Abstract
The purpose of this study was to determine the impact of peripheral chemoreflex inhibition with low-dose dopamine on maximal apnea time, and the related hemodynamic and cerebrovascular responses in elite apnea divers. In a randomized order, participants performed a maximal apnea while receiving either intravenous 2 μg·kg−1·min−1 dopamine or volume-matched saline (placebo). The chemoreflex and hemodynamic response to dopamine was also assessed during hypoxia [arterial O2 tension, (PaO2) ∼35 mmHg] and mild hypercapnia [arterial CO2 tension (PaCO2) ∼46 mmHg] that mimicked the latter parts of apnea. Outcome measures included apnea duration, arterial blood gases (radial), heart rate (HR, ECG), mean arterial pressure (MAP, intra-arterial), middle (MCAv) and posterior (PCAv) cerebral artery blood velocity (transcranial ultrasound), internal carotid (ICA) and vertebral (VA) artery blood flow (ultrasound), and the chemoreflex responses. Although dopamine depressed the ventilatory response by 27 ± 41% (vs. placebo; P = 0.01), the maximal apnea duration was increased by only 5 ± 8% (P = 0.02). The PaCO2 and PaO2 at apnea breakpoint were similar (P > 0.05). When compared with placebo, dopamine increased HR and decreased MAP during both apnea and chemoreflex test (P all <0.05). At rest, dopamine compared with placebo dilated the ICA (3.0 ± 4.1%, P = 0.05) and VA (6.6 ± 5.0%, P < 0.01). During apnea and chemoreflex test, conductance of the cerebral vessels (ICA, VA, MCAv, PCAv) was increased with dopamine; however, flow (ICA and VA) was similar. At least in elite apnea divers, the small increase in apnea time and similar PaO2 at breakpoint (∼31 mmHg) suggest the apnea breakpoint is more related to PaO2, rather than peripheral chemoreflex drive to breathe.
Keywords: blood pressure, breath hold, carotid body, cerebral
a voluntary breath hold breakpoint in the untrained but motivated person normally occurs within 1 to 2 min and is generally associated with an autonomic drive to breathe that provokes involuntary contractions of inspiratory muscles (involuntary breathing movements, IBMs) (17). The IBM onset is aptly termed the “physiological breakpoint” (24). In the untrained breath holder, the IBM onset and therefore apnea duration may be doubled in duration by phrenic and glossopharyngeal nerve blockade (22, 24), and in turn is in large part mediated by powerful chemosensory and type III and IV diaphragmatic afferents (24, 25). In contrast, the underlying mechanism(s) of an extreme apnea breakpoint remains poorly characterized (4, 9, 24, 25), since it occurs well beyond the physiological breakpoint, evidenced by the current apnea world record of 11:35 min. The apneic period beyond the physiological breakpoint, termed the “struggle phase,” is hallmarked by frequent IBMs, and comprises roughly 50% of the total breath hold duration in the elite apnea diver (6).
The carotid and aortic bodies (peripheral chemosensors) are highly sensitive to the catecholamine dopamine. At low doses, dopamine inhibits the calcium currents in chemoreceptor glomus cells (10, 34, 35). The suppression of peripheral chemoreflex activity with low-dose dopamine infusion (<5 μg·kg−1·min−1) prolongs the physiological breakpoint and is reflected by reductions in oxygen saturations at apnea termination in untrained volunteers (30). In the elite apnea diver, however, delaying the physiological breakpoint (onset of IBMs) may have no benefit in prolonging breath hold time. That is, the elite apnea breakpoint may be governed by cerebral metabolic needs (4), and therefore consciousness, rather than the autonomic drive to breathe per se. For example, although lower arterial oxygen partial pressure (PaO2) values have been reported (7), loss of consciousness in humans is suggested to occur at a PaO2 threshold of ∼27 mmHg (23, 24), values on average very similar to the PaO2 breakpoint previously recorded in elite apnea divers, ranging from 23 to 37 mmHg, with an average of ∼30 mmHg (31). That the elite divers indicate that prolonging their breath hold any further (by seconds) will, and has (e.g., during competition and training), result in unconsciousness corroborates the notion of a threshold consciousness breakpoint, i.e., the point whereby any further drop in PaO2 will result in unconsciousness. Notably, this average breakpoint PaO2 closely reflects the 50% oxygen hemoglobin saturation.
With low-dose dopamine, an alternate impact on the elite maximal breath hold may be via the known cardiovascular adjustments (19, 21). For example, low-dose dopamine likely interferes with the normal hemodynamic responses associated with the mammalian dive reflex [e.g., peripheral vasoconstriction and attenuated heart rate (HR)], and prevents a rise in cerebral blood flow that maintains the cerebral oxygen delivery (31). As such, the primary aim of the study was to determine if the maximal breath hold duration in elite apnea divers was altered by the administration of low-dose (2 μg·kg−1·min−1) dopamine. In a randomized order, elite apnea divers performed a maximal breath hold while receiving either low-dose dopamine or placebo. The cerebrovascular, hemodynamic, and arterial blood gas responses were quantified throughout. The chemoreflex and hemodynamic response was further independently assessed via the ventilatory response to hypoxia with mild hypercapnia. It was hypothesized that 1) low-dose dopamine infusion in elite apnea divers would decrease the ventilatory response to hypoxia, and in turn delay the onset of IBMs during a maximal breath hold; however, apnea duration would be unaltered; and 2) the termination of apnea in the placebo and dopamine condition would occur at identical arterial oxygen tensions, suggesting that it is a threshold PaO2 and therefore consciousness that determines actual breakpoint in elite apnea divers, rather than drive to breathe.
METHODS
Participants
Thirteen elite apnea divers (1 female) were recruited from the National Croatian Apnea team. Subject specifications and training history are presented in Table 1. All participants were normotensive and free from any cardiovascular and respiratory disease. The experimental procedures were approved by the ethical committee of the University of Split School of Medicine and by the Clinical Research Ethics Board of the University of British Columbia and conformed to the standards set by the Declaration of Helsinki.
Table 1.
Subject specifications
| Age | Height, cm | Weight, kg | FVC, l | FEV1, l | Years Competing | PB Static Apnea, s | |
|---|---|---|---|---|---|---|---|
| Median | 26 | 187.5 | 79.8 | 7.4 | 5.6 | 3.5 | 394 |
| Minimum | 20 | 164 | 56.4 | 5.0 | 4.0 | 1.5 | 296 |
| Maximum | 48 | 194 | 106.4 | 8.6 | 6.2 | 14.0 | 560 |
PB Static apnea indicates the personal best record static (resting) apnea while face down in water.
FVC, forced vital capacity (standard spirometry, without glossopharyngeal insufflation); FEV1, forced expired volume in 1 s.
Experimental Design
Experimentation for a single subject was completed on a single day, following strict adherence to pretesting protocol, including abstinence from vigorous exercise and alcohol at least 48 h, and from caffeine at least 12 h before arriving to the laboratory. All testing was performed at the University of Split, School of Medicine, Department of Integrative Physiology. Upon arrival to the laboratory, a medical history and standard anthropometric and pulmonary functioning metrics were assessed. After instrumentation, participants rested supine and randomly received either 2 μg·kg−1·min−1 of dopamine or volume-matched saline (placebo) through an intravenous catheter placed in the left arm. The apnea coach and participants were blinded to the experimental condition. The study drug (dopamine or placebo) was continually administered throughout the apnea and hypoxic breathing. To assure complete dopamine clearance (half-life of 8 min) and recovery from the first apnea, participants were given a 75-min break before completing the second condition.
Apnea protocol.
After 20 min from the start of infusion, baseline measures were collected. The participants then underwent a preparatory phase that included two submaximal practice apneas. The first preparatory apnea was performed at the end of a normal expiration, until reaching six IBMs. After a 2-min rest, the second preparatory apnea was performed at total lung capacity, until reaching 10 IBMs. The practice apneas were performed as a well-accepted method for prolonging the maximal apnea time. After 6 min rest from the end of the second preparatory apnea, participants performed the maximal apnea. Participants were permitted to lung pack (glossopharyngeal insufflation) for the apnea. Identical preparatory phases were completed in each experimental condition (dopamine or placebo). Participants were instructed to break the maximal breath hold before losing consciousness. The national apnea coach was the respective coach for each participant and was present at all times.
Chemoreflex test.
After ∼15 min recovery from the maximal apnea, the hypoxic breathing test was performed using dynamic end-tidal forcing (29). The gas delivery consisted of 1 min baseline (eucapnic and normoxic) followed by 5 min of mild hypercapnic hypoxia. All outcome variables were collected during the last 30 s of the test (from minutes 4.5 to 5.0). The assigned values of arterial CO2 tension (PaCO2) and PaO2 were selected on an individual basis from the serial arterial blood gas samples taken during the maximal apnea, as the PaCO2 recorded at 50% and average PaO2 value over the last 1.5 min of the first performed maximal apnea (whether placebo or drug). The average levels of PaCO2 were 45.9 ± 3.3 mmHg and PaO2 were 36.1 ± 6.3 mmHg and were kept equal during the dopamine and placebo trials (average difference of −0.4 ± 2.0 and −0.3 ± 2.8 mmHg, respectively). In both conditions, the PaCO2 was on average 5.0 mmHg higher from baseline resting conditions.
Measurements
Blood gases.
PaO2, PaCO2, and O2 saturation (SaO2, %), were sampled from a 20-gauge radial arterial catheter (Arrow, Markham, Ontario, Canada) placed in the right radial artery. The catheter was attached to an in-line wasteless sampling system (Edwards Lifesciences VAMP, Irvine, CA) and a pressure transducer located at the height of the right atrium (TruWave transducer; Edwards Lifesciences). During the apnea, blood samples (2 ml) were collected at baseline, then every 30-s into the apnea, and immediately at apnea termination. During the hypoxic breathing test, blood samples were collected at baseline, and during the last 30 s of the test.
Cardiovascular.
HR was obtained from the R-R intervals measured from a three-lead ECG. Beat-to-beat arterial blood pressure was measured by finger photoplethysmography (Finometer PRO; Finapress Medical Systems, Amsterdam, The Netherlands) normalized to intra-arterial pressures of the radial artery. Because of the frequency of blood sampling, intra-arterial pressure measures were available only for ∼5- to 10-s data bins around each blood draw. End-apnea values for mean arterial pressure (MAP) and HR were acquired from an average of the last 30 s immediately before termination of the apnea.
Cerebrovascular.
Cerebral blood velocity envelope of the right middle cerebral artery (MCAv) and left posterior cerebral artery (PCAv) were measured using a 2-MHz pulsed transcranial Doppler ultrasound system (Spencer Technologies, Seattle, WA). A specialized headband fixation device (model M600 bilateral head frame; Spencer Technologies) was used to secure the probes in position. Signal quality was optimized using standardized search techniques as previously described that produce test-retest reliability of ∼3 and 2% for MCAv and PCAv, respectfully (32). The MCAv was insonated through the left temporal window, at a depth 1 cm distal to the MCA-anterior cerebral artery bifurcation. The PCAv was insonated at the P1 segment through the right temporal window. End-apnea values for MCAv and PCAv were acquired from an average of the last 30 s immediately before termination of the apnea.
Internal carotid and vertebral artery flow.
Volumetric blood flow of the right internal carotid artery (ICA, n = 7) and left vertebral artery (VA, n = 10) was simultaneously measured using duplex vascular ultrasound (Terason 3200; Teratech, Burlington, MA). The right ICA was insonated ∼2 cm from the carotid bifurcation, and the left VA was insonated at the C5–C6 or C5–C4 vertebral space depending on individual anatomy. Care was taken to standardize the insonation location between measures within subjects between placebo and dopamine. The steering angle was fixed to 60 degrees among all trials, and the sample volume was placed in the center of the vessel adjusted to cover the entire vascular lumen. All files were collected as an AVI file at 30 Hz for offline analysis using edge detection software (33). Simultaneous measures of luminal diameter and velocity (envelope velocity divided by 2) over a minimum of 10 cardiac cycles were used to calculate flow. The sample size for ICA and VA measures was reduced from 13 to 7 and 10, respectively, due to images that did not meet inclusion criteria standards, e.g., a visible angle change was observed and because of the removal of the outlier (see results). Within-subject data for all conditions were rejected if an unreliable image was observed at any time, so that the averaged data across conditions reflect the same participants, within measurement. Images were rejected before performing any analysis as to not bias rejection criteria.
Because of the involuntary breathing movements, concomitant images of ICA and VA velocity and diameter were unattainable up to the breath hold breakpoint in five subjects for the VA and four subjects for the ICA. In these subjects, the vessel diameter only (not velocity) was measured during the latter parts of the breath hold. The end breath hold (i.e., 80 and 100%, or just 100%) VA and ICA flow was subsequently calculated from the change in PCAv and ICAv, respectively. Specifically, velocity of the ICA and VA was calculated from the velocity of the last successful ICA/VA velocity measurement multiplied by the percent change in the respective MCAv/PCAv of the next stage. Peak (envelope) flow was subsequently calculated from: 0.25 × Π × (diameter2) × velocity. During the first 50% of the breath hold, the average within-subject r2 value between the ICA velocity and MCAv was ∼0.90, and ∼0.95 for the VA velocity and PCAv. Nevertheless, estimations of ICA and VA flow during the latter part of the breath hold were performed in the same subjects between conditions, to avoid potential within-subject trial differences attributed to measurement technique.
Ventilatory data.
The chemoreflex test was performed using end-tidal forcing (29). The custom-built end-tidal forcing system uses independent gas solenoid valves for oxygen, carbon dioxide, and nitrogen for delivery of each gas. The system controls the volume of each gas delivered to the inspiratory reservoir through a mixing and humidification chamber. With use of feedback information regarding end-tidal pressures for CO2 and O2, and inspiratory/expiratory tidal volumes, the system prospectively targets the inspirate to bring end-tidal gas to the desired millimeters mercury, while allowing participants to breath spontaneously. Gas control was fine-tuned using a feedback control and error reduction algorithm (LabView, Austin, TX). Respired gases were sampled at the mouth by securing a sample line in the mouthpiece attached to a calibrated online gas analyzer (model ML206; AD Instruments, Colorado Springs, CO). Respiratory flow for minute ventilation (V̇e), tidal volume, and respiratory rate was measured at the mouth using pneumotachography (model HR 800L; HansRudolph, Shawnee, KS). Outcome variables during the hypoxic ventilatory tests were ventilatory, blood pressure, cerebrovascular (ICA, VA, MCAv, and PCAv), and HR responses for a given SaO2.
Involuntary breathing movements.
The IBM onset was recorded in real time during the apnea by the apnea coach. The presence of IBMs was further verified from a chest plethysmography belt integrated into Labchart.
Statistical Methods
Mean values ± SD are presented. To normalize the apnea data between participants, comparisons were made at 20% increments from the start to termination of the apnea. Values between baseline and 100% apnea were derived from 20-s averages around the blood draw that best corresponded with the 20% increment (i.e., 20, 40, 60, and 80%). Normal distribution of variables was confirmed with the Shapiro-Wilk normality test. To compare differences in ICA, VA, MCAv, PCAv, HR, MAP, PaCO2, and PaO2 throughout the apnea, a 2 × 6 repeated-measures ANOVA using the factors of condition (placebo and dopamine) and time [baseline (0%), 20, 40, 60, and 80%, and apnea termination (100%)] was employed. Degrees of freedom were modified using the Greenhouse-Geisser correction when sphericity could not be assumed. When a significant main effect or interaction was observed, post hoc comparisons were made using Student's paired t-tests corrected for multiple comparisons using the Holm's sequential Bonferroni method. Post hoc comparisons for significant interactions were made using the changes from baseline. Simple a priori Student's paired t-tests were used to compare differences in apnea time, PaCO2, and PaO2 at the IBM onset, IBM onset time, and percent of apnea in the struggle phase (with IBMs) between placebo and dopamine. Differences in the ventilatory, PCAv, MCAv, ICA, VA, MAP, and HR responses to mild hypercapnic hypoxia between placebo and dopamine were also compared using a priori Student's t-tests. Significance was set at a confidence level of 95% (P < 0.05). Statistical measures were performed using the statistical software package IBM SPSS 20 for Mac (SPSS, Chicago, IL).
RESULTS
Outlier
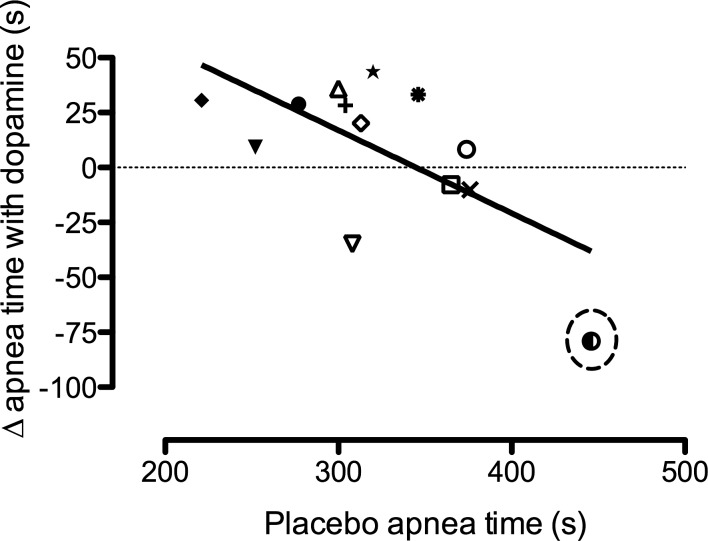
A statistical outlier (confirmed with a Dixon's Q test, with >95% confidence) was found in the apnea protocol. This male subject was therefore removed from analysis for both apnea and chemoreflex tests; thus, analyzed data were based on 12 subjects. However, the outlier sets a trend (Fig. 1), suggesting that dopamine prolongs apnea time most in the less trained, while it may in fact reduce apnea time in the best (as determined by the placebo breath hold time). When this participant is removed from the correlation in Fig. 1, the r2 is reduced from 0.418 (P = 0.017) to 0.113 (P = 0.286).
Fig. 1.
Relationship between the changes in apnea time with dopamine compared with placebo (y-axis), over the placebo apnea time; r2 = 0.418 with the outlier, and 0.113 without. Although heavily skewed by the outlier (circled), this relationship highlights the potential for low-dose dopamine to in fact shorten or have no impact on apnea time in the ultraelite, where the peripheral chemoreflex drive to breathe likely has little to no role on the actual maximal breakpoint. Dopamine may attenuate the maximal apnea time in the ultraelite by preventing the reduction in heart rate (HR) and metabolism associated with the mammalian dive reflex. See discussion for details.
Apnea
Apnea time and IBM onsets.
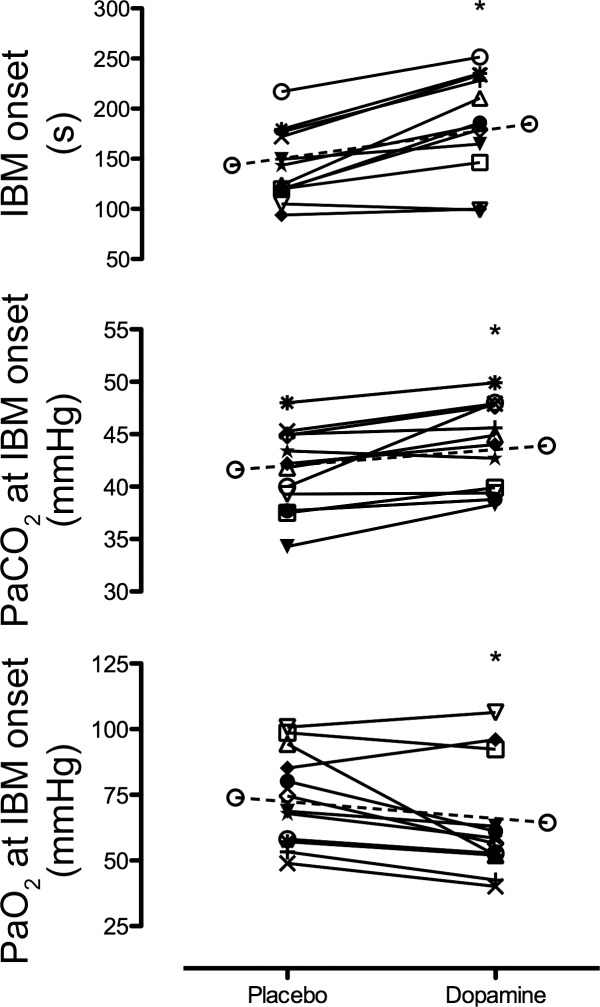
Maximal apnea duration was increased (P = 0.02) by 5 ± 8% with dopamine compared with placebo (328 ± 46 vs. 313 ± 48 s, respectively; Fig. 1). The IBM onset time was also significantly (P < 0.01) delayed by 29 ± 22% with dopamine compared with placebo (185 ± 51 vs. 143 ± 37 s, respectively; Fig. 2, top). In turn, the percent of apnea spent in the struggle phase (with IBMs) was significantly reduced with dopamine compared with placebo (44 ± 11 vs. 54 ± 9%; P < 0.01).
Fig. 2.
Involuntary breathing movement (IBM) onset time (top), partial pressure of arterial CO2 (PaCO2) at the IBM onset (middle), and partial pressure of arterial O2 (PaO2) at the IBM onset (bottom) during the maximal apnea with placebo and dopamine. The elongated dashed bar with ○ denotes mean data. *Significant difference from placebo.
Arterial blood gases.
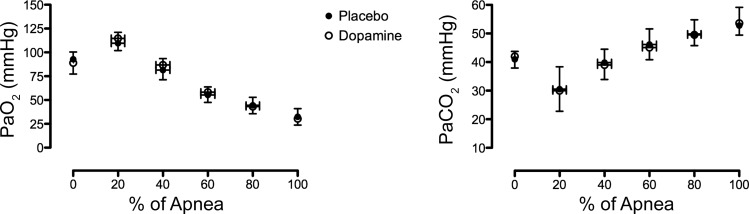
At the onset of IBMs, PaO2 and PaCO2 were significantly decreased (P = 0.02) and increased (P < 0.01), respectively, with dopamine compared with placebo (Fig. 2, bottom and middle, respectively). However, there was no main effect or condition × time interaction between dopamine and placebo in PaO2 or PaCO2 throughout the apnea (P all >0.05) (Fig. 3). Of note, a more liberal statistical analysis with simple a priori single-tailed t-tests of PaO2 and PaCO2 between dopamine and placebo at the termination of apnea further revealed no significant differences (P = 0.100 and 0.360, respectively).
Fig. 3.
Dynamics of PaO2 (left) and PaCO2 (right) throughout a maximal apnea with placebo (●) and dopamine (○).
Hemodynamics.
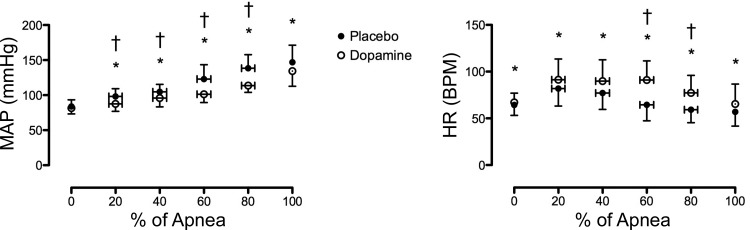
Values for HR and MAP throughout the apneas are presented in Fig. 4. There was a significant main effect (P < 0.01) and condition × time interaction (P < 0.01) for HR during the dopamine compared with placebo apneas. Post hoc tests revealed a significant elevation in HR at each time point with dopamine compared with placebo (P all <0.05). The change (increase) in HR from baseline was significantly greater at 60 and 80% of the apnea in the dopamine compared with the placebo trial (P both <0.01). A significant main effect (P < 0.01) and condition × time interaction (P < 0.01) was also observed for values of MAP between placebo and dopamine apneas. Post hoc comparisons revealed significantly lower MAP values at 20% (P = 0.02), 40% (P = 0.02), 60% (P < 0.01), 80% (P < 0.01), and 100% (P = 0.02), but not at baseline (P = 0.243) with dopamine compared with placebo. The MAP change from baseline was significantly lower with dopamine at 20% (P = 0.01), 40% (P = 0.03), 60% (P < 0.01), and 80% (P < 0.01) compared with placebo.
Fig. 4.
Mean arterial pressure (MAP, left) and HR response throughout a maximal apnea with placebo (●) and dopamine (○). bpm, beats/min. *Significant difference between placebo and dopamine. †Significant difference in the change from baseline between placebo and dopamine.
Intracerebral hemodynamics.
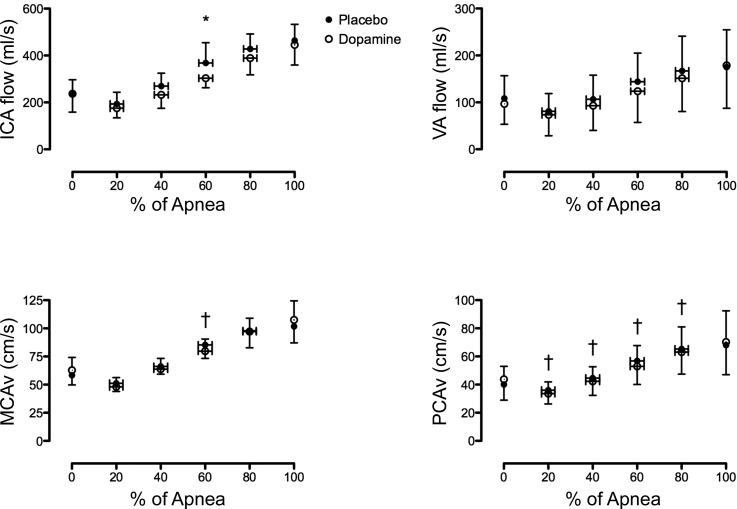
Values for MCAv and PCAv throughout the apneas are presented in Fig. 5, bottom. In two participants who did not respond to standard landmarking, compression, or visual tests, reliable PCA recordings were unattainable; therefore, the sample size for PCAv was reduced to 10. There was no significant main effect of condition for MCAv (P = 0.891) or PCAv (P = 0.271). However, there was a significant condition × time interaction for both MCAv (P = 0.01) and PCA (P = 0.04). Post hoc comparison revealed an attenuated increase from baseline in MCAv at 60% (P = 0.05) and in PCAv at 20% (P = 0.03), 40% (P = 0.03), 60% (P < 0.01), and 80% (P = 0.04) of the apnea with dopamine compared with placebo. There was a significant main effect (P = 0.015) and condition × time interaction (P = 0.023) in MCAv conductance (i.e., MCAv/MAP) between dopamine and placebo. The post hoc comparisons revealed a significantly elevated MCAv conductance at 80% (0.86 ± 0.13 vs. 0.72 ± 0.13 cm·s−1·mmHg−1, P < 0.01) and at 100% (0.82 ± 0.21 vs. 0.70 ± 0.12 cm·s−1·mmHg−1, P = 0.04) with placebo compared with dopamine. Post hoc testing revealed no significant differences at any time point in the change from baseline MCAv conductance between placebo and dopamine. In contrast to the MCAv conductance, there was no significant main effect (P = 0.102) or condition × time interaction (P = 0.193) in PCAv conductance between placebo and dopamine.
Fig. 5.
Cerebrovascular responses throughout a maximal apnea with placebo (●) and dopamine (○). ICA, internal carotid artery blood flow; VA, vertebral artery blood flow; MCAv, middle cerebral artery velocity; PCAv, posterior cerebral artery velocity. *Significant difference between placebo and dopamine. †Significant difference in the change from baseline between placebo and dopamine.
Extracerebral hemodynamics.
There was no main effect (P = 0.151) or condition × time interaction (P = 0.318) in VA flow during the apnea between dopamine and placebo (Fig. 5). In contrast, there was a significant main effect of condition (P = 0.027) but no interaction (P = 0.209) in ICA flow between dopamine and placebo (Fig. 5). Post hoc analysis revealed a significantly lower ICA flow at 60% of the apnea with dopamine compared with placebo (P = 0.05, Fig. 5). There was no main effect (P = 0.711) or condition × time interaction (P = 0.401) in the VA conductance (i.e., VA/MAP) between dopamine and placebo. Likewise, there was no main effect (P = 0.357) or condition × time interaction (P = 0.334) in ICA conductance between dopamine and placebo.
Chemoreflex Test
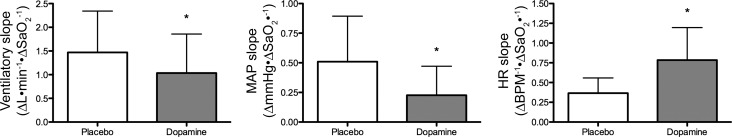
Because of the relatively low level of hypercapnia (average PaCO2 of 45.9 ± 3.3 mmHg) but extreme level of hypoxia (average PaO2 of 36.1 ± 6.3 mmHg), measures during the chemoreflex test are expressed as changes relative to absolute reduction in SaO2 only. Presenting data over SaO2 and not the PaO2 was chosen because of the linear relationship between V̇e and SaO2, to account for the hypercapnia-induced Bohr effect, and to compare with existing literature. As predicted, the ventilatory response to hypoxia at the 4.5 to 5.0 time bin (ΔV̇e/ΔSaO2) was significantly depressed by 27 ± 41% with dopamine compared with placebo (P = 0.01; Fig. 6, left). Because of the known temporal V̇e pattern to steady-state hypoxia, we performed a secondary assessment of V̇e at the peak response. The peak V̇e response occurred at 180 ± 66 s with dopamine, and earlier with placebo, at 140 ± 70 s, P = 0.003. Similarly to the 4.5- to 5.0-min time bin, the magnitude of the V̇e was blunted with dopamine (39.2 ± 11.2 l/min) compared with placebo (48.7 ± 10.5 l/min), P = 0.01.
Fig. 6.
Ventilatory slope (left), MAP slope (middle), and HR slope (right) to severe hypoxic (PaO2 = 36.1 ± 6.3 mmHg) and mild hypercapnic (PaCO2 = 45.9 ± 3.3 mmHg) breathing with placebo vs. dopamine. SaO2, O2 saturation. *Significant difference from placebo.
Hemodynamics.
The MAP (ΔmmHg/ΔSaO2) and HR [Δbeats·min−1·ΔSaO2−1] responses to hypoxic breathing with dopamine and placebo are depicted in Fig. 6. Dopamine significantly reduced the MAP response by 52 ± 46% compared with placebo (P < 0.01). In contrast, dopamine significantly increased the HR response by 200 ± 336% compared with placebo (P < 0.01). Because the HR response follows the V̇e response to hypoxia, we further assessed the HR at the peak V̇e. Similarly to the 4.5- to 5.0-min time bin, the peak HR response was increased with dopamine (91.3 ± 13.2 beats/min) compared with placebo (87.9 ± 11.3 beats/min), P < 0.01.
Intracerebral hemodynamics.
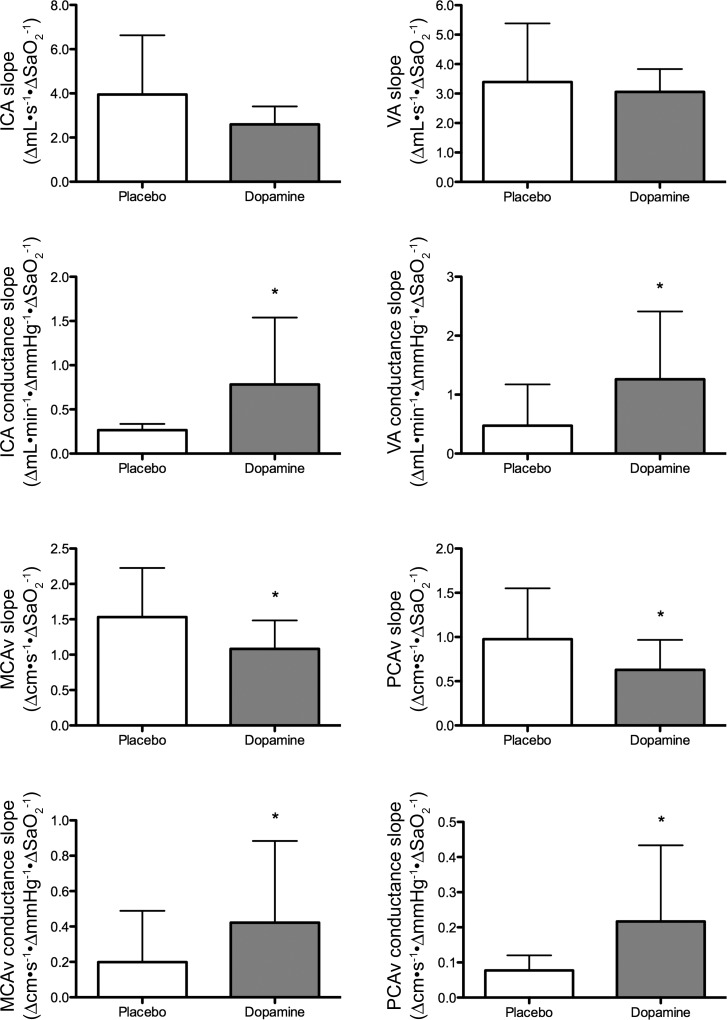
The responses in MCAv (Δcm·s−1·ΔSaO2−1) and PCAv (Δcm·s−1·ΔSaO2−1) to hypoxic breathing with dopamine and placebo are depicted in Fig. 7. Dopamine significantly reduced the MCAv response by 24 ± 25% (P < 0.01) and PCAv response by 26 ± 40% (P = 0.01) compared with placebo. However, the MCAv (P = 0.02) and PCAv conductance (Δcm·s−1·ΔSaO2−1) response to hypoxia is increased with dopamine compared with placebo (P = 0.02 and 0.03, respectively; Fig. 7).
Fig. 7.
Cerebrovascular response to severe hypoxic (PaO2 = 36.1 ± 6.3 mmHg) and mild hypercapnic (PaCO2 = 45.9 ± 3.3 mmHg) breathing with placebo vs. dopamine. *Significantly different from placebo. Although the MCAv and PCAv response to hypoxia is reduced with dopamine, this is confounded by an increased compliance of the cerebral vascular bed (middle). Therefore, true cerebral volumetric flow (ICA and VA) response to hypoxia is largely unaltered with dopamine.
Extracerebral hemodynamics.
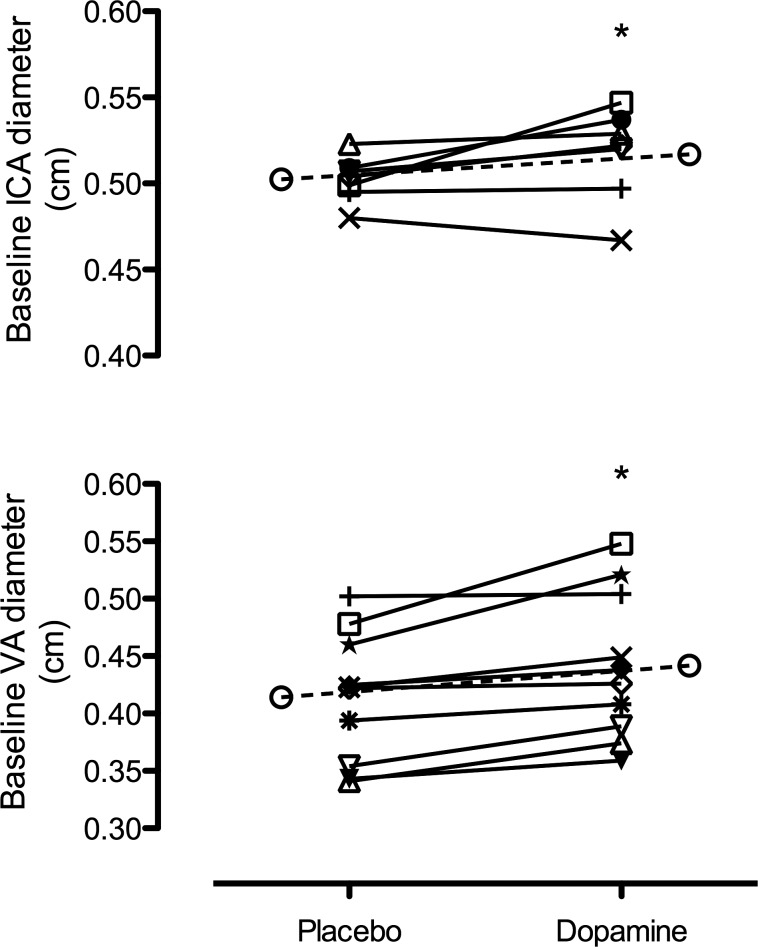
At baseline, dopamine caused a significant dilation in the ICA (3.0 ± 4.1%, P = 0.05; Fig. 8) and VA (6.6 ± 5.0%, P < 0.01; Fig. 8) compared with placebo. Although flow was unchanged, velocity was lower with dopamine compared with placebo (P both <0.05). The ICA and VA diameter and flow changes to hypoxia with dopamine compared with placebo were similar (P all >0.05; Fig. 7). Similarly to the MCAv and PCAv conductance, dopamine significantly increased the ICA (P = 0.05) and VA (P = 0.01) conductance to hypoxia (Fig. 7).
Fig. 8.
Baseline ICA diameter (top) and VA diameter (bottom) with placebo vs. dopamine. *Significantly different from placebo.
DISCUSSION
The novel findings in this study are threefold. First, blunting the peripheral chemoreflex by ∼27% with low-dose dopamine in elite breath holders only marginally (∼5%) increased maximal breath hold time, and the apnea breakpoint occurred at a similar millimeter mercury of PaO2. The apnea breakpoint in the elite diver is therefore more determined by a threshold PaO2 than it is by the peripheral chemoreflex drive to breathe. Second, low-dose dopamine during an apnea and hypoxic breathing mitigated the rise in arterial blood pressure and caused a reflex increase in HR. Last, although dopamine blunted the rise in MAP for a given decrease in arterial oxygen saturation, the cerebral blood flow response was minimally altered, during both hypoxic breathing and apnea, likely because of an increased compliance of the cerebral vascular bed.
Apnea Breakpoint
The mechanism(s) of an apnea breakpoint is no doubt integrative. Central to the current apnea breakpoint hypothesis, however, is a critical integration of diaphragmatic (phrenic nerve) and to a lesser extent chemoreceptor (for PaO2 and PaCO2) afferents arriving at the respiratory center in the medulla (reviewed in Ref. 25). Central hypoxia- and oxygen-sensing neurons in the central nervous system (e.g., in the thalamus, hypothalamus, pons, and medulla) are likely also involved, although experimental study in humans is limited (20). Of relevance to this study, lending credence to the role of chemoreception is the fact that the hypoxic ventilatory response is a significant predictor of breath-holding performance in the untrained breath holder (8); the ventilatory response to hypoxia (18) and hypercapnia (11) is generally depressed in elite apnea divers (see Ref. 9 for review); and breath hold time in the untrained is approximately doubled with glossopharyngeal and vagus nerve blockade (22, 24).
While the current apnea breakpoint hypothesis is dominated by autonomic input (from both chemoreception and lung afferents) (24), some elite athletes often hold a maximal apnea until unconsciousness, a grounds for disqualification from competition in accordance with the governing body for international apnea competition (Association Internationale pour le Développement de L'Apnée). In the ultraelite diver, as examined in this study, it therefore seems reasonable to posit that the motivated breakpoint is dependent on a functional limitation for consciousness, rather than from an autonomic drive to breath, per se. The current data indicate this breakpoint likely falls just below a threshold PaO2 of ∼30 mmHg (see Ref. 31 and Fig. 3). The present data do not offer any interpretation on the molecular or neurological underpinnings of this threshold PaO2 breakpoint although some cerebral metabolic theories have been proposed (4).
If the apnea breakpoint in the ultraelite is indeed dependent on consciousness, prolonging the apnea breakpoint in elite breath holders must fundamentally rely on oxygen conservation. This may be achieved either by slowing oxidative metabolism (e.g., with β-blockers; unpublished observations) or by supersaturating the tissue Po2 before commencing an apnea (e.g., with hyperoxic prebreathing, which can double maximal breath hold time, with the current world record at 23:01 min). The similar arterial blood gases despite an average 15 ± 23 s longer apnea time with dopamine compared with placebo may in turn point to a metabolic shift, that is, delaying the onset of IBMs with dopamine may have prolonged breath hold time by mitigating the oxygen cost of inspiratory muscle contractions. Indeed, with the outlier excluded, a simple linear regression between the change in IBM onset time and breakpoint yields an r2 of 0.262 (P = 0.089). With the outlier included, this relationship is stronger (r2 of 0.688, P < 0.001). Alternatively, chemoreceptor silencing with dopamine may have contributed to a psychophysical relaxation and reduction of skeletal muscle tone. A blunted PaCO2 and PaCO2 slope was not observed in the current study (absolute times), however, perhaps a result of the confounding elevated HR (discussed below) and marginal changes in breath hold time.
Cardiovascular Effects of Low-Dose Dopamine
During a maximal breath hold, elite apnea divers rely heavily on the mammalian dive reflex. The peripheral vessels are constricted, cerebral blood flow is maximized, and the HR is reduced (3, 13). In effect, the cerebral oxygen delivery is maintained (31), and metabolism is slowed. The mammalian dive reflex in humans is in large part the result of trigeminal nerve activation following cold water facial immersion (15, 27). Although this effect was absent in the conditions of the current study, the dive reflex in humans is also mediated by carotid and aortic body activation via the glossopharyngeal and vagus nerve, respectively (12).
Low-dose dopamine infusion aimed to inhibit the carotid and aortic body blunted the MAP increase during the maximal apnea (Fig. 4), and hypoxic breathing (Fig. 6). A blunted MAP response in transient hypoxia with low dose-dopamine infusion has been previously reported in healthy humans (21). The present data extend these findings by observing a compensatory increase in HR during the apnea (Fig. 4), and hypoxic breathing (Fig. 6). The HR response is in contrast to the findings by Niewinski et al. (21) and is likely explained by the differences in temporal hypoxic stimulation. For example, the hypoxic stimulus in Niewinski et al. (21) was acute and transient (10–45 s of 100% nitrogen breathing) compared to following 4.5-min steady-state exposure in the present study. The separate effect of dopamine to blunt the vasomotor, but increase the cardiac, response to hypoxia is likely consequent to the direct vasodilatory properties of dopamine (see discussion below), that is, vasodilation (Fig. 8) likely led to a baroreflex-mediated increase in HR. The cardiac baroreflex may have further been potentiated from carotid body chemoreflex inhibition (26, 28). Alternatively, chemoreflex activation in the absence of ventilatory feedback increases both sympathetic and parasympathetic activity (5). The increased HR response to dopamine may therefore have also been consequent to decreased parasympathetic activity, at least during the apnea.
Cerebrovascular Effects of Low-Dose Dopamine
Although its clinical use is now largely abandoned (16), low-dose dopamine infusion was traditionally used to improve renal blood flow via its direct D1 receptor vasodilatory effects. Indirectly, and more systemically, low-dose dopamine also causes vasodilation via prejunctional D2 receptors on postganglionic sympathetic nerve terminals that inhibit release of norepinephrine (19). This latter vasodilatory effect was directly quantified by observing a 3.0 ± 4.1 and 6.6 ± 5.0% increase in resting ICA and VA diameter, respectively, with dopamine compared with placebo (Fig. 8). The lack of any considerable drop in resting MAP despite such pronounced vasodilation (although peripheral vascular resistance is unknown) was likely due to the elevated resting HR (Fig. 5) (i.e., MAP = cardiac output × total peripheral resistance). Nevertheless, the increased cerebral conductance, proportional to the fourth power of the vessel radius, with dopamine is clearly observed with the increased conductance of the ICA, VA, MCAv, and PCAv with the hypoxic breathing (Fig. 7). The reduced cerebrovascular response to hypoxia with dopamine (Fig. 7) was therefore confounded by the increased compliance of the cerebral vascular bed and highlights the need for concomitant diameter and velocity flow measures during experimentations prone to alter vessel diameter (2). In the end, the cerebral volumetric flow response in the hypoxic breathing trial, as demonstrated by ICA and VA flow, was negligibly lower with dopamine (Fig. 7, top) even despite the attenuated MAP response. On the other hand, the attenuated MAP response with dopamine compared with placebo during the apnea caused a significantly lower ICA flow at 60% (Fig. 5, top left). The decreased MAP response during the dopamine apnea was likely accentuated by the delayed onset of IBMs. Specifically, the IBMs result in sinusoidal surges in venous return (negative thoracic pressures) and consequently arterial pressure (6).
A blunted cerebral blood flow response during the apnea will theoretically increase the drive to breathe by reducing the CO2 washout at the central chemoreceptors (1). Moreover, a blunted cerebral blood flow response will reduce cerebral oxygen delivery. However, cerebral oxygen delivery does not appear to be a factor in determining the elite apnea breakpoint (Ref. 31 and unpublished observations). In the present study, the moderate difference in ICA flow exclusively at 60% between dopamine and placebo is unlikely to have majorly affected cerebral CO2 washout or the apnea breakpoint. Nevertheless, the interplay between cerebral oxygen delivery, tissue Po2, tissue pH, and local metabolism (4) in determining the apnea breakpoint, and ultimately consciousness, remains to be determined.
Considerations
The apnea outcome of peripheral chemoreflex inhibition is selective to the unique population tested. In the untrained breath holder, blunting the peripheral chemoreflex with low-dose dopamine may double maximal apnea and proffer a dramatically reduced peripheral oxygen saturation at the apnea breakpoint [e.g., from 78 to 63% (30)]. In the current study, breath-hold time was increased by only ∼5% (with the outlier excluded); however, the variability of the response (Fig. 1) must be acknowledged. From previous studies in the untrained breath holder (30), and in Fig. 1, it is evident that the underlying variations (aside from inherent genetic ability) seem to be influenced by level of training, which in turn may affect level of motivation. As exemplified in the outlier displayed in Fig. 1 (who at the time of writing is the world record holder for a dynamic apnea and apnea with oxygen prebreathing), low-dose dopamine infusion may in fact decrease maximal breath hold time. Here, the dopamine-induced elevation in HR combined with the decreased psychophysical relaxation and therefore potentially increased muscular tone negatively impacted breath hold time by increasing the metabolic rate. Indeed, in this participant the maximal apnea time was reduced by 79 s with dopamine compared with placebo, yet the end apnea PaO2 (25.2 mmHg with placebo vs. 27.0 mmHg with dopamine) was similar. These data, again, support the notion of a threshold PaO2 for the elite apnea breakpoint.
A growing body of literature now supports differential cardiovascular control between sexes (14). In this study, the single female displayed cardiovascular responses and changes with dopamine that closely reflected the mean group responses. Specifically, in the dopamine compared with placebo apnea trial, apnea time was increased by 3.7%, while MAP was lower by 24 mmHg, and HR was increased by 4 beats/min at the breakpoint. The sole female participant was therefore kept within the group analysis. Future study will be required to establish cardiovascular differences (if any) in prolonged breath holding between the sexes.
An obvious caveat to this study is the lack of a control group. Nevertheless, the primary goal of this study was to test the impact of carotid body inhibition on prolonged apnea that yields extreme levels of arterial hypoxia and hypercapnia. As such, a separate independent control group would be irrelevant, given that control subjects would not tolerate such levels of hypoxia and hypercapnia. A second study limitation is the difficulty in completely standardizing the apnea conditions. For example, the protocol for the preparatory phase before each maximal apnea was identical between placebo and dopamine conditions; however, because dopamine delayed the onset of IBMs, the preparatory phase was generally longer in the dopamine condition. Participants were instructed to perform an identical glossopharyngeal insufflation and preapnea breathing pattern for both trials. Although there is inherent within-subject variability in the glossopharyngeal insufflation and prebreathing patterns between conditions, this was small given that the within-subject PaCO2 at 30 s into the maximal apneas was nearly identical between conditions (difference of 0.42 ± 2.5 mmHg).
Perspectives and Significance
The apnea breakpoint in elite apnea divers seems to be determined by a threshold PaO2 of ∼30 mmHg, with little influence of the peripheral chemoreflex drive to breathe. Although we did not partition the drive to breathe from lung afferents, increasing apnea time in the elite diver must rely most on either a reduction in metabolism or an increase in the oxygen availability (e.g., via increased lung volume and hematocrit, improved splenic contraction, better O2 extraction at the tissue level, increased mitochondrial bioenergetics, etc.). While highlighting the apnea breakpoint in elite divers, this study further provides a unique insight to the cardiovascular responses to hypoxia that is modified with low-dose dopamine. Specifically, low-dose dopamine blunted the MAP but increased the HR response to steady-state hypoxia. These differences may be attributable to the vasodilatory properties of low-dose dopamine, a heighted cardiac baroreflex, and potentially reduced parasympathetic activity. Finally, low-dose dopamine caused a dilation of the cerebral arteries; however, this did not affect the cerebral blood flow response (change) to 5 min of steady-state hypoxia.
GRANTS
This study was funded through a Canadian Research Chair and Natural Sciences and Engineering Research Council (NSERC) Discovery grant held by P. N. Ainslie. Z. Dujic, O. F. Barak, and P. N. Ainslie were also funded through the Croatian Science Foundation. A. R. Bain was funded through a postgraduate NSERC scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.R.B., I.D., and P.N.A. conception and design of research; A.R.B., Z.D., R.L.H., O.F.B., D.M., I.D., M.S., D.B.M., D.M.M., and P.N.A. performed experiments; A.R.B. analyzed data; A.R.B., Z.D., R.L.H., O.F.B., I.D., M.S., D.B.M., and P.N.A. interpreted results of experiments; A.R.B. prepared figures; A.R.B. drafted manuscript; A.R.B., Z.D., R.L.H., O.F.B., I.D., M.S., D.B.M., D.M.M., and P.N.A. edited and revised manuscript; A.R.B., Z.D., R.L.H., O.F.B., D.M., I.D., M.S., D.B.M., D.M.M., and P.N.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the apnea divers from the Croatia National Apnea team for their participation.
REFERENCES
- 1.Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 296: R1473–R1495, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Ainslie PN, Hoiland RL. Transcranial Doppler ultrasound: valid, invalid, or both? J Appl Physiol 117: 1081–1083, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Butler PJ, Jones DR. Physiology of diving of birds and mammals. Physiol Rev 77: 837–899, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Dangmann R. An insulin based model to explain changes and interactions in human breath-holding. Med Hypotheses 84: 532–538, 2015. [DOI] [PubMed] [Google Scholar]
- 5.de Burgh Daly M, Kirkman E, Wood LM. Cardiovascular responses to stimulation of cardiac receptors in the cat and their modification by changes in respiration. J Physiol 407: 349–362, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dujic Z, Uglesic L, Breskovic T, Valic Z, Heusser K, Marinovic J, Ljubkovic M, Palada I. Involuntary breathing movements improve cerebral oxygenation during apnea struggle phase in elite divers. J Appl Physiol 107: 1840–1846, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Ernsting J. The effect of brief profound hypoxia upon the arterial and venous oxygen tensions in man. J Physiol 169: 292–311, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feiner JR, Bickler PE, Severinghaus JW. Hypoxic ventilatory response predicts the extent of maximal breath-holds in man. Respir Physiol 100: 213–222, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Ferretti G. Extreme human breath-hold diving. Eur J Appl Physiol 84: 254–271, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 74: 829–898, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Grassi B, Ferretti G, Costa M, Ferrigno M, Panzacchi A, Lundgren CE, Marconi C, Cerretelli P. Ventilatory responses to hypercapnia and hypoxia in elite breath-hold divers. Respir Physiol 97: 323–332, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Gross PM, Whipp BJ, Davidson JT, Koyal SN, Wasserman K. Role of the carotid bodies in the heart rate response to breath holding in man. J Appl Physiol 41: 336–340, 1976. [DOI] [PubMed] [Google Scholar]
- 13.Heusser K, Dzamonja G, Tank J, Palada I, Valic Z, Bakovic D, Obad A, Ivancev V, Breskovic T, Diedrich A, Joyner MJ, Luft FC, Jordan J, Dujic Z. Cardiovascular regulation during apnea in elite divers. Hypertension 53: 719–724, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Joyner MJ, Barnes JN, Hart EC, Wallin BG, Charkoudian N. Neural control of the circulation: how sex and age differences interact in humans. Compr Physiol 5: 193–215, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawakami Y, Natelson BH, DuBois AR. Cardiovascular effects of face immersion and factors affecting diving reflex in man. J Appl Physiol 23: 964–970, 1967. [DOI] [PubMed] [Google Scholar]
- 16.Lauschke A, Teichgraber UK, Frei U, Eckardt KU. “Low-dose” dopamine worsens renal perfusion in patients with acute renal failure. Kidney Int 69: 1669–1674, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Lin YC. Breath-hold diving in terrestrial mammals. Exercise Sport Sci Rev 10: 270–307, 1982. [PubMed] [Google Scholar]
- 18.Masuda Y, Yoshida A, Hayashi F, Sasaki K, Honda Y. The ventilatory responses to hypoxia and hypercapnia in the Ama. Jpn J Physiol 31: 187–197, 1981. [DOI] [PubMed] [Google Scholar]
- 19.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev 78: 189–225, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Neubauer JA, Sunderram J. Oxygen-sensing neurons in the central nervous system. J Appl Physiol 96: 367–374, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Niewinski P, Tubek S, Banasiak W, Paton JF, Ponikowski P. Consequences of peripheral chemoreflex inhibition with low-dose dopamine in humans. J Physiol 592: 1295–1308, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noble MI, Eisele JR, Frankel HL, Else W, Guz A. The role of the diaphragm in the sensation of holding the breath. Clin Sci 41: 275–283, 1971. [DOI] [PubMed] [Google Scholar]
- 23.Nunn JF. Applied Respiratory Physiology (3rd ed). London, UK: Butterworth, 1987. [Google Scholar]
- 24.Parkes MJ. Breath-holding and its breakpoint. Exp Physiol 91: 1–15, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Parkes MJ. The limits of breath holding. Sci Am 306: 74–79, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Ponikowski P, Chua TP, Piepoli M, Ondusova D, Webb-Peploe K, Harrington D, Anker SD, Volterrani M, Colombo R, Mazzuero G, Giordano A, Coats AJ. Augmented peripheral chemosensitivity as a potential input to baroreflex impairment and autonomic imbalance in chronic heart failure. Circulation 96: 2586–2594, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Schaller BJ. Trigeminocardiac reflex. J Neurosurg 107: 243–244, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Somers VK, Mark AL, Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest 87: 1953–1957, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tymko MM, Ainslie PN, Macleod DB, Willie CK, Foster GE. End-tidal-to-arterial CO2 and O2 gas gradients at low- and high-altitude during dynamic end-tidal forcing. Am J Physiol Regul Integr Comp Physiol 308: R895–R906, 2015. [DOI] [PubMed] [Google Scholar]
- 30.van de Borne P, Oren R, Somers VK. Dopamine depresses minute ventilation in patients with heart failure. Circulation 98: 126–131, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Willie CK, Ainslie PN, Drvis I, MacLeod DB, Bain AR, Madden D, Maslov PZ, Dujic Z. Regulation of brain blood flow and oxygen delivery in elite breath-hold divers. J Cereb Blood Flow Metab 35: 66–73, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, Haykowsky MJ, Bellapart J, Ogoh S, Smith KJ, Smirl JD, Day TA, Lucas SJ, Eller LK, Ainslie PN. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 196: 221–237, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol 91: 929–937, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Zapata P. Effects of dopamine on carotid chemo- and baroreceptors in vitro. J Physiol 244: 235–251, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zapata P, Hess A, Bliss EL, Eyzaguirre C. Chemical, electron microscopic and physiological observations on the role of catecholamines in the carotid body. Brain Res 14: 473–496, 1969. [DOI] [PubMed] [Google Scholar]