Abstract
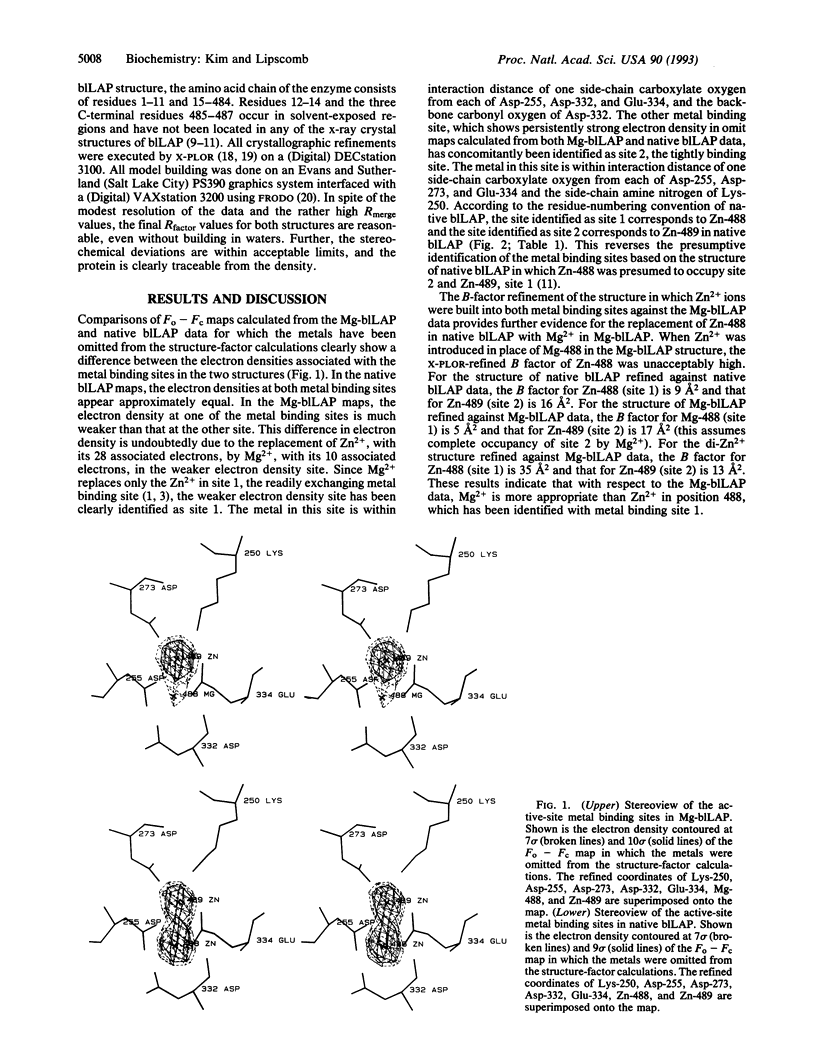
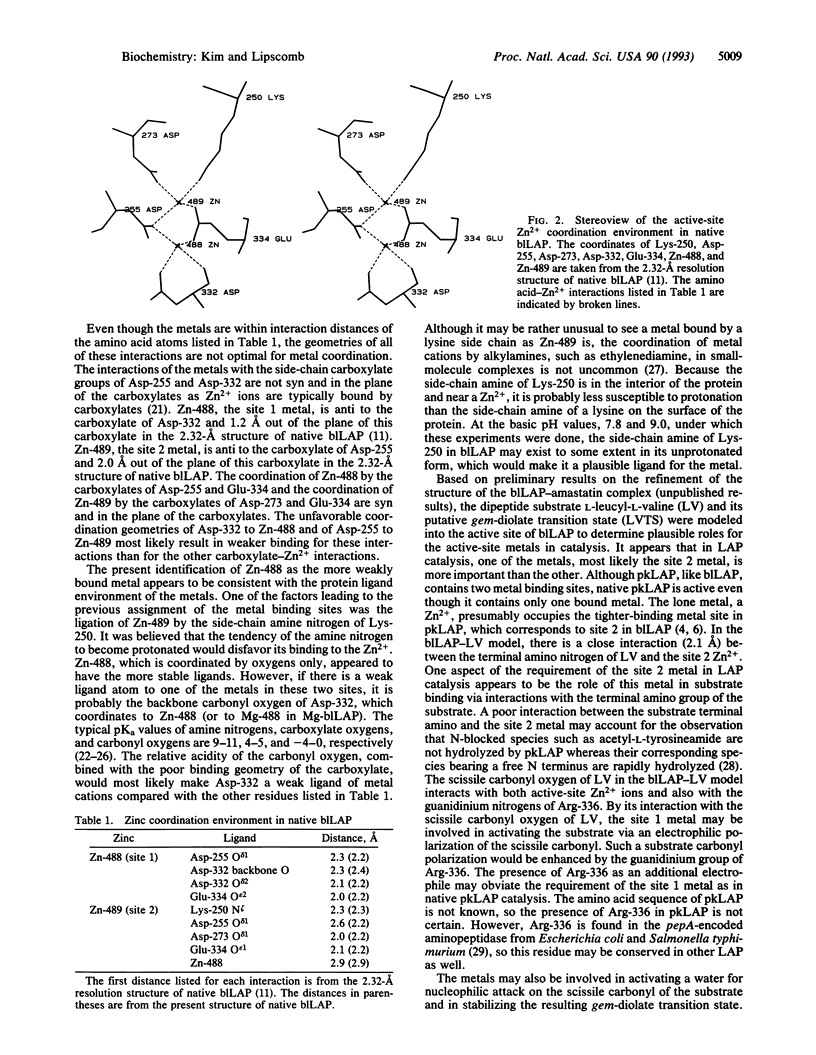
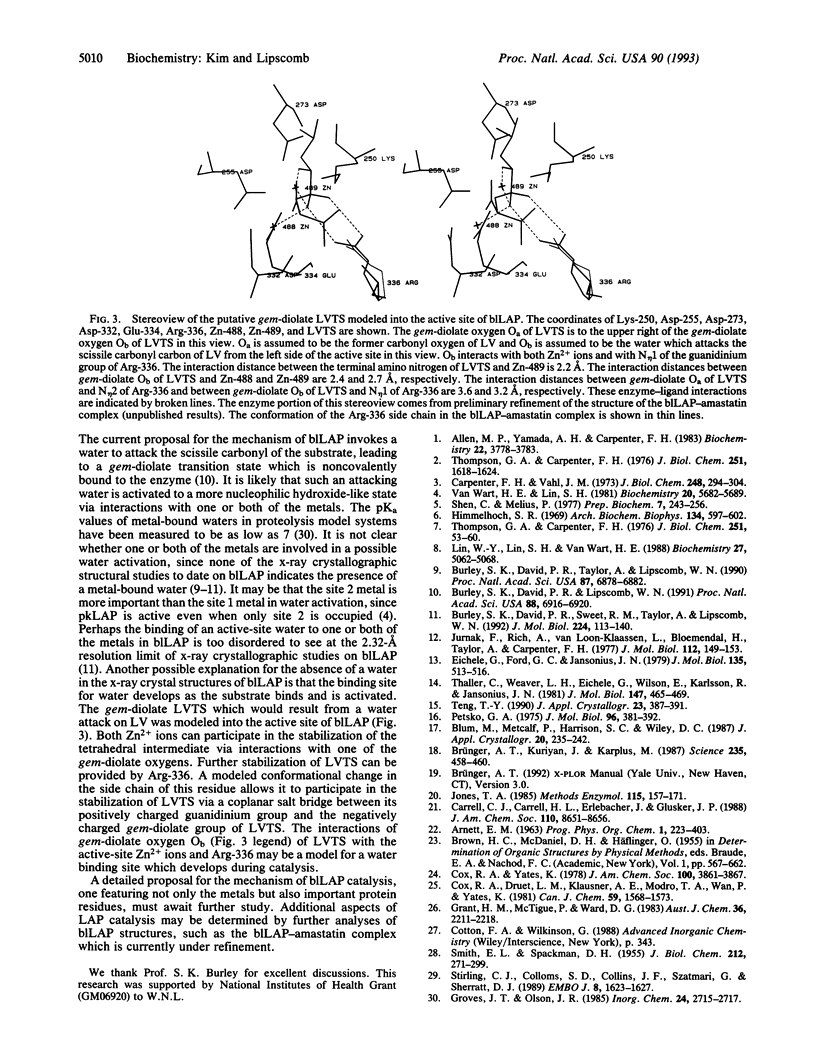
The tightly binding and readily exchanging metal binding sites in the active site of bovine lens leucine aminopeptidase (blLAP; EC 3.4.11.1) have been differentiated and identified by x-ray crystallography. In native blLAP,Zn2+ occupies both binding sites. In solution, site 1 readily exchanges Zn2+ for other divalent cations, including Mg2+. The Zn2+ in site 2 is unavailable for metal exchange under conditions which allow exchange at site 1. The Zn2+/Mg2+ metal hybrid of blLAP (Mg-blLAP) was prepared in solution and crystallized. X-ray diffraction data to 2.9-A resolution were collected at -150 degrees C from single crystals of Mg-blLAP and native blLAP. Comparisons of omit maps calculated from the Mg-blLAP data with analogous maps calculated from the native blLAP data show electron density in one of the metal binding sites in Mg-blLAP which is much weaker than the electron density in the other binding site. Since there are fewer electrons associated with Mg2+ than with Zn2+, the difference in electron density between the two metal binding sites is consistent with occupancy of the weaker electron density site by Mg2+ and identifies this metal binding site as site 1, defined as the readily exchanging site. The present identification of the metal binding sites reverses the previous presumptive assignment of the metal binding sites which was based on the structure of native blLAP [Burley, S. K., David, P. R., Sweet, R. M., Taylor, A. & Lipscomb, W. N. (1992) J. Mol. Biol. 224, 113-140]. According to the residue-numbering convention of native blLAP, the new assignment of the metal binding sites identifies the readily exchanging site 1 with Zn-488, which is within interaction distance of one side-chain carboxylate oxygen from each of Asp-255, Asp-332, and Glu-334 and the main-chain carbonyl oxygen of Asp-332. The more tightly binding site 2 is identified with Zn-489, which is within interaction distance of one side-chain carboxylate oxygen from each of Asp-255, Asp-273, and Glu-334 and the side-chain amine nitrogen of Lys-250.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. P., Yamada A. H., Carpenter F. H. Kinetic parameters of metal-substituted leucine aminopeptidase from bovine lens. Biochemistry. 1983 Aug 2;22(16):3778–3783. doi: 10.1021/bi00285a010. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Burley S. K., David P. R., Lipscomb W. N. Leucine aminopeptidase: bestatin inhibition and a model for enzyme-catalyzed peptide hydrolysis. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6916–6920. doi: 10.1073/pnas.88.16.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley S. K., David P. R., Sweet R. M., Taylor A., Lipscomb W. N. Structure determination and refinement of bovine lens leucine aminopeptidase and its complex with bestatin. J Mol Biol. 1992 Mar 5;224(1):113–140. doi: 10.1016/0022-2836(92)90580-d. [DOI] [PubMed] [Google Scholar]
- Burley S. K., David P. R., Sweet R. M., Taylor A., Lipscomb W. N. Structure determination and refinement of bovine lens leucine aminopeptidase and its complex with bestatin. J Mol Biol. 1992 Mar 5;224(1):113–140. doi: 10.1016/0022-2836(92)90580-d. [DOI] [PubMed] [Google Scholar]
- Burley S. K., David P. R., Taylor A., Lipscomb W. N. Molecular structure of leucine aminopeptidase at 2.7-A resolution. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6878–6882. doi: 10.1073/pnas.87.17.6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichele G., Ford G. C., Jansonius J. N. Crystallization of pig mitochondrial aspartate aminotransferase by seeding with crystals of the chicken mitochondrial isoenzyme. J Mol Biol. 1979 Dec 5;135(2):513–516. doi: 10.1016/0022-2836(79)90451-0. [DOI] [PubMed] [Google Scholar]
- Himmelhoch S. Leucine aminopeptidase: a zinc metloenzyme. Arch Biochem Biophys. 1969 Nov;134(2):597–602. doi: 10.1016/0003-9861(69)90322-1. [DOI] [PubMed] [Google Scholar]
- Jones T. A. Diffraction methods for biological macromolecules. Interactive computer graphics: FRODO. Methods Enzymol. 1985;115:157–171. doi: 10.1016/0076-6879(85)15014-7. [DOI] [PubMed] [Google Scholar]
- Jurnak F., Rich A., van Loon-Klaassen A., Bloemendal H., Taylor A., Carpenter F. H. Preliminary X-ray study of leucine aminopeptidase (bpvine lens), and oligomeric metalloenzyme. J Mol Biol. 1977 May 5;112(1):149–153. doi: 10.1016/s0022-2836(77)80162-9. [DOI] [PubMed] [Google Scholar]
- Lin W. Y., Lin S. H., Van Wart H. E. Steady-state kinetics of hydrolysis of dansyl-peptide substrates by leucine aminopeptidase. Biochemistry. 1988 Jul 12;27(14):5062–5068. doi: 10.1021/bi00414a017. [DOI] [PubMed] [Google Scholar]
- Petsko G. A. Protein crystallography at sub-zero temperatures: cryo-protective mother liquors for protein crystals. J Mol Biol. 1975 Aug 15;96(3):381–392. doi: 10.1016/0022-2836(75)90167-9. [DOI] [PubMed] [Google Scholar]
- SMITH E. L., SPACKMAN D. H. Leucine aminopeptidase. V. Activation, specificity, and mechanism of action. J Biol Chem. 1955 Jan;212(1):271–299. [PubMed] [Google Scholar]
- Stirling C. J., Colloms S. D., Collins J. F., Szatmari G., Sherratt D. J. xerB, an Escherichia coli gene required for plasmid ColE1 site-specific recombination, is identical to pepA, encoding aminopeptidase A, a protein with substantial similarity to bovine lens leucine aminopeptidase. EMBO J. 1989 May;8(5):1623–1627. doi: 10.1002/j.1460-2075.1989.tb03547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaller C., Weaver L. H., Eichele G., Wilson E., Karlsson R., Jansonius J. N. Repeated seeding technique for growing large single crystals of proteins. J Mol Biol. 1981 Apr 15;147(3):465–469. doi: 10.1016/0022-2836(81)90496-4. [DOI] [PubMed] [Google Scholar]
- Thompson G. A., Carpenter F. H. Leucine aminopeptidase (bovine lens). Effect of pH on the relative binding of Zn2+ and Mg2+ to and on activation of the enzyme. J Biol Chem. 1976 Jan 10;251(1):53–60. [PubMed] [Google Scholar]
- Thompson G. A., Carpenter F. H. Leucine aminopeptidase (bovine lens). The relative binding of cobalt and zinc to leucine aminopeptidase and the effect of cobalt substitution on specific activity. J Biol Chem. 1976 Mar 25;251(6):1618–1624. [PubMed] [Google Scholar]
- Van Wart H. E., Lin S. H. Metal binding stoichiometry and mechanism of metal ion modulation of the activity of porcine kidney leucine aminopeptidase. Biochemistry. 1981 Sep 29;20(20):5682–5689. doi: 10.1021/bi00523a007. [DOI] [PubMed] [Google Scholar]


