Abstract
Antifungal vaccines have recently engendered considerable excitement for counteracting the resurgence of fungal infections. In this context, β-glucan, which is abundantly expressed on all fungal cell surfaces, functionally necessary for fungi and immunologically active, is an attractive target antigen. Aiming at the development of effective antifungal vaccines based on β-glucan, a series of its oligosaccharide derivatives were designed, synthesized, and coupled with a carrier protein, keyhole limpet hemocyanin (KLH), to form new semi-synthetic glycoconjugate vaccines. In this paper, a convergent and effective synthetic strategy using pre-activation-based iterative glycosylation was developed for the designed oligosaccharides. The strategy can be widely useful for rapid construction of large oligo-β-glucans with shorter oligosaccharides as building blocks. The KLH conjugates of the synthesized β-glucan hexa-, octa-, deca- and dodecasaccharides were demonstrated to elicit high titers of antigen-specific total and IgG antibodies in mice, suggesting the induction of functional T cell-mediated immunity. Moreover, it was revealed that octa-, deca-, and dodeca-β-glucans were much more immunogenic than the hexamer, while the octamer was the best. The results suggested that the optimal oligosaccharide sequence of β-glucan required for exceptional immunogenicity was a hepta- or octamer and that longer glucans are not necessarily better antigens, a finding that may be of general importance. Most importantly, the octa-β-glucan-KLH conjugate provoked protective immunities against Candida albicans infection in a systemic challenge model in mice, suggesting the great potential of this glycoconjugate as a clinically useful immunoprophylactic antifungal vaccine.
Keywords: fungus, Candida, vaccine, carbohydrate, β-glucan, glycoconjugate
Graphical abstract
Introduction
Fungal infection poses a great threat to the human health, and its cases grow rapidly year by year1 owing to the limitations of current antifungal drugs and, especially, the emergence of drug-resistant strains. As a result, deep-seated infections in nosocomial settings have a high mortality even after treatment with antifungal drugs.2, 3 Moreover, many commensal and opportunistic fungi, previously thought to be nonpathogenic, have emerged as pathogens in immunocompromised patients.4,5 To meet the urgent medical need for antifungal therapies, development of prophylactic and/or therapeutic antifungal vaccines is increasingly considered as one of the most attractive and appropriate strategies.6
Beta-(1,3)-glucan (β-glucan) with sporadic branches linked to the 6-O-position is an essential cell wall component of various fungi.7 This biopolymer is exposed on the surface of fungal cells and is functionally necessary, thus it is an excellent target antigen for the development of broadly useful antifungal vaccines. It has been demonstrated that β-glucan could provoke immunogenic protection against Candida albicans in mice.8 Therefore, a series of vaccines based on natural β-glucans, such as their conjugates with diphtheria toxin CMR197, have been explored and shown to elicit protections against Candida in a mouse model.9–11 Recent studies suggested that linear β-glucan and its short oligosaccharides could elicit immune responses and protections against C. albicans.12–14 Furthermore, it has been demonstrated that the CMR197 conjugate of a synthetic β-glucan hexasaccharide elicited stronger glucan-specific antibody responses than the conjugate of natural β-glucan. The hexasaccharide conjugate as an antifungal vaccine was proposed to be more promising than other synthetic or natural β-glucan conjugates.12–14
Developing conjugate vaccines using synthetic oligosaccharide antigens is a relatively new concept. One of the successful stories was a semi-synthetic anti-Haemophilus influenzae type b (Hib) vaccine.15 This type of vaccines has some advantages. For example, their synthetic antigens have defined chemical structures, which would facilitate detailed immunological and structure-activity relationship studies to help gain more insights into the function of vaccines and optimize vaccine design. The reaction sites and/or linkage positions of the carbohydrate antigens are well defined and predictable, which would improve vaccine quality control. Therefore, as promising candidate antigens for vaccine design, oligomeric β-glucans have received considerable attention. For example, several groups have recently synthesized linear tetra,16 penta,17 hexa,14,18,19 dodeca20 and hexadeca,21 and a branched heptadeca oligosaccharides21 of β-glucans by various methods. However, most conjugate vaccines studied so far are made of heterogeneous natural β-glucans or oligosaccharides derived from natural β-glucans, and there are very few reports about conjugate vaccines derived from synthetic β-glucan derivatives.12–14
In an effort to systematically explore the structure-immunogenicity relationships of linear β-glucans and optimize the antigen design for creating more efficacious antifungal vaccines, we have: (1) developed a highly convergent and effective method for the synthesis of oligosaccharides of β-glucan with varied chain lengths, (2) coupled them with keyhole limpet hemocyanin (KLH), and (3) evaluated the immunological properties of resulting glycoconjugates and their capability to elicit protective immune responses against C. albicans in mice.
Results and Discussion
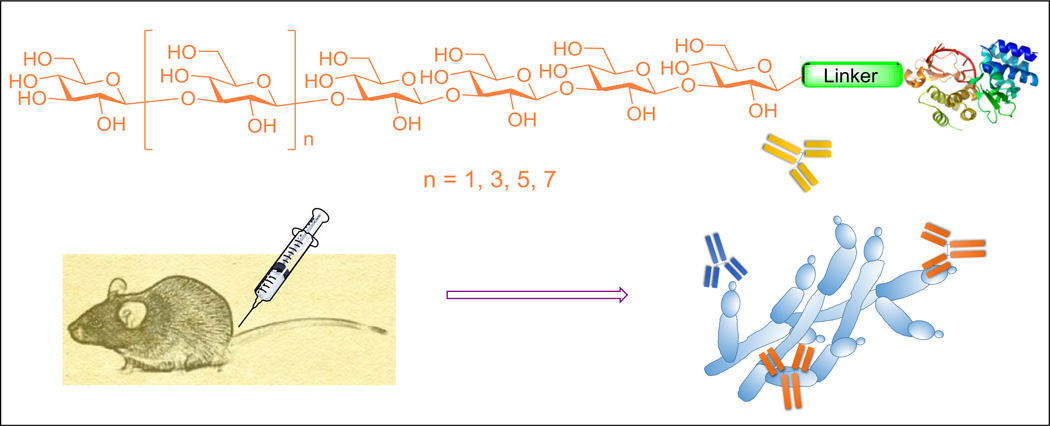
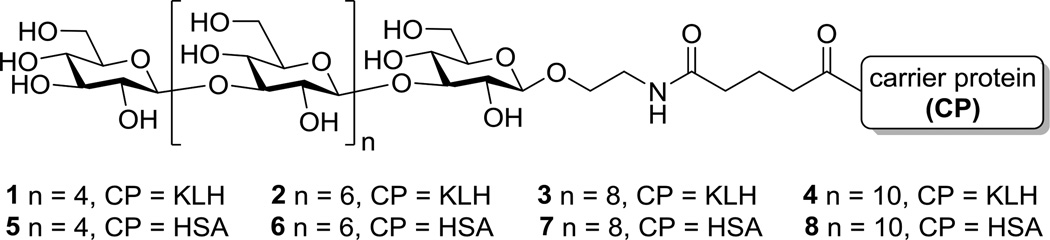
Based on reports that a hexasaccharide of β-glucan was highly immunogenic12–14 and that at least an octa- or nonasaccharide may be required to generate special 3D structures,22 we planned to prepare and compare hexa-, octa-, deca- and dodecasaccharides of β-glucan (Figure 1). These oligosaccharides were coupled with KLH to form vaccines 1–4. KLH is one of most commonly used and effective protein carriers for glycoconjugate vaccine development.23 In the meantime, the oligosaccharides were also coupled with human serum albumin (HSA) to provide conjugates 5–8 that were used as capture reagents for detecting β-glucan-specific antibodies by the enzyme-linked immunosorbent assay (ELISA).
Figure 1.
The structure of designed β-glucan oligosaccharides and their protein conjugates 1–8
Oligosaccharide synthesis
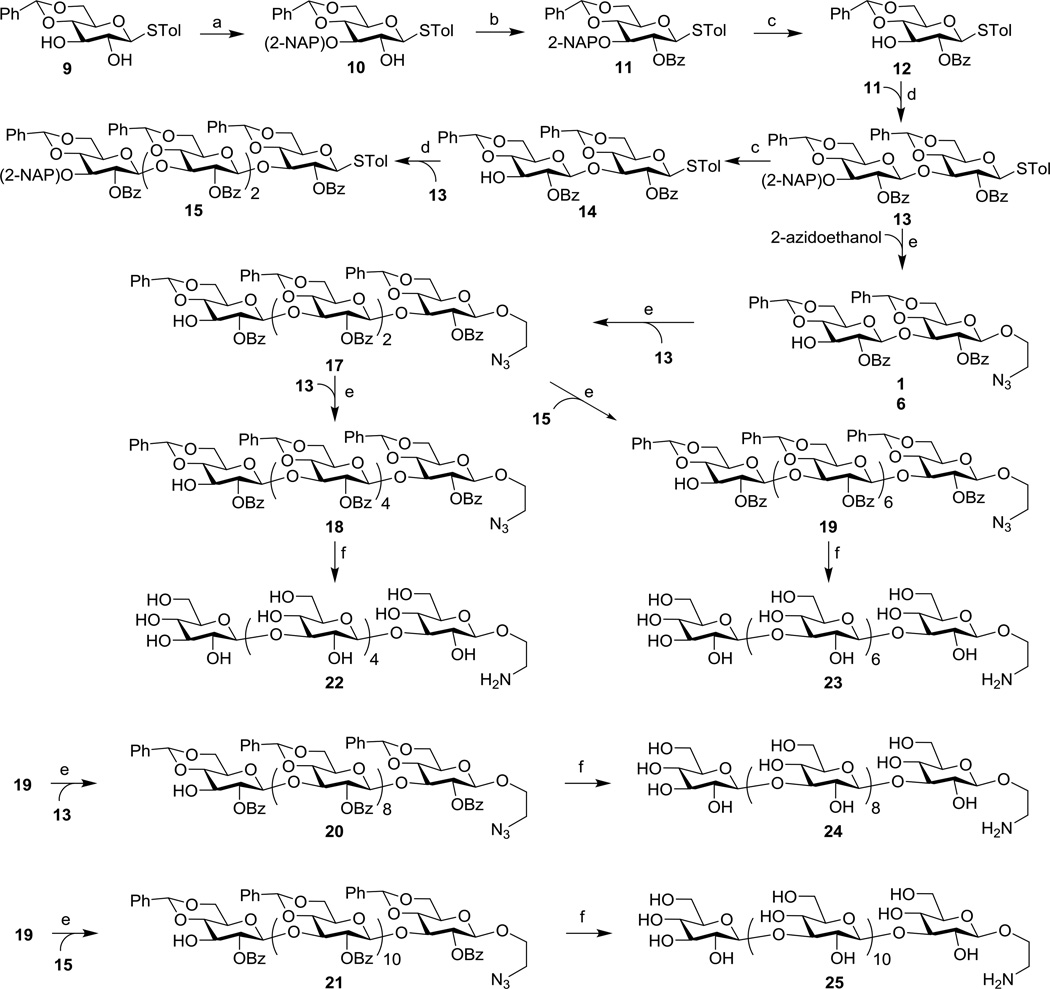
As depicted in Scheme 1, the designed β-glucan oligosaccharides were achieved through pre-activation-based iterative glycosylation with p-toluenethioglycosides as glycosyl donors and disaccharide 13 as a key building block. The synthesis was commenced with the preparation of 9 from d-glucose according to a literature procedure in four steps and in a 40% overall yield.24, 25 Treatment of 9 with dibutyltin oxide to furnish the stannylene acetal-directed regioselective protection of 3-O-position with a 2-naphthylmethyl (NAP) group26, 27 was followed by 2-O-benzoylation of the resultant 10 to afford thioglycoside donor 11. Here, the NAP group was employed as a temporary protection instead of the common para-methoxybenzyl (PMB) group because the former is more stable to acidic conditions involved in glycosylation reactions, although both groups can be readily removed with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ).22,23 Removal of the 3-O-NAP group in 11 with DDQ was straightforward to give 12 in an excellent yield (92%).26, 27 Thereafter, 11 was coupled with 12 via pre-activation glycosylation28, 29 to get 13. Specifically, glycosyl donor 11 was first activated with p-toluenesulfenyl triflate (p-TolSOTf) that was generated in situ from the reaction between p-toluenesulfenyl chloride (p-TolSCl) and silver triflate (AgOTf) at −78 °C. Then, glycosyl acceptor 12 was added to furnish glycosylation, resulting in the desired β-disaccharide 13 (J1,2 = 7.5 Hz, 90% yield) in a stereospecific manner, due to neighboring group participation. Compound 13 was used as one of the common glycosyl donors for subsequent carbohydrate chain elongation. On the other hand, removal of the NAP group in 13 with DDQ provided 14. A convergent [2+2] glycosylation reaction between 13 and 14 by the same pre-activation protocol yielded tetrasaccharide 15 (86%) as a glycosyl donor for more complex oligosaccharide assembly. Pre-activated glycosylation of 2-azidoethanol with 13, followed by removal of the NAP group with DDQ, afforded 16 (91%), which carried an azido group at the non-reducing end. The azido group would be reduced to form a primary amine later on to enable a selective reaction with the linker and then coupling with carrier proteins. Moreover, since the pre-activation-based glycosylation reaction was clean and high yielding and the donor and acceptor were almost completely consumed, this allowed us to move on to the next step after glycosylation, i.e., removal of the NAP group, without purification of the reaction intermediate. Similarly, pre-activation-based glycosylation of 16 with 13 and then removal of the NAP group produced tetrasaccharide 17. On the basis of 17, the sugar chain was further elongated successfully via pre-activation-based glycosylation to achieve all of the designed β-glucan oligosaccharides. Coupling of 17 with disaccharide 13 and tetrasaccharide 15, followed by selective removal of the NAP group, afforded hexasaccharide 18 and octasaccharide 19, respectively. Subsequently, 19 was coupled with 13 and 15, which was followed by NAP group removal to produce decasaccharide 20 and dodecasaccharide 21. Notably, the glycosylation yields were not significantly affected by the increased size of involved building blocks. All of the synthetic intermediates and final products were fully characterized, proving that the glycosylation reactions were β-specific.
Scheme 1.
Synthesis of β-glucan oligosaccharides 22–25
Reagents and conditions: a) Bu2SnO, toluene, reflux, 6 h; then 2-naphthylmethyl bromide, CsF, DMF, 70 °C, 12 h, 72%; b) BzCl, Et3N, CH2Cl2, rt, 12 h, 96%; c) DDQ, CH2Cl2/H2O (18:1), rt, 8 h, 92% for 12, 95% for 14; d) AgOTf, TTBP, p-TolSCl, CH2Cl2, −78 °C to rt, 4 h, 90% for 13, 86% for 15 ; e) AgOTf, TTBP, p-TolSCl, CH2Cl2, −78 °C, rt, 4 h; then DDQ, CH2Cl2/H2O (18:1), rt, 8 h, 91% for 16, 90% for 17, 87% for 18, 81% for 19, 80% for 20, 85% for 21; f) Zn, AcOH, CH2Cl2, 24 h, rt; then AcOH/H2O (5:1), 60 °C, 24 h; finally NaOH, t-BuOH:H2O, 40 °C, 24 h, 80% for 22, 88% for 23, 85% for 24, 88% for 25.
Attempts to globally deprotect 18–21 via saponification followed by hydrogenation or via hydrogenation followed by saponification in one pot were unsuccessful, mainly due to the poor mutual solubility of 18–21 and the partially deprotected products in various solvent combinations. Eventually, they were fully deprotected in a stepwise manner using the proper solvent or solvent combination for each transformation, following the order of Zn-mediated reduction of the azido group in dichloromethane (DCM), acidic cleavage of all benzylidene groups in acetic acid and water (5:1), and finally sodium hydroxide-promoted removal of all benzoate groups in t-butyl alcohol and water (4:1). The final products were purified with a Sephadex-G25 size exclusion column with distilled water as the eluent to afford 22–25 as white fluff solids upon lyophilization. The final products were fully characterized by both NMR and MS.
Conjugation of oligosaccharides 22–25 with carrier proteins
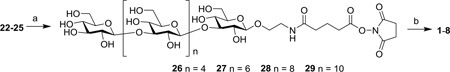
Free oligosaccharides 22–25 were conjugated with carrier proteins KLH and HSA through the bifunctional glutaryl group, as this conjugation can be easily and effectively realized via activated glutaryl ester and, not like other complex linkers, this simple linker would not affect the immunological activity of conjugates.30,31 A two-step procedure was used to furnish the conjugation (Scheme 2). First, reaction between the free amino group in 22–25 and a large excess of active ester disuccinimidal glutarate (DSG) gave the corresponding mono-activated esters 26–29 in quantitative yields. Then, 26–29 were coupled with KLH or HSA in 0.1 M phosphate-buffered saline (PBS) to afford the desired glycoprotein conjugates 1–8, which were purified with a Biogel A0.5 column to remove remaining free sugars. The conjugate-containing fractions were dialyzed against distilled water and lyophilized to give 1–8. Finally, the glucose content of each conjugate was analyzed by the phenol-sulfuric acid method following a reported protocol.32 The glucose contents of the KLH and HSA conjugates were 7.5–9.1% and 10.5–25.8%, respectively (Table 1), showing that the coupling reactions were efficient and the antigen loading levels were in the desired range for glycoconjugate vaccines.33 The sugar loadings of HSA conjugates were also confirmed by MALDI-TOF mass spectrometry.
Scheme 2.
Preparation of oligo-β-glucan-protein conjugates 1–8
Reagents and conditions: a) DSG, DMF and PBS buffer (4:1), rt, 4 h; b) KLH or HSA, PBS buffer, rt, 2.5 days.
Table 1.
Carbohydrate loadings of glycoconjugates 1–8
| Sample | KLH conjugates | HSA conjugates | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Loading (%) | 8.3 | 7.8 | 7.5 | 9.1 | 10.5 | 11.0 | 14.3 | 25.8 |
Immunological studies of glycoconjugate vaccines 1–4
The immunological properties of KLH conjugates 1–4 were investigated in female C57BL/6J mice. For this purpose, each conjugate was thoroughly mixed with Titermax Gold adjuvant, and the resulting emulsion was then injected intramuscularly (i.m.) into mice. Following the initial immunization, mice were boosted 4 times on days 14, 21, 28 and 38 by subcutaneous (s.c.) injection of the same vaccine emulsion. Blood samples of each mouse were collected through the leg veins prior to the initial immunization on day 0 and after immunizations on days 27, 38 and 48. Antisera were obtained from clotted blood samples and were stored at −80 °C before use. ELISA using the corresponding HSA conjugates as capture reagents for plate coating was employed to determine antibody titers, which reflected the elicited immune responses. Antibody titers were defined as the dilution number yielding an OD value of 0.2, and the results are shown in Figure 2.
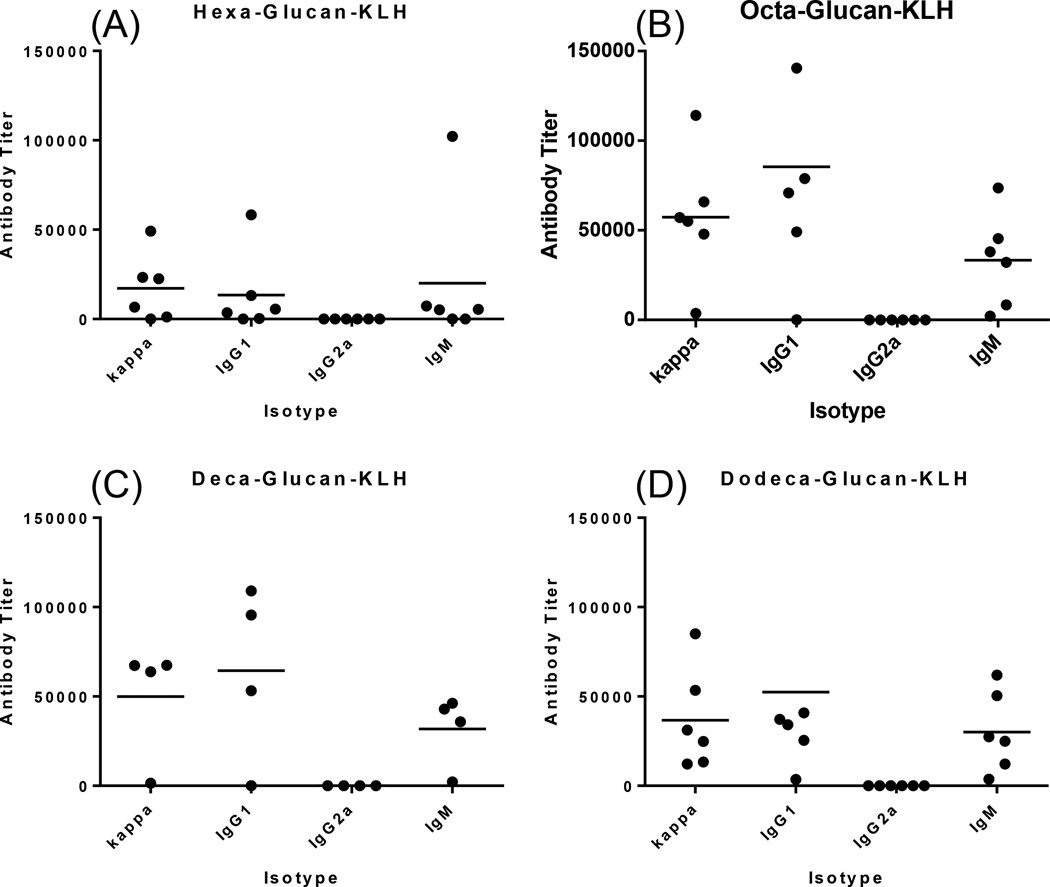
Figure 2.
ELISA results of the day 48 antisera obtained with conjugates 1 (A), 2 (B), 3 (C) and 4 (D) combined with Titermax Gold adjuvant, respectively. The titers of corresponding antigen-specific antibodies are displayed. Each dot represents the antibody titer of an individual mouse, and the black bar shows the average titer.
Evidently, all of the KLH conjugates 1–4 elicited high titers of antigen-specific total (kappa) antibodies, indicating strong immune responses. More importantly, high titers of IgG1 antibodies were observed for all glycoconjugates, suggesting memorable T cell-dependent immunities.34, 35 IgG1 antibody is usually considered as the protective antibody isotype,36, 37 thus these conjugates were believed to elicit protective immune responses and have great potential for being developed into clinically functional vaccines against fungal infections.
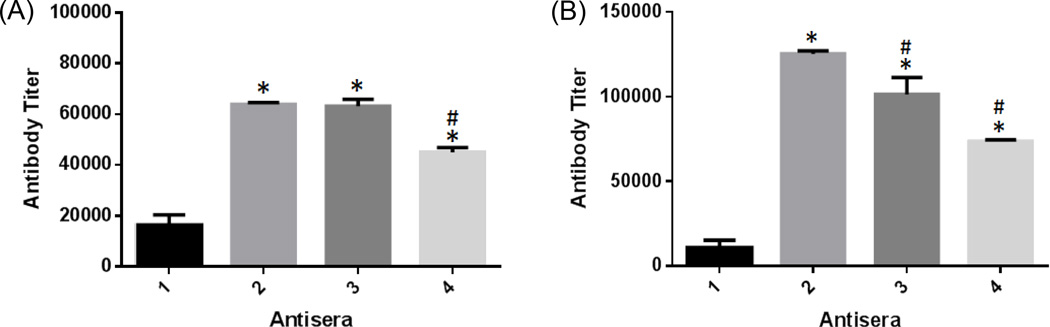
The above immunological results revealed that, overall, conjugates 2–4 induced significantly higher titers of both total (anti-kappa) and IgG1 antibodies than 1 (P << 0.01, Figure 3), indicating that 2–4 were much more immunogenic and provoked much stronger immune responses in mice than 1. Further analysis of the immune responses showed that the IgG1 antibody titer induced by 2 was significantly higher than that induced by 3 and 4 (P < 0.01, Figure 3B) as well. Although the total antibody titer for 2 was also slightly higher than that for 3 and 4 (Figure 3A), this difference was less significant (P > 0.05 and < 0.01, respectively). There are several factors that may affect the immune response to a glycoconjugate, such as carbohydrate loading,38, 39 conjugation method, immunization protocol, and carbohydrate antigen structure. The carbohydrate loadings of 1–4 were very similar, and their conjugation method and immunization protocol were identical. Therefore, the different immunological properties for these glycoconjugates were because of their different carbohydrate structures, and among the oligosaccharides investigated here octa-β-glucan seemed to be the most immunogenic and the most promising antigen for vaccine development.
Figure 3.
Comparison of the average antibody titers of corresponding antigen-specific (A) total (anti-kappa) antibodies and (B) IgG1 antibodies in the day 48 pooled antisera of mice immunized with conjugates 1–4, respectively. Each error bar is the standard deviations for three parallel experiments. * P << 0.01 as compared to 1; # P < 0.05 as compared to 2.
Protection against fungal infection in mouse
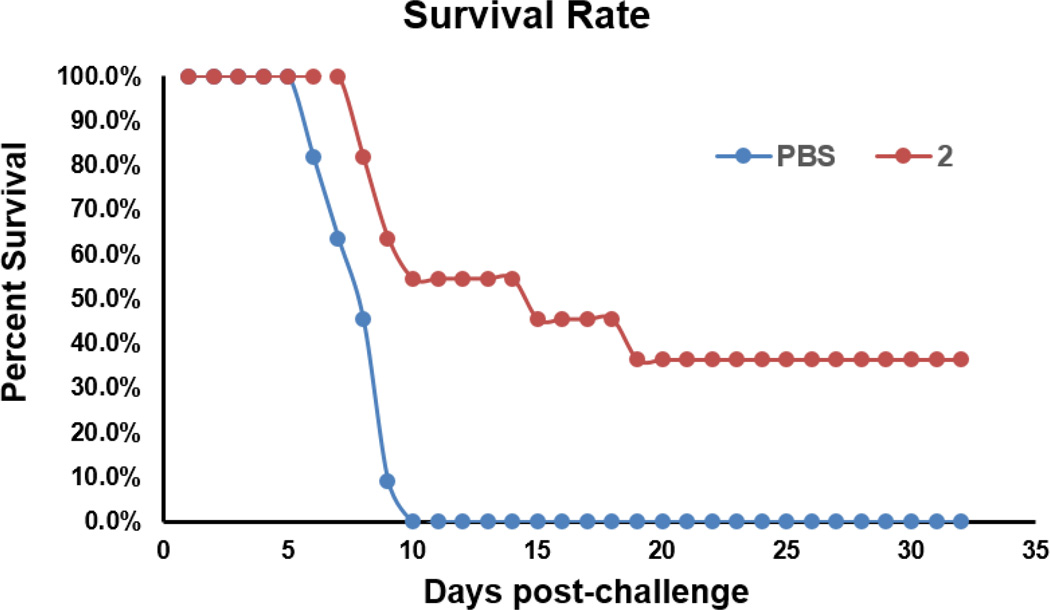
To ultimately prove the efficacy of the new glycoconjugate vaccine to protect against fungal infections, conjugate 2 that elicited the strongest immune responses in above studies was evaluated in a fungal challenge experiment in mice. The fungus used was C. albicans (strain SC5314), one of the most common and important pathogenic fungi in clinic.40 In this experiment, each group of 11 mice were immunized with 2 or PBS (the control group) 4 times on days 1, 14, 21, and 28 according to above-mentioned protocols. On day 38, a pre-determined lethal dose of C. albicans (7.5 ×105 cells/mouse in 200 µL PBS) was given by i.v. injection to each mouse. The responses of these mice were observed under normal feeding and care conditions. As shown in Figure 4, mice in the control group started to die of infection on day 6 after the fungal injection, and all died within 4 days (on day 10). In comparison, mice in the 2-immunized group did not have fatal incident until day 8, and on day 14 the animal survival rate was about 55%. At the end of this experiment (on day 32), there were still 4 mice (about 34%) in the immunized group unaffected, suggesting complete protection of these mice from C. albicans infection. These results proved that glycoconjugate 2 could elicit protective immunity in mice against lethal systemic challenge with C. albicans.
Figure 4.
Survival time of mice immunized with 2 compared with mice immunized with PBS after i.v. injection of C. albicans (7.5 × 105 cells per mouse and 11 mice per group).
Conclusion
In summary, a series of β-glucan oligosaccharides were synthesized and coupled with KLH to generate glycoconjugates that contained structurally well-defined carbohydrate antigens. These glycoconjugates were shown to elicit robust T cell-dependent and protective immune responses in mice, which helped identify some promising antifungal vaccines. This work is distinguished from previous studies in the area in several aspects. First, a highly convergent, effective and potentially broadly applicable strategy was developed for the synthesis of structurally well-defined β-glucans. Large oligosaccharides could be rapidly assembled from short oligosaccharide segments by the pre-activation-based glycosylation protocol that had significantly reduced the number of steps for anomeric manipulation. It was further observed that the size of the oligosaccharide segments used for the synthesis had little influence on the glycosylation efficiency. Furthermore, with the help of neighboring group participation, all of the glycosylations were highly stereoselective to create the desired β-anomer. Therefore, this synthetic strategy can be widely applicable to larger and more complex β-glucan derivatives via [n+n] or [n+(n+1)] glycosylations.
Second, the synthesized oligosaccharides had a reactive amino group at their reducing ends, enabling their effective coupling with carrier proteins, such as KLH, through a bifunctional linker. The resultant conjugates contained structurally defined carbohydrate antigens to facilitate detailed and in-depth investigation of glycoconjugate vaccines, such as their structure-activity relationship analysis. Although a number of β-glucan oligosaccharides have been synthetized previously, only a few have been conjugated with a carrier protein and investigated as vaccines. On the other hand, conjugate vaccines currently employed for biological studies are typically made of heterogeneous natural β-glucans or oligosaccharides derived from natural β-glucans.
Third, immunological studies of glycoconjugate vaccines 1–4 revealed that while all of them could elicit robust T cell-dependent immune responses, octa-, deca-, and dodeca-β-glucans were much more immunogenic than hexasa-β-glucan, which was different from the literature results.14 Close structure-activity relationship analysis further revealed that the immunogenicity decreased in the order of octa-, deca- and dodeca-β-glucans. These results suggest that at least an octamer is necessary for oligo-β-glucans as optimal antigens for elicitation of functional immune responses. However, this does not necessarily mean that the longer the better for an oligosaccharide antigen. As a result, an octa- or nona-β-glucan was identified as the most promising antigen for designing and developing β-glucan-based antifungal vaccines, which is of general significance.
Finally and most importantly, we have demonstrated in a mouse model that the conjugate of KLH and octa-β-glucan, namely 2, could elicit protective immune responses against the deadly pathogen C. albicans. This result is highly relevant to clinic application. Therefore, this work has paved the foundation for developing an effective and clinically useful antifungal vaccine.
Experimental Section
General Experimental Methods
Chemicals and materials were obtained from commercial sources and were used as received without further purification unless otherwise noted. MS 4 Å was flame-dried under high vacuum and used immediately after cooling under a N2 atmosphere. Analytical TLC was carried out on silica gel 60Å F254 plates with detection by a UV detector and/or by charring with 15% (v/v) H2SO4 in EtOH. NMR spectra were recorded on a 400, 500, or 600 MHz machine with chemical shifts reported in ppm (δ) downfield from tetramethylsilane (TMS) that was used as an internal reference.
p-Tolyl 4,6-O-Benzylidene-3-O-(naphthalene-2-ylmethyl)-1-thio-β-d-glucopyranoside (10)
A mixture of diol 9 (9.0 g, 24.03 mmol) and Bu2SnO (7.18 g, 28.84 mmol) in anhydrous toluene (400 mL) was refluxed in a flask equipped with a Dean-Stark device for 6 h. After the mixture was cooled to room temperature, the residual solvent was removed under vacuum. CsF (7.99 g, 52.87 mmol), 2-bromomethylnaphthalene (10.10 g, 45.66 mmol) and DMF (60 mL) were added, and the resulting solution was stirred at 70 °C for 12 h when TLC showed completion of reaction. After DMF was removed under vacuum, the residue was dissolved in CH2Cl2 and washed with 1 M NaF aqueous solution. The organic phase was dried over Na2SO4 and purified by flash column chromatography (toluene/EtOAc 10:1) to offer give 10 (8.9 g, 72%) as a white solid. 1H-NMR (400 MHz, CDCl3) δ: 7.85-7.70 (m, 4H), 7.51-7.40 (m, 10H), 7.15 (d, J = 7.83 Hz, 2H), 5.60 (s, 1H Ph-CH), 5.12 (d, J = 11.74 Hz, 1H), 4.99 (d, J = 11.74 Hz, 1H), 4.57 (d, J = 9.29 Hz, 1H, H-1), 4.40 (dd, J = 10.27, 4.89 Hz, 1H), 3.81 (t, J = 10.27 Hz, 1H), 3.75 (t, J = 9.29 Hz, 1H), 3.68 (t, J = 9.29 Hz, 1H), 3.56-3.48 (m, 2H), 2.61 (br s, 1H), 2.36 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 138.8, 137.2, 135.6, 133.8, 135.3, 133.0, 129.9, 129.1, 128.3, 128.3, 127.9, 127.7, 127.1, 126.9, 126.1, 126.0, 125.9, 101.3 (Ph-CH), 88.64 (C-1), 81.4, 81.1, 74.8, 72.2, 70.7, 68.6, 21.2. [α]D25 = −27.2° (c 0.5, CHCl3). HRMS (ESI-TOF) m/z: calcd. for C31H30NaO5S [M+Na] +, 537.1712; found, 537.1709.
p-Tolyl 2-O-Benzoyl-4,6-O-benzylidene-3-O-(naphthalene-2-ylmethyl)-1-thio-β-d-glucopyranoside (11)
A solution of 10 (8.6 g, 16.71 mmol), triethyl amine (5.8 mL, 41.77 mmol), benzoyl chloride (2.33 mL, 20.05 mmol) and a catalytic amount of N, N-dimethylaminopyridine in anhydrous CH2Cl2 (150 mL) was stirred at room temperature overnight. The reaction mixture was washed with saturated aqueous NaHCO3 solution (3 × 150 mL), and the organic layer was dried over Na2SO4. The desired product 11 was obtained (9.9 g, 96%) after purification by flash column chromatography (hexanes/EtOAc/CH2Cl2 6:1:1) as a white solid. 1H-NMR (400 MHz, CDCl3) δ: 7.99 (d, J = 7.34 Hz, 2H), 7.71-7.68 (d, J = 8.80 Hz, 1H), 7.62-7.54 (m, 5H), 7.47-7.34 (m, 10H), 7.23 (d, J = 8.31 Hz, 1H), 7.10 (d, J = 7.83 Hz, 2H), 5.65 (s, 1H Ph-CH), 5.32 (dd, J = 9.78, 8.80 Hz, 1H), 4.99 (d, J = 12.23 Hz, 1H), 4.86 (d, J = 12.23 Hz, 1H), 4.78 (d, J = 9.78 Hz, 1H, H-1), 4.45 (dd, J = 10.76, 5.38 Hz, 1H), 3.95 (t, J = 8.80 Hz, 1H), 3.87 (t, J = 10.27 Hz, 1H), 3.86 (t, J = 9.29 Hz, 1H), 3.60-3.54 (m, 1H), 2.34 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 165.0, 138.5, 137.2, 135.2, 133.6, 133.2, 133.0, 132.9, 129.9, 129.8, 129.7, 129.1, 128.3, 128.3, 128.2, 128.0, 127.8, 127.6, 127.0, 126.2, 126.1, 125.9, 125.7, 101.3 (Ph-CH), 87.2 (C-1), 81.5, 79.1, 74.3, 72.0, 70.6, 68.6, 21.2. [α]D25 = +19.5° (c 0.5, CHCl3). HRMS (ESI-TOF) m/z: calcd. for C38H34NaO6S [M+Na] +, 641.1974; found, 641.1979.
General procedure for deprotection of 2-naphthylmethyl ethers
To the stirred solution of a 2-naphthylmethyl ether compound (1 mmol) in CH2Cl2 (18 mL) and water (1 mL) was added DDQ (2 mmol) at room temperature. After the reaction was stirred for 8 h, saturated aqueous NaHCO3 solution was added, and the product was extracted with CH2Cl2. The combined organic layers were washed three times with saturated aqueous NaHCO3 solution and dried over Na2SO4. After removal of the solvent in vacuum, the product was purified by silica gel chromatography.
p-Tolyl 2-O-Benzoyl-4,6-O-benzylidene-1-thio-β-d-glucopyranoside (12)
Compound 12 (5.6 g, 92%) was prepared from 11 (7.88 g, 12.73 mmol) and DDQ (5.78 g, 25.47 mmol) according to the general procedure for deprotection of naphthylmethyl ethers and was purified by flash column chromatography (toluene/EtOAc 15:1 to 10:1). 1H-NMR (500 MHz, CDCl3) δ: 8.12 (d, J = 8.24 Hz, 2H), 7.64-7.61 (m, 1H), 7.51-7.48 (m, 4H), 7.40-7.36 (m, 5H), 7.13 (d, J = 7.93 Hz, 2H), 5.56 (s, 1H Ph-CH), 5.14 (dd, J = 9.77, 8.85 Hz, 1H), 4.84 (d, J = 10.07 Hz, 1H, H-1), 4.43 (dd, J = 10.68, 4.88 Hz, 1H), 4.06-4.02 (m, 1H), 3.82 (t, J = 10.38 Hz, 1H), 3.62-3.53 (m, 2H), 2.83 (br s, 1H), 2.36 (s, 3H). 13C-NMR (125 MHz, CDCl3) δ 165.9, 138.7, 136.8, 133.8, 133.5, 130.1, 129.8, 129.6, 129.4, 128.5, 128.4, 127.9, 126.3, 101.9 (Ph-CH), 86.8 (C-1), 80.6, 73.7, 73.3, 70.4, 68.5, 21.2. [α]D25 = −45.2° (c 0.5, CHCl3). HRMS (ESI-TOF) m/z: calcd. for C27H26NaO6S [M+Na] +, 501.1348; found, 501.1345.
General procedure for pre-activation-based glycosylation reactions
After the mixture of a glycosyl donor (1 mmol) and 4Å MS (1.5 g) in CH2Cl2 (20 mL) was stirred at room temperature for 1 h, it was cooled to −78 °C, and AgOTf (3 mol in 6 mL dry acetonitrile) was added, followed by addition of p-toluene sulfenyl chloride (p-TolSCl) (1 mmol) via a micro-syringe 10 min later. The mixture was stirred at −78 °C for an additional 15 min, when TLC showed that the donor was completely consumed. A solution of the acceptor (1 mmol) and 2,4,6-tri-tert-butyl pyrimidine (TTBP) (1 mmol) in CH2Cl2 (5 mL) was added. The resulting mixture was stirred for 20 min and warmed up to room temperature, followed by filtration to remove MS. The filtrate was washed with saturated aqueous NaHCO3 solution and brine, dried over Na2SO4, and concentrated under vacuum. The resultant crude product was purified by silica gel flash column chromatography to get the desired compound.
p-Tolyl [2-O-Benzoyl-4,6-O-benzylidene-3-O-(naphthalene-2-ylmethyl)-β-d-glucopyranosyl]-(1→3)-2-O-benzoyl-4,6-O-benzylidene-1-thio-β-d-glucopyranoside (13)
Compound 13 (6.15 g, 90%) was prepared from glycosyl donor 11 (4.37 g, 7.05 mmol) and acceptor 12 (3.375 g, 7.05 mmol) according to the general procedure for pre-activation-based glycosylation and was purified by flash column chromatography (toluene/ EtOAc 15:1). 1H-NMR (500 MHz, CDCl3) δ 7.85 (d, J = 7.32 Hz, 2H), 7.70 (d, J = 7.93 Hz, 1H), 7.58-7.48 (m, 8H), 7.46-7.33 (m, 12H), 7.30-7.16 (m, 6H), 7.08 (d, J = 7.93 Hz, 2H), 5.58 (s, 1H ph-CH), 5.33 (s, 1H ph-CH), 5.29 (t, J = 7.02 Hz, 1H), 5.28 (dd, J = 9.77, 9.16 Hz, 1H), 4.89 (d, J = 7.52 Hz, 1H H-1’), 4.87 (d, J = 12.51 Hz, 1H), 4.79 (d, J = 11.60 Hz, 1H), 4.78 (d, J = 10.07 Hz, 1H, H-1), 4.41 (dd, J = 10.38, 4.88 Hz, 1H), 4.21 (t, J = 9.16 Hz, 1H), 4.17 (dd, J = 10.68, 4.88 Hz, 1H), 3.95 (dd, J = 9.46, 9.16 Hz, 1H), 3.84 (t, J = 10.38 Hz, 1H), 3.81 (dd, J = 9.46, 9.16 Hz, 1H), 3.75 (dd, J = 8.55, 7.63 Hz, 1H), 3.70 (dd, J = 10.38, 10.07 Hz, 1H), 3.61-3.56 (m, 1H), 3.42-3.37 (m, 1H), 2.34 (s, 3H). 13C-NMR (125 MHz, CDCl3) δ 164.7, 164.6, 138.5, 137.3, 137.1, 135.3, 133.5, 133.0, 132.8, 132.7, 129.9, 129.7, 129.7, 129.5, 129.5, 129.4, 129.1, 129.0, 128.4, 128.4, 128.3, 128.2, 128.1, 127.9, 127.6, 126.7, 126.2, 126.1, 126.0, 125.8, 125.7, 101.6 (Ph-CH), 101.0 (Ph-CH), 100.5 (C-1’), 87.4 (C-1), 80.7, 79.3, 79.0, 78.2, 73.7, 73.4, 72.3, 70.7, 68.7, 68.6, 65.9, 21.2. [α]D25 = +40.0° (c 0.5, CHCl3). HRMS (ESI-TOF) m/z: calcd. For C58H52NaO12S [M+Na] +, 995.3077; found, 995.3040.
p-Tolyl [2-O-Benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-2-O-benzoyl-4,6-O-benzylidene-1-thio-β-d-glucopyranoside (14)
Compound 14 (1.63 g, 95%) was prepared from 13 (2.0 g, 2.05 mmol) and DDQ (0.93 g, 4.11 mmol) according to the general procedure for naphthylmethyl ether deprotection and purified by flash column chromatography (toluene/EtOAc 7:1).1H-NMR (500 MHz, CDCl3) δ 7.85 (d, J = 8.24 Hz, 2H), 7.70 (d, J = 8.24 Hz, 2H), 7.56-7.50 (m, 4H), 7.43-7.27 (m, 14H), 7.08 (d, J = 7.63 Hz, 2H), 5.57 (s, 1H ph-CH), 5.33 (s, 1H ph-CH), 5.26 (dd, J = 9.77, 8.85 Hz, 1H), 5.09 (dd, J = 7.63, 7.32 Hz, 1H), 4.93 (d, J = 7.02 Hz, 1H, H-1’), 4.79 (d, J = 10.07 Hz, 1H, H-1), 4.41 (dd, J = 10.38, 4.88 Hz, 1H), 4.25 (t, J = 8.85 Hz, 1H), 4.17 (dd, J = 10.38, 4.88 Hz, 1H), 3.86-3.77 (m, 3H), 3.70-3.66 (m, 2H), 3.61-3.56 (m, 1H), 3.39-3.34 (m, 1H), 2.63 (br s, 1H), 2.33 (s, 3H). 13C-NMR (125 MHz, CDCl3) δ 165.5, 164.7, 138.5, 137.1, 136.9, 133.5, 133.1, 133.0, 129.8, 129.8, 129.7, 129.5, 129.4, 129.3, 129.2, 128.3, 128.3, 128.2, 126.3, 126.2, 101.6 (ph-CH), 101.56 (ph-CH), 100.30 (C-1’), 87.36 (C-1), 80.4, 79.3, 79.1, 75.4, 72.6, 72.3, 70.7, 68.6, 68.6, 66.0, 21.2. HRMS (ESI-TOF) m/z: calcd. For C47H44NaO12S [M+Na] +, 855.2451; found, 855.2425.
p-Tolyl [2-O-Benzoyl-4,6-O-benzylidene-3-O-(naphthalene-2-ylmethyl)-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-2-O-benzoyl-4,6-O-benzylidene-3-O-1-thio-β-d-glucopyranoside (15)
Compound 15 (3.3 g, 86%) was prepared from glycosyl donor 13 (2.23 g, 2.29 mmol) and acceptor 14 (1.91 g, 2.29 mmol) according to the general procedure for pre-activation-based glycosylation and was purified by flash column chromatography (toluene/EtOAc 12:1). 1H-NMR (500 MHz, CDCl3) δ 7.89 (d, J = 7.63 Hz, 2H), 7.82 (d, J = 7.93 Hz, 2H), 7.78 (d, J = 7.93 Hz, 2H), 7.71 (d, J = 7.93 Hz, 1H), 7.65 (d, J = 7.63 Hz, 2H), 7.59-7.44 (m, 11H), 7.43-7.25 (m, 25H), 7.23-7.19 (m, 4H), 7.09 (d, J = 7.93 Hz, 2H), 5.55 (s, 1H ph-CH), 5.46 (s, 1H ph-CH), 5.35 (dd, J = 7.93, 7.63 Hz, 1H), 5.19 (dd, J = 5.80, 5.49 Hz, 1H), 5.01 (d, J = 6.41 Hz, 1H anomeric), 4.99 (d, J = 7.93 Hz, 1H anomeric), 4.97 (s, 1H), 4.92 (d, J = 12.21 Hz, 1H), 4.87-4.79 (m, 4H, 1H-anomeric), 4.69 (d, J = 10.07 Hz, 1H, H-1), 4.63 (s, 1H ph-CH), 4.37 (dd, J = 10.38, 4.58 Hz, 1H), 4.23 (dd, J = 10.38, 4.58 Hz, 1H), 4.16-4.07 (m, 4H), 3.97 (dd, J = 8.24, 7.93 Hz, 1H), 3.93-3.90 (m, 2H), 3.85 (dd, J = 8.85, 8.24 Hz, 1H), 3.77-3.71 (m, 2H), 3.62-3.57 (m, 3H), 3.49-3.41 (m, 4H), 3.27 (dd, J = 9.46, 9.16 Hz, 1H), 2.34 (s, 3H). 13C-NMR (125 MHz, CDCl3) δ 165.0, 164.8, 164.6, 164.6, 138.3, 137.4, 137.3, 137.3, 137.9, 135.4, 133.5, 133.3, 133.2, 133.1, 133.1, 132.9, 132.8, 129.8, 129.8, 129.8, 129.7, 129.7, 129.6, 129.5, 129.4, 129.4, 129.1, 129.0, 129.0, 128.9, 128.6, 128.5, 128.4, 128.4, 128.3, 128.1, 128.0, 127.9, 127.9, 127.6, 126.7, 126.4, 126.3, 126.1, 126.0, 125.8, 125.6, 102.0, 101.1, 100.9, 100.9, 99.3 (anomeric), 98.0 (anomeric), 97.0 (anomeric), 87.9 (C-1), 81.3, 78.6, 78.5, 78.2, 77.1, 75.7, 74.3, 73.7, 73.5, 73.2, 73.0, 72.7, 70.7, 68.7, 68.7, 68.5, 66.0, 65.7, 65.4, 21.2. [α]D25 = +43.0° (c 0.5, CHCl3). HRMS (ESI-TOF) m/z: calcd. For C98H89O24S [M+H] +, 1681.5465; found, 1681.5461.
2-Azidoethyl [2-O-Benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranoside (16)
The reaction between 13 (3.5 g, 3.60 mmol) and 2-azidoethanol (0.5 g, 5.65 mmol) was carried out according to the general procedure for pre-activation-based glycosylation, and the crude product was directly subjected to deprotection by the general procedure to remove the naphthylmethyl group to afford 16 (2.5 g, 91%), which was purified by flash column chromatography (toluene/EtOAc 5:1). 1H-NMR (400 MHz, CDCl3) δ 7.84 (d, J = 7.83 Hz, 2H), 7.74 (d, J = 7.83 Hz, 2H), 7.55-7.49 (m, 4H), 7.43-7.24 (m, 12H), 5.56 (s, 1H ph-CH), 5.36 (s, 1H ph-CH), 5.27 (dd, J = 8.31, 7.34 Hz, 1H), 5.12 (dd, J = 8.31, 7.34 Hz, 1H), 4.95 (d, J = 6.85 Hz, 1H, H-1’), 4.67 (d, J = 7.34 Hz, 1H, H-1), 4.37 (dd, J = 10.27, 4.89 Hz, 1H), 4.24-4.19 (m, 2H), 3.88-3.79 (m, 4H), 3.70 (dd, J = 10.76, 10.27 Hz, 1H), 3.66 (t, J = 9.29 Hz, 1H), 3.61-3.49 (m, 2H), 3.43-3.37 (m, 1H), 3.28-3.18 (m, 2H), 2.60 (br d, J = 3.91 Hz, 1H). 13C-NMR (100 MHz, CDCl3) δ 165.6, 164.6, 137.1, 136.9, 133.1, 133.0, 129.8, 129.7, 129.3, 129.3, 129.2, 129.0, 128.3, 128.3, 128.2, 126.3, 126.1, 101.7 (ph-CH), 101.4 (ph-CH), 101.1 (C-1), 100.29 (C-1’), 80.5, 79.1, 77.8, 75.2, 73.2, 72.6, 68.7, 68.6, 67.7, 66.5, 66.0, 50.6. [α]D25 = −22.0° (c 1, CHCl3). HRMS (ESI-TOF) m/z: calcd. For C42H42N3O13 [M+H] +, 796.2718; found, 796.2697.
2-Azidoethyl 3-O-[2-O-Benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranoside (17)
Compound 17 (1.53 g, 90%) was prepared from glycosyl donor 13 (1.10 g, 1.13 mmol) and acceptor 16 (0.905 g, 1.13 mmol) according to the general procedure for pre-activation-based glycosylation and was purified by flash column chromatography (toluene/EtOAc 4:1). 1H-NMR (400 MHz, CDCl3) δ 7.92 (d, J = 7.83 Hz, 2H), 7.87 (d, J = 7.83 Hz, 2H), 7.79 (d, J = 7.83 Hz, 2H), 7.67 (d, J = 7.83 Hz, 2H), 7.57-7.44 (m, 7H), 7.42-7.30 (m, 17H), 7.28-7.18 (m, 8H), 5.54 (s, 1H), 5.42 (s, 1H), 5.18 (t, J = 7.34 Hz, 1H), 5.17 (t, J = 8.80 Hz, 1H), 5.05 (d, J = 7.83 Hz, 1H anomeric), 5.00 (d, J = 5.38 Hz, 1H anomeric), 4.94 (dd, J = 8.31, 7.83 Hz, 1H), 4.87 (s, 1H), 4.83 (t, J = 8.80 Hz, 1H), 4.82 (d, J = 5.38 Hz, 1H anomeric), 4.76 (s, 1H), 4.56 (d, J = 7.83 Hz, 1H anomeric), 4.34 (dd, J = 10.27, 4.40 Hz, 1H), 4.21 (dd, J = 10.76, 4.89 Hz, 1H), 4.15-4.06 (m, 4H), 4.00-3.92 (m, 3H), 3.89-3.84 (m, 1H), 3.76-3.63 (m, 3H), 3.60-3.42 (m, 8H), 3.37 (t, J = 9.29 Hz, 1H), 3.30-3.20 (m, 2H), 2.66 (br d, J = 3.42 Hz, 1H). 13C-NMR (100 MHz, CDCl3) δ 165.8, 164.7, 164.6, 164.6, 137.3, 137.2, 137.1, 137.0, 133.6, 133.4, 133.2, 133.1, 129.9, 129.8, 129.7, 129.7, 129.4, 129.3, 129.3, 129.1, 128.9, 128.6, 128.6, 128.4, 128.4, 128.3, 128.3, 128.2, 128.1, 126.4, 126.3, 126.3, 126.1, 125.3, 101.8, 101.8, 101.3, 101.1, 100.8 (anomeric), 98.8 (anomeric), 98.4 (anomeric), 97.0 (anomeric), 80.8, 78.7, 78.3, 77.5, 76.9, 75.0, 74.8, 74.4, 73.7, 73.4, 72.6, 72.5, 68.7, 68.6, 67.9, 66.5, 66.0, 65.5, 50.6. [α]D25 = +6.0° (c 0.5, CHCl3). HRMS (ESI-TOF) m/z: calcd. For C82H81N4O25 [M+Na] +, 1521.5190; found, 1521.5153.
2-Azidoethyl [2-O-Benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranoside (18)
Compound 18 (0.606 g, 87%) was prepared from glycosyl donor 13 (0.306 g, 0.315 mmol) and acceptor 17 (0.475 g, 0.315 mmol) by the same synthetic procedure for 17 and was purified by flash column chromatography (toluene/EtOAc 10:1 to 6:1). 1H NMR (600 MHz, CDCl3) δ 7.95 (d, J = 7.2 Hz, 2H), 7.86 (d, J = 7.3 Hz, 2H), 7.81 – 7.77 (m, 5H), 7.65 (d, J = 7.3 Hz, 2H), 7.59 (d, J = 7.4 Hz, 2H), 7.51 (m, 2H), 7.47 – 7.17 (m, 45H), 5.52 (s, 1H), 5.43 (s, 1H), 5.22 – 5.17 (m, 2H), 5.10 (d, J = 7.5 Hz, 1H), 5.03 – 4.96 (m, 2H), 4.94 – 4.85 (m, 6H), 4.79 (m, 4H), 4.55 (d, J = 7.6 Hz, 1H), 4.33 (dd, J = 10.4, 4.8 Hz, 1H), 4.22 (dd, J = 10.3, 4.9 Hz, 1H), 4.18 – 4.07 (m, 6H), 4.00 (m, 4H), 3.92 (dd, J = 6.8, 4.4 Hz, 1H), 3.88 – 3.84 (m, 1H), 3.69 (m, 3H), 3.60 (m, 1H), 3.56 – 3.38 (m, 12H), 3.33 – 3.27 (m, 2H), 3.23 (m, 2H). 13C NMR (150 MHz, CDCl3) δ 165.86, 164.84, 164.6, 164.5, 137.4, 137.27, 137.25, 137.1, 137.0, 133.8, 133.6, 133.56, 133.5, 133.2, 133.1, 129.9, 129.74, 129.7, 129.6, 129.5, 129.4, 129.34, 129.31, 129.23, 129.17, 129.2, 129.1, 129.0, 128.97, 128.8, 128.7, 128.6, 128.58, 128.55, 128.4, 128.3, 128.27, 128.2, 128.1, 127.99, 126.4, 126.36, 126.33, 126.3, 126.1, 125.3, 101.9, 101.8, 101.3, 101.24, 101.21, 101.19, 100.7, 98.43, 98.39, 97.2, 97.1, 96.9, 80.8, 79.1, 78.7, 78.1, 77.9, 77.88, 77.5, 76.6, 75.0, 74.8, 74.6, 74.3, 73.7, 73.4, 73.2, 72.9, 72.5, 72.4, 68.7, 68.6, 67.9, 66.5, 66.0, 65.6, 65.5, 65.48, 50.6. [α]D25 = +22.0° (c 0.5, CHCl3). MS (MALDI TOF) m/z: calcd. For C122H114N3O37 [M+H] +, 2212.7131; found, 2212.7119.
2-Azidoethyl [2-O-Benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranoside (19)
Compound 19 (0.74 g, 81%) was prepared from glycosyl donor 15 (0.53 g, 0.315 mmol) and acceptor 17 (0.47 g, 0.315 mmol) by the same synthetic procedure for 17 and purified by flash column chromatography (toluene/EtOAc 8:1 to 4:1). 1H-NMR (400 MHz, CDCl3) δ 7.97 (d, J = 6.85 Hz, 2H), 7.87-7.77 (m, 12H), 7.66 (d, J = 7.83 Hz, 2H), 7.58 (d, J = 7.34 Hz, 2H), 7.55-7.16 (m, 62H), 5.51 (s, 1H), 5.45 (s, 1H), 5.22 (t, J = 7.83 Hz, 1H), 5.21 (t, J = 8.31 Hz, 1H), 5.12 (d, J = 7.83 Hz, 1H), 5.02 (d, J = 4.89 Hz, 1H), 4.94-4.79 (m, 16H), 4.77 (d, J = 8.31 Hz, 1H), 4.55 (d, J = 7.34 Hz, 1H), 4.33 (dd, J = 10.76, 4.89 Hz, 1H), 4.23 (dd, J = 10.27, 4.89 Hz, 1H), 4.16-4.08 (m, 8H), 4.04-3.96 (m, 6H), 3.91-3.84 (m, 2H), 3.75-3.67 (m, 3H), 3.62-3.34 (m, 19H), 3.32-3.23 (m, 4H), 2.61 (br s, 1H). 13C-NMR (100 MHz, CDCl3) δ 165.9, 164.9, 164.8, 164.7, 164.7, 164.7, 164.6, 137.4, 137.4, 137.4, 137.3, 137.2, 137.1, 133.8, 133.8, 133.7, 133.7, 133.9, 133.7, 133.64, 133.63, 133.56, 133.55, 133.22, 133.20, 133.19, 123.0, 129.8, 129.7, 129.66, 129.56, 129.44, 129.41, 129.34, 129.27, 129.25, 129.2, 129.13, 129.09, 129.06, 129.0, 128.9, 128.7, 128.6, 128.58, 128.44, 128.4, 128.33, 128.30, 128.2, 128.1, 128.05, 126.5, 126.48, 126.42, 126.38, 126.2, 125.4, 102.0, 101.8, 101.4, 101.39, 101.32, 101.27, 101.2, 100.7, 98.5, 98.32, 97.2, 97.2, 97.14, 97.05, 97.0, 96.9, 80.9, 79.0, 78.7, 78.1, 78.0, 77.9, 77.9, 76.6, 74.9, 74.8, 74.6, 74.5, 74.4, 74.3, 74.2, 73.8, 73.5, 73.2, 73.12, 73.11, 73.05, 72.95, 72.94, 72.5, 68.8, 68.7, 68.66, 68.6, 68.0, 66.5, 66.1, 65.6, 65.58, 65.51, 50.7. [α]D25 = +27.0° (c 0.5, CHCl3). MS (MALDI TOF) m/z: calcd. For C162H149N3NaO49 [M+Na] +, 2944.89; found, 2944.71.
2-Azidoethyl [2-O-Benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranoside (20)
Compound 20 (0.156 g, 80%) was prepared from glycosyl donor 13 (108 mg, 0.11 mmol) and acceptor 19 (158 mg, 0.05 mmol) by the same synthetic procedure for 17 and was purified by flash column chromatography (toluene/EtOAc 10:1 to 4:1). 1H-NMR (500 MHz, CDCl3) δ 7.99 (d, J = 7.63 Hz, 2H), 7.89-7.79 (m, 15H), 7.68 (d, J = 7.93 Hz, 2H), 7.61 (d, J = 7.63 Hz, 2H), 7.57-7.17 (m, 79H), 5.53 (s, 1H), 5.46 (s, 1H), 5.24 (t, J = 7.93 Hz, 1H), 5.23 (t, J = 7.93 Hz, 1H), 5.15 (d, J = 7.63 Hz, 1H), 5.05 (d, J = 4.58 Hz, 1H), 5.00-4.77 (m, 23H), 4.56 (d, J = 7.63 Hz, 1H), 4.35 (dd, J = 9.77, 4.27 Hz, 1H), 4.25 (dd, J = 10.38, 4.58 Hz, 1H), 4.18-4.09 (m, 10H), 4.07-3.96 (m, 8H), 3.94-3.86 (m, 2H), 3.76-3.68 (m, 3H), 3.66-3.41 (m, 22H), 3.40-3.23 (m, 7H), 2.64 (br s, 1H). 13C-NMR (125 MHz, CDCl3) δ 165.9, 164.9, 164.8, 164.77, 164.75, 164.72, 164.70, 164.68, 164.5, 137.8, 137.4, 137.36, 137.34, 137.31, 137.2, 137.1, 133.8, 133.7, 133.6, 133.58, 133.52, 133.19, 133.16, 129.9, 129.8, 129.7, 129.6, 129.5, 129.4, 129.37, 129.33, 129.23, 129.19, 129.17, 129.1, 128.8, 128.7, 128.65, 128.58, 128.4, 128.3, 128.26, 128.18, 128.14, 128.1, 128.0, 126.5, 126.39, 126.36, 126.33, 126.1, 125.3, 105.0, 102.0, 101.8, 101.4, 101.33, 101.29, 101.26, 101.2, 101.1, 100.6, 98.4, 98.2, 97.2, 97.1, 97.02, 96.98(2C), 96.91, 96.86, 96.8, 80.9, 78.7, 78.1, 78.0, 77.94, 77.91, 77.88, 77.8, 77.5, 76.6, 74.8, 74.5, 74.4, 74.4, 74.2, 74.1, 73.8, 73.5, 73.1, 73.0, 72.95, 72.9, 72.6, 72.4, 68.7, 68.6, 68.0, 66.48, 66.06, 65.60, 65.55, 65.50, 65.47, 50.67. [α]D25 = +31.2° (c 0.5, CHCl3). MS (MALDI TOF) m/z: calcd. For C202H185N3NaO61 [M+Na] +, 3651.13; found, 3651.50.
2-Azidoethyl [2-O-Benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-[2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranosyl]-(1→3)-2-O-benzoyl-4,6-O-benzylidene-β-d-glucopyranoside (21)
Compound 21 (0.380 g, 85%) was prepared from glycosyl donor 15 (218 mg, 0.13 mmol) and acceptor 19 (300 mg, 0.10 mmol) by the same synthetic procedure for 17 and was purified by flash column chromatography (toluene/EtOAc 12:1 to 3:1). 1H-NMR (500 MHz, CDCl3) δ 7.97 (d, J = 7.02 Hz, 2H), 7.87-7.77 (m, 17H), 7.66 (d, J = 7.32 Hz, 2H), 7.59 (d, J = 7.32 Hz, 2H), 7.55-7.15 (m, 97H), 5.51 (s, 1H), 5.45 (s, 1H), 5.22 (t, J = 8.55 Hz, 1H), 5.21 (t, J = 7.32 Hz, 1H), 5.13 (d, J = 7.63 Hz, 1H), 5.02 (d, J = 5.19 Hz, 1H), 4.92-4.73 (m, 29H), 4.55 (d, J = 7.63 Hz, 1H), 4.33 (dd, J = 10.38, 4.88 Hz, 1H), 4.24 (dd, J = 10.38, 4.88 Hz, 1H), 4.16-4.07 (m, 11H), 4.04-3.95 (m, 10H), 3.91-3.84 (m, 2H), 3.75-3.67 (m, 3H), 3.64-3.34 (m, 30H), 3.33-3.20 (m, 7H). 13C-NMR (125 MHz, CDCl3) δ 165.9, 164.9, 164.8, 164.79, 164.78, 164.77, 164.76, 164.74, 164.72, 164.7, 164.6, 164.5, 137.9, 137.4, 137.33, 137.30, 137.2, 137.0, 133.8, 133.7, 133.6, 133.2, 129.9, 129.8, 129.7, 129.6, 129.5, 129.38, 129.35, 129.3, 129.23, 129.2, 129.1, 129.0, 128.8, 128.7, 128.6, 128.4, 128.3, 128.2, 128.18, 128.13, 128.08, 127.9, 126.5, 126.4, 126.35, 126.32, 126.1, 125.3, 102.0, 101.8, 101.4, 101.33, 101.30, 101.26, 101.24, 101.22, 101.20, 101.18, 101.1, 100.6, 98.4, 98.2, 97.1, 97.12, 97.09, 96.98(2C), 96.91, 96.88, 96.85, 96.84, 96.8, 80.9, 78.7, 78.1, 78.0, 77.96, 77.9, 77.88, 77.8, 77.5, 77.3, 76.59, 76.58, 74.8, 74.5, 74.4, 74.3, 74.2, 73.8, 73.5, 73.2, 73.1, 73.0, 72.9, 72.8, 72.6, 72.4, 68.8, 68.7, 68.67, 68.62, 68.0, 66.5, 66.1, 65.6, 65.6, 65.5, 50.7. [α]D25 = +36.0° (c 0.5, CHCl3). MS (MALDI TOF) m/z: calcd. For C242H221N3NaO73 [M+H] +, 4359.35; found, 4360.53.
General procedure for global deprotection of 18–21
To a solution of 18, 19, 20 or 21 (23 µmol) in CH2Cl2 (12 mL) was added acetic acid (8 drops) and zinc powder (80 mg). The mixture was vigorously stirred at room temperature for 24 h and then filtered through a pad of Celite plug. The filtrate was condensed in vacuum, and the resulting residue was dissolved in AcOH and H2O (5:1, 60 mL) and heated at 60 °C for 24 h. The solvents were removed in vacuum and the residue was co-evaporated with toluene 5 times. After the product was dissolved in tBuOH and H2O (4:1, 60 mL), NaOH (120 mg) in H2O (6.0 mL) was added in portions. The mixture was stirred at 40 °C for 24 h, neutralized with 0.25 N HCl at 0 °C, and lyophilized. The crude product was purified on a sephadex G-25 gel filtration column using water as the eluent, and the product fractions were lyophilized to afford the desired free oligosaccharides.
2-Aminoethyl β-d-Glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-β-d-glucopyranoside (22)
Compound 22 (18.7 mg) was prepared from 18 (50.0 mg, 23 µmol) by the above general procedure in an 80% yield. 1H-NMR (600 MHz, D2O) δ 4.59 (m, 5H), 4.39 (d, J = 8.1 Hz, 1H), 3.97 (dd, J = 10.9, 5.1 Hz, 1H, ½OCH2CH2), 3.85 – 3.74 (m, 6H), 3.68 – 3.55 (m, 10H), 3.47 – 3.30 (m, 13H), 3.29 – 3.16 (m, 4H), 3.15 – 3.09 (m, 2H, CH2N3). 13C NMR (150 MHz, D2O) δ 102.7, 102.5, 101.8, 84.2, 84.0, 76.0, 75.6, 75.5, 73.4, 73.2, 72.7, 69.5, 68.0, 65.8, 60.6, 60.5, 60.03, 60.02, 39.4. HRMS (ESI-TOF,) m/z: calcd. for C50H88NO41 [M+H] +, 1304.3775; found, 1034.3727.
2-Aminoethyl β-d-Glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d- glucopyranosyl-(1→3)-β-d-glucopyranoside (23)
Compound 23 (10.2 mg) was prepared from 19 (25 mg, 8.5 µmol) by the above general procedure in an 88% yield. 1H-NMR (600 MHz, D2O) δ 4.57 (m, 7H), 4.35 (d, J = 8.0 Hz, 1H), 3.93 (m, 1H), 3.78 – 3.71 (m, 8H), 3.62 – 3.50 (m, 16H), 3.40 – 3.26 (m, 22H), 3.19 (m, 3H), 3.08 (t, J = 4.9 Hz, 2H). 13C NMR (150 MHz, D2O) δ 102.7, 102.4, 101.8, 84.1, 84.1, 84.0, 75.9, 75.5, 75.5, 73.4, 73.2, 73.1, 72.7, 69.5, 68.0, 67.9, 65.8, 60.6, 60.4, 39.3. HRMS (ESI-TOF) m/z: calcd. for C50H88NO41 [M+H] +, 1358.4832; found, 1358.4775.
2-Aminoethyl β-d-Glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl]-(1→3)-β-d-glucopyranoside (24)
Compound 24 (11.0 mg) was prepared from 20 (27.8 mg, 7.6 µmol) by the above general procedure in an 85% yield. 1H-NMR (600 MHz, D2O) δ 4.66-4.59 (m, 9H), 4.39 (d, J = 8.24 Hz, 1H), 4.00-3.96 (m, 1H), 3.82-3.74 (m, 12H), 3.65-3.55 (m, 19H), 3.42-3.31 (m, 26H), 3.27-3.19 (m, 4H), 3.13-3.11 (m, 2H). 13C NMR (150 MHz, D2O) δ 102.7, 102.5, 101.8, 84.1, 84.0, 75.9, 75.6, 73.4, 73.2, 72.7, 71.6, 69.5, 69.4, 68.0, 65.78, 60.6, 60.3, 60.0, 39.4. HRMS (ESI-TOF) m/z: calcd. for C62H108NO51 [M+H] +, 1682.5888; found, 1682.5787.
2-Aminoethyl β-d-Glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl]-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl-(1→3)-β-d-glucopyranoside (25)
Compound 25 (10.4 mg) was prepared from 21 (25.6 mg, 5.9 µmol) by the above general procedure in an 88% yield. 1H-NMR (600 MHz, D2O) δ 4.65-4.59 (m, 11H), 4.39 (d, J = 7.83 Hz, 1H), 3.98-3.93 (m, 1H), 3.80-3.74 (m, 13H), 3.65-3.54 (m, 24H), 3.42-3.31 (m, 32H), 3.27-3.18 (m, 4H), 3.08-3.06 (m, 2H). 13C NMR (151 MHz, D2O) δ 102.7, 102.4, 101.8, 84.1, 84.1, 83.9, 75.9, 75.5, 73.4, 73.2, 72.7, 71.6, 69.6, 69.5, 69.3, 68.0, 65.8, 64.8, 62.4, 60.6, 60.4, 60.3, 60.0, 39.3. HRMS (ESI-TOF) m/z: calcd. for C74H128NNaO61 [M+H+Na] 2+, 1014.8421; found, 1014.8417.
General procedure for activation of amino oligosaccharides 22–25
Each oligosaccharide was dissolved in DMF and 0.1M PBS buffer (4:1, 0.5 mL), and then disuccinimidal glutarate (15 eq) was added to the solution. The reaction was kept under gentle stirring at room temperature for 4 h, followed by removal of the solvents under vacuum. The excessive reagent was removed from the reaction by precipitation with 9 volumes of EtOAc, and the precipitates were washed 10 times with EtOAc followed by drying under vacuum to give activated oligosaccharides 26–29.
General procedure for conjugating activated oligosaccharides 26–29 with KLH and HSA
After solutions of 26–29 and KLH or HSA in a molar ratio of 30:1 in 0.1 M PBS buffer (0.35 mL) were gently stirred at room temperature for 3 days, they were applied to a Biogel A0.5 column to separate the resultant glycoconjgates 1–8 from unreacted oligosaccharides sing 0.1 M PBS buffer (I = 0.1, pH = 7.8) as the eluent. Fractions containing glycoconjugates, which were confirmed by the bicinchoninic acid (BCA) assay for protein and the phenol-sulfuric acid assay for carbohydrate, were combined and dialyzed against distilled water for 2 days. The solutions were lyophilized to get 1–8 as white solids.
Analysis of the carbohydrate loadings of glycoconjugates 1–8:32
Aliquots of a standard d-glucose solution (1 mg/mL) in water were added in ten dry 10-mL test tubes in 5 µL increment to form standard samples that contained 0 to 50 µg of glucose. In another test tube, an accurately weighed sample of the to-be-analyzed glycoconjugate 1–8 was placed. The content of glucose in each tested sample was estimated to be also in the range of 0 to 50 µg. To all of the tubes were then added 500 µL of 4% phenol and 2.5 mL of 96% sulfuric acid at room temperture. About 20 min later, the resultant colored solutions were transferred into cuvettes, and their absorptions at 490 nm wavelength (A490) were measured. A sugar calibration curve was created by plotting the A490 values of standard samples against their glucose contents (in µg), which was employed to calculate the glucose content of each tested glycoconjugate sample based on its A490 value. The carbohydrate loading of each glycoconjugate was calculated according to the following equation, and the results are shown in Table 1.
Carbohydrate loading% = sugar weight in a tested sample/total weight of the sample × 100%
Immunization of mouse
After each glycoconjugate (2.17 mg of 1, 2.32 mg of 3, 2.40 mg of 5 or 1.98 mg of 7) was dissolved in 0.3 mL of 10 × PBS buffer, it was diluted with water to form a 2 × PBS solution (1.5 mL). The solution was well mixed with 1.5 mL of Titermax Gold adjuvant (1:1, v/ v) to form an emulsion according to the protocols given by the manufacturer. Each group of six female C57BL/6J mice were initially immunized (day 1) by i.m. injection of 0.1 mL of the emulsion described above. Following the initial immunization, mice were boosted 4 times on days 14, 21, 28, and 38 by s.c. injection of the same conjugate emulsion. Therefore, each injected dose of glycoconjugate contained about 6 µg of the carbohydrate antigen. Mouse blood samples were collected through the leg veins of each mouse on day 0 prior to the initial immunization and on days 27, 38 and 48 after the boost immunizations. Finally, antisera were obtained from the clotted blood samples and stored at −80 °C before use.
The ELISA protocol
Each well of ELISA plates was treated with 100 µl of a solution of an individual HSA conjugate 5, 6, 7 or 8 (2 µg/ml) dissolved in coating buffer (0.1 M bicarbonate, pH 9.6) at 4 °C overnight and then at 37 °C for 1 h, which was followed by washing (3 times) with PBS buffer containing 0.05% Tween-20 (PBST) and treatment with blocking buffer (10% BSA in PBS buffer containing NaN3) at room temperature for 1 h. After 3 times of washing with PBST, half-log serially diluted solutions (from 1:300 to 1:656100) of a pooled or an individual mouse antiserum in PBS were added to the coated ELISA plates (100 µL/well), followed by incubation at 37 °C for 2 h. The plates were then washed with PBST and incubated at room temperature for another 1 h with a 1:1000 diluted solution of alkaline phosphatase (AP)-linked goat anti-mouse kappa, IgG1, IgG2a or IgM antibody (100 µL/well), respectively. Finally, the plates were washed with PBST and developed with 100 µL of p-nitrophenylphosphate (PNPP) solution (1.67 mg/mL in buffer) for 30 min at room temperature, which was followed by colorimetric readout at 405 nm wavelength using a microplate reader. The optical density (OD) values were plotted against the logarithmic scale of antiserum dilution values, and a best-fit line was obtained. The equation of the line was employed to calculate the dilution value at which an OD of 0.2 was achieved, and the antibody titer was obtained as the inverse of the dilution value.
In vivo evaluation of the new vaccine 2 to elicit protections against fungal infection
A group of 11 female C57BL/6J mice were immunized with an emulsion of conjugate 2 (containing 6 µg of octasaccharide antigen per dose) and Titermax Gold adjuvant prepared according to the protocol described above or with PBS (control group) on days 1, 14, 21, and 28. Thereafter, C. albicans (strain SC5314) cells (7.5 × 105 per mouse) in 200 µL of PBS were injected in the mice by i.v. administration on day 38. C. albican cells used in this experiment were cultured in YEPD medium at 28 °C for 24 h, and before injection, they were centrifuged and washed 3 times with PBS. The mice were checked on a daily basis, and the observation continued for 32 days after the injection of C. albican cells. Note: Animal protocols for the immunization and fungal challenge experiments were approved by the Institutional Animal Use and Care Committees of Wayne State University and Second Military Medical University.
Supplementary Material
Acknowledgement
This work was supported in parts by an NIH/NCI grant (R01 CA95142) and by the National Major Scientific and Technological Special Project for “Significant New Drugs Development” of China (Grant No. 2012ZX09502001-005) and the National High Technology Research and Development Program of China (Grant No. 2012AA021500). The authors thank Dr. B Ksebati for some of the 2D NMR measurements. The 600 MHz NMR instrument used in this study was financed by an NSF grant (CHE-0840413).
References
- 1.Limper AH, Knox KS, Sarosi GA, Ampel NM, Bennett JE, Catanzaro A, Davies SF, Dismukes WE, Hage CA, Marr KA, Mody CH, Perfect JR, Stevens DA American Thoracic Society Fungal Working, G. An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patients. A. J. Res. Crit. Care Med. 2011;183:96–128. doi: 10.1164/rccm.2008-740ST. [DOI] [PubMed] [Google Scholar]
- 2.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 3.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson MA, Bundle DR. Designing a new antifungal glycoconjugate vaccine. Chem. Soc. Rev. 2013;42:4327–4344. doi: 10.1039/c2cs35382b. [DOI] [PubMed] [Google Scholar]
- 5.Cassone A. Development of vaccines for Candida albicans: fighting a skilled transformer. Nat. Rev. Microbiol. 2013;11:884–891. doi: 10.1038/nrmicro3156. [DOI] [PubMed] [Google Scholar]
- 6.Cutler JE, Deepe GS, Jr, Klein BS. Advances in combating fungal diseases: vaccines on the threshold. Nat. Rev. Microbiol. 2007;5:13–28. doi: 10.1038/nrmicro1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowman SM, Free SJ. The structure and synthesis of the fungal cell wall. Bioessays. 2006;28:799–808. doi: 10.1002/bies.20441. [DOI] [PubMed] [Google Scholar]
- 8.Bromuro C, Torosantucci A, Chiani P, Conti S, Polonelli L, Cassone A. Interplay between protective and inhibitory antibodies dictates the outcome of experimentally disseminated Candidiasis in recipients of a Candida albicans vaccine. Infect. Immun. 2002;70:5462–5470. doi: 10.1128/IAI.70.10.5462-5470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, Norelli F, Bellucci C, Polonelli L, Costantino P, Rappuoli R, Cassone A. A novel glyco-conjugate vaccine against fungal pathogens. J. Exp. Med. 2005;202:597–606. doi: 10.1084/jem.20050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torosantucci A, Chiani P, Bromuro C, De Bernardis F, Palma AS, Liu Y, Mignogna G, Maras B, Colone M, Stringaro A, Zamboni S, Feizi T, Cassone A. Protection by anti-beta-glucan antibodies is associated with restricted beta-1,3 glucan binding specificity and inhibition of fungal growth and adherence. PLoS One. 2009;4:e5392. doi: 10.1371/journal.pone.0005392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bromuro C, Romano M, Chiani P, Berti F, Tontini M, Proietti D, Mori E, Torosantucci A, Costantino P, Rappuoli R, Cassone A. Beta-glucan-CRM197 conjugates as candidates antifungal vaccines. Vaccine. 2010;28:2615–2623. doi: 10.1016/j.vaccine.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Hu Q-Y, Allan M, Adamo R, Quinn D, Zhai H, Wu G, Clark K, Zhou J, Ortiz S, Wang B, Danieli E, Crotti S, Tontini M, Brogioni G, Berti F. Synthesis of a well-defined glycoconjugate vaccine by a tyrosine-selective conjugation strategy. Chem. Sci. 2013;4:3827. [Google Scholar]
- 13.Adamo R, Hu Q-Y, Torosantucci A, Crotti S, Brogioni G, Allan M, Chiani P, Bromuro C, Quinn D, Tontini M, Berti F. Deciphering the structure–immunogenicity relationship of anti-Candida glycoconjugate vaccines. Chem. Sci. 2014;5:4302–4311. [Google Scholar]
- 14.Adamoa R, Tontinia M, Brogionia G, Romanoa MR, Costantinia G, Danielia E, Proiettia D, Bertia F, Costantinoa P. Synthesis of Laminarin Fragments and Evaluation of a β-(1,3) Glucan Hexasaccaride-CRM197 Conjugate as Vaccine Candidate against Candida albicans. J. Carbohydr. Chem. 2011;30:249–280. [Google Scholar]
- 15.Verez-Bencomo V, Fernandez-Santana V, Hardy E, Toledo ME, Rodriguez MC, Heynngnezz L, Rodriguez A, Baly A, Herrera L, Izquierdo M, Villar A, Valdes Y, Cosme K, Deler ML, Montane M, Garcia E, Ramos A, Aguilar A, Medina E, Torano G, Sosa I, Hernandez I, Martinez R, Muzachio A, Carmenates A, Costa L, Cardoso F, Campa C, Diaz M, Roy R. A synthetic conjugate polysaccharide vaccine against Haemophilus influenzae type b. Science. 2004;305:522–525. doi: 10.1126/science.1095209. [DOI] [PubMed] [Google Scholar]
- 16.Yu H, Williams DL, Ensley HE. 4-Acetoxy-2,2-dimethylbutanoate: a useful carbohydrate protecting group for the selective formation of beta-(1->3)-D-glucans. Tetrahedron Lett. 2005;46:3417–3421. doi: 10.1016/j.tetlet.2005.03.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamois F, Ferrieres V, Guegan JP, Yvin JC, Plusquellec D, Vetvicka V. Glucan-like synthetic oligosaccharides: iterative synthesis of linear oligo-beta-(1,3)-glucans and immunostimulatory effects. Glycobiology. 2005;15:393–407. doi: 10.1093/glycob/cwi020. [DOI] [PubMed] [Google Scholar]
- 18.Zeng Y, Ning J, Kong F. Remote control of alpha- or beta-stereoselectivity in (1-->3)-glucosylations in the presence of a C-2 ester capable of neighboring-group participation. Carbohydr. Res. 2003;338:307–311. doi: 10.1016/s0008-6215(02)00455-x. [DOI] [PubMed] [Google Scholar]
- 19.Mo KF, Li H, Mague JT, Ensley HE. Synthesis of the beta-1,3-glucan, laminarahexaose: NMR and conformational studies. Carbohydr. Res. 2009;344:439–447. doi: 10.1016/j.carres.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Weishaupt MW, Matthies S, Seeberger PH. Automated solid-phase synthesis of a beta-(1,3)-glucan dodecasaccharide. Chemistry. 2013;19:12497–12503. doi: 10.1002/chem.201204518. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka H, Kawai T, Adachi Y, Ohno N, Takahashi T. beta(1,3) Branched heptadeca- and linear hexadeca-saccharides possessing an aminoalkyl group as a strong ligand to dectin-1. Chem. Commun. 2010;46:8249–8251. doi: 10.1039/c0cc03153d. [DOI] [PubMed] [Google Scholar]
- 22.Anish C, Schumann B, Pereira CL, Seeberger PH. Chemical biology approaches to designing defined carbohydrate vaccines. Chemistry & biology. 2014;21:38–50. doi: 10.1016/j.chembiol.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Yin Z, Huang X. Recent Development in Carbohydrate Based Anti-cancer Vaccines. J. Carbohydr. Chem. 2012;31:143–186. doi: 10.1080/07328303.2012.659364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liptak A, Jodal I, Harangi J, Nanasi P. Hydrogenolysis of the Benzylidene Acetals of Thioglycosides with the Lialh4-Alcl3 Reagent - Synthesis of Partially Benzylated Thioglycoside Derivatives. Acta Chim. Hung. 1983;113:415–422. [Google Scholar]
- 25.Ellervik U, Grundberg H, Magnusson G. Synthesis of lactam and acetamido analogues of sialyl Lewis × tetrasaccharide and Lewis × trisaccharide. J. Org. Chem. 1998;63:9323–9338. [Google Scholar]
- 26.de Jong AR, Hagen B, van der Ark V, Overkleeft HS, Codee JDC, van der Marel GA. Exploring and Exploiting the Reactivity of Glucuronic Acid Donors. J. Org. Chem. 2012;77:108–125. doi: 10.1021/jo201586r. [DOI] [PubMed] [Google Scholar]
- 27.Wang CC, Kulkarni SS, Lee JC, Luo SY, Hung SC. Regioselective one-pot protection of glucose. Nat. Protoc. 2008;3:97–113. doi: 10.1038/nprot.2007.493. [DOI] [PubMed] [Google Scholar]
- 28.Huang X, Huang L, Wang H, Ye XS. Iterative one-pot synthesis of oligosaccharides. Angew. Chem. Int. Ed. 2004;43:5221–5224. doi: 10.1002/anie.200460176. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Zhou L, El-Boubbou K, Ye XS, Huang X. Multi-component one-pot synthesis of the tumor-associated carbohydrate antigen Globo-H based on preactivation of thioglycosyl donors. J. Org. Chem. 2007;72:6409–6420. doi: 10.1021/jo070585g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q, Ekanayaka SA, Wu J, Zhang J, Guo Z. Synthetic and immunological studies of 5’-N-phenylacetyl sTn to develop carbohydrate-based cancer vaccines and to explore the impacts of linkage between carbohydrate antigens and carrier proteins. Bioconjugate Chem. 2008;19:2060–2068. doi: 10.1021/bc800243f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buskas T, Li Y, Boons GJ. The immunogenicity of the tumor-associated antigen Lewis (y) may be suppressed by a bi-functional cross-linker required for coupling to a carrier protein. Chem. Eur. J. 2004;10:3517–3524. doi: 10.1002/chem.200400074. [DOI] [PubMed] [Google Scholar]
- 32.Wrolstad RE, Acree TE, Decker EA. Current Protocols in Food Analytical Chemistry. John Wiley & Sons, Inc.; 2001. [Google Scholar]
- 33.Jennings HJ, Sood RK. Neoglycoconjugates, preparation and applications. Academic Press: San Diego, San Diego; 1994. Synthetic Glycoconjugates as Human Vaccines. [Google Scholar]
- 34.Markham RB, Pier GB, Schreiber JR. The Role of Cytophilic Igg3 Antibody in T-Cell-Mediated Resistance to Infection with the Extracellular Bacterium, Pseudomonas-Aeruginosa. J. Immunol. 1991;146:316–320. [PubMed] [Google Scholar]
- 35.Gavin AL, Barnes N, Dijstelbloem HM, Hogarth PM. Cutting edge: Identification of the mouse IgG3 receptor: Implications for antibody effector function at the interface between innate and adaptive immunity. J. Immunol. 1998;160:20–23. [PubMed] [Google Scholar]
- 36.Casadevall A. Antibody immunity and invasive fungal infections. Infect. Immun. 1995;63:4211–4218. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan RR, Casadevall A, Spira G, Scharff MD. Isotype Switching from Igg3 to Igg1 Converts a Nonprotective Murine Antibody to Cryptococcus-Neoformans into a Protective Antibody. J. Immunol. 1995;154:1810–1816. [PubMed] [Google Scholar]
- 38.Mawas F, Niggemann J, Jones C, Corbel MJ, Kamerling JP, Vliegenthart JF. Immunogenicity in a mouse model of a conjugate vaccine made with a synthetic single repeating unit of type 14 pneumococcal polysaccharide coupled to CRM197. Infect. Immun. 2002;70:5107–5114. doi: 10.1128/IAI.70.9.5107-5114.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pozsgay V, Chu C, Pannell L, Wolfe J, Robbins JB, Schneerson R. Protein conjugates of synthetic saccharides elicit higher levels of serum IgG lipopolysaccharide antibodies in mice than do those of the O-specific polysaccharide from Shigella dysenteriae type 1. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5194–5197. doi: 10.1073/pnas.96.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Sudbery P. Candida albicans, a major human fungal pathogen. J. Microbiol. 2011;49:171–177. doi: 10.1007/s12275-011-1064-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.