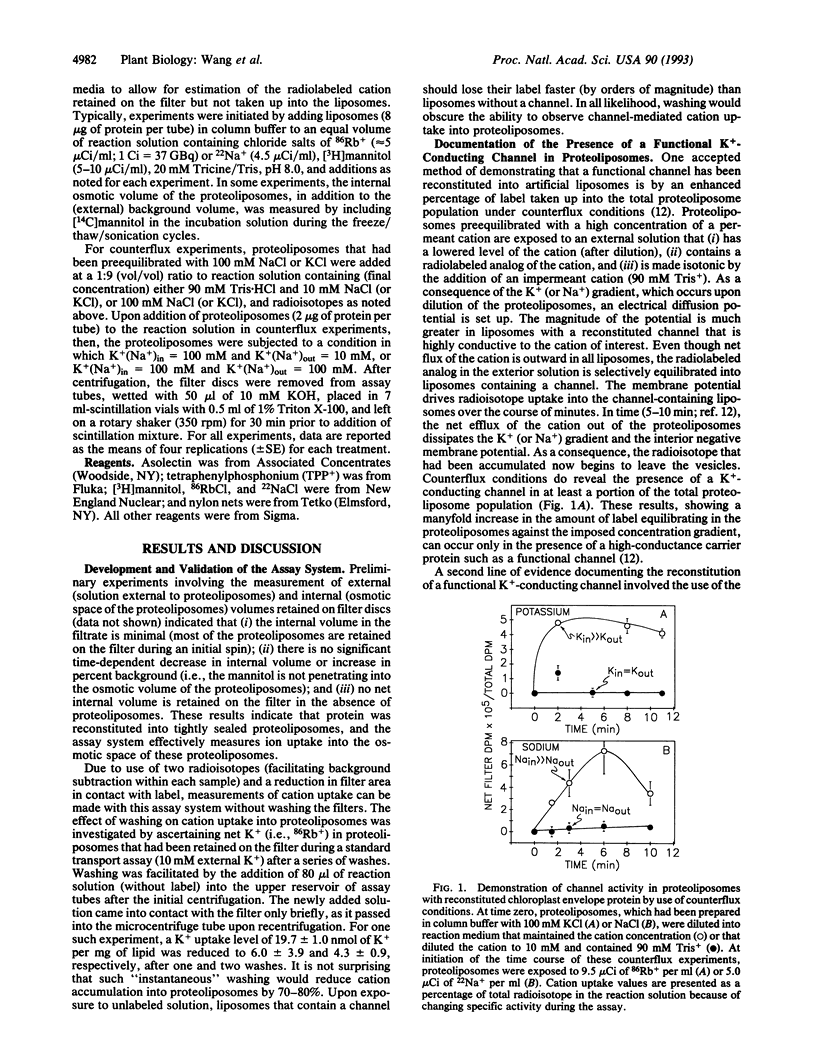
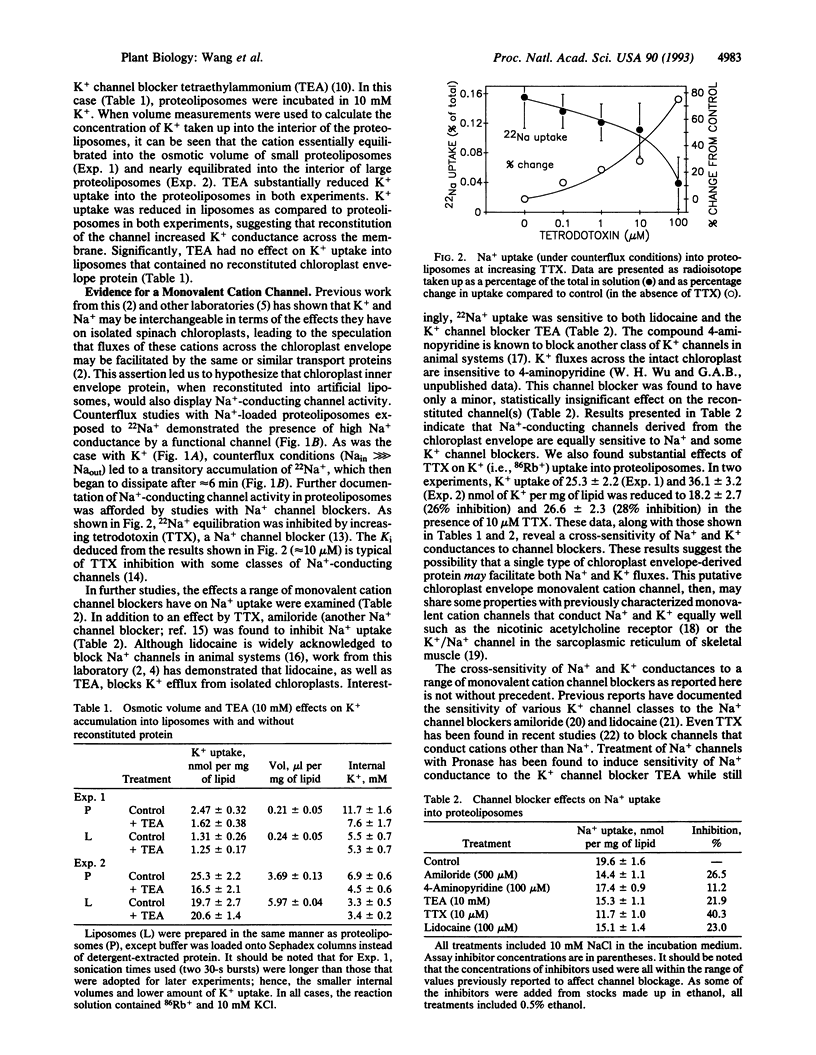
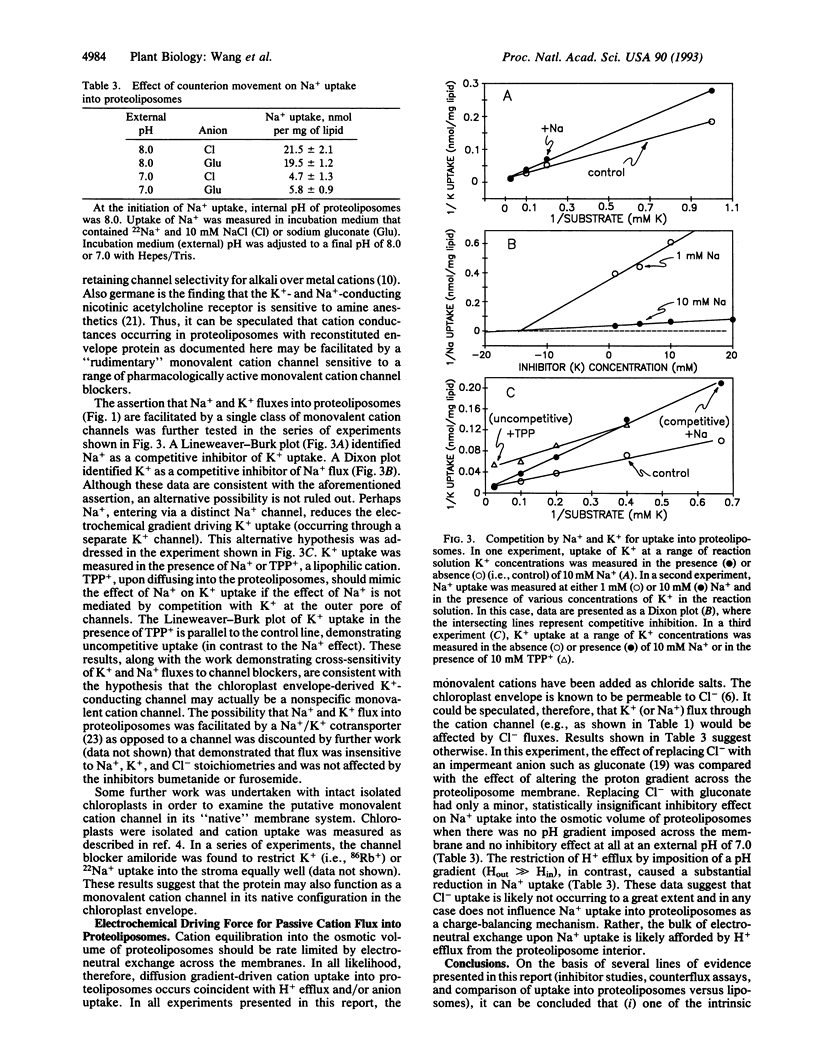
Abstract
Potassium flux between the chloroplast stroma and cytoplasm is known to be indirectly linked to H+ countertransport and, hence, stromal pH and photosynthetic capacity. The specific molecular mechanism that facilitates K+ flux across the chloroplast envelope is not known and has been a source of controversy for well over a decade. The objective of this study was to elucidate the nature of this envelope protein. To this end, solubilized protein in detergent extracts of purified chloroplast inner envelope vesicles was reconstituted into artificial liposomes, and cation fluxes into these proteoliposomes were measured. Results of inhibitor studies and counterflux experiments indicated that a K+-conducting ion channel was solubilized and functionally reconstituted into the proteoliposomes. This transport protein may be a nonspecific monovalent cation channel. This report represents a direct demonstration of ion channel activity associated with the limiting (inner) membrane of the chloroplast envelope.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Takahashi K. Tetrodotoxin-sensitive calcium-conducting channels in the rat hippocampal CA1 region. J Physiol. 1992 May;450:529–546. doi: 10.1113/jphysiol.1992.sp019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B., Gimmler H. Properties of the Isolated Intact Chloroplast at Cytoplasmic K Concentrations : I. Light-Induced Cation Uptake into Intact Chloroplasts is Driven by an Electrical Potential Difference. Plant Physiol. 1983 Sep;73(1):169–174. doi: 10.1104/pp.73.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty H., Rudy B., Karlish S. J. A simple and sensitive procedure for measuring isotope fluxes through ion-specific channels in heterogenous populations of membrane vesicles. J Biol Chem. 1983 Nov 10;258(21):13094–13099. [PubMed] [Google Scholar]
- Gellens M. E., George A. L., Jr, Chen L. Q., Chahine M., Horn R., Barchi R. L., Kallen R. G. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):554–558. doi: 10.1073/pnas.89.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz K. T., McCarty R. E. Solubilization, partial purification, and reconstitution of the glycolate/glycerate transporter from chloroplast inner envelope membranes. Plant Physiol. 1991 Aug;96(4):1060–1069. doi: 10.1104/pp.96.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyman T. R., Cragoe E. J., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988 Oct;105(1):1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Lauf P. K., McManus T. J., Haas M., Forbush B., 3rd, Duhm J., Flatman P. W., Saier M. H., Jr, Russell J. M. Physiology and biophysics of chloride and cation cotransport across cell membranes. Fed Proc. 1987 May 15;46(7):2377–2394. [PubMed] [Google Scholar]
- Maury W. J., Huber S. C., Moreland D. E. Effects of Magnesium on Intact Chloroplasts : II. CATION SPECIFICITY AND INVOLVEMENT OF THE ENVELOPE ATPase IN (SODIUM) POTASSIUM/PROTON EXCHANGE ACROSS THE ENVELOPE. Plant Physiol. 1981 Dec;68(6):1257–1263. doi: 10.1104/pp.68.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley D., Meissner G. Evidence for a K+, Na+ permeable channel in sarcoplasmic reticulum. J Membr Biol. 1978 Dec 15;44(2):159–186. doi: 10.1007/BF01976037. [DOI] [PubMed] [Google Scholar]
- Peters J. S., Berkowitz G. A. Studies on the System Regulating Proton Movement across the Chloroplast Envelope : Effects of ATPase Inhibitors, Mg, and an Amine Anesthetic on Stromal pH and Photosynthesis. Plant Physiol. 1991 Apr;95(4):1229–1236. doi: 10.1104/pp.95.4.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. Tetraethylammonium ions and the potassium permeability of excitable cells. Rev Physiol Biochem Pharmacol. 1983;97:1–67. doi: 10.1007/BFb0035345. [DOI] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]
- Wu W., Berkowitz G. A. K stimulation of ATPase activity associated with the chloroplast inner envelope. Plant Physiol. 1992 Jun;99(2):553–560. doi: 10.1104/pp.99.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Berkowitz G. A. Lidocaine and ATPase inhibitor interaction with the chloroplast envelope. Plant Physiol. 1991 Dec;97(4):1551–1557. doi: 10.1104/pp.97.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Berkowitz G. A. Stromal pH and Photosynthesis Are Affected by Electroneutral K and H Exchange through Chloroplast Envelope Ion Channels. Plant Physiol. 1992 Feb;98(2):666–672. doi: 10.1104/pp.98.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]


