Abstract
Effective function of the locomotor system in the Drosophila larva requires a continuous adjustment of synaptic architecture and neurotransmission at the neuromuscular junction (NMJ). This feature has made the larval NMJ a favorite model to study the genetic and molecular mechanisms underlying synapse plasticity. This chapter will review experimental strategies used to study plasticity at the NMJ, the cellular parameters affected during plastic changes, and many of the known molecules involved in plastic changes. In addition, signal transduction pathways activated during plasticity will be discussed.
I. Introduction
Plasticity, in the broadest sense, is the ability to be molded. Neurobiologists have co-opted this term to mean the capacity of genetically identical organisms to vary in developmental pattern, phenotype, or behavior in response to varying local or global environmental conditions. The original definition, however, is quite evocative of the way the larval neuromuscular junction (NMJ) responds to change: the forces of electrical activity, secreted and environmental factors and a myriad of other internal and external conditions push and shove the structure and the function of this synapse to shape it and to maintain an output adequate to support larval locomotion. Because of its robust response to change, the larval NMJ has been a workhorse for investigators interested in the signal transduction pathways that subserve plasticity.
Why is this synapse so plastic? From the time of hatching to the time of pupation, roughly 4 days, the larval body wall muscle fiber surface area increases by a factor of more than 100-fold (Gorczyca et al., 1993; Keshishian et al., 1993; Schuster et al., 1996b; Fig. 1). To allow the animal to maintain control of its musculature, the structure and functional properties of the glutamatergic motor neurons have to be rapidly and continuously updated. The larval NMJ therefore has a large number of signal transduction pathways that sense internal and environmental changes and trigger homeostatic adjustment of synaptic strength and size. Interestingly, these transduction pathways are almost all important in the short- and long-term behavioral plasticity that occurs in the central nervous system of both invertebrates and vertebrates. While the NMJ is clearly not a synapse central to learning, the parsimonious nature of evolution has resulted in learning-relevant biochemical events being particularly easy to study there.
Figure 1.
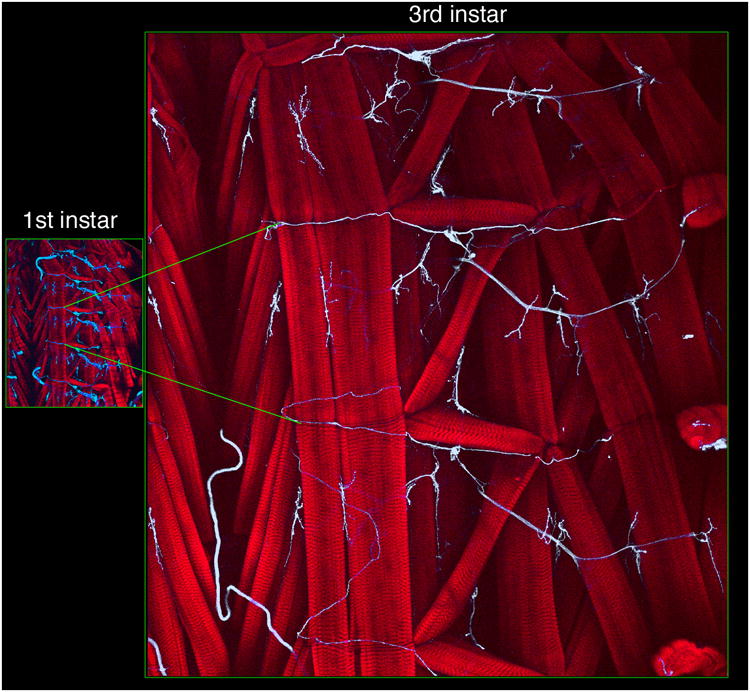
Changes in body wall muscle size during the larva period. Micrographs show comparative regions of the larval body wall in (left) first, and (right) third instar stages in preparations stained with texas red-conjugated phalloidin (red) to visualize muscles, and antibodies to the neuronal membrane marker HRP (blue) to visualize nerve trunks and NMJs. Green lines across the two panels mark two segmental boundaries.
This chapter will outline first some of the experimental paradigms for the study of plasticity at the NMJ and discuss what cellular parameters are believed to be altered by plasticity at the NMJ. The second part of this chapter will discuss a number of the known molecules that can effect plastic changes at the NMJ by activation of signal transduction pathways and how they initiate these processes. The last part of the chapter will look at the internal machinery of signal transduction and discuss what happens inside the cell when plasticity-inducing pathways are activated.
II. Manifestations of plasticity at the larval neuromuscular junction
1. Functional plasticity
The term functional plasticity, as used here, refers to changes in the magnitude of synaptic transmission that occur without gross structural change, usually on a short time scale. The Drosophila NMJ is a glutamatergic synapse. Release of glutamate into the synaptic cleft activates ionotropic glutamate receptors (GluRs) permeable to cations (Schuster et al., 1991; Qin et al., 2005). The resulting glutamate-induced depolarization of the muscle membrane also leads to the opening of a variety of voltage-dependent channels (Suzuki and Kano, 1977; Singh and Wu, 1989). The combined currents activated by stimulation of the motor axon give rise to the characteristic amplitude and kinetic profile of the postsynaptic response. The binding of glutamate to postsynaptic receptors can be detected by recording from the muscle in either current- or voltage-clamp, and the amplitude of the excitatory junctional potentials (EJPs) or excitatory junctional currents (EJCs) can be quantified. Typically at the larval NMJ, short-term plastic changes are detected as activity-dependent alterations in these evoked responses.
There are several types of potentiating short-term plasticity events that can measured at the NMJ (Jan and Jan, 1978). Depression has also been measured at this synapse, but it was believed largely to reflect depletion of glutamate-containing vesicles and has therefore often been used as a measure of vesicle recycling. Recent evidence suggests that perhaps this assumption should be reevaluated (Wu et al., 2005). A frequency-dependent long-term depression-type phenomenon can be seen at the NMJ, and interestingly, its molecular basis appears to be quite distinct from potentiating plasticity (Guo and Zhong, in press). In this chapter, however, we will focus exclusively on potentiating short-term plasticity.
Paradigms that are variations on facilitation or post-tetanic potentiation (PTP) are most commonly employed to look at short-term plasticity at the NMJ. Facilitation is the increase in EJP/EJC amplitude observed between two stimuli. Paired-pulse facilitation (PPF) occurs at low calcium concentrations on a msec timescale and is characterized by an increased EJP/EJC amplitude after the second stimulation in a closely spaced stimulus pair. The enhancement of the second EJP/EJC is believed to be a result of residual presynaptic calcium in response to the first stimulus (Beaumont et al., 2002). PTP occurs on a time scale of minutes and is also believed to be due to residual calcium (Beaumont et al., 2002), although the cAMP pathway has also been implicated in PTP (Zhong and Wu, 1991). The amplitude of EJP/EJCs evoked by low-frequency stimulation is increased for several minutes after a high-frequency (5-10 Hz) tetanus. The early increase in amplitude during the high frequency train has been called augmentation and has a time course in the range of seconds.
While all of these forms of plasticity are believed to be due to increased probability of release, and therefore presynaptic, the biochemical details of how this happens in the different fast (msec to min) time regimes cannot be identical. As will be detailed below, mutations in many signal transduction pathways have differential effects on these types of short-term plasticity. Because of their time course, the mechanisms by which these pathways affect activity are believed to involve post-translational modification of the presynaptic apparatus.
There are also postsynaptic contributions to functional plasticity. Activity can signal synthesis of glutamate receptors on a time scale of hours (Sigrist et al., 2000; Sigrist et al., 2002; Sigrist et al., 2003) and clustering of glutamate receptors on a longer time scale (Marrus and DiAntonio, 2004). Because of the intrinsic linkage of structure and function, and the fact that many signal transduction pathways affect both, the boundary between strictly functional and structural plasticity begins to breakdown in this temporal regime.
2. Structural plasticity
Structural plasticity is believed to require new protein synthesis and therefore occurs on a longer time scale than the short-term plasticity described above. The larval NMJ is in a continuous state of growth and remodeling. Muscles grow in size and the number of synaptic boutons increases over 10-fold between first and third instars (Gorczyca et al., 1993; Keshishian et al., 1993). Over the course of hours to days, the NMJ can alter its size and shape dramatically; the area of these terminal arbors scales to muscle size to optimize neuromuscular transmission (Lnenicka and Keshishian, 2000). Both intrinsic and activity-dependent signal transduction pathways drive these changes in the wild type animal. Activity-dependent changes in gene expression will be discussed in Chapter 14, so they will not be considered here in detail.
Both genetic perturbations of the signals that engage the above processes or of components of the intracellular machinery that is turned on by these signals can result in structural changes in NMJ morphology. These changes can occur at many levels. On the presynaptic side, the number and order of branches and the number and size of boutons are easily observed under the light microscope. More subtle changes in the number of active zones or the number of vesicle profiles require the use of electron microscopy. On the postsynaptic side, changes in the amount or localization of molecules such as receptors, scaffolding proteins and signal transduction molecules can be measured immunocytochemically, and structural changes in the size of the postsynaptic membrane, postsynaptic densities, or subsynaptic structures are observed at the ultrastructural level.
These morphological changes often have functional consequences that can be measured. Spontaneous release rates can be an indicator of an increase in the number of active zones. The number of functional glutamate receptors can be assessed by measurements of muscle responses to evoked activity and glutamate. Optical imaging techniques are also being used to address this class of changes. The releasable pool of vesicles can be assessed by styryl dye release and electrophysiological methods (Kidokoro et al., 2004) and postsynaptic responses can be followed by calcium imaging (Dawson-Scully et al., 2000; Guerrero et al., 2005). The number of new tools for analyzing the state of the NMJ is growing rapidly. Many of these tools are genetically encodable, allowing for more precise and detailed information on the functional consequences of plasticity than ever before (White et al., 2001; Mosca et al., 2005).
III. Plasticity-inducing signals at the larval neuromuscular junction
1. Synaptic activity
Synaptic activity is perhaps the major endogenous regulator of structure and function at the larval NMJ (Budnik et al., 1990). During normal growth, mismatches between the pre- and postsynaptic cells are detected by alterations in the effectiveness of synaptic transmission and both retrograde and anterograde signals help to coordinate the growth of pre- and postsynaptic compartments. Alterations in the balance of these signals by mutation or misexpression of pathway components results in aberrant functional and structural plasticity.
Alterations in synaptic activity induced by mutations in channel proteins have been used to probe the role of activity in the development of this synapse. Hyperexcitability can be produced by mutations in potassium channels or their regulatory subunits [Hyperkinetic (Hk), Shaker (Sh) and ether-a-go-go (eag)] or by duplication of the sodium channel gene paralytic (para). Such manipulations alter the morphology of the NMJ in a predictable manner: hyperexcitable mutants have increased branching and an increase in the number of boutons (Budnik et al., 1990). These mutants also exhibit profound ultrastructural changes with vesicle depletion and changes in the number of synaptic densities (Jia et al., 1993). Hypoexcitability can be produced by disrupting para splicing with no action potential (napts) mutants. In these animals, there is a slight reduction in the number and complexity of branches, and when this mutation is present on an eag, Sh background, it suppresses the increase in branching and bouton number (Budnik et al., 1990). These experiments argue strongly that he NMJ is integrating activity to determine it optimum structure. Because sodium channels are only present in the presynaptic neurons and not in muscles the above observations suggest that presynaptic activity signal the changes in NMJ arborization. This is supported by cell-specific expression of transgenes that alter activity (Mosca et al., 2005). For example, inducing hyperexcitability by expressing a Shaker dominant-negative transgene in neurons but not in muscles mimics the increase in arborization and bouton number observed in eag Sh mutants.
2. Glutamate
Glutamate is the major excitatory transmitter at the Drosophila NMJ. As such, its release is the signal of presynaptic neuronal activity, a potent inducer of both functional and structural plasticity at this synapse. Glutamate release is detected by glutamate receptors formed by at least 5 different subunits at the larval NMJ and these receptors are discussed in detail in Chapter 8. The best characterized are the ionotropic DGluRIIA and DGluRIIB subunits that are localized by activity-dependent processes to the postsynaptic densities in the muscle membrane (Broadie and Bate, 1993). Recently it has been shown that other family members, DGluRIII, DGluRIID and DGluRIIE are obligate members of the active DGluRIIA or DGluRIIB containing complexes at the NMJ (Marrus and DiAntonio, 2004; Qin et al., 2005). These receptors are all related to the vertebrate AMPA/Kainate or non-NMDA family of glutamate receptors. The synthesis of DGluRIIA is a critical variable in long-term plasticity (Sigrist et al., 2002). Drosophila also expresses an NMDA-type glutamate receptor presynaptically that enhances vesicle release to potentiate transmission secondary to high levels of locomotor activity (C. Schuster, personal communication). Activation of both of these classes of ionotropic receptors can directly admit calcium and activate downstream calcium-dependent signal transduction pathways. Postsynaptic activation of the ionotropic DGluR receptors also depolarizes the muscle membrane and leads to the opening of voltage-gated calcium channels.
Glutamate can also activate type II metabotropic mGluRs (DmGluRA) at the NMJ to modulate transmission. DmGluRA is expressed presynaptically, and null mutants have normal basal transmission and PPF, but enhanced facilitation to high frequency stimulation (Bogdanik et al., 2004). These mutants also have a mild reduction in bouton number and size. In first instar larvae, mGluR agonists increase mEJCs. This effect is mimicked by forskolin and CPT-cAMP, a cAMP analog, and blocked by an adenylate cyclase inhibitor (Zhang et al., 1999). In rutabaga (rut) flies, which lack Ca2+/calmodulin-dependent adenylate cyclase, the effects of DmGluR agonists are greatly attenuated. These results suggest that DmGluRA couples to adenylate cyclase presynaptically to effect short-term changes in transmission. Interestingly, this coupling to cyclase in embryos does not appear to be mediated by Gsa (Hou et al., 2003).
3. Octopamine
Glutamate is not the only small molecule transmitter released at the NMJ. Octopamine, a transmitter important in learning (Schwaerzel et al., 2003), and other behaviors (Roeder, 2005) is a modulator of transmission at the NMJ of many insects. At the NMJ, octopamine is found only in the type II boutons (Monastirioti et al., 1995). Unlike type I neurons which usually innervate a single muscle, type II synapses are widely distributed; three type II motor neurons in each segment provide synaptic input to all but 8 muscles of the body wall musculature (Monastirioti et al., 1995; Hoang and Chiba, 2001). These neurons therefore can provide broad modulation of the motor pattern (Fox et al., 2006).
The Drosophila octopamine receptors all appear to be G-protein coupled and it was shown very early on that octopamine was a good stimulator of adenylate cyclase in adult head extracts (Uzzan and Dudai, 1982). At the first instar larval NMJ, octopamine decreases the size of EJCs and has no effect on mEJCs (Nishikawa and Kidokoro, 1999), but in embryos it increases the frequency of mEJCs in a manner consistent with it acting via increasing cAMP (Hou et al., 2003). The exact identity of the octopamine receptors involved in these effects and their G-protein effectors have not been determined.
4. Peptides and secreted proteins
Peptide signaling molecules are important neuromodulators in both vertebrates and invertebrates. The estimated number of such peptides in Drosophila is large: close to three dozen based on number of peptide coding genes and G-protein-coupled receptors found in the genome (Nassel, 2002). Peptides can reach the larval NMJ by two routes: they can be released as co-transmitters from motor neuron endings e.g. CCAP (D. Park and L.C. Griffith, unpublished), Proctolin (Anderson et al., 1988), and Insulin-like peptides (Gorczyca et al., 1993), or they can be released into the larval hemolymph which bathes the musculature (Hewes et al., 1998). Only a few of the peptides that have been extensively characterized as to their effects on NMJ function in Drosophila are considered here.
a. Amnesiac/PACAP
One of the most widely studied neuropeptides in Drosophila is the product of the amnesiac (amn) locus. Mutations in this gene specifically block consolidation of memory (Quinn et al., 1979; Waddell et al., 2000). The amn gene product has high homology to the mammalian pituitary Adenylate Cyclase-activating peptide PACAP-38 (Feany and Quinn, 1995). Application of PACAP-38 to third instar larval NMJ elicits an immediate depolarization that is followed by a delayed enhancement of potassium currents (Zhong, 1995).
The signal transduction machinery activated by PACAP-38 at the NMJ are diverse and include both the Ras/Raf/MAPK and cAMP pathways (Zhong and Pena, 1995). The cAMP branch of this pathway involved the rut cyclase and the Drosophila homolog of the neurofibromatosis type 1 (NF1) gene (Guo et al., 1997). L-type calcium channels are also activated by PACAP-38 via a protein kinase A (PKA)-dependent mechanism (Bhattacharya et al., 2004).
b. Insulin
Insulin-like immunoreactivity has been found in a limited number of terminals at the third instar NMJ. Antibodies to vertebrate Insulin stain the type III boutons of muscle 12. Insulin receptor immunoreactivity and Insulin binding are much more widely distributed, occurring at almost all type I boutons of the NMJs (Gorczyca et al., 1993). The function of Insulin at the NMJ is unknown, but mutations in the Insulin receptor and other components of the Insulin pathway result in alterations in animal size and lifespan. Ablation of Insulin-producing neurons in the adult abolishes sex-specific differences in locomotor activity, but the source of this defect is still unknown (Belgacem and Martin, 2006). In addition, Insulin receptors have been shown to be involved in axon guidance in the fly visual system (Song et al., 2003).
c. FMRFamide-related peptides
The Drosophila FMRFamide gene encodes as many as 15 neuropeptides (Nambu et al., 1988) which are synthesized, and potentially secreted into the hemolymph from neurosecretory organs localized in the dorsal region of the CNS, but not from nerve terminals at the NMJ (Schneider et al., 1993). Assay of a large group of these peptides at the NMJ showed that they had essentially redundant functionality, enhancing twitch tension presumably by increasing EJC amplitude (Hewes et al., 1998). At muscle 6/7, application of one of the peptides, DPKQDFMRFamide, enhanced EJPs produced by stimulation of the MN6/7-Ib motor neuron, but not EJPs produced by stimulation of the MNSNb/d-Is neuron. Genetic and pharmacological inhibition of CaMKII blocked the potentiation of Ib neuron stimulation, suggesting that this peptide acts through CaMKII to increase EJP amplitude (Dunn and Mercier, 2005).
d. Glass bottom boat/BMPs
The product of the glass bottom boat (gbb) gene is a member of the BMP subfamily of the TGF-b superfamily of morphogens, and at the NMJ is secreted by muscle cells to promote presynaptic growth and to regulate neurotransmitter release (Marques, 2005). The signal transduction pathway activated by Gbb release from the muscles is thought to provide a retrograde signal to motorneurons, thus coupling muscle growth to changes in strength of the presynaptic input to maintain synaptic strength. The presence of such a retrograde signaling pathway has been suggested by a number of observations. First, there is a tight correlation between the size of muscle fibers and the number of synaptic boutons, suggesting that muscles provide information to motorneurons to adjust the size of their terminal arborization in a manner correlated with muscle growth (Gorczyca et al., 1993; Keshishian et al., 1993). Second, elimination of GluRIIA, which results in a decrease in mEJP/mEJC size, leads to an increase in the amount of neurotransmitter released. The final result of these homeostatic compensations are EJPs/EJCs with amplitudes indistinguishable from wild type (Petersen et al., 1997).
Work from several labs has implicated a canonical TGF-ß pathway as a mediator of these retrograde processes (Aberle et al., 1997; McCabe et al., 2003; Rawson et al., 2003). Briefly, Gbb binds to the BMP type I receptors encoded by Thick veins (Tkv) and Saxophone (Sax) and BMP type II receptor encoded by Wishful thinking (Wit). Activation of these receptors by Gbb binding results in phosphorylation of the receptor Smad Mothers against dpp (Mad), which complexes with the Co-Smad Medea (Med). The complex is imported into the nucleus where it is presumed to regulate transcription of genes involved in synaptic growth. Loss of any of the above components interferes with normal synapse homeostasis and result in NMJ arbors that are smaller than normal. In addition, these presynaptic arbors have reduced neurotransmitter release, leading to a reduction in EJP/EJC amplitude. Thus, in the absence of the Gbb-mediated retrograde pathway, muscle growth appears to become functionally and structurally uncoupled from the presynaptic motorneuron, preventing the maintenance of synaptic efficacy.
The above model is strongly supported by the genetic data, including the analysis of the distribution of phospho-Smad to the nucleus of motorneurons, the absence of this nuclear translocation in mutants affecting the Gbb ligand and its receptors, and rescue experiments. However, an intriguing report shows that phospho-Mad is also present in colocalization with GluRs at postsynaptic muscles as well as in muscle nuclei (Dudu et al., 2006). Although the significance of the postsynaptic localization of activated Mad to postsynaptic densities and muscle nucleus is at present unknown, these observations suggest that the TGFß pathway may not function exclusively as a retrograde pathway. A more thorough understanding of the complexities of this pathway in anterograde and retrograde signaling will be better assessed once the distribution of the receptors is better established.
e. Wingless
Wingless (Wg), a member of the Wnt family of secreted glycoproteins, is also a morphogen that plays important roles during the development of the larval NMJ (Packard et al., 2003b; Fig. 2). The evidence suggests that Wg functions in an anterograde, and perhaps an autocrine fashion. At the NMJ, Wg is observed both at the pre- and at the postsynaptic aspect of type I boutons (Packard et al., 2002). However, several lines of evidence suggest that Wg is synthesized and secreted by motorneurons and deposited at the postsynaptic membrane. A Wg receptor, DFrizzled-2 (DFz2) is localized at the postsynaptic muscle membrane. Overexpressing Wg in the pre- but not in the postsynaptic cell results in increased Wg accumulation in the postsynaptic membrane. In addition, structural defects induced by reducing Wg secretion can be rescued by expressing a wild type Wg transgene in the presynaptic cell (Packard et al., 2002).
Figure 2.
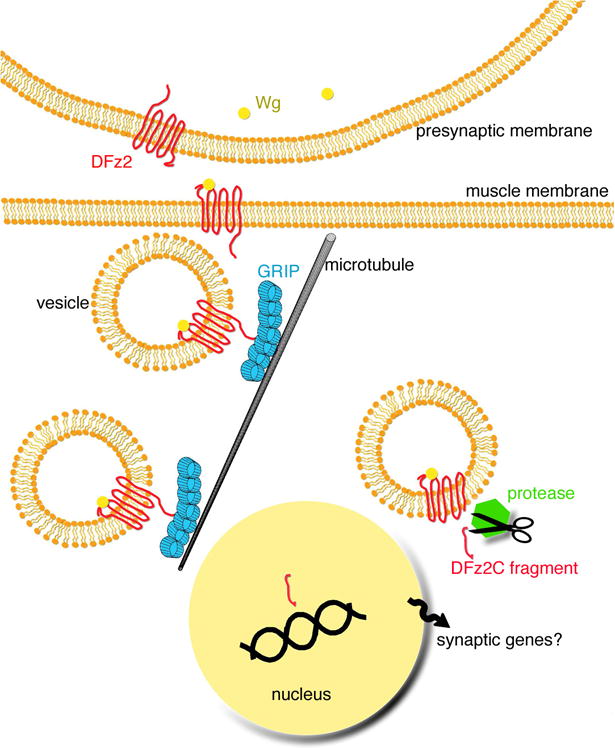
The Wg cleavage pathway. The diagram shows a model of known events during Wg signaling at the NMJ. Wg is secreted by the presynaptic motoneuron and it binds to postsynaptic DFz2 receptors. DFz2, perhaps still bound to Wg is internalized, and it is transported to the perinuclear area via an interaction between its C-terminal tail and the PDZ4-5 domains of dGRIP. dGRIP is present in trafficking intracellular vesicles that move along microtubules. Once DFz2 reaches the perinuclear area, the C-terminal tail is cleaved and imported into the muscle nuclei, where it is postulated to regulate the transcription of genes required for the differentiation of new synapses.
The role of Wg at the NMJ has been investigated independently from the role of Wg in early morphogenesis by using a temperature sensitive wingless allele (wgts). In this mutant Wg is not processed properly and becomes trapped in the ER (van den Heuvel et al., 1993). Eliminating Wg secretion during the last day of larval development results in stunted NMJs containing about 50% fewer boutons than normal (Packard et al., 2002). Ultrastructural analysis of NMJs in these mutants reveals drastic defects in synapse structure. Many boutons are devoid of active zones, pre- and postsynaptic densities, and subsynaptic reticulum. Despite these abnormalities, these boutons still contain synaptic vesicles. Other boutons have intermediate phenotypes, with grossly abnormal active zones and postsynaptic structures. These observations suggest that Wg has an essential function in the development of the most basic structures required for synaptic function, and that it might be one of the initial signals for new synapse formation. Similar morphological abnormalities are elicited by hypomorphic dfz2 alleles (Mathew et al., 2005). In addition, the serine/threonine kinase Gsk3-ß/Shaggy is enriched at presynaptic boutons (Packard et al., 2002), and defects similar to those observed in wg and dfz2 mutants are observed in shaggy mutants (Franco et al., 2004).
Several alternative Wnt transduction pathways have been described during the development of organisms (Ciani and Salinas, 2005; DasGupta et al., 2005). These include the canonical pathway, in which Frizzled activation by Wnts results in an elevation of cytoplasmic ß-Catenin by preventing its degradation by the proteasome. Stabilized ß-Catenin translocates into the nucleus where it forms transcriptionally active complexes with lymphoid enhancer factor (LEF)/T cell-specific transcription factor (TCF) proteins and regulates cell fate. In the planar cell polarity pathway, Wg binding to Frizzled receptors leads to activation of the small G-proteins Rho and Rac, and JNK signaling. This process is thought to regulate the cytoskeleton during the development of cell and tissue polarity and during the formation of dendrites. In the calcium pathway, activation of Frizzled receptors by Wnts results in calcium increase and subsequent activation of Protein Kinase C (PKC) and Calcium/Calmodulin dependent protein kinase II (CaMKII). This pathway is also believed to regulate the transcription of genes required for cell fate determination and cell movements (Ciani and Salinas, 2005; DasGupta et al., 2005).
Besides Wnts and Frizzled receptors, a common component of all of the above pathways is the scaffolding protein Disheveled (Dvl), a PDZ-containing protein. Curiously however, Dvl is not observed at detectable levels at the NMJ, and hypomorphic dvl mutants do not show obvious NMJ defects (Mathew et al., 2005). Similarly, Armadillo, the ß-Catenin homolog in flies, is not observed at the NMJ, although it has been reported to be present in neurons during embryonic development (Loureiro and Peifer, 1998). These observations and the ones described below have led to the conclusion that the Wnt pathway at the NMJ utilizes a yet alternative transduction pathway, the receptor cleavage pathway (Mathew et al., 2005).
In the receptor cleavage pathway, DFz2 is internalized and transported into the perinuclear region. Along the way, the C-terminal fragment of the receptor is cleaved and imported into the nucleus where it is postulated to regulate transcription (Mathew et al., 2005; Fig. 2). While the cleavage and import of the C-terminal DFz2 fragment is necessary for NMJ differentiation and growth, the nature of the events downstream of nuclear DFz2-C import are still unknown. However, more recently another component of this pathway, which appears to be required for trafficking the receptor from the synaptic membrane to the muscle nuclei, has been identified (Ataman et al., in press). This corresponds to the 7 PDZ protein dGRIP (Fig. 2). dGRIP interacts with the C-terminal tail of DFz2, and is required to transport vesicles containing the receptor towards the nucleus. Mutations in dgrip or genetically encoded dGRIP-RNAi phenocopy wg and dfz2 mutant phenotypes at the NMJ, and in these mutants, the DFz2 C-terminal fragment is not imported into the nucleus. Like wg and dfz2 mutants, dgrip mutants have stunted NMJs, and a proportion of boutons (called ghost boutons) fail to develop pre- and postsynaptic structures, and are devoid of all postsynaptically localized proteins. This phenotype appears to be the opposite of that observed in certain alterations of the TGF-ß pathway and retrograde transport at the NMJ (Eaton et al., 2002; Eaton and Davis, 2005), in which presynaptic membranes are reported to retract, leaving remnants of postsynaptic proteins (footprints) behind.
While the above studies document the transduction pathway activated in postsynaptic muscles, mutations in wg, dfz2, and dgrip clearly affect the development of both pre- and postsynaptic structures. Whether this is mediated by Wg binding to presynaptic DFz2 receptors in an autocrine fashion remains to be determined. In this regard, it is important to note that in mammals a retrograde control of presynaptic differentiation by Wnts has been reported (Hall et al., 2000; Krylova et al., 2002).
5. Cell adhesion molecules
Membrane bound signaling molecules are important in both the development and plasticity of cellular junctions. Reducing cell adhesion may be permissive for synaptic expansion, while increasing it can block growth. Cell adhesion molecules can also, in some contexts, function as receptors or ligands to activate intracellular cascades.
a. Fasciclin II
Fasciclin II (FasII) is a Drosophila homolog of the mammalian NCAM cell adhesion molecule. FasII has been shown to behave as a signaling molecule in early development (Garcia-Alonso et al., 1995) and to be required for memory formation in the adult (Cheng et al., 2001). In the complete absence of FasII, synapses initially form but retract early in development suggesting an essential role in NMJ maintenance (Schuster et al., 1996b). Alterations in the level of FasII during development have profound consequences for the structure and function of the synapse. Reduction to ca. 10% of wild type levels causes a reduction in branching and bouton number (Schuster et al., 1996a) which is accompanied by functional alterations that maintain synaptic strength (Stewart et al., 1996). In contrast, a milder reduction, to ca. 50% of wild type levels, causes overgrowth of boutons (Schuster et al., 1996a) and functional changes that decrease quantal content to normalize synaptic transmission (Schuster et al., 1996a). This has led to the idea that many pathways that alter synaptic structure at the NMJ do so by modulating the levels of FasII (Packard et al., 2003a; Fig. 3B). The structural effects of hyperexcitability mutants such as eagSh are mediated by FasII (Schuster et al., 1996a) as are the effects of alterations in the Ras/MAPK cascade (Koh et al., 2002) and CaMKII (Koh et al., 1999). The trafficking of FasII to and from the postsynaptic cell surface is accomplished by SNARE-mediated fusion and requires the Drosophila homolog of Amphiphysin (Mathew et al., 2003) and it is localized in the postsynaptic membrane by binding to Dlg, a member of the MAGUK family of proteins which includes PSD95 (Thomas et al., 1997).
Figure 3.
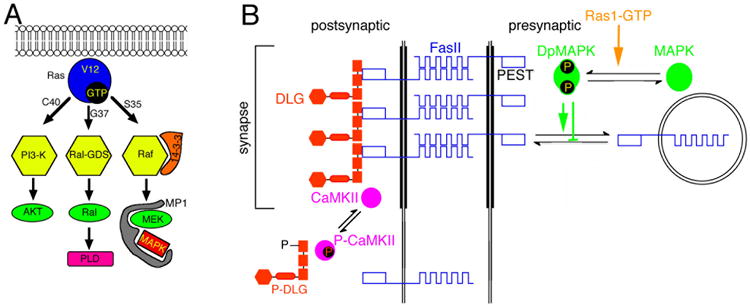
Regulation of synaptic FasII by CaMKII and the MAPKinase pathway. (A) Diagram of signal transduction pathways constitutively activated by Ras1 mutant constructs. Substitution of glycine-12 to valine (Ras1V12) renders Ras1 constitutively active. Additional substitution of either threonine-35 to serine (Ras1V12S35), glutamic acid-37 to glycine (Ras1V12G37), or tyrosine-40 to cysteine (Ras1V12C40), results in the activation of a single pathway. (B) Postulated model summarizing the regulation of synaptic FasII by both activation of CaMKII, and by recruitment and activation of the Ras-MAPKinase pathway. According to this model, electrical activity activates CaMKII, which phosphorylates Dlg detaching it from the synaptic complex and therefore reducing its ability to cluster FasII. An additional pathway involved in the downregulation of synaptic FasII might be the endocytic pathway based on work in flies and Aplysia. Ras-MAPKinase pathway activation in this scheme induces the endocytosis of FasII-rich membrane, presumably by phosphorylation of the PEST sequence at the FasII cytoplasmic region which would target it for proteolytic cleavage. Note that for simplicity we have drawn the CaMKII pathway at the postsynaptic site, and the Ras-MAPKinase pathway at the presynaptic site. However the CaMKII mechanism may operate at both sides of the synapse.
Recent studies suggest that FasII-mediated cell adhesion at the NMJ may activate a transduction pathway that involves APPL the Drosophila homolog of Amyloid Precursor Protein (APP; Ashley et al., 2005). These studies show that the ability of FasII to stimulate or inhibit the proliferation of boutons depends on the symmetry of transmembrane FasII levels in the presynaptic and postsynaptic cell and requires the presence of the fly homolog of (APPL). In turn, APPL is regulated by direct interactions with the PDZ-containing protein dX11/Mint/Lin-10, which also regulates synapse expansion downstream of FasII.
b. Integrins
Integrins, like NCAMs, function as both structural and signaling molecules (Arnaout et al., 2005). In Drosophila, Integrins are present both pre- and postsynaptically at the NMJ. Mutation of the volado (vol) gene, which encodes an aPS Integrin, causes defects in adult short-term memory that are not due to developmental defects since they can be rescued by acute expression of Vol (Grotewiel et al., 1998). At the NMJ, vol mutants have large boutons, larger than normal evoked currents and defects in presynaptic short-term plasticity. Pharmacological disruption of Integrin interactions in wild type animals by application the RGD peptide, which competes for the Integrin ligand site, phenocopies aspects of the mutant phenotype (Rohrbough et al., 2000). Hypomorphic mutations in bPS Integrins also have complex effects on NMJ structure and function (Beumer et al., 1999). These molecules can activate CaMKII and via this kinase modulate FasII levels (Beumer et al., 2002). Integrins are also involved in hypertonicity-induced vesicle fusion and require an intact cAMP cascade to modulate release (Suzuki et al., 2002).
c. Proteoglycans
The extracellular compartment of most cells contains proteins that have long polymeric sugar side chains called proteoglycans. The two major heparan sulfate proteoglycan families, glypicans and syndecans differ in their sugars and their modes of attachment to the cell surface: glypicans are linked by a glycosylphosphatidylinositol tail, while syndecans are transmembrane proteins (Lander and Selleck, 2000). The function of these molecules has been mysterious, but in the last several years it has become clear that they are important components of known signal transduction pathways. Syndecan has been shown to be a ligand for Dlar, a membrane-bound tyrosine phosphatase (Fox and Zinn, 2005; Johnson et al., 2006). Dlar is important in axon guidance at the NMJ (Johnson et al., 2004). Dally-like protein, which is a glypican, has been shown to be important in Wnt signaling, helping to present Wnt to its receptor and creating extracellular gradients of this morphogen (Baeg et al., 2001; Kirkpatrick et al., 2004). As noted above, Wg has important roles in plasticity at the NMJ.
IV. Major intracellular effectors of plasticity at the neuromuscular junction
1. cAMP
Among the initial wave of learning mutants isolated in Drosophila, there were several genes that encoded proteins involved in cAMP-dependent signal transduction. The identification of this first plasticity-associated signal transduction pathway paved the way for molecular studies of learning and memory in many systems. In Drosophila, the analysis of this cascade has been facilitated by both traditional and reverse genetic approaches and cAMP appears to be a common mediator of plasticity across phyla.
cAMP is generated from ATP by adenylate cyclases. This is a large family of enzymes, most of which are stimulated by release of Gas from trimeric G proteins by G protein-coupled receptors (GPCRs). Some adenylate cyclases can also respond to additional signals, integrating GPCR activation with another pathway and in effect becoming molecular coincidence detectors. Mammalian neurons contain a voltage-sensitive adenylate cyclase (Reddy et al., 1995) and at lease two Ca2+/CaM stimulable cyclases (Ferguson and Storm, 2004). The cyclase encoded by the Drosophila rut gene is stimulated by binding of Ca2+/CaM (Livingstone et al., 1984). In the PACAP-stimulated pathway, NF1 appears to modulate the activation of rut (Guo et al., 1997; Tong et al., 2002) adding yet another level of complexity to the integration.
Intracellular levels of most second messengers are under tight control, and cAMP is no exception. Cyclic nucleotides are degraded to AMP by a family of enzymes called phosphodiesterases. The product of the dunce (dnc) gene is a cAMP-specific phosphodiesterase (Walter and Kiger, 1984) and mutations in this gene would be expected to elevate cAMP, the opposite of the rut biochemical effect. Indeed rut alleles that have intact ability to be stimulated by calcium, but lowered basal activity, can suppress the female sterility of dnc and its behavioral phenotype (Feany, 1990) indicating that for these functions, the calcium-sensitivity of Rut is important. Other functions do not seem to require this feature of the enzyme since the rut1 allele, which has no calcium-stimulated activity, is capable of rescuing the morphological defects of dnc (Zhong et al., 1992).
dnc and rut have been studied extensively with regard to larval functional plasticity to assess the effects of bidirectional manipulation of cAMP. In dnc mutants, there is an increase in evoked EJC amplitude, but no alteration in mEJC size (Zhong and Wu, 1991). In the same study rut larvae did not show alterations in evoked EJCs, but both mutants had weak or non-existent facilitation, PPF and PTP. Mutation of the dgs gene, which encodes the Drosophila Gas protein, also blocks PTP and facilitation (Wolfgang et al., 2004). These data suggested that cAMP has profound effects on presynaptic function, and that increases in presynaptic cAMP enhance release. This conclusion is supported by studies that overexpress dnc to lower cAMP levels and find a decrease in evoked EJP size (Cheung et al., 1999) and studies in which the recruitment of vesicles from the reserve pool by high frequency stimulation was examined. It was found that rut mutants were impaired in reserve pool access, while dnc mutants showed enhanced mobilization (Kuromi and Kidokoro, 2000).
The morphological effects of chronically altering cAMP levels have also been studied. dnc mutants have an increased number of branches and boutons, while rut is relatively normal (Zhong et al., 1992). The overgrowth of dnc mutants were further stimulated on either an eag or Sh background, suggesting that activity and cAMP can act synergistically. Structural studies carried out at the EM level demonstrate that the docking of vesicles is altered in these mutants in a way that is consistent with their functional presynaptic phenotypes: rut larva have fewer docked vesicles while dnc animals have more (Renger et al., 2000).
The mechanisms by which these functional and structural changes come about are likely to be complex. The presynaptic functional changes in release found in dnc occur via activation of CREB (Davis et al., 1996), while the structural changes are secondary to decreases in FasII (Schuster et al., 1996a). In the postsynaptic cell, alteration of cAMP can alter excitability and affect the response to neuronal activity. In dnc mutants there are specific increase in the IA and IK potassium currents. In rut, ICS is increased (Zhong and Wu, 1993). Postsynaptic activation of PKA can also directly modulate the expression of DGluRIIA and activate a retrograde signaling pathway to alter quantal content in larvae (Davis et al., 1998). Activity-dependent retrograde signaling at the embryonic NMJ requires presynaptic cAMP pathways to modulate release (Yoshihara et al., 2005).
2. cGMP
The actions of cGMP in the larva have been investigated both from the behavioral and the synaptic point of view. Genetic polymorphism in the foraging (for) gene which encodes cGMP protein kinase (PKG) has effects on larval feeding behavior; animals with the Rover allele will wander far afield to eat, while sitter animals feed in one place (Osborne et al., 1997). At the NMJ, fors animals are hyperexcitable, showing spontaneous activity after stimulation. In addition, they have aberrant targeting and architecture. In culture, fors neurons have reduced potassium currents (Renger 1999). Presynaptic function can clearly be modulated by PKG and this produces behavioral effects in the larva.
3. Calcium
Calcium is an important second messenger in both the motor neuron and the muscle at the larval NMJ. In muscle, calcium influx is the major determinant of membrane depolarization and is responsible for activating the Myosin contractile apparatus. In both the pre-and postsynaptic cells calcium can activate signal transduction pathways. Intracellular calcium can be increased by either the opening of calcium-permeable channels in the plasma membrane or by release from intracellular stores. Not surprisingly, these functions are vital: mutations in the ER calcium pump, SERCA are lethal (Sanyal et al., 2005) as are mutations in the a subunit of the voltage-gated L-type calcium channel (Eberl et al., 1998), the Ryanodine receptor (Sullivan et al., 2000), the IP3 receptor (Venkatesh and Hasan, 1997) and the major calcium-binding effector protein, CaM (Nelson et al., 1997). Calcium has many downstream targets; only two major kinases are considered here.
a. PKC
Protein kinase C (PKC) is a family of kinases that differ in their requirements for activation. Classical or conventional PKC (cPKC) requires calcium, diacylglycerol and a phospholipid. Novel PKC (nPKC) requires lipid/diacylglycerol but not calcium. Atypical PKC (aPKC) is activated by lipid and interaction with specific partners such as Par-6, and can also be proteolysed into an active form. In Drosophila, two cPKCs, one aPKC and two nPKCs have been identified (Shieh et al., 2002).
Both cPKCs and nPKCs are activated downstream of Phospholipases that generate diacylglycerol. In the eye, PLC and a cPKC are critical to signal transduction (Shieh et al., 2002). At the NMJ, norpA mutants, which disrupt PLC-b function, have a reduction in muscle L-type calcium currents (Gu and Singh, 1997). Application of PKC activators can rescue current levels in the mutant, but have no effect in wild type, suggesting that PKC is a regulator of the basal levels of calcium current in the larval muscle.
aPKC has been implicated in memory maintenance (Drier et al., 2002) and establishment of cell polarity (Johnson and Wodarz, 2003) in Drosophila. At the larval NMJ, this kinase is expressed both pre- and postsynaptically and is critical to organization of the Actin and Microtubule cytoskeletons (Ruiz-Canada et al., 2004). It also serves to mediate localization of other cell polarity genes such as the fly homologs of Par-3, Par-6. Alterations in the level of aPKC affect mEJP amplitude and DGluRIIA levels and distribution. dapkc mutants have an increase in mEJP amplitude and an increase in the intensity of postsynaptic DGluRIIA staining. Postsynaptic overexpression of activated aPKC decreased mEJP amplitude and causes aberrant localization of the receptor. mEJP frequency was reduced in both types of animals leading to an profoundly decreased evoked EJP in the overexpresser and a partially compensated increase in the EJP of the dapkc mutant.
At the structural level, both increasing and decreasing aPKC activity lead to a reduction in bouton number (Ruiz-Canada et al., 2004). Both types of manipulations also disrupt the organization of the cytoskeleton, suggesting that normal dynamics and interactions between pre-and postsynaptic compartments are necessary for normal growth of the bouton arbor.
b. CaMKII
Ca2+/calmodulin protein kinase II (CaMKII) is an abundant signaling molecule in both vertebrate and invertebrate nervous systems. Binding of Ca2+/CaM allows substrate phosphorylation, but also stimulates a fast phosphorylation of T287 in the autoregulatory domain. This phosphorylation makes the kinase constitutively active. The constitutive activity of CaMKII has been shown to be important for learning and plasticity in both flies (Mehren and Griffith, 2004) and mammals (Giese et al., 1998). At the NMJ, CaMKII regulates presynaptic excitability and plasticity (Wang et al., 1994; Park et al., 2002). One mechanism by which excitability can be regulated is by modulation of potassium channels, including the EAG protein (Griffith et al., 1994; Wang et al., 2002). Postsynaptic CaMKII has a role in regulation of Dlg and FasII localization (Koh et al., 2000; Fig. 3B) and can also participate in generation of an activity-dependent retrograde signal that modulates presynaptic function (Haghighi et al., 2003; Kazama et al., 2003; Morimoto-Tanifuji et al., 2004).
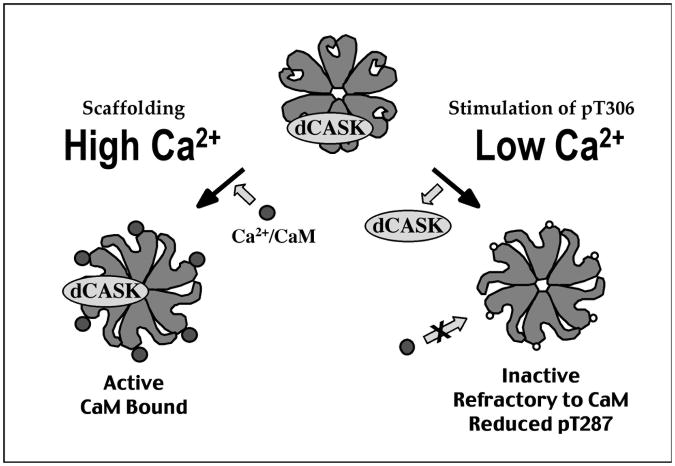
Activation of postsynaptic CaMKII, as measured by phosphorylation of T287, is stimulated by neuronal activity (J. Hodge, P. Mullasseril and L.C. Griffith, submitted; Fig. 4). The ability of calcium influx to activate the kinase is modulated by an interaction of CaMKII with the Drosophila homolog of CASK (dCASK) a mammalian MAGUK scaffolding protein (Lu et al., 2003). dCASK, which is also known as caki or Camguk, physically interacts with CaMKII in the presence of Ca2+/CaM to localize the kinase's activity. In the absence of Ca2+/CaM, dCASK dissociates from CaMKII and stimulates the kinase to autophosphorylate at T306 in its CaM-binding domain. This pT306 kinase is no longer able to bind to Ca2+/CaM and is therefore inactive until it is dephosphorylated by protein phosphatases 2A. Inhibition of CaM binding would also be expected to blunt generation of the constitutive form of the kinase since autophosphorylation of T287 requires binding of Ca2+/CaM to two neighboring subunits (Wang et al., 1998).
Figure 4.
Activity-dependent interactions of CaMKII and dCASK. CaMKII and dCASK associate tightly under physiological conditions. At synapses where activity and intracellular calcium are high (left), calcium/calmodulin (Ca2+/CaM) can bind to the CaMKII:dCASK complex. In this tripartite complex, CaMKII is active and can phosphorylate substrates and autophosphorylate at T287. Phosphorylation of T306 is blocked by the presence of bound CaM. At synapses where activity and intracellular calcium are low (right), dCASK stimulates CaMKII autophosphorylation of T306. This causes the complex to dissociate, releasing dCASK and pT306 CaMKII. Subunits with pT306 are unable to bind Ca2+/CaM and are therefore refractory to activation by calcium influx and cannot be substrates for intersubunit phosphorylation of T287.
The fact that the dCASK-stimulated phosphorylation of T306 only occurs at low calcium suggested that it might be associated with synapses that were inactive and could serve to differentiate them from more active synapses by depleting their activatable CaMKII. Genetically manipulating synaptic activity and immunocytochemically measuring pT287 (the activating autophosphorylation) and pT306 (the inactivating autophosphorylation) at the NMJ supports this idea (Lu et al., 2003, and J. Hodge and L.C. Griffith, upublished). Behavioral manipulation of activity in the adult brain indicates that the dCASK interaction is also an indirect regulator of pT287 phosphorylation (J Hodge, P. Mullasseril and L.C. Griffith, submitted). Both the fidelity and the dynamic range of circuit-specific changes in CaMKII autophosphorylation are lessened in dCASK mutant animals.
4. Ras/MAPK
Ras proteins are small GTPases with well-known functions in cell proliferation and differentiation. In these processes they play key roles as molecular switches that can trigger distinct signal transduction pathways, such as the Mitogen-activated protein kinase (MAPKinase) pathway, the Phosphoinositide-3 kinase pathway (PI3-K), and the Ral-GDS pathway (Fig. 3A). Ras proteins are highly conspicuous in developing and adult brains (Leon et al., 1987), and maintenance of long-term potentiation (LTP) is critically dependent on MAPKinase activation (English and Sweatt, 1997). Mutations in genes encoding members of the MAPKinase pathway, such as Mitogen-activated-protein-kinase-kinase (MEK), Ras-guanine-nucleotide-releasing factor (GRE) or H-Ras cause defects in learning and LTP (Brambilla et al., 1997; Atkins et al., 1998; Manabe et al., 2000).
In Aplysia, ApMAPKinase, the homolog of P44/42-extracellular-signal-regulated-kinase (ERK), plays a major role in long-term facilitation (LTF; Bailey et al., 1997). LTF elicits translocation of activated ApMAPKinase into the neuronal nucleus, and the internalization of ApCAM, a homolog of Neuronal-Cell-Adhesion-Molecule (NCAM) in mouse, and FasII in flies (Mayford et al., 1992). Mutations in MAPKinase or MAPKinase phosphorylation targets in ApCAM block internalization of ApCAM, preventing synaptic growth (Bailey et al., 1997; Martin et al., 1997).
Both Ras1 and MAPKinase are expressed at the NMJ, and modification in their activity levels results in an altered number of synaptic boutons (Koh et al., 2002). As discussed above, synapse stability and synapse expansion during muscle growth at the NMJ are regulated by changes in FasII expression at pre- and postsynaptic membranes, and FasII expression is in part controlled by electrical activity (Schuster et al., 1996b; Ashley et al., 2005). One mechanism through which electrical activity alters FasII levels is by regulating its synaptic clustering via CaMKII-dependent phosphorylation of Dlg (Thomas et al., 1997; Koh et al., 1999). An additional mechanism by which the levels of FasII at the presynaptic terminal are modified is by the activation of the Ras/MAPKinase pathway (Koh et al., 2002). This redundant mechanism may serve the differential regulation of FasII localization at the pre- and postsynaptic site, or may represent FasII regulation in response to different signals. While activation of CaMKII is elicited by an increase in electrical activity, activation of the MAPKinase pathway may be triggered by activity or a yet unknown but different signaling mechanism. Expression of constitutively active Ras drastically increased the number of synaptic boutons at the NMJ. This change was indistinguishable from the increase in boutons observed in a Ras variant that selectively activates the MAPKinase pathway, and by constitutively active RafF179, suggesting that these changes were induced by activation of the MAPKinase pathway. Consistent with these results, a hypomorphic mutation in ras1 had the opposite phenotype, a decrease in bouton number, and a gain of function mutation in the fly MAPKinase gene rolled (rl) led to an increase in bouton number (Koh et al., 2002). These results are in agreement with the studies in Aplysia dissociated neurons, which show that ApMAPKinase is involved in the internalization of ApCAM (Bailey et al., 1997; Martin et al., 1997).
Further support that the changes in bouton number elicited by alterations in Ras1 and MAPKinase activity are mediated by alterations in FasII levels was demonstrated by examining the overall expression of FasII in MAPKinase gain or loss of function alleles, by examining the distribution of FasII within single synaptic boutons in relationship to active MAPKinase, and by using hypomorphic fasII mutants (Koh et al., 2002). The studies with rl mutants demonstrated that there was an inverse relationship between levels of synaptic FasII and MAPKinase activity. Further, active MAPKinase localization coincided with active zones, regions of the bouton that have no or low FasII levels. These results provide evidence for a Ras-dependent signaling cascade that regulates FasII-mediated cell adhesion at synaptic terminals during synapse growth.
Further support for the above model and for a relationship between MAPKinase activation and level of synaptic activity was provided by studies of flies with conditional mutations resulting in abnormally high bursts of neural activity including comatosets and Kumts mutants, with conditional defects in N-ethylmaleimide-sensitive fusion factor 1 and sarco-endoplasmic reticulum Ca2+ ATPase, respectively (Hoeffer et al., 2003). Shifting the larvae to restrictive temperature resulted in persistent activation of neuronal extracellular signal-regulated kinase (ERK). ERK activation in turn coincided with a rapid reduction of synaptic Fasciclin II. In addition, there was nuclear translocation of activated ERK in neuronal somata together with increased transcription of the immediate-early genes Fos and c/EBP (CCAAT element binding protein). This effect was found to require neural activity and was mediated through activation of MEK (MAPK/erk kinase), the MAPKK (mitogen-activated protein kinase kinase) that functions upstream of ERK (Hoeffer et al., 2003).
V. Conclusion
The Drosophila neuromuscular junction has proven to be fertile ground for the study of plasticity and signal transduction. Molecules that are involved in invertebrate and vertebrate behavior almost inevitably have a role in the development or modulation of this beautiful and experimentally accessible synapse.
Acknowledgments
LCG was supported by NIH grants MH067284 and GM54408.
References
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. Embo J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MS, Halpern ME, Keshishian H. Identification of the neuropeptide transmitter proctolin in Drosophila larvae: characterization of muscle fiber-specific neuromuscular endings. J Neurosci. 1988;8:242–255. doi: 10.1523/JNEUROSCI.08-01-00242.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- Ashley J, Packard M, Ataman B, Budnik V. Fasciclin II signals new synapse formation through amyloid precursor protein and the scaffolding protein dX11/Mint. J Neurosci. 2005;25:5943–5955. doi: 10.1523/JNEUROSCI.1144-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128:87–94. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Kaang BK, Chen M, Martin KC, Lim CS, Casadio A, Kandel ER. Mutation in the phosphorylation sites of MAP kinase blocks learning-related internalization of apCAM in Aplysia sensory neurons [see comments] Neuron. 1997;18:913–924. doi: 10.1016/s0896-6273(00)80331-1. [DOI] [PubMed] [Google Scholar]
- Beaumont V, Zhong N, Froemke RC, Ball RW, Zucker RS. Temporal synaptic tagging by I(h) activation and actin: involvement in long-term facilitation and cAMP-induced synaptic enhancement. Neuron. 2002;33:601–613. doi: 10.1016/s0896-6273(02)00581-0. [DOI] [PubMed] [Google Scholar]
- Belgacem YH, Martin JR. Disruption of insulin pathways alters trehalose level and abolishes sexual dimorphism in locomotor activity in Drosophila. J Neurobiol. 2006;66:19–32. doi: 10.1002/neu.20193. [DOI] [PubMed] [Google Scholar]
- Beumer K, Matthies HJ, Bradshaw A, Broadie K. Integrins regulate DLG/FAS2 via a CaM kinase II-dependent pathway to mediate synapse elaboration and stabilization during postembryonic development. Development. 2002;129:3381–3391. doi: 10.1242/dev.129.14.3381. [DOI] [PubMed] [Google Scholar]
- Beumer KJ, Rohrbough J, Prokop A, Broadie K. A role for PS integrins in morphological growth and synaptic function at the postembryonic neuromuscular junction of Drosophila. Development. 1999;126:5833–5846. doi: 10.1242/dev.126.24.5833. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Lakhman SS, Singh S. Modulation of L-type calcium channels in Drosophila via a pituitary adenylyl cyclase-activating polypeptide (PACAP)-mediated pathway. J Biol Chem. 2004;279:37291–37297. doi: 10.1074/jbc.M403819200. [DOI] [PubMed] [Google Scholar]
- Bogdanik L, Mohrmann R, Ramaekers A, Bockaert J, Grau Y, Broadie K, Parmentier ML. The Drosophila metabotropic glutamate receptor DmGluRA regulates activity-dependent synaptic facilitation and fine synaptic morphology. J Neurosci. 2004;24:9105–9116. doi: 10.1523/JNEUROSCI.2724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Gnesutta N, Minichiello L, White G, Roylance AJ, Herron CE, Ramsey M, Wolfer DP, Cestari V, Rossi-Arnaud C, Grant SG, Chapman PF, Lipp HP, Sturani E, Klein R. A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature. 1997;390:281–286. doi: 10.1038/36849. [DOI] [PubMed] [Google Scholar]
- Broadie K, Bate M. Activity-dependent development of the neuromuscular synapse during Drosophila embryogenesis. Neuron. 1993;11:607–619. doi: 10.1016/0896-6273(93)90073-z. [DOI] [PubMed] [Google Scholar]
- Budnik V, Zhong Y, Wu CF. Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci. 1990;10:3754–3768. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Endo K, Wu K, Rodan AR, Heberlein U, Davis RL. Drosophila fasciclinII is required for the formation of odor memories and for normal sensitivity to alcohol. Cell. 2001;105:757–768. doi: 10.1016/s0092-8674(01)00386-5. [DOI] [PubMed] [Google Scholar]
- Cheung US, Shayan AJ, Boulianne GL, Atwood HL. Drosophila larval neuromuscular junction's responses to reduction of cAMP in the nervous system. J Neurobiol. 1999;40:1–13. doi: 10.1002/(sici)1097-4695(199907)40:1<1::aid-neu1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Boutros M, Perrimon N. Drosophila Wnt/Fz pathways. Sci STKE. 2005;2005:cm5. doi: 10.1126/stke.2832005cm5. [DOI] [PubMed] [Google Scholar]
- Davis GW, Schuster CM, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. III. CREB is necessary for presynaptic functional plasticity [see comments] Neuron. 1996;17:669–679. doi: 10.1016/s0896-6273(00)80199-3. [DOI] [PubMed] [Google Scholar]
- Davis GW, DiAntonio A, Petersen SA, Goodman CS. Postsynaptic PKA controls quantal size and reveals a retrograde signal that regulates presynaptic transmitter release in Drosophila. Neuron. 1998;20:305–315. doi: 10.1016/s0896-6273(00)80458-4. [DOI] [PubMed] [Google Scholar]
- Dawson-Scully K, Bronk P, Atwood HL, Zinsmaier KE. Cysteine-string protein increases the calcium sensitivity of neurotransmitter exocytosis in Drosophila. J Neurosci. 2000;20:6039–6047. doi: 10.1523/JNEUROSCI.20-16-06039.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drier EA, Tello MK, Cowan M, Wu P, Blace N, Sacktor TC, Yin JC. Memory enhancement and formation by atypical PKM activity in Drosophila melanogaster. Nat Neurosci. 2002;5:316–324. doi: 10.1038/nn820. [DOI] [PubMed] [Google Scholar]
- Dudu V, Bittig T, Entchev E, Kicheva A, Julicher F, Gonzalez-Gaitan M. Postsynaptic mad signaling at the Drosophila neuromuscular junction. Curr Biol. 2006;16:625–635. doi: 10.1016/j.cub.2006.02.061. [DOI] [PubMed] [Google Scholar]
- Dunn TW, Mercier AJ. Synaptic modulation by a Drosophila neuropeptide is motor neuron-specific and requires CaMKII activity. Peptides. 2005;26:269–276. doi: 10.1016/j.peptides.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Eaton BA, Davis GW. LIM Kinase1 controls synaptic stability downstream of the type II BMP receptor. Neuron. 2005;47:695–708. doi: 10.1016/j.neuron.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Eaton BA, Fetter RD, Davis GW. Dynactin is necessary for synapse stabilization. Neuron. 2002;34:729–741. doi: 10.1016/s0896-6273(02)00721-3. [DOI] [PubMed] [Google Scholar]
- Eberl DF, Ren D, Feng G, Lorenz LJ, Van Vactor D, Hall LM. Genetic and developmental characterization of Dmca1D, a calcium channel alpha1 subunit gene in Drosophila melanogaster. Genetics. 1998;148:1159–1169. doi: 10.1093/genetics/148.3.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J Biol Chem. 1997;272:19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- Feany MB. Rescue of the learning defect in dunce, a Drosophila learning mutant, by an allele of rutabaga, a second learning mutant. Proc Natl Acad Sci U S A. 1990;87:2795–2799. doi: 10.1073/pnas.87.7.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany MB, Quinn WG. A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science. 1995;268:869–873. doi: 10.1126/science.7754370. [DOI] [PubMed] [Google Scholar]
- Ferguson GD, Storm DR. Why calcium-stimulated adenylyl cyclases? Physiology (Bethesda) 2004;19:271–276. doi: 10.1152/physiol.00010.2004. [DOI] [PubMed] [Google Scholar]
- Fox AN, Zinn K. The heparan sulfate proteoglycan syndecan is an in vivo ligand for the Drosophila LAR receptor tyrosine phosphatase. Curr Biol. 2005;15:1701–1711. doi: 10.1016/j.cub.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Fox LE, Soll DR, Wu CF. Coordination and modulation of locomotion pattern generators in Drosophila larvae: effects of altered biogenic amine levels by the tyramine Beta hydroxlyase mutation. J Neurosci. 2006;26:1486–1498. doi: 10.1523/JNEUROSCI.4749-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco B, Bogdanik L, Bobinnec Y, Debec A, Bockaert J, Parmentier ML, Grau Y. Shaggy, the homolog of glycogen synthase kinase 3, controls neuromuscular junction growth in Drosophila. J Neurosci. 2004;24:6573–6577. doi: 10.1523/JNEUROSCI.1580-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alonso L, VanBerkum MF, Grenningloh G, Schuster C, Goodman CS. Fasciclin II controls proneural gene expression in Drosophila. Proc Natl Acad Sci U S A. 1995;92:10501–10505. doi: 10.1073/pnas.92.23.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Gorczyca M, Augart C, Budnik V. Insulin-like receptor and insulin-like peptide are localized at neuromuscular junctions in Drosophila. J Neurosci. 1993;13:3692–3704. doi: 10.1523/JNEUROSCI.13-09-03692.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith LC, Wang J, Zhong Y, Wu CF, Greenspan RJ. Calcium/calmodulin-dependent protein kinase II and potassium channel subunit eag similarly affect plasticity in Drosophila. Proc Natl Acad Sci U S A. 1994;91:10044–10048. doi: 10.1073/pnas.91.21.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewiel MS, Beck CD, Wu KH, Zhu XR, Davis RL. Integrin-mediated short-term memory in Drosophila. Nature. 1998;391:455–460. doi: 10.1038/35079. [DOI] [PubMed] [Google Scholar]
- Gu GG, Singh S. Modulation of the dihydropyridine-sensitive calcium channels in Drosophila by a phospholipase C-mediated pathway. J Neurobiol. 1997;33:265–275. doi: 10.1002/(sici)1097-4695(199709)33:3<265::aid-neu5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Guerrero G, Reiff DF, Agarwal G, Ball RW, Borst A, Goodman CS, Isacoff EY. Heterogeneity in synaptic transmission along a Drosophila larval motor axon. Nat Neurosci. 2005;8:1188–1196. doi: 10.1038/nn1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo HF, The I, Hannan F, Bernards A, Zhong Y. Requirement of Drosophila NF1 for activation of adenylyl cyclase by PACAP38-like neuropeptides. Science. 1997;276:795–798. doi: 10.1126/science.276.5313.795. [DOI] [PubMed] [Google Scholar]
- Haghighi AP, McCabe BD, Fetter RD, Palmer JE, Hom S, Goodman CS. Retrograde control of synaptic transmission by postsynaptic CaMKII at the Drosophila neuromuscular junction. Neuron. 2003;39:255–267. doi: 10.1016/s0896-6273(03)00427-6. [DOI] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Hewes RS, Snowdeal EC, 3rd, Saitoe M, Taghert PH. Functional redundancy of FMRFamide-related peptides at the Drosophila larval neuromuscular junction. J Neurosci. 1998;18:7138–7151. doi: 10.1523/JNEUROSCI.18-18-07138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang B, Chiba A. Single-cell analysis of Drosophila larval neuromuscular synapses. Dev Biol. 2001;229:55–70. doi: 10.1006/dbio.2000.9983. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Sanyal S, Ramaswami M. Acute induction of conserved synaptic signaling pathways in Drosophila melanogaster. J Neurosci. 2003;23:6362–6372. doi: 10.1523/JNEUROSCI.23-15-06362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou D, Suzuki K, Wolfgang WJ, Clay C, Forte M, Kidokoro Y. Presynaptic impairment of synaptic transmission in Drosophila embryos lacking Gs(alpha) J Neurosci. 2003;23:5897–5905. doi: 10.1523/JNEUROSCI.23-13-05897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Genetic dissection of short-term and long-term facilitation at the Drosophila neuromuscular junction. Proc Natl Acad Sci U S A. 1978;75:515–519. doi: 10.1073/pnas.75.1.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia XX, Gorczyca M, Budnik V. Ultrastructure of neuromuscular junctions in Drosophila: comparison of wild type and mutants with increased excitability [published erratum appears in J Neurobiol 1994 Jul; 25(7):893-5] J Neurobiol. 1993;24:1025–1044. doi: 10.1002/neu.480240804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K, Wodarz A. A genetic hierarchy controlling cell polarity. Nat Cell Biol. 2003;5:12–14. doi: 10.1038/ncb0103-12. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Ghose A, Epstein E, Lincecum J, O'Connor MB, Van Vactor D. Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr Biol. 2004;14:499–504. doi: 10.1016/j.cub.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Tenney AP, Ghose A, Duckworth AM, Higashi ME, Parfitt K, Marcu O, Heslip TR, Marsh JL, Schwarz TL, Flanagan JG, Van Vactor D. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006;49:517–531. doi: 10.1016/j.neuron.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Kazama H, Morimoto-Tanifuji T, Nose A. Postsynaptic activation of calcium/calmodulin-dependent protein kinase II promotes coordinated pre- and postsynaptic maturation of Drosophila neuromuscular junctions. Neuroscience. 2003;117:615–625. doi: 10.1016/s0306-4522(02)00923-5. [DOI] [PubMed] [Google Scholar]
- Keshishian H, Chiba A, Chang TN, Halfon MS, Harkins EW, Jarecki J, Wang L, Anderson M, Cash S, Halpern ME, et al. Cellular mechanisms governing synaptic development in Drosophila melanogaster [published erratum appears in J Neurobiol 1993 Aug; 24(8):1130] J Neurobiol. 1993;24:757–787. doi: 10.1002/neu.480240606. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y, Kuromi H, Delgado R, Maureira C, Oliva C, Labarca P. Synaptic vesicle pools and plasticity of synaptic transmission at the Drosophila synapse. Brain Res Brain Res Rev. 2004;47:18–32. doi: 10.1016/j.brainresrev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick CA, Dimitroff BD, Rawson JM, Selleck SB. Spatial regulation of Wingless morphogen distribution and signaling by Dally-like protein. Dev Cell. 2004;7:513–523. doi: 10.1016/j.devcel.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Koh YH, Gramates LS, Budnik V. Drosophila larval neuromuscular junction: molecular components and mechanisms underlying synaptic plasticity. Microsc Res Tech. 2000;49:14–25. doi: 10.1002/(SICI)1097-0029(20000401)49:1<14::AID-JEMT3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Koh YH, Ruiz-Canada C, Gorczyca M, Budnik V. The Ras1-Mitogen-Activated Protein Kinase Signal Transduction Pathway Regulates Synaptic Plasticity through Fasciclin II-Mediated Cell Adhesion. J Neurosci. 2002;22:2496–2504. doi: 10.1523/JNEUROSCI.22-07-02496.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh YH, Popova E, Thomas U, Griffith LC, Budnik V. Regulation of DLG localization at synapses by CaMKII-dependent phosphorylation. Cell. 1999;98:353–363. doi: 10.1016/s0092-8674(00)81964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylova O, Herreros J, Cleverley KE, Ehler E, Henriquez JP, Hughes SM, Salinas PC. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron. 2002;35:1043–1056. doi: 10.1016/s0896-6273(02)00860-7. [DOI] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Tetanic stimulation recruits vesicles from reserve pool via a cAMP-mediated process in Drosophila synapses. Neuron. 2000;27:133–143. doi: 10.1016/s0896-6273(00)00015-5. [DOI] [PubMed] [Google Scholar]
- Lander AD, Selleck SB. The elusive functions of proteoglycans: in vivo veritas. J Cell Biol. 2000;148:227–232. doi: 10.1083/jcb.148.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon J, Guerrero I, Pellicer A. Differential expression of the ras gene family in mice. Mol Cell Biol. 1987;7:1535–1540. doi: 10.1128/mcb.7.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Sziber PP, Quinn WG. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984;37:205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- Lnenicka GA, Keshishian H. Identified motor terminals in Drosophila larvae show distinct differences in morphology and physiology. J Neurobiol. 2000;43:186–197. [PubMed] [Google Scholar]
- Loureiro J, Peifer M. Roles of Armadillo, a Drosophila catenin, during central nervous system development. Curr Biol. 1998;8:622–632. doi: 10.1016/s0960-9822(98)70249-0. [DOI] [PubMed] [Google Scholar]
- Lu CS, Hodge JJ, Mehren J, Sun XX, Griffith LC. Regulation of the Ca2+/CaM-responsive pool of CaMKII by scaffold-dependent autophosphorylation. Neuron. 2003;40:1185–1197. doi: 10.1016/s0896-6273(03)00786-4. [DOI] [PubMed] [Google Scholar]
- Manabe T, Aiba A, Yamada A, Ichise T, Sakagami H, Kondo H, Katsuki M. Regulation of long-term potentiation by H-Ras through NMDA receptor phosphorylation. J Neurosci. 2000;20:2504–2511. doi: 10.1523/JNEUROSCI.20-07-02504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques G. Morphogens and synaptogenesis in Drosophila. J Neurobiol. 2005;64:417–434. doi: 10.1002/neu.20165. [DOI] [PubMed] [Google Scholar]
- Marrus SB, DiAntonio A. Preferential localization of glutamate receptors opposite sites of high presynaptic release. Curr Biol. 2004;14:924–931. doi: 10.1016/j.cub.2004.05.047. [DOI] [PubMed] [Google Scholar]
- Martin KC, Casadio A, Zhu H, E Y, Rose JC, Chen M, Bailey CH, Kandel ER. Synapse-specific, long-term facilitation of aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- Mathew D, Popescu A, Budnik V. Drosophila amphiphysin functions during synaptic Fasciclin II membrane cycling. J Neurosci. 2003;23:10710–10716. doi: 10.1523/JNEUROSCI.23-33-10710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D, Ataman B, Chen J, Zhang Y, Cumberledge S, Budnik V. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science. 2005;310:1344–1347. doi: 10.1126/science.1117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Barzilai A, Keller F, Schacher S, Kandel ER. Modulation of an NCAM-related adhesion molecule with long-term synaptic plasticity in Aplysia. Science. 1992;256:638–644. doi: 10.1126/science.1585176. [DOI] [PubMed] [Google Scholar]
- McCabe BD, Marques G, Haghighi AP, Fetter RD, Crotty ML, Haerry TE, Goodman CS, O'Connor MB. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron. 2003;39:241–254. doi: 10.1016/s0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- Mehren JE, Griffith LC. Calcium-independent calcium/calmodulin-dependent protein kinase II in the adult Drosophila CNS enhances the training of pheromonal cues. J Neurosci. 2004;24:10584–10593. doi: 10.1523/JNEUROSCI.3560-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastirioti M, Gorczyca M, Rapus J, Eckert M, White K, Budnik V. Octopamine immunoreactivity in the fruit fly Drosophila melanogaster. J Comp Neurol. 1995;356:275–287. doi: 10.1002/cne.903560210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto-Tanifuji T, Kazama H, Nose A. Developmental stage-dependent modulation of synapses by postsynaptic expression of activated calcium/calmodulin-dependent protein kinase II. Neuroscience. 2004;128:797–806. doi: 10.1016/j.neuroscience.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Mosca TJ, Carrillo RA, White BH, Keshishian H. Dissection of synaptic excitability phenotypes by using a dominant-negative Shaker K+ channel subunit. Proc Natl Acad Sci U S A. 2005;102:3477–3482. doi: 10.1073/pnas.0406164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu JR, Murphy-Erdosh C, Andrews PC, Feistner GJ, Scheller RH. Isolation and characterization of a Drosophila neuropeptide gene. Neuron. 1988;1:55–61. doi: 10.1016/0896-6273(88)90209-7. [DOI] [PubMed] [Google Scholar]
- Nassel DR. Neuropeptides in the nervous system of Drosophila and other insects: multiple roles as neuromodulators and neurohormones. Prog Neurobiol. 2002;68:1–84. doi: 10.1016/s0301-0082(02)00057-6. [DOI] [PubMed] [Google Scholar]
- Nelson HB, Heiman RG, Bolduc C, Kovalick GE, Whitley P, Stern M, Beckingham K. Calmodulin point mutations affect Drosophila development and behavior. Genetics. 1997;147:1783–1798. doi: 10.1093/genetics/147.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K, Kidokoro Y. Octopamine inhibits synaptic transmission at the larval neuromuscular junction in Drosophila melanogaster. Brain Res. 1999;837:67–74. doi: 10.1016/s0006-8993(99)01676-5. [DOI] [PubMed] [Google Scholar]
- Osborne KA, Robichon A, Burgess E, Butland S, Shaw RA, Coulthard A, Pereira HS, Greenspan RJ, Sokolowski MB. Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science. 1997;277:834–836. doi: 10.1126/science.277.5327.834. [DOI] [PubMed] [Google Scholar]
- Packard M, Mathew D, Budnik V. FASt remodeling of synapses in Drosophila. Curr Opin Neurobiol. 2003a;13:527–534. doi: 10.1016/j.conb.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Packard M, Mathew D, Budnik V. Wnts and TGF beta in synaptogenesis: old friends signalling at new places. Nat Rev Neurosci. 2003b;4:113–120. doi: 10.1038/nrn1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V. The Drosophila wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Coleman MJ, Hodge JJ, Budnik V, Griffith LC. Regulation of neuronal excitability in Drosophila by constitutively active CaMKII. J Neurobiol. 2002;52:24–42. doi: 10.1002/neu.10066. [DOI] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–1248. doi: 10.1016/s0896-6273(00)80415-8. [DOI] [PubMed] [Google Scholar]
- Qin G, Schwarz T, Kittel RJ, Schmid A, Rasse TM, Kappei D, Ponimaskin E, Heckmann M, Sigrist SJ. Four different subunits are essential for expressing the synaptic glutamate receptor at neuromuscular junctions of Drosophila. J Neurosci. 2005;25:3209–3218. doi: 10.1523/JNEUROSCI.4194-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn WG, Sziber PP, Booker R. The Drosophila memory mutant amnesiac. Nature. 1979;277:212–214. doi: 10.1038/277212a0. [DOI] [PubMed] [Google Scholar]
- Rawson JM, Lee M, Kennedy EL, Selleck SB. Drosophila neuromuscular synapse assembly and function require the TGF-beta type I receptor saxophone and the transcription factor Mad. J Neurobiol. 2003;55:134–150. doi: 10.1002/neu.10189. [DOI] [PubMed] [Google Scholar]
- Reddy R, Smith D, Wayman G, Wu Z, Villacres EC, Storm DR. Voltage-sensitive adenylyl cyclase activity in cultured neurons. A calcium-independent phenomenon. J Biol Chem. 1995;270:14340–14346. doi: 10.1074/jbc.270.24.14340. [DOI] [PubMed] [Google Scholar]
- Renger JJ, Ueda A, Atwood HL, Govind CK, Wu CF. Role of cAMP cascade in synaptic stability and plasticity: ultrastructural and physiological analyses of individual synaptic boutons in Drosophila memory mutants. J Neurosci. 2000;20:3980–3992. doi: 10.1523/JNEUROSCI.20-11-03980.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder T. Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol. 2005;50:447–477. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Grotewiel MS, Davis RL, Broadie K. Integrin-mediated regulation of synaptic morphology, transmission, and plasticity. J Neurosci. 2000;20:6868–6878. doi: 10.1523/JNEUROSCI.20-18-06868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Canada C, Ashley J, Moeckel-Cole S, Drier E, Yin J, Budnik V. New Synaptic Bouton Formation Is Disrupted by Misregulation of Microtubule Stability in aPKC Mutants. Neuron. 2004;42:567–580. doi: 10.1016/s0896-6273(04)00255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, Consoulas C, Kuromi H, Basole A, Mukai L, Kidokoro Y, Krishnan KS, Ramaswami M. Analysis of conditional paralytic mutants in Drosophila sarco-endoplasmic reticulum calcium ATPase reveals novel mechanisms for regulating membrane excitability. Genetics. 2005;169:737–750. doi: 10.1534/genetics.104.031930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H, Trimmer BA, Rapus J, Eckert M, Valentine DE, Kravitz EA. Mapping of octopamine-immunoreactive neurons in the central nervous system of the lobster. J Comp Neurol. 1993;329:129–142. doi: 10.1002/cne.903290109. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity [see comments] Neuron. 1996a;17:655–667. doi: 10.1016/s0896-6273(00)80198-1. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth [see comments] Neuron. 1996b;17:641–654. doi: 10.1016/s0896-6273(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Ultsch A, Schloss P, Cox JA, Schmitt B, Betz H. Molecular cloning of an invertebrate glutamate receptor subunit expressed in Drosophila muscle. Science. 1991;254:112–114. doi: 10.1126/science.1681587. [DOI] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh BH, Parker L, Popescu D. Protein kinase C (PKC) isoforms in Drosophila. J Biochem (Tokyo) 2002;132:523–527. doi: 10.1093/oxfordjournals.jbchem.a003252. [DOI] [PubMed] [Google Scholar]
- Sigrist SJ, Thiel PR, Reiff DF, Schuster CM. The postsynaptic glutamate receptor subunit DGluR-IIA mediates long-term plasticity in Drosophila. J Neurosci. 2002;22:7362–7372. doi: 10.1523/JNEUROSCI.22-17-07362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist SJ, Reiff DF, Thiel PR, Steinert JR, Schuster CM. Experience-dependent strengthening of Drosophila neuromuscular junctions. J Neurosci. 2003;23:6546–6556. doi: 10.1523/JNEUROSCI.23-16-06546.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist SJ, Thiel PR, Reiff DF, Lachance PE, Lasko P, Schuster CM. Postsynaptic translation affects the efficacy and morphology of neuromuscular junctions. Nature. 2000;405:1062–1065. doi: 10.1038/35016598. [DOI] [PubMed] [Google Scholar]
- Singh S, Wu CF. Complete separation of four potassium currents in Drosophila. Neuron. 1989;2:1325–1329. doi: 10.1016/0896-6273(89)90070-6. [DOI] [PubMed] [Google Scholar]
- Song J, Wu L, Chen Z, Kohanski RA, Pick L. Axons guided by insulin receptor in Drosophila visual system. Science. 2003;300:502–505. doi: 10.1126/science.1081203. [DOI] [PubMed] [Google Scholar]
- Stewart BA, Schuster CM, Goodman CS, Atwood HL. Homeostasis of synaptic transmission in Drosophila with genetically altered nerve terminal morphology. J Neurosci. 1996;16:3877–3886. doi: 10.1523/JNEUROSCI.16-12-03877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KM, Scott K, Zuker CS, Rubin GM. The ryanodine receptor is essential for larval development in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2000;97:5942–5947. doi: 10.1073/pnas.110145997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Grinnell AD, Kidokoro Y. Hypertonicity-induced transmitter release at Drosophila neuromuscular junctions is partly mediated by integrins and cAMP/protein kinase A. J Physiol. 2002;538:103–119. doi: 10.1113/jphysiol.2001.012901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Kano M. Development of action potential in larval muscle fibers in Drosophila melanogaster. J Cell Physiol. 1977;93:383–388. doi: 10.1002/jcp.1040930309. [DOI] [PubMed] [Google Scholar]
- Thomas U, Kim E, Kuhlendahl S, Koh YH, Gundelfinger ED, Sheng M, Garner CC, Budnik V. Synaptic clustering of the cell adhesion molecule fasciclin II by discs-large and its role in the regulation of presynaptic structure. Neuron. 1997;19:787–799. doi: 10.1016/s0896-6273(00)80961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Hannan F, Zhu Y, Bernards A, Zhong Y. Neurofibromin regulates G protein-stimulated adenylyl cyclase activity. Nat Neurosci. 2002;5:95–96. doi: 10.1038/nn792. [DOI] [PubMed] [Google Scholar]
- Uzzan A, Dudai Y. Aminergic receptors in Drosophila melanogaster: responsiveness of adenylate cyclase to putative neurotransmitters. J Neurochem. 1982;38:1542–1550. doi: 10.1111/j.1471-4159.1982.tb06631.x. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M, Harryman-Samos C, Klingensmith J, Perrimon N, Nusse R. Mutations in the segment polarity genes wingless and porcupine impair secretion of the wingless protein [published erratum appears in EMBO J 1994 Jun 15;13(12):2950] Embo J. 1993;12:5293–5302. doi: 10.1002/j.1460-2075.1993.tb06225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh K, Hasan G. Disruption of the IP3 receptor gene of Drosophila affects larval metamorphosis and ecdysone release. Curr Biol. 1997;7:500–509. doi: 10.1016/s0960-9822(06)00221-1. [DOI] [PubMed] [Google Scholar]
- Waddell S, Armstrong JD, Kitamoto T, Kaiser K, Quinn WG. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell. 2000;103:805–813. doi: 10.1016/s0092-8674(00)00183-5. [DOI] [PubMed] [Google Scholar]
- Walter MF, Kiger JA., Jr The Dunce gene of Drosophila: roles of Ca2+ and calmodulin in adenosine 3′:5′-cyclic monophosphate-specific phosphodiesterase activity. J Neurosci. 1984;4:495–501. doi: 10.1523/JNEUROSCI.04-02-00495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Renger JJ, Griffith LC, Greenspan RJ, Wu CF. Concomitant alterations of physiological and developmental plasticity in Drosophila CaM kinase II-inhibited synapses. Neuron. 1994;13:1373–1384. doi: 10.1016/0896-6273(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Palmer G, Griffith LC. Regulation of Drosophila Ca2+/calmodulin-dependent protein kinase II by autophosphorylation analyzed by site-directed mutagenesis. J Neurochem. 1998;71:378–387. doi: 10.1046/j.1471-4159.1998.71010378.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wilson GF, Griffith LC. Calcium/calmodulin-dependent protein kinase II phosphorylates and regulates the Drosophila eag potassium channel. J Biol Chem. 2002;277:24022–24029. doi: 10.1074/jbc.M201949200. [DOI] [PubMed] [Google Scholar]
- White B, Osterwalder T, Keshishian H. Molecular genetic approaches to the targeted suppression of neuronal activity. Curr Biol. 2001;11:R1041–1053. doi: 10.1016/s0960-9822(01)00621-2. [DOI] [PubMed] [Google Scholar]
- Wolfgang WJ, Clay C, Parker J, Delgado R, Labarca P, Kidokoro Y, Forte M. Signaling through Gs alpha is required for the growth and function of neuromuscular synapses in Drosophila. Dev Biol. 2004;268:295–311. doi: 10.1016/j.ydbio.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Wu Y, Kawasaki F, Ordway RW. Properties of short-term synaptic depression at larval neuromuscular synapses in wild-type and temperature-sensitive paralytic mutants of Drosophila. J Neurophysiol. 2005;93:2396–2405. doi: 10.1152/jn.01108.2004. [DOI] [PubMed] [Google Scholar]
- Yoshihara M, Adolfsen B, Galle KT, Littleton JT. Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science. 2005;310:858–863. doi: 10.1126/science.1117541. [DOI] [PubMed] [Google Scholar]
- Zhang D, Kuromi H, Kidokoro Y. Activation of metabotropic glutamate receptors enhances synaptic transmission at the Drosophila neuromuscular junction. Neuropharmacology. 1999;38:645–657. doi: 10.1016/s0028-3908(98)00232-9. [DOI] [PubMed] [Google Scholar]
- Zhong Y. Mediation of PACAP-like neuropeptide transmission by coactivation of Ras/Raf and cAMP signal transduction pathways in Drosophila. Nature. 1995;375:588–592. doi: 10.1038/375588a0. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Wu CF. Altered synaptic plasticity in Drosophila memory mutants with a defective cyclic AMP cascade. Science. 1991;251:198–201. doi: 10.1126/science.1670967. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Wu CF. Differential modulation of potassium currents by cAMP and its long-term and short-term effects: dunce and rutabaga mutants of Drosophila. J Neurogenet. 1993;9:15–27. doi: 10.3109/01677069309167273. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Pena LA. A novel synaptic transmission mediated by a PACAP-like neuropeptide in Drosophila. Neuron. 1995;14:527–536. doi: 10.1016/0896-6273(95)90309-7. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Budnik V, Wu CF. Synaptic plasticity in Drosophila memory and hyperexcitable mutants: role of cAMP cascade. J Neurosci. 1992;12:644–651. doi: 10.1523/JNEUROSCI.12-02-00644.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]