Abstract
The formation of more polar and toxic polycyclic aromatic hydrocarbon (PAH) transformation products is one of the concerns associated with the bioremediation of PAH-contaminated soils. Soil contaminated with coal tar (pre-bioremediation) from a former manufactured gas plant (MGP) site was treated in a laboratory scale bioreactor (post-bioremediation) and extracted using pressurized liquid extraction. The soil extracts were fractionated, based on polarity, and analyzed for 88 PAHs (unsubstituted, oxygenated, nitrated, and heterocyclic PAHs). The PAH concentrations in the soil tested, post-bioremediation, were lower than their regulatory maximum allowable concentrations (MACs), with the exception of the higher molecular weight PAHs (BaA, BkF, BbF, BaP, and IcdP), most of which did not undergo significant biodegradation. The soil extract fractions were tested for genotoxicity using the DT40 chicken lymphocyte bioassay and developmental to xicity using the embryonic zebrafish (Danio rerio) bioassay. A statistically significant increase in genotoxicity was measured in the unfractionated soil extract, as well as in four polar soil extract fractions, post-bioremediation (p < 0.05). In addition, a statistically significant increase in developmental toxicity was measured in one polar soil extract fraction, post-bioremediation (p < 0.05). A series of morphological abnormalities, including peculiar caudal fin malformations and hyperpigmentation in the tail, were measured in several soil extract fractions in embryonic zebrafish, both pre- and post-bioremediation. The increased toxicity measured post-bioremediation is not likely due to the 88 PAHs measured in this study (including quinones), because most were not present in the toxic polar fractions and/or because their concentrations did not increase post-bioremediation. However, the increased toxicity measured post-bioremediation is likely due to hydroxylated and carboxylated transformation products of the 3- and 4-ring PAHs (PHE, 1MPHE, 2MPHE, PRY, BaA, and FLA) that were most degraded.
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are a group of environmental contaminants formed through the incomplete combustion of organic matter. PAHs are of concern because some are toxic, suspected or known mutagens and/or carcinogens, and some tend to be persistent in the environment.1–3 These pollutants are primary constituents in soils at manufactured gas plant (MGP) sites, where sources of PAHs often include coal tar.4 Due to the relative stability and hydrophobic character of PAHs, soil ultimately acts as a major sink for these compounds.5,6
Bioremediation uses microorganisms to decrease PAH concentrations in soil, thus reducing their associated risks.7 However, under certain conditions, reductions in PAH concentrations do not necessarily correspond with decreased soil toxicity.8,9 Incomplete degradation, or oxidation, of PAHs may lead to the formation of more polar and mobile PAH transformation products, which may include PAH derivatives containing oxygen groups (OPAHs), and nitro groups (NPAHs). These more polar PAH compounds are not as well-studied in bioremediation systems, and could be present alongside PAHs, serving both as co-contaminants and/or remedial transformation products. Additionally, they may be more reactive and potentially more toxic due to the presence of electronegative atoms.10–14 For instance, some OPAHs and NPAHs are known to exhibit greater toxicity than their corresponding unsubstituted PAH precursors and do not require enzymatic activation to express toxicity.12–16 Heterocyclic PAHs, HPAHs (PAH derivatives containing heteroatoms oxygen, nitrogen, or sulphur), have been shown to contribute significantly to toxicity at contaminated sites, and their metabolites have been linked to endocrine disruption.17,18
Beyond monitoring PAHs, chiefly those labeled as the 16 United States Environmental Protection Agency (U.S. EPA) PAH priority pollutants, the formation of PAH transformation products is not commonly measured at remediation sites. In complex and dynamic biological systems, it can be difficult to reliably predict the transformation products that will be formed. Additionally, environmental analysis of PAH transformation products, and more polar PAHs, is more challenging than that of the PAHs because they may be present in lower concentrations, are more reactive, and are strongly influenced by matrix interferences from soil organic matter and unresolved complex mixtures.19 Compared with PAHs, there is also a lack of labeled standards and certified reference materials for these compounds.
Previous studies have used an effects-directed analysis (EDA) approach to assess toxicity changes during or after remediation. These previous studies have predominantly used bacterial and in vitro mammalian-cell assays,20–23 which can be marred by high false positives and negatives, as well as limited sensitivities.24,25 The DT40 bioassay uses DNA damage repair-deficient mutants of the parental DT40 cell line to measure genotoxicity, and the response to mutagenic chemicals in these repair-deficient mutants is marked by an increase in chromosomal aberrations relative to the parental DT40 cell line.26–28 The advantages of this assay include quick proliferation rates, a resemblance to higher eukaryotic cells, and high gene targeting efficiencies necessary in the production of deficient-repair mutants.28 Another unique feature of DT40 cells is their apparent lack of a functional p53 protein, which can induce apoptosis in the presence of cell stress. The lack of a functioning p53 protein ensures that the cell death observed is due to failures in specific DNA-damage repair pathways rather than from apoptosis activated by the cell in response to DNA damage.29 While many assays can determine whether a toxin is mutagenic or not, the DT40 bioassay provides information on the mode of action, which can shed more light in understanding how certain chemicals are likely to behave in human exposure scenarios.26
The embryonic zebrafish assay (Danio rerio) is an effective in vivo model to assess the developmental toxicity of environmental toxicants.30,31 Zebrafish share significant genetic and physiological homology with humans, and there is growing evidence that zebrafish can rival or exceed rodent models in predicting human disease outcomes.32,33 To the best of our knowledge, no studies have used the embryonic zebrafish assay to study the effect of bioremediation on PAH contaminated soils. However, a recent study by Wincent et al. investigated the developmental toxicity in zebrafish in soil from multiple industrial sites, and found that in gas contaminated soil, there was greater developmental toxicity associated with the relatively more polar oxygenated fraction than with the PAH fraction.34
While some studies on the bioremediation of PAH contaminated soils measured a general decrease in soil toxicity following bioremediation,35–37 other studies measured an increase, suggesting the formation of toxic transformation products and/or metabolites.8,20–22,36 However, an in depth investigation into potentially toxic PAH transformation products has not been carried out. The objectives of this study were to (1) use an EDA approach to begin to identify potentially toxic PAH transformation products, as well as eliminate non-toxic PAH transformation products, in bioremediated soil; and (2) use changes in PAH, OPAH, NPAH, and HPAH concentrations, pre- and post-bioremediation, as a possible explanation for changes in soil toxicity. Soil contaminated with coal tar was extracted pre- and post-bioremediation, the extract was fractionated based on polarity, and the fractions were evaluated for changes in PAH, OPAH, NPAH, and HPAH concentrations, as well as for genotoxicity and developmental toxicity using the DT40 and zebrafish bioassays, respectively.
MATERIALS AND METHODS
Chemicals
Standard solutions of PAHs and methyl PAHs were purchased from AccuStandard (New Haven, CT) and Chem Service (West Chester, PA), OPAHs from Sigma Aldrich (St. Louis, MO), HPAHs from AccuStandard (New Haven, CT) and Sigma Aldrich (St. Louis, MO), and NPAHs from AccuStandard (New Haven, CT). All 88 PAHs studied and their abbreviations are listed in Table 1. Isotopically labeled standards used as surrogates and internal standards for PAHs and methyl PAHs, OPAHs, HPAHs, and NPAHs were purchased from CDN Isotopes (Point-Claire, Quebec) and are listed in the supporting information.
Table 1.
PAHs measured, their abbreviations, and the soil extracts that contained them. Where more than two fractions are listed, the first fraction was the primary fraction. Nitrated PAHs were not detected in study above LOD 0.3 ng g−1.
| Unsubstituted, methyl PAHs |
Abbr. | Primary Fraction |
OPAHs | Abbr. | Primary Fraction |
NPAHs | Abbr. | Primary Fraction |
|---|---|---|---|---|---|---|---|---|
| Naphthalene | NAP | A | 9-Fluorenone | 9FLO | C | 1-Nitronaphthalene | 1NNAP | B |
| 2-Methylnaphthalene | 2MNAP | A | 1,4-Naphthoquinone | 1,4NQ | C | 2-Nitronaphthalene | 2NNAP | B |
| 1-Methylnaphthalene | 1MNAP | A | Acenaphthenequinone | ACEN | B | 2-Nitrobiphenyl | 2NBP | B |
| 2,6-Dimethylnaphthalene | 2,6MNAP | A | Phenanthrene-1,4-dione | 1,4PD | B | 3-Nitrobiphenyl | 3NBP | B |
| 1,3-Dimethylnaphthalene | 1,3MNAP | A | 9,10-Anthraquinone | 9,10AQ | B | 4-Nitrobiphenyl | 4NBP | B |
| Acenaphthylene | ACEY | A | 1,4-Anthraquinone | 1,4AQ | B | 3-Nitrodibenzofuran | 3NBF | B |
| Acenaphthene | ACE | A | 2-methyl-9,10-anthraquinone | 2M9,10AQ | C | 5-Nitroacenaphthene | 5NACE | B |
| Fluorene | FLU | A | 2-Ethyl-9,10-Anthraquinone | 2E9,10AQ | B | 2-Nitrofluorene | 2NF | B |
| Phenanthrene | PHE | A | 9,10-Phenanthrenequinone | 9,10PQ | C | 9-Nitroanthracene | 9NANT | B |
| Anthracene | ANT | A | Benzo[a]fluorenone | BaF | B | 9-Nitrophenanthrene | 9NPHE | B |
| 2-Methylphenanthrene | 2MPHE | A | Benzanthrone | BZ | B | 2-Nitrodibenzothiophene | 2DBT | B |
| 2-Methylanthracene | 2MANT | A | Aceanthrenequinone | ACEAN | C | 3-Nitrophenanthrene | 3NPHE | B |
| 1-Methylphenanthrene | 1MPHE | A | Benzo[c]phenanthrene-[1,4]quinone | Bc1,4Q | B | 2-Nitroanthracene | 2NANT | B |
| 3,6-Dimethylphenanthrene | 3,6MPHE | A | 7,12-Benzo[a]anthracene dione | 7,12BaAD | B | 2-Nitrofluoranthene | 2NF | B |
| Fluoranthene | FLA | A | Benzo[cd]pyrenone | BcdP | B | 3-Nitrofluoranthene | 3NF | B |
| Pyrene | PYR | A | 5,12-Napthacenequinone | 5,12NQ | C | 1-Nitropyrene | 1-NP | B |
| Retene | RET | A | 1,6-Benzo[a]pyrene quinone | 1,6BaPQ | C | 2-Nitropyrene | 2NP | B |
| Benz[c]fluorene | BcF | A | HPAHs | 2,8-Dinitrodibenzothiophene | 2-NP | B | ||
| 1-Methylpyrene | 1MPYR | A | 2-Methylbenzofuran | 2MBZ | C | 7-Nitrobenz[a]anthracene | 2NBaA | B |
| Cyclopenta[cd]pyrene | CdeP | A | Thianapthene | THN | B | 1-Nitrotriphenylene | 1-NTRI | B |
| Benzo(a)anthracene | BaA | A | Quinoline | QUI | E, F | 6-Nitrochyrsene | 6NChr | B |
| Chrysene + Triphenylene | CHR+TRI | A | Indole | IND | E, F | 3-Nitrobenzanthrone | 3NBZ | B |
| 6-Methylchrysene | 6MCHR | A | 8-Methylquinoline | 8MQ | C | 2-Nitrotriphenylene | 2NTRI | B |
| Benzo(b)fluoranthene | BbF | A | Dibenzofuran | DBF | A | 1,3-Dinitropyrene | 1,3NP | B |
| Benzo(k)fluoranthene | BkF | A | Xanthene | XAN | B | 1,6-Dinitropyrene | 1,6NP | B |
| Benz[j][e]aceanthrylene | BjeA | A | 5,6-Benzoquinoline | 5,6BQ | A | 1,8-Dinitropyrene | 1,8NP | B |
| Benz(e)pyrene | BeP | A | Acridine | ACR | B | 6-Nitrobenzo(a)pyrene | 6-NBaP | B |
| Benzo(a)pyrene | BaP | A | Carbazole | CAR | A, B | |||
| Dibenz(a,c)anthracene | DacP/DahP | A | Dibenzothiophene | DBZ | A, B | |||
| Indeno(1,2,3-cd)pyrene | IcdP | A | ||||||
| Benzo(ghi)perylene | BghiP | A | ||||||
| Anthranthrene | ANTH | A |
Study Area and Soil Samples
Soil contaminated with coal tar was collected from a former MGP site in Salisbury, North Carolina.8 The soil was treated in an aerobic laboratory-scale bioreactor under conditions previously described.8,38 The contaminated soil before treatment was labeled as “pre-bioremediation” and after treatment as “post-bioremediation.”
Pressurized Liquid Extraction (PLE)
Approximately 0.5 g wet weight soil was extracted in 100 mL cells using an Accelerated Solvent Extractor (ASE) (Dionex ASE 350) in hexane:acetone (75:25, v/v) (1500 psi, 100 °C, 3 cycles, 240 s purge). ASE is an exhaustive extraction technique that is useful for extracting the majority of PAHs, OPAHs, NPAHs, and HPAHs from the soil samples.39 However, it is a worst case scenario in terms of estimating bioavailable concentrations.4,40 The extract was then split 75% for toxicity testing and 25% for chemical analysis and the portion undergoing chemical analysis was spiked with isotopically labeled surrogate standards. This was done so that the DT40 cells and zebrafish embryos were not exposed to potentially toxic isotopically labeled PAHs and to ensure that the extracts being chemically analyzed were the same as the extracts undergoing toxicity testing. Dry weights of soil were obtained after drying at 120 °C for 24 h. All concentrations are reported on a dry weight basis.
Fractionation
The toxicological and chemical portions of the extract were fractionated into fourteen 25 mL fractions using 20 g silica solid phase extraction (SPE) cartridges from Agilent (Santa Clara, CA) (Table 2). However, due to the intensive fractionation and to ensure there was enough soil residue to elicit a response in the DT40 assay, these fractions were combined into six composite fractions A, B, C, D, E, and F, as shown in Table 2. Soil was also extracted, and not fractionated (“unfractionated”), and analyzed with the fractionated soil extracts. Lab blanks consisting of sodium sulfate were extracted and analyzed for target PAHs and toxicity alongside soil extracts. The extracts undergoing chemical analysis were evaporated down to a final volume of 300 µL. The extracts undergoing toxicological analysis were evaporated just to dryness under a flow of nitrogen in pre-weighed vials. The mass of the dry residue was measured using an analytical balance, and the residue was re-dissolved in dimethyl sulfoxide (DMSO) (Sigma, St. Louis, MO) to a concentration of approximately 10,000 µg soil residue per mL DMSO.
Table 2.
Silica solid phase extraction solvent elution composition for soil extract fractions A–F.
| Soil fraction | Composite Solvent Elution [v/v] |
|---|---|
| A (least polar) | 100% Hexane |
| 90:10 Hexane:Dichloromethane | |
| 80:20 Hexane:Dichloromethane | |
| 70:30 Hexane:Dichloromethane | |
| B | 60:40 Hexane:Dichloromethane |
| 50:50 Hexane:Dichloromethane | |
| 40:60 Hexane:Dichloromethane | |
| C | 30:70 Hexane:Dichloromethane |
| 20:80 Hexane:Dichloromethane | |
| D | 10:90 Hexane:Dichloromethane |
| 100% Dichloromethane | |
| E | 100% Ethyl acetate |
| F (most polar) | 100% Acetone (2 cycles) |
Chemical Analysis
Gas chromatographic/mass spectrometry (GC/MS) analysis was carried out using an Agilent 6890 GC system, equipped with a mass selective detector on a DB-5MS (30 m × 0.25 mm I.D. × 0.25 µm film thickness) capillary column. The soil extracts were spiked with isotopically labeled internal standards prior to GC/MS analysis. PAHs and methyl PAHs, and HPAHs were analyzed in electron impact ionization (EI) mode, while OPAHs and NPAHs were analyzed in electron capture negative ionization (ECNI) mode.41–43 CHR and DahA were not resolved from TRI and DacA, respectively, and were reported as a sum (i.e. CHR+TRI and Dah+acA).
DT40 Bioassay
The toxicological soil extracts were stored at −80 °C prior to exposure. They were serially diluted with phosphate-buffered saline (PBS) (Life Technologies, Grand Island, NY) and administered to the DT40 cell line and the mutant Rad54−/− and Rev1−/− cells. A DMSO blank, diluted with PBS, was used as a negative control. The cells were incubated at 39.5 °C for at least 48 h, at 5% CO2 and 95% relative humidity.28 After incubation, the cells were treated with 2, 3-bis [2-methoxy-4-nitro-5-sulfo-phenyl]-2H-tetrazolium-5-carbox-anilide salt (XTT dye) (Sigma, St. Louis, MO) and returned to the incubator to allow for dye metabolism. Once the dye was metabolized and the cells had developed sufficient color (approximately after 4 to 6 h), the absorbance was determined using a Vmax kinetic microplate reader (Molecular Devices, Sunnyvale, CA) and related to percentage cell survival.8 Details on the DT40 bioassay cell culturing, exposure method, and maintenance are reported elsewhere.28
Embryonic Zebrafish Bioassay
The toxicological soil extracts were stored at −20 °C until 1 h prior to exposure. They were diluted in DMSO in a 96-well plate to 1171 µg residue per mL DMSO, then diluted further 8 times in a 5-fold serial dilution. Ten microliters were taken from the initial dilution to create a 10% DMSO in embryo media (EM) dilution row. Ten microliters were taken from the second dilution and added to the embryo-loaded 90 uL of EM. Ten microliters were added to each row of 4 exposure plates. The final DMSO concentration was 1% (v/v). A 1% DMSO vehicle control was used on every exposure plate. If mortality and morbidity, combined, were greater than 15% in the vehicle control, the exposures were re-run. Further details of the zebrafish method are reported elsewhere.31,44
Statistical Analysis
Median lethal concentrations (LC50) were determined using Graphpad PRISM software, while statistical analyses were conducted using Microsoft® Excel 2013 and JMP (Statistical Discovery™ from SAS) software. Student t-tests were used to identify statistically significant changes in PAH concentrations and toxicity, post-bioremediation (p < 0.05).
RESULTS AND DISCUSSION
Chemical Analysis
Unfractionated Soil Extracts
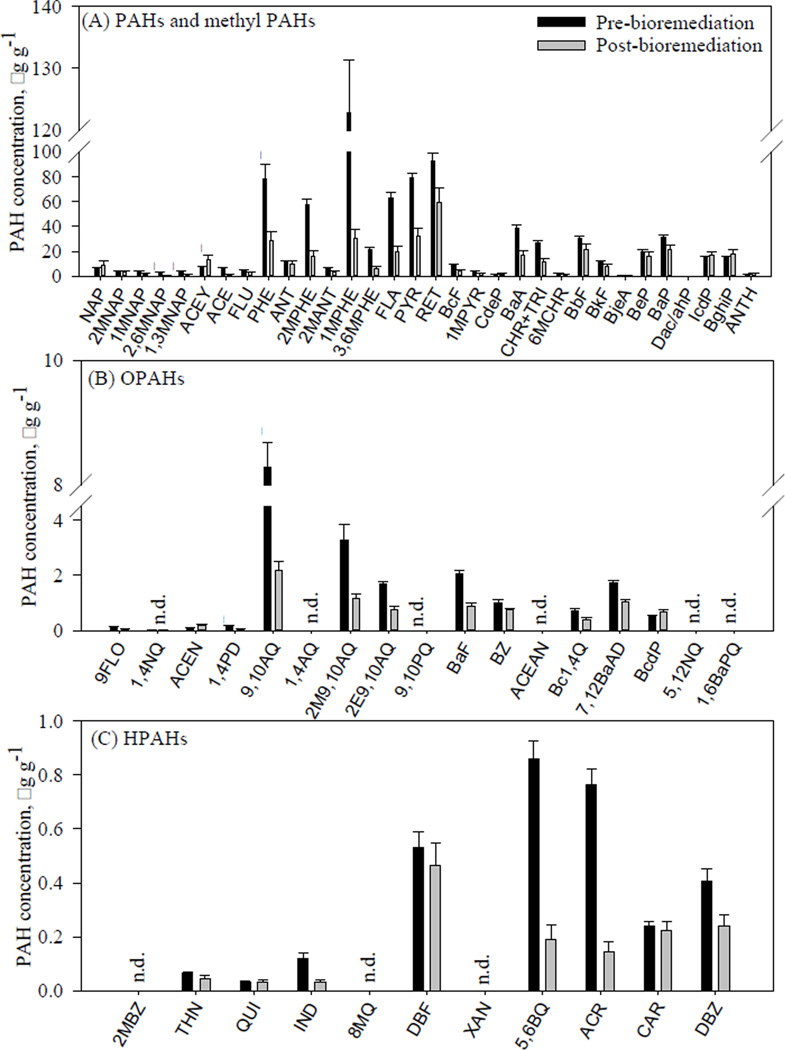
Pre-bioremediation, the total PAH (PAHs and methyl PAHs, OPAHs, and HPAHs) concentrations in the unfractionated soil extract ranged from 0.01 to 123 µg g−1, while concentrations post-bioremediation ranged from 0.03 to 60 µg g−1 (Figure 1, Table S1). No NPAHs were detected above the limit of detection (LOD) of 0.3 ng g−1. The sum of PAH and methyl PAH concentrations accounted for about 97% of the total PAH, OPAH and HPAH concentration, with 3- and 4-ring PAHs (including PHE, 1MPHE, 2MPHE, PYR, BaA, and FLA), having the highest concentrations and showing the greatest reduction in concentration, post-bioremediation (Figure 1A). The higher molecular weight 5- and 6-ring PAHs (ANTH, BghiP, IcdP, BaP, and BeP) were not biodegraded (Figure 1A).45,46 Because higher molecular PAHs are more hydrophobic, they tend to sorb strongly to organic matter and may not be available to microorganisms for biodegradation.6,45–47
Figure 1.
Mean concentrations in dry weight (with standard errors bars, n = 3) of investigated (A) PAHs and methyl PAHs, (B) OPAHs and, (C) HPAHs pre- and post-bioremediation in the unfractionated soil extract. Compounds with asterisks (*) showed significant changes in concentration post-bioremediation (p < 0.05). No NPAHs were detected above the limit of detection (0.3 ng g−1). (n.d. = not detected).
The sum 16 U.S. EPA PAH priority pollutants (excluding CHR and DahA) concentration was reduced 45% post-bioremediation, and is comparable to previous studies, where removal percentages for these compounds were between 40 and 77%.8,20,22,45,47 Maximum allowable concentrations (MACs) for priority PAHs in industrial soils have been proposed by regulatory agencies and governments, including the U.S. EPA, the Canadian Council of Ministers of the Environment (CCME), and the German Federal Government (Table S2).48–50 The PAH concentrations in the soil, post-bioremediation, were lower than their corresponding MACs, with the exception of the higher molecular weight PAHs (BaA, BkF, BbF, BaP, and IcdP) (Table S2). The higher molecular weight PAHs have the lowest regulated MACs (0.29 – 12 µg g−1), likely because of their classification as B2 probable human carcinogens by the U.S. EPA.51
The sum of OPAHs accounted for about 2% of the total PAH, OPAH, and HPAH concentration, both pre- and post-bioremediation (Figure 1B). The sum of OPAH concentration was reduced 58%, post-bioremediation, with 9,10AQ, 2M9,10AQ, E9,10AQ, and BaF concentrations significantly reduced (p < 0.05). Though other studies have noted increases post-bioremediation in certain OPAHs, including 9FLO,23,52 we did not measure any significant increases in OPAH concentrations, post-bioremediation.
The HPAHs were measured at the lowest concentrations, accounting for about 0.3% of the total PAH, OPAH, and HPAH concentration. Of the HPAHs, IND, 5,6BQUI, and ACR concentrations were significantly reduced post-bioremediation (p < 0.05) (Figure 1C). Previous studies have shown that the presence of HPAHs can inhibit the degradation of PAHs.53,54
The formation of polar PAH transformation products during bioremediation may vary depending on a number of factors, including: degree of contamination, bioremediation conditions, microbial community composition, and soil properties.55 In addition, compared to unsubstituted PAHs, less is known about the degradation pathways and microorganisms that can degrade these polar PAHs. For instance, Rodgers-Vieira et. al recently identified the first bacterial strain capable of degrading 9,10AQ, but noted that this strain differed from the ANT degrading strain, implying that, while bacteria may be equipped to degrade the unsubstituted PAHs, they might not necessarily be equipped to degrade corresponding OPAHs.56
Fractionated Soil Extracts
The soil extracts were fractionated into six fractions based on polarity, A to F (Table 2), and analyzed to identify which fractions contained the PAHs and methyl PAHs, OPAHs, HPAHs, and NPAHs (Table 1). The purpose of fractionating the soil extract was not to isolate the different PAH classes, but to simplify the complex mixture of PAHs in the soil extract and to better link the measured toxicity of a fraction to the chemistry of a fraction. The PAHs and methyl PAHs, the least polar of the PAH classes, were primarily contained in fraction A. The majority of the individual OPAHs, which are more polar than the PAHs and methyl PAHs, were primarily contained in fractions B and C. This includes the potential quinone products of the 3- and 4-ring PAHs that biodegraded, such as 9FLO. The polarities of the HPAHs vary depending on the heteroatom and the number of rings. The least polar HPAHs were contained in fractions A and B, while the more polar HPAHs were contained in fractions E and F. Though NPAHs were not measured above the LOD in the soil, a spike and recovery experiment showed that they would be contained primarily in fraction B.
DT40 Bioassay
DNA damage repair-deficient mutants Rad54−/− and Rev1−/− were used to evaluate DNA damage in the soil extracts, pre- and post-bioremediation. Rad54−/− and Rev1−/− are both sensitive to a wide range of DNA damaging agents and indicate w hether the formation of DNA double-strand breaks (Rad54−/−) or translesion synthesis (Rev1−/−) DNA damage has occurred.57,58
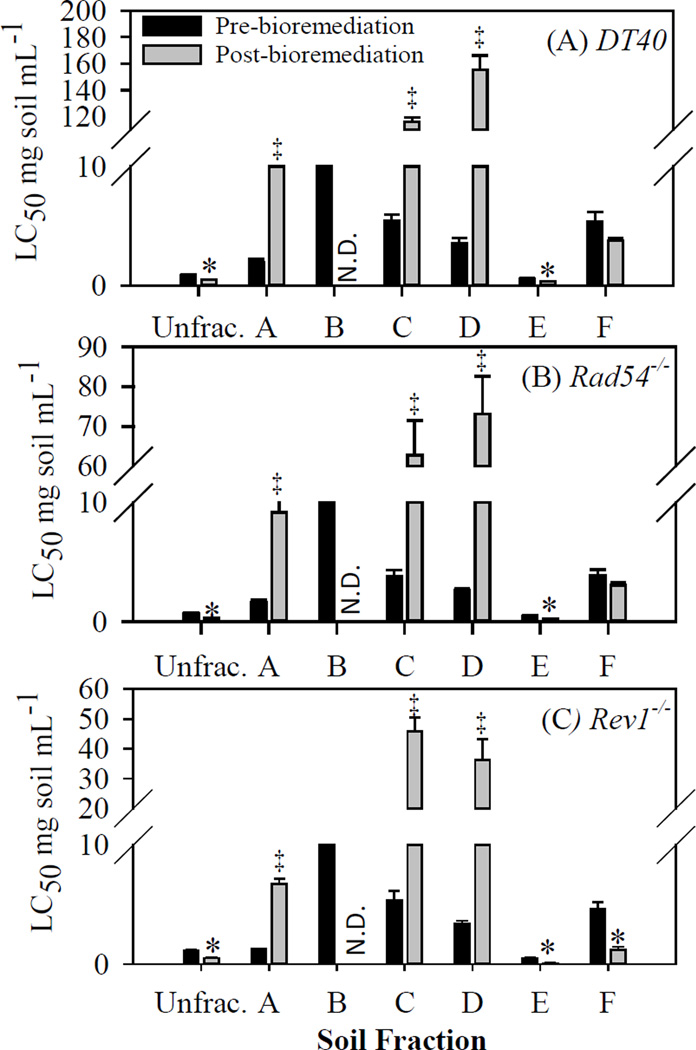
In the unfractionated soil extracts, a significant decrease in median lethal concentration (LC50), associated with increased toxicity, was measured post-bioremediation for the parental DT40 (p < 0.001) and mutants Rad54−/− (p < 0.001) and Rev1−/− (p < 0.01) (Figure 2, Table S3). The effect on both mutants suggests that compounds affecting the double-strand breaks and translesion DNA damage repair pathways likely contribute to the measured toxicity in the parental DT40 cells, post-bioremediation. These results are consistent with earlier work on this system by Hu et al.,8 who noted an increase in genotoxicity in DT40 cells and mutant Rad54−/− cell lines, post-bioremediation.
Figure 2.
Mean of the median lethal concentrations (LC50) (with standard errors bars, n = 4) of unfractionated soil extract (Unfrac.) and soil extract fractions (A – F) pre- and post-bioremediation for (A) DT40, (B) Rad54−/−, and (C) Rev1−/− cells in mg soil residue per mL DMSO. LC50 values with asterisks (*) showed a significant decrease post-bioremediation (increased toxicity), while (‡) showed a significant increase post-bioremediation (decreased toxicity) (p < 0.05). The LC50 for soil extract fraction B post-bioremediation could not be determined because the full dose-response curve could not be captured from the exposure concentrations (N.D. = not determined).
In the fractionated soil extracts, a significant decrease in LC50 was measured post240 bioremediation in fraction E for DT40 (p < 0.05), Rad54−/− (p < 0.01), and Rev1−/− (p < 0.001), and in fraction F for Rev1−/− (p < 0.01), suggesting that compounds in fractions E and F contribute to the increased toxicity measured post-bioremediation in the unfractionated soil extracts (Figure 2, Table S3). In fractions A, C, and D, we measured a significant increase in LC50 post244 bioremediation (p < 0.05), indicating a decrease in toxicity from compounds in these fractions after bioremediation.
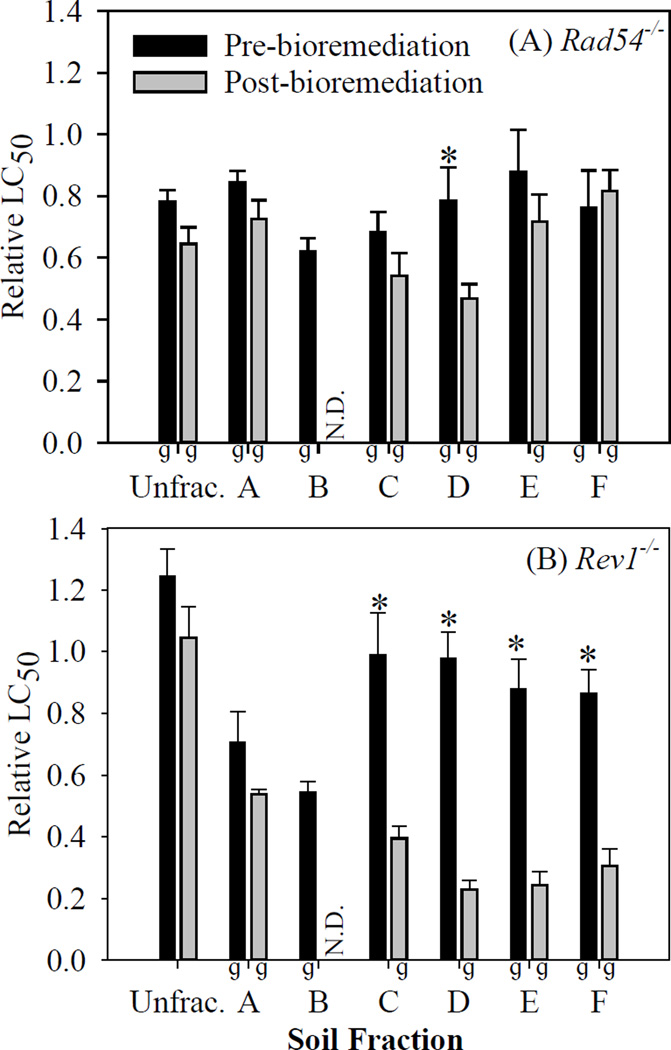
While the LC50 provides information on general toxicity, the relative LC50 is a quantitative measure of how sensitive a DNA repair-deficient mutant is in relation to the parental DT40 cell line (which has all functioning repair pathways). The relative LC50 was calculated by dividing the LC50 of the mutant (Rad54−/− or Rev1−/−) by the LC50 of the parental DT40. A ratio less than 1 (and p < 0.05) signified the mutant was more sensitive to the soil extract than the parental DT40, and the soil extract could be considered genotoxic.27,59 The smaller the LC50 of the mutant, the more toxic the soil extract is to the mutant, and the smaller the relative LC50.
Rad54−/− was more sensitive than the parental DT40 (relative LC50 < 1 and p < 0.05) to all soil extract fractions pre- and post-bioremediation, except for fraction E pre-bioremediation. This suggests that these fractions contained genotoxic compounds that affected the DNA double-strand repair pathway (Figure 3A). The unfractionated extract was also genotoxic to Rad54−/−, pre-bioremediation, with no significant change post-bioremediation. However, we measured a significant decrease in relative LC50 for Rad54−/− in fraction D post-bioremediation (p < 0.05), suggesting increased genotoxicity after bioremediation.
Figure 3.
Mean of the relative LC50 values (with standard errors bars, n = 4) of unfractionated soil extract (Unfrac.) and soil extract fractions (A – F) pre- and post-bioremediation for (A) DT40, (B) Rad54−/− and (C) Rev1−/− cells. “ɡ” indicates the fraction was genotoxic (i.e. mean relative LC50 < 1.0 and p < 0.05). Relative LC50 values with asterisks (*) showed a significant decrease post-bioremediation (increased toxicity), while (‡) showed a significant increase post-bioremediation (decreased toxicity) (p < 0.05). The relative LC50 for soil extract fraction B post-bioremediation could not be determined because the full dose-response curve could not be captured from the exposure concentrations (N.D. = not determined).
Rev1−/− was more sensitive than the parental DT40 (relative LC50 < 1 and p < 0.05) to all soil extract fractions pre- and post-bioremediation, except for fractions C and D pre-bioremediation, suggesting that these fractions contained genotoxic compounds that affected the DNA translesion repair pathway (Figure 3B). It is important to note that fractions C and D were not genotoxic pre-bioremediation, but were post-bioremediation. This suggests that bioremediation resulted in the formation and/or increased concentration of genotoxic compounds in these fractions. We measured a significant decrease in relative LC50 for Rev1−/− in fractions C, D, E, and F post-bioremediation (p < 0.05), suggesting increased genotoxicity after bioremediation. Since Rev1−/− is involved in error prone translesion DNA synthesis, the increased sensitivity to Rev1−/− compared to the parental DT40 suggests that those soil extract fractions may include mutagenic chemicals.60 However, Rev1−/− was not more sensitive than the parental DT40 to the unfractionated soil extracts, pre- and post-bioremediation. This may be due to antagonistic effects from the complex mixture of compounds in the unfractionated extracts that were not present in the fractions.
The vast majority of PAHs, OPAHs, HPAHs measured in t his study, including those with known genotoxicity,61–64 were contained in fractions A, B, and C (Table 1). Though these compounds may have accounted for the observed genotoxicity in fractions A, B, and C (Figure 3), the increased genotoxicity in fractions D, E, and F cannot be attributed to these compounds because they were not contained in these fractions and/or did not increase in concentration post-bioremediation (Figure 1, Table S1). The degradation pathways of these PAHs have been studied and transformation products often include hydroxylated, carboxylated, and quinone PAH transformation products, such as 9-fluorenone (9FLO), 9-hydroxyfluorenone, 1-indanone, 1-hydroxynaphthoic acid, cis-4,5-dihydroxy-4,5-dihydropyrene, pyrene-4,5-dione, 2-carboxybenzaldehyde, 9-fluorenone-1-carboxylic acid, 9-carboxymethylene-9H-fluorene-1-carboxylic acid, and fluoranthene-2,3-dione etc.11,65–68 Some potential transformation products of 3- and 4-ring PAHs (9FLO, 1,4PD, 9,10PQ, and 7,12BaAD) were measured in this study but they were either not detected above the LOD (0.3 ng g−1), or their concentrations decreased or did not change post-bioremediation (Figure 1, Table S1). This suggests that these transformation products did not contribute to the observed toxicity. However, the increased toxicity measured post-bioremediation is likely due to transformation products, including those of the 3- and 4-ring PAHs (PHE, 1MPHE, 2MPHE, PRY, BaA, and FLA) that were most degraded. Future work will focus on identifying, characterizing, and quantifying the potential hydroxylated and carboxylated 3- and 4-ring PAH transformation products responsible for the increased genotoxicity and developmental toxicity post-bioremediation.
Embryonic Zebrafish Bioassay
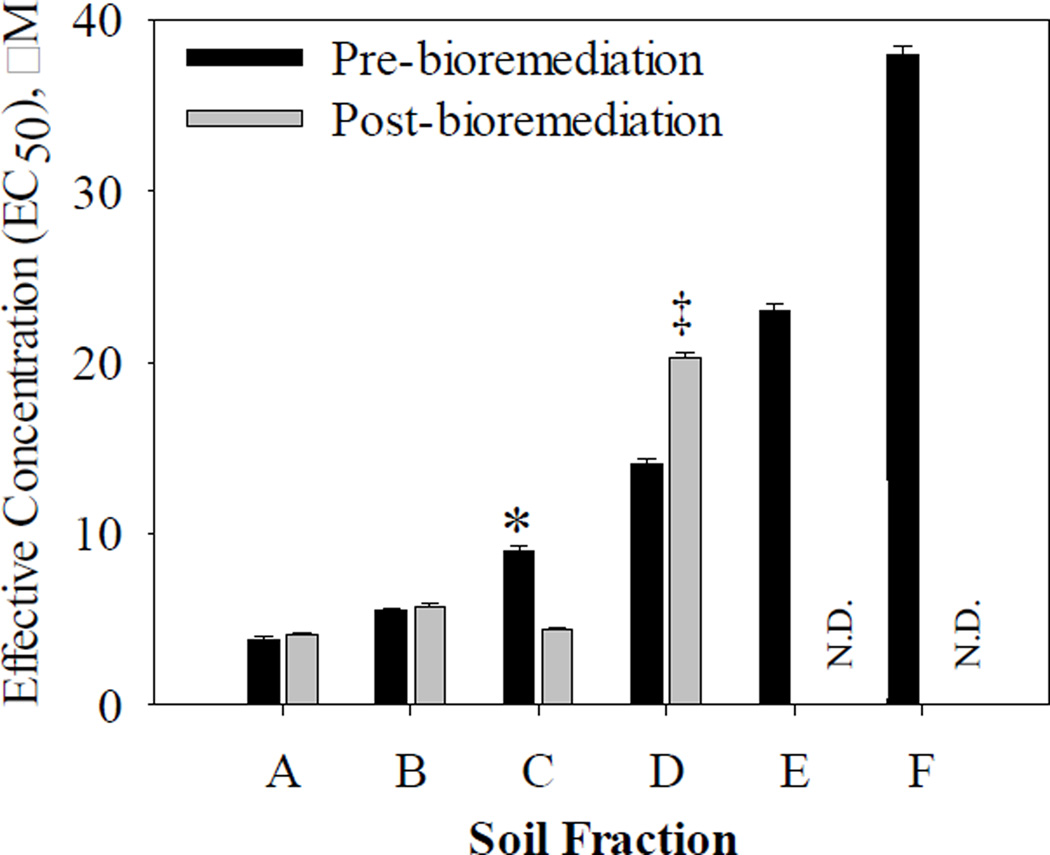
The embryonic zebrafish bioassay was used to assess the soil extract fractions for developmental toxicity, both pre- and post-bioremediation. Soil extract fractions A, B, and C had lower median effective concentrations (EC50) (were more developmentally toxic) than fractions D, E, and F (Figure 4, Table S4). The EC50 for fractions E and F, post-bioremediation, were unable to be calculated because the concentrations tested were too low to capture the full concentration-response curve.
Figure 4.
Mean of the median effective concentrations (EC50) (with standard errors bars, n = 32) of fractionated soil extracts (A–F) pre- and post-bioremediation in embryonic zebrafish. EC50 values with asterisks (*) showed a significant decrease post-bioremediation (increased developmental toxicity), while (‡) showed a significant increase post-bioremediation (decreased developmental toxicity) (p < 0.05). The EC50s of fractions E and F post-bioremediation were unable to be calculated because the concentrations tested were too low to capture the full concentration-response curve (N.D. = not determined).
Fractions A, B, and C primarily contained the PAHs and methyl PAHs, OPAHs, and HPAHs in this study (Table 1). This suggests that the PAHs and methyl PAHs, OPAHs, and HPAHs measured in this study contributed significantly to the developmental toxicity of the zebrafish in these fractions. No significant change in EC50 was measured post-bioremediation in fractions A and B, suggesting the developmental toxicity potential of these fractions did not change after remediation. A statistically significant decrease in EC50 post-bioremediation was measured in fraction C (p < 0.001), indicating an increase in developmental toxicity after bioremediation. Fraction C contained 9FLO (Table 1), but 9FLO is unlikely to have caused the increase in developmental toxicity in this fraction because its concentration did not increase post-bioremediation (Figure 1 and Table S1). It should be noted that though we measured increased genotoxicity in the DT40 bioassay in fraction D (Figure 3), we measured a significant increase in EC50 post-bioremediation (p < 0.001) in fraction D, suggesting that the compounds causing developmental toxicity in the embryonic zebrafish bioassay in this fraction were bio-transformed and/or decreased in concentration after bioremediation.
Although genotoxicity increased post-bioremediation in fraction D (Figure 3), and developmental toxicity decreased (Figure 4) in fraction D, this is not inconsistent because the two different assays provide information on different toxicological endpoints. While the DT40 bioassay provides a measure of DNA damage, the embryonic zebrafish bioassay provides a comprehensive overview of any effect that can interfere with the normal development of the zebrafish.
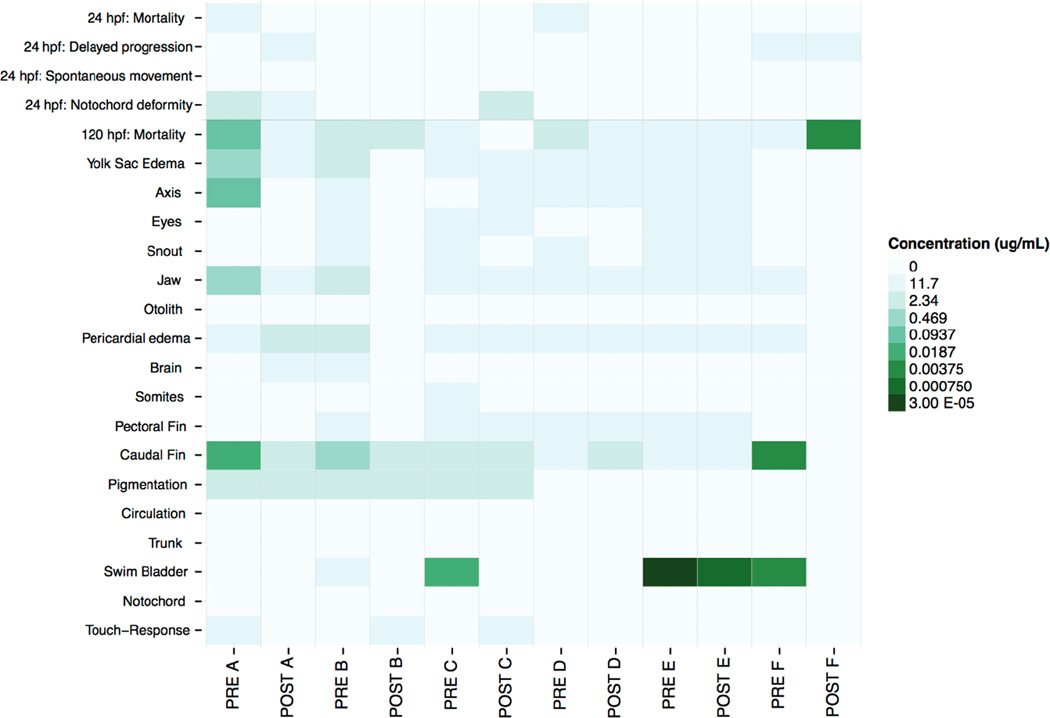
In addition to EC50, we evaluated 22 endpoints in the embryonic zebrafish, including swim bladder, pericardial edema, caudal and pectoral fin malformations. The malformations induced by each concentration level of the individual soil extract fractions, compared with the 1% DMSO vehicle control, are presented as a heat map of lowest effect levels (LELs) in Figure 5. Axis, jaw, caudal fin, and yolk sac edema malformations were measured pre-bioremediation in fraction A and were reduced post-bioremediation. Fraction B had a similar malformation profile to fraction A, except that the malformations were less pronounced. We measured a dominant swim bladder malformation in fraction C pre-bioremediation and this malformation was also reduced post-bioremediation. Compared to all other fractions, fraction D had the lowest number of malformations, both pre- and post-bioremediation. A swim bladder malformation was measured in fractions E and F and was reduced post-bioremediation. We also measured mortality at 120 hours post fertilization (hpf) in fraction F post-bioremediation, which was not present pre-bioremediation, suggesting that bioremediation produced larval mortality in the zebrafish (Figure 5).
Figure 5.
Heat map of Lowest Effect Levels (LELs) for each of the 22 evaluated endpoints in 24 hours post fertilization (hpf) and 120 hpf embryonic zebrafish. Darker color indicates lower LEL. (Pre = pre-bioremediation; post = post-bioremediation, concentration “0” indicates no measured effect).
Although we measured an increase in the LELs (decreased developmental toxicity) in individual malformations post-bioremediation in fractions A and B (Figure 5), the EC50’s for fractions A and B did not increase (developmental toxicity unchanged) post-bioremediation (Figure 4). This suggests that the severity of the 22 malformations induced by the post-bioremediation extracts for these fractions were reduced (i.e. while the number of fish with at least one of the 22 evaluated malformations were the same pre- and post-bioremediation, the number of fish with more than one of the 22 evaluated malformations decreased post-bioremediation). This may also be the case for fraction C, where the EC50 decreased (increased developmental toxicity) post-bioremediation (Figure 4) even though there was an increase in LELs (decreased developmental toxicity) overall in measured malformations in this fraction post-bioremediation (Figure 5) (i.e. while the number of fish with at least one of the twenty-two evaluated malformations increased post-bioremediation, the number of fish with more than 22 of the evaluated decreased post-bioremediation).
Implications
One of the implications of this research for sites contaminated with PAHs, including many U.S. Superfund sites, is that the higher molecular weight PAHs (including BaA, BkF, BbF, BaP, and IcdP) are not significantly decreased in concentration post-bioremediation and may exceed regulatory MACs in the U.S., Germany, and Canada, even after bioremediation of the contaminated soil.8,23,47 Another implication is that the genotoxicity and developmental toxicity of the soils may increase after bioremediation due to the formation of hydroxylated, carboxylated, and quinone PAH transformation products,66–70 that have not yet been positively identified. While the formation of polar transformation products merits attention due to their potential accumulation and toxicity,11,52,56,71 their likely increased bioavailability needs to be accounted for as well.11,72 Future work will focus on identifying, characterizing, and quantifying the potential hydroxylated and carboxylated 3- and 4-ring PAH transformation products responsible for the increased genotoxicity and developmental toxicity post-bioremediation using non-targeted comprehensive two dimensional gas chromatography coupled to time of flight mass spectrometry (GCxGC/ToF-MS)19,73 (with and without derivatization) and liquid chromatography-tandem mass spectrometry (LC/MS-MS).74
Supplementary Material
ACKNOWLEDGMENTS
This publication was made possible in part by grant number P30ES00210 from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), and NIEHS Grants P42 ES016465, P42 ES005948, and P30-ES10126. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIEHS, NIH. We thank Julian Sale and Shunichi Takeda for providing the DT40 mutants, Core D at the Oregon State University (OSU) Superfund Research Program for supplying standards, Alden Adrion at the University of North Carolina at Chapel Hill for the laboratory-scale bioreactor experiments.
Footnotes
The authors declare no competing financial interest.
Tables S1–S4: PAH concentrations in unfractionated soil extracts, maximum allowable concentrations (MACs, median lethal concentrations (LC50) in DT40 bioassay, median effective concentrations (EC50) in embryonic fish assay,
This information is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Armstrong B, Hutchinson E, Unwin J, Fletcher T. Lung Cancer Risk after Exposure to Polycyclic Aromatic Hydrocarbons: A Review and Meta-Analysis. Environ. Health Perspect. 2004;112(9):970–978. doi: 10.1289/ehp.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baird WM, Hooven LA, Mahadevan B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ. Mol. Mutagen. 2005;45(2–3):106–114. doi: 10.1002/em.20095. [DOI] [PubMed] [Google Scholar]
- 3.Shimada T, Guengerich FP. Inhibition of Human Cytochrome P450 1A1-, 1A2-, and 1B1-Mediated Activation of Procarcinogens to Genotoxic Metabolites by Polycyclic Aromatic Hydrocarbons. Chem. Res. Toxicol. 2006;19(2):288–294. doi: 10.1021/tx050291v. [DOI] [PubMed] [Google Scholar]
- 4.Hawthorne SB, Poppendieck DG, Grabanski CB, Loehr RC. Comparing PAH Availability from Manufactured Gas Plant Soils and Sediments with Chemical and Biological Tests. 1. PAH Release during Water Desorption and Supercritical Carbon Dioxide Extraction. Environ. Sci. Technol. 2002;36(22):4795–4803. doi: 10.1021/es020626k. [DOI] [PubMed] [Google Scholar]
- 5.Weissenfels WD, Klewer H-J, Langhoff J. Adsorption of polycyclic aromatic hydrocarbons (PAHs) by soil particles: influence on biodegradability and biotoxicity. Appl. Microbiol. Biotechnol. 1992;36(5):689–696. doi: 10.1007/BF00183251. [DOI] [PubMed] [Google Scholar]
- 6.Chiou CT, McGroddy SE, Kile DE. Partition Characteristics of Polycyclic Aromatic Hydrocarbons on Soils and Sediments. Environ. Sci. Technol. 1998;32(2):264–269. [Google Scholar]
- 7.Bamforth SM Federal Soil Protection. Bioremediation of polycyclic aromatic hydrocarbons: current knowledge and future directions. J. Chem. Technol. Biotechnol. 2005;80(7):723–736. [Google Scholar]
- 8.Hu J, Nakamura J, Richardson SD, Aitken MD. Evaluating the Effects of Bioremediation on Genotoxicity of Polycyclic Aromatic Hydrocarbon-Contaminated Soil Using Genetically Engineered, Higher Eukaryotic Cell Lines. Environ. Sci. Technol. 2012;46(8):4607–4613. doi: 10.1021/es300020e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson E, Rotander A, Kronhelm T, von Berggren A, Ivarsson P, Hollert H, Engwall M. AhR agonist and genotoxicant bioavailability in a PAH-contaminated soil undergoing biological treatment. Environ. Sci. Pollut. Res. 2009;16(5):521–530. doi: 10.1007/s11356-009-0121-9. [DOI] [PubMed] [Google Scholar]
- 10.Wischmann H, Steinhart H, Hupe K, Montresori G, Stegmann R. Degradation of Selected PAHs in Soil/Compost and Identification of Intermediates. Int. J. Environ. Anal. Chem. 1996;64(4):247–255. [Google Scholar]
- 11.Lundstedt S, White PA, Lemieux CL, Lynes KD, Lambert IB, Öberg L, Haglund P, Tysklind M. Sources, Fate, and Toxic Hazards of Oxygenated Polycyclic Aromatic Hydrocarbons (PAHs) at PAH- contaminated Sites. AMBIO. J. Hum. Environ. 2007;36(6):475–485. doi: 10.1579/0044-7447(2007)36[475:sfatho]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Chesis PL, Levin DE, Smith MT, Ernster L, Ames BN. Mutagenicity of quinones: pathways of metabolic activation and detoxification. Proc. Natl. Acad. Sci. 1984;81(6):1696–1700. doi: 10.1073/pnas.81.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flowers-Geary L, Bleczinski W, Harvey RG, Penning TM. Cytotoxicity and mutagenicity of polycyclic aromatic hydrocarbon o-quinones produced by dihydrodiol dehydrogenase. Chem. Biol. Interact. 1996;99(1–3):55–72. doi: 10.1016/0009-2797(95)03660-1. [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa K, Kawaguchi Y, Murahashi T, Miyazaki M. Distribution of nitropyrenes and mutagenicity in airborne particulates collected with an Andersen sampler. Mutat. Res. Lett. 1995;348(2):57–61. doi: 10.1016/0165-7992(95)00046-1. [DOI] [PubMed] [Google Scholar]
- 15.Rosenkranz HS, Mermelstein R. Mutagenicity and genotoxicity of nitro-arenes: All nitro-containing chemicals were not created equal. Mutat. Res. Genet. Toxicol. 1983;114(3):217–267. doi: 10.1016/0165-1110(83)90034-9. [DOI] [PubMed] [Google Scholar]
- 16.Arey J, Harger WP, Helmig D, Atkinson R. Bioassay-directed fractionation of mutagenic PAH atmospheric photooxidation products and ambient particulate extracts. Mutat. Res. Lett. 1992;281(1):67–76. doi: 10.1016/0165-7992(92)90038-j. [DOI] [PubMed] [Google Scholar]
- 17.Brinkmann M, Maletz S, Krauss M, Bluhm K, Schiwy S, Kuckelkorn J, Tiehm A, Brack W, Hollert H. Heterocyclic Aromatic Hydrocarbons Show Estrogenic Activity upon Metabolization in a Recombinant Transactivation Assay. Environ. Sci. Technol. 2014;48(10):5892–5901. doi: 10.1021/es405731j. [DOI] [PubMed] [Google Scholar]
- 18.Blum P, Sagner A, Tiehm A, Martus P, Wendel T, Grathwohl P. Importance of heterocylic aromatic compounds in monitored natural attenuation for coal tar contaminated aquifers: A review. J. Contam. Hydrol. 2011;126(3–4):181–194. doi: 10.1016/j.jconhyd.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Manzano C, Hoh E, Simonich SLM. Improved Separation of Complex Polycyclic Aromatic Hydrocarbon Mixtures Using Novel Column Combinations in GC × GC/ToF-MS. Environ. Sci. Technol. 2012;46(14):7677–7684. doi: 10.1021/es301790h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks LR, Hughes TJ, Claxton LD, Austern B, Brenner R, Kremer F. Bioassay-directed fractionation and chemical identification of mutagens in bioremediated soils. Environ. Health Perspect. 1998;106(Suppl 6):1435–1440. doi: 10.1289/ehp.98106s61435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J, Ball LM, Richardson SD, Zhu H-B, Aitken MD. Oxidative mutagenicity of polar fractions from polycyclic aromatic hydrocarbon-contaminated soils. Environ. Toxicol. Chem. 2008;27(11):2207–2215. doi: 10.1897/07-572.1. [DOI] [PubMed] [Google Scholar]
- 22.Lemieux CL, Lynes KD, White PA, Lundstedt S, Öberg L, Lambert IB. Mutagenicity of an aged gasworks soil during bioslurry treatment. Environ. Mol. Mutagen. 2009;50(5):404–412. doi: 10.1002/em.20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundstedt S, Haglund P, Öberg L. Degradation and formation of polycyclic aromatic compounds during bioslurry treatment of an aged gasworks soil. Environ. Toxicol. Chem. 2003;22(7):1413–1420. [PubMed] [Google Scholar]
- 24.Fowler P, Smith K, Young J, Jeffrey L, Kirkland D, Pfuhler S, Carmichael P. Reduction of misleading (“false”) positive results in mammalian cell genotoxicity assays. I. Choice of cell type. Mutat. Res. 2012;742(1–2):11–25. doi: 10.1016/j.mrgentox.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Kirkland D, Aardema M, Henderson L, Müller L. Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens: I. Sensitivity, specificity and relative predictivity. Mutat. Res. Toxicol. Environ. Mutagen. 2005;584(1–2):1–256. doi: 10.1016/j.mrgentox.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Evans TJ, Yamamoto KN, Hirota K, Takeda S. Mutant cells defective in DNA repair pathways provide a sensitive high-throughput assay for genotoxicity. DNA Repair. 2010;9(12):1292–1298. doi: 10.1016/j.dnarep.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Ji K, Kogame T, Choi K, Wang X, Lee J, Taniguchi Y, Takeda S. A Novel Approach Using DNA-Repair-Deficient Chicken DT40 Cell Lines for Screening and Characterizing the Genotoxicity of Environmental Contaminants. Environ. Health Perspect. 2009;117(11):1737–1744. doi: 10.1289/ehp.0900842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridpath JR, Takeda S, Swenberg JA, Nakamura J. Convenient, multi-well plate-based DNA damage response analysis using DT40 mutants is applicable to a high-throughput genotoxicity assay with characterization of modes of action. Environ. Mol. Mutagen. 2011;52(2):153–160. doi: 10.1002/em.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulrich E, Boehmelt G, Bird A, Beug H. Immortalization of conditionally transformed chicken cells: loss of normal p53 expression is an early step that is independent of cell transformation. Genes & development. 1992;6(5):876–887. doi: 10.1101/gad.6.5.876. [DOI] [PubMed] [Google Scholar]
- 30.Scholz S, Fischer S, Gündel U, Küster E, Luckenbach T, Voelker D. The zebrafish embryo model in environmental risk assessment—applications beyond acute toxicity testing. Environ. Sci. Pollut. Res. 2008;15(5):394–404. doi: 10.1007/s11356-008-0018-z. [DOI] [PubMed] [Google Scholar]
- 31.Knecht AL, Goodale BC, Truong L, Simonich MT, Swanson AJ, Matzke MM, Anderson KA, Waters KM, Tanguay RL. Comparative developmental toxicity of environmentally relevant oxygenated PAHs. Toxicol. Appl. Pharmacol. 2013;271(2):266–275. doi: 10.1016/j.taap.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat. Rev. Genet. 2007;8(5):353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- 33.Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, et al. Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, et al. Beverley Mortimore, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, et al. Christine Lloyd. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wincent E, Jönsson ME, Bottai M, Lundstedt S, Dreij K. Aryl Hydrocarbon Receptor Activation and Developmental Toxicity in Zebrafish in Response to Soil Extracts Containing Unsubstituted and Oxygenated PAHs. Environ. Sci. Technol. 2015;49(6):3869–3877. doi: 10.1021/es505588s. [DOI] [PubMed] [Google Scholar]
- 35.Mendonça E, Picado A. Ecotoxicological monitoring of remediation in a coke oven soil. Environ. Toxicol. 2002;17(1):74–79. doi: 10.1002/tox.10034. [DOI] [PubMed] [Google Scholar]
- 36.Phillips TM, Liu D, Seech AG, Lee H, Trevors JT. Monitoring bioremediation in creosote-contaminated soils using chemical analysis and toxicity tests. J. Ind. Microbiol. Biotechnol. 2000;24(2):132–139. [Google Scholar]
- 37.Sayles GD, Acheson CM, Kupferle MJ, Shan Y, Zhou Q, Meier JR, Chang L, Brenner RC. Land Treatment of PAH-Contaminated Soil: Performance Measured by Chemical and Toxicity Assays. Environ. Sci. Technol. 1999;33(23):4310–4317. [Google Scholar]
- 38.Singleton DR, Richardson SD, Aitken MD. Pyrosequence analysis of bacterial communities in aerobic bioreactors treating polycyclic aromatic hydrocarbon-contaminated soil. Biodegradation. 2011;22(6):1061–1073. doi: 10.1007/s10532-011-9463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seiler T-B, Schulze T, Hollert H. The risk of altering soil and sediment samples upon extract preparation for analytical and bio-analytical investigations—a review. Anal. Bioanal. Chem. 2008;390(8):1975–1985. doi: 10.1007/s00216-008-1933-z. [DOI] [PubMed] [Google Scholar]
- 40.Hu J, Adrion AC, Nakamura J, Shea D, Aitken MD. Bioavailability of (Geno)toxic Contaminants in Polycyclic Aromatic Hydrocarbon-Contaminated Soil Before and After Biological Treatment. Environ. Eng. Sci. 2014;31(4):176–182. doi: 10.1089/ees.2013.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Jariyasopit N, Schrlau J, Jia Y, Tao S, Yu T-W, Dashwood RH, Zhang W, Wang X, Simonich SLM. Concentration and Photochemistry of PAHs, NPAHs, and OPAHs and Toxicity of PM2.5 during the Beijing Olympic Games. Environ. Sci. Technol. 2011;45(16):6887–6895. doi: 10.1021/es201443z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jariyasopit N, Zimmermann K, Schrlau J, Arey J, Atkinson R, Yu T-W, Dashwood RH, Tao S, Simonich SLM. Heterogeneous Reactions of Particulate Matter-Bound PAHs and NPAHs with NO3/N2O5, OH Radicals, and O3 under Simulated Long-Range Atmospheric Transport Conditions: Reactivity and Mutagenicity. Environ. Sci. Technol. 2014;48(17):10155–10164. doi: 10.1021/es5015407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zimmermann K, Jariyasopit N, Massey Simonich SL, Tao S, Atkinson R, Arey J. Formation of Nitro-PAHs from the Heterogeneous Reaction of Ambient Particle-Bound PAHs with N2O5/NO3/NO2. Environ. Sci. Technol. 2013;47(15):8434–8442. doi: 10.1021/es401789x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Truong L, Reif DM, Mary LS, Geier MC, Truong HD, Tanguay RL. Multidimensional In Vivo Hazard Assessment Using Zebrafish. Toxicol. Sci. 2014;137(1):212–233. doi: 10.1093/toxsci/kft235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lors C, Ryngaert A, Périé F, Diels L, Damidot D. Evolution of bacterial community during bioremediation of PAHs in a coal tar contaminated soil. Chemosphere. 2010;81(10):1263–1271. doi: 10.1016/j.chemosphere.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 46.Tabak HH, Lazorchak JM, Lei L, Khodadoust AP, Antia JE, Bagchi R, Suidan MT. Studies on bioremediation of polycyclic aromatic hydrocarbon-contaminated sediments: Bioavailability, biodegradability, and toxicity issues. Environ. Toxicol. Chem. 2003;22(3):473–482. [PubMed] [Google Scholar]
- 47.Haeseler F, Blanchet D, Druelle V, Werner P, Vandecasteele J-P. Ecotoxicological Assessment of Soils of Former Manufactured Gas Plant Sites: Bioremediation Potential and Pollutant Mobility. Environ. Sci. Technol. 1999;33(24):4379–4384. [Google Scholar]
- 48.Regional Screening Levels | Region 9: Superfund | US EPA. [accessed Apr 29, 2015]; http://www.epa.gov/region9/superfund/prg/
- 49.Government of Canada, E. C. Guidelines - Acts & Regulations - Environment Canada. [accessed May 6, 2015]; http://www.ec.gc.ca/lcpe-cepa/default.asp?lang=En&n=E9DBBC31-1.
- 50.Lehmphul K. Soil protection law. [accessed Apr 27, 2015]; http://www.umweltbundesamt.de/en/topics/soil-agriculture/soil-protection/soil-protection-law. [Google Scholar]
- 51.US EPA, O. Integrated Risk Information System (IRIS) [accessed May 5, 2015]; http://www.epa.gov/iris/
- 52.Wilcke W, Kiesewetter M, Musa Bandowe BA. Microbial formation and degradation of oxygen-containing polycyclic aromatic hydrocarbons (OPAHs) in soil during short-term incubation. Environ. Pollut. 2014;184:385–390. doi: 10.1016/j.envpol.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 53.Meyer S, Steinhart H. Effects of heterocyclic PAHs (N, S, O) on the biodegradation of typical tar oil PAHs in a soil/compost mixture. Chemosphere. 2000;40(4):359–367. doi: 10.1016/s0045-6535(99)00237-4. [DOI] [PubMed] [Google Scholar]
- 54.Lantz SE, Montgomery MT, Schultz WW, Pritchard PH, Spargo BJ, Mueller JG. Constituents of an Organic Wood Preservative That Inhibit the Fluoranthene-Degrading Activity of Sphingomonas paucimobilis Strain EPA505. Environ. Sci. Technol. 1997;31(12):3573–3580. [Google Scholar]
- 55.Neilson AH, Allard A-S. Environmental Degradation and Transformation of Organic Chemicals. CRC Press; 2007. [Google Scholar]
- 56.Rodgers-Vieira EA, Zhang Z, Adrion AC, Gold A, Aitken MD. Identification of Anthraquinone-Degrading Bacteria in Soil Contaminated with Polycyclic Aromatic Hydrocarbons. Appl. Environ. Microbiol. 2015 doi: 10.1128/AEM.00033-15. AEM.00033–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takata M, Sasaki MS, Sonoda E, Morrison C, Hashimoto M, Utsumi H, Yamaguchi-Iwai Y, Shinohara A, Takeda S. Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J. 1998;17(18):5497–5508. doi: 10.1093/emboj/17.18.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Groote FH, Jansen JG, Masuda Y, Shah DM, Kamiya K, de Wind N, Siegal G. The Rev1 translesion synthesis polymerase has multiple distinct DNA binding modes. DNA Repair. 2011;10(9):915–925. doi: 10.1016/j.dnarep.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 59.Ji K, Seo J, Liu X, Lee J, Lee S, Lee W, Park J, Khim JS, Hong S, Choi Y, et al. Genotoxicity and Endocrine-Disruption Potentials of Sediment near an Oil Spill Site: Two Years after the Hebei Spirit Oil Spill. Environ. Sci. Technol. 2011;45(17):7481–7488. doi: 10.1021/es200724x. [DOI] [PubMed] [Google Scholar]
- 60.Ross A-L, Sale JE. The catalytic activity of REV1 is employed during immunoglobulin gene diversification in DT40. Mol. Immunol. 2006;43(10):1587–1594. doi: 10.1016/j.molimm.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 61.White PA. The genotoxicity of priority polycyclic aromatic hydrocarbons in complex mixtures. Mutat. Res. Toxicol. Environ. Mutagen. 2002;515(1–2):85–98. doi: 10.1016/s1383-5718(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 62.Bleeker EAJ, Geest HGVD, Klamer HJC, Voogt PD, Wind E, Kraak MHS. Toxic and Genotoxic Effects of Azaarenes: Isomers and Metabolites. Polycycl. Aromat. Compd. 1999;13(3):191–203. [Google Scholar]
- 63.Gurbani D, Bharti SK, Kumar A, Pandey AK, Ana GREE, Verma A, Khan AH, Patel DK, Mudiam MKR, Jain SK, et al. Polycyclic aromatic hydrocarbons and their quinones modulate the metabolic profile and induce DNA damage in human alveolar and bronchiolar cells. Int. J. Hyg. Environ. Health. 2013;216(5):553–565. doi: 10.1016/j.ijheh.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 64.Brinkmann M, Blenkle H, Salowsky H, Bluhm K, Schiwy S, Tiehm A, Hollert H. Genotoxicity of Heterocyclic PAHs in the Micronucleus Assay with the Fish Liver Cell Line RTL-W1. PLoS ONE. 2014;9(1):e85692. doi: 10.1371/journal.pone.0085692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kanaly RA, Harayama S. Advances in the field of high-molecular-weight polycyclic aromatic hydrocarbon biodegradation by bacteria. Microbial biotechnology. 2010;3(2):136–164. doi: 10.1111/j.1751-7915.2009.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boldrin B, Tiehm A, Fritzsche C. Degradation of phenanthrene, fluorene, fluoranthene, and pyrene by a Mycobacterium sp. Appl. Environ. Microbiol. 1993;59(6):1927–1930. doi: 10.1128/aem.59.6.1927-1930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kazunga C, Aitken MD, Gold A, Sangaiah R. Fluoranthene-2,3- and-1,5-diones Are Novel Products from the Bacterial Transformation of Fluoranthene. Environ. Sci. Technol. 2001;35(5):917–922. doi: 10.1021/es001605y. [DOI] [PubMed] [Google Scholar]
- 68.Kazunga C, Aitken MD. Products from the Incomplete Metabolism of Pyrene by Polycyclic Aromatic Hydrocarbon-Degrading Bacteria. Appl. Environ. Microbiol. 2000;66(5):1917–1922. doi: 10.1128/aem.66.5.1917-1922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kanaly RA, Harayama S. Biodegradation of High-Molecular-Weight Polycyclic Aromatic Hydrocarbons by Bacteria. J. Bacteriol. 2000;182(8):2059–2067. doi: 10.1128/jb.182.8.2059-2067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heitkamp MA, Freeman JP, Miller DW, Cerniglia CE. Pyrene degradation by a Mycobacterium sp.: identification of ring oxidation and ring fission products. Appl. Environ. Microbiol. 1988;54(10):2556–2565. doi: 10.1128/aem.54.10.2556-2565.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Donnelly KC, Huebner HJ, Claxton LD, Calvin JA, Vos GA, Cizmas L, He L. Biodegradation of simple chemical mixtures in soil. Environ. Toxicol. Chem. 2005;24(11):2839–2845. doi: 10.1897/04-630r.1. [DOI] [PubMed] [Google Scholar]
- 72.Ortega-Calvo JJ, Tejeda-Agredano MC, Jimenez-Sanchez C, Congiu E, Sungthong R, Niqui-Arroyo JL, Cantos M. Is it possible to increase bioavailability but not environmental risk of PAHs in bioremediation? J. Hazard. Mater. 2013;261:733–745. doi: 10.1016/j.jhazmat.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 73.Manzano C, Hoh E, Simonich SLM. Quantification of complex polycyclic aromatic hydrocarbon mixtures in standard reference materials using comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry. J. Chromatogr. A. 2013;1307:172–179. doi: 10.1016/j.chroma.2013.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lintelmann J, Fischer K, Matuschek G. Determination of oxygenated polycyclic aromatic hydrocarbons in particulate matter using high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2006;1133(1–2):241–247. doi: 10.1016/j.chroma.2006.08.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.