Abstract
The present work investigated correlations between cartilage and subchondral bone repair, facilitated by a growth factor-delivering scaffold, in a rabbit osteochondral defect model. Histological scoring indices and microcomputed tomography morphological parameters were used to evaluate cartilage and bone repair, respectively, at 6 and 12 weeks. Correlation analysis revealed significant associations between specific cartilage indices and subchondral bone parameters that varied with location in the defect (cortical vs. trabecular region), time point (6 vs. 12 weeks), and experimental group (insulin-like growth factor-1 only, bone morphogenetic protein-2 only, or both growth factors). In particular, significant correlations consistently existed between cartilage surface regularity and bone quantity parameters. Overall, correlation analysis between cartilage and bone repair provided a fuller understanding of osteochondral repair and can help drive informed studies for future osteochondral regeneration strategies.
Introduction
The regeneration of osteochondral tissue remains a difficult challenge due to the inability for articular cartilage to self-regenerate and the need to consider the multiphasic nature of the osteochondral unit.1,2 Although the osteochondral unit comprises different tissues, cartilage and bone, homeostasis is predicated by an innate biochemical and biomechanical interplay between these two tissue types.3–7 There is an increasing body of knowledge in the fields of osteoarthritis and osteochondral tissue engineering on the importance of the subchondral bone on the pathogenesis as well as regeneration of the articulating cartilage.8–14 Yet, while many studies have examined the associated structural changes of subchondral bone with changes in cartilage tissue morphology in an osteoarthritic model,15–18 little is known about how the repair of subchondral bone and its associated structural changes are related to the repair of articular cartilage in an osteochondral defect.
Previously, Qiu et al.19 and Orth et al.20 investigated the healing of subchondral bone and articular cartilage in an empty rabbit osteochondral defect. In particular, it was found that there was no significant correlation between upward subchondral bone migration and cartilage degeneration over time.20 In addition, Zhang et al. characterized cartilage and bone repair using a biphasic hydrogel/ceramic scaffold in a critical-sized rabbit osteochondral defect in a year-long study and saw that the gross appearance of cartilage positively correlated with subchondral bone volume.21 However, the rise of tissue engineering strategies for osteochondral repair necessitates further research on the associated changes in bone and cartilage repair, especially when facilitated by an implanted biomaterial scaffold. To determine these associations, correlation modeling offers a statistical analysis for these relationships. By understanding how specific morphological parameters for bone and cartilage are correlated, valuable information can be gathered on the repair process.
As a result, the current report utilized growth factor-delivering, bilayered hydrogel composites to facilitate osteochondral repair in a rabbit model. Specifically, the hydrogel composites were based on the synthetic macromer oligo(poly(ethylene glycol) fumarate) (OPF) and contained gelatin microparticles (GMPs) as drug delivery vehicles. Insulin-like growth factor-1 (IGF-1) and bone morphogenetic protein-2 (BMP-2) were delivered separately or together to facilitate tissue formation. In a previous publication, the effect of growth factor treatment option on osteochondral repair was presented as a comparison of means between experimental groups, as is typically done in an outcome-based analysis.22 In this technical report, correlation analysis is used on the same raw data to present direct associations between cartilage and bone repair. While causality cannot be determined through correlation analysis (e.g., better bone repair causes better cartilage repair), specific relationships between cartilage and bone parameters can be discovered. In turn, these specific relationships can be the foundation for future mechanistic studies investigating cause and effect.
Thus, the main objective of this report was to examine correlations between cartilage indices, as determined by histological scoring, and subchondral bone parameters, as determined by microcomputed tomography (micro-CT). In addition, correlations between cartilage and bone repair were compared at 6- and 12-week time points, and the influence of growth factor delivery treatment on trends in tissue repair was investigated.
Materials and Methods
Experimental study design
The materials and methods used for the current report have previously been published.22 Briefly, dried GMPs (50–100 μm in diameter) were loaded with a growth factor solution, IGF-1 or BMP-2, and encapsulated in bilayered OPF hydrogel composites. Three different growth factor-loaded GMP combinations were encapsulated in bilayered hydrogel composites: (1) IGF-1 only in the chondral layer, (2) BMP-2 only in the subchondral layer, or (3) IGF-1 and BMP-2 in the chondral and subchondral layer, respectively. The hydrogel composites were then implanted in skeletally mature New Zealand White Rabbits using a medial femoral condyle osteochondral defect model (3 mm in diameter and 3 mm in thickness). A total of 36 rabbits were used, with each rabbit receiving bilateral implants and 10–12 hydrogels for each group at each time point (6 or 12 weeks).
Following 6 or 12 weeks, rabbits were euthanized, and condyle samples, including the defect site, underwent micro-CT imaging and histological preparation. Due to the presence of two distinct bone morphologies in the subchondral bone, two volumes of interest (VOIs) were analyzed separately for the bone plate and the trabecular bone. The upper 0.85 mm of the defect was selected as the cartilage and cortical (C&C) region and the lower 2.15 mm was selected as the trabecular region. The cartilage region was included with the cortical region to cover all possible neobone formation as well as due to the potential for upward subchondral bone plate migration during osteochondral repair.20 Morphological parameters analyzed for both the C&C and trabecular VOI include bone mineral density (BMD), percent bone volume (BV/TV), intersection surface (i.S), bone-specific surface (BS/BV), and object number (Obj.N). Additional parameters analyzed for the trabecular VOI include trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp). Each of these parameters is further explained in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/tec). For histology, sections from the center and peripheral edges were stained with hematoxylin and eosin, Safranin O/Fast Green, and van Gieson's Picrofuchsin. One center and two opposing peripheral histological sections were then blindly and independently scored by three evaluators (J.L., J.E.T., and F.K.K.) using a previously established histological scoring system for osteochondral defects as seen in Supplementary Table S2.22–24
Statistical analysis
A significance level of p < 0.05 was used for all statistical analysis through JMP Pro v11.0.0. To determine the presence and strength of correlation between key micro-CT parameters and histological scoring indices, Spearman's rank correlation test was used due to the nonparametric and ordinal nature of the histological scoring data sets. For each sample, three histological sections were scored (one center and two peripheral) for each of the six cartilage indices. The three histological scores were then averaged for each cartilage index (n = 70), and the mean scores for each cartilage index were compared with the associated micro-CT measurements for both C&C (n = 70) and trabecular VOIs (n = 70). As a result, correlation analysis was performed between six cartilage scores and five (C&C) or eight (trabecular) bone morphological measurements, at each time point (n = 34 or 36), and for each growth factor group (n = 10 or 12) as seen in Supplementary Tables S3 and S4. Correlation coefficients with a significance level of p < 0.05 were graded on a scale from 0.00 to |1.00| as seen in Table 1.25,26
Table 1.
Interpretation of Correlation Coefficient
| Correlation coefficient value | Strength of correlation |
|---|---|
| 0.00 to |0.30| | Negligible correlation |
| |0.30| to |0.50| | Weak correlation |
| |0.50| to |0.70| | Moderate correlation |
| |0.70| to |0.90| | Strong correlation |
| |0.90| to |1.00| | Very strong correlation |
The sign of the correlation coefficient (i.e., positive or negative) indicates the direction of the relationship.
Results
Correlations between micro-CT and histological scoring parameters for all samples
Except for cartilage thickness, all other histological scoring parameters had significant correlations with one or more micro-CT parameters as seen in Table 2. Cartilage morphology had a weak correlation with BMD and Tb.Sp in the trabecular VOI. In addition, neocartilage cell and glycosaminoglycan (GAG) content had a weak correlation with Obj.N in the C&C VOI. Notably, cartilage regularity exhibited moderate correlations with p < 0.0001 with BMD, BV/TV, and i.S in the trabecular VOI.
Table 2.
Correlations Between Microcomputed Tomography Morphological and Histological Scoring Parameters for All Samples and Both Volumes of Interest
| BMD | BV/TV | i.S | BS/BV | Tb.Th | Tb.N | Tb.Sp | Obj.N | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C&C | Trabec | C&C | Trabec | C&C | Trabec | C&C | Trabec | C&C | Trabec | C&C | Trabec | C&C | Trabec | C&C | Trabec | |
| Cartilage morphology | 0.30 | 0.33 | 0.25 | 0.27 | 0.27 | — | — | — | −0.35 | |||||||
| Cartilage thickness | — | — | — | |||||||||||||
| Cartilage regularity | 0.37 | 0.56 | 0.33 | 0.50 | 0.33 | 0.57 | −0.35 | −0.29 | — | 0.24 | — | 0.40 | — | −0.32 | ||
| Chondrocyte clustering | — | — | — | 0.25 | 0.26 | |||||||||||
| Neocartilage cell and GAG | −0.27 | −0.26 | 0.28 | 0.30 | — | −0.30 | — | — | 0.38 | 0.31 | ||||||
| Adjacent cell and GAG | 0.32 | 0.29 | 0.26 | 0.30 | 0.27 | −0.31 | — | — | 0.29 | — | −0.30 | |||||
Highlighted cells represent p < 0.0001; blank cells represent nonsignificant correlations (p > 0.05); n = 70.
BMD, bone mineral density; BS/BV, bone-specific surface; BV/TV, percent bone volume; C&C, cartilage and cortical; GAG, glycosaminoglycan; i.S, intersection surface; Obj.N, object number; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness; Trabec, trabecular.
Correlations between micro-CT and histological scoring parameters for 6- and 12-week samples
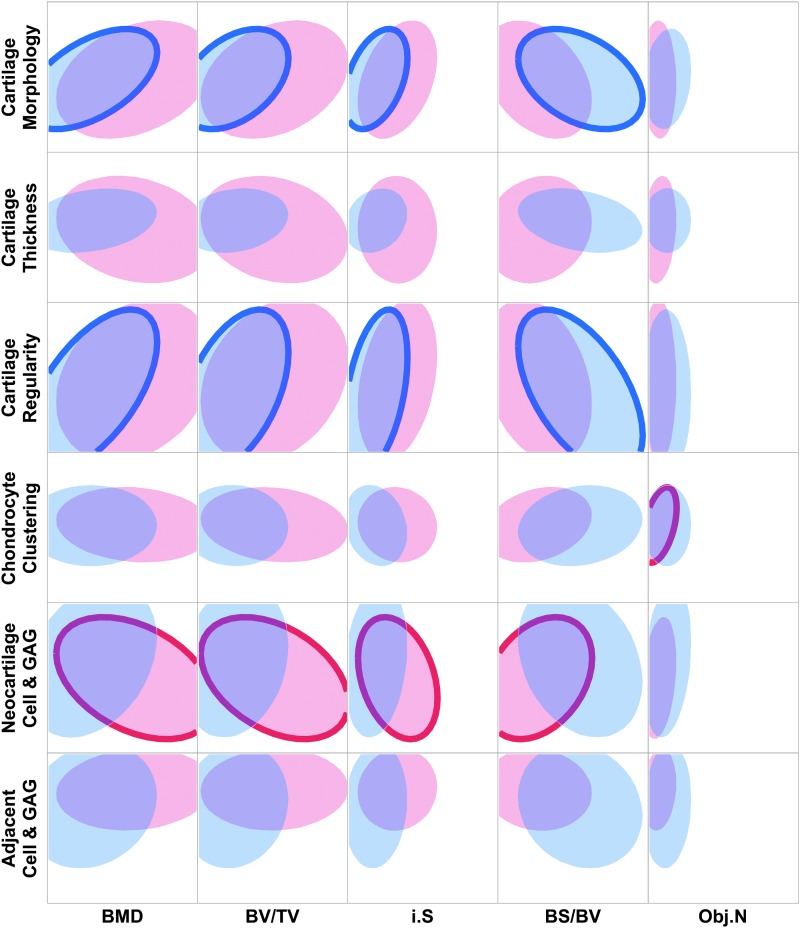
In the C&C VOI, differences in correlations between 6- and 12-week samples could be seen (Fig. 1). Significant 6-week correlations existed between micro-CT parameters BMD, BV/TV, i.S, and BS/BV and histological scoring parameter cartilage morphology and cartilage regularity. In particular, cartilage morphology had a moderate correlation with BMD, and cartilage regularity had moderate correlations with BMD, BV/TV, and BS/BV (p < 0.001). At 12 weeks, neocartilage cell and GAG had weak correlations with all micro-CT parameters, except for Obj.N, and chondrocyte clustering had a moderate correlation with Obj.N.
FIG. 1.
Scatterplot matrix showing density ellipses for 95% of the data between cartilage histological scoring indices and bone morphological parameters in the cartilage and cortical VOI. Density ellipses were computed from a bivariate normal distribution, fit to the histological scoring and micro-CT measurements, and are a function of the means and standard deviations of the variables, as well as the correlation between them. Six-week samples are represented by blue ellipses, and 12-week samples are represented by red ellipses. Significant correlations between cartilage and bone parameters are indicated by bolded outlines (p < 0.05), where n = 34 for 6-week correlations and n = 36 for 12-week correlations. BMD, bone mineral density; BS/BV, bone-specific surface; BV/TV, percent bone volume; GAG, glycosaminoglycan; i.S, intersection surface; micro-CT, microcomputed tomography; Obj.N, object number; VOI, volume of interest.
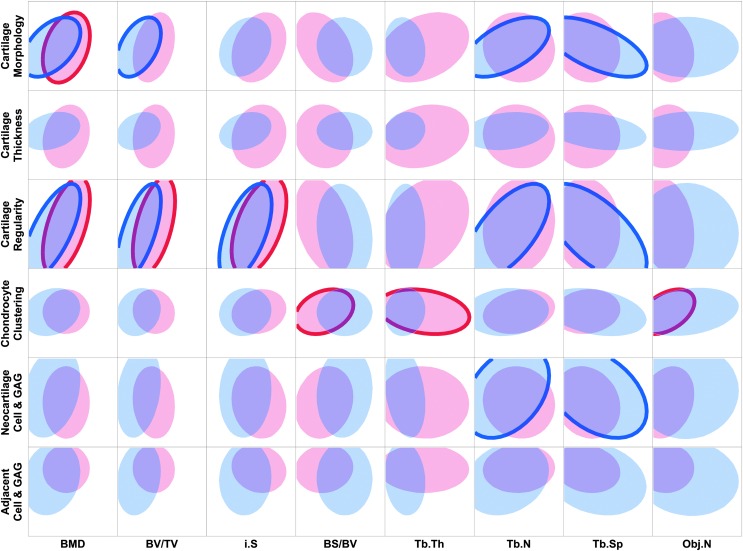
The trabecular VOI displayed similar differences with the C&C VOI in correlations between 6- and 12-week samples, with more significant correlations present at 6 weeks compared to 12 weeks (Fig. 2). At 6 weeks, cartilage morphology and regularity had moderate correlations with BMD and BV/TV, with cartilage regularity also having a significant correlation with i.S. Trabecular-specific parameters, Tb.N and Tb.Sp, were seen to have significant associations with cartilage morphology, cartilage regularity, and neocartilage cell and GAG. At 12 weeks, cartilage morphology exhibited a significant correlation with BMD, and cartilage regularity was moderately correlated with BMD, BV/TV, and i.S (p < 0.01). This trend can also be seen in Figures 3 and 4, where the regularity of the neocartilage surface is even with the adjacent cartilage in the presence of repaired trabecular bone (Fig. 4), and a depression in the neocartilage surface is seen with minimal subchondral bone repair (Fig. 3). Chondrocyte clustering was also weakly correlated with BS/BV and Tb.Th, and similar to the C&C VOI, was moderately correlated with Obj.N at 12 weeks.
FIG. 2.
Scatterplot matrix showing density ellipses for 95% of the data between cartilage histological scoring indices and bone morphological parameters in the trabecular VOI. Density ellipses were computed from a bivariate normal distribution fit to the histological scoring and micro-CT measurements and are a function of the means and standard deviations of the variables, as well as the correlation between them. Six-week samples are represented by blue ellipses, and 12-week samples are represented by red ellipses. Significant correlations between cartilage and bone parameters are indicated by bolded outlines (p < 0.05), where n = 34 for 6-week correlations and n = 36 for 12-week correlations. Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness.
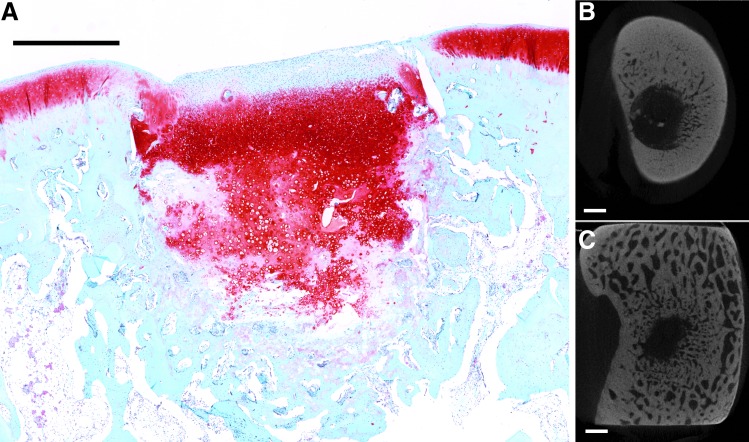
FIG. 3.
Representative histological section with Safranin O/Fast Green staining of osteochondral tissue repair after 12 weeks of implantation (A). (B, C) Representative transverse micro-CT images of the bone plate and trabecular bone, respectively. This sample from the insulin-like growth factor-1 only group demonstrated minimal bone formation in both cortical and trabecular regions and had a depressed cartilage surface (scale bar: 1000 μm).
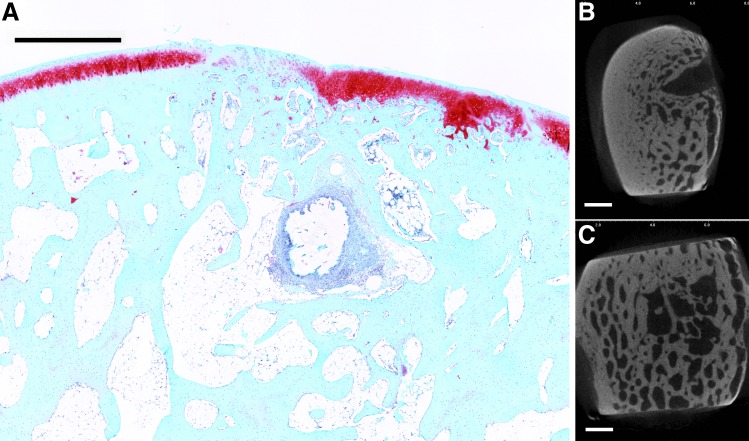
FIG. 4.
Representative histological section with Safranin O/Fast Green staining of osteochondral tissue repair after 12 weeks of implantation (A). (B, C) Representative transverse micro-CT images of the bone plate and trabecular bone, respectively. This sample from the Both group had well-remodeled trabecular structures even with the presence of remaining hydrogel. In addition, the bone plate has formed and the cartilage surface is regular (scale bar: 1000 μm).
Correlations between micro-CT and histological scoring parameters for each experimental group
At 6 weeks, in the C&C VOI, differences in correlations among the three experimental groups are seen (Table 3). In general, histological scoring indices had positive correlations with BMD, BV/TV, i.S, and Obj.N and negative correlations with BS/BV. Interestingly, there were no correlations between cartilage indices and bone parameters for the group delivering IGF-1 only. In contrast, associations were seen in the BMP-2 only and Both groups. Cartilage morphology, cartilage regularity, and neocartilage cell and GAG had significant correlations with BMD, BV/TV, i.S, and BS/BV for groups with BMP-2 only. In addition, group Both had significant correlations between cartilage regularity and BMD, BV/TV, i.S, and BS/BV. Adjacent cell and GAG also had significant correlations with BS/BV and Obj.N in the Both group. At 12 weeks, in the C&C VOI, most correlations occurred in the IGF-1 and BMP-2 groups with the exception of a positive correlation between cartilage morphology and i.S in the Both group (p < 0.01) (Table 4). In addition, there existed strong negative correlations with a p < 0.01 between neocartilage cell and GAG and BMD, BV/TV, and i.S in the BMP-2 group.
Table 3.
Cartilage and Cortical Volume of Interest, 6-Week Correlation Between Microcomputed Tomography Morphological and Histological Scoring Parameters for Each Experimental Group
| BMD | BV/TV | i.S | BS/BV | Obj.N | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IGF | BMP | Both | IGF | BMP | Both | IGF | BMP | Both | IGF | BMP | Both | IGF | BMP | Both | |
| Cartilage morphology | 0.75 | 0.75 | 0.70 | −0.86 | |||||||||||
| Cartilage thickness | −0.71 | ||||||||||||||
| Cartilage regularity | 0.70 | 0.70 | 0.73 | 0.80 | 0.64 | 0.68 | −0.96 | −0.81 | |||||||
| Chondrocyte clustering | −0.64 | ||||||||||||||
| Neocartilage cell and GAG | 0.67 | 0.70 | −0.78 | 0.67 | |||||||||||
| Adjacent cell and GAG | 0.61 | 0.77 | |||||||||||||
Highlighted cells represent p < 0.01; blank cells represent nonsignificant correlations (p > 0.05); n = 10–12.
BMP, bone morphogenetic protein; IGF, insulin-like growth factor.
Table 4.
Cartilage and Cortical Volume of Interest, 12-Week Correlation Between Microcomputed Tomography Morphological and Histological Scoring Parameters for Each Experimental Group
| BMD | BV/TV | i.S | BS/BV | Obj.N | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IGF | BMP | Both | IGF | BMP | Both | IGF | BMP | Both | IGF | BMP | Both | IGF | BMP | Both | |
| Cartilage morphology | 0.76 | ||||||||||||||
| Cartilage thickness | |||||||||||||||
| Cartilage regularity | 0.62 | −0.60 | 0.70 | −0.60 | |||||||||||
| Chondrocyte clustering | −0.58 | 0.60 | 0.68 | ||||||||||||
| Neocartilage cell and GAG | −0.79 | −0.81 | −0.82 | 0.62 | 0.69 | ||||||||||
| Adjacent cell and GAG | |||||||||||||||
Highlighted cells represent p < 0.01; blank cells represent nonsignificant correlations (p > 0.05); n = 12.
At 6 weeks in the trabecular VOI, strong correlations between cartilage scores and micro-CT parameters existed predominantly in the BMP-2 group, similar to the C&C VOI at 6 weeks (Table 5). In particular, cartilage morphology, cartilage regularity, and neocartilage cell and GAG were strongly correlated with BMD, BV/TV, and Tb.N in the BMP-2 group (p < 0.01). Cartilage regularity was also strongly correlated with i.S and Tb.Sp in the BMP group. At 12 weeks in the trabecular VOI, strong correlations are seen between cartilage regularity and BMD, BV/TV, and i.S for IGF-1 and Both groups (Table 6). In addition, adjacent cell and GAG were strongly negatively correlated with Tb.Sp in the Both group.
Table 5.
Trabecular Volume of Interest, 6-Week Correlation Between Microcomputed Tomography Morphological and Histological Scoring Parameters for Each Experimental Group
| BMD | BV/TV | i.S | BS/BV | Tb.Th | Tb.N | Tb.Sp | Obj.N | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IGF | BMP | Both | IGF | BMP | Both | IGF | BMP | Both | IGF | BMP | Both | IGF | BMP | Both | IGF | BMP | Both | IGF | BMP | Both | IGF | BMP | Both | |
| Cartilage morphology | 0.86 | 0.82 | 0.76 | 0.89 | −0.67 | −0.74 | ||||||||||||||||||
| Cartilage thickness | 0.76 | |||||||||||||||||||||||
| Cartilage regularity | 0.92 | 0.80 | 0.59 | 0.88 | 0.80 | −0.92 | −0.69 | |||||||||||||||||
| Chondrocyte clustering | 0.64 | −0.65 | ||||||||||||||||||||||
| Neocartilage cell and GAG | 0.82 | 0.79 | 0.79 | 0.87 | −0.66 | 0.63 | ||||||||||||||||||
| Adjacent cell and GAG | 0.68 | |||||||||||||||||||||||
Highlighted cells represent p < 0.01; blank cells represent nonsignificant correlations (p > 0.05); n = 10–12.
Table 6.
Trabecular Volume of Interest, 12-Week Correlation Between Microcomputed Tomography Morphological and Histological Scoring Parameters for Each Experimental Group
| BMD | BV/TV | i.S | BS/BV | Tb.Th | Tb.N | Tb.Sp | Obj.N | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IGF | BMP | Both | IGF | BMP | Both | IGF | BMP | Both | IGF | BMP | Both | IGF | BMP | Both | IGF | BMP | Both | IGF | BMP | Both | IGF | BMP | Both | |
| Cartilage morphology | 0.63 | 0.62 | 0.58 | |||||||||||||||||||||
| Cartilage thickness | 0.64 | |||||||||||||||||||||||
| Cartilage regularity | 0.69 | 0.81 | 0.72 | 0.78 | 0.75 | 0.73 | −0.58 | |||||||||||||||||
| Chondrocyte clustering | 0.62 | |||||||||||||||||||||||
| Neocartilage cell and GAG | −0.69 | |||||||||||||||||||||||
| Adjacent cell and GAG | −0.83 | |||||||||||||||||||||||
Highlighted cells represent p < 0.01; blank cells represent nonsignificant correlations (p > 0.05); n = 12.
Discussion
The primary objective of this report was to examine correlations between the repair of articular cartilage and the repair of subchondral bone in an osteochondral defect, facilitated by a hydrogel composite scaffold. Osteochondral repair was evaluated at 6 and 12 weeks with histological scoring indices for cartilage evaluation and micro-CT structural parameters for subchondral bone repair. Specifically, we investigated (1) if the degree of cartilage repair could be associated with the degree of subchondral bone repair through correlation analysis, (2) whether the correlations between cartilage and bone would change from an early to a later time point, and (3) if the delivery of different growth factor combinations would impact trends between cartilage and bone repair.
When all samples at both time points were analyzed, significant correlations were found between cartilage histological scoring indices and subchondral bone micro-CT parameters. Although most correlations were negligible to weak in strength (<|0.50|), cartilage surface regularity was moderately correlated with bone volume and bone formation at the defect edges in the trabecular region (p < 0.0001). This can be seen in histological images where cartilage depressions were seen in samples with low bone formation (Fig. 3) and smooth intact surfaces were seen with greater bone repair (Fig. 4). Indeed, the subchondral bone provides a mechanical support for the overlying cartilage, and without sufficient bone formation, fissures can develop in the surface cartilage.27
When this association was examined separately at 6 and 12 weeks, moderate correlations still existed between surface regularity and bone formation parameters in the trabecular region (p < 0.01). However, similar correlations were only present in the C&C region at 6 weeks. In addition, significant correlations were present between bone formation parameters and cartilage morphology and regularity in the C&C region at 6 weeks, but not at 12 weeks. These changes in correlations from an early to a later time point may be a result of the different rates at which bone and cartilage remodel, as seen previously.20,28 Indeed, the more rapid appearance of skeletal changes due to abnormal mechanical loading on cartilage highlights the differential adaptive capacity and metabolic activity between bone and cartilage.16
Interestingly, cartilage thickness did not correlate with any bone parameters, possibly indicating that the thickness of neocartilage tissue was not related to the volume of bone formed or the extent of bone remodeling. However, thinning of neocartilage has previously been seen to be affected by thickening and upward migration of the subchondral bone plate.13,29 In addition, thinning of repaired cartilage was seen at 16 weeks, but not 8 weeks, in the healing of an empty osteochondral defect.19 While the current study saw only two samples with the presence of the subchondral bone plate above the tidemark of the surrounding tissue, but below the surface of the articulating cartilage at 12 weeks,22 further study with longer time points may see more associations of cartilage thickness with subchondral bone parameters.
Correlation analysis for each experimental group at each time point was also performed. While the sample size for these analyses was low (n = 10–12), prudent interpretation of these statistically significant results is still relevant. In addition, greater credence is placed on correlations with a significance level of p < 0.01. However, future studies with larger sample sizes would be needed to confirm the significant correlations, rule out false positives, or find false negatives. In the C&C VOI at 6 weeks, correlations only existed in the two groups that delivered BMP-2 (i.e., BMP-2 only and both IGF-1 and BMP-2). Group BMP-2 also predominantly had strong correlations between bone parameters and cartilage morphology, regularity, and cell and GAG content in the trabecular VOI at 6 weeks. These strong associations could be attributed to the potent osteoconductive effects of BMP-2 on early bone formation.22,27,30
In contrast, in the trabecular VOI at 12 weeks, strong positive correlations were seen between cartilage regularity and bone formation for the two groups delivering IGF-1. Indeed, IGF-1 has been shown to decrease synovial inflammation in comparison to growth factor-free controls when delivered with chondrocytes in an equine model.31 IGF-1 can also increase proteoglycan synthesis and slow proteoglycan catabolism in a dose-dependent manner.32–34 This is corroborated by the strong negative correlations between neocartilage cell and GAG content and bone formation at 12 weeks in the C&C group for the BMP-2 only delivering group. Interestingly, this chondroprotective quality of IGF-1 is not clearly apparent in the outcome-based publication, since a comparison of means for cartilage scoring indices revealed no differences between experimental groups at either time point.22
Overall, correlation analysis gave valuable insight in the repair process of osteochondral tissue and can be a useful tool in supplementing traditional outcome-based analysis. In the present report, moderate to strong correlations were consistently seen between cartilage regularity and bone formation parameters, especially in the trabecular region. By simply comparing the means of cartilage and bone repair data sets, as performed in a previous publication,22 this unique relationship could not have been construed. Although causality could not be determined with correlation analysis in the present report (e.g., greater bone formation in the trabecular region improves cartilage regularity), knowing specific correlations can be a preliminary step in inspiring informed studies for future osteochondral repair strategies. For example, scaffold stiffness in the subchondral bone region could be modulated to examine its effects on cartilage regularity. In addition, whether or not early bone repair improves cartilage repair could be directly tested by using minimally invasive techniques for examining bone and cartilage repair, and subsequently performing correlation and/or regression analysis on early and late time point measurements.
Conclusion
The repair of articular cartilage and subchondral bone, facilitated by a growth factor-delivering hydrogel composite scaffold, was evaluated in an osteochondral defect at 6 and 12 weeks. Associations between specific cartilage indices and bone parameters were tested through correlations analysis. Overall, there were significant associations between the repair of cartilage and the repair of subchondral bone. Significant correlations were consistently seen between cartilage surface regularity and bone quantity parameters, which may confirm the role of mechanical support that the subchondral bone plays for the overlying articular cartilage. Bone and cartilage correlations also changed from 6 to 12 weeks of repair, an indication of the different rates at which cartilage and bone repair and remodel. In addition, the three experimental groups had different trends in correlations, revealing stronger correlations between cartilage repair and bone formation at an earlier time point when BMP-2 was involved, and suggesting the chondroprotective nature of IGF-1 at a later time point, highlighting the differing effects of IGF-1 and BMP-2 on osteochondral repair. Altogether, the repair of cartilage is associated with the repair of subchondral bone for specific structural parameters, and through correlation analysis, a complete understanding of the repair of both tissues can help drive informed studies for future osteochondral repair strategies.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (R01-AR048756). The authors acknowledge the technical assistance of Ms. Natasja van Dijk with the histological preparation of specimens and Mr. Brian C. Dawson with micro-CT evaluation. The authors would also like to thank the registered veterinary technicians at the University of Texas Health Science Center for their support during the animal procedures.
Disclosure Statement
No competing financial interests exist.
References
- 1.Vinatier C., Bouffi C., Merceron C., Gordeladze J., Brondello J.M., Jorgensen C., et al. . Cartilage tissue engineering: towards a biomaterial-assisted mesenchymal stem cell therapy. Curr Stem Cell Res Ther 4, 318, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinatier C., Mrugala D., Jorgensen C., Guicheux J., and Noel D. Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol 27, 307, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Goldring S.R. Cross-talk between subchondral bone and articular cartilage in osteoarthritis. Arthritis Res Ther 14, A7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radin E.L., Ehrlich M.G., Chernack R., Abernethy P., Paul I.L., and Rose R.M. Effect of repetitive impulsive loading on knee joints of rabbits. Clin Orthop Relat Res 131, 288, 1978 [PubMed] [Google Scholar]
- 5.Serink M.T., Nachemson A., and Hansson G. Effect of impact loading on rabbit knee joints. Acta Orthop Scand 48, 250, 1977 [DOI] [PubMed] [Google Scholar]
- 6.de Vries-van Melle M.L., Narcisi R., Kops N., Koevoet W.J., Bos P.K., Murphy J.M., et al. . Chondrogenesis of mesenchymal stem cells in an osteochondral environment is mediated by the subchondral bone. Tissue Eng Part A 20, 23, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funck-Brentano T., and Cohen-Solal M. Crosstalk between cartilage and bone: when bone cytokines matter. Cytokine Growth Factor Rev 22, 91, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Santo V.E., Gomes M.E., Mano J.F., and Reis R.L. Controlled release strategies for bone, cartilage, and osteochondral engineering-part I: recapitulation of native tissue healing and variables for the design of delivery systems. Tissue Eng Part B Rev 19, 308, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keeney M., and Pandit A. The osteochondral junction and its repair via bi-phasic tissue engineering scaffolds. Tissue Eng Part B Rev 15, 55, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Lajeunesse D. The role of bone in the treatment of osteoarthritis. Osteoarthritis Cartilage 12Suppl A, S34, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Tat S.K., Lajeunesse D., Pelletier J.P., and Martel-Pelletier J. Targeting subchondral bone for treating osteoarthritis: what is the evidence? Best Pract Res Clin Rheumatol 24, 51, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radin E.L., and Rose R.M. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res 213, 34, 1986 [PubMed] [Google Scholar]
- 13.Orth P., Cucchiarini M., Kohn D., and Madry H. Alterations of the subchondral bone in osteochondral repair—translational data and clinical evidence. Eur Cell Mater 25, 299, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Karsdal M.A., Leeming D.J., Dam E.B., Henriksen K., Alexandersen P., Pastoureau P., et al. . Should subchondral bone turnover be targeted when treating osteoarthritis? Osteoarthritis Cartilage 16, 638, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Baker-LePain J.C., and Lane N.E. Role of bone architecture and anatomy in osteoarthritis. Bone 51, 197, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldring M.B., and Goldring S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci 1192, 230, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Hayami T., Pickarski M., Zhuo Y., Wesolowski G.A., Rodan G.A., and Duong le T. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone 38, 234, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Mansell J.P., Collins C., and Bailey A.J. Bone, not cartilage, should be the major focus in osteoarthritis. Nat Clin Pract Rheumatol 3, 306, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Qiu Y.S., Shahgaldi B.F., Revell W.J., and Heatley F.W. Observations of subchondral plate advancement during osteochondral repair: a histomorphometric and mechanical study in the rabbit femoral condyle. Osteoarthritis Cartilage 11, 810, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Orth P., Cucchiarini M., Kaul G., Ong M.F., Graber S., Kohn D.M., et al. . Temporal and spatial migration pattern of the subchondral bone plate in a rabbit osteochondral defect model. Osteoarthritis Cartilage 20, 1161, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Zhang W.J., Lian Q., Li D.C., Wang K.Z., Hao D.J., Bian W.G., et al. . Cartilage repair and subchondral bone migration using 3D printing osteochondral composites: a one-year-period study in rabbit trochlea. Biomed Res Int 2014, 746138, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu S., Lam J., Trachtenberg J.E., Lee E.J., Seyednejad H., van den Beucken J.J., et al. . Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials 35, 8829, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim K., Lam J., Lu S., Spicer P.P., Lueckgen A., Tabata Y., et al. . Osteochondral tissue regeneration using a bilayered composite hydrogel with modulating dual growth factor release kinetics in a rabbit model. J Control Release 168, 166, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam J., Lu S., Lee E.J., Trachtenberg J.E., Meretoja V.V., Dahlin R.L., et al. . Osteochondral defect repair using bilayered hydrogels encapsulating both chondrogenically and osteogenically pre-differentiated mesenchymal stem cells in a rabbit model. Osteoarthritis Cartilage 22, 1291, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukaka M.M. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J 24, 69, 2012 [PMC free article] [PubMed] [Google Scholar]
- 26.Zou K.H., Tuncali K., and Silverman S.G. Correlation and simple linear regression. Radiology 227, 617, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Tokuhara Y., Wakitani S., Imai Y., Kawaguchi A., Fukunaga K., Kim M., et al. . Repair of experimentally induced large osteochondral defects in rabbit knee with various concentrations of Escherichia coli-derived recombinant human bone morphogenetic protein-2. Int Orthop 34, 761, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomoll A.H., Madry H., Knutsen G., van Dijk N., Seil R., Brittberg M., et al. . The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc 18, 434, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orth P., Cucchiarini M., Zurakowski D., Menger M.D., Kohn D.M., and Madry H. Parathyroid hormone [1–34] improves articular cartilage surface architecture and integration and subchondral bone reconstitution in osteochondral defects in vivo. Osteoarthritis Cartilage 21, 614, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Sellers R.S., Peluso D., and Morris E.A. The effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) on the healing of full-thickness defects of articular cartilage. J Bone Joint Surg Am 79A, 1452, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Fortier L.A., Mohammed H.O., Lust G., and Nixon A.J. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br 84B, 276, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Hickey D.G., Frenkel S.R., and Di Cesare P.E. Clinical applications of growth factors for articular cartilage repair. Am J Orthop (Belle Mead NJ) 32, 70, 2003 [PubMed] [Google Scholar]
- 33.Schmidt M.B., Chen E.H., and Lynch S.E. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthritis Cartilage 14, 403, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Lam J., Lu S., Kasper F.K., and Mikos A.G. Strategies for controlled delivery of biologics for cartilage repair. Adv Drug Deliv Rev 84, 123, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.