Abstract
It is virtually undisputed that IGF-I promotes cell growth and survival. However, the presence of several IGF-I isoforms, vast numbers of intracellular signaling components, and multiple receptors results in a complex and highly regulated system by which IGF-I actions are mediated. IGF-I has long been recognized as one of the critical factors for coordinating muscle growth, enhancing muscle repair, and increasing muscle mass and strength. How to optimize this panoply of pathways to drive anabolic processes in muscle as opposed to aberrant growth in other tissues is an area that deserves focus. This review will address how advances in the bioavailability, potency, and tissue response of IGF-I can provide new potential directions for skeletal muscle therapeutics.
Keywords: anabolism, hypertrophy, satellite cells, isoforms, post-translational processing
Introduction
Insulin-like growth factor I (IGF-I) is critical for the growth and development of many tissues. For skeletal muscle, IGF-I coordinates with additional growth factors to promote myoblast proliferation, differentiation, and fiber formation during normal growth as well as during regeneration after injury. These actions can also result in muscle hypertrophy, and this boost in muscle mass improves the functional capacity of skeletal muscle. Thus, IGF-I is a central therapeutic target for enhancing muscle function in aging and disease, and for accelerating repair following acute damage.
To increase IGF-I levels for promoting increased muscle growth and regenerative capacity, the most straightforward approach is to deliver IGF-I systemically. Several clinical trials have assessed the efficacy of systemic delivery of recombinant IGF-I in patients who could benefit from strength gains, summarized in Table 1. These include the aging population, patients with growth hormone deficiency, and those who suffer from amyotrophic lateral sclerosis and myotonic dystrophy1–11. In addition, recombinant GH has also been utilized in a subset of these patient groups to allow for both direct actions of GH as well as those mediated through IGF-I to provide benefit. For all of the above indications, IGF-I has been delivered in limiting amounts, for chronic treatment with such a potent growth factor poses a potential carcinogenic risk. Thus, these trials have produced mixed results, because the ability for IGF-I to provide any benefit to skeletal muscle is constrained by both the low level of protein administered as well as the limited distribution of IGF-I to the muscle by the circulation4,5,12. While short-term treatments for rehabilitation from acute injury can introduce higher doses with lower long-term consequences, efficient delivery to the target is still a hurdle. Therefore, new strategies are needed to allow for heightened levels of IGF-I where it is needed, while avoiding the systemic risks. This review will cover the existing evidence supporting new strategies for IGF-I therapies, focusing on bioavailability, potency, and tissue response.
Table 1.
Clinical trials for IGF-I and muscle therapy
| Therapeutic Target | Subjects | IGF-I Isoform | Delivery Method | Outcome |
|---|---|---|---|---|
| Sarcopenia | ||||
| Elderly women (N=14) | rhIGF-I | 15–60 ug/kg SQ BID 1 month | Increased whole body and muscle protein synthesis2 | |
| Healthy, nonobese, postmenopausal women over 60 yr of age (N=16) | rhIGF-I | 15 ug/kg SQ BID 1 year | No change in body composition, bone density, strength, or memory4 | |
| GH Deficiency | ||||
| Growth hormone deficient young adults (N=8) | rhIGF-I | 60 ug/kg SQ BID 8 weeks | Increased fat-free mass and protein synthesis rates; no effect on skeletal muscle strength90 | |
| Muscle Wasting/Cachexia | ||||
| Patients with AIDS-associated wasting (N=60) | rhIGF-I and GH | 0 or 5 mg IGF SQ BID 0 or 1.4 mg GH SQ 1D 12 weeks | Single and combined treatments increased lean body mass, with combined GH/rhIGF-I providing the most benefit; GH only improved muscle strength91 | |
| Diabetes/Metabolism | ||||
| Obese postmenopausal women (N=33) | rhIGF-I and GH | 0 or 15 ug/kg IGF SQ BID 0 or 25 ug/kg GH SQ 1D 12 weeks | Enhanced fat loss92 | |
| Type 2 diabetes mellitus subjects (N=8) | rhIGF-I | 80 ug/kg SQ BID 7 days | Improved hepatic and muscle insulin sensitivity3 | |
| Neuromuscular Disease | ||||
| Amyotrophic lateral sclerosis (ALS) patients (N=266) | rhIGF-I | 5 or 10 ug/kg SQ ID 9 months | Dose-dependent deceleration of pathological progression5 | |
| Amyotrophic lateral sclerosis (ALS) patients (N=124) | rhIGF-1 | 10 ug/kg SQ ID 9 months | No significant differences in disease progression1 | |
| Amyotrophic lateral sclerosis (ALS) patients (N=9) | rhIGF-I | 0.5–3 μg/kg Intrathecal every 2 weeks for 40 weeks | Modest but significant beneficial functional effects8 | |
| Amyotrophic lateral sclerosis (ALS) patients (N=330) | rhIGF-I | 3 μg/kg SQ BID 2 years | No differences between treatment & placebo groups; Major effect of hypoglycemia9 | |
| Myotonic dystrophy type 1 patients (N=15) | rhIGF-I/rhIGFBP3 (IPLEX) | 0.5–2 mg/kg SQ 1D 24 weeks | Increased lean body mass; improved metabolism; no increased muscle strength93 | |
The myriad of IGF-I forms: clues to optimization or a red herring?
Alternative splicing of the highly conserved Igf1 gene produces multiple isoforms of IGF-I (Figure 1). All isoforms bear the same mature IGF-I protein, but differ in the carboxy-terminal extensions, called the E-peptides13–16. Utilization of exons 1 and 2 results in class I and II precursors, respectively. These exons encode, apart from the 5′ untranslated region, a portion of the signal peptide(s) for secretion, and their use seems dependent on two different promoters that are regulated in a tissue-specific manner17. Exons 3 and 4 are invariant, and encode the remaining part of the signal peptide(s), the mature IGF-I peptide, and a portion of the E peptide(s). The remaining sequence of the E peptides is encoded by exons 5 or 6. Class A IGF-I transcripts skip exon 5 and retain exon 6, and are the most prominent class. In humans, Class B transcripts utilize only exon 5, while class C is produced by the inclusion of a portion of human exon 5 and an internal splice site within it that causes a frame shift and premature termination in exon 6. The resultant peptide shares a high homology to rodent class B IGF-I, which includes all of the rodent exon 5 followed by exon 614,15,18. All possible combinations between N-terminal signal peptide usage and C-terminal E peptide can occur in different IGF-I precursors.
Figure 1.
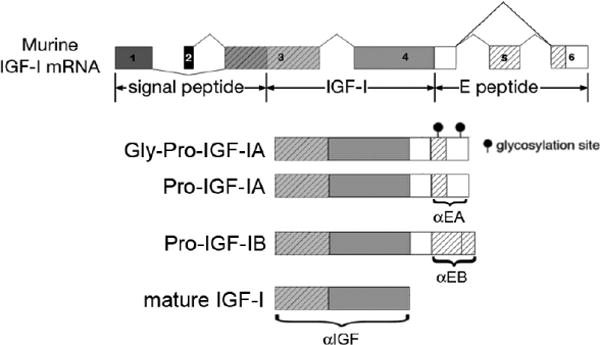
Alternative splicing of igf1 produces multiple isoforms of the protein. However, all transcripts encode for the same mature IGF-I. IGF-IA has 2 N-glycosylation sites in the rodent E peptide, and 1 sire is conserved in humans. Antibodies recognizing the E-peptide (Philippou et al. 2008; Brisson and Barton, 2012) or IGF-I are noted, which can be used to examine specific detection of the IGF-I isoforms.
Regardless of the isoform transcribed, a pre-pro-peptide is translated, which consists of a Class I or II signal peptide directing secretion, the mature IGF-I peptide, and a C-terminal E-peptide extension19. Following cleavage of the signal peptide, the pro-IGF-I (mature IGF-I plus an E-peptide) can be subjected to additional processing prior to secretion. This includes cleavage of the E-peptide by intracellular proteases of the pro-protein convertase family to release mature IGF-I for secretion20, maintenance of pro-IGF-I to be secreted without cleavage21–24, or N-glycosylation in the E-peptide of the predominant IGF-I isoform (IGF-IA)25, and then secretion. Hence, three forms of IGF-I protein could exist in the extracellular milieu: mature IGF-I, non-glycosylated pro-IGF-I, and glycosylated-pro-IGF-I. Figure 2 schematizes the post-translational processing steps associated with production of the IGF-I forms.
Figure 2.
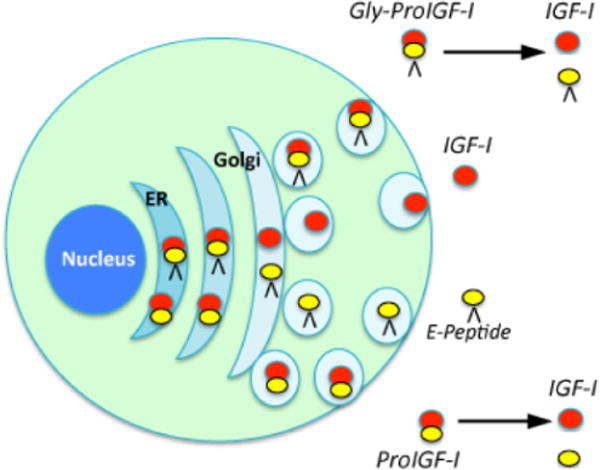
Pro-IGF-IA can be glycosylated in the endoplasmic reticulum (ER), followed by pro-protein convertase cleavage in the Golgi to release mature IGF-I from the C-terminal E peptide. Mature IGF-I is then secreted. Alternate pathways could avoid glycosylation or cleavage and result in additional secreted forms of IGF-I. These, upon secretion, could be cleaved in the ECM to release mature IGF-I.
The splicing and processing of the gene encoding IGF-I is well characterized and it is generally accepted that the mature IGF-I peptide is the main mediator of IGF-I actions via the type 1 IGF receptor (IGF-IR)26,27. There has been an ongoing debate for the last 25 years regarding the biological significance of the E-peptides, and whether or not they represent additional growth factors generated from the same Igf1 gene, or if they are simply a by-product of the post-translational processing for IGF-I. The dialog has been particularly heated in the muscle community, for the actions of IGF-I are critical to the formation and growth of this tissue, and one E-peptide (human EC/rodent EB) has been implicated as a highly potent growth factor that acts independently of IGF-I. The early and conflicting observations of E-peptide activity have plagued forward progress to understand why the E-peptides have been retained across all species studied. Like a vestigial organ, it is possible that there is little evolutionary pressure on this sequence. However, if this were the case, then the E-peptides, particularly the most abundant EA peptide, would lack significant conservation across species. Sequence homology is strong for EA, although other splice forms do not exhibit the same level of similarity18. Thus, the retention of this sequence argues for a biological use. Proposed actions of the E peptides include improved localization, increased potency, and independent activity28, and this possibility may establish new potential ways to optimize IGF-I activity, where novel actions of IGF-I and the E-peptides could have significant impact on muscle mass and repair. We will discuss each of these proposed properties in the context of strategies to improve IGF-I actions in skeletal muscle.
Muscle’s potential for retaining IGF-I
Many tissues in the body produce IGF-I, and skeletal muscle is no exception. We know that both liver and muscle contribute to circulating IGF-I29–31, and that once IGF-I arrives in the bloodstream, it is difficult to imagine how one particular form could target a specific tissue. Most of the circulating IGF-I is produced by the liver, yet ablation of this source in mice (the LID mouse, for “Liver IGF-I Deficient”) does not have a dramatic effect on muscle size, nor on general postnatal body growth30,31, indicating that skeletal muscles can rely on other pools of IGF-I. The general consensus is that limiting liver IGF-I, or the proteins in its circulating ternary complex does not control the growth of many of the tissues that make their own IGF-I, such as skeletal muscles, long bones, or brain, supporting the hypothesis that local IGF-I is sufficient to sustain normal growth of these tissues. Muscles themselves produce both IGF-I and IGF-II32, which are most likely sufficient for maintaining muscle mass. However, the capacity of muscle utilization surpasses its normal production. Studies in which muscle IGF-I production is increased by transgenic or viral over-expression33–35 take advantage of the mismatch between the capacity of muscle to use IGF-I and its production to promote functional hypertrophy. In a complementary experiment, we have shown that retention of locally produced IGF-I is important for muscle growth. By ablating the stress protein Glucose Regulated Protein 94 (GRP94; gene name HSP90B1) in striated muscle, which is required for IGF-I and IGF-II production36, muscle and body mass decreased dramatically29, presumably because the local source of IGF-I is key to maintaining normal growth. Finally, boosting circulating levels of IGF-I by increasing liver production37 or through daily injections of recombinant IGF-I increases body (and muscle) weight, supporting the premise that circulating IGF-I can enter the local tissue environment and have enhanced anabolic effects. Likewise, we can rescue the growth defect in GRP94 null muscle through intraperitoneal injections of recombinant IGF-I29. These studies confirm that skeletal muscle is both a sink and source for IGF-I, and this raises the possibility that enhancing either production or retention of IGF-I in this tissue could lead to increased muscle mass.
Improving IGF-I storage in the extracellular matrix
In order for IGF-I to drive muscle hypertrophy, it must be in close proximity to IGF-I receptors. Granted, it is clear that muscle produces a local supply of IGF-I that is secreted from the fibers to the extracellular matrix (ECM). What are the features of the protein that retain it in the ECM? Classically, the most prominent stabilizer and localizer is the pool of IGF binding proteins (IGFBPs). Because mature IGF-I half-life is brief, its association with IGFBPs keeps the muscle pool local. The IGFBPs, in turn, are thought to bind to the ECM. The affinity of IGF-I for the IGFBPs is sufficiently high that the binding proteins must undergo protease cleavage to release an IGF-I molecule for receptor binding. The IGFBPs have a range of affinities for IGF-I, and have different proteases for their cleavage (reviewed in38). These properties can then fine-tune the availability of IGF-I for activity.
An emerging concept is the ability for some of the IGF-I forms to bind directly to the ECM, potentially without the use of IGFBP stabilization. In one study, forms of IGF-I delivered to de-cellularized matrix resulted in the enhanced association of pro-IGF-I, but not mature IGF-I, on sections39. The authors asserted that it was the charge of the E-peptide that bound to matrix, which is highly positive for both of the isoforms tested (IGF-IA and IGF-IC). They went further to show that the glycosylation of the IGF-IA C-terminus disrupted this association. This observation provides one explanation for why the C-terminal extension has been preserved: to improve bioavailability of IGF-I through increased ECM retention. However, there are multiple issues that conflict with this model. First, when production of E peptides is performed by viral mediated gene transfer, neither of these forms accumulate in the matrix, but instead are found within muscle fibers40. Second, the E peptide derived from the human IGF-IB isoform appears in the nucleus, not the ECM, even though it is even more positively charged than other E peptides41. Finally, the disruption of IGF-I/ECM association by glycosylation cannot not explain why there is an enrichment of glycosylated pro-IGF-I in muscle extracts23. Although there is no direct evidence to date, both forms, pro-IGF-I and glycosylated pro-IGF-I, may enhance localization of IGF-I, and aid in the paracrine actions of IGF-I for muscle, rather than mature IGF-I. Further, because pro-IGF-I in the circulation originates, in part, from skeletal muscle, it suggests that glycosylated pro-IGF-I may have better retention in the tissue than the non-glycosylated form. These observations, while preliminary, raise the possibility for improving the storage of IGF-I in muscle to provide greater bioavailability. This is a new avenue for therapeutic strategies to build muscle.
Altering Potency of IGF-I
In addition to enhancing retention of IGF-I in the ECM, the E-peptides may alter IGF-I receptor activation in other ways. We have found in cell-based assays the pro-IGF-I causes ~30% more IGF-IR phosphorylation than equivalent amounts of mature IGF-I23. This is a modest improvement, to be sure. However, this property is independent of any alterations in ECM localization. Thus, considering the increased potency combined with the greater availability of pro-IGF-I39, there is potential for optimizing therapies through the delivery or expression of the pro-IGF-I form. Exposure of myoblasts or muscle to the E-peptides themselves has had mixed results depending upon the nature of the experiment. On one hand, the E-peptides provide a boost to IGF-I mediated signaling42, but only nonexistent to minimal hypertrophy occurs in vivo40,43. Further, in our hands, overexpression of EB causes diminished force production, suggesting that pursuing an E-peptide-only strategy for driving increased muscle mass may not be beneficial for function.
In contrast to the activity of mature and pro-IGF-I, we found that glycosylated pro-IGF-I was inefficient at receptor activation in vitro23. How, then, can we explain the multipronged benefits to muscle mass, strength and regenerative capacity in mice expressing IGF-IA, in which the predominant form that is stored is glycosylated pro-IGF-I33,35? We have speculated that when the C-terminus is glycosylated it must be cleaved from the mature growth factor to release it for receptor binding. The storage of glycosylated pro IGF-I can exist because the proteases responsible for removing it may be active for only a brief, but necessary, period. We have recently reported indirect evidence for this model. Using muscle reloading after disuse as a trigger for promoting muscle recovery, we found that while both mature IGF-I and glycosylated IGF-I could enhance rescue, mature IGF-I did so more rapidly44. Further, mature IGF-I was evident in the glycosylated pro-IGF-I muscles after 1 week of reloading, which was the point when force and mass improved. These proteases may differ from those that release mature IGF-I from IGFBPs. Our working hypothesis is that they are part of the family of pro-protein convertases (PCs) that are also responsible for intracellular processing of IGF-I45. As previously described43, a pentabasic motif is retained in all classes of pro-IGF-I which contains two putative cleavage sites recognized by PCs, Arg71 and Arg77. The remaining Arginine residue at position 71 is thought to be removed by a carboxypeptidase. Of the 9 PCs, 7 are secreted or shed, and are possible candidates for cleaving IGF-I outside of the cell. However, there is no direct evidence that pro-IGF-I cleavage occurs extracellularly. Two candidates are at the forefront. Furin, an ubiquitous PC, is expressed constitutively in muscle cells and can be found in the Golgi as well as in the extracellular space, so it could cleave pro-IGF-I in either area. A second protease of this family, proprotein convertase subtilisin/kexin type 6 (PACE4), also exists both intra- and extracellularly. Previous work in 293 cells showed that furin efficiently cleaves pro-IGF-IA intracellularly at Arg71, but that PACE4 also cleaves Pro-IGF-IA at Arg71 and Arg7720. In addition, PACE4 is critical to myoblast differentiation process, in which it cleaves IGF-II46. Therefore, a role for PACE4 is plausible in the release of IGFs from the pro-IGF forms stored in the ECM. One difficulty in studying the actions of PCs on IGF-I ligand release is the fact that these proteases have several targets. In particular, IGF-I receptor processing relies on this protease family, and so care must be taken to devise strategies for cleanly separating the actions of the PC family on ligand or receptor. Ultimately, the fact that there are potentially multiple pools of IGF-I in the ECM, as well as an array of proteases which can cleave either IGFBPs or IGF-I itself, provides a way to release and mobilize a subset of the stored IGF-I for a variety of needs, including reloading, repair, normal growth, among others.
Modulating IGF-I receptor activation
For IGF-I to promote growth, it must bind to and activate receptors on the membrane surface. IGF-I acts predominantly via the IGF-I receptor (IGF-IR), a transmembrane protein consisting of two extracellular α-subunits, which contain the IGF-I binding site, and two transmembrane β-subunits that have a cluster of three tyrosine residues, which undergo phosphorylation and activation upon IGF-I binding to mediate canonical signaling pathways necessary for cell survival and growth. The receptor tetramer can also be composed of both insulin and IGF-I hemireceptor αβ dimers, which are called hybrid receptors. The separation and overlap of IGF-I and insulin functions are, in part, due to the ability for both ligands to bind to these highly homologous receptors with different affinities. In skeletal muscle, hybrid receptors comprise at least half of the insulin receptor (IR) and IGF-IR population47, where hybrid receptors preferentially bind IGF-I compared to insulin, and bind IGF-I with equivalent affinity as IGF-IR48,49. It is not clear, however, how downstream actions of the hybrid receptor differ from IGF-IR, nor how this alters the biological actions of IGF-I. Some clues to their distinctions have come from studying chimeric receptors, in which domains within each hemireceptor were exchanged between the IR and IGF-IR49–54. The main conclusion from these experiments was that signal transduction pathways are governed by the β subunit, and most specifically by the C-terminal domain. Chimeras containing the C-terminal domain of IGF-IR were more effective in promoting mitogenic responses, whereas signaling associated with metabolism was linked to the C-terminal of IR. Therefore, the intracellular domains of each receptor direct a significant portion of their specific actions. The intracellular signaling upon ligand binding helps to amplify IGF-I actions through two signaling arms. However, a major divergence between the IGF-I and insulin receptor populations is the preferential coupling to members of the insulin receptor substrate (IRS) gene family. The adaptor protein IRS-1 tends to bind to IGF-IR, and IRS-2 tends to bind to the insulin receptor55–57. Muscle, in particular, requires IRS-1 for IGF-I mediated hypertrophy, because a transgenic cross of IRS-1+/− mice with a mouse line overexpressing IGF- I blocked the anticipated increase in skeletal muscle mass, even though IGF-I could rescue the growth deficits in other tissues (brain, intestine, and heart)55. This indicates that absolute or partial IRS-1 deficiency impairs IGF-I-induced muscle growth. The use of specific IRS family members is not exclusive to each receptor, but may modulate the downstream signaling (reviewed in58). Thus the tendency for IGF-IR activation to drive anabolic, rather than metabolic signals, appears to be tuned by this family of adaptor proteins. In addition, other factors can alter the efficiency of IGF-I signaling through targeting IRS-1 stability. Specifically, the ubiquitin E3 ligase, Fbxo40, targets IRS-1 for degradation59. This provides another level of control of IGF-I activity, where the negative feedback of increased Fbxo40 will shut down IGF-I mediated hypertrophy. Hence, Fbxo40 targeting could increase downstream signaling via the IGF-I receptors driving muscle growth, yet potentially leave hybrid receptor signaling untouched, retaining metabolic actions of IGF-I via this receptor. The requirement of IGF-IR activation for skeletal muscle hypertrophy has been addressed in various models, such as overload-dependent or follistatin-induced muscle hypertrophy, showing conflicting findings60,61. Specifically, by utilizing a transgenic mouse model where a dominant negative IGF-I receptor is expressed specifically in skeletal muscle (MKR), it has been shown that increased mechanical load can induce muscle hypertrophy and activate Akt and p70S6K independently of a functional IGF-I receptor, implying that IGF-I may not be a limiting factor in the induction of hypertrophy with muscle overloading61. However, in the same animal model, another study has recently shown that the IGF-IR/Akt pathway plays a critical role in muscle hypertrophy induced by a myostatin inhibitor, follistatin60. Clearly, more studies are needed to identify the upstream mechanisms responsible for activation of Akt-mediated signaling in response to loading and other growth stimuli, and whether IGF-I is part of those mechanisms61.
Satellite cells and IGF-I
Since muscle fibers are post-mitotic, the mitogenic actions of IGF-I must rely on mononucleated satellite cells. These cells are a stem cell like population located between the basal lamina of the muscle and the sarcolemma of myofibers62 and consist a source for replenishing the nuclear content of the muscle fibers63. After their activation, by factors such as hepatocyte growth factor (HGF) or epidermal growth factor (EGF)64–66, they express IGF-IR67. The activation, proliferation and fusion of satellite cells to existing myofibers has been thought to be a predominant mechanism that leads to increase in muscle mass63,68,69, and they are essential for muscle repair. The satellite cells divide and then can undergo differentiation, fusing to damaged sites on muscle fibers, or forming new fibers, thus providing the extra power for the increased protein synthesis during the repair70–76, or hypertrophy process63,68,77–80. IGF-I immunoreactivity has been detected in satellite cell and in cytoplasm of myoblasts and myotubes during skeletal muscle regeneration81–83, while IGF-I mRNA and protein expression have been detected in newly replicating skeletal muscle following injury in rats84.
Exogenous administration of IGF-I is able to induce muscle hypertrophy33,78,85,86, and high levels of IGF-I can enhance satellite cell division, increasing the pool of cells available for repair and growth67. In transgenic animals expressing IGF-I in skeletal muscle, hypertrophy is associated with satellite cell incorporation into existing fibers, for the myonuclear domain is maintained even though the fibers are significantly larger87. Previous studies showed that satellite cells were responsible for approximately half of the increased muscle mass following viral delivery of IGF-I67. This is consistent with the diminished hypertrophic response of mature or aged muscle to IGF-I, where there is a reduction in the pool of activated satellite cells compared to young, growing muscle88. Although they certainly contribute to muscle hypertrophy, recent evidence supports that satellite cells are not necessary for skeletal muscle fiber hypertrophy in general. In the novel mouse strain (Pax7-DTA), conditional ablation of >90% of satellite cells in mature skeletal muscle could be achieved89, and yet a robust hypertrophic response to mechanical overload was achieved. Taken together, development of strategies enhancing satellite cell mediated hypertrophy may only be useful for a subset of therapeutic applications.
Who can benefit from IGF-I therapies?
Results from several animal models have laid the groundwork to move new potential strategies for IGF-I dependent actions on muscle into people (Table 2). As stated initially, patients suffering from sarcopenia, muscle disease, or acute injury could benefit significantly. In the context of the strategies proposed in this review, what are additional factors to consider? For aging and genetic disease, therapies are likely to be administered for the long-term. Thus, safety is a primary issue for any growth promoting therapy. For IGF-I, it is absolutely imperative that carcinogenic side effects are eliminated. This may be achieved through strategies that target IGF-I retention, regardless of the potency, because optimizing the activity of the IGF-I ligand is likely to drive growth in every tissues indiscriminately. In therapies for acute muscle injury, safety considerations should still be part of the equation, but because the time course of repair lasts only a few weeks, it is less of an issue than in prolonged exposure to IGF-I.
Table 2.
Preclinical trials for IGF-I and muscle therapy
| Therapeutic Target | Animal Model | IGF-I Isoform | Delivery Method | Outcome |
|---|---|---|---|---|
| Sarcopenia | ||||
| 27 mo mice | IGF-IA | AAV | Maintenance of muscle mass and function33 | |
| 20–24 mo mice | IGF-IA | Transgenic | Maintenance of muscle mass and regenerative capacity35 | |
| Disuse Atrophy | ||||
| Hindlimb Suspension | IGF-IA | Transgenic | No protection against atrophy96 | |
| Hindlimb Suspension & reloading | IGF-IA vs. mature IGF-I | AAV | Enhanced recovery more with mature IGF-I, functional protection by mature IGF-I44 | |
| Cast immobilization & reloading | IGF-IA | AAV | Enhanced recovery, no atrophy protection94,95 | |
| Muscle Wasting | ||||
| Angiotensin II Mice | IGF-IA | Transgenic | Block of apoptosis and muscle specific ubiquitin ligases97 | |
| Glucocorticoid Mice | E-peptides | AAV | No protection40 | |
| Acute Injury | ||||
| Mice Cardiotoxin Injection | IGF-IA vs. rhIGF-I | Plasmid vs osmotic pump | Accelerated repair by plasmid IGF-IA86 | |
| PEG-IGF-I vs. rhIGF-I | IM/SQ injection | Improved functional recovery of IM PEG-IGF-I vs. rhIGF-I or SQ PEG-IGF-I98 | ||
| IGF-IA | Transgenic | Enhanced repair; limited fibrosis99 | ||
| Mice Laceration | IGF-IA | Plasmid | Improved regeneration100 | |
| rhIGF-I | IM Injection | Improved healing101 | ||
| Rat Ischemia Reperfusion | PEG-IGF-I | fibrin gel injection | Improved functional recovery102 | |
| Neuromuscular Disease | ||||
| ALS (G93A mouse) | rhIGF-I | Intrathecal | improved survival103 | |
| IGF-IA | Transgenic | Improved survival104 | ||
| PEG-IGF-I | SQ injection | Mild symptoms improved; severe symptoms no protection105 | ||
| DMD (mdx mouse) | IGF-IA | Transgenic | Improved function; reduced fibrosis; Increased muscle mass; reduction of myofiber necrosis106,107 | |
| PEG-IGF | SQ injection | Reduced contraction -induced injury108,109 | ||
| rhIGF-I | SQ injection | Improved fatigue resistance and contractile function110,111 | ||
| LR-IGF-I | SQ injection | Improved function112 | ||
Satellite cells are central to the repair process, and so both in acute injury as well as in the chronic damage associated with neuromuscular disease, they are likely to play a significant role in driving maintenance of muscle mass and improved regeneration. Certainly, there are many therapeutic strategies under development that focus on satellite cell recruitment into the repair process. Combining these therapies with IGF-I administration will be important to address their interaction. For aging, the involvement of satellite cells in prevention of sarcopenia should be considered. However, because IGF-I is not a trigger for their activation, it can only improve muscle mass through anabolic actions in the muscle fibers, or in satellite cells that have been activated through some other mechanism.
Finally, the receptor pool that IGF-I uses for driving hypertrophy may provide another potential avenue for therapeutic strategies. Since muscle maintains IGF-I and hybrid receptors, as well as insulin receptors to a lesser extent, modulation of either activation of these populations or the ligand interactions with the range of receptors are areas with untapped potential. As much as we know about the actions of IGF-I, there is much more to explore in terms of its optimization for targeting muscle and other tissues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borasio GD, Robberecht W, Leigh PN, et al. A placebo-controlled trial of insulin-like growth factor-I in amyotrophic lateral sclerosis. European ALS/IGF-I Study Group. Neurology. 1998;51:583–586. doi: 10.1212/wnl.51.2.583. [DOI] [PubMed] [Google Scholar]
- 2.Butterfield GE, Thompson J, Rennie MJ, Marcus R, Hintz RL, Hoffman AR. Effect of rhGH and rhIGF-I treatment on protein utilization in elderly women. Am J Physiol. 1997;272:E94–99. doi: 10.1152/ajpendo.1997.272.1.E94. [DOI] [PubMed] [Google Scholar]
- 3.Cusi K, DeFronzo R. Recombinant human insulin-like growth factor I treatment for 1 week improves metabolic control in type 2 diabetes by ameliorating hepatic and muscle insulin resistance. J Clin Endocrinol Metab. 2000;85:3077–3084. doi: 10.1210/jcem.85.9.6827. [DOI] [PubMed] [Google Scholar]
- 4.Friedlander AL, Butterfield GE, Moynihan S, et al. One year of insulin-like growth factor I treatment does not affect bone density, body composition, or psychological measures in postmenopausal women. J Clin Endocrinol Metab. 2001;86:1496–1503. doi: 10.1210/jcem.86.4.7377. [DOI] [PubMed] [Google Scholar]
- 5.Lai EC, Felice KJ, Festoff BW, et al. Effect of recombinant human insulin-like growth factor-I on progression of ALS. A placebo-controlled study. The North America ALS/IGF-I Study Group. Neurology. 1997;49:1621–1630. doi: 10.1212/wnl.49.6.1621. [DOI] [PubMed] [Google Scholar]
- 6.Mauras N, O’Brien KO, Welch S, et al. Insulin-like growth factor I and growth hormone (GH) treatment in GH-deficient humans: differential effects on protein, glucose, lipid, and calcium metabolism. J Clin Endocrinol Metab. 2000;85:1686–1694. doi: 10.1210/jcem.85.4.6541. [DOI] [PubMed] [Google Scholar]
- 7.Moxley RT., 3rd Potential for growth factor treatment of muscle disease. Curr Opin Neurol. 1994;7:427–434. doi: 10.1097/00019052-199410000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Nagano I, Shiote M, Murakami T, et al. Beneficial effects of intrathecal IGF-1 administration in patients with amyotrophic lateral sclerosis. Neurol Res. 2005;27:768–772. doi: 10.1179/016164105X39860. [DOI] [PubMed] [Google Scholar]
- 9.Sorenson EJ, Windbank AJ, Mandrekar JN, et al. Subcutaneous IGF-1 is not beneficial in 2-year ALS trial. Neurology. 2008;71:1770–1775. doi: 10.1212/01.wnl.0000335970.78664.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson JL, Butterfield GE, Gylfadottir UK, et al. Effects of human growth hormone, insulin-like growth factor I, and diet and exercise on body composition of obese postmenopausal women. J Clin Endocrinol Metab. 1998;83:1477–1484. doi: 10.1210/jcem.83.5.4826. [DOI] [PubMed] [Google Scholar]
- 11.Waters D, Danska J, Hardy K, et al. Recombinant human growth hormone, insulin-like growth factor 1, and combination therapy in AIDS-associated wasting. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1996;125:865–872. doi: 10.7326/0003-4819-125-11-199612010-00001. [DOI] [PubMed] [Google Scholar]
- 12.Heatwole CR, Eichinger KJ, Friedman DI, et al. Open-label trial of recombinant human insulin-like growth factor 1/recombinant human insulin-like growth factor binding protein 3 in myotonic dystrophy type 1. Arch Neurol. 2011;68:37–44. doi: 10.1001/archneurol.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adamo ML, Neuenschwander S, LeRoith D, Roberts CT., Jr Structure, expression, and regulation of the IGF-I gene. Adv Exp Med Biol. 1993;343:1–11. doi: 10.1007/978-1-4615-2988-0_1. [DOI] [PubMed] [Google Scholar]
- 14.Barton ER. The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair. Appl Physiol Nutr Metab. 2006;31:791–797. doi: 10.1139/h06-054. [DOI] [PubMed] [Google Scholar]
- 15.Rotwein P, Pollock KM, Didier DK, Krivi GG. Organization and sequence of the human insulin-like growth factor I gene. Alternative RNA processing produces two insulin-like growth factor I precursor peptides. J Biol Chem. 1986;261:4828–4832. [PubMed] [Google Scholar]
- 16.Shimatsu A, Rotwein P. Mosaic evolution of the insulin-like growth factors. Organization, sequence, and expression of the rat insulin-like growth factor I gene. J Biol Chem. 1987;262:7894–7900. [PubMed] [Google Scholar]
- 17.Adamo ML, Neuenschwander S, LeRoith D, Roberts CT. Structure, Expression, And Regulation Of The IGF-1 Gene. Adv Exp Med Biol. 1993;343:1–11. doi: 10.1007/978-1-4615-2988-0_1. [DOI] [PubMed] [Google Scholar]
- 18.Wallis M. New insulin-like growth factor (IGF)-precursor sequences from mammalian genomes: the molecular evolution of IGFs and associated peptides in primates. Growth Horm IGF Res. 2009;19:12–23. doi: 10.1016/j.ghir.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Duguay SJ. Post-translational processing of insulin-like growth factors. Horm Metab Res. 1999;31:43–49. doi: 10.1055/s-2007-978697. [DOI] [PubMed] [Google Scholar]
- 20.Duguay SJ, Milewski WM, Young BD, Nakayama K, Steiner DF. Processing of Wild-type and Mutant Proinsulin-like Growth Factor-IA by Subtilisin-related Proprotein Convertases. The Journal of Biological Chemistry. 1997;272:6663–6670. doi: 10.1074/jbc.272.10.6663. [DOI] [PubMed] [Google Scholar]
- 21.Conover CA, Baker BK, Hintz RL. Cultured human fibroblasts secrete insulin-like growth factor IA prohormone. J Clin Endocrinol Metab. 1989;69:25–30. doi: 10.1210/jcem-69-1-25. [DOI] [PubMed] [Google Scholar]
- 22.Conover CA, Baker BK, Bale LK, Clarkson JT, Liu F, Hintz RL. Human hepatoma cells synthesize and secrete insulin-like growth factor Ia prohormone under growth hormone control. Regul Pept. 1993;48:1–8. doi: 10.1016/0167-0115(93)90330-b. [DOI] [PubMed] [Google Scholar]
- 23.Durzynska J, Philippou A, Brisson BK, Nguyen-McCarty M, Barton ER. The pro-forms of insulin-like growth factor I (IGF-I) are predominant in skeletal muscle and alter IGF-I receptor activation. Endocrinology. 2013;154:1215–1224. doi: 10.1210/en.2012-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson HE, Westwood M, White A, Clayton PE. Monoclonal antibodies to the carboxyterminal Ea sequence of pro-insulin-like growth factor-IA (proIGF-IA) recognize proIGF-IA secreted by IM9 B-lymphocytes. Growth Horm IGF Res. 2001;11:10–17. doi: 10.1054/ghir.2000.0182. [DOI] [PubMed] [Google Scholar]
- 25.Bach MA, Roberts CT, Jr, Smith EP, LeRoith D. Alternative splicing produces messenger RNAs encoding insulin-like growth factor-I prohormones that are differentially glycosylated in vitro. Mol Endocrinol. 1990;4:899–904. doi: 10.1210/mend-4-6-899. [DOI] [PubMed] [Google Scholar]
- 26.Adams TE, Epa VC, Garrett TP, Ward CW. Structure and function of the type 1 insulinlike growth factor receptor. Cell Mol Life Sci. 2000;57:1050–1093. doi: 10.1007/PL00000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan C, Ren H, Gao S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen Comp Endocrinol. 2010;167:344–351. doi: 10.1016/j.ygcen.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Brisson BK, Barton ER. New Modulators for IGF-I Activity within IGF-I Processing Products. Front Endocrinol (Lausanne) 2013;4:42. doi: 10.3389/fendo.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barton ER, Park S, James JK, et al. Deletion of muscle GRP94 impairs both muscle and body growth by inhibiting local IGF production. FASEB J. 2012;26:3691–3702. doi: 10.1096/fj.11-203026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sjogren K, Liu JL, Blad K, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yakar S, Liu JL, Stannard B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner JD, Rotwein P, Novakofski J, Bechtel PJ. Induction of mRNA for IGF-I and -II during growth hormone-stimulated muscle hypertrophy. Am J Physiol. 1988;255:E513–517. doi: 10.1152/ajpendo.1988.255.4.E513. [DOI] [PubMed] [Google Scholar]
- 33.Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci U S A. 1998;95:15603–15607. doi: 10.1073/pnas.95.26.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coleman ME, DeMayo F, Yin KC, et al. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem. 1995;270:12109–12116. doi: 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- 35.\Musaro A, McCullagh K, Paul A, et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 36.Ostrovsky O, Eletto D, Makarewich C, Barton ER, Argon Y. Glucose regulated protein 94 is required for muscle differentiation through its control of the autocrine production of insulinlike growth factors. Biochimica et Biophysica Acta. 2010;1803:333–341. doi: 10.1016/j.bbamcr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elis S, Wu Y, Courtland HW, et al. Increased serum IGF-1 levels protect the musculoskeletal system but are associated with elevated oxidative stress markers and increased mortality independent of tissue igf1 gene expression. Aging Cell. 2011;10:547–550. doi: 10.1111/j.1474-9726.2011.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 39.Hede MS, Salimova E, Piszczek A, et al. E-peptides control bioavailability of IGF-1. PLoS One. 2012;7:e51152. doi: 10.1371/journal.pone.0051152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brisson BK, Spinazzola J, Park S, Barton ER. Viral expression of insulin-like growth factor I E-peptides increases skeletal muscle mass but at the expense of strength. Am J Physiol Endocrinol Metab. 2014;306:E965–974. doi: 10.1152/ajpendo.00008.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durzynska J, Wardzinski A, Koczorowska M, Gozdzicka-Jozefiak A, Barton ER. Human Eb peptide: not just a by-product of pre-pro-IGF1b processing? Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2013;45:415–422. doi: 10.1055/s-0032-1331699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brisson BK, Barton ER. Insulin-like growth factor-I E-peptide activity is dependent on the IGF-I receptor. PLoS One. 2012;7:e45588. doi: 10.1371/journal.pone.0045588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fornaro M, Hinken AC, Needle S, et al. Mechano-growth factor peptide, the COOH terminus of unprocessed insulin-like growth factor 1, has no apparent effect on myoblasts or primary muscle stem cells. American journal of physiology. Endocrinology and metabolism. 2014;306:E150–156. doi: 10.1152/ajpendo.00408.2013. [DOI] [PubMed] [Google Scholar]
- 44.Park S, Brisson BK, Liu M, Spinazzola JM, Barton ER. Mature IGF-I excels in promoting functional muscle recovery from disuse atrophy compared with pro-IGF-IA. J Appl Physiol (1985) 2014;116:797–806. doi: 10.1152/japplphysiol.00955.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duguay SJ, Milewski WM, Young BD, Nakayama K, Steiner DF. Processing of wild-type and mutant proinsulin-like growth factor-IA by subtilisin-related proprotein convertases. J Biol Chem. 1997;272:6663–6670. doi: 10.1074/jbc.272.10.6663. [DOI] [PubMed] [Google Scholar]
- 46.Yuasa K, Masuda T, Yoshikawa C, Nagahama M, Matsuda Y, Tsuji A. Subtilisin-like proprotein convertase PACE4 is required for skeletal muscle differentiation. J Biochem. 2009;146:407–415. doi: 10.1093/jb/mvp090. [DOI] [PubMed] [Google Scholar]
- 47.Bailyes EM, Nave BT, Soos MA, Orr SR, Hayward AC, Siddle K. Insulin receptor/IGF-I receptor hybrids are widely distributed in mammalian tissues: quantification of individual receptor species by selective immunoprecipitation and immunoblotting. Biochem J. 1997;327(Pt 1):209–215. doi: 10.1042/bj3270209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Entingh-Pearsall A, Kahn CR. Differential roles of the insulin and insulin-like growth factor-I (IGF-I) receptors in response to insulin and IGF-I. J Biol Chem. 2004;279:38016–38024. doi: 10.1074/jbc.M313201200. [DOI] [PubMed] [Google Scholar]
- 49.Kristensen C, Wiberg FC, Andersen AS. Specificity of insulin and insulin-like growth factor I receptors investigated using chimeric mini-receptors. Role of C-terminal of receptor alpha subunit. J Biol Chem. 1999;274:37351–37356. doi: 10.1074/jbc.274.52.37351. [DOI] [PubMed] [Google Scholar]
- 50.Christoffersen CT, Bornfeldt KE, Rotella CM, et al. Negative cooperativity in the insulinlike growth factor-I receptor and a chimeric IGF-I/insulin receptor. Endocrinology. 1994;135:472–475. doi: 10.1210/endo.135.1.8013387. [DOI] [PubMed] [Google Scholar]
- 51.Mothe I, Tartare S, Kowalski-Chauvel A, Kaliman P, Van Obberghen E, Ballotti R. Tyrosine kinase activity of a chimeric insulin-like-growth-factor-1 receptor containing the insulin receptor C-terminal domain. Comparison with the tyrosine kinase activities of the insulin and insulin-like-growth-factor-1 receptors using a cell-free system. Eur J Biochem. 1995;228:842–848. doi: 10.1111/j.1432-1033.1995.0842m.x. [DOI] [PubMed] [Google Scholar]
- 52.Tartare S, Mothe I, Kowalski-Chauvel A, Breittmayer JP, Ballotti R, Van Obberghen E. Signal transduction by a chimeric insulin-like growth factor-1 (IGF-1) receptor having the carboxyl-terminal domain of the insulin receptor. J Biol Chem. 1994;269:11449–11455. [PubMed] [Google Scholar]
- 53.Urso B, Cope DL, Kalloo-Hosein HE, et al. Differences in signaling properties of the cytoplasmic domains of the insulin receptor and insulin-like growth factor receptor in 3T3-L1 adipocytes. J Biol Chem. 1999;274:30864–30873. doi: 10.1074/jbc.274.43.30864. [DOI] [PubMed] [Google Scholar]
- 54.Urso B, Niesler CU, O’Rahilly S, Siddle K. Comparison of anti-apoptotic signalling by the insulin receptor and IGF-I receptor in preadipocytes and adipocytes. Cell Signal. 2001;13:279–285. doi: 10.1016/s0898-6568(01)00130-9. [DOI] [PubMed] [Google Scholar]
- 55.Pete G, Fuller CR, Oldham JM, et al. Postnatal growth responses to insulin-like growth factor I in insulin receptor substrate-1-deficient mice. Endocrinology. 1999;140:5478–5487. doi: 10.1210/endo.140.12.7219. [DOI] [PubMed] [Google Scholar]
- 56.Sesti G, Federici M, Hribal ML, Lauro D, Sbraccia P, Lauro R. Defects of the insulin receptor substrate (IRS) system in human metabolic disorders. Faseb J. 2001;15:2099–2111. doi: 10.1096/fj.01-0009rev. [DOI] [PubMed] [Google Scholar]
- 57.Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Na Genet. 1999;23:32–40. doi: 10.1038/12631. [DOI] [PubMed] [Google Scholar]
- 58.Siddle K. Molecular basis of signaling specificity of insulin and IGF receptors: neglected corners and recent advances. Front Endocrinol (Lausanne) 2012;3:34. doi: 10.3389/fendo.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi J, Luo L, Eash J, Ibebunjo C, Glass DJ. The SCF-Fbxo40 complex induces IRS1 ubiquitination in skeletal muscle, limiting IGF1 signaling. Developmental cell. 2011;21:835–847. doi: 10.1016/j.devcel.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 60.Kalista S, Schakman O, Gilson H, et al. The type 1 insulin-like growth factor receptor (IGF-IR) pathway is mandatory for the follistatin-induced skeletal muscle hypertrophy. Endocrinology. 2012;153:241–253. doi: 10.1210/en.2011-1687. [DOI] [PubMed] [Google Scholar]
- 61.Spangenburg EE, Le Roith D, Ward CW, Bodine SC. A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. The Journal of physiology. 2008;586:283–291. doi: 10.1113/jphysiol.2007.141507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol (1985) 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- 64.Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol. 1995;165:307–312. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- 65.Golding JP, Calderbank E, Partridge TA, Beauchamp JR. Skeletal muscle stem cells express anti-apoptotic ErbB receptors during activation from quiescence. Exp Cell Res. 2007;313:341–356. doi: 10.1016/j.yexcr.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 66.Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- 67.Barton-Davis ER, Shoturma DI, Sweeney HL. Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand. 1999;167:301–305. doi: 10.1046/j.1365-201x.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- 68.Adams GR. Invited Review: Autocrine/paracrine IGF-I and skeletal muscle adaptation. J Appl Physiol (1985) 2002;93:1159–1167. doi: 10.1152/japplphysiol.01264.2001. [DOI] [PubMed] [Google Scholar]
- 69.Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971;170:421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- 70.Garry DJ, Meeson A, Elterman J, et al. Myogenic stem cell function is impaired in mice lacking the forkhead/winged helix protein MNF. Proc Natl Acad Sci U S A. 2000;97:5416–5421. doi: 10.1073/pnas.100501197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hurme T, Kalimo H. Activation of myogenic precursor cells after muscle injury. Med Sci Sports Exerc. 1992;24:197–205. [PubMed] [Google Scholar]
- 72.Karalaki M, Fili S, Philippou A, Koutsilieris M. Muscle regeneration: cellular and molecular events. In Vivo. 2009;23:779–796. [PubMed] [Google Scholar]
- 73.Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol. 2003;35:1151–1156. doi: 10.1016/s1357-2725(03)00042-6. [DOI] [PubMed] [Google Scholar]
- 74.Robertson TA, Papadimitriou JM, Grounds MD. Fusion of myogenic cells to the newly sealed region of damaged myofibres in skeletal muscle regeneration. Neuropathol Appl Neurobiol. 1993;19:350–358. doi: 10.1111/j.1365-2990.1993.tb00451.x. [DOI] [PubMed] [Google Scholar]
- 75.Schultz E. Satellite cell behavior during skeletal muscle growth and regeneration. Med Sci Sports Exerc. 1989;21:S181–186. [PubMed] [Google Scholar]
- 76.Seale P, Rudnicki MA. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev Biol. 2000;218:115–124. doi: 10.1006/dbio.1999.9565. [DOI] [PubMed] [Google Scholar]
- 77.Adams GR, Haddad F. The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol (1985) 1996;81:2509–2516. doi: 10.1152/jappl.1996.81.6.2509. [DOI] [PubMed] [Google Scholar]
- 78.Adams GR, McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol (1985) 1998;84:1716–1722. doi: 10.1152/jappl.1998.84.5.1716. [DOI] [PubMed] [Google Scholar]
- 79.Grounds MD, White JD, Rosenthal N, Bogoyevitch MA. The role of stem cells in skeletal and cardiac muscle repair. J Histochem Cytochem. 2002;50:589–610. doi: 10.1177/002215540205000501. [DOI] [PubMed] [Google Scholar]
- 80.McCall GE, Allen DL, Linderman JK, et al. Maintenance of myonuclear domain size in rat soleus after overload and growth hormone/IGF-I treatment. J Appl Physiol (1985) 1998;84:1407–1412. doi: 10.1152/jappl.1998.84.4.1407. [DOI] [PubMed] [Google Scholar]
- 81.Jennische E, Hansson HA. Regenerating skeletal muscle cells express insulin-like growth factor I. Acta Physiol Scand. 1987;130:327–332. doi: 10.1111/j.1748-1716.1987.tb08144.x. [DOI] [PubMed] [Google Scholar]
- 82.Jennische E, Skottner A, Hansson HA. Satellite cells express the trophic factor IGF-I in regenerating skeletal muscle. Acta Physiol Scand. 1987;129:9–15. doi: 10.1111/j.1748-1716.1987.tb08034.x. [DOI] [PubMed] [Google Scholar]
- 83.Tollefsen SE, Lajara R, McCusker RH, Clemmons DR, Rotwein P. Insulin-like growth factors (IGF) in muscle development. Expression of IGF-I, the IGF-I receptor, and an IGF binding protein during myoblast differentiation. J Biol Chem. 1989;264:13810–13817. [PubMed] [Google Scholar]
- 84.Caroni P, Schneider C. Signaling by insulin-like growth factors in paralyzed skeletal muscle: rapid induction of IGF1 expression in muscle fibers and prevention of interstitial cell proliferation by IGF-BP5 and IGF-BP4. J Neurosci. 1994;14:3378–3388. doi: 10.1523/JNEUROSCI.14-05-03378.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barton ER, DeMeo J, Lei H. The insulin-like growth factor (IGF)-I E-peptides are required for isoform-specific gene expression and muscle hypertrophy after local IGF-I production. J Appl Physiol (1985) 2010;108:1069–1076. doi: 10.1152/japplphysiol.01308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schertzer JD, Lynch GS. Comparative evaluation of IGF-I gene transfer and IGF-I protein administration for enhancing skeletal muscle regeneration after injury. Gene Ther. 2006;13:1657–1664. doi: 10.1038/sj.gt.3302817. [DOI] [PubMed] [Google Scholar]
- 87.Qaisar R, Renaud G, Morine K, Barton ER, Sweeney HL, Larsson L. Is functional hypertrophy and specific force coupled with the addition of myonuclei at the single muscle fiber level? Faseb J. 2012;26:1077–1085. doi: 10.1096/fj.11-192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barton ER. Viral expression of insulin-like growth factor-I isoforms promotes different responses in skeletal muscle. J Appl Physiol (1985) 2006;100:1778–1784. doi: 10.1152/japplphysiol.01405.2005. [DOI] [PubMed] [Google Scholar]
- 89.McCarthy JJ, Mula J, Miyazaki M, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mauras N, Martinez V, Rini A, Guevara-Aguirre J. Recombinant human insulin-like growth factor I has significant anabolic effects in adults with growth hormone receptor deficiency: studies on protein, glucose, and lipid metabolism. J Clin Endocrinol Metab. 2000;85:3036–3042. doi: 10.1210/jcem.85.9.6772. [DOI] [PubMed] [Google Scholar]
- 91.Waters D, Danska J, Hardy K, et al. Recombinant human growth hormone, insulin-like growth factor 1, and combination therapy in AIDS-associated wasting. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1996;125:865–872. doi: 10.7326/0003-4819-125-11-199612010-00001. [DOI] [PubMed] [Google Scholar]
- 92.Thompson JL, Butterfield GE, Gylfadottir UK, et al. Effects of human growth hormone, insulin-like growth factor I, and diet and exercise on body composition of obese postmenopausal women. The Journal of clinical endocrinology and metabolism. 1998;83:1477–1484. doi: 10.1210/jcem.83.5.4826. [DOI] [PubMed] [Google Scholar]
- 93.Heatwole CR, Eichinger KJ, Friedman DI, et al. Open-label trial of recombinant human insulin-like growth factor 1/recombinant human insulin-like growth factor binding protein 3 in myotonic dystrophy type 1. Arch Neurol. 2011;68:37–44. doi: 10.1001/archneurol.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stevens-Lapsley JE, Ye F, Liu M, et al. Impact of viral-mediated IGF-I gene transfer on skeletal muscle following cast immobilization. Am J Physiol Endocrinol Metab. 2010;299:E730–740. doi: 10.1152/ajpendo.00230.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ye F, Mathur S, Liu M, et al. Overexpression of insulin-like growth factor-1 attenuates skeletal muscle damage and accelerates muscle regeneration and functional recovery after disuse. Exp Physiol. 2013;98:1038–1052. doi: 10.1113/expphysiol.2012.070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Criswell DS, Booth FW, DeMayo F, Schwartz RJ, Gordon SE, Fiorotto ML. Overexpression of IGF-I in skeletal muscle of transgenic mice does not prevent unloading-induced atrophy. Am J Physiol. 1998;275:E373–379. doi: 10.1152/ajpendo.1998.275.3.e373. [DOI] [PubMed] [Google Scholar]
- 97.Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest. 2005;115:451–458. doi: 10.1172/JCI22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martins KJ, Gehrig SM, Naim T, et al. Intramuscular administration of PEGylated IGF-I improves skeletal muscle regeneration after myotoxic injury. Growth Horm IGF Res. 2013;23:128–133. doi: 10.1016/j.ghir.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 99.Pelosi L, Giacinti C, Nardis C, et al. Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. Faseb J. 2007;21:1393–1402. doi: 10.1096/fj.06-7690com. [DOI] [PubMed] [Google Scholar]
- 100.Takahashi T, Ishida K, Itoh K, et al. IGF-I gene transfer by electroporation promotes regeneration in a muscle injury model. Gene Ther. 2003;10:612–620. doi: 10.1038/sj.gt.3301900. [DOI] [PubMed] [Google Scholar]
- 101.Menetrey J, Kasemkijwattana C, Day CS, et al. Growth factors improve muscle healing in vivo. The Journal of bone and joint surgery. British. 2000;82:131–137. doi: 10.1302/0301-620x.82b1.8954. [DOI] [PubMed] [Google Scholar]
- 102.Hammers DW, Sarathy A, Pham CB, Drinnan CT, Farrar RP, Suggs LJ. Controlled release of IGF-I from a biodegradable matrix improves functional recovery of skeletal muscle from ischemia/reperfusion. Biotechnol Bioeng. 2012;109:1051–1059. doi: 10.1002/bit.24382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nagano I, Ilieva H, Shiote M, et al. Therapeutic benefit of intrathecal injection of insulinlike growth factor-1 in a mouse model of Amyotrophic Lateral Sclerosis. J Neurol Sci. 2005;235:61–68. doi: 10.1016/j.jns.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 104.Dobrowolny G, Giacinti C, Pelosi L, et al. Muscle expression of a local Igf-1 isoform protects motor neurons in an ALS mouse model. J Cell Biol. 2005;168:193–199. doi: 10.1083/jcb.200407021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saenger S, Goeldner C, Frey JR, et al. PEGylation enhances the therapeutic potential for insulin-like growth factor I in central nervous system disorders. Growth Horm IGF Res. 2011;21:292–303. doi: 10.1016/j.ghir.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 106.Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol. 2002;157:137–148. doi: 10.1083/jcb.200108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shavlakadze T, White J, Hoh JF, Rosenthal N, Grounds MD. Targeted expression of insulin-like growth factor-I reduces early myofiber necrosis in dystrophic mdx mice. Mol Ther. 2004;10:829–843. doi: 10.1016/j.ymthe.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 108.Gehrig SM, van der Poel C, Hoeflich A, Naim T, Lynch GS, Metzger F. Therapeutic potential of PEGylated insulin-like growth factor I for skeletal muscle disease evaluated in two murine models of muscular dystrophy. Growth Horm IGF Res. 2012;22:69–75. doi: 10.1016/j.ghir.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 109.Metzger F, Sajid W, Saenger S, et al. Separation of fast from slow anabolism by site-specific PEGylation of insulin-like growth factor I (IGF-I) J Biol Chem. 2011;286:19501–19510. doi: 10.1074/jbc.M110.172189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gregorevic P, Plant DR, Leeding KS, Bach LA, Lynch GS. Improved contractile function of the mdx dystrophic mouse diaphragm muscle after insulin-like growth factor-I administration. Am J Pathol. 2002;161:2263–2272. doi: 10.1016/S0002-9440(10)64502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gregorevic P, Plant DR, Lynch GS. Administration of insulin-like growth factor-I improves fatigue resistance of skeletal muscles from dystrophic mdx mice. Muscle Nerve. 2004;30:295–304. doi: 10.1002/mus.20082. [DOI] [PubMed] [Google Scholar]
- 112.Gehrig SM, Ryall JG, Schertzer JD, Lynch GS. Insulin-like growth factor-I analogue protects muscles of dystrophic mdx mice from contraction-mediated damage. Exp Physiol. 2008;93:1190–1198. doi: 10.1113/expphysiol.2008.042838. [DOI] [PubMed] [Google Scholar]


