Abstract
Background
Sleep disturbance is associated with inflammatory disease risk and all-cause mortality. Here, we assess global evidence linking sleep disturbance, sleep duration, and inflammation in adult humans.
Methods
A systematic search of English language publications was performed, with inclusion of primary research articles that characterized sleep disturbance and/or sleep duration or performed experimental sleep deprivation, and assessed inflammation by levels of circulating markers. Effect sizes (ES) and 95% confidence intervals (CI) were extracted and pooled using a random effect model.
Results
A total of 72 studies (n>50000) were analyzed with assessment of C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor α (TNF). Sleep disturbance was associated with higher levels of CRP (ES 0.12; 95% CI 0.05 – 0.19) and IL-6 (ES 0.20; 95% CI 0.08 – 0.31). Shorter sleep duration, but not the extreme of short sleep, was associated with higher levels of CRP (ES 0.09; 95% CI 0.01 – 0.17) but not IL-6 (ES 0.03; 95% CI −0.09 – 0.14). The extreme of long sleep duration was associated with higher levels of CRP (ES 0.17; 95% CI 0.01 – 0.34) and IL-6 (ES 0.11; 95% CI 0.02 – 0.20). Neither sleep disturbances nor sleep duration was associated with TNF. Neither experimental sleep deprivation nor sleep restriction was associated with CRP, IL-6, or TNF. Some heterogeneity among studies was found, but no evidence of publication bias.
Conclusions
Sleep disturbance and long sleep duration, but not short sleep duration, are associated with increases in markers of systemic inflammation.
Keywords: Insomnia, Inflammation, Sleep Disturbance, Sleep Duration, Sleep Deprivation, C-reactive protein, Interleukin-6, Meta-Analysis
INTRODUCTION
Over the past decade, compelling evidence has demonstrated that disturbances of sleep such as insomnia complaints and extremes of sleep duration adversely influence risk of inflammatory disease and contribute to all-cause mortality (1–4). Because about 25% of the population of the United States report insomnia complaints (5), and nearly 10% fulfil diagnostic criteria for chronic insomnia (6, 7), which is persistent for as long as three years in nearly 50% (6), the burden of insomnia has substantial public health implications. Indeed, the Center for Diseases Control has identified insufficient sleep as a public health epidemic (www.cdc.gov/features/dssleep/). Increasingly, research on “sleep health” (8) has focused on the biological mechanisms underlying these effects, with substantial interest in the role of sleep disturbance on measures of innate immunity (9).
Inflammatory mechanisms contribute to the risk of a wide spectrum of medical conditions. Increases in circulating markers of inflammation, such as high sensitivity C-reactive protein (CRP) and interleukin-6 (IL-6), predict cardiovascular events (10, 11), hypertension (12), weight gain in older adults (13), and type 2 diabetes (14, 15). However, it is difficult to draw robust conclusions about the effects of sleep on inflammation, given the variety of studies with differences in the characterization of sleep disturbance, varying assessment methods used to evaluate sleep disturbance (i.e., sleep quality, sleep complaints) and sleep duration, and various markers of inflammation (9). Systematic evaluation of the associations between sleep disturbance and sleep duration, as well as experimental sleep deprivation on inflammatory outcomes, and related effect sizes has not been previously undertaken. Moreover, understanding the magnitude and specificity of different aspects of sleep (i.e., sleep disturbance, sleep duration) on inflammation has further health implications, because inflammation appears to be amenable to modification by way of treatments that target insomnia complaints (16–18).
The aims of this study were to (a) systematically review published studies evaluating sleep and inflammatory outcomes, and (b) carry out a meta-analysis to assess the global evidence that links sleep disturbance, sleep duration, or experimental sleep loss with circulating markers of inflammation in adult humans. This meta-analysis focuses on CRP and IL-6, because the vast majority of research on sleep and inflammation has predominantly measured these markers of systemic inflammation, and because these markers have been consistently found to have health relevance (9–13, 15). Effects on tumor necrosis factor-α (TNF) are also explored. This meta-analysis considers the combination of results across different studies, increasing the overall statistical power as well as precision of estimates with evaluation of bias and random error.
METHODS
Study Selection
A search strategy was developed to identify studies that examined the relationship between sleep disturbance and/or sleep duration, including experimental sleep deprivation, and inflammation. The following databases were searched for primary studies: MEDLINE, PsycINFO, EMBASE, PsycArticles, and Scopus through September 2013. The MEDLINE search strategy used PubMed medical subject heading terms and the text words of key articles that we identified a priori, with a similar strategy for other electronic sources. The following search terms were used “Sleep or insomnia or sleep initiation and maintenance disorders or sleep deprivation”, and “inflammation or inflammatory or proinflammatory or C-reactive protein or CRP or C-Reactive Protein or interferon or Interferons or interleukin-6 or Interleukin-6 or tumor necrosis factor or tumor necrosis factor-α or interleukin-8 or Interleukin-8.” In addition, reference lists of included articles, relevant review articles, and related systematic reviews were used to identify articles, which might have been missed in the database searches. Limits were imposed based on English language, but not on date of publication, although all identified articles were found since 1989. Studies that evaluated the effects of sleep apnea and/or restless legs syndrome on inflammation were excluded, as these associations have been previously reported (19).
Inclusion criteria and screening review
Three trained investigators independently reviewed titles and abstracts; studies were excluded as not being relevant in a consensus meeting (MRI, JEC, RO). Criteria for inclusion were: 1) indication of number of subjects studied and sample characteristics; 2) sleep disturbance (i.e., poor sleep quality, insomnia complaints) was characterized by either survey items, questionnaire, interview, and/or standard diagnostic criteria using the International Classification of Disorders (ICD)-10, Diagnostic and Statistical Manual (DSM)-III, IIIR, or IV; 2) sleep duration was characterized by survey items, questionnaire, interview, and/or objective measures including actigraphy or polysomnography; 3) sleep deprivation was performed by an experimental manipulation of sleep duration over one- or several nights; 4) assessment of inflammation as an outcome by levels of circulating markers of inflammation; and 5) primary research articles (i.e., review articles or abstracts were not included). If multiple published reports from the same study were available, we included only the one with the most detailed information for both sleep and inflammation.
Data extraction
Three investigators (MRI, RO, JC) independently extracted data; discussion and additional consensus meetings resolved differences. Relevant data included the first author’s surname, title of article, year of publication, number of participants, participants age and gender, study design (i.e., epidemiologic, naturalistic, prospective, case-control, and experimental), number of participants, methods used to evaluate sleep disturbance (i.e., single survey item, multiple symptoms reporting, validated questionnaire, or diagnosis), methods used to evaluate sleep duration (i.e., single survey item, validated questionnaire, sleep diary, actigraphy, or polysomnography), methods used to manipulate experimentally sleep duration (i.e., partial night sleep deprivation over one night, sleep restriction over several nights, total sleep deprivation over one or more nights, but not sleep fragmentation); and circulating inflammatory markers (i.e., CRP, IL-6, TNF, or other).
Definition of sleep disturbance and sleep duration categories
Studies evaluating sleep disturbance data were categorized into three groups as determined by the assessment method: symptom reporting (single- or multiple items) (20–32), questionnaire (33–57), or diagnosis (34, 58–60). Studies evaluating sleep duration were grouped into those that treated sleep duration as a continuous measure subjectively (24, 31, 38, 45, 54, 61–66) or objectively (21, 22, 25, 34, 39, 54, 65, 67–70) vs. those that categorized sleep duration as short- or long sleep (27, 38, 62, 71–75). Consistent with prior meta-analyses (76, 77), the reference category for sleep duration was 7–8 h per night in the majority of studies. Hence, short sleep was defined as < 7 h per night, and long sleep was defined as > 8 h per night. Additionally, for sleep duration the assessment method was considered, i.e., self-report or objective. Finally, we evaluated studies that experimentally manipulated sleep duration over one night (78–88) or multiple nights (89–94), analyzing the sample obtained first in the morning.
Statistical analyses
The quality of the studies included in the meta-analysis was evaluated by the Downs & Black Quality Index score system (95), a validated checklist for assessing the quality of both randomized and non-randomized studies (cohort and case-control studies), which consists of 5 subscales (i.e., reporting, external validity, bias, confounding, and power) with a maximum score of 14 for non-randomized, non-prospective studies. Included studies scored between 12–14.
For all sources that met inclusion criteria, methods provided by Wilson (96) were utilized to calculate effect sizes in the Cohen’s d metric and associated standard errors. For sources in which the study’s methods section indicated that the relationship between selected sleep and immune measures were tested but either were not reported or reported as non-significant without sufficient information to calculate effect size, the effect size was assumed to be zero with appropriate standard errors for the sample. If multiple estimates of effects size were possible, based upon underlying distributional assumptions, the smallest effect size was included. For some studies, effects were reported for separate groups, most commonly by gender. Rather than create pooled effect sizes, effects were treated separately as independent groups. Some studies also provided effect size estimates in more than one category (e.g., sleep disturbance and sleep duration, CRP and IL-6). The heterogeneity among studies was tested by Q-statistic and quantified by H-statistic and I2-statistic (97). Funnel plot asymmetry was used to detect publication bias, and Egger’s regression test was applied to measure funnel plot asymmetry (98).
A priori, the set of studies selected was assumed to represent a random effects model, thus pooled effect size estimates were calculated as such; estimates of heterogeneity supported this decision. Pooled effect size estimates were generated according to the categories noted above, as well as overall pooled estimates. Based upon the availability of information, meta-regression (96) examined the impact of mean/median age of the sample and proportion of females vs. males upon the effect size estimates; the experimental studies were not tested as both the age and gender distributions were largely restricted.
RESULTS
As shown in Figure S1, a total of 1,802,699 articles were retrieved; 2206 articles were identified that included both sleep and inflammation terms. A total of 340 were duplicates, yielding 1866 articles for abstract review. An additional 1751 were excluded as not being relevant by review of title and abstract. Hence, 156 articles underwent full-text review with discussion during consensus meetings. Additional studies were excluded for the following reasons: review articles (n=6); absence of sleep assessment (n=13); absence of inflammation assessment (n=3); absence of analyses evaluating the relationship between sleep and inflammation (n=20); absence of assessment of circulating markers of inflammation (i.e., studies that included only cellular or genomic markers of inflammation were excluded) (n=22); circadian only studies (n=5); and sleep disturbance was the target of a treatment intervention with inflammation as a secondary outcome (n=4). Specifically, none of the intervention studies tested the relationship between change in sleep and inflammation; only main effects of the intervention were reported.
Following careful scrutiny of each article during data extraction, 11 additional studies were excluded for the following reasons: 1) statistics could not be estimated for associations, which reduced the Down and Black Quality index to below the minimum threshold of 12 (n=3) (43, 99, 100); 2) sleep, rather than inflammation, was the outcome (n=5) (101–105); and 3), no assessment of at least one of the selected measures of inflammation (i.e., CRP, IL-6, TNF) (n=3) (106–108). In addition to CRP, IL-6, or TNF, studies assessed other circulating markers of inflammation including interleukin-1β (IL-1) (n=8); IL-1 receptor antagonist (IL-1ra) (n=4); soluble IL-6 receptor (sIL6R) (n=3); IL-8 (n=2); tumor necrosis factor receptor I (TNFRI) (n=5); or TNFRII (n=2), but analyses related to these additional markers were not performed given the limited number of studies. Hence, 72 empirical studies were included, of which 28 evaluated sleep disturbance; 14 evaluated sleep duration; 13 evaluated both sleep disturbance and sleep duration, and 17 were experimental sleep deprivation or sleep restriction studies. Tables S1–3 summarize the characteristics of the included studies.
Sleep disturbance and inflammation
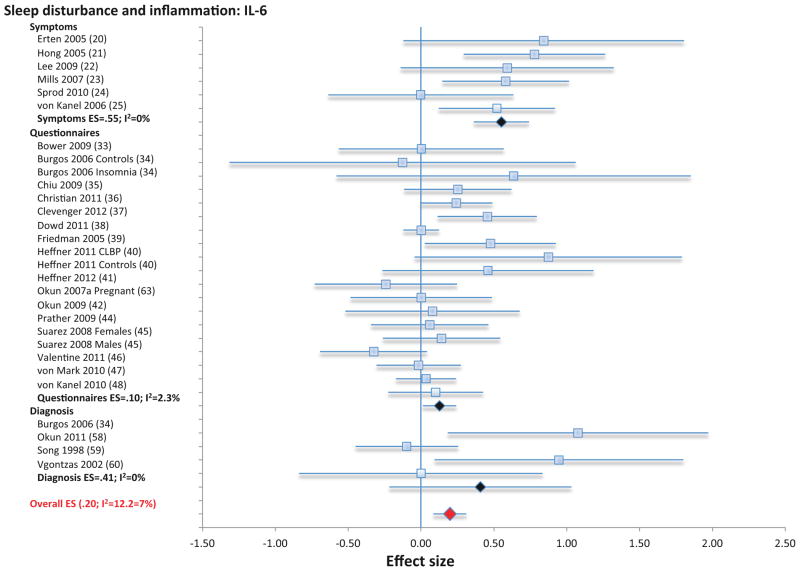
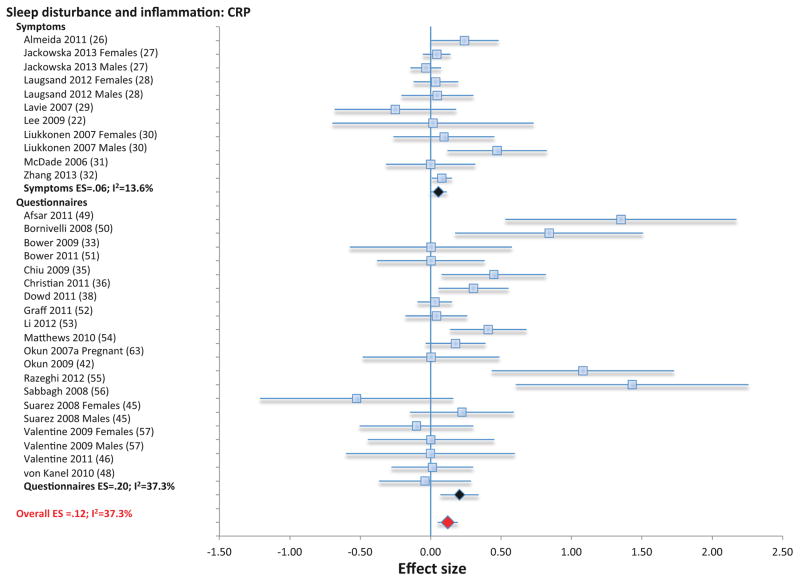
Three categories of assessment of sleep disturbance (i.e., symptom reporting using single- or multiple items; questionnaire; diagnosis) were used to evaluate the link between sleep disturbance and CRP, IL-6, and TNF; these varying assessment methods were analyzed separately and in combination for CRP and IL-6. For TNF, only effects of combined assessment categories were analyzed due to the few studies. First, symptom reporting of sleep disturbance was not associated with CRP (11 samples; N=31569; ES 0.06; 95% CI −0.005 – 0.12; Qv=10.9; p=.37; I2 = 7.9; Figure 1), but was associated with higher levels of IL-6 (6 samples, N=380; ES 0.55; 95% CI 0.36 – 0.74; Qv=4.8; p=.44; I2 =0; Figure 2). Second, sleep disturbance as assessed by questionnaire was associated with higher levels of CRP (20 samples; N= 3374; ES 0.20; 95% CI 0.07 – 0.33; Qv=30.3; p=.05; I2 = 37.7; Figure 1) and with higher levels of IL-6 (19 samples; N=2785; ES 0.10; 95% CI 0.01 – 0.20; Qv=18.4; p=.43; I2 =2.3; Figure 2). Third, sleep disturbance as assessed using diagnostic criteria for insomnia disorder was not associated with IL-6 (4 samples; N=174; ES 0.41; 95% CI −0.22 – 1.03; Qv=2.8; p=.42; I2 = 0.00); the association between diagnostic insomnia and CRP has not been determined. Finally, when all available methods of assessment were combined, sleep disturbance was associated with higher levels of CRP (31 samples; n=34943; ES 0.12; 95% CI 0.05 – 0.19; Qv=47.9; p=.02; I2 = 37.3) and with higher levels of IL-6 (29 samples; N= 3339; ES 0.20; 95% CI 0.08 – 0.31; Qv=29.6; p=.29; I2 = 12.2), but not TNF (8 samples; N=672; ES 0.07=; 95% CI −0.13–0.28, Qv=8.0; p=34; I2 =12.1%l; Figure S2).
Figure 1.
Forest plot of sleep disturbance associated with inflammation as indexed by C-reactive. Sleep disturbance is assessed by self-reported symptoms and questionnaires. Results are expressed as effect sizes (ES) and 95% confidence intervals (95% CI).
Figure 2.
Forest plot of sleep disturbance associated with inflammation as indexed by circulating levels of interleukin-6. Sleep disturbance is assessed by self-reported symptoms and questionnaires. Results are expressed as effect sizes (ES) and 95% confidence intervals (95% CI).
Sleep duration and inflammation
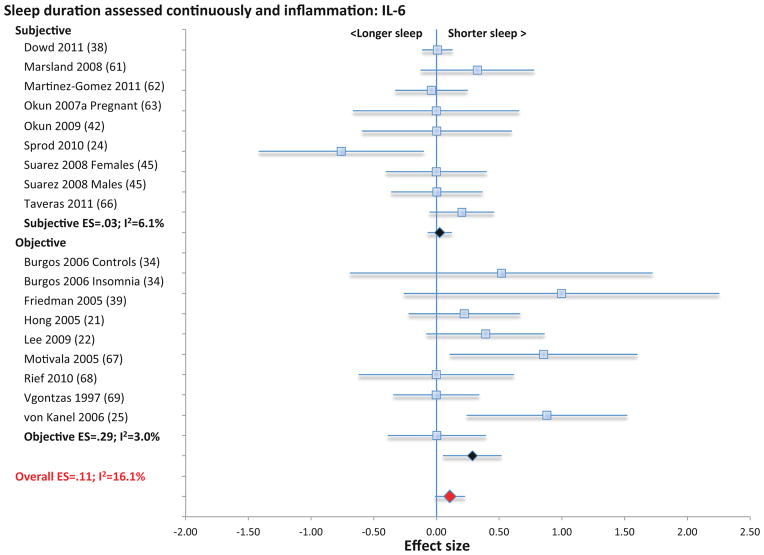
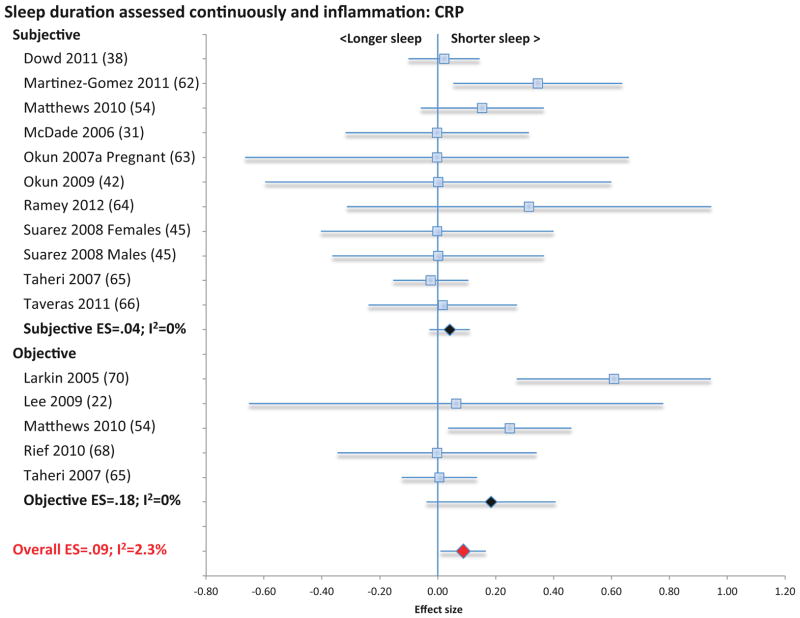
Two categories of assessment of sleep duration (i.e., sleep duration as a continuous variable using either subjective or objective measures; or short- and long sleep duration compared to reference normal of 7–8 h) (76, 77) were identified for evaluation in relation to CRP and IL-6. Sleep duration as a continuous variable using subjective measures was not associated with CRP (11 samples; N= 3490; ES 0.04; 95% CI −.03 – .11; Qv=7.3; p=.70; I2 = 0.00; Figure 3), or IL-6 (9 samples; N=2084; ES 0.03; 95% CI −0.09 – 0.14; Qv=8.5; p=.39; I2 = 6.1; Figure 4).
Figure 3.
Forest plot of sleep duration associated with inflammation as indexed by C-reactive. Sleep duration is assessed continuously by subjective and objective measures. Results are expressed as effect sizes (ES) and 95% confidence intervals (95% CI).
Figure 4.
Forest plot of sleep duration associated with inflammation as indexed by circulating levels of interleukin-6. Sleep duration is assessed continuously by subjective and objective measures. Results are expressed as effect sizes (ES) and 95% confidence intervals (95% CI).
When sleep duration was treated continuously using objective measures, sleep duration was also not associated with CRP (5 samples; N= 1550; ES 0.18; 95% CI −.04 – .41; Qv=4.0; p=.41; I2 = 0.00; Figure 3), although short sleep duration was associated with higher levels of IL-6 (9 samples; N=489; ES 0.29; 95% CI 0.05 – 0.52; Qv=8.2; p=.41; I2 = 3.0; Figure 4). In analyses that combined subjective and objective measures, short sleep duration was associated with higher levels of CRP (16 samples; N=5040; ES 0.09; 95% CI 0.01 – 0.17; Qv=15.4; p=.43; I2 =2.3; Figure 3) but not with IL-6 (18 samples; N=2573; ES 0.11; 95% CI −0.01 – 0.23; Qv=203; p=.26; I2 = 16.1; Figure 4) or TNF (4 samples; N=157; ES=0.29; 95% CI −0.27 – 0.84; Qv=3.1; p=.38; I2=3.2%; Figure S3)
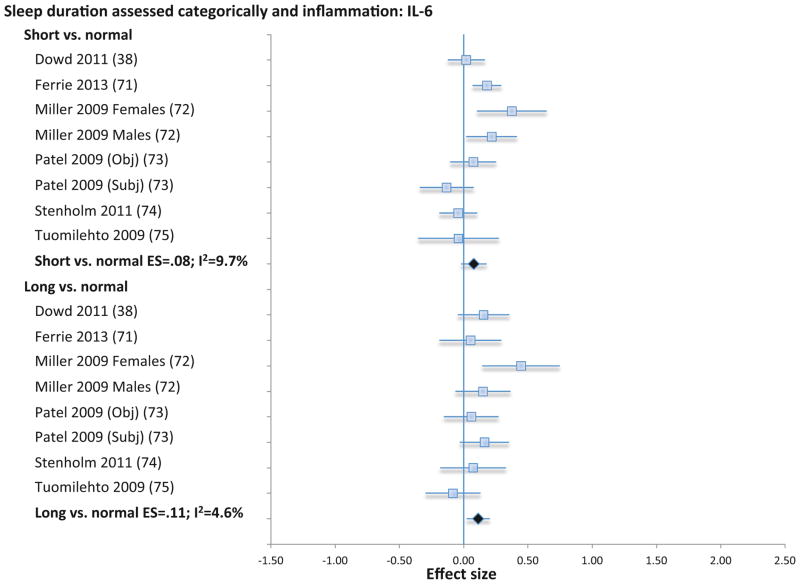
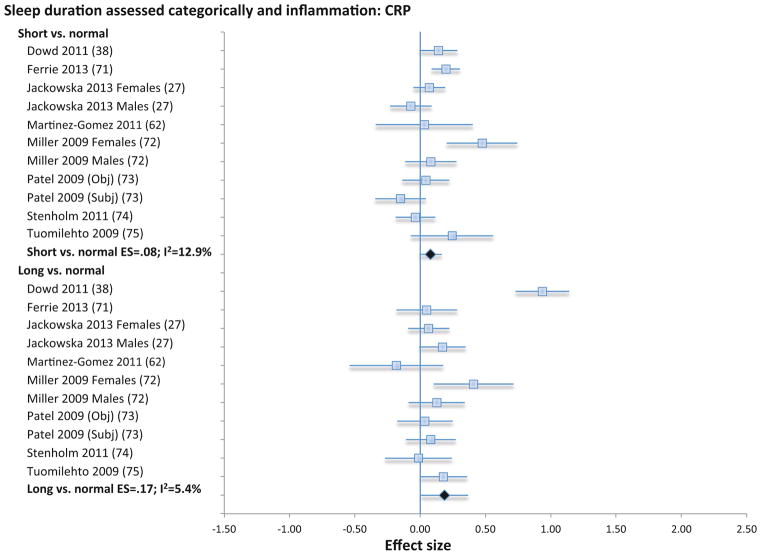
When extremes of sleep duration (i.e., short vs. long sleep duration) were analyzed as compared to normal sleep reference (7–8 h), short sleep duration was not associated with CRP (11 samples; N=19573; ES 0.08; 95% CI −0.01 – 0.16; Qv=11.5; p=.32; I2 = 12.9; Figure 5) IL-6 (8 samples; N=12925; ES 0.08; 95% CI −0.02 – 0.18; Qv=7.8; p=.35; I2 =9.7; Figure 6), or TNF (3 samples; N=1979; ES=0.11; 95% CI −.01 – .22; Qv=0.1; p=.97; I2=0%; Figure S4). However, long sleep duration was associated with higher levels of CRP (11 samples; N=19573; ES 0.17; 95% CI 0.01 – 0.34; Qv=10.6; p=.39; I2 = 5.4; Figure 5) and with higher levels of IL-6 (8 samples; N=12925; ES 0.11; 95% CI 0.02 – 0.20; Qv=7.3; p=.39; I2 = 4.6; Figure 6), but not TNF (3 samples; N=1979; ES=.08; CI −.06 – .22; Qv=2.2; p=.34; I2=8.0%; Figure S4). Subjective and objective methods for assessment of sleep duration were combined because there were too few studies that objectively evaluated sleep duration objectively.
Figure 5.
Forest plot of sleep duration associated with inflammation as indexed by C-reactive. Sleep duration is assessed categorically with normal sleep being defined by sleep duration 7–8 h per night, and short sleep as < 7 h per night, and long sleep as > 8 h per night. Results are expressed as effect sizes (ES) and 95% confidence intervals (95% CI).
Figure 6.
Forest plot of sleep duration associated with inflammation as indexed by circulating levels of interleukin-6. Sleep duration is assessed categorically with normal sleep being defined by sleep duration 7–8 h per night, and short sleep as < 7 h per night, and long sleep as > 8 h per night. Results are expressed as effect sizes (ES) and 95% confidence intervals (95% CI).
Overall, meta-regression results suggested that larger effect sizes were associated younger age and greater proportion of females within the sample; however these findings were only statistically significant for two subsamples: sleep disturbance predicting IL-6 (Female percentage of sample beta=0.36, p=.03) and sleep duration continuously predicting CRP (Age beta= −0.54, p=.02).
Experimental sleep deprivation and inflammation
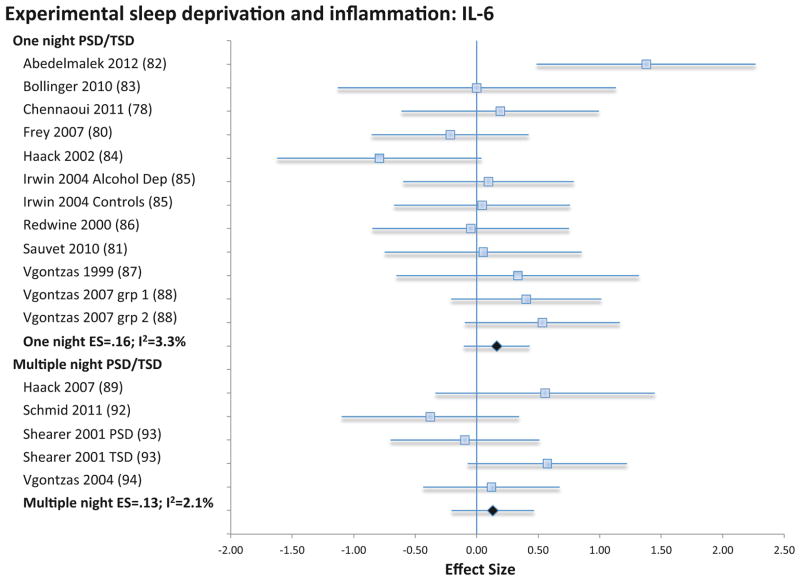
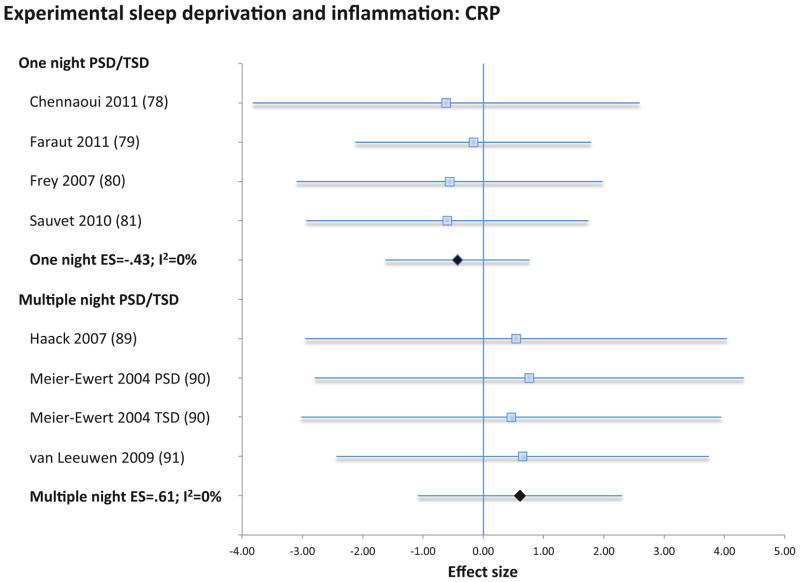
Experimental sleep deprivation, either for partial or total night, was not associated with CRP (4 samples; N=30; ES −0.43; 95% CI −1.62 – 0.77; Qv=0.1; p=.99; I2 = 0.0; Figure 7), IL-6 (12 samples; N=165; ES 0.16; 95% CI −0.11 – 0.43; Qv=11.4; p=.41; I2 = 3.3; Figure 8), or TNF (5 samples; N=61, ES=.04; 95% CI −0.32 – 0.39; Qv=0.4; p=.98; I2=0%; Figure S5). Likewise, sleep restriction over several days was not associated with CRP (4 samples; N=188; ES 0.61; 95% CI −1.09 – 2.30; Qv=0.1.; p=.99; I2 = 0.0; Figure 7), IL-6 (5 samples; N=98; ES 0.13; 95% CI −0.21 – 0.47; Qv=4.1; p=.39; I2 = 2.1; Figure 8), or TNF (4 samples; N=48, ES=.06; 95% CI −0.34 – 0.46; Qv=0.8; p=.86; I2=0%; Figure S5).
Figure 7.
Forest plot of experimentally shortened sleep duration associated with inflammation as indexed by C-reactive. Sleep duration was shortened by either partial- or total night sleep deprivation for one night or for multiple nights. Results are expressed as effect sizes (ES) and 95% confidence intervals (95% CI).
Figure 8.
Forest plot of experimentally shortened sleep duration associated with inflammation as indexed by circulating levels of interleukin-6. Sleep duration was shortened by either partial- or total night sleep deprivation for one night or for multiple nights. Results are expressed as effect sizes (ES) and 95% confidence intervals (95% CI).
Publication bias
There was some indication of publication bias, although this was evidenced only for sleep disturbance with outliers in the funnel plot. Trimming studies with effect sizes greater than 1.0 in absolute value eliminated this bias (Egger’s Regression test p>.20), and the relevant findings were largely unchanged: sleep disturbance by questionnaire remained associated with CRP (revised ES 0.12; 95% CI 0.02 – 0.22; Qv=16.5; p=.34; I2 =9.5) and with IL-6 (revised ES 0.10; 95% CI 0.003 – 0.21; Qv=17.6; p=.41; I2 =3.6), and sleep disturbance by diagnosis remained associated with IL-6 (revised ES 0.20; 95% CI −0.40 – 0.80; Qv=2.1; p=.35; I2 =5.3).
Furthermore, when all methods to assess sleep disturbance were combined, sleep disturbance remained associated with CRP (ES 0.09 (95% CI 0.03 – 0.14; Qv=30.1; p=.26; I2 =13.7) and with IL-6 (ES 0.19 (95% CI 0.08 – 0.30; Qv=26.7; p=.32; I2 =10.3). Though there was little evidence of publication bias for the TNF findings due both to the smaller number of studies and the larger percentage of null results.
DISCUSSION
This study provides a comprehensive review and quantitative estimates of the associations between sleep disturbance, as well as extremes of sleep duration, and inflammation in population-based samples, and varying clinical samples, around the world. It adds to a growing body of evidence that sleep disturbance is associated with inflammatory disease risk and all-cause mortality, possibly by effects of sleep disturbance on inflammatory mechanisms.
These results confirm the presence of an association between sleep disturbance and two markers of systemic inflammation, CRP and IL-6, with some heterogeneity among studies, no presence of publication bias, and a high statistical power conferred by nearly 34,000 participants for CRP and over 3000 participants for IL-6. Whereas sleep disturbance was not related to TNF, this conclusion is tempered by low statistical power with only 672 participants. The effect sizes linking sleep disturbance with IL-6 were larger than those found for CRP. Sleep disturbance is thought to have proximal effects on IL-6; in turn, IL-6 induces CRP. Hence, increases of CRP might due to more persistent or severe sleep disturbance (109). Evidence also showed that assessment of sleep disturbance by validated questionnaires was associated with increases in CRP and in IL-6, whereas assessment by symptom reporting had mixed effects. Questionnaires provide comprehensive assessment of sleep disturbance, and symptom reporting often relies on a single question.
The effects of sleep disturbance on inflammation were not associated with age, and relationships were comparable in men and women, although individual high quality studies showed that women, as compared to men, may be more vulnerable to the effects of sleep disturbance and show greater increases of CRP and IL-6 (45, 110), greater increases in Toll-like receptor 4 (TLR-4) stimulated monocyte production of inflammatory cytokines, and greater increases of nuclear factor (NF)-κB (111, 112). During undisturbed sleep, women also show greater TLR-4 stimulated production of IL-6 than men, a difference that is moderated by sex differences in tonic sympathovagal activity (113). Together these findings have implications for understanding the differential risk profile for inflammatory disorders between the sexes (114). For example, subjective symptoms of disturbed sleep are associated with a greater risk of cardiovascular disease in women than men, even after control for relevant confounders (115, 116).
In contrast to the associations between sleep disturbance and inflammation, sleep duration, as measured as a continuous variable using either subjective or objective methods, showed no significant association with IL-6, although a small effect was noted for CRP. When the extremes of sleep duration were evaluated, long sleep duration, but not short sleep duration, was associated with increases in CRP and with increases in IL-6. Shortening of sleep duration by experimental sleep deprivation was also not associated with CRP or IL-6, and this was the case for studies that either deprived participants of sleep for one night, or for part of a single night or for several consecutive nights. Interestingly, the associations between sleep duration and inflammation parallel the findings linking sleep and mortality; prior meta-analytic findings on sleep duration and mortality have found a U-shaped association, in which long sleepers (> 8 hours per night) have a 30% greater risk, whereas short sleepers (<7 hours per night) have a 12% greater risk of dying than those who sleep 7 to 8 h per night (76).
It is not known what aspect of sleep disturbance contributes to increases in inflammation. Sleep disturbance when combined with short duration are thought to be particularly caustic for health outcomes (4, 117–120), although studies of inflammation have predominantly examined sleep disturbance and sleep duration in separate models. Sleep fragmentation, as opposed to shortened sleep amounts, might also contribute to sleep disturbance, and such disruption of sleep continuity is uniquely associated with daytime dysfunction (121) and increases rates of mortality (1).
Experimental sleep loss did not alter circulating markers of inflammation, which stands in sharp contrast with findings that have evaluated upstream pathways of cellular and genomic markers of inflammation. For example, cellular production of IL-6 and TNF is due in part through activation of TLR activity, and partial night sleep deprivation induces an increase in TLR-4-stimulated production of inflammatory cytokines (122), as well as activation of the key transcription control pathway in the inflammatory signaling cascade, nuclear factor (NF)-κB (111), which in turn drives effects on transcriptome dynamics with an upregulation of a gene ensemble that includes the master circadian regulator, several immediate early genes marking cellular signal transduction, and multiple inflammatory response genes (122). More persistent disturbances of sleep may be needed to translate inflammatory signaling into increases in systemic markers of inflammation.
The mechanisms that might explain the associations between sleep disturbance and inflammation are relatively unexplored. Sleep influences two primary effector systems, the hypothalamus-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS), which together shift the basal gene expression profile toward increased proinflammatory skewing (14, 123). Activation of β-adrenergic signaling induces increases in NF-κB, inflammatory gene expression, production of proinflammatory cytokines, and markers of systemic inflammation (14). Given that normal nocturnal sleep is associated with a drop in sympathetic outflow (124), activation of the sympathetic effector pathway is one biologically plausible mechanism to explain the associations between sleep disturbance, short sleep duration, and increases in markers of inflammation. The association between long sleep and inflammation may be the result of underlying comorbidities, which were not fully controlled.
The quality of these meta-analytic data cannot go beyond the quality of the individual studies included. Although all studies fulfilled a minimum threshold of quality and the majority of studies considered confounding variables, a meta-analysis of observational data is open to residual confound and bias. Whereas we made an attempt to allow for multiple confounding by including adjusted estimates from multivariate models from each contributing study, many studies did not report adjusted estimates. Second, the results can only be representative of the studies that have been included, although there was no evidence of publication bias for the primary findings. Third, the vast majority of studies used sleep questionnaires, as opposed to diagnostic criteria or objective measures such as actigraphy or polysomnography, to assess sleep disturbance and sleep duration. Nevertheless, sleep diaries, actigraphy, and polysomnography from some large population and small-scale investigations have shown high correlations with subjective-estimates of sleep duration (125, 126). Fourth, sleep disturbance and/or sleep duration was assessed at one point in time in all studies, which neglects testing the possibility that persistent sleep problems may have more robust effects on inflammation. Fifth, insufficient data were available to examine systematically whether those with clinically severe insomnia (34) or extreme short sleep duration (<5 h) (27, 71, 72) were more likely to evidence inflammation. Finally, none of these studies were prospective in design which limits any conclusions about the direction of the associations as acute elevations in markers of inflammation can also alter sleep amounts and sleep depth (9).
Whereas no study has systematically evaluated whether elevated levels of inflammation mediate the association between sleep disturbance and cardiovascular disease or other diseases that have an inflammatory component including cancer and depression, extensive data show that sleep disturbance, as well as extremes of sleep duration, are linked to multiple morbidities and mortality (9). Sleep disturbance, and long sleep duration, should be regarded as additional behavioral risk factors for inflammation, which are amenable to modification through treatments that target sleep behaviors. Indeed, treatment of insomnia been found to reduce inflammation (17, 18), and together with diet and physical activity, represent a third component in the promotion of sleep health.
Supplementary Material
Acknowledgments
Supported by grant R01-AG034588 from the National Institute of Aging, other grant support from the National Institutes of Health to MRI including R01-CA119159; R01-HL079955; R01 HL095799; P30-AG028748; P30-AG017265; UL RR 033176; the Cousins Center for Psychoneuroimmunology, and UCLA Claude D. Pepper Older Americans Independence Center.
Footnotes
FINANCIAL DISCLOSURES
All authors report no biomedical financial interests or potential conflicts of interest.
The National Institutes of Health had no role in the design and conduct of the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dew MA, Hoch CC, Buysse DJ, Monk TH, Begely AE, Houck PR, Hall M, Kupper DJ, Reynold CF. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 2.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 3.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251:207–16. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- 4.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17:241–54. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeBlanc M, Merette C, Savard J, Ivers H, Baillargeon L, Morin CM. Incidence and risk factors of insomnia in a population-based sample. Sleep. 2009;32:1027–37. doi: 10.1093/sleep/32.8.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morin CM, Belanger L, LeBlanc M, Ivers H, Savard J, Espie CA, Merette C, Baillargeon L, Gregoire JP. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Internal Medicine. 2009;169:447–53. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 7.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 8.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37:9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Ann Rev Psychol. 2015;66:2.1–2.30. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–7. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 12.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–51. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 13.Barzilay JI, Forsberg C, Heckbert SR, Cushman M, Newman AB. The association of markers of inflammation with weight change in older adults: the Cardiovascular Health Study. Int J Obes (Lond) 2006;30:1362–7. doi: 10.1038/sj.ijo.0803306. [DOI] [PubMed] [Google Scholar]
- 14.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nature Rev Immunol. 2011;11:625–32. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunner EJ, Kivimaki M, Witte DR, Lawlor DA, Davey Smith G, Cooper JA, Miller M, Lowe GD, Rumley A, Casas JP, Shah T, Humphries SE, Hingorani AD, Marmot MG, Timpson NJ, Kumari M. Inflammation, insulin resistance, and diabetes--Mendelian randomization using CRP haplotypes points upstream. PLoS Med. 2008;5:e155. doi: 10.1371/journal.pmed.0050155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irwin M, Olmstead R, Breen E, Witarama T, Carrillo C, NS, Arevalo J, Ma J, Nicassio P, Ganz P, Bower J, Cole S. Tai Chi, cellular inflammation, and transcriptome dynamics in breast cancer survivors with insomnia: a randomized controlled trial. J Natl Cancer Inst. 2014;50:295–301. doi: 10.1093/jncimonographs/lgu028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Breen EC, Witarama T, Yokomizo M, Lavretsky H, Carroll JE, Motivala SJ, Bootzin R, Nicassio P. Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: a randomized controlled comparative efficacy trial. Sleep. 2014;37:1543–52. doi: 10.5665/sleep.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irwin MR, Olmstead R, Breen EC, Witarama T, Carrillo C, Sadeghi N, Arevalo JM, Ma J, Nicassio P, Bootzin R, Cole S. Cognitive behavioral therapy and Tai Chi reverse cellular and genomic markers of inflammation in late life insomnia: a randomized controlled trial. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie X, Pan L, Ren D, Du C, Guo Y. Effects of continuous positive airway pressure therapy on systemic inflammation in obstructive sleep apnea: a meta-analysis. Sleep Med. 2013;14:1139–50. doi: 10.1016/j.sleep.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Erten Y, Kokturk O, Yuksel A, Elbeg S, Ciftci TU, Pasaoglu H, Ozkan S, Bali M, Arinsoi T, Sindel S. Relationship between sleep complaints and proinflammatory cytokines in haemodialysis patients. Nephrology (Carlton) 2005;10:330–5. doi: 10.1111/j.1440-1797.2005.00418.x. [DOI] [PubMed] [Google Scholar]
- 21.Hong S, Mills PJ, Loredo JS, Adler KA, Dimsdale JE. The association between interleukin-6, sleep, and demographic characteristics. Brain Behav Immun. 2005;19:165–72. doi: 10.1016/j.bbi.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Kim SJ, Jung HH. Nocturnal sleep related with metabolic markers in end-stage renal disease patients receiving hemodialysis. Psychiatry Investig. 2009;6:34–8. doi: 10.4306/pi.2009.6.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills PJ, von Kanel R, Norman D, Natarajan L, Ziegler MG, Dimsdale JE. Inflammation and sleep in healthy individuals. Sleep. 2007;30:729–35. doi: 10.1093/sleep/30.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sprod LK, Palesh OG, Janelsins MC, Peppone LJ, Heckler CE, Adams MJ, Morrow GR, Mustian KM. Exercise, sleep quality, and mediators of sleep in breast and prostate cancer patients receiving radiation therapy. Community Oncol. 2010;7:463–471. doi: 10.1016/s1548-5315(11)70427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Kanel R, Dimsdale JE, Ancoli-Israel S, Mills PJ, Patterson TL, McKibbin CL, Archuleta C, Grant I. Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin D-dimer in older caregivers of people with Alzheimer’s disease. J Am Geriatr Soc. 2006;54:431–7. doi: 10.1111/j.1532-5415.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 26.Almeida OP, Alfonso H, Yeap BB, Hankey G, Flicker L. Complaints of difficulty to fall asleep increase the risk of depression in later life: the health in men study. Journal of affective disorders. 2011;134:208–16. doi: 10.1016/j.jad.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 27.Jackowska M, Kumari M, Steptoe A. Sleep and biomarkers in the English Longitudinal Study of Ageing: associations with C-reactive protein, fibrinogen, dehydroepiandrosterone sulfate and hemoglobin. Psychoneuroendocrin. 2013;38:1484–93. doi: 10.1016/j.psyneuen.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Laugsand LE, Vatten LJ, Bjorngaard JH, Hveem K, Janszky I. Insomnia and high-sensitivity C-reactive protein: the HUNT study, Norway. Psychosom Med. 2012;74:543–53. doi: 10.1097/PSY.0b013e31825904eb. [DOI] [PubMed] [Google Scholar]
- 29.Lavie L, Lavie P. Elevated plasma homocysteine in older shift-workers: a potential risk factor for cardiovascular morbidity. Chronobiol Int. 2007;24:115–28. doi: 10.1080/07420520601139797. [DOI] [PubMed] [Google Scholar]
- 30.Liukkonen T, Rasanen P, Ruokonen A, Laitinen J, Jokelainen J, Leinonen M, Meyer-Rochow VB, Timonen M. C-reactive protein levels and sleep disturbances: observations based on the Northern Finland 1966 Birth Cohort study. Psychosom Med. 2007;69:756–61. doi: 10.1097/PSY.0b013e318157cb96. [DOI] [PubMed] [Google Scholar]
- 31.McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosom Med. 2006;68:376–81. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Lamers F, Hickie IB, He JP, Feig E, Merikangas KR. Differentiating nonrestorative sleep from nocturnal insomnia symptoms: demographic, clinical, inflammatory, and functional correlates. Sleep. 2013;36:671–9. doi: 10.5665/sleep.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, Cole S, Aziz N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15:5534–40. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgos I, Richter L, Klein T, Fiebich B, Feige B, Lieb K, Voderholzer U, Riemann D. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain Behav Immun. 2006;20:246–53. doi: 10.1016/j.bbi.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Chiu YL, Chuang YF, Fang KC, Liu SK, Chen HY, Yang JY, Pai MF, Peng YS, Wu KD, Tsai TJ. Higher systemic inflammation is associated with poorer sleep quality in stable haemodialysis patients. Nephrol Dial Transplant. 2009;24:247–51. doi: 10.1093/ndt/gfn439. [DOI] [PubMed] [Google Scholar]
- 36.Christian LM, Glaser R, Porter K, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK. Poorer self-rated health is associated with elevated inflammatory markers among older adults. Psychoneuroendocrin. 2011;36:1495–504. doi: 10.1016/j.psyneuen.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clevenger L, Schrepf A, Christensen D, DeGeest K, Bender D, Ahmed A, Goodheart MJ, Penedo F, Lubaroff DM, Sood AK, Lutgendorf SK. Sleep disturbance, cytokines, and fatigue in women with ovarian cancer. Brain Behav Immun. 2012;26:1037–44. doi: 10.1016/j.bbi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dowd JB, Goldman N, Weinstein M. Sleep duration, sleep quality, and biomarkers of inflammation in a Taiwanese population. Ann Epidemiol. 2011;21:799–806. doi: 10.1016/j.annepidem.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman EM, Hayney MS, Love GD, Urry HL, Rosenkranz MA, Davidson RJ, Singer BH, Ryff CD. Social relationships, sleep quality, and interleukin-6 in aging women. Proc Natl Acad Sci U S A. 2005;102:18757–62. doi: 10.1073/pnas.0509281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heffner KL, France CR, Trost Z, Ng HM, Pigeon WR. Chronic low back pain, sleep disturbance, and interleukin-6. Clin J Pain. 2011;27:35–41. doi: 10.1097/ajp.0b013e3181eef761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heffner KL, Ng HM, Suhr JA, France CR, Marshall GD, Pigeon WR, Moynihan JA. Sleep disturbance and older adults’ inflammatory responses to acute stress. Am J Geriatr Psychiatry. 2012;20:744–52. doi: 10.1097/JGP.0b013e31824361de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okun ML, Coussons-Read M, Hall M. Disturbed sleep is associated with increased C-reactive protein in young women. Brain Behav Immun. 2009;23:351–4. doi: 10.1016/j.bbi.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okun ML, Hall M, Coussons-Read ME. Sleep disturbances increase interleukin-6 production during pregnancy: implications for pregnancy complications. Reprod Sci. 2007b;14:560–7. doi: 10.1177/1933719107307647. [DOI] [PubMed] [Google Scholar]
- 44.Prather AA, Marsland AL, Hall M, Neumann SA, Muldoon MF, Manuck SB. Normative variation in self-reported sleep quality and sleep debt is associated with stimulated pro-inflammatory cytokine production. Biol Psychol. 2009;82:12–7. doi: 10.1016/j.biopsycho.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav Immun. 2008;22:960–8. doi: 10.1016/j.bbi.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valentine RJ, Woods JA, McAuley E, Dantzer R, Evans EM. The associations of adiposity, physical activity and inflammation with fatigue in older adults. Brain Behav Immun. 2011;25:1482–90. doi: 10.1016/j.bbi.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 47.van Mark A, Weiler SW, Schroder M, Otto A, Jauch-Chara K, Groneberg DA, Spallek M, Kessel R, Kalsdorf B. The impact of shift work induced chronic circadian disruption on IL-6 and TNF-alpha immune responses. J Occup Med Toxicol. 2010;5:18. doi: 10.1186/1745-6673-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Kanel R, Ancoli-Israel S, Dimsdale JE, Mills PJ, Mausbach BT, Ziegler MG, Patterson TL, Grant I. Sleep and biomarkers of atherosclerosis in elderly Alzheimer caregivers and controls. Gerontology. 2010;56:41–50. doi: 10.1159/000264654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Afshar R, Emany A, Saremi A, Shavandi N, Sanavi S. Effects of intradialytic aerobic training on sleep quality in hemodialysis patients. Iran J Kidney Dis. 2011;5:119–23. [PubMed] [Google Scholar]
- 50.Bornivelli C, Alivanis P, Giannikouris I, Arvanitis A, Choustoulakis I, Georgopoulou K, Karvouniaris N, Zervos A. Relation between insomnia mood disorders and clinical and biochemical parameters in patients undergoing chronic hemodialysis. J Nephrol. 2008;21(Suppl 13):S78–83. [PubMed] [Google Scholar]
- 51.Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517–22. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graff LA, Vincent N, Walker JR, Clara I, Carr R, Ediger J, Miller N, Rogala L, Rawsthorne P, Lix L, Bernstein CN. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1882–9. doi: 10.1002/ibd.21580. [DOI] [PubMed] [Google Scholar]
- 53.Li J, Guo Q, Ye X, Lin J, Yi C, Mao H, Yang X, Yu X. Prevalence and risk factors of sleep disturbance in continuous ambulatory peritoneal dialysis patients in Guangzhou, southern China. Int Urol Nephrol. 2012;44:929–36. doi: 10.1007/s11255-011-0060-5. [DOI] [PubMed] [Google Scholar]
- 54.Matthews KA, Zheng H, Kravitz HM, Sowers M, Bromberger JT, Buysse DJ, Owens JF, Sanders M, Hall M. Are inflammatory and coagulation biomarkers related to sleep characteristics in mid-life women?: Study of Women’s Health across the Nation sleep study. Sleep. 2010;33:1649–55. doi: 10.1093/sleep/33.12.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Razeghi E, Sahraian MA, Heidari R, Bagherzadeh M. Association of inflammatory biomarkers with sleep disorders in hemodialysis patients. Acta Neurol Belg. 2012;112:45–9. doi: 10.1007/s13760-012-0003-7. [DOI] [PubMed] [Google Scholar]
- 56.Sabbagh R, Iqbal S, Vasilevsky M, Barre P. Correlation between physical functioning and sleep disturbances in hemodialysis patients. Hemodial Int. 2008;12(Suppl 2):S20–4. doi: 10.1111/j.1542-4758.2008.00319.x. [DOI] [PubMed] [Google Scholar]
- 57.Valentine RJ, McAuley E, Vieira VJ, Baynard T, Hu L, Evans EM, Woods JA. Sex differences in the relationship between obesity, C-reactive protein, physical activity, depression, sleep quality and fatigue in older adults. Brain Behav Immun. 2009;23:643–8. doi: 10.1016/j.bbi.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 58.Okun ML, Reynolds CF, 3rd, Buysse DJ, Monk TH, Mazumdar S, Begley A, Hall M. Sleep variability, health-related practices, and inflammatory markers in a community dwelling sample of older adults. Psychosom Med. 2011;73:142–50. doi: 10.1097/PSY.0b013e3182020d08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song C, Lin A, Bonaccorso S, Heide C, Verkerk R, Kenis G, Bosmans E, Scharpe S, Whelan A, Cosyns P, de Jongh R, Maes M. The inflammatory response system and the availability of plasma tryptophan in patients with primary sleep disorders and major depression. J Affect Disord. 1998;49:211–9. doi: 10.1016/s0165-0327(98)00025-1. [DOI] [PubMed] [Google Scholar]
- 60.Vgontzas AN, Zoumakis M, Papanicolaou DA, Bixler EO, Prolo P, Lin HM, Vela-Bueno A, Kales A, Chrousos GP. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism. 2002;51:887–92. doi: 10.1053/meta.2002.33357. [DOI] [PubMed] [Google Scholar]
- 61.Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64:484–90. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinez-Gomez D, Eisenmann JC, Gomez-Martinez S, Hill EE, Zapatera B, Veiga OL, Marcos A, Group AS. Sleep duration and emerging cardiometabolic risk markers in adolescents. The AFINOS study. Sleep Med. 2011;12:997–1002. doi: 10.1016/j.sleep.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 63.Okun ML, Coussons-Read ME. Sleep disruption during pregnancy: how does it influence serum cytokines? J Reprod Immunol. 2007a;73:158–65. doi: 10.1016/j.jri.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 64.Ramey SL, Perkhounkova Y, Moon M, Budde L, Tseng HC, Clark MK. The effect of work shift and sleep duration on various aspects of police officers’ health. Workplace Health Saf. 2012;60:215–22. doi: 10.1177/216507991206000505. [DOI] [PubMed] [Google Scholar]
- 65.Taheri S, Austin D, Lin L, Nieto FJ, Young T, Mignot E. Correlates of serum C-reactive protein (CRP)--no association with sleep duration or sleep disordered breathing. Sleep. 2007;30:991–6. doi: 10.1093/sleep/30.8.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taveras EM, Rifas-Shiman SL, Rich-Edwards JW, Mantzoros CS. Maternal short sleep duration is associated with increased levels of inflammatory markers at 3 years postpartum. Metabolism. 2011;60:982–6. doi: 10.1016/j.metabol.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosom Med. 2005;67:187–94. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- 68.Rief W, Mills PJ, Ancoli-Israel S, Ziegler MG, Pung MA, Dimsdale JE. Overnight changes of immune parameters and catecholamines are associated with mood and stress. Psychosom Med. 2010;72:755–62. doi: 10.1097/PSY.0b013e3181f367e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 70.Larkin EK, Rosen CL, Kirchner HL, Storfer-Isser A, Emancipator JL, Johnson NL, Zambito AM, Tracy RP, Jenny NS, Redline S. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111:1978–84. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- 71.Ferrie JE, Kivimaki M, Akbaraly TN, Singh-Manoux A, Miller MA, Gimeno D, Kumari M, Davey Smith G, Shipley MJ. Associations between change in sleep duration and inflammation: findings on C-reactive protein and interleukin 6 in the Whitehall II Study. Am J Epidemiol. 2013;178:956–61. doi: 10.1093/aje/kwt072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller MA, Kandala NB, Kivimaki M, Kumari M, Brunner EJ, Lowe GD, Marmot MG, Cappuccio FP. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep. 2009;32:857–64. [PMC free article] [PubMed] [Google Scholar]
- 73.Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, Redline S. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–4. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stenholm S, Kronholm E, Bandinelli S, Guralnik JM, Ferrucci L. Self-reported sleep duration and time in bed as predictors of physical function decline: results from the InCHIANTI study. Sleep. 2011;34:1583–93. doi: 10.5665/sleep.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tuomilehto H, Peltonen M, Partinen M, Lavigne G, Eriksson JG, Herder C, Aunola S, Keinanen-Kiukaanniemi S, Ilanne-Parikka P, Uusitupa M, Tuomilehto J, Lindstrom J Finnish Diabetes Prevention Study G. Sleep duration, lifestyle intervention, and incidence of type 2 diabetes in impaired glucose tolerance: The Finnish Diabetes Prevention Study. Diabetes Care. 2009;32:1965–71. doi: 10.2337/dc08-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 78.Chennaoui M, Sauvet F, Drogou C, Van Beers P, Langrume C, Guillard M, Gourby B, Bourrilhon C, Florence G, Gomez-Merino D. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-α) levels in healthy men. Cytokine. 2011;56:318–324. doi: 10.1016/j.cyto.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 79.Faraut B, Boudjeltia KZ, Dyzma M, Rousseau A, David E, Stenuit P, Franck T, Van Antwerpen P, Vanhaeverbeek M, Kerkhofs M. Benefits of napping and an extended duration of recovery sleep on alertness and immune cells after acute sleep restriction. Brain Behav Immun. 2011;25:16–24. doi: 10.1016/j.bbi.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 80.Frey DJ, Fleshner M, Wright KP., Jr The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21:1050–7. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 81.Sauvet F, Leftheriotis G, Gomez-Merino D, Langrume C, Drogou C, Van Beers P, Bourrilhon C, Florence G, Chennaoui M. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol. 2010;108:68–75. doi: 10.1152/japplphysiol.00851.2009. [DOI] [PubMed] [Google Scholar]
- 82.Abedelmalek S, Chtourou H, Aloui A, Aouichaoui C, Souissi N, Tabka Z. Effect of time of day and partial sleep deprivation on plasma concentrations of IL-6 during a short-term maximal performance. Eur J Appl Physiol. 2013;113:241–248. doi: 10.1007/s00421-012-2432-7. [DOI] [PubMed] [Google Scholar]
- 83.Bollinger T, Bollinger A, Oster H, Solbach W. Sleep, immunity, and circadian clocks: a mechanistic model. Gerontology. 2010;56:574–80. doi: 10.1159/000281827. [DOI] [PubMed] [Google Scholar]
- 84.Haack M, Kraus T, Schuld A, Dalal M, Koethe D, Pollmacher T. Diurnal variations of interleukin-6 plasma levels are confounded by blood drawing procedures. Psychoneuroendocrinology. 2002;27:921–31. doi: 10.1016/s0306-4530(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 85.Irwin M, Rinetti G, Redwine L, Motivala S, Dang J, Ehlers C. Nocturnal proinflammatory cytokine-associated sleep disturbances in abstinent African American alcoholics. Brain Behav Immun. 2004;18:349–60. doi: 10.1016/j.bbi.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 86.Redwine L, Hauger RL, Gillin JC, Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000;85:3597–603. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- 87.Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, Prolo P, Wong ML, Licinio J, Gold PW, Hermida RC, Mastorakos G, Chrousos GP. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84:2603–7. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- 88.Vgontzas AN, Pejovic S, Zoumakis E, Lin HM, Bixler EO, Basta M, Fang J, Sarrigiannidis A, Chrousos GP. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am J Physiol Endocrinol Metab. 2007;292:E253–61. doi: 10.1152/ajpendo.00651.2005. [DOI] [PubMed] [Google Scholar]
- 89.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–52. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 91.van Leeuwen WM, Lehto M, Karisola P, Lindholm H, Luukkonen R, Sallinen M, Harma M, Porkka-Heiskanen T, Alenius H. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One. 2009;4:e4589. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Lehnert H, Born J, Schultes B. Disturbed glucoregulatory response to food intake after moderate sleep restriction. Sleep. 2011;34:371–7. doi: 10.1093/sleep/34.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, Szuba MP, Van Dongen HP, Dinges DF. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–70. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 94.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 95.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilson DB, Lipsey MW. The role of method in treatment effectiveness research: evidence from meta-analysis. Psychol Methods. 2001;6:413–29. [PubMed] [Google Scholar]
- 97.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Foster SB, Lu M, Glaze DG, Reuben JM, Harris LL, Cohen EN, Lee BN, Zhao E, Paul ME, Schwarzwald H, McMullen-Jackson C, Clark C, Armstrong FD, Brouwers PY, Miller TL, Colin AA, Scott GB, Shahzeidi S, Willen EJ, Asthana D, Lipshultz SE, Thompson BW, Shearer WT. Associations of cytokines, sleep patterns, and neurocognitive function in youth with HIV infection. Clin Immunol. 2012;144:13–23. doi: 10.1016/j.clim.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Main LC, Dawson B, Heel K, Grove JR, Landers GJ, Goodman C. Relationship between inflammatory cytokines and self-report measures of training overload. Res Sports Med. 2010;18:127–39. doi: 10.1080/15438621003627133. [DOI] [PubMed] [Google Scholar]
- 101.Capuron L, Schroecksnadel S, Féart C, Aubert A, Higueret D, Barberger-Gateau P, Layé S, Fuchs D. Chronic Low-Grade Inflammation in Elderly Persons Is Associated with Altered Tryptophan and Tyrosine Metabolism: Role in Neuropsychiatric Symptoms. Biol Psychiatry. 2011;70:175–182. doi: 10.1016/j.biopsych.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 102.Cho HJ, Bower JE, Kiefe CI, Seeman TE, Irwin MR. Early life stress and inflammatory mechanisms of fatigue in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Brain Behav Immun. 2012;26:859–65. doi: 10.1016/j.bbi.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu L, Mills PJ, Rissling M, Fiorentino L, Natarajan L, Dimsdale JE, Sadler GR, Parker BA, Ancoli-Israel S. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav Immun. 2012;26:706–13. doi: 10.1016/j.bbi.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.von Käanel R, Mausbach BT, Ancoli-Israel S, Dimsdale JE, Mills PJ, Patterson TL, Ziegler MG, Roepke SK, Chattillion EA, Allison M, Grant I. Sleep in Spousal Alzheimer Caregivers: A Longitudinal Study with a Focus on the Effects of Major Patient Transitions on Sleep. Sleep. 2012 doi: 10.5665/sleep.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vgontzas AN, Zoumakis M, Bixler EO, Lin HM, Prolo P, Vela-Bueno A, Kales A, Chrousos GP. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J Clin Endocrinol Metab. 2003;88:2087–95. doi: 10.1210/jc.2002-021176. [DOI] [PubMed] [Google Scholar]
- 106.Carpagnano GE, Spanevello A, Sabato R, Depalo A, Palladino GP, Bergantino L, Foschino Barbaro MP. Systemic and airway inflammation in sleep apnea and obesity: the role of ICAM-1 and IL-8. Transl Res. 2010;155:35–43. doi: 10.1016/j.trsl.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 107.Hohagen F, Timmer J, Weyerbrock A, Ritsch-Montero R, Ganter U, Krieger S, Berger M, Bauer J. Cytokine production during sleep and wakefulness and its relationship to cortisol in healthy humans. Neuropsychobiol. 1993;28:9–16. doi: 10.1159/000118993. [DOI] [PubMed] [Google Scholar]
- 108.Sakami S, Ishikawa T, Kawakami N, Haratani T, Fukui A, Kobayashi F, Fujita O, Araki S, Kawamura N. Coemergence of insomnia and a shift in the Th1/Th2 balance toward Th2 dominance. Neuroimmunomodulation. 2002;10:337–43. doi: 10.1159/000071474. [DOI] [PubMed] [Google Scholar]
- 109.Cole SW, Arevalo JM, Manu K, Telzer EH, Kiang L, Bower JE, Irwin MR, Fuligni AJ. Antagonistic pleiotropy at the human IL6 promoter confers genetic resilience to the pro-inflammatory effects of adverse social conditions in adolescence. Develop Psychol. 2011;47:1173–80. doi: 10.1037/a0023871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prather AA, Epel ES, Cohen BE, Neylan TC, Whooley MA. Gender differences in the prospective associations of self-reported sleep quality with biomarkers of systemic inflammation and coagulation: findings from the Heart and Soul Study. J Psychiatr Res. 2013;47:1228–35. doi: 10.1016/j.jpsychires.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, Martinez-Maza O, Cole S. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–40. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010;24:54–7. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.O’Connor MF, Motivala SJ, Valladares EM, Olmstead R, Irwin MR. Sex differences in monocyte expression of IL-6: role of autonomic mechanisms. Am J Physiol Regul Integr Comp Physiol. 2007;293:R145–51. doi: 10.1152/ajpregu.00752.2006. [DOI] [PubMed] [Google Scholar]
- 114.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–80. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 115.Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M, Ferrie JE, Shipley MJ, Brunner EJ, Marmot MG. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Newman AB, Spiekerman CF, Enright P, Lefkowitz D, Manolio T, Reynolds CF, Robbins J. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. The Cardiovascular Health Study Research Group. J Am Geriatr Soc. 2000;48:115–23. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- 117.Carroll JE, Seeman TE, Olmstead R, Melendez G, Sadakane R, Bootzin R, Nicassio P, Irwin MR. Improved sleep quality in older adults with insomnia reduces biomarkers of disease risk: Pilot results from a randomized controlled comparative efficacy trial. Psychoneuroendocrin. 2015;55:184–92. doi: 10.1016/j.psyneuen.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carroll JE, Irwin MR, Stein Merkin S, Seeman TE. Sleep and multisystem biological risk: a population-based study. PLoS One. 2015;10:e0118467. doi: 10.1371/journal.pone.0118467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Basta M, Fernandez-Mendoza J, Bixler EO. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33:1159–64. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kierlin L, Olmstead R, Yokomizo M, Nicassio P, Irwin MR. Diagnostic and Statistical Manual criteria for insomnia related impairment in daytime functioning: Polysomnographic correlates in older adults. Sleep Med. 2012;13:958–60. doi: 10.1016/j.sleep.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 123.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrin Metab. 1999;84:1979–85. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- 125.Signal TL, Gale J, Gander PH. Sleep measurement in flight crew: comparing actigraphic and subjective estimates to polysomnography. Aviat Space Environ Med. 2005;76:1058–63. [PubMed] [Google Scholar]
- 126.Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, Stampfer MJ, Hu FB. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–4. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.