Abstract
Recent studies indicate that mindfulness meditation training interventions reduce stress and improve stress-related health outcomes, but the neural pathways for these effects are unknown. The present research evaluates whether mindfulness meditation training alters resting state functional connectivity (rsFC) of the amygdala, a region known to coordinate stress processing and physiological stress responses. We show in an initial discovery study that higher perceived stress over the past month is associated with greater bilateral amygdala-subgenual anterior cingulate cortex (sgACC) rsFC in a sample of community adults (n = 130). A follow-up, single-blind randomized controlled trial shows that a 3-day intensive mindfulness meditation training intervention (relative to a well-matched 3-day relaxation training intervention without a mindfulness component) reduced right amygdala-sgACC rsFC in a sample of stressed unemployed community adults (n = 35). Although stress may increase amygdala-sgACC rsFC, brief training in mindfulness meditation could reverse these effects. This work provides an initial indication that mindfulness meditation training promotes functional neuroplastic changes, suggesting an amygdala-sgACC pathway for stress reduction effects.
Keywords: amygdala, cingulate, health, mindfulness, stress
Introduction
There has been significant recent interest in identifying interventions and pathways for stress resilience in at-risk individuals (Feder et al., 2009; Bonanno et al., 2011). Recent studies suggest that mindfulness meditation interventions, which train the capacity to be more open and aware of present-moment experience, may increase stress resilience (Ludwig and Kabat-Zinn, 2008; Creswell, 2014). For example, RCTs show that mindfulness meditation training interventions reduce reactivity to acute stressors (Nyklíček et al., 2013; Creswell et al., 2014) and improve health outcomes in stress-related disorders and diseases (e.g., anxiety, depression, PTSD, HIV-infection: Teasdale et al., 2000; Creswell et al., 2009; Hölzel et al., 2013; King et al., 2013). Moreover, cumulative evidence has linked individual differences in stress reactivity and the experience of stress to a broad range of physiological health outcomes (Everson-Rose and Lewis, 2005; Cohen et al., 2007; Gianaros and Sheu, 2009; Miller et al., 2009; McEwen and Gianaros, 2010). Although there has been significant progress in understanding the neurobiology of mindfulness meditation training effects (Tang et al., 2015), there is still relatively little work directly relating the neural mechanisms of mindfulness training and stress resilience.
Resting state functional connectivity (rsFC) methods provide one approach for interrogating how mindfulness alters neural dynamics among stress-responsive brain regions. RsFC metrics of inter-regional dynamics specifically afford the advantage of being task-independent, providing reliable estimates of neural circuit functionality corresponding to structural topography (Fox and Raichle, 2007; Greicius, 2008). Here, we tested the extent to which mindfulness alters stress-related amygdala rsFC for three reasons: (i) The amygdala is a cell complex that is centrally involved in processing psychological stressors and coordinating physiological stress responses (LeDoux 1994; Arnsten 2009). (ii) Recent studies show that greater reported mindfulness is associated with reduced amygdala volumes and task-based amygdala activation (Goldin and Gross 2010; Hölzel et al., 2010; Way et al., 2010; Taren et al. , 2013; Desbordes et al., 2012). (iii) Anatomical studies in animal models indicate robust amygdala connectivity to other brain regions considered to be integral for processing stressors and orchestrating stress reactions [e.g. anterior cingulate cortex (ACC) and medial prefrontal cortex, hypothalamus, periaqueductal gray, and pontine/medullary autonomic control regions] (Ulrich-Lai and Herman, 2009; McEwen and Gianaros, 2010). Moreover, recent human studies have shown that amygdala functional connectivity with the ACC is associated with greater stressor-evoked physiological reactivity (Gianaros et al., 2008) and amygdala-ACC functional connectivity is enhanced after acute stress exposures (Van Marle et al., 2010; Veer et al., 2011).
Accordingly, to test whether mindfulness reduces stress-related amygdala-ACC rsFC, we conducted two studies: (i) an initial discovery study evaluated whether self-reported perceived stress was associated with greater amygdala-ACC rsFC in a large sample of community volunteers (n = 130). Although some human studies implicate increased stress-related amygdala rsFC with spatially adjacent areas of the ACC (e.g. Gianaros et al., 2008), the discovery study tested for associations between perceived stress (and mindfulness) with amygdala rsFC across the whole brain. (ii) We then conducted a randomized controlled trial (RCT) of mindfulness meditation training (compared with a matched relaxation training intervention without a mindfulness component) in a high-stress unemployed community sample to test whether mindfulness training prospectively reduces stress-related amygdala-ACC rsFC.
Methods
Discovery study
Participants, procedure and analysis
133 healthy adults were recruited from the community by mass mailings to residents of Allegheny County, PA (3 participants were removed due to poor co-registration during preprocessing, resulting in a final sample of n = 130). This discovery study was drawn from a larger ongoing NIH-funded parent study focused on understanding the neurobiological, psychosocial and behavioral correlates of health among community adults (the Pittsburgh Imaging Project). Demographic characteristics of this study sample are provided in Table 1. All participants gave written informed consent as part of protocols approved by the Institutional Review Boards of the University of Pittsburgh and Carnegie Mellon University. Inclusion criteria included no history of (i) cardiovascular disease (including treatment for or diagnoses of hypertension, stroke, myocardial infarction, congestive heart failure and atrial or ventricular arrhythmias); (ii) prior neurosurgery or neurological disorder; (iii) current treatment for or self-reported psychiatric disorder; (iv) typical consumption of >15 alcoholic beverages per week; (v) daily use of corticosteroid inhaler; (vi) current use of psychotropic, lipid lowering or any cardiovascular medication, including any medication to control blood pressure; (vii) metal implants or exposure; (viii) colorblindness; and (ix) claustrophobia. All participants were right-handed, as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971). Women were excluded if pregnant (verified by urine test). Participants completed a psychosocial survey battery, which included the Perceived Stress Scale (PSS). The PSS is a 10-item questionnaire measuring the frequency of stressful feelings and thoughts within the last month (0 = Never, 4 = Very Often). Four of the items are reverse-scored and an overall composite measure is computed, with higher values indicating a greater perceived stress (Cohen et al., 1983).
Table 1.
Discovery study subject characteristics (n = 130)
| Variable | Mean | s.d. |
|---|---|---|
| Age | 40.15 | 6.14 |
| Gender | 71 male, 59 female | |
| Household Income | $39 199 | $17 713 |
| Years of School | 17.31 | 3.21 |
| PSS-10: perceived stress | 1.33 (range 0.1–3.2) | 0.61 |
PSS, Perceived Stress Scale.
All participants completed a separate neuroimaging session, which included a 5-min rsFC scan where participants were asked to rest quietly with their eyes open. Images were acquired on a 3 Tesla Trio whole-body scanner (Siemens, Erlangen, Germany), equipped with a 12-channel phased-array head coil. Three-dimensional magnetization prepared rapid gradient echo (MPRAGE) high-resolution T1-weighted neuroanatomical images were acquired for each subject over 7 min 17 s by these parameters: field of view = 256 × 208 mm, matrix size = 256 × 208 mm, time to repetition = 2100 ms, time-to-inversion = 1100 ms, time to echo = 3.29 ms and flip angle = 8° (192 slices, 1-mm thick, no gap). For each subject, a single 300 s run of resting state BOLD data was acquired with a repetition time of 2 s. Preprocessing of images was conducted in SPM8 (Welcome Department of Cognitive Neurology, London, UK; run on MATLAB, MathWorks, Inc., Natick, MA, USA) and rsFC analysis was conducted using the CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012). BOLD images for each participant were realigned to the first image of the series, corrected for distortion due to movement, and spatial normalized to the Montreal Neurological Institute 152 template. Normalization was conducted using the structural gray matter image segmented from the T1-weighted MPRAGE image; the structural gray matter image was co-registered with the mean realigned BOLD image, and registered to the Montreal Neuroimaging (MNI) template by non-linear affine transformation. The normalized BOLD images were smoothed with a 6-mm full-width at half-maximum isotropic Gaussian kernel for statistical analysis. In the CONN toolbox, structural MPRAGE images were segmented to define gray matter, white matter and cerebrospinal fluid areas. A time series was extracted from each region of interest using functional BOLD fMRI resting state data. Additional preprocessing was carried out in the CONN toolbox to account for further temporal confounding factors, including BOLD signal from the subject-specific white matter and CSF masks, motion parameters (six dimensions), and the effect of rest (an average across the session) to account for global signal. A covariate for each subject’s head motion was entered at the first level. A band-pass filter of 0.008–0.09 Hz was used. The CONN toolbox estimates orthogonal time series using principal component analysis of the BOLD signal in each noise ROI.
At the single subject level, functional connectivity was measured by calculating the average BOLD time series across all voxels in each seed region and calculating a bivariate correlation between each seed region of interest and every other voxel. A hemodynamic response function was used to down weight the initial scans within each resting state block to minimize potential ramping effects. Left and right amygdala seeds were anatomically defined using the Wake Forest University (WFU) pickatlas (Maldjian et al., 2003) for generating whole-brain rsFC maps in CONN, which were then related to PSS scores as a covariate of interest in separate group-level general linear model analyses. Whole-brain analyses relating PSS to left and right amygdala rsFC were conducted using a discovery threshold of uncorrected P < 0.001 and k > 30.
RCT of mindfulness meditation training
Participants
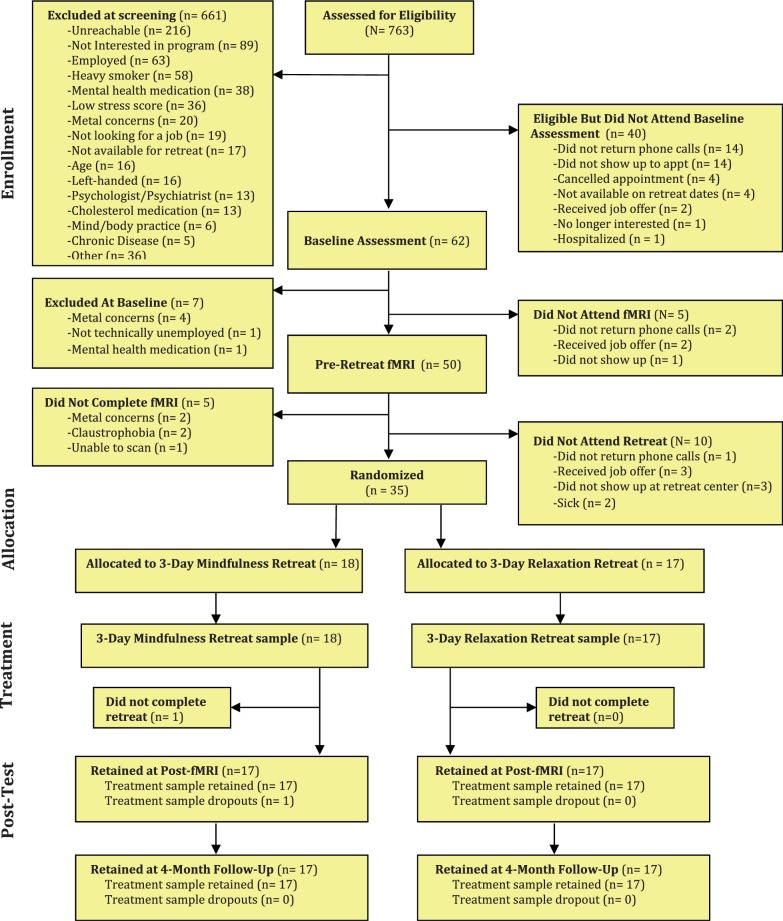
Thirty-five stressed unemployed job-seeking community adults (who indicated moderate to high levels of perceived job-seeking stress over the past month, scoring > 9 on an adapted four-item PSS for job-seeking stress; α = 0.55) participated in a single-blind RCT of 3-day intensive mindfulness meditation or relaxation training intervention (see Table 2 for participant characteristics). Participants were recruited via newspaper advertisements and through employment agencies in Pittsburgh, PA. Participants were English-speaking, had no pre-existing health conditions, were willing and available to participate in all study assessments, and were willing to be randomly assigned to one of two study conditions. Callers who met these qualifications were invited to come to Carnegie Mellon University for an in-person screening interview and baseline assessment. A more in-depth screening interview followed, including assessments of basic cognitive ability, right- or left-handedness and internal metal content (for fMRI eligibility), employment background (to probe for unemployment-related stress), medical history and health behavior. Subjects taking psychotropic medications were excluded. Figure 1 depicts the flow of participants through the RCT. This study was approved by the Carnegie Mellon University Internal Review Board and all participants provided informed consent.
Table 2.
Baseline characteristics of RCT participants
| Characteristic | Mindfulness group | Relaxation group | Difference statistic |
|---|---|---|---|
| Age [mean years (SD)] | 37.94 (10.96) | 41.00 (9.55) | t(33) = −0.48, P = 0.64 |
| Gender | χ2(1) = 0.24, P = 0.63 | ||
| Male | 11 | 9 | |
| Female | 7 | 8 | |
| Ethnicity | χ2(3) = 4.36, p = 0.23 | ||
| Caucasian | 10 | 13 | |
| African American | 6 | 2 | |
| Asian American | 1 | 0 | |
| Latino(a) | 0 | 0 | |
| Native American | 0 | 0 | |
| Other | 0 | 1 | |
| Years unemployed | 8.17 (12.48) | 10.58 (20.31) | t(33) = −0.43, P = 0.67 |
| Education | χ2(8) = 8.43, P = 0.39 | ||
| No high school degree | 1 | 0 | |
| GED | 1 | 0 | |
| High school degree | 1 | 2 | |
| Technical training | 3 | 2 | |
| Some college | 4 | 3 | |
| Associate degree | 2 | 0 | |
| Bachelor’s degree | 2 | 7 | |
| Master’s degree | 3 | 3 | |
| MD/PhD/JD/PharmD | 1 | 0 |
Notes: SD values are provided in parentheses. Mindfulness group refers to the 3-day HEM intervention. Relaxation group refers to the 3-day HER intervention.
Fig. 1.
CONSORT flowchart of participants retained at each stage of the Mindfulness Meditation Training RCT.
Procedure
We conducted this RCT between December 2010 and October 2011. Beginning 4 weeks before the 3-day training intervention, participants completed a baseline neuroimaging session. All participants began with a 5-min resting state scan (where they passively viewed a fixation cross), followed by three functional tasks in counterbalanced order (Multi-Source Interference Task, Affect Labeling and a Personalized Stress Task), and a 7-min perfusion MRI scan with a guided awareness of breathing task (the results of these functional tasks are not reported here). After neuroimaging, participants were invited to a nearby residential retreat center where they were randomized to either a 3-day intensive mindfulness meditation training (n = 18) or matched 3-day relaxation residential retreat intervention (n = 17). Only the participant, project manager, treatment program staff members and the treatment program instructor were aware of the participant’s study condition. All other study personnel responsible for collecting study assessments remained blinded. Participants returned for a neuroimaging assessment within 2 weeks of completing the 3-day intervention and completed an identical scanning procedure as at baseline, including the same 5-min resting state scan. At both neuroimaging sessions, participants were instructed to passively view a fixation cross during the resting state scan period and not to sleep or engage in any meditation or relaxation practices (which were verbally confirmed in all participants at the conclusion of the neuroimaging session). In total 97% of randomized participants were retained at the post-intervention neuroimaging assessment (3% study attrition, see Figure 1). As part of the larger study, participants completed a comprehensive battery of psychosocial measures and provided a blood draw at baseline and at 4-month follow-up; the present report focuses on testing how mindfulness meditation training changes rsFC patterns using the 5-min resting state BOLD scan at baseline and in the 2 weeks following the 3-day intensive training period (post-intervention). In order to measure cumulative hypothalamic-pituitary-adrenal (HPA) axis activation over the 4-month follow-up period, participants were invited to provide a hair sample at the 4-month follow-up appointment (Dettenborn et al., 2010; Stalder and Kirschbaum, 2012).
Interventions
We adapted the standardized and manualized 8-week Mindfulness-Based Stress Reduction (MBSR) program (which includes a day-long retreat) (Kabat-Zinn, 1982, 1990) into a condensed 3-day residential retreat format, entitled Health Enhancement through Mindfulness (HEM). Delivery of the HEM program in a structured residential retreat format improves compliance with training, reduces treatment attrition and greater experimental control is afforded by offering a parallel matched relaxation training retreat (in a separate wing of the retreat center). The HEM instructor was a doctoral level psychologist with 7 years of MBSR teaching experience. Subjects were not informed that the mindfulness intervention was called HEM, so as to avoid any non-specific demand characteristics. Briefly, the HEM program consists of mindfulness training through body scan awareness exercises, sitting and walking meditations, mindful eating and mindful movement (gentle hatha yoga postures). After each formal meditation period, participants engaged in discussion of their observations about themselves and the practices. The instructor modeled and encouraged attitudes to foster mindfulness such as letting go of judgment and expectations, cultivating self-care, patience and friendly curiosity toward present moment experience. On the third day, formal meditation practices were extended to discussions about how participants could use mindful awareness for their unemployment and job-seeking stress.
We developed a structurally matched Health Enhancement through Relaxation (HER) program that included similar behavioral training activities (e.g. walking, stretching and didactics) as HEM, but all trainings emphasized participation in these activities in a restful way rather than a mindful way. The HER program instructor was a licensed social worker with over 2 decades of clinical experience in stress management. The use of a structurally matched active comparison group was designed to control for non-mindfulness specific factors such as positive treatment expectancies, group support, teacher attention, physical activity and mental engagement. An hour-by-hour outline of interventions is provided in Supplementary Materials.
Image acquisition
Structural and functional images were acquired on a Siemens Verio 3 T scanner using a 32-channel head coil. High-resolution T1-weighted gradient-echo images were acquired at the start of the scanning session, with a slice orientation of AC-PC aligned, temporal lobes up (TR = 1800 ms, TE = 2.22 ms, flip angle = 9°, matrix size = 256 × 256, number of slices = 256, FOV = (205 mm, 0.8-mm thick slices), GRAPPA accel. factor PE = 2, voxel size = 0.8 × 0.8 × 0.8 mm). Four functional echo-planar imaging runs were acquired, including a 300 s resting state scan (TR = 2000 ms, TE = 30 ms, flip angle = 79°, matrix size = 64 × 64, number of slices = 36, FOV = 205 mm, 3.2-mm thick slices EPI with rate 2 GRAPPA, voxel size = 3.2 × 3.2 × 3.2 mm).
Image pre-processing
Functional BOLD data were processed using SPM8 (Welcome Department of Cognitive Neurology, London, UK; implemented by MATLAB, MathWorks, Inc., Natick, MA, USA). First, the data were realigned to the mean image of the first run and then smoothed with a 4-mm FWHM Gaussian kernel to be in the preferred format for the motion correction program, ArtRepair. Data were then submitted to motion correction using the ArtRepair utility (Mazaika et al., 2007, 2009), an interpolation-based motion correction utility program. Motion correction in ArtRepair follows a two-step process. In the first step, an algorithm is applied to each run of data to suppress interpolation errors due to large motion. The algorithm applies a larger correction to edge-wise voxels than to central voxels, since the effects of motion on BOLD signal are most pronounced in these areas. In the second step, TRs with large amounts of fast motion or large global signal variation are flagged for repair. A default motion threshold of 1 mm was used, so that TRs with motion >1 mm were flagged for repair. Repair of the data was done through linear interpolation, so that volumes flagged for repair were filled in with the average signal value from the two nearest unrepaired TRs. To quantify head motion that could appreciably impact artifactual signal in our rsFC analyses, we had a hypothesis- and condition-blind coder count total fast head motion TRs for each subject at each resting state scan time point (quantified as a TR–TR change of 0.25 mm or 0.25° rotation in any plane). A mixed linear model analysis indicated there were no head motion differences between groups (condition main effect P = 0.97) and no differential fast head motion change over time (condition × time interaction P = 0.98). After motion correction the functional data was normalized to the standard MNI Template T1 template using indirect normalization, in which the functionals are first coregistered to the MPRAGE, and then the MPRAGE is normalized to the T1 template. Finally, the images were smoothed a second time with a 7-mm FWHM kernel, resulting in an overall FWHM smoothing of 8 mm (Mazaika et al., 2007).
Connectivity and data analyses
Left and right amygdala seeded resting state BOLD fMRI images were generated in the CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012) (using the same procedures as the discovery study), which were then applied in a group-level flexible factorial analysis in SPM8 with two factors specified, time (pre- and post-intervention) and group (HEM vs HER groups). We generated a time-by-group spreading (or ordinal) interaction contrast that tested for baseline to post-intervention decreases in rsFC in the HEM program (relative to the HER program, in which we did not expect to see this rsFC decrease) using contrast weights: [1(pre, HEM), 1(pre, HER), −3(post, HEM), 1(post, HER)]. As our primary interest was in testing the hypothesis that the mindfulness training program group would show connectivity change from baseline to post-treatment, relative to no change in the relaxation control training group, this approach is superior to approaches that compare the two treatment groups at post-treatment only (such as using post only contrast weights 1, −1), as they neglect baseline values and fail to evaluate change over time. The discovery study indicated stress-related bilateral amygdala-sgACC rsFC, thus we pursued amygdala rsFC analyses with a bilateral anatomical (AAL atlas) ACC ROI [defined by the WFU Pickatlas (Maldjian et al., 2003)] in the mindfulness meditation training RCT. Cluster-level correction for multiple comparisons was obtained using a Monte Carlo simulation implemented by AlphaSim (National Institute of Mental Health, Bethesda, MD). AlphaSim was implemented with a bilateral ACC mask generated from the AAL atlas using an 8 mm smoothing kernel and 10 000 iterations. Significant clusters (P < 0.05, corrected) were defined as those involving k > 27 contiguous voxels, each at P < 0.005.
Measuring stress in the mindfulness meditation training RCT: hair sampling
In order to evaluate whether changes in amygdala-ACC rsFC prospectively predict stress-related biomarkers after mindfulness meditation training, we conducted an exploratory analysis testing whether pre- to post-intervention changes in rsFC were associated with hair-derived cumulative measures of HPA-axis activation during the 4-month follow-up period (Stalder and Kirschbaum, 2012). Hair samples have been used to measure cumulative HPA-axis activation, with approximately 1 cm of hair length corresponding to one month of HPA-axis activity. Recent studies indicate higher HPA-axis activation among the unemployed relative to employed adults in hair samples (Dettenborn et al., 2010). Hair was acquired and assayed using standard procedures described by Kirschbaum and colleagues (2009, 2010). Briefly, about 40 hairs were cut with scissors as close to the back of the scalp as possible from a posterior vertex position. The follicle end of each hair sample was labeled and clipped to a piece of aluminum foil and then sent to a specialized laboratory in Dresden, Germany for assay. Each sample was washed, dried, and spun for steroid extraction (see Dettenborn et al., 2010). Cortisone and cortisol determination was then assessed using a commercially available immunoassay (with chemiluminescence detection, CLIA-IBL-Hamburg, Germany). The lab reports intra-assay and inter-assay coefficients of variance as below 8%.
Data on demographic and lifestyle factors that consistently affect hair glucocorticoid levels is limited, but previous studies suggest that age, gender and exercise volume (as vigorous exercise is associated with HPA axis activation) may be associated with hair cortisol (Raul et al., 2004; Russell et al., 2012; Skoluda et al., 2012; Stalder and Kirschbaum, 2012). Thus, participant age, gender and frequency of vigorous exercise were included as covariates in hair analyses in order to reduce noise attributable to these potential confounding factors. We analyzed both hair cortisone and cortisol; both are glucocorticoids released by the adrenal glands, with similar physiologic functions. Although cortisol is the more active form; previous research reports that hair cortisone may be higher than hair cortisol (as opposed to in plasma, where cortisol concentrations are higher than cortisone) due to increased activity of the enzyme responsible for converting cortisol to cortisone in the hair bulb (Tiganescu et al., 2011; Russell et al., 2012). We first conducted a one-way ANCOVA comparing the mindfulness to relaxation groups on hair-derived measures of cortisone and cortisol at the 4-month follow-up appointment. Parameter estimates were then extracted in SPM8 using cluster analysis centered on the peak voxel in subgenual ACC at baseline and post-training. Exploratory analyses then tested whether greater pre-post (change score) training-related changes in amygdala-ACC rsFC co-varied with lower levels of these hair-derived markers of cumulative HPA-axis activation at 4-month follow-up. Specifically, associations between hair cortisone, cortisol and post-pre training change in functional connectivity across groups were examined using bivariate Pearson correlation analyses. In total n = 4 participants were unable or unwilling to provide hair samples at the 4-month follow-up time point, and were excluded from these analyses. Statistical analyses were carried out in SPSS with a significance threshold of P < 0.05.
Results
Discovery study results
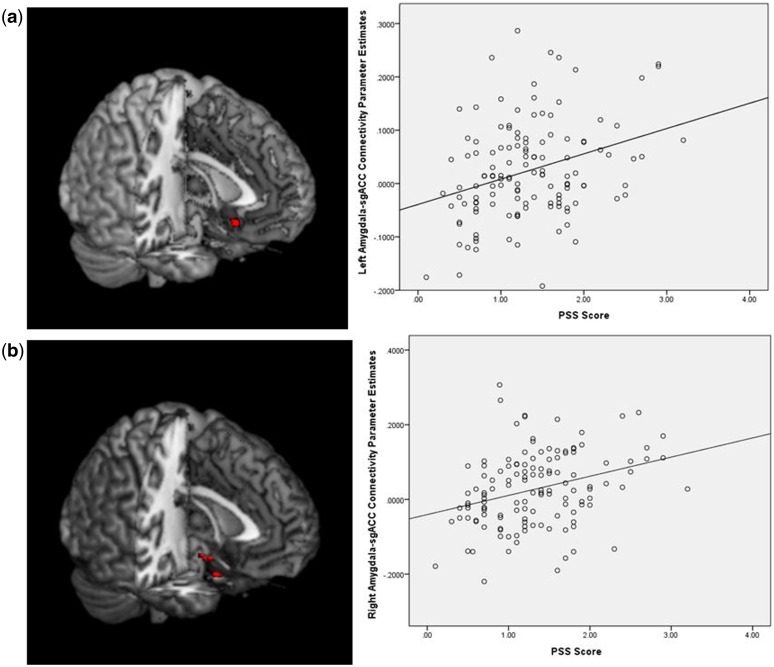
A whole-brain analysis indicated a positive association between perceptions of stress over the previous month and greater amygdala coupling with the sgACC. Specifically, higher levels of stress were associated with increased rsFC between right amygdala and subgenual ACC (sgACC) (MNI: 0, 16, −13, k = 120 voxels), the only region of significant correlation with the right amygdala in a whole-brain analysis (P < 0.001, uncorrected) (Figure 2a, Table 3). Although smaller in spatial extent, greater self-reported stress was also associated with increased left amygdala-sgACC (MNI: 1, 22, −4, k = 24 voxels) rsFC (P < 0.001, uncorrected) (Figure 2b). Higher levels of self-reported perceived stress were also associated with greater left amygdala rsFC with perigenual right ACC, left parahippocampal gyrus, left insula, and right superior temporal cortex in the discovery study (Table 4).
Fig. 2.
Discovery study (a, top left panel) Greater self-reported perceived stress on the PSS (item average) is associated with greater rsFC between right amygdala and subgenual ACC (P < 0.001). (a, top right panel) Scatterplot between perceived stress and right amygdala-sgACC rsFC parameter estimates (n = 130, R = −0.15). (b, bottom left panel) Greater self-reported perceived stress on the PSS (item average) is associated with greater rsFC between left amygdala and ACC (P < 0.001). (b, bottom right panel) Scatterplot between perceived stress and left amygdala-sgACC rsFC parameter estimates (n = 130, R = −0.101).
Table 3.
Subgenual ACC clusters identified in amygdala rsFC analyses
| Analysis | Subgenual ACC MNI peak coordinates | k | P | t | Z |
|---|---|---|---|---|---|
| Study 1, PSS, right amygdala | (0, 15.5, −13) | 120 | <0.001, uncorrected | 3.71 | 3.61 |
| Study 1, PSS, left amygdala | (1, 21.5, −4) | 24 | <0.001, uncorrected | 3.35 | 3.28 |
| Study 2, spreading interaction HEM < HER at post-intervention relative to baseline | (0, 18, −12) | 28 | <0.05, corrected | 2.93 | 2.82 |
Table 4.
Discovery study. PSS left amygdala seed connectivity, whole-brain analysis
| Increased coupling associated with higher PSS score, P < 0.001, k > 30 | Region (AAL) | Peak MNI coordinate | Cluster Size | t | Z |
|---|---|---|---|---|---|
| Left parahippocampal gyrus | −13, −11, −22 | 152 | 3.69 | 3.59 | |
| Right superior temporal | 56, −7, 3 | 80 | 3.77 | 3.66 | |
| Left insula | −39, −13, 11 | 112 | 3.92 | 3.80 | |
| Right perigenual anterior cingulum | 11, 30, 14 | 236 | 4.48 | 4.31 |
Mindfulness meditation training RCT Results
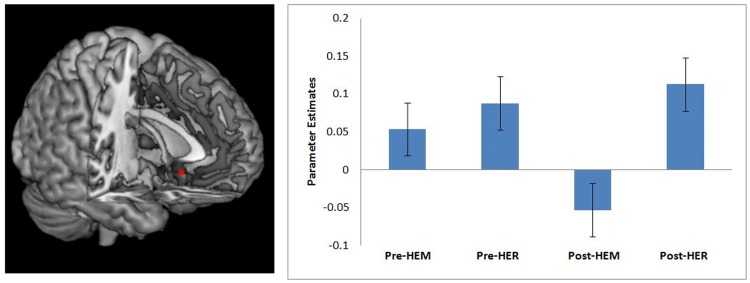
Building on the discovery study findings showing that stress was associated with greater amygdala-sgACC rsFC, it was predicted that mindfulness meditation training (relative to a well-matched relaxation training program without a mindfulness component), would decrease right amygdala-sgACC rsFC in stressed, unemployed community adults. Consistent with this prediction, a flexible factorial random effects analysis showed that a 3-day intensive mindfulness meditation training program reduced right amygdala-sgACC (MNI: 0, 18, −12) rsFC, compared with relaxation training without a mindfulness component (time × treatment interaction P < 0.05, k = 28, corrected for multiple comparisons) (Table 3, Figure 3). As shown in Figure 3, we observed positive right amygdala-sgACC rsFC pre-intervention in this high stress unemployed sample, which is consistent with the discovery study findings suggesting that stress is associated with elevated amygdala-sgACC rsFC (there were no significant difference in right amygdala-sgACC connectivity between the HEM and HER groups at baseline, P = 0.877). But notably this was a spreading interaction driven by mindfulness meditation training reductions in right amygdala rsFC at post-treatment (Figure 3). A parallel flexible factorial random effects analysis also tested for training related changes in left amygdala-ACC rsFC, but there were no significant differential decreases in left amygdala-ACC rsFC pre-post training (no significant time × treatment interaction). Although the primary intent was to test for stress-related amygdala-ACC rsFC in an ACC ROI analysis, exploratory uncorrected whole-brain results are provided in the Supplementary Materials.
Fig. 3.
Mindfulness Meditation Training RCT. The left panel depicts the region of sgACC that showed decreased rsFC with right amygdala from before to after mindfulness meditation training (HEM) relative to relaxation training (HER) (P < 0.05, corrected for multiple comparisons). The right panel depicts the mean percent signal change for subgenual ACC cluster for the mindfulness (HEM) and relaxation (HER) training groups at each of the two time points (pre- and post-intervention). Error bars depict ± 1 standard error. Parameter estimates were extracted in SPM8 and plotted in a random effects mixed model conducted in SPSS.
Change in amygdala rsFC co-vary with biomarkers of stress
An exploratory analysis evaluated whether hair-derived markers of cumulative HPA-axis were associated with brain activation during the 4-month follow-up period. There were no statistically significant differences between the mindfulness meditation and relaxation groups in cortisone [one-way ANCOVA F(1,25) = 0.08, P = 0.779; mindfulness group M = 54.99, SE = 43.63; relaxation group M = 72.12, SE = 40.75] or cortisol [one-way ANCOVA F(1,25) = 0.783, P = 0.385; mindfulness group M = 52.80, SE = 12.31; relaxation group M = 37.72, SE = 11.50] at 4-month follow-up. However, pre- or post-intervention decreases in amygdala-sgACC rsFC were associated with less cumulative HPA-axis activation in hair across all subjects. Specifically, training-related decreases for right amygdala-sgACC rsFC were inversely associated with hair cortisone [r(24) = −0.39, P = 0.049] and showed a trending association with hair cortisol [r(24) = −0.31, P = 0.102].
Despite recommendations to continue home practice after the 3-day retreat with customized compact discs, the high stress participants in the HEM and HER programs did not complete much formal guided practice in the 4-month follow-up period. HEM group participants reported using their home practice CD an average of 1.24 times per week (SD = 1.28) over the last month (at 4-month follow-up), while HER group participants reported using their home practice CD an average of 0.382 times per week (SD = 0.86) over the past month (t31 = 2.272, P = 0.03).
Discussion
We provide an initial indication that alterations in amygdala-ACC rsFC track with perceived stress and can be altered by a mindfulness meditation intervention. Specifically, self-reported perceived stress was found to be associated with greater amygdala-sgACC rsFC. Moreover, mindfulness meditation training decreased amygdala-sgACC functional coupling relative to a well-matched comparison relaxation treatment without a mindfulness component. These findings agree with a neural circuitry-based account of previous studies suggesting that mindfulness alters amygdala structure and function (Creswell et al., 2007; Goldin and Gross, 2010; Hölzel et al., 2010; Way et al., 2010; Taren et al., 2013), identifying a candidate amygdala-sgACC pathway that may link mindfulness training with reduced stress and stress-related health outcomes (Ludwig and Kabat-Zinn, 2008; Creswell, 2014). Although brain regions other than the amygdala are involved in stress reactivity, our approach was to first identify a candidate stress processing region that is central for gating stress responding (i.e. the amygdala) (Arnsten, 2009; Ulrich-Lai and Herman, 2009; McEwen and Gianaros, 2010) and test how trained mindfulness alters stress-related rsFC.
This discovery study indicates a positive association between perceived stress and amygdala–sgACC coupling. This finding is in accord with several studies, which link the right amygdala and ACC to stress. Specifically, the amygdala has well-known anatomical connectivity with regions of ACC (Carmichael and Price, 1996; Ongür et al., 1998; Freedman et al., 2000; Johansen-Berg et al., 2008), including sgACC (Freedman et al., 2000; Johansen-Berg et al., 2008)—and neurobiological accounts highlight the importance of amygdala and ACC/medial PFC networks in driving central fight-or-flight stress response cascades via activation of the HPA and sympathetic-adrenal-medullary axes (Arnsten, 2009). ACC regions spatially similar to regions identified in this study have been implicated in stress responding; stressor-evoked physiological reactivity has been positively associated with right amygdala-perigenual ACC connectivity (Gianaros et al., 2008). One important question for the present work is whether mindfulness training related changes in amygdala-sgACC rsFC could be prospectively driving changes in stress. We provide some initial suggestive evidence that pre-post training related decreases in right amygdala-sgACC are associated with lower cumulative markers of HPA-axis activation over the 4-month follow-up period in the mindfulness meditation RCT study. However, this exploratory analysis should be viewed with some caution (and tested in new studies) given the lack of any robust training differences between the mindfulness meditation and relaxation conditions in these cumulative HPA-axis activation biomarkers, and the small subsample who provided hair samples at 4-month follow-up in the RCT study (n = 30).
One limitation of the present research is that there were no stress-related disease outcomes in the RCT study with high stress community adults, and thus no way to evaluate whether mindfulness training changes in amygdala rsFC might drive potential improvements in stress-related disease. Nonetheless, this work offers a novel candidate pathway for explaining how mindfulness interventions benefit stress sensitive psychiatric populations. Previous studies have implicated disrupted rsFC in stress-related psychiatric disorders (e.g. PTSD, generalized anxiety disorder, major depressive disorder) (Mayberg, 2003; Bluhm et al., 2009; Etkin et al., 2009; Lanius et al., 2010), and recent RCTs indicate robust effects of mindfulness training on improving psychiatric outcomes in these same patient groups (Ma and Teasdale, 2004; Bhatnagar et al., 2013; Hoge et al., 2013; King et al., 2013; Zeidan et al., 2013). Moreover, higher levels of self-reported perceived stress have been linked to poorer mental and physical health outcomes (Keller et al., 2012). This research offers a testable new prediction for future studies; namely, mindfulness interventions may reduce amygdala-sgACC rsFC, which serves as a neurobiological mechanism for improvements in emotion regulation, stress reactivity and improved stress-related health and disease outcomes.
There has been a recent interest in how mindfulness meditation alters structural and functional activity in the ACC. For example, integrative mind-body meditation training has been shown to increase network efficiency and connectivity of the ACC (Xue et al., 2011) and recent work has shown that this form of meditation training increases white matter integrity in the corona radiata—the major tract projecting through ACC (Tang et al., 2010). Notably, sgACC is thought to be an important hub in networks for negative affect and mood disorders (Mayberg, 2003; Seminowicz et al., 2004) and anatomical studies have shown direct connections between sgACC and the amygdala, hypothalamus, nucleus accumbens and orbitofrontal cortex (whereas pregenual ACC is more strongly connected to medial prefrontal and mid-cingulate cortex, sgACC and subcortical regions) (Chiba et al., 2001). In combination, these studies and our work here suggest potential dissociable effects of mindfulness meditation training on spatially distinct regions of ACC; such as mindfulness meditation training increasing rsFC on more dorsal and pregenual ACC tracts associated with enhanced self-regulation (Tang et al., 2010; cf. Hasenkamp and Barsalou, 2012) and pain modulation (Zeidan et al., 2011), while also decreasing stress-related functional connectivity of the sgACC with the amygdala. Together, this work suggests the possibility that mindfulness meditation training fosters dorsal/pregenual ACC connectivity for attention monitoring while decoupling stress-related sgACC connectivity for stress resilience.
An important limitation of the present seed-based rsFC analytic approach is that it precludes inferences about the directionality of amygdala-sgACC connectivity; in the future, effective connectivity analyses (e.g. with dynamic causal modeling) offer opportunities to test causal interactions between these stress-sensitive nodes. Moreover, we utilized 5-min resting state scans; longer scans periods as well as cardiorespiratory data can be used to reduce measurement error in future studies (Birn et al., 2006, 2013). Our aim in this study was to reveal stable stress-related rsFC changes by using the PSS as a measure of stress symptoms over the past month (Liston et al., 2009); however, the scanner environment itself can be stress-inducing, and the lack of a state-stress scanner measure in this study does not allow us to fully exclude potential state-stress effects. An additional limitation is the lack of inclusion of a usual care group, which would provide an additional level of comparison to the HER and HEM intervention groups, allowing examination of the effects of attending a 3-day relaxation retreat and the potential stress-buffering gained from this (above and beyond no intervention). Given prohibitive cost and subject burden concerns, hair samples were not collected at time 1, only at 4-month follow-up; thus, our analyses are necessarily limited to relating change in amygdala-ACC rsFC to cortisone and cortisol levels at follow-up. Finally, this work more broadly suggests a new amygdala rsFC pathway for stress resilience; future work should evaluate whether similar changes in amygdala rsFC can be achieved with other clinically impactful psychological interventions (e.g. cognitive behavioral therapy) or anxiolytic pharmacological treatments in at-risk stressed populations.
Conclusions
The present findings significantly advance our understanding of rsFC in stress and mindfulness training interventions, and implicate decoupling of the amygdala and sgACC as a potential neurobiological mechanism underlying mindfulness training intervention effects.
Supplementary Material
Acknowledgements
The RCT is registered on clinicaltrials.gov (NCT01628809). Laura Pacilio, Baldwin Way, Lei Sheu and Shinzen Young provided advice the design, analysis, and interpretation of results. We thank the Scientific Imaging and Brain Research (SIBR) center for neuroimaging support and the Kearns Spirituality Center for use of their retreat center.
Funding
This research was supported by funding from the Pittsburgh Life Sciences Greenhouse Opportunity Fund and NIH R01 HL-089850.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Arnsten A.F.T. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience 10(6), 410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar R., Phelps L., Rietz K., Juergens T., Russell D., Miller N., et al. (2013). the effects of mindfulness training on post-traumatic stress disorder symptoms and heart rate variability in combat veterans. Journal of Alternative and Complementary Medicine, 19(11), 860–1. [DOI] [PubMed] [Google Scholar]
- Birn R.M., Diamond J.B., Smith M.A., Bandettini P.A. (2006). Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage, 31(4), 1536–48. [DOI] [PubMed] [Google Scholar]
- Birn R.M., Molloy E.K., Patriat R., Parker T., Meier T.B., Kirk G.R., et al. (2013). The effect of scan length on the reliability of resting-state fMRI connectivity estimates. NeuroImage, 83, 550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm R.L., Williamson P.C., Osuch E.A., Frewen P.A., Stevens T.K., Boksman K., et al. (2009). Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. Journal of Psychiatry and Neuroscience, 34(3), 187–94. [PMC free article] [PubMed] [Google Scholar]
- Bonanno G.A., Westphal M., Mancini A.D. (2011). Resilience to Loss and Potential Trauma. Annual Review of Clinical Psychology, 7, 511–35. [DOI] [PubMed] [Google Scholar]
- Carmichael S.T., Price J.L. (1996). Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. The Journal of Comparitive Neurology, 371(2), 179–207. [DOI] [PubMed] [Google Scholar]
- Chiba T., Kayahara T., Nakano K. (2001). Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Research, 888(1), 83–101. [DOI] [PubMed] [Google Scholar]
- Cohen S., Janicki-Deverts D., Miller G.E. (2007). Psychological stress and disease. Journal of the American Medical Association, 298(14), 1685–7. [DOI] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–96. [PubMed] [Google Scholar]
- Creswell J.D. (2014). Biological pathways linking mindfulness with health. In: Brown K.W., Creswell J.D., editors. Handbook of Mindfulness: Theory, Research, and Practice. New York, NY: Guilford Press. [Google Scholar]
- Creswell J.D., Myers H.F., Cole S.W., Irwin M. R. (2009). Mindfulness meditation training effects on CD4+ T lymphocytes in HIV-1 infected adults: a small randomized controlled trial. Brain, Behavior and Immunity, 23(2), 184–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell J.D., Pacilio L.E., Lindsay E.K., Brown K.W. (2014). Brief mindfulness meditation training alters psychological and neuroendocrine responses to social evaluative stress. Psychoneuroendocrinology , 44, 1–12. [DOI] [PubMed] [Google Scholar]
- Creswell J.D., Way B.M., Eisenberger N.I., Lieberman M.D. (2007). Neural correlates of dispositional mindfulness during affect labeling. Psychosomatic Medicine, 69(6), 560–5. [DOI] [PubMed] [Google Scholar]
- Desbordes G., Negi L.T., Pace T.W.W., Wallace B.A., Raison C.L., Schwartz E.L. (2012). Effects of mindful-attention and compassion meditation training on amygdala response to emotional stimuli in an ordinary, non-meditative state. Frontiers in Human Neuroscience, 6, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettenborn L., Tietze A., Bruckner F., Kirschbaum C. (2010). Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology, 35(9), 1404–9. [DOI] [PubMed] [Google Scholar]
- Etkin A., Prater K.E., Schatzberg A.F., Menon V., Greicius M.D. (2009). DIsrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of General Psychiatry, 66(12), 1361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson-Rose S.A., Lewis T.T. (2005). Psychosocial factors and cardiovascular diseases. Annu Rev Public Health, 26, 469–500. [DOI] [PubMed] [Google Scholar]
- Feder A., Nestler E.J., Charney D.S. (2009). Psychobiology and molecular genetics of resilience. Nature Reviews Neuroscience, 10(6), 446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience, 8(9), 700–11. [DOI] [PubMed] [Google Scholar]
- Freedman L.J., Insel T.R., Smith Y. (2000). Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. The Journal of Comparative Neurology, 421(2), 172–88. [PubMed] [Google Scholar]
- Gianaros P.J., Sheu L.K. (2009). A review of neuroimaging studies of stressor-evoked blood pressure reactivity: Emerging evidence for a brain-body pathway to coronary heart disease risk. NeuroImage, Brain Body Medicine, 47(3), 922–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros P.J., Sheu L.K., Matthews K.A., Jennings J.R., Manuck S.B., Hariri A.R. (2008). Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. Journal of Neuroscience, 28(4), 990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P.R., Gross J.J. (2010). Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion, 10(1), 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. (2008). Resting-state functional connectivity in neuropsychiatric disorders. Current Opinion in Neurology, 24, 424–30. [DOI] [PubMed] [Google Scholar]
- Hasenkamp W., Barsalou L.W. (2012). Effects of meditation experience on functional connectivity of distributed brain networks. Frontiers in Human Neuroscience, 6, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge E.A., Bui E., Marques L., Metcalf C.A., Morris L.K., Robinaugh D.J., et al. 2013. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. Journal of Clinical Psychiatry, 74(8), e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel B.K., Carmody J., Evans K.C., Hoge E.A., Dusek J.A., Morgan L., et al. (2010). Stress reduction correlates with structural changes in the amygdala. Social Cognitive and Affective Neuroscience, 5(1), 11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel B.K., Hoge E.A., Greve D.N., Gard T., Creswell J.D., Brown K.W., et al. (2013). Neural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training. NeuroImage: Clinical, 2, 448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H., Gutman D.A., Behrens T.E.J., Matthews P.M., Rushworth M.F.S., Katz E., et al. (2008). Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cerebral Cortex, 18(6), 1374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J. (1982). An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. General Hospital Psychiatry, 4(1), 33–47. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. (1990). Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. New York, NY: Delta. [Google Scholar]
- Keller A., Litzelman K., Wisk L.E., Maddox T., Cheng E.R., Creswell P.D., et al. (2012). Does the perception that stress affects health matter? The association with health and mortality. Health Psychology, 31(5), 677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A.P., Erickson T.M., Giardino N.D., Favorite T., Rauch S.A.M., Robinson E., et al. (2013). A pilot study of group mindfulness-based cognitive therapy (MBCT) for combat veterans with posttraumatic stress disorder (PTSD). Depression and Anxiety, 30(7), 638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C., Tietze A., Skoluda N., Dettenborn L. (2009). Hair as a retrospective calendar of cortisol production—increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology, 34(1), 32–7. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Bluhm R.L., Coupland N.J., Hegadoren K.M., Rowe B., Théberge J., et al. 2010. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatrica Scandivica, 121(1), 33–40. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. (1994). The amygdala: contributions to fear and stress. Seminars in Neuroscience, 6, 231–7. [Google Scholar]
- Liston C., McEwen B.S., Casey B.J. (2009). Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences, 106(3), 912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig D.S., Kabat-Zinn J. (2008). Mindfulness in medicine. Journal of the American Medical Association, 300(11), 1350–2. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19(3), 1233–9. [DOI] [PubMed] [Google Scholar]
- Ma S.H., Teasdale J.D. (2004). Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. Journal of Consulting and Clinical Psychology, 72(1), 31–40. [DOI] [PubMed] [Google Scholar]
- Mayberg H.S. (2003). Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. British Medical Bulletin, 65, 193–207. [DOI] [PubMed] [Google Scholar]
- Mazaika P., Hoeft F., Glover G., Reiss A. (2009). Methods and Software for fMRI Analysis for Clinical Subjects. San Francisco, CA: Human Brain Mapping. [Google Scholar]
- Mazaika P., Whitfield-Gabrieli S., Reiss A. (2007). Artifact repair for fMRI data from motion clinical subjects. Presented at the Organization for Human Brain Mapping Annual Conference, Chicago, IL. [Google Scholar]
- McEwen B.S., Gianaros P.J. (2010). Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences, 1186, 190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Chen E., Cole S.W. (2009). Health psychology: developing biologically plausible models linking the social world and physical health. Annual Review of Psychology, 60, 501–24. [DOI] [PubMed] [Google Scholar]
- Nyklíček I., Mommersteeq P.M., Van Beugen S., Ramakers C., Van Boxtel G.J. (2013). Mindfulness-based stress reduction and physiological activity during acute stress: a randomized controlled trial. Health Psychology, 32(10), 1110–3. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Ongür D., An X., Price J.L. (1998). Prefrontal cortical projections to the hypothalamus in macaque monkeys. Journal of Comparative Neurology, 401(4), 480–505. [PubMed] [Google Scholar]
- Raul J.-S., Cirimele V., Ludes B., Kintz P. (2004). Detection of physiological concentrations of cortisol and cortisone in human hair. Clinical Biochemistry, 37(12), 1105–11. [DOI] [PubMed] [Google Scholar]
- Russell E., Koren G., Rieder M., Van Uum S. (2012). Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology, 37(5), 589–601. [DOI] [PubMed] [Google Scholar]
- Seminowicz D., Mayberg H., McIntosh A., Goldapple K., Kennedy S., Segal Z., et al. (2004). Limbic–frontal circuitry in major depression: a path modeling metanalysis. NeuroImage, 22(1), 409–18. [DOI] [PubMed] [Google Scholar]
- Skoluda N., Dettenborn L., Stalder T., Kirschbaum C. (2012). Elevated hair cortisol concentrations in endurance athletes. Psychoneuroendocrinology, 37(5), 611–7. [DOI] [PubMed] [Google Scholar]
- Stalder T., Kirschbaum C. (2012). Analysis of cortisol in hair—state of the art and future directions. Brain, Behavior and Immunity, 26(7), 1019–29. [DOI] [PubMed] [Google Scholar]
- Tang Y.-Y., Hölzel B.K., Posner M.I. (2015). The neuroscience of mindfulness meditation. Nature Reviews Neurosciecne, 16(4), 213–25. [DOI] [PubMed] [Google Scholar]
- Tang Y.-Y., Lu Q., Geng X., Stein E.A., Yang Y., Posner M.I. (2010). Short-term meditation induces white matter changes in the anterior cingulate. Proceedings of the National Academy of Sciences, 107(35), 15649–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taren A.A., Creswell J.D., Gianaros P.J. (2013). Dispositional mindfulness co-varies with smaller amygdala and caudate volumes in community adults. PLoS One, 8(5), e64574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor V.A., Grant J., Daneault V., Scavone G., Breton E., Roffe-Vidal S., et al. (2011). Impact of mindfulness on the neural responses to emotional pictures in experienced and beginner meditators. NeuroImage, 57(4), 1524–33. [DOI] [PubMed] [Google Scholar]
- Teasdale J.D., Segal Z.V., Mark J., Ridgeway V.A., Soulsby J.M., Lau M.A. (2000). Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology, 68(4), 615–23. [DOI] [PubMed] [Google Scholar]
- Tiganescu A., Walker E.A., Hardy R.S., Mayes A.E., Stewart P.M. (2011). Localization, age- and site-dependent expression, and regulation of 11β-hydroxysteroid dehydrogenase type 1 in skin. Journal of Investigative Dermatology, 131(1), 30–6. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y.M., Herman J.P. (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neurosciences, 10(6), 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Marle H.J.F., Hermans E.J., Qin S., Fernández G. (2010). Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. NeuroImage, 53(1), 348–54. [DOI] [PubMed] [Google Scholar]
- Veer I.M., Oei N.Y.L., Spinhoven P., van Buchem M.A., Elzinga B.M., Rombouts S.A.R.B. (2011). Beyond acute social stress: increased functional connectivity between amygdala and cortical midline structures. NeuroImage, 57(4), 1534–41. [DOI] [PubMed] [Google Scholar]
- Way B.M., Creswell J.D., Eisenberger N.I., Lieberman M.D. (2010). Dispositional mindfulness and depressive symptomatology: correlations with limbic and self-referential neural activity during rest. Emotion, 10(1), 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–41. [DOI] [PubMed] [Google Scholar]
- Xue S., Tang Y.-Y., Posner M.I. (2011). Short-term meditation increases network efficiency of the anterior cingulate cortex. Neuroreport, 22(12), 570–4. [DOI] [PubMed] [Google Scholar]
- Zeidan F., Martucci K.T., Kraft R.A., Gordon N.S., McHaffie J.G., Coghill R.C. (2011). Brain mechanisms supporting the modulation of pain by mindfulness meditation. Journal of Neuroscience, 31(14), 5540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F., Martucci K.T., Kraft R.A., McHaffie J.G., Coghill R.C. (2013). Neural correlates of mindfulness meditation-related anxiety relief. Social Cognitive and Affective Neuroscience , 9(6), 751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.