Abstract
Aging is strongly correlated with decreases in neurogenesis, the process by which neural stem and progenitor cells proliferate and differentiate into new neurons. In addition to stem-cell-intrinsic factors that change within the aging stem-cell pool, recent evidence emphasizes new roles for systemic and microenvironmental factors in modulating the neurogenic niche. This article focuses on new insights gained through the use of heterochronic parabiosis models, in which an old mouse and a young circulatory system are joined. By studying the brains of both young and old mice, researchers are beginning to uncover circulating proneurogenic “youthful” factors and “aging” factors that decrease stem-cell activity and neurogenesis. Ultimately, the identification of factors that influence stem-cell aging may lead to strategies that slow or even reverse age-related decreases in neural-stem-cell (NSC) function and neurogenesis.
Heterochronic parabiosis models, in which an old mouse and a young mouse partially share a circulatory system, are leading to the identification of “youthful” and “aging” factors that influence stem-cell activity and neurogenesis.
Aging is a process by which cells alter their biochemical and genetic functions through cell-intrinsic and cell-extrinsic (microenvironment and systemic) factors. Aging manifests in many ways including dysregulation of tissue homeostasis and the gradual loss of regenerative capacity (Lopez-Otin et al. 2013). One of the main goals of regenerative medicine and stem-cell biology is to overcome the deleterious cellular effects of aging and, ultimately, to reverse them. Stem cells play a two-pronged role in tissue maintenance through divisions: on one hand, stem cells divide asymmetrically to produce a daughter cell that can differentiate and maintain tissue homeostasis and repair tissue damage; on the other hand, stem cells must divide asymmetrically to maintain themselves (“self-renewal”) and to provide a long-lasting source of cells with stem-like potential. To this end, one of the long-term effects associated with aging is the loss of cell “stemness” in aging tissue, either through stem cells dividing symmetrically into two new daughter cells and thus depleting the stem-cell pool, or by replicative senescence, whereby cells with stem-like potential exit the cell cycle and no longer contribute to tissue maintenance. In the either case, loss of stem cells can occur through cell-intrinsic effects or from loss of the microenvironmental niche that normally facilitates continued asymmetric divisions of stem cells and maintenance of homeostasis.
In the adult brain, stem cells persist in several discrete areas, contributing to adult neurogenesis. Neurogenesis is the process by which a proliferating cell exits the cell cycle and differentiates into a neuron, ultimately incorporating into the neuronal circuitry. Although it is widespread during embryogenesis, neurogenesis becomes increasingly restricted as the animal ages. Specifically in mice and humans, neurogenesis within the cortex of the brain is complete during the early postnatal period. However, there are at least two areas of the brain with well-established and substantial neurogenesis throughout the life of most mammals: the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the hippocampal dentate gyrus. Despite ongoing research into the cellular origins of neurogenesis, debate continues as to the stem-like cell within each of these two regions (Carlen et al. 2009; Ma et al. 2009; Bonaguidi et al. 2011, 2012; Encinas et al. 2011; Goritz and Frisen 2012; DeCarolis et al. 2013). Although the identity of the stem cell remains controversial, one thing is clear: Cells with stem-like and neurogenic potential persist in the SVZ and SGZ and new neurons are born throughout the mammalian life, including in humans (Eriksson et al. 1998; Sanai et al. 2004, 2011; Curtis et al. 2007). In rodent models, SGZ stem-like populations give rise to new neurons that migrate a short distance in to the dentate gyrus granular layer and become new granule cells. In contrast, new neuroblasts derived from SVZ stem cells migrate a long way in what is known as the rostral migratory stream, from the SVZ to the olfactory bulb (OB), where they become new inhibitory neurons. In the adult hippocampus, new immature neurons are highly plastic and hypothesized to have crucial roles in memory function (Clelland et al. 2009; Sahay et al. 2011; Aimone et al. 2014; Rangel et al. 2014). New olfactory neurons may play a role in olfactory memory or discrimination (Lazarini and Lledo 2011).
Some aspects of these adult neurogenic systems appear to be conserved in humans. In the human, the dentate gyrus has decreased levels of neurogenesis with age, but recent work by Frisen and colleagues suggests that the age-related decline is much more gradual than previously thought (Spalding et al. 2013). Neurogenic precursor cells have been observed in the dentate gyrus of humans up to 100 years of age (Knoth et al. 2010). On the other hand, in the human OB, recent evidence suggests negligible amounts of adult neurogenesis (Sanai et al. 2011; Wang et al. 2011; Bergmann et al. 2012; Ernst et al. 2014), despite robust neurogenesis in mouse, rat, and nonhuman primates (Kornack and Rakic 1999; Pencea et al. 2001). Additional work is needed in humans to observe and characterize stem-cell function and neurogenesis.
To this end, we define neural-stem-like cells as cells, which can divide asymmetrically to produce a daughter cell with neurogenic potential while maintaining itself in an undifferentiated state. Our current understanding is that stem-like cells divide infrequently in vivo (Morshead et al. 1994). We define progenitor cells as rapidly dividing cells with neurogenic potential that cannot divide continuously and thus deplete after multiple rounds of successive division (Encinas et al. 2011).
Regarding the important intersections of aging and neurogenesis, two key features are well established. First, as an animal ages, there are fewer dividing cells and fewer neurogenic precursor cells in neurogenic regions in rodents and in humans (Kuhn et al. 1996; Rao et al. 2005, 2006; Ben Abdallah et al. 2010; Spalding et al. 2013). Second, as an animal ages, fewer cells maintain neurogenic potential as they differentiate (Ahlenius et al. 2009; Encinas et al. 2011; Villeda et al. 2011); in other words, as an animal gets older, fewer cells become neurons and more cells become astrocytes, the major cell fate alternative for neural precursors that are differentiating after cell cycle exit (although see Rao et al. 2005; Hattiangady and Shetty 2008). There are a number of possible explanations for the age-related decrease in neurogenesis, including the progressive loss of stem cells (as suggested by Encinas et al. 2011 and others), the potentially reversible loss of replicative activity in stem cells (Lugert et al. 2010), or the decrease in permissive microenvironment surrounding the stem cells (as suggested by Bernal and Peterson 2011; Villeda et al. 2011). It has also recently been found that stem and progenitor cells may also regulate their own microenvironment via secreted factors (Mosher et al. 2012; Butti et al. 2014; Kirby et al. 2015). For example, Wyss-Coray and colleagues recently showed that undifferentiated adult neural stem and progenitor cells secrete up to a third of the vascular endothelial growth factor (VEGF) in the young adult dentate gyrus, and that loss of VEGF from just the stem and progenitor population causes long-term depletion of the neurogenic pool (Kirby et al. 2015). VEGF is a neurogenic niche factor known to decrease with aging, and this recent work suggests the possibility that the loss of local growth factors that regulate neurogenesis may not be solely caused by local astrocytes. However, the secretome of stem and progenitor cells and its contribution to aging of the brain remain relatively unexplored.
In this review, we focus on changes associated with the neural-stem-cell (NSC) niches in the brain. Our current understanding is limited regarding the cellular and molecular mechanisms behind the diminished capacity for neurogenesis in the adult brain, but likely there is strong interplay between the stem-like cells in the brain and their permissive neurogenic niche. Recent studies have attributed the decline in neuron production with loss of NSCs in the hippocampus (Encinas et al. 2011) and in the SVZ (Maslov et al. 2004). However, other studies have argued that the number of stem-like cells (Hattiangady and Shetty 2008; Lugert et al. 2010) and the number of neurosphere-forming cells remains constant with aging (Ahlenius et al. 2009). These results suggest that the neurogenic niche becomes less supportive (Luo et al. 2006; Ahlenius et al. 2009; Bouab et al. 2011) and/or that the NSCs shift into a quiescent state (Hattiangady and Shetty 2008; Lugert et al. 2010; Bouab et al. 2011). It is likely that a combination of factors contribute to the diminished neurogenic potential of the brain neurogenic niches, and we highlight what is known and emphasize that there is still much that we do not understand about aging and stem cells in the brain.
Although there has been a surge in research and reviews on aging and stem cells (Pollina and Brunet 2011; Artegiani and Calegari 2012; Conboy and Rando 2012; Lee et al. 2012; Lopez-Otin et al. 2013; Rolando and Taylor 2014), unfortunately, relatively little is known about aging NSCs, in part, because of technical challenges. Within the context of the adult mammalian brain, it has been difficult to identify stem cells from progenitor cells (DeCarolis et al. 2013; Knobloch et al. 2014). Further, protocols have been established to grow cells with stem-like potential in vitro as neurospheres or in monolayers (Reynolds and Weiss 1992; Morshead et al. 1994; Babu et al. 2007), but these protocols require removing cells from their in vivo microenvironmental niche. Given the complex interplay between neurogenic cells and their niches (Palmer et al. 2000; Shen et al. 2008; Tavazoie et al. 2008), new paradigms have emerged to explore age-related changes in neurogenesis within the complex stem-cell niche. The parabiosis model is one such method recently applied to the adult stem-cell niche and revealed numerous novel insights to how stem cells respond to an aging environment. In parabiosis, two mice are surgically connected and their circulatory systems become partially shared (Conboy et al. 2005). By pairing a young mouse with an older mouse in “heterochronic” pairings (Conboy and Rando 2012; Paul and Reddy 2014), researchers have explored age-associated changes in stem-cell function in muscle (Conboy et al. 2005; Brack et al. 2007), the heart (Loffredo et al. 2013), and, recently, the brain (Ruckh et al. 2012; Katsimpardi et al. 2014; Villeda et al. 2011, 2014). This work will explore briefly the cell-intrinsic changes associated with stem-cell decline in aging, then focus more broadly on the niche-specific effects that suggest that systemic circulation and the vasculature interposed within the neurogenic niche.
CELL-INTRINSIC CHANGES
Accumulating evidence suggests a battery of changes within stem cells that correlate with aging. For example, in nonneuronal systems like the hematopoietic system, stem cells reduce proliferation and differentiation capacity, accumulate marks of DNA damage, reduce activity of telomerase, change epigenetic marks, and alter transcription factor profiles (DeCarolis et al. 2008; Jaskelioff et al. 2011; Lopez-Otin et al. 2013). Importantly, although these age-related changes have been described in peripheral stem-cell pools, aging factors in the brain and NSC pools remain largely unexplored and poorly understood.
The best-characterized change related to neurogenesis is the significant decrease in proliferative capacity of the neurogenic regions with increasing age. The precipitous drop in proliferation of neurogenic cells was first characterized in the dentate gyrus of the rodent (Kuhn et al. 1996) and later in the OB (Molofsky et al. 2006), as noted above.
There is some limited evidence for cell-intrinsic aging of NSCs in adults. For example, recent studies suggest the accumulation of DNA damage (as indicated by genomic marks like γ-H2AX and 53BP1) in stem-cell populations like the hematopoietic system (Rossi et al. 2007) and in the dentate gyrus neurogenic niche (DeCarolis et al. 2014). Other reports have suggested the accumulation of mutations in DNA and genomic instability within the NSC pool with age (Mikheev et al. 2012; Dong et al. 2014) but additional research is needed. Other cell-intrinsic facets of aging have been characterized in peripheral stem-cell pools (Lopez-Otin et al. 2013), including decreased telomerase activity associated with shortened telomeres (Sahin and Depinho 2010), epigenetics changes (Webb et al. 2013; Brunet and Berger 2014), and asymmetric nonrandom chromosome segregation (Charville and Rando 2011). However, similar changes in NSC pools are under investigation. Specifically, telomerase activity has been an active area of research, and promoting telomerase activity increases neurogenesis in vitro and in vivo (Caporaso et al. 2003; Ferron et al. 2009; Jaskelioff et al. 2011; Liu et al. 2012). These studies suggest that reversing the declines in telomerase activity can prevent aging-associated declines in stem-cell function and cognition.
SYSTEMIC FACTORS AND THE “NICHE”
The neurogenic niche that surrounds adult neural stem and progenitor cells is populated by a variety of cell types, including astrocytes, microglia, mature neurons, and endothelial cells. All of these cell types show age-related changes that could impact adult NSCs. Both astrocytes and microglia show increased activation with age (Conde and Streit 2006; Norden and Godbout 2013; Sierra et al. 2014; Rodriguez-Arellano et al. 2015) potentially as a part of increased immune activation with aging (termed “inflammaging”). This activation likely changes their secretory profile. For example, secretion of neurogenic growth factors commonly derived from glia, such as fibroblast growth factor 2 (FGF-2) and VEGF, decreases prominently with age (Shetty et al. 2005; Bernal and Peterson 2011). The vasculature also may deteriorate with age, occupying less volume and providing less blood flow to brain regions, such as the SVZ (Katsimpardi et al. 2014). If any of these changes are necessary or sufficient for inducing age-related neurogenic decline remains unclear. However, the potential involvement of the vasculature and immune responses strongly suggests a third player in inducing the aging phenotype in NSCs: the systemic environment.
SYSTEMIC CIRCULATION
The role of systemic circulation in aging of NSCs appears to be particularly potent. Like aging muscle, the aging brain has a persistent population of resident stem cells that lose proliferative activity with age. Using the heterochronic parabiosis model, Villeda, Wyss-Coray, and colleagues (Villeda et al. 2011) have recently shown that this proliferative capacity can be bidirectionally modulated by the “age” of the systemic circulation. Heterochronic young mice have significantly reduced numbers of new, doublecortin (DCX+)-labeled neurons in the hippocampus, whereas their older counterpart shows partial restoration of new DCX+ neuron number (Villeda et al. 2011). A similar increase in progenitor proliferation with parabiosis to a younger mouse has been recently found in the SVZ (Katsimpardi et al. 2014), suggesting that the rejuvenating effects of young blood extend to both neurogenic niches. However, it remains unknown which neurogenic populations are most affected by systemic factors. Are these stem cells or progenitors (or both) that are induced to proliferate? The answer to this question has relevance to the sustainability of young blood as a therapeutic intervention because stimulation of only progenitors could wane over repeated treatments if the stem-cell population does not replenish the progenitor pool.
Although parabiosis allows for sharing of both soluble blood-derived proteins and circulating cell populations, it appears that the operative components of the blood for aging of stem cells are soluble plasma proteins. Parabiotic pairing with a GFP+ mouse reveals very little infiltration of GFP+ cells in the brain of a wild-type parabionts (Villeda et al. 2011), suggesting that direct cellular contribution is unlikely. Moreover, the antineurogenic effects of heterochronic pairing with an old mouse can be recapitulated by injection with plasma isolated from aged mice (Villeda et al. 2011). Several recent studies have implicated growth differentiation factor 11 (GDF11), a transforming growth factor β (TGF-β) family member, as a key blood-derived “youthful” factor that reverses aging of SVZ proliferation, vascular deterioration, skeletal muscle, and heart (Loffredo et al. 2013; Katsimpardi et al. 2014; Sinha et al. 2014). Recent work suggests that the role of systemic GDF11 in aging of other tissues may differ from the brain (Egerman et al. 2015). CCL11, a circulating immune cytokine, has also been shown to be associated with age-related decline in hippocampal neurogenesis (Villeda et al. 2011).
The question of which factors drive the rejuvenating properties of young blood remains open. Most likely, the rejuvenating cocktail is complex and possibly tissue-specific such that factors or mechanisms that rejuvenate myogenic progenitors may not be the same ones that rejuvenate neural progenitors. More broadly, the reliance on soluble factors may even differ for different tissues and cell types. Although muscle cell aging is rejuvenated by young serum-derived proteins much like CNS stem cells (Conboy et al. 2005; Brack et al. 2007), oligodendrocyte progenitor cells (OPCs) appear to be rejuvenated by the cellular component of young blood (Ruckh et al. 2012). Heterochronic parabiosis with a young mouse rescues age-related deficits in OPC-mediated remyelination after injury, but the rescue relies on recruitment of circulating young monocytes to clear myelin debris that then allows resident old OPCs to function better (Ruckh et al. 2012).
Part of determining what factors mediate systemic regulation of CNS stem cells is determining which cells respond directly to soluble factors. For example, although effects of CCL11 on isolated hippocampal NSCs suggest that these cells respond negatively to this “aging” factor (Villeda et al. 2011), numerous other aspects of hippocampal function are impacted by plasma-borne factors and aging, including hippocampal long-term potentiation (LTP) (Villeda et al. 2011, 2014), dendritic spine density (Villeda et al. 2014), immediate early gene expression (Villeda et al. 2014), and vascularization (Katsimpardi et al. 2014). Where do plasma proteins act first? The NSCs? The vasculature? The mature neurons and astrocytes? All these pieces of the neurogenic niche rely on each other extensively, and alterations in one cell population will likely echo through the others. The order of cause and effect is not yet clear. Teasing apart the varied players in maintaining a neurogenic niche on an aging systemic background remains a challenge.
THE VASCULAR NICHE
The reliance of the aging of adult NSCs on circulating plasma-borne proteins is perhaps not surprising given the highly vascularized nature of both the SVZ and SGZ neurogenic niches. Adult NSCs in both the SVZ and SGZ reside in complex, multicell niches in close association with local capillaries.
The SVZ is covered by a planar vascular plexus spanning its entire length, which is quite distinct from the more segmented capillary supply found in other, nonneurogenic brain regions, such as the cortex (Tavazoie et al. 2008). Proliferating SVZ progenitors and stem cells are found in close association with this vascular supply, especially when repopulating after depletion by antimitotic treatment. The SVZ may also show unique permeability of the blood–brain barrier, allowing small molecules to diffuse through gaps between the astrocytic endfeet that typically insulate the brain from the circulating factors (Tavazoie et al. 2008).
In the SGZ, proliferating cells are similarly found in close apposition to blood vessels (Palmer et al. 2000). There is an intimate association between the blood vasculature and neurogenic progenitors in the SGZ (Palmer et al. 2000; Pereira et al. 2007), which has been tied to the neurogenic response after exercise. Specifically, circulating increases in both VEGF and insulin-like growth factor 1 (IGF-1) have been suggested to drive exercise-induced increases in progenitor proliferation (Trejo et al. 2001; Fabel et al. 2003).
The vasculature may not simply be a means of conveying blood-borne factors, though, as it shows prominent age-related degradation that may be a driving factor in cognitive decline and neurodegenerative disease (Tarumi and Zhang 2014). Recent work by Katsimpardi, Rubin, and colleagues (2014) suggests that this decline in vascularization in the SVZ is closely linked with the decline in neurogenesis in this area and that both declines can be rescued by exposure to a young systemic environment. However, it remains unclear whether vascular endothelial cells and neural progenitors are both responding to systemic factors directly or to some indirect signal from each other or another niche cell type. The enhanced contact of adult neural stem and progenitor cells with the vasculature increases progenitor cell exposure both to the systemic circulation and to the vascular endothelium itself, which secretes a variety of proteins that impact NSC maintenance (Shen et al. 2004). The secretory profile of endothelium could change depending on the composition of the blood supply and thereby drive changes in closely associated stem and progenitor cells.
CONCLUSIONS
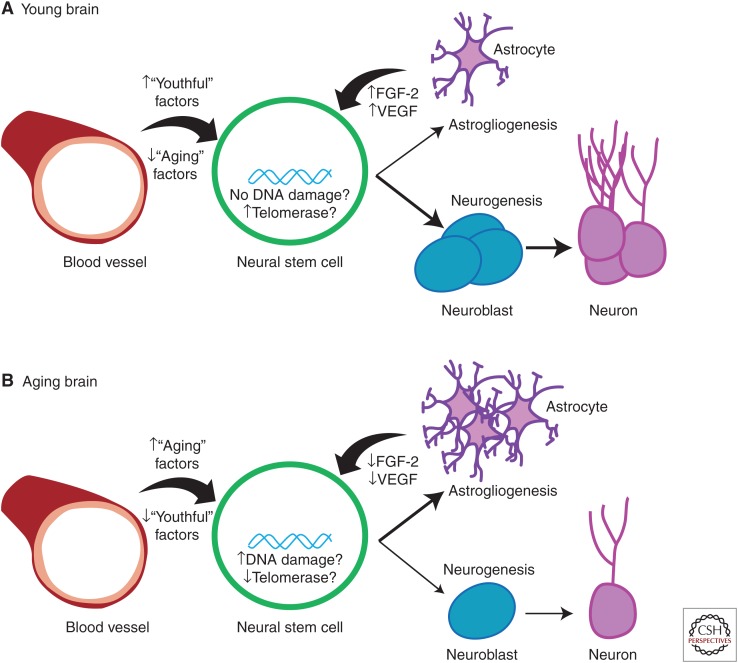
Taken together, current literature supports that a combination of factors contribute to changes in NSC function in aging, summarized in Figure 1. In the young brain, the local microenvironment combined with young stem cells produce an abundance of new neurons. Circulating “youthful” factors lead to the activation of cAMP-response element-binding protein (Creb), which promotes neurogenesis (Villeda et al. 2011). In a similar vein, Rubin and colleagues identified GDF11 as a circulating factor promoting vascularization and neurogenesis (Katsimpardi et al. 2014). The niche astrocytes in the young brain also provide abundant trophic support to promote neurogenesis, including FGF-2, VEGF, and IGF (Shetty et al. 2005); as the brain ages, growth factor secretion decreases and, consequently, so does neurogenesis. Taken together, these results suggest that some (though likely not all) age-mediated decreases in neurogenesis can be rescued and perhaps even reverse the cognitive decline associated with aging.
Figure 1.
Hypothesized aging effects on neural stem cells. (A) In the young brain, neural stem cells (green) have low amounts of DNA damage but high amounts of telomerase activity. Astrocytes (purple) provide trophic support and circulating “youthful” factors from the blood (red) support a neurogenic environment, in which neural stem cells divide into neuroblasts (blue), which mature into neurons (magenta). (B) In contrast, in the aging brain, neural stem cells accumulate DNA damage and show decreased telomerase activity. Further, astrocytes provide less trophic support and “aging” factors increase in concentration in blood. In the aging brain, therefore, fewer neuroblasts are produced and neurogenesis is decreased; on the other hand, more astrocytes are produced. FGF, Fibroblast growth factor; VEGF, vascular endothelial growth factor.
Footnotes
Editors: S. Jay Olshansky, George M. Martin, and James L. Kirkland
Additional Perspectives on Aging available at www.perspectivesinmedicine.org
REFERENCES
- Ahlenius H, Visan V, Kokaia M, Lindvall O, Kokaia Z. 2009. Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. J Neurosci 29: 4408–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH. 2014. Regulation and function of adult neurogenesis: From genes to cognition. Physiol Rev 94: 991–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artegiani B, Calegari F. 2012. Age-related cognitive decline: Can neural stem cells help us? Aging 4: 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu H, Cheung G, Kettenmann H, Palmer TD, Kempermann G. 2007. Enriched monolayer precursor cell cultures from micro-dissected adult mouse dentate gyrus yield functional granule cell-like neurons. PLoS ONE 2: e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP. 2010. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging 31: 151–161. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Liebl J, Bernard S, Alkass K, Yeung MS, Steier P, Kutschera W, Johnson L, Landen M, Druid H, et al. 2012. The age of olfactory bulb neurons in humans. Neuron 74: 634–639. [DOI] [PubMed] [Google Scholar]
- Bernal GM, Peterson DA. 2011. Phenotypic and gene expression modification with normal brain aging in GFAP-positive astrocytes and neural stem cells. Aging Cell 10: 466–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. 2011. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145: 1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Song J, Ming GL, Song H. 2012. A unifying hypothesis on mammalian neural stem cell properties in the adult hippocampus. Curr Opin Neurobiol 22: 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouab M, Paliouras GN, Aumont A, Forest-Berard K, Fernandes KJ. 2011. Aging of the subventricular zone neural stem cell niche: Evidence for quiescence-associated changes between early and mid-adulthood. Neuroscience 173: 135–149. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. 2007. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317: 807–810. [DOI] [PubMed] [Google Scholar]
- Brunet A, Berger SL. 2014. Epigenetics of aging and aging-related disease. J Gerontol A Biol Sci Med Sci 69: S17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butti E, Cusimano M, Bacigaluppi M, Martino G. 2014. Neurogenic and non-neurogenic functions of endogenous neural stem cells. Front Neurosci 8: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso GL, Lim DA, Alvarez-Buylla A, Chao MV. 2003. Telomerase activity in the subventricular zone of adult mice. Mol Cell Neurosci 23: 693–702. [DOI] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Goritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabe-Heider F, Yeung MS, Naldini L, et al. 2009. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci 12: 259–267. [DOI] [PubMed] [Google Scholar]
- Charville GW, Rando TA. 2011. Stem cell ageing and non-random chromosome segregation. Philos Trans R Soc Lond B Biol Sci 366: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, et al. 2009. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325: 210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. 2012. Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches. Cell Cycle 11: 2260–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. 2005. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433: 760–764. [DOI] [PubMed] [Google Scholar]
- Conde JR, Streit WJ. 2006. Microglia in the aging brain. J Neuropathol Exp Neurol 65: 199–203. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, van Roon-Mom WM, Bjork-Eriksson T, Nordborg C, et al. 2007. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science 315: 1243–1249. [DOI] [PubMed] [Google Scholar]
- DeCarolis NA, Wharton KA Jr, Eisch AJ. 2008. Which way does the Wnt blow? Exploring the duality of canonical Wnt signaling on cellular aging. Bioessays 30: 102–106. [DOI] [PubMed] [Google Scholar]
- DeCarolis NA, Mechanic M, Petrik D, Carlton A, Ables JL, Malhotra S, Bachoo R, Gotz M, Lagace DC, Eisch AJ. 2013. In vivo contribution of nestin- and GLAST-lineage cells to adult hippocampal neurogenesis. Hippocampus 23: 708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarolis NA, Rivera PD, Ahn F, Amaral WZ, LeBlanc JA, Malhotra S, Shih HY, Petrik D, Melvin N, Chen BP, et al. 2014. 56Fe particle exposure results in a long-lasting increase in a cellular index of genomic instability and transiently suppresses adult hippocampal neurogenesis. Life Sci Space Res (Amst) 2: 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CM, Wang XL, Wang GM, Zhang WJ, Zhu L, Gao S, Yang DJ, Qin Y, Liang QJ, Chen YL, et al. 2014. A stress-induced cellular aging model with postnatal neural stem cells. Cell Death Dis 5: e1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, Mallozzi C, Jacobi C, Jennings LL, Clay I, et al. 2015. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab 22: 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. 2011. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8: 566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. 1998. Neurogenesis in the adult human hippocampus. Nat Med 4: 1313–1317. [DOI] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisen J. 2014. Neurogenesis in the striatum of the adult human brain. Cell 156: 1072–1083. [DOI] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. 2003. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci 18: 2803–2812. [DOI] [PubMed] [Google Scholar]
- Ferron SR, Marques-Torrejon MA, Mira H, Flores I, Taylor K, Blasco MA, Farinas I. 2009. Telomere shortening in neural stem cells disrupts neuronal differentiation and neuritogenesis. J Neurosci 29: 14394–14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goritz C, Frisen J. 2012. Neural stem cells and neurogenesis in the adult. Cell Stem Cell 10: 657–659. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. 2008. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging 29: 129–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadinanos J, et al. 2011. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 469: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. 2014. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344: 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby ED, Kuwahara AA, Messer RL, Wyss-Coray T. 2015. Adult hippocampal neural stem and progenitor cells regulate the neurogenic niche by secreting VEGF. Proc Natl Acad Sci 112: 4128–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch M, von Schoultz C, Zurkirchen L, Braun SM, Vidmar M, Jessberger S. 2014. SPOT14-positive neural stem/progenitor cells in the hippocampus respond dynamically to neurogenic regulators. Stem Cell Rep 3: 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, Kempermann G. 2010. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 5: e8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. 1999. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci 96: 5768–5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. 1996. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci 16: 2027–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarini F, Lledo PM. 2011. Is adult neurogenesis essential for olfaction? Trends Neurosci 34: 20–30. [DOI] [PubMed] [Google Scholar]
- Lee SW, Clemenson GD, Gage FH. 2012. New neurons in an aged brain. Behav Brain Res 227: 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Hu Y, Zhu L, Chen C, Zhang Y, Sun W, Zhou Q. 2012. Overexpression of the mTERT gene by adenoviral vectors promotes the proliferation of neuronal stem cells in vitro and stimulates neurogenesis in the hippocampus of mice. J Biomed Res 26: 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL, et al. 2013. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153: 828–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. 2013. The hallmarks of aging. Cell 153: 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G, Taylor V, Giachino C. 2010. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 6: 445–456. [DOI] [PubMed] [Google Scholar]
- Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. 2006. The aging neurogenic subventricular zone. Aging Cell 5: 139–152. [DOI] [PubMed] [Google Scholar]
- Ma DK, Bonaguidi MA, Ming GL, Song H. 2009. Adult neural stem cells in the mammalian central nervous system. Cell Res 19: 672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. 2004. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J Neurosci 24: 1726–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheev AM, Ramakrishna R, Stoll EA, Mikheeva SA, Beyer RP, Plotnik DA, Schwartz JL, Rockhill JK, Silber JR, Born DE, et al. 2012. Increased age of transformed mouse neural progenitor/stem cells recapitulates age-dependent clinical features of human glioma malignancy. Aging Cell 11: 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. 2006. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 443: 448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. 1994. Neural stem cells in the adult mammalian forebrain: A relatively quiescent subpopulation of subependymal cells. Neuron 13: 1071–1082. [DOI] [PubMed] [Google Scholar]
- Mosher KI, Andres RH, Fukuhara T, Bieri G, Hasegawa-Moriyama M, He Y, Guzman R, Wyss-Coray T. 2012. Neural progenitor cells regulate microglia functions and activity. Nat Neurosci 15: 1485–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Godbout JP. 2013. Review: Microglia of the aged brain: Primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol 39: 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. 2000. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425: 479–494. [DOI] [PubMed] [Google Scholar]
- Paul SM, Reddy K. 2014. Young blood rejuvenates old brains. Nat Med 20: 582–583. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Freedman LJ, Luskin MB. 2001. Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp Neurol 172: 1–16. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. 2007. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci 104: 5638–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollina EA, Brunet A. 2011. Epigenetic regulation of aging stem cells. Oncogene 30: 3105–3126. [DOI] [PubMed] [Google Scholar]
- Rangel LM, Alexander AS, Aimone JB, Wiles J, Gage FH, Chiba AA, Quinn LK. 2014. Temporally selective contextual encoding in the dentate gyrus of the hippocampus. Nat Commun 5: 3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. 2005. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci 21: 464–476. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. 2006. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell 5: 545–558. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. 1992. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255: 1707–1710. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A. 2015. Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience 10.1016/j.neuroscience.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Rolando C, Taylor V. 2014. Neural stem cell of the hippocampus: Development, physiology regulation, and dysfunction in disease. Curr Top Dev Biol 107: 183–206. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. 2007. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447: 725–729. [DOI] [PubMed] [Google Scholar]
- Ruckh JM, Zhao JW, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJ. 2012. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 6: 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. 2011. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472: 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E, Depinho RA. 2010. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 464: 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, et al. 2004. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 427: 740–744. [DOI] [PubMed] [Google Scholar]
- Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, et al. 2011. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 478: 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. 2004. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304: 1338–1340. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. 2008. Adult SVZ stem cells lie in a vascular niche: A quantitative analysis of niche cell–cell interactions. Cell Stem Cell 3: 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. 2005. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: Role of astrocytes. Glia 51: 173–186. [DOI] [PubMed] [Google Scholar]
- Sierra A, Beccari S, Diaz-Aparicio I, Encinas JM, Comeau S, Tremblay ME. 2014. Surveillance, phagocytosis, and inflammation: How never-resting microglia influence adult hippocampal neurogenesis. Neural Plast 2014: 610343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, et al. 2014. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 344: 649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, et al. 2013. Dynamics of hippocampal neurogenesis in adult humans. Cell 153: 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarumi T, Zhang R. 2014. Cerebral hemodynamics of the aging brain: Risk of Alzheimer disease and benefit of aerobic exercise. Front Physiol 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. 2008. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 3: 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. 2001. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci 21: 1628–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, et al. 2011. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477: 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, et al. 2014. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med 20: 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Liu F, Liu YY, Zhao CH, You Y, Wang L, Zhang J, Wei B, Ma T, Zhang Q, et al. 2011. Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res 21: 1534–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AE, Pollina EA, Vierbuchen T, Urban N, Ucar D, Leeman DS, Martynoga B, Sewak M, Rando TA, Guillemot F, et al. 2013. FOXO3 shares common targets with ASCL1 genome-wide and inhibits ASCL1-dependent neurogenesis. Cell Rep 4: 477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]