Km)app value for 5-methoxytryptamine, 1.8 – 3.5-fold, relative to tryptamine and the other 5-substituted tryptamines resulted from an increase in the Km,app value. The kcat,app value was generally the same for all the tryptamine analogs we included, except for a ~2-fold higher value for tryptamine. In all, there were minimal differences between the kinetic constants for tryptamine and the 5-substituted tryptamine substrates, indicating that AANATL2 can readily accommodate even a large benzyloxy group at the 5-position of the indole ring. Accommodation could occur via a region in the AANATL2 active site or flexibility in the active site that does not significantly affect the effective positioning of the amine for nucleophilic attack at the carbonyl of the acetyl-CoA thioester moiety.
Phenethylamine, C6H5-CH2-CH2-NH2, is a AANATL2 substrate exhibiting a similar (kcat/Km)app to that we measured for tryptamine. Included in our assessment of amine substrates were six ring-substituted phenethylamines: 3-(trifluoromethyl)phenethylamine, 4-methoxyphenethylamine, 3-methoxyphenethylamine, 4-hydroxyphenethylamine (tyramine), 3,4—dihydroxyphenethylamine (dopamine), and 3,4-methylenedioxyphenethylamine (Table 1). We found limited variation in the (kcat/Km)app values between the six phenethylamines included in our study; the largest difference being the ratio of (kcat/Km)app, 3-(trifluoromethyl)phenethylamine/(kcat/Km)app,dopamine ~ 3.
Another class of phenethylamine analogs we evaluated as AANATL2 substrates, were variants in the –CH2-CH2- spacer between the phenyl ring and the amine. Included in this group of phenethylamines are octopamine and norepinephrine with a β-hydroxyl group attached to the spacer, β-methylphenethylamine with a β-methyl group attached to the spacer, 4-phenylbutylamine with a longer spacer (-CH2-CH2-CH2-CH2-), and benzylamine with a shorter spacer (-CH2-). For two of the phenylethylamine pairs that only differ in a β-substituent on the spacer, dopamine/norepinephrine and phenethylamine/β-methylphenethylamine, we found little difference in the (kcat/Km)app values. However, we did measure a ~10-fold difference in (kcat/Km)app values for the tyramine/octopamine pair – a result that is difficult to reconcile with our other data. The decrease in the spacer length has a dramatic effect on catalytic efficiency as benzylamine is a very poor substrate when compared to phenethylamine, the ratio of (kcat/Km)app,benzylamine to (kcat/Km)app,phenethylamine is ~0.002. In contrast, the increase in the spacer length from two methylene groups to four has a limited effect on catalytic efficiency, evidence being the ratio of (kcat/Km)app,phenylbutylamine to (kcat/Km)app,phenethylamine of is ~0.50. In considering our data on the phenethylamines as AANATL2 substrates, we found that decoration of the phenyl ring or -CH2-CH2- spacer and an small increase in the spacer length has limited effect on the (kcat/Km)app values. These results, again, point toward flexibility in the AANATL2 active site to accommodate structural differences between the phenethylamine substrates, included in our study.
An obvious set of amine substrates to consider in our evaluation of potential amine substrates are the amino acids, possessing a carboxylate moiety at the α-position of the spacer. Tyrosine, phenylalanine, and tryptophan, the amino acids most related to arylalkylamine substrates reported in Table 1, were not AANATL2 substrates at a concentration of 1.0 mM, suggesting that the modification of the α-position of the spacer group precludes catalysis by either a steric effect, an electrostatic effect, or a combination of the two effects. To evaluate between these possibilities, we evaluated tyrosine methyl ester as a substrate and, again, found no activity at 1.0 mM, suggesting that the arylalkylamines with a modification at the α-position of the spacer group will not serve as substrates prominently due to a steric effect. We further found no inhibition of the AANATL2-catalyzed formation of N-acetyltyramine from acetyl-CoA and tyramine for tyrosine, phenylalanine, tryptophan, and tyrosine methyl ester at 1.0 mM, confirming that these α-position modified arylalkylamines do not bind or bind weakly to AANATL2 (Kd ≥ 10 mM).
In completing our studies of potential amine substrates for AANATL2, we examined two non-arylalkylamines as substrates: histamine and ethanolamine. Histamine is substrate with a relatively high kcat/Km for AANATL7, a related arylalkylamine N-acyltransferase in D. melanogaster (Dempsey et al., 2015) and the acylation of ethanolamine would lead to the bioactive N-acylethanolamines (Farrell and Merkler, 2008). We found that AANATL2 will catalyze the N-acetylation of histamine and ethanolamine, albeit with high (Km)app and low kcat,app values relative to the arylalkylamine substrates. For example, when compared to the arylalkylamine with the highest (kcat/Km)app value, the (kcat/Km)app values for histamine and ethanolamine are only 0.06% and 0.002% of that for 3-(trifluoromethyl)phenethylamine, respectively. We also determined that AANATL2 does not catalyze the N-acetylation of isoniazid, which is a first line drug for the treatment of tuberculosis (Chang et al., 2013; Vernon, 2013; Zumla et al., 2013). In sum, our data suggest that AANATL2 predominantly catalyzes the N-acetylation of biologically relevant arylalkyamines.
3.2 Structure-activity relationships: acyl-CoA thioester substrates AANATL2
Previous data shows that the amine specificity of AANATL2 is dependent on the chain length of the acyl-CoA co-substrate, as the acyl-chain length increased from acetyl-CoA to oleoyl-CoA (Dempsey et al., 2014b). We found that tyramine and octopamine are AANATL2 substrates when acetyl-CoA is the acyl donor; however, tyramine and octopamine will not as substrates when the acyl donor is oleoyl-CoA. Two possible explanations for these data are: (a) longer chain acyl-CoAs will serve as substrates in a different enzyme form that is specific for serotonin or dopamine, or (b) the acyl group of the long-chain acyl-CoAs will occupy the amine binding pocket, resulting in the perturbed and non-productive binding of amine substrates. The Km,app value increased >25-fold for both serotonin and dopamine when a longer chain acyl-CoA (C16 – C20) is used in place of acetyl-CoA. Coupling these data to the dead-end inhibition data of Section 3.2, it is more likely that the longer acyl group is perturbing amine substrate binding, meaning that only certain amines (serotonin and dopamine) will function as substrates when the co-substrate is a long-chain acyl-CoA thioester (Dempsey et al., 2014b).
Overall, the data from the substrate specificity studies reported here and published elsewhere (Dempsey et al., 2014b) show that AANATL2 has significant substrate promiscuity for both acyl-CoA and amine substrates. The three major structural features that affect binding and/or catalysis for AANATL2 substrates were the nature of the amine (arylalkylamine or non-arylalkylamine), the length and α-modification of the spacer in the phenethylamines and the length of the acyl group for the acyl-CoA thioesters. The structure-activity results show that effective AANATL2 catalysis, characterized by a relatively high (kcat/Km)app value, requires an optimum positioning of the amine for nucleophilic attack at the carbonyl of the acyl-CoA thioester. The ratio of ~10-fold between the (kcat/Km)app values for the tyramine/octopamine pair and that of ~500 for the benzylamine/phenethylamine pair is consistent with our hypothesis, illustrating that subtle interactions between the substrate and active site amino acids can significantly impact effective catalysis.
3.3 Kinetic mechanism
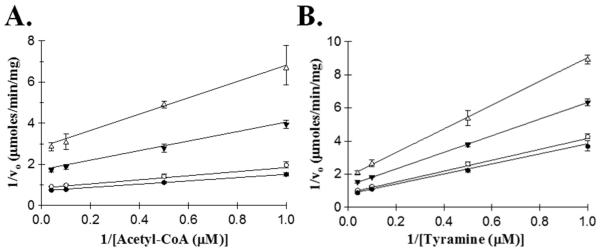
Defining the kinetic mechanism for AANATL2 could provide insight into the broad specificity for the acyl-CoA substrates and acyl-CoA-dependence of the amine specificity. Using acetyl-CoA and tyramine as substrates, we varied the concentration of one substrate at different, but fixed concentrations of the second substrate. The intersecting double reciprocal plots for both substrates were the outcome of these experiments (Figure 1) strongly suggesting that AANATL2 will catalyze the formation of N-acetyltyramine by a sequential mechanism. This means that a ternary AANATL2•acetyl-CoA•tyramine complex forms prior to catalysis.
Figure 1.
Double reciprocal plots of initial velocities for acetyl-CoA and tyramine. (A) Initial velocities measured at a fixed concentration of tyramine: 25 μM (●), 10 μM (○), 2 μM (▼), 1 μM (Δ). (B) Velocities measured at a fixed concentration of acetyl-CoA: 25 μM (●), 10 μM (○), 2 μM (▼), 1 μM (Δ).
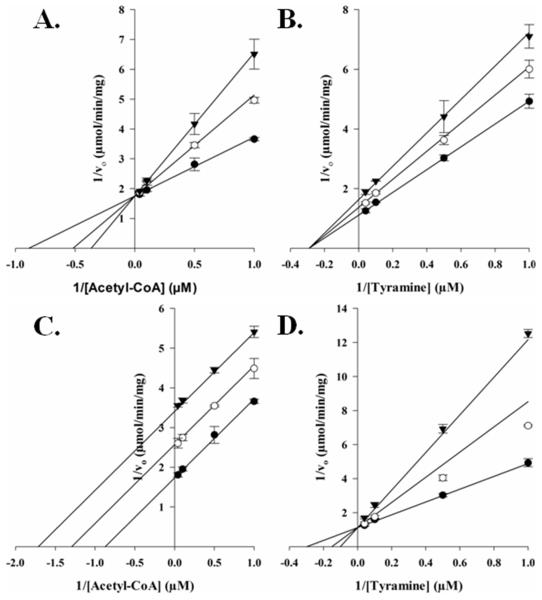
Next, we sought to delineate between random or ordered substrate binding for acetyl-CoA and tyramine. We accomplished this using a structural analog for each substrate as an AANATL2 inhibitor. Previous data showed that oleoyl-CoA would not serve as a substrate for AANATL2 when tyramine was the amine substrate (Dempsey et al., 2014b); therefore, we decided to use oleoyl-CoA as an acetyl-CoA analog. Long-chain acyl-CoAs have been used as inhibitors for GNAT enzymes (Dempsey et al., 2014c; Ferry et al., 2000; Khalil and Cole, 1998) and as dead-end inhibitors in elucidating the kinetic mechanism for other D. melanogaster AANATL enzymes (Dempsey et al., 2014a, 2015). We employed tyrosol as the other dead-end inhibitor because tyrosol, is an analog of tyramine in which the amino group is replaced with a hydroxyl group. In addition, O-acetylation of tyrosol is not catalyzed by AANATL2 because we found no measurable rate of CoA release above background at 1.0 mM acetyl-CoA and concentrations of tyramine as high as 10 mM. Oleoyl-CoA is competitive vs. acetyl-CoA, Ki = 180 ± 30 nM (Figure 2A), and noncompetitive vs. tyramine, Ki = 540 ± 40 nM (Figure 2B). Tyrosol is uncompetitive vs. acetyl-CoA, Ki = 104 ± 6 μM (Figure 2C), and a competitive vs. tyramine, Ki = 52 ± 9 μM (Figure 2D). These data indicate that AANATL2 catalyzes the formation of N-acetyltyramine via an ordered sequential mechanism, in which acetyl-CoA binds first followed by tyramine to generate the AANATL2•acetyl-CoA•tyramine ternary complex prior to any product formation. An ordered sequential mechanism identified for AANATL2 is consistent with other D. melanogaster AANATL enzymes and with the mammalian ortholog, sheep serotonin N-acetyltransferase (De Angelis et al., 1998). The fact that the acyl-CoA binds first could provide an explanation for dependence of the amine specificity on the acyl-chain length of the acyl-CoA substrate. Since an AANATL2•acetyl-CoA complex is required prior to tyramine binding, it is likely that the longer-chain the acyl group of oleoyl-CoA can, at least partially, occupy the amine substrate binding site, perturbing the ability for specific amines to bind properly for effective catalysis to occur. This model is supported by the significant increase in the Km,app for serotonin (7.2 μM → 490 μM) and dopamine (42 μM → 1.2 mM), when comparing oleoyl-CoA to acetyl-CoA as the saturating acyl-CoA substrate (Dempsey et al., 2014b). The increase in the Km,app and decrease in the kcat,app values (acetyl-CoA → oleoyl-CoA) suggest that as the length of the acyl-CoA aliphatic chain is increased, it begins to occupy the binding site for the amine substrate, leading to non-productive amine binding which results in a decrease in the rate of catalysis.
Figure 2.
Dead-end inhibtion analysis of AANATL2. (A) Initial velocities measured at a fixed concentration of tyramine (3.9 μM), varying the concentration of acetyl-CoA, and varying the concentration of the inhibitor, oleoyl-CoA: 0 nM (●), 125 nM (○), 250 nM (▼); Ki = 180 ± 30 nM. (B) Initial velocities measured at a fixed concentration of acetyl-CoA (7.2 μM), varying the concentration of tyramine, and varying the concentration of the inhibitor, oleoyl-CoA: 0 nM (●), 125 nM (○), 250 nM (▼); Ki = 540 ± 40 nM. (C) Initial velocities measured at a fixed concentration of tyramine (3.9 μM), varying the concentration of acetyl-CoA, and varying the concentration of the inhibitor, tyrosol: 0 μM (●), 50 μM (○), 100 μM (▼); Ki = 104 ± 6 μM. (D) Initial velocities measured at a fixed concentration of acetyl-CoA (7.2 μM), varying the concentration of tyramine, and varying the concentration of the inhibitor, tyrosol: 0 μM (●), 50 μM (○), 100 μM (▼); Ki = 52 ± 9 μM.
3.4 AANATL2: acid/base catalysis and the amino acid residues important to this chemistry
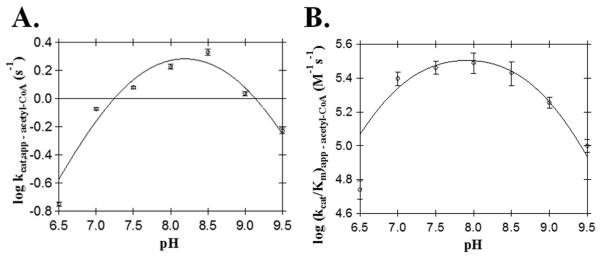
We used a series of pH-activity profiles, primary sequence alignments to other D. melanogaster AANATL enzymes, and site-directed mutagenesis experiments to generate data that was used to produce a proposed chemical mechanism for AANATL2-mediated catalysis. First, we determined the pH-dependence of the apparent steady-state kinetic constants for acetyl-CoA by varying [acetyl-CoA] at constant [tyramine] as a function of pH (Figure 3). Bell-shaped curves were obtained for both, the kcat,app – acetyl-CoA and (kcat/Km)app – acetyl-CoA profiles yielding two apparent pKa values. The pKa,app values from the kcat,app – acetyl-CoA profile were 7.4 ± 0.2 and 8.9 ± 0.2, whereas the pKa,app values from the (kcat/Km)app – acetyl-CoA profile were 6.8 ± 0.2 and 9.0 ± 0.1. The pKa,app values of 7.4 and 6.8 from both profiles, likely represents the same ionizable group - the general base in catalysis. The differences in these two pKa,app values most likely results from either the kinetic stickiness of acetyl-CoA or from the difficulty in measuring the acetyl-CoA kinetic constants with varying concentrations that range at the low end of the linear response for the DTNB assay. The second pKa,app values of 8.9 and 9.0 represents the same ionizable group, either the general acid in catalysis or the thiol group of coenzyme A with a slightly perturbed pKa. The pKa for the thiol group of coenzyme A has been reported to range from 9.6 to 10.4 (Pitman and Morris, 1980; The Merck Index, 1996).
Figure 3.
AANATL2 wild-type pH-rate profiles. (A) kcat,app for acetyl-CoA. (B) (kcat/Km)app for acetyl-CoA.
Studying the pH-dependence of kinetic constants for the AANATL2 wild-type provides information regarding acid/base catalysis, but does not define the specific ionizable groups that account for the measured pKapp values. Therefore, we used a combination of primary sequence alignment with other D. melanogaster AANATL enzymes (Dempsey et al., 2014a) and site-directed mutagenesis of conserved residues to aid our identification of the respective amino acids that function in catalysis and/or substrate binding. A primary sequence alignment of the eight D. melanogaster AANATL enzymes pointed towards five amino acid residues of AANATL2 that could function in catalysis and/or substrate binding: Glu-29, Pro-30, Arg-138, Ser-171, and His-206 (Dempsey et al., 2014b). The amino acids that correlate to these five amino acids from AANATL2 in AANATA (Dempsey et al., 2014a) and AANATL7 (Dempsey et al., 2015) were evaluated by site-directed mutagenesis. Therefore, we can index the data from the site-directed alanine mutants (Table 2) to the corresponding residues from the previously studied D. melanogaster AANATL enzymes to delineate a respective function in AANATL2 (Dempsey et al., 2014a, 2015).
Table 2.
Steady-state kinetic constants for AANATL2 mutant enzymesa
| Mutant b | Acetyl-CoA | |||
|---|---|---|---|---|
| Km, app (μM) |
kcat, app (s−1) |
(kcat/Km)app (M−1s−1) |
||
| % | ||||
| Wild-type | 5 ± 1 | 1.6 ± 0.1 | (3.1 ± 0.4) × 105 | 100 |
| E29A | 5.1 ± 0.2 | 0.37 ± 0.01 | (7.2 ± 0.3) × 104 | 23 |
| P30A | 3.3 ± 0.4 | 0.032 ± 0.001 | (10 ± 1) × 103 | 3.2 |
| R138A | 1.6 ± 0.1 | 0.313 ± 0.004 | (1.9 ± 0.1) × 105 | 61 |
| S167A | 40 ± 2 | 0.75 ± 0.01 | (1.9 ± 0.1) × 104 | 6.1 |
| S171A | ndc | ndc | ndc | ndc |
| H206A | ndc | ndc | ndc | ndc |
| Mutant d | Tyramine | |||
| Km, app (μM) |
kcat, app (s−1) |
(kcat/Km)app (M−1s−1) |
||
| % | ||||
| Wild-type | 5 ± 1 | 1.47 ± 0.03 | (2.9 ± 0.3) × 105 | 100 |
| E29A | 85 ± 5 | 0.37 ± 0.01 | (4.4 ± 0.3) × 103 | 1.5 |
| P30A | 52 ± 7 | 0.043 ± 0.001 | 820 ± 110 | 0.28 |
| R138A | 6 ± 1 | 0.26 ± 0.01 | (4.5 ± 0.7) × 104 | 16 |
| S167A | 19 ± 2 | 1.03 ± 0.04 | (5.3 ± 0.7) × 104 | 18 |
| S171A | ndc | ndc | ndc | ndc |
| H206A | ndc | ndc | ndc | ndc |
Kinetic constants are reported as ± standard error (n = 3).
The reaction rate was measured at a fixed saturating concentration of tyramine while varying the concentration of acetyl-CoA.
The notation of “nd” is used for a mutant that did not display a rate of coenzyme A release at 412 nm above the baseline hydrolysis rate.
The reaction rate was measured at a fixed saturating concentration of acetyl-CoA while varying the concentration of tyramine.
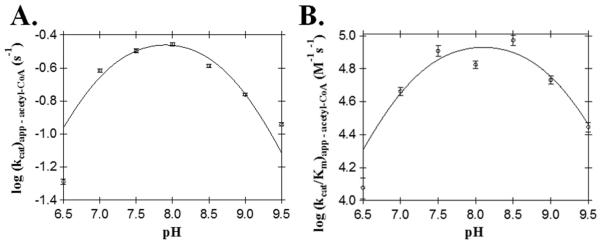
In both AANATA and AANATL7, a glutamate has been proposed as the general base in catalysis. The corresponding glutamate in AANATL2 is Glu-29; thus, this amino acid may serve as the general base in catalysis. The kcat,app value for the E29A mutant was 23% - 25% of the wild-type enzyme, whereas the (kcat/Km)app for acetyl-CoA and tyramine decreased 4.3- and 66-fold, respectively (Table 2). The Km,app,acetyl-CoA for E29A has the same value as wild type AANATL2, indicating that Glu-29 does not function in acetyl-CoA binding. Interestingly, the small decrease in the kcat,app value suggests that Glu-29 is not the general base in catalysis; however, the large increase in the tyramine Km,app (17-fold) indicates that this residue is important to amine substrate binding. Since the corresponding glutamates found in D. melanogaster AANATA (Glu-47) (Dempsey et al., 2014a) and AANATL7 (Glu-26) (Dempsey et al., 2015), were identified as the general base in catalysis, we wanted to confirm that Glu-29 in AANATL2 does not have the same function in catalysis. This was accomplished by evaluating the pH-dependence on the acetyl-CoA kinetic constants for the E29A mutant (Figure 4). Both, the kcat,app – acetyl-CoA and (kcat/Km)app – acetyl-CoA profiles for E29A produced a bell-shaped curve with two apparent pKa values, similar to the wild-type. The pKa,app values for the E29A mutant were: kcat,app – acetyl-CoA = 7.0 ± 0.1 and 8.8 ± 0.1, whereas the pKa,app values for the (kcat/Km)app – acetyl-CoA profile = 7.1 ± 0.2 and 9.1 ± 0.2. The pKa,app value of ~7.0 for the E29A mutant corresponds to the general base in catalysis, whereas the pKa,app value of ~9.0 is either the general acid in catalysis or the thiol group of the coenzyme A product. The similarity in the pKa,app value of ~7.0 for both the E29A mutant and wild type enzymes strongly implies that that Glu-29 is not the general base in AANATL2 catalysis. This is a striking difference from the two other D. melanogaster AANATL enzymes, where the corresponding glutamate functions as the general base (Dempsey et al., 2014a, 2015). When the appropriate glutamate was mutated to an alanine in these enzymes, the pH-rate profile for the alanine mutant has a linear dependence on pH (instead of a bell-shaped profile) with the disappearance of the pKa,app for the general base (Cheng et al., 2012; Dempsey et al., 2014a, 2015) – a very different results than what we found for the E29A mutant. Another possibility to account for our data for the E29A mutant would be that Glu-29 does function as the general base in AANATL2 catalysis, but in a redundant role with another active site amino acid. In this manner, mutation of Glu-29 to Ala would have a relativity minimal effect on catalysis. There is literature precedent for two residues functioning in a redundant role as the general base in catalysis, as observed for sheep serotonin N-acetyltransferase (His-120 and His-122) (Hickman et al., 1999a, 1999b; Klein, 2007; Scheibner et al., 2002; Zheng and Cole, 2003). Further evaluation of the redundancy that active site amino acid residues might have with respect to functioning as a general base requires the availability of a crystal structure.
Figure 4.
AANATL2 E29A pH-rate profiles. (A) kcat,app for acetyl-CoA. (B) (kcat/Km)app for acetyl-CoA.
The second pKa,app observed in the wild-type pH-rate profiles (pKa,app ~9.0) is either the general acid in catalysis or the thiol group of the CoA-SH product. The decrease in the activity at high pH could result from CoA-S− having greater affinity to AANATL2 than the CoA-SH form (Kd, CoA-SH > CoA-S−). Two serine residues in AANATA, Ser-182 and Ser-186, were proposed to function in catalysis, with Ser-186 suggested as the general acid (Cheng et al., 2012). In AANATL2, Ser-167 and Ser-171 corresponds to Ser-182 and Ser-186 in AANATA. We prepared both the S167A and S171A mutants and determined the effect of these mutations on catalysis (Table 2). The S167A mutant yielded a kcat,app value that is 47% of the wild-type and a Km,app - acetyl-CoA and Km,app - tyramine that were increased 8- and 4-fold, respectively. The minimal decrease in the S167A kcat,app value suggests that this residue probably does not function directly in catalysis. However, the increase in both substrates Km,app values indicates that this residue has an effect on substrate binding, possibly by functioning in the proper organization of the active site. The second serine mutant, S171A, was completely deficient of catalysis, in which there was not a rate observed above the baseline rate of acetyl-CoA. This could indicate that Ser-171 is the general acid in catalysis or that this residue has a critical function in one (or more) of the following: (a) structural component in the formation of the active site, (b) participates in a “proton wire” by shuttling protons out of the active site (Dempsey et al., 2014a; Hegde et al., 2007; Hickman et al., 1999b; Vetting et al., 2005), or (c) required for the proper positioning of the catalytic residues. Since S171A is catalytically deficient, we could not evaluate the mutant pH-rate profile to determine if this residue corresponds to the second pKa,app value observed in the wild-type enzyme. It seems unlikely that Ser-171 functions as a general acid, since it is thermodynamically unfavorable to transfer a proton from a serine hydroxyl to the thiolate anion of CoA. The serine residue requires a substantial depression in the pKa value (3-5 pH units) to function in the protonation of a thiolate. It is more likely that this residue has a critical structural function that when disrupted, completely abolishes catalysis.
3.5 Evaluation of AANATL2 amino acid residues that are conversed between other D. melanogaster AANATLs
We further evaluated the other conserved residues to understand their respective function in AANATL2. The amino acid residues, Pro-30, Arg-138, and His-220, were each mutated to an alanine and their respective kinetic constants evaluated. To further understand the function of these residues in the absence of a crystal structure, we indexed the data to that generated for the corresponding residue in D. melanogaster AANATA (Pro-48) and AANATL7 (Pro-27) (Dempsey et al., 2014a, 2015). Pro-30 corresponds to Pro-48 and Pro-27 in AANATA and AANATL7, respectively. The P30A mutant produced a kcat,app value that is 2% - 3% of the wild-type enzyme, whereas the Km,app for tyramine increased 10-fold. In contrast to the data generated for tyramine, the Km,app for acetyl-CoA was similar to the wild-type enzyme. These data suggest that Pro-30 has an important function in regulating catalysis and the binding of the amine substrate; however, has limited impact on acetyl-CoA binding. The corresponding residue in the mammalian ortholog, sheep serotonin N-acetyltransferase (Pro-64) and D. melanogaster AANATA is located on a “floppy loop” that occupies the binding site of acetyl-CoA (Dempsey et al., 2014a; Pavlicek et al., 2008). After acetyl-CoA binding, the proline residue migrates ~ 8 Å to open up the amine substrate binding pocket for the sheep ortholog, in addition to having a direct π-stacking interaction with the indole ring of the tryptamine-acetyl-CoA bisubstrate inhibitor. The movement of the “floppy loop” results in the conversion of the enzyme from a low to high affinity state and this particular proline residue is the catalytic switch serving to regulate this conversion (Dempsey et al., 2014a; Pavlicek et al., 2008). The large decrease in the kcat,app value and increase in the Km,app – tyramine suggests, but does not prove, that Pro-30 of AANATL2 is, also, a catalytic switch regulating loop movement in this enzyme, as well. The proposed movement of Pro-30 is important to the formation of the amine substrate binding pocket, providing a structural rational to the ordered sequential mechanism. Acetyl-CoA binds first, resulting in the Pro-30-dependent movement of the loop to convert AANATL2 from a low affinity state to high affinity state for the amine substrate, followed by the binding of the amine substrate (tyramine, in our specific experiments). This proposed function of Pro-30 as the catalytic switch is consistent with our data and that for the other GNAT enzymes characterized to date (Dempsey et al., 2014a, 2015; Pavlicek et al., 2008). One puzzling aspect in the data for the P30A mutant is lack of difference for the Km,app – acetyl-CoA for the P30A mutant of AANATL2, as was observed for both corresponding proline to alanine mutants constructed for AANATA (Dempsey et al, 2014a) and AANATL7 (Dempsey et al., 2015). The relative position of Pro-30 in the low affinity state does not hinder acetyl-CoA binding for AANATL2 is one possible explanation for our results, as observed for the other AANAT enzymes (Dempsey et al., 2014a; Hickman et al., 1999a; Pavlicek et al., 2008). However, our data for the P30A mutant for D. melanogaster AANATL2 clearly shows that Pro-30 does function to regulate catalysis and in the binding of the amine substrate.
Creation of the R138A mutant yielded an enzyme with a kcat,app value that is ~20% of the wild-type enzyme and Km,app values for acetyl-CoA and tyramine that were similar to that of wild-type enzyme. Mutation of the corresponding arginine to alanine in D. melanogaster AANATA (Arg-153) and D. melanogaster AANATL7 (Arg-138) yielded active mutant enzymes with kinetic constants, kcat,app and the Km,app for both substrates, that were increased relative to wild-type (Dempsey et al., 2014a, 2015). In AANATA, we proposed that the corresponding arginine, Arg-153, formed a salt bridge with Asp-46 and that the Arg-153:Asp-46 was critical to a conformation responsible for the relatively tight-binding of CoA-S− product. Thus, in AANATA, CoA-SH release is partially rate-determining and elimination of the Arg-153:Asp-46 in the R153A mutant is consistent with the observed increase in kcat,app (Dempsey et al., 2014a). While there in no reported structure for AANATL7, the similarity in the data between AANATA and AANATL7 suggests that Arg-153 in AANATA and Arg-138 in AANATL7 fulfill the same role in both acyltransferases. The differences in the kinetic constants from the R138A mutant in AANATL2 relative to those obtained for the corresponding arginine to alanine mutants in AANATA and AANATL7 indicate that Arg-138 may serve a different role in AANATL2. Without a structure, it is not clear why Arg-138 may have a different function in AANATL2, but some possibilities include: (a) product release might not be rate-limiting for AANATL2 as observed in other GNAT enzymes (Khalil et al, 1998; Farazi et al, 2000; Draker et al, 2003; Vetting et al, 2005), (b) Arg-138 is not within the acyl-CoA binding pocket or in the AANATL2 active site at all, and/or (c) Arg-138 is not found in a salt bridge with any residue in AANATL2. Sequence alignment of AANATL2 to AANATA reveals that the salt bridge partner to Arg-153 in AANATA (Dempsey et al, 2014a), Asp-46, is a glutamine, Gln-28, in AANATL2. A structure of AANATL2 is required to more clearly define the role of Arg-138 in AANATL2 catalysis.
The final AANATL2 amino acid residue evaluated in our study was His-206. The H206A mutant of AANATL2 is inactive with no rate of CoA-SH release above the background acetyl-CoA hydrolysis. The corresponding residues in D. melanogaster AANATA (His-220) and AANATL7 (His-206) are important to the active site organization (Dempsey et al., 2014a, 2015). Mutation of both to alanine, H220A and H206A, yields catalytically defective enzymes with (kcat/Km)app values that are 3%-6% and kcat,app values that are ~22%-30% of wild-type. His-206 may function similarly in AANATL2; however, this residue must be even more critical to active site organization in this enzyme. His-206 could function to organize the active site by positioning a catalytic amino acid residue(s) in the optimal position(s) for catalysis. Also, His-206 could be the general base in AANATL2 catalysis, since our data indicates that Glu-29 cannot serve this role. Again, an AANATL2 structure is required to more definitively define the function of His-206 in AANATL2.
We choose to mutate Glu-29, Pro-30, Arg-138, Ser-167, Ser-171, and His-206 in D. melanogaster AANATL2 based on previous research, sequence alignment to related acyltransferases, and structural information from AANATA (Cheng et al., 2012; Dempsey et al., 2014a). Our results for all the AANATL2 amino acids evaluated herein show differences relative to what was has been reported for corresponding residues in D. melanogaster AANATA and AANATL7. Why are there differences between AANATL2, AANATA, and AANATL7 when the enzymes catalyze a similar reaction with similar substrates (Dempsey et al., 2014a, 2015) – an intriguing question? One possible explanation for the differences is that AANATL2 evolved to have a greater promiscuity for amine or acyl-CoA substrates, especially functioning in the biosynthesis of long-chain N-acyldopamines and N-acylserotonins (Dempsey et al., 2014b). Could the differences we find for AANATL2 relative to AANATA and AANATL7 be related to structural differences “forced” upon AANATL2 so that this enzyme would accept long-chain acyl-CoA substrates? This question can only be addressed when structures of all the enzymes have been solved.
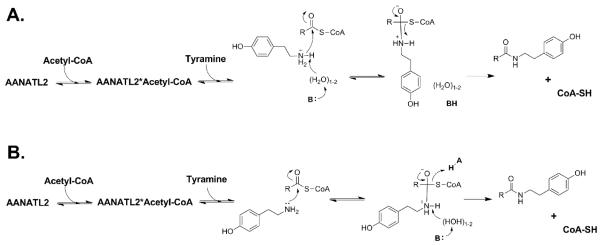
3.6 Proposed chemical mechanism for AANATL2
Two possible chemical mechanisms consistent with the data reported herein are illustrated in Scheme 2. Currently, our data does not delineate between these two possible mechanisms. The first possible mechanism (Scheme 2A) features: (a) ordered substrate binding to generate the AANATL2•acetyl-CoA•tyramine ternary complex prior to catalysis, (b) deprotonation of the positively charged amine of tyramine by a catalytic base, possibly through ordered water molecules (“proton wire”), (c) nucleophilic attack of the carbonyl of the acetyl-CoA thioester by the deprotonated tyramine to generate the zwitterionic tetrahedral intermediate, and (d) the final breakdown of the zwitterionic tetrahedral intermediate with the concomitant protonation of coenzyme A. The pKa,app values of ~7.0 and 9.0 for this possible mechanism correspond to an undefined general base (His-206?) and the thiolate of CoA-SH. The second possible mechanism (Scheme 2B) consists of: (a) a sequential ordered mechanism with acetyl-CoA binding first followed by tyramine to generate the AANATL2•acetyl-CoA•tyramine ternary complex prior to catalysis, (b) nucleophilic attack of the carbonyl of the acetyl-CoA thioester by tyramine to generate the zwitterionic tetrahedral intermediate, and (c) collapse of the zwitterionic tetrahedral intermediate by a general base catalyzed deprotonation of the positively charge amine intermediate, in addition to a general acid catalyzed protonation of coenzyme A. The pKa,app values of ~7.0 and 9.0 in this possible mechanism corresponds to an undefined general base and general acid in catalysis. Both mechanisms of Scheme 2 are consistent with mechanisms that have been proposed for other GNAT enzymes (Berndsen and Denu, 2008; Farazi et al., 2001; Hegde et al., 2007; Hickman et al., 1999b; Klein, 2007; Thoden et al., 2009; Thoden and Holden, 2010; Vetting et al., 2005) and, in particular, the mechanism depicted in Scheme 2A has been proposed for D. melanogaster AANATA and AANATL7 (Dempsey et al., 2014a, 2015). In addition to this chemical mechanism, we highlight that Pro-30, Ser-171, and His-206 are critical in regulating the AANATL2-catalyzed reaction. A structure for AANATL2 is necessary to better define the function of the amino acid residues described herein and to aid in our understanding of its chemical mechanism.
Scheme 2.
Proposed chemical mechanisms for D. melanogaster AANATL2. R = different acyl chain length for the acyl-CoA thioester.
Supplementary Material
AANATL2 has promiscuous substrate specificity for acyl-CoA and amine substrates.
AANATL2 catalyzes the formation of N-acetyltyramine through an ordered sequential mechanism.
Acetyl-CoA binds first, followed by tyramine to generate an AANATL2•acetyl-CoA•tyramine complex prior to catalysis.
pH-activity profiles highlight an acid/base chemical mechanism with two ionizable groups important to catalysis.
Functions for certain amino acids residues have been proposed in relation to regulating substrate binding and/or catalysis.
Acknowledgements
This work has been supported, in part, by grants from the Florida Center for Excellence for Biomolecular and Targeted Therapeutics (FCoE-BITT grant no. GALS020), the University of South Florida (a Research Scholarship grant from the College of Arts and Sciences), the Shirley W. and William L. Griffin Foundation, National Institute of Drug Abuse at the National Institutes of Health (R03-DA034323), and National Institute of General Medical Science of the National Institutes of Health (R15-GM107864) to D.J.M, and a Dissertation Completion Fellowship from the Office of Graduate Studies at the University of South Florida to D.R.D.
Abbreviations and Textual Footnotes
- AANATL
arylalkylamine N-acetyltransferase
- AANATL2
arylalkylamine N-acyltransferase like 2
- AANATA
arylalkylamine N-acetyltransferase variant A
- AANATL7
arylalkylamine N-acetyltransferase like 7
- GNAT
GCN5-related N-acetyltransferase.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Amherd R, Hintermann E, Walz D, Affolter M, Meyer UA. Purification, cloning, and characterization of a second arylalkylamine N-acetyltransferase from Drosophila melanogaster. DNA Cell Biol. 2000;19:697–705. doi: 10.1089/10445490050199081. [DOI] [PubMed] [Google Scholar]
- Andersen SO. Insect cuticular sclerotization: a review. Insect Biochem. Mol. Biol. 2010;40:166–178. doi: 10.1016/j.ibmb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Berndsen CE, Denu JM. Catalysis and substrate selection by histone/protein lysine acetyltransferases. Curr. Opin. Struct. Biol. 2008;18:682–689. doi: 10.1016/j.sbi.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenau W, Baumann A. Molecular and pharmacological properties of insect biogenic amine receptors: lessons from Drosophila melanogaster and Apis mellifera. Arch. Insect Biochem. Physiol. 2001;48:13–38. doi: 10.1002/arch.1055. [DOI] [PubMed] [Google Scholar]
- Blumenthal EM. Isoform- and cell-specific function of tyrosine decarboxylase in the Drosophila malpighian tubule. J. Exp. Biol. 2009;212:3802–3809. doi: 10.1242/jeb.035782. [DOI] [PubMed] [Google Scholar]
- Brodbeck D, Amherd R, Callaerts P, Hintermann E, Meyer UA, Affolter M. Molecular and biochemical characterization of the aaNAT1 (Dat) locus in Drosophila melanogaster: differential expression of two gene products. DNA Cell Biol. 1998;17:621–633. doi: 10.1089/dna.1998.17.621. [DOI] [PubMed] [Google Scholar]
- Brunet PCJ. The metabolism of the aromatic amino acids concerned in the cross-linking of insect cuticle. Insect. Biochem. 1980;10:467–500. [Google Scholar]
- Cazaméa-Catalan D, Besseau L, Falcón J, Magnanou E. The timing of timezyme diversification in vertebrates. PLoS One. 2014;9:e112380. doi: 10.1371/journal.pone.0112380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K-C, Yew WW, Tam CM, Leung CC. WHO group 5 drugs and difficult multidrug-resistant tuberculosis: a systematic review with cohort analysis and meta-analysis. Antimicrob. Agents Chemother. 2013;57:4097–4104. doi: 10.1128/AAC.00120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K-C, Liao J-N, Lyu P-C. Crystal structure of the dopamine N-acetyltransferase-acetyl-CoA complex provides insights into the catalytic mechanism. Biochem. J. 2012;446:395–404. doi: 10.1042/BJ20120520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J. Biol. Chem. 2005;280:14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- Coleman CM, Neckameyer WS. Substrate regulation of serotonin and dopamine synthesis in Drosophila. Invert. Neurosci. 2004;5:85–96. doi: 10.1007/s10158-004-0031-y. [DOI] [PubMed] [Google Scholar]
- Coleman CM, Neckameyer WS. Serotonin synthesis by two distinct enzymes in Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2005;59:12–31. doi: 10.1002/arch.20050. [DOI] [PubMed] [Google Scholar]
- Coon SL, Klein DC. Evolution of arylalkylamine N-acetyltransferase: emergence and divergence. Mol. Cell. Endocrinol. 2006;252:2–10. doi: 10.1016/S0303-7207(06)00135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis J, Gastel J, Klein DC, Cole PA. Kinetic analysis of the catalytic mechanism of serotonin N-acetyltransferase (EC 2.3.1.87) J. Biol. Chem. 1998;273:3045–3050. doi: 10.1074/jbc.273.5.3045. [DOI] [PubMed] [Google Scholar]
- Dempsey DR, Jeffries KA, Bond JD, Carpenter AM, Rodriguez-Ospina S, Breydo L, Caswell KK, Merkler DJ. Mechanistic and structural analysis of Drosophila melanogaster arylalkylamine N-acetyltransferases. Biochemistry. 2014a;53:7777–7793. doi: 10.1021/bi5006078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey DR, Jeffries KA, Anderson RL, Carpenter AM, Rodriquez Opsina S, Merkler DJ. Identification of an arylalkylamine N-acyltransferase from Drosophila melanogaster that catalyzes the formation of long-chain N-acylserotonins. FEBS Lett. 2014b;588:594–599. doi: 10.1016/j.febslet.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey DR, Bond JD, Carpenter AM, Rodriguez Ospina S, Merkler DJ. Expression, purification, and characterization of mouse glycine N-acyltransferase in Escherichia coli. Protein Expr. Purif. 2014c;97:23–28. doi: 10.1016/j.pep.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey DR, Jeffries KA, Handa S, Carpenter AM, Rodriguez Ospina S, Breydo L, Merkler DJ. Mechanistic and structural analysis of Drosophila melanogaster arylalkylamine N-acetyltransferase like 7, an enzyme that catalyses the formation of N-acetylarylalkylamides and N-acetylhistamine. Biochemistry. 2015;54:2644–2658. doi: 10.1021/acs.biochem.5b00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhurst SA, Croker SG, Ikeda K, McCaman RE. Metabolism of biogenic amines in Drosophila nervous tissue. Comp. Biochem. Physiol. B. 1972;43:975–981. doi: 10.1016/0305-0491(72)90241-6. [DOI] [PubMed] [Google Scholar]
- Draker KA, Northrop DB, Wright GD. Kinetic mechanism of the GCN5-related chromosomal aminoglycoside acetyltransferase AAC(6′)-Ii from Enterococcus faecium: Evidence of dimer subunit cooperativity. Biochemistry. 2003;42:6565–6574. doi: 10.1021/bi034148h. [DOI] [PubMed] [Google Scholar]
- Dyda F, Klein DC, Hickman AB. GCN5-related N-acetyltransferases: a structural overview. Annu. Rev. Biophys. Biomol. Struct. 2000;29:81–103. doi: 10.1146/annurev.biophys.29.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Falcón J, Coon SL, Besseau L, Cazaméa-Catalan D, Fuentès M, Magnanou E, Paulin C-H, Boeuf G, Sauzet S, Jørgensen EH, Mazan S, Wolf YI, Koonin EV, Steinbach PJ, Hyodo S, Klein DC. Drastic neofunctionalization associated with evolution of timezyme AANAT 500 Mya. Proc. Natl. Acad. Sci USA. 2014;111:314–319. doi: 10.1073/pnas.1312634110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farazi TA, Manchester JK, Gordon JI. Transient-state kinetic analysis of Saccharomyces cerevisiae myristoyl-CoA:protein N-myristoyltransferase reveals that a step after chemical transformation is rate limiting. Biochemistry. 2000;39:15807–15816. doi: 10.1021/bi002074t. [DOI] [PubMed] [Google Scholar]
- Farazi TA, Manchester JK, Waksman G, Gordon JI. Pre-steady-state kinetic studies of Saccharomyces cerevisiae myristoylCoA:protein N-myristoyltransferase mutants identify residues involved in catalysis. Biochemistry. 2001;40:9177–9186. doi: 10.1021/bi0107997. [DOI] [PubMed] [Google Scholar]
- Farrell EK, Merkler DJ. Biosynthesis, degradation and pharmacological importance of the fatty acid amides. Drug Discov. Today. 2008;13:558–568. doi: 10.1016/j.drudis.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry G, Loynel A, Kucharczyk N, Bertin S, Rodriguez M, Delagrange P, Galizzi J-P, Jacoby E, Volland J-P, Lesieur D, Renard P, Canet E, Fauchere J-L, Boutin JA. Substrate specificity and inhibition studies of human serotonin N-acetyltransferase. J. Biol. Chem. 2000;275:8794–8805. doi: 10.1074/jbc.275.12.8794. [DOI] [PubMed] [Google Scholar]
- Fezza F, Bari M, Florio R, Talamonti E, Feole M, Maccarrone M. Endocannabinoids, related compounds and their metabolic routes. Molecules. 2014;19:17078–17106. doi: 10.3390/molecules191117078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finocchiaro L, Callebert J, Launay JM, Jallon JM. Melatonin biosynthesis in Drosophila: its nature and its effects. J. Neurochem. 1988;50:382–387. doi: 10.1111/j.1471-4159.1988.tb02923.x. [DOI] [PubMed] [Google Scholar]
- Ganguly S, Mummaneni P, Steinbach PJ, Klein DC. Characterization of the Saccharomysces cerevisiae homolog of the melatonin rhythm enzyme arylalkylamine N-acetyltransferase (EC 2.3.1.87) J. Biol. Chem. 2001;276:47239–47247. doi: 10.1074/jbc.M107222200. [DOI] [PubMed] [Google Scholar]
- Gray EE, Small SN, McGuirl MA. Expression and characterization of recombinant tyramine β-monooxygenase from Drosophila: a monomeric copper-containing hydroxylase. Protein Expr. Purif. 2006;47:162–170. doi: 10.1016/j.pep.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Han Q, Ding H, Robinson H, Christensen BM, Li J. Crystal structure and substrate specificity of Drosophila 3,4-dihydroxyphenylalanine decarboxylase. PLoS One. 2010;5:e8826. doi: 10.1371/journal.pone.0008826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde SS, Chandler J, Vetting MW, Yu M, Blanchard JS. Mechanistic and structural analysis of human spermidine/spermine N1-acetyltransferase. Biochemistry. 2007;46:7187–7195. doi: 10.1021/bi700256z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess CR, McGuirl MM, Klinman JP. Mechanism of the insect enzyme, tyramine β-monooxygenase, reveals differences from the mammalian enzyme, dopamine β-monooxygenase. J. Biol. Chem. 2008;283:3042–3049. doi: 10.1074/jbc.M705911200. [DOI] [PubMed] [Google Scholar]
- Hickman AB, Klein DC, Dyda F. Melatonin biosynthesis: the structure of serotonin N-acetyltransferase at 2. A resolution suggests a catalytic mechanism. Mol. Cell. 1999a;3:23–32. doi: 10.1016/s1097-2765(00)80171-9. [DOI] [PubMed] [Google Scholar]
- Hickman AB, Namboodiri MAA, Klein DC, Dyda F. The structural basis of ordered substrate binding by serotonin N-acetyltransferase: enzyme complex at 1.8 A resolution with a bisubstrate analog. Cell. 1999b;97:361–369. doi: 10.1016/s0092-8674(00)80745-x. [DOI] [PubMed] [Google Scholar]
- Hiragaki S, Suzuki T, Mohamed AAM, Takeda M. Structures and functions of insect arylalkylamine N-acetyltransferase (iaaNAT); a key enzyme for physiological and behavioral switch in arthropods. Front. Physiol. 2015;6:1–16. doi: 10.3389/fphys.2015.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- The Merck Index. 12th ed Merck and Co.; White House Station, NJ: 1996. [Google Scholar]
- Jeffries KA, Dempsey DR, Behari AL, Anderson RL, Merkler DJ. Drosophila melanogaster as a model system to study long-chain fatty acid amide metabolism. FEBS Lett. 2014;588:1596–1602. doi: 10.1016/j.febslet.2014.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil EM, Cole PA. A potent inhibitor of the melatonin rhythm enzyme. J. Am. Chem. Soc. 1998;120:6195–6196. [Google Scholar]
- Klein DC. Evolution of the vertebrate pineal gland: the AANAT hypothesis. Chronobiol. Int. 2006;23:5–20. doi: 10.1080/07420520500545839. [DOI] [PubMed] [Google Scholar]
- Klein DC. Arylalkylamine N-acetyltransferase: “the timezyme”. J Biol Chem. 2007;282:4233–4237. doi: 10.1074/jbc.R600036200. [DOI] [PubMed] [Google Scholar]
- Maranda B, Hodgetts R. Characterization of dopamine acetyltransferase in Drosophila-melanogaster. Insect Biochem. 1977;7:33–43. [Google Scholar]
- Marsh JL, Wright TRF. Developmental relationship between dopa decarboxylase, dopamine acetyltransferase, and ecdysone in Drosophila. Dev. Biol. 1980;80:379–387. doi: 10.1016/0012-1606(80)90412-1. [DOI] [PubMed] [Google Scholar]
- Mehere P, Han Q, Christensen BM, Li J. Identification and characterization of two arylalkylamine N-acetyltransferases in the yellow fever mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 2011;41:707–714. doi: 10.1016/j.ibmb.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkler DJ, Merkler KA, Stern W, Fleming FF. Fatty acid amide biosynthesis: a possible new role for peptidylglycine α-amidating monooxygenase. Arch. Biochem. Biophys. 1995;330:430–434. doi: 10.1006/abbi.1996.0272. [DOI] [PubMed] [Google Scholar]
- Monastirioti M, Linn CE, Jr., White K. Characterization of Drosophila tyramine β-hydroxylase gene and isolation of mutant flies lacking octopamine. J. Neurosci. 1996;16:3900–3911. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JF, Uhr ML. The function of bivalent metal ions in the reaction catalysed by ATP:creatine phosphotransferase. Biochim. Biophys. Acta. 1966;122:57–74. doi: 10.1016/0926-6593(66)90091-9. [DOI] [PubMed] [Google Scholar]
- Pavlicek J, Coon SL, Ganguly S, Weller JL, Hassan SA, Sackett DL, Klein DC. Evidence that proline focuses movement of the floppy loop of arylalkylamine N-acetyltransferase (EC 2.3.1.87) J. Biol. Chem. 2008;283:14552–14558. doi: 10.1074/jbc.M800593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlicek J, Sauzet S, Besseau L, Coon SL, Weller JL, Boeuf G, Gaildrat P, Omelchenko MV, Koonin EV, Falcón J, Klein DC. Evolution of AANAT: expansion of the gene family in the cephalochalochordate amphioxus. BMC Evol. Biol. 2010;10:154. doi: 10.1186/1471-2148-10-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxon TL, Powell PR, Lee HG, Han KA, Ewing AG. Microcolumn separation of amine metabolites in the fruit fly. Anal. Chem. 2005;77:5349–5355. doi: 10.1021/ac050474m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman IH, Morris IJ. Coenzyme A: pKa and gamma values. Aust. J. Chem. 1980;33:1625–1630. [Google Scholar]
- Rauschenbach IY, Adonyeva NV, Alekseev AA, Chentsova NA, Gruntenko NE. Role of arylalkylamine N-acetyltransferase in regulation of biogenic amines levels by gonadotropins in Drosophila. J. Comp. Physiol. B. 2008;178:315–320. doi: 10.1007/s00360-007-0224-x. [DOI] [PubMed] [Google Scholar]
- Rauschenbach IY, Bogomolova EV, Karpova EK, Adonyeva NV, Faddeeva NV, Menshanov PN, Gruntenko NE. Mechanisms of age-specific regulation of dopamine metabolism by juvenile hormone and 20-hydroxyecdysone in Drosophila females. J. Comp. Physiol., B. 2011;181:19–26. doi: 10.1007/s00360-010-0512-8. [DOI] [PubMed] [Google Scholar]
- Scheibner KA, De Angelis J, Burley SK, Cole PA. Investigation of the roles of catalytic residues in serotonin N-acetyltransferase. J. Biol. Chem. 2002;277:18118–18126. doi: 10.1074/jbc.M200595200. [DOI] [PubMed] [Google Scholar]
- Sukhanova M, Shumnaya LV, Grenback LG, Gruntenko NE, Khlebodarova TM, Rauschenbach IY. Tyrosine decarboxylase and dopa decarboxylase in Drosophila virilis under normal conditions and heat stress: genetic and physiological aspects. Biochem. Genet. 1997;35:91–103. doi: 10.1023/a:1022209707655. [DOI] [PubMed] [Google Scholar]
- Thoden JB, Cook PD, Schäffer C, Messner P, Holden HM. Structural and functional studies of QdtC: an N-acetyltransferase required for the biosynthesis of dTDP-3-acetamido-3,6-dideoxy-α-D-glucose. Biochemistry. 2009;48:2699–2709. doi: 10.1021/bi802313n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoden JB, Holden HM. Molecular structure of WlbB, a bacterial N-acetyltransferase involved in the biosynthesis of 2,3-diacetamido-2,3-dideoxy-D-mannuronic acid. Biochemistry. 2010;49:4644–4653. doi: 10.1021/bi1005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortoriella G, Rhodes BP, Takacs SM, Stuart JM, Basnet A, Raboune S, Widlanski TS, Doherty P, Harkany T, Bradshaw HB. Targeted lipidomics in Drosophila melanogaster identifies novel 2-monoacylglycerols and N-acyl amides. PLoS One. 2013;8:e67865. doi: 10.1371/journal.pone.0067865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon A. Treatment of latent tuberculosis infection. Semin. Respir. Crit. Care Med. 2013;34:67–86. doi: 10.1055/s-0032-1333544. [DOI] [PubMed] [Google Scholar]
- Vetting MW, S., de Carvalho LP, Yu M, Hegde SS, Magnet S, Roderick SL, Blanchard JS. Structure and functions of the GNAT superfamily of acetyltransferases. Arch. Biochem. Biophys. 2005;433:212–226. doi: 10.1016/j.abb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Vömel M, Wegener C. Neuroarchitecture of aminergic systems in the larval ventral ganglion of Drosophila melanogaster. PLoS One. 2008;3:e1848. doi: 10.1371/journal.pone.0001848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waluk DP, Schultz N, Hunt MC. Identification of glycine N-acyltransferase-like 2 (GLYATL2) as a transferase that produces N-acyl glycines in humans. FASEB J. 2010;24:2795–2803. doi: 10.1096/fj.09-148551. [DOI] [PubMed] [Google Scholar]
- Wittich R-M, Walter RD. Putrescine N-acetyltransferase in Onchocerca volvulus and Ascaris suum, an enzyme which is involved in polyamine degradation and release of N-acetylputrescine. Mol. Biochem. Parasitol. 1990;38:13–17. doi: 10.1016/0166-6851(90)90199-v. [DOI] [PubMed] [Google Scholar]
- Wright TR. The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Adv. Genet. 1987;24:127–222. [PubMed] [Google Scholar]
- Zheng W, Cole PA. Serotonin N-acetyltransferase: mechanism and inhibition. Curr. Med. Chem. 2002;9:1187–1199. doi: 10.2174/0929867023370013. [DOI] [PubMed] [Google Scholar]
- Zheng W, Cole PA. Novel bisubstrate analog inhibitors of serotonin N-acetyltransferase: the importance of being neutral. Bioorg. Chem. 2003;31:398–411. doi: 10.1016/s0045-2068(03)00081-6. [DOI] [PubMed] [Google Scholar]
- Zumla A, Raviglione M, Hafner R, von Reyn CF. Tuberculosis. N. Engl. J. Med. 2013;368:745–755. doi: 10.1056/NEJMra1200894. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.