Abstract
Modification of histones is one of the important mechanisms of epigenetics, in which genetic control is determined by factors other than an individual's DNA sequence. Sirtuin family proteins, which are class III histone deacetylases, were originally identified as gene silencers that affect the mating type of yeast, leading to the name “silent mating-type information regulation 2” (SIR2). They are characterized by their requirement of nicotinamide adenine dinucleotide for their enzyme activity, unlike other classes of histone deacetylases. Sirtuins have been traditionally linked to longevity and the beneficial effects of calorie restriction and DNA damage repair. Recently, sirtuins have been shown to be involved in a wide range of physiological and pathological processes, including aging, energy responses to low calorie availability, and stress resistance, as well as apoptosis and inflammation. Sirtuins can also regulate mitochondrial biogenesis and circadian clocks. Seven sirtuin family proteins (Sirt1-7) have been identified as mammalian SIR2 orthologs, localized in different subcellular compartments, namely, the cytoplasm (Sirt1, 2), the mitochondria (Sirt3, 4, 5), and the nucleus (Sirt1, 2, 6, 7). Sirt1 is evolutionarily close to yeast SIR2 and has been the most intensively investigated in the cardiovascular system. Endogenous Sirt1 plays a pivotal role in mediating the cell death/survival process and has been implicated in the pathogenesis of cardiovascular disease. Downregulation of Sirt2 is protective against ischemic-reperfusion injury. Increased Sirt3 expression has been shown to correlate with longevity in humans. In addition, Sirt3 protects cardiomyocytes from aging and oxidative stress and suppresses cardiac hypertrophy. Sirt6 has also recently been demonstrated to attenuate cardiac hypertrophy, and Sirt7 is known to regulate apoptosis and stress responses in the heart. On the other hand, the roles of Sirt4 and Sirt5 in the heart remain largely uncharacterized.
Keywords: longevity, cell death/survival, FoxO, sirtuin-activating compounds
modification of histones is one of the important mechanisms of epigenetics, in which genetic control is determined by factors other than an individual's DNA sequence. DNA is structurally wrapped around histones, whose acetylation and deacetylation regulate gene expression. Histone acetyltransferases are enzymes that transfer an acetyl group (O=C—CH3) from acetyl CoA to lysine to form ε-N-acetyl lysine, thus acetylating conserved lysine amino acids on histone proteins. In contrast, histone deacetylases (HDACs) are enzymes that allow the DNA to wrap around histones more tightly by removing acetyl groups from ε-N-acetyl lysine amino acids on the histones. In mammals, there are four classes of HDACs (class I-IV).
Class III HDACs are sirtuin family proteins. Sirtuins were first identified as silencers of genes affecting the mating type of yeast and were, therefore, named “silent mating type information regulation 2” (SIR2) (115). They are characterized by their requirement of nicotinamide (NAM) adenine dinucleotide (NAD+) for their enzyme activity, unlike other classes of HDACs (44). Sirtuins have traditionally been linked to longevity and the beneficial effects of calorie restriction (CR) and DNA damage repair. Recently, sirtuins have been shown to be involved in a wide range of physiological and pathological processes, including aging, energy responses to low calorie availability, and stress resistance, as well as apoptosis and inflammation. In addition, sirtuins can also regulate mitochondrial biogenesis and circadian clocks (76).
So far, seven sirtuin family proteins (Sirt1-7) have been identified as mammalian SIR2 orthologs, occupying different subcellular compartments, namely, the cytoplasm (Sirt1, 2), the mitochondria (Sirt3, 4, 5), and the nucleus (Sirt1, 2, 6, 7) (26). Of these, Sirt1 is evolutionarily closest to yeast SIR2 and has been the most extensively investigated in the cardiovascular system. Sirt1 knockout mice (Sirt−/1−) demonstrated perinatal death and retinal, bone, and cardiac defects (17, 26). Endogenous Sirt1 plays a pivotal role in mediating cell death/survival and has been implicated in the pathogenesis of heart failure. Beneficial effects of Sirt1 against oxidative stress and aging in the heart were investigated by using transgenic mice with cardiac-specific overexpression of Sirt1 (5). Downregulation of Sirt2 is protective against ischemia-reperfusion (I/R) injury (67). Increased Sirt3 expression has been shown to correlate with longevity in humans. In addition, Sirt3 protects cardiomyocytes from aging and oxidative stress and suppresses cardiac hypertrophy (126). Sirt6 was also recently demonstrated to attenuate cardiac hypertrophy (127), and Sirt7 regulates apoptosis and stress responses in the heart (137). The functions of Sirt4 and Sirt5, however, have been poorly investigated in the heart in vivo. In this review, we summarize the most recent findings regarding the physiological and pathological roles of sirtuins in the heart and discuss how sirtuins can be targets of treatment for cardiovascular disease.
Regulation and Targets of Sirtuins
Sirt1 is widely expressed in mammalian cells. Although Sirt1 was originally identified as a nuclear-localizing protein, recently, its subcellular localization has been shown to depend on cell type. In embryonic mouse hearts, Sirt1 is highly expressed in the nucleus but declines with further organogenesis. The expression level of Sirt1 in adult hearts is about 20% of that in embryonic hearts (132). Sirt3 was originally identified as a mitochondria-localizing protein. However, it is also found in the nucleus and cytoplasm (119, 126).
The expression and activity of Sirt1 are controlled by several pathophysiological stresses, transcription factors and cofactors, and post-translational modification. Several regulatory factors of Sirt1 have been identified in non-cardiac cells. Sirt1 is upregulated by E2F1, Forkhead box class O (FoxO) 1, and FoxO3 (92, 140) in mammalian cells (PC cells) and human non-small cell lung carcinoma cells (H1299 cells), whereas it is repressed by hypermethylated in cancer and COOH-terminal binding protein (16, 154) in fibroblasts. Hu antigen R stabilizes Sirt1 mRNA(1) in HeLa cells, and several microRNAs, such as miR-34a, miR-134, miR-199a, and miR-217, regulate Sirt1 expression in nueronal cells and HeLa cells (30, 146). Cyclin B/cyclin-dependent kinase 1 and c-Jun NH2-terminal kinase 1 (JNK1) phosphorylate Sirt1, thereby enhancing its deacetylase activity in C2C12 cells and HEK293 cells (91). In addition, sumoylation represents another important regulatory mechanism. Sirt1 deacetylase activity is increased by sumoylation of Sirt1 at K734, which is repressed by sentrin-specific peptidase 1 (148), in cancer cell lines. Sumoylation enhances Sirt1 activity during ischemic preconditioning (IPC) in the mouse heart (87). Active regulator of Sirt1 and deleted in breast cancer 1 are known as positive and negative regulators in cancer cell lines, respectively (51, 155). Furthermore, some caspases suppress the activity of Sirt1 through cleavage and degradation in HeLa cells (96).
The deacetylase activity of Sirt1 is regulated by the availability of NAD+. Nicotinamide phosphoribosyltransferase (Nampt) catalyzes the transfer of a phosphoribosyl pyrophosphate to NAM to produce NAM mononucleotide, thereby serving as a rate-limiting enzyme in the mammalian NAD+ salvage pathway. Recent studies have shown that Nampt is regulated according to the circadian rhythm by clock genes, including BMAL1 and PER2, via Sirt1-mediated deacetylation, which, in turn, positively regulates Sirt1 activity in NIH3T3 cells, mouse embryonic fibroblasts (MEFs), and hepatocytes (8, 89, 113). Pressure overload, nutrient starvation, exercise, and acute IPC upregulate Sirt1 (5, 39) in the heart. Nucleocytoplasmic shuttling may also regulate the function of Sirt1, since Sirt1 has both nuclear localization and nuclear export signals, which are regulated via phosphorylation by phosphatidylinositol 3-kinase (PI3K) in C2C12 cells (132). Sirt1 is thought to localize in both the cytoplasm and the nucleus at baseline and move to the nucleus in response to stress in the heart (132).
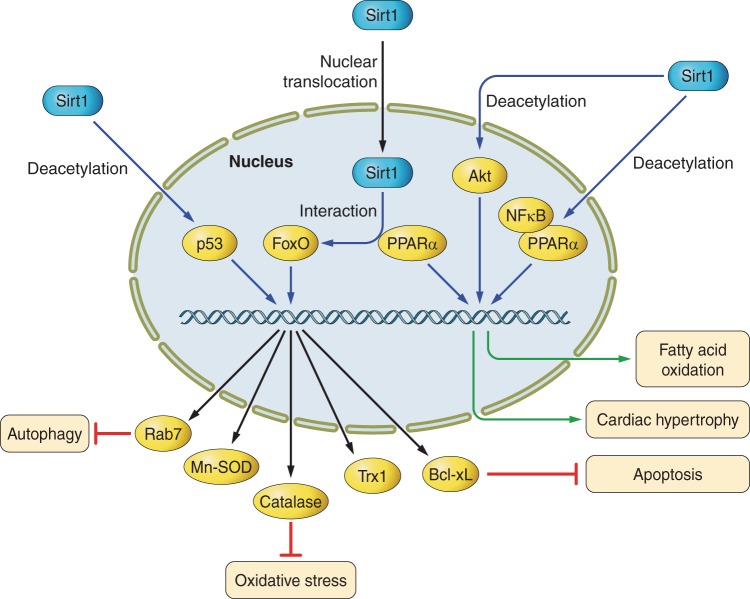
Only a few proteins have been demonstrated to be directly deacetylated and functionally regulated by Sirt1. These include FoxOs, p53, and poly-adenosine 5′-diphosphate (ADP) -ribose polymerase (PARP) 1 (5, 102, 111, 131). FoxO, a mammalian homolog of Daf16, which is involved in DNA repair, cell cycle, cell death/survival, and regulation of reactive oxygen species (ROS), is both positively and negatively regulated by Sirt1. Sirt1 deacetylates and activates FoxOs to upregulate expression of antioxidants, including Mn-superoxide dismutase (MnSOD), catalase, and thioredoxin-1 (Trx1), and antiapoptotic factors, like B-cell lymphoma-extra large (Bcl-xL), in cardiomyocytes (14, 20, 39). Sirt1 also activates Akt/PI3K signaling, leading to enhancement of the cardiac hypertrophic response (125).
As described above, the regulatory mechanisms and targets of Sirt1 have been well investigated. However, less is known regarding those of other sirtuin family proteins. Several studies have raised the possibility that sirtuin proteins are regulated by a common pathway and that the sirtuin proteins regulate one another. AMP-activated protein kinase (AMPK) increased expression of Sirt1, Sirt2, Sirt3, and Sirt6, whereas Sirt5 mRNA was downregulated, in mouse hepatocytes (15). Sirt1 can bind and activate the Sirt6 promoter, which may amplify the response to stress in hepatocytes (50). Recently, fatty acyl modification of TNF-α was also found to be regulated by Sirt6 in MEF cells (46). Sirt6 interacts with CLOCK/BMAL1 and governs their chromatin recruitment to circadian gene promoters in hepatocytes (75). The roles of Sirt proteins and their signaling mechanisms in the cardiovascular system are discussed below.
Regulation of Energy Metabolism by Sirtuins
The heart is characterized by high energy demand because its main function is to pump against blood pressure. Cardiomyocytes play a central role in contraction of the heart and contain a substantial number of mitochondria, which mediate energy production. Mitochondria are ATP-producing organelles that play a critical role in energy metabolism in the heart. Among the sirtuin family proteins, Sirt1 regulates mitochondrial function by deacetylating nuclear proteins, whereas Sirt3 does so by deacetylating mitochondrial proteins (130). The heart predominantly uses free fatty acid as a substrate for adenosine triphosphate (ATP) production under physiological conditions. In failing hearts, the preferred substrate switches from free fatty acid to glucose to produce more ATP per molecule of oxygen consumed. However, the advanced failing heart develops insulin resistance in the myocardium and undergoes a decline in glucose utilization, limiting ATP production (94).
Peroxisome proliferator-activated receptor (PPAR)-γ coactivator 1α (PGC-1α), a transcriptional coactivator of nuclear receptors, including PPAR-α and estrogen-related receptor (ERR)-α, is a master regulator of metabolism and mitochondrial biogenesis. Sirt1 directly binds to PGC-1α and deacetylates it in 293T cells (116) and PC12 cells (93). Sirt1 stimulates the ability of PGC-1α to coactivate hepatocyte nuclear factor 4α, thereby positively regulating gluconeogenic genes in response to pyruvate in hepatic cells (116). In the same cell type, Sirt1 also enhances the ability of PGC-1α to inhibit glycolytic genes in response to pyruvate (116). In PC12 cells, wild-type Sirt1, but not a Sirt1 mutant that cannot interact with PGC-1α, negatively regulates the transcriptional activity of PGC-1α (93). Thus the functional consequences of PGC-1α deacetylation appear diverse and cell type or transcription factor dependent. Furthermore, Krishnan et al. (54) demonstrated that Sirt2, but not Sirt1, affects fatty acid oxidation in adipocytes and that knockdown of PGC-1α cancels the effect of overexpression of Sirt2 upon fatty acid oxidation. Thus the availability of other family members also contributes to the net effect of sirtuins upon PGC-1α. Systemic deletion of Sirt1 in mice induces the development of dilated cardiomyopathy, which is accompanied by mitochondrial dysfunction (104). However, Sirt1 is upregulated in the failing heart and contributes to downregulation of genes involved in mitochondrial function that are regulated by ERRs (97). Thus the effect of Sirt1 upon mitochondrial function and metabolism in the heart is also complex and possibly dose dependent.
Overexpression of Sirt1 in pancreatic β-cells enhances insulin secretion in response to glucose and improves glucose metabolism by increasing ATP production via suppression of uncoupling protein-2 expression (84). Resveratrol, a Sirt1 activator, inhibits vacuolization, degeneration, and inflammation in the heart in an insulin resistance model (11). Akt is also a target of Sirt1 in insulin signaling. Akt is acetylated in various tissues, including the heart, under basal conditions, and acetylation of Akt at K14 and K20 suppresses Akt activity. Deacetylation of Akt by Sirt1 is necessary for the binding of Akt to phosphatidylinositol 3,4,5-trisphosphate (PIP3) in the heart (125). It should be noted, however, that one group reported that they were unable to detect acetylation of Akt in HEK 293T cells (112). Thus it is possible that under some experimental conditions, the effect of sirtuins upon Akt may not be through direct deacetylation. In cardiomyocytes, overexpression of Sirt1 inhibited phenylephrine-induced downregulation of fatty acid oxidation genes by enhancing PPAR-α binding to the p65 subunit of NF-κB (105) (Fig. 1).
Fig. 1.
Signaling pathways of silent mating-type information regulation (Sirt) 1 in the heart. Sirt1 can modulate fatty acid oxidation, cardiac hypertrophy, apoptosis, oxidative stress, and autophagy through deacetylation of NF-κB, Akt, Forkhead box class O (FoxO), and p53. PPAR-α, peroxisome proliferator-activated receptor-α; Trx1, thioredoxin-1; Bcl-xL, B-cell lymphoma-extra large.
Sirt3 localizes in mitochondria and regulates a diverse array of mitochondrial functions, suggesting that Sirt3 is a mitochondrial fidelity protein. Although Sirt3 knockout mice show an almost normal phenotype at birth, they exhibit hyperacetylation of mitochondrial proteins (64). Target proteins of Sirt3 in mitochondria in the liver include MnSOD, NADH dehydrogenase 1α subcomplex 9, and succinate dehydrogenase complex subunit A (4, 133), suggesting that Sirt3 is closely tied to energy metabolism. Sirt3 also deacetylates and activates acetyl-CoA synthetase 2, a key enzyme in acetyl-CoA production, and long-chain acyl-CoA dehydrogenase, a key enzyme of fatty acid oxidation, in the liver (36). Knockout of Sirt3 exhibited a 50% decrease in basal ATP content in the heart (4). Together, these data indicate that Sirt3 increases energy production by activating the mitochondrial electron transport chain (ETC) and upregulating fatty acid oxidation.
Reduced Sirt2 function directly diminishes deacetylation of PGC-1α and expression of β-oxidation and mitochondrial genes in adipocytes (54). Ramakrishnan et al. (112) reported that although Sirt2 interacted with Akt and activated it in insulin-responsive cell lines, they could not detect acetylation of Akt. So far, the role of Sirt2 in mitochondrial function and energy metabolism in the heart remains unclear. Inhibition of Sirt4 increases fatty acid oxidation in liver and mitochondrial function in muscle. Sirt4 also inhibits glutamate dehydrogenase and impairs insulin secretion in pancreatic β-cells (32). Overexpression of Sirt5 increases oxygen consumption and ATP synthesis in human hepatocellular carcinoma cells but is not involved in mitochondrial biogenesis. Importantly, however, knockout of either Sirt4 or Sirt5 does not induce mitochondrial protein hyperacetylation (32). Sirt6 is involved in hepatic glucose production, inducing PGC-1α acetylation in a general control of amino-acid synthesis 5 (acetyltransferase)-dependent manner and suppressing hepatic glucose production (23). Sirt6 also controls circadian chromatin recruitment of sterol regulatory element-binding protein 1, resulting in the cyclic regulation of genes implicated in the metabolism of cholesterol and fatty acids in the liver (75). In the liver, Sirt7 positively regulates the protein level of nuclear receptor testicular receptor 4/transforming growth factor-β-activated kinase 1, involved in lipid metabolism, thereby activating testicular receptor-4/target genes to increase uptake of fatty acids and synthesis/storage of triglyceride (149).
Role of Sirtuins in Cardiac Hypertrophy
In general, cardiac hypertrophy is the primary response of the heart to hemodynamic overload, such as pressure or volume overload, and is characterized by enhanced protein synthesis and sarcomeric reorganization, leading to increased cardiomyocyte size. Hypertrophic growth initially reduces wall stress, maintains cardiac function, and serves as a compensatory response to hypertrophic stimuli, but it eventually initiates the development of cardiac remodeling and heart failure. Pathological hypertrophy is recognized as a strong independent factor in heart failure. Reversible modification of the acetylation status of proteins by histone acetyltransferases and HDACs critically regulates the activity of signaling molecules mediating cardiac hypertrophy (34). Class I HDACs, including HDAC1, 2, 3, and 8, positively regulate cardiac hypertrophy, whereas class II HDACs, including HDAC4, 5, 7, and 9, negatively regulate cardiac hypertrophy (152). Class II HDACs are expressed only in nonproliferative cells, including cardiomyocytes. The export of class II HDACs from the nucleus to the cytosol in the heart directly induces transcription of nuclear factor of activated T cell and MEF2, master positive regulators of cardiac hypertrophy (9). Class II HDACs are regulated by several forms of post-translational modification, including phosphorylation, ubiquitination, and sumoylation (9, 52, 108). Recently, it was shown that Trx1 regulates the nucleocytoplasmic shuttling of HDAC4 through a redox-dependent mechanism (3). By forming a multiprotein complex with Trx1-binding protein-2 and DnaJb5, Trx1 reduces HDAC4 at C667 and C669, which are easily oxidized to form a disulfide bond in response to hypertrophic stimuli. The redox status of these cysteines plays a critical role in the localization of HDAC4, thereby regulating cardiac hypertrophy (3).
Sirtuins have been demonstrated to be involved in cardiac hypertrophy, although their effects vary depending upon experimental conditions. Resveratrol attenuates the phenylephrine-induced hypertrophic response in cardiomyocytes and pressure overload-induced cardiac hypertrophy. Pharmacological inhibition of Sirt1 (with NAM or sirtinol) decreases, whereas overexpression of Sirt1 increases the size of cardiomyocytes (6). Overexpression of Sirt1 attenuates endothelin-1-induced cardiomyocyte hypertrophy in vitro via degradation of H2A.z. Upregulation and activation of Sirt1 induced by phenylephrine (an α-adrenergic agonist) are blocked by inhibition or downregulation of AMPK, leading to attenuated cardiac hypertrophy. Phenylephrine-induced hypertrophy in cardiomyocytes is attenuated by resveratrol and overexpression of Sirt1. This antihypertrophic effect is canceled by downregulation of PPAR-α. On the other hand, pressure overload coupregulates Sirt1 and PPAR-α in the heart, which coordinately suppress genes that are regulated by ERRs, thereby leading to the development of cardiac hypertrophy and heart failure (Fig. 1) (97). Importantly, haploinsufficiency of PPAR-α and Sirt1 attenuates cardiac hypertrophy in response to pressure overload (97). Interestingly, although low (2.5-fold) to moderate (7.5-fold) levels of overexpression of Sirt1 in the heart attenuate age-dependent cardiac hypertrophy, a high level (12.5-fold) of Sirt1 increases it (5). Thus the effect of Sirt1 upon cardiac hypertrophy may be affected by a balance of interaction with other factors, such as PPAR-α, or severity and type of stress. In addition, cardiac hypertrophy is a multifactorial disease and may also be affected by angiogenesis, apoptosis, and fibrosis. Akt is one of the key molecules regulating cardiac hypertrophy. A recent study demonstrated that Sirt1 critically regulates Akt activation by deacetylation at lysine residues in the pleckstrin homology domains of Akt. Acetylation of Akt and phosphoinositide-dependent protein kinase 1 (PDK1) blocks binding of Akt and PDK1 to PIP3. Deacetylation of Akt by Sirt1 enhances binding of Akt and PDK1 to PIP3 and promotes their activation, thereby leading to cardiac hypertrophy (Fig. 1) (125). On the other hand, Sirt1 knockout mice have smaller hearts than wild-type mice and exhibit resistance to the development of cardiac hypertrophy in response to hypertrophic stimuli. Overall, these findings lead to speculation that the role of Sirt1 as a progrowth or antigrowth factor for cardiomyocytes is tightly controlled by the magnitude of Sirt1 expression.
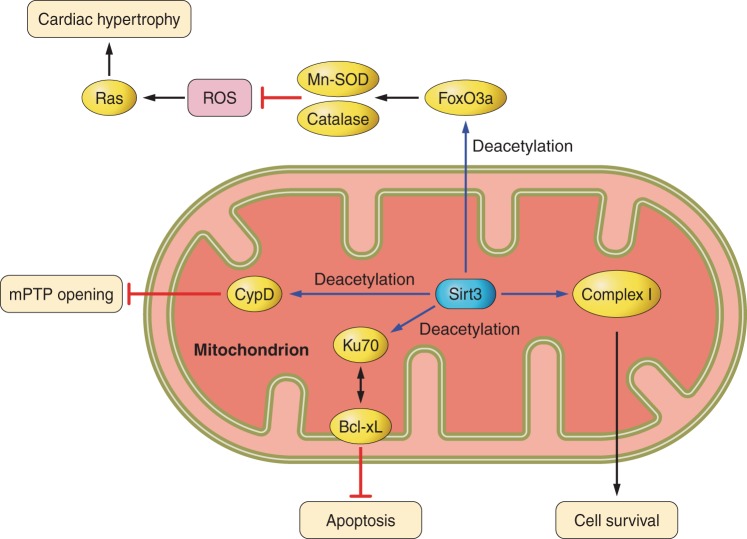
Sirt3 has also been shown to confer resistance to cardiac hypertrophy. Sirt3 is upregulated during mild cardiac hypertrophy, whereas it is decreased in severe cardiac hypertrophy (126). Overexpression of Sirt3 causes suppression of agonist-induced cardiac hypertrophy. Conversely, Sirt3 knockout mice exhibit exacerbation of the hypertrophic response. The beneficial effects of Sirt3 are mediated by the activation of FoxO3-dependent transcription of catalase and MnSOD, suppression of ROS-induced Ras activity, MAPK/ERF, and Akt/PI3K signaling (Fig. 2) (124). Exogenous NAD+ treatment blocks antihypertrophic liver kinase B1-AMPK signaling, which is accompanied by activation of Sirt3 but not Sirt1(103), indicating the critical role of Sirt3 in cardiac hypertrophy and redundancy in Sirt1 targets.
Fig. 2.
Signaling pathways of Sirt3 in the heart. Sirt3 regulates Mitochondrial permeability transition pore (mPTP) opening, apoptosis, and cell survival, mainly through deacetylation of mitochondrial proteins, including cyclophilin D (CypD), Ku70, and complex I, independently of transcription. Sirt3 also deacetylates FoxO, leading to transcriptional upregulation of MnSOD and catalase and inhibition of cardiac hypertrophy.
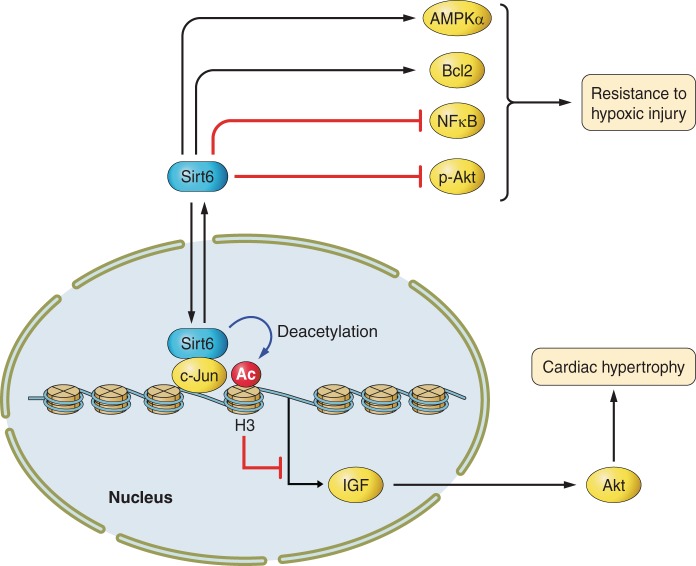
The role of Sirt6 in cardiac hypertrophy has been investigated using loss- and gain-of-function models (127). Sirt6 knockout mice exhibited cardiac hypertrophy and heart failure, whereas overexpression of Sirt6 attenuated cardiac hypertrophy, indicating that Sirt6 negatively regulates cardiac hypertrophy. Unlike Sirt1 and Sirt3, Sirt6 functions to directly attenuate insulin-like growth factor (IGF)-Akt signaling at the chromatin level (Fig. 3) (127).
Fig. 3.
Signaling pathways of Sirt6 in the heart. Sirt6 deacetylates histones at Lys9, thereby suppressing the insulin-like growth factor (IGF)-Akt pathway and cardiac hypertrophy. Sirt6 increases the resistance to hypoxic injury in the heart by stimulating AMPK-α and Bcl2 and inhibiting NK-κB and Akt. pAkt, phospho-Akt; Ac, acetylated.
Whereas a recent study demonstrated that Sirt2 is required for optimal Akt activation in insulin- and growth factor-responsive cells, the role of Sirt2 in cardiac hypertrophy is still unclear. Likewise, although Sirt4, Sirt5, and Sirt7, like Sirt1 and Sirt3, appear to be protective against stress in the heart, there has been no report thus far regarding the role of these proteins in cardiac hypertrophy.
Role of Sirtuins in Cardiac Function and Heart Failure
Heart failure represents a condition in which cardiac output is insufficient to meet the needs of peripheral organs. Heart failure is caused by almost all cardiac diseases, including myocardial infarction, hypertension, valvular heart disease, cardiomyopathy, and arrhythmia, and is one of the leading causes of death worldwide. These cardiac diseases frequently provoke left ventricular dilatation and hypertrophy, which are collectively referred to as ventricular remodeling and contribute to the development of depressed cardiac performance (101). Pathophysiological left ventricular remodeling is characterized by cardiomyocyte hypertrophy and death and interstitial fibrosis. The mechanisms mediating these processes are triggered by hemodynamic stress, as well as by the activation of neurohumoral factors, including the autonomic nervous system and the renin-angiotensin system, and ROS. Given the effects of sirtuins in cardiac hypertrophy, cell death/survival, and oxidative stress, sirtuins appear to be closely involved in cardiac remodeling and heart failure.
Sirt1-deficient mice exhibit notable developmental defects of the heart and only infrequently survive postnatally (17). Adult hearts from Sirt1 heterozygous knockout mice show dilated cardiomyopathy, a phenotype characterized by an absence of fibrosis and reduction of cardiomyocyte size, indicating that Sirt1 primarily affects cardiomyocyte hypertrophy. Expression of Sirt1 is upregulated in dog hearts undergoing failure induced by rapid pacing (6). Several studies examining the effects of resveratrol indicate that Sirt1 may have a beneficial role in failing hearts and that endogenous Sirt1 upregulation is a protective mechanism in the early stage of heart failure. Administration of resveratrol (2.5 mg·kg−1·day−1, 10 wk) prevents the development of concentric hypertrophy and cardiac dysfunction in the spontaneously hypertensive rat without affecting blood pressure, which may be partially mediated by suppression of ROS (135). In Dahl salt-sensitive rats fed with a high-salt diet, resveratrol (18 mg·kg−1·day−1, 8 wk) improves survival and counteracts the development of cardiac dysfunction without regression of hypertension or cardiac hypertrophy (114). Resveratrol also preserves mitochondrial fatty acid oxidation and PPAR-α expression in Dahl salt-sensitive rats (114). Administration of resveratrol (∼145 mg/kg, 24 wk) to the TO-2 hamster, a model of dilated cardiomyopathy, suppresses cardiac fibrosis, preserves cardiac function, and improves survival by increasing MnSOD expression and reducing oxidative stress (131). Although effects of resveratrol on cardiac remodeling such as cardiac hypertrophy and fibrosis may depend on the dose of resveratrol, period of administration of resveratrol, or model of heart failure, Sirt1 is thought be a potential target for treatment of heart failure by reducing oxidative stress and preserving mitochondrial fatty acid oxidation.
Recent studies, however, including ours, demonstrated that upregulation of Sirt1 is not necessarily protective in the heart in the presence of pressure overload. In the mouse model of heart failure, endogenous Sirt1 is upregulated by pressure overload and, together with simultaneous upregulation of PPAR-α in the nucleus, Sirt1 exacerbates cardiac dysfunction (5, 49, 95). In this situation, Sirt1 interacts with PPAR-α, thereby inhibiting expression of genes related to the mitochondrial ETC and tricarboxylic acid cycle that are normally regulated by ERR-α, through their binding to the ERR element. Haploinsufficiency of either Sirt1 or PPAR-α attenuates not only cardiac hypertrophy but also cardiac dysfunction induced by pressure overload (95). Further investigations are needed to clarify the function of Sirt1 in heart failure caused by different types of stress and the underlying molecular mechanisms mediating the effect of Sirt1 in the heart.
Little is known regarding the role of other sirtuins in heart failure. Sirt3-deficient mice exhibit signs of cardiac hypertrophy and interstitial fibrosis at baseline and severe cardiac hypertrophy in response to hypertrophic stimuli (116). In addition, overexpression of Sirt3 prevented isoproterenol-induced cardiac hypertrophy and dysfunction by augmenting FoxO3a-induced expression of MnSOD and catalase and reducing ROS levels. Reduction of ROS inhibited Ras activation and its downstream targets, such as MAPK/ERK and the PI3K/Akt pathway, thereby repressing the activity of GATA4, nuclear factor of activated T cell, eukaryotic initiation factor 4E, and S6 ribosomal protein (116).
The expression level of Sirt6 is decreased in the failing heart in humans, and a recent study demonstrated the beneficial role of Sirt6 in heart failure (127). Deletion of Sirt6 induces cardiac hypertrophy and heart failure in mice, whereas overexpression of Sirt6 attenuates development of cardiac hypertrophy in response to hypertrophic stimuli. Sirt6 binds to and suppresses the promoters of genes involved in IGF-signaling by interacting with c-Jun and deacetylating histone H3 at K9, thereby inhibiting the abnormal activation of IGF-Akt signaling implicated in the progression of heart failure.
Another important aspect of cardiac remodeling is angiogenesis. Dissociation between angiogenesis and cardiac muscle growth triggers the development of pathological hypertrophy and eventually causes heart failure (123). Thus the effect of sirtuins on vascular phenotype is also implicated in the pathogenesis of heart failure. Sirt1 plays a critical role in sprouting angiogenesis during vascular development, and Sirt1 in endothelial cells impairs the formation of new blood vessels in ischemic tissues (107). Sirt1 interacts with and deacetylates FoxO1, which plays an essential role in the negative regulation of blood vessel development, in human umbilical vein endothelial cells (HUVECs) (107). Inhibition of Sirt1 blocks nitric oxide (NO) production and endothelium-dependent vasodilation (79), and resveratrol activates endothelial NO synthase and increases NO production, thereby protecting the heart from I/R injury (42). In addition, resveratrol induces angiogenesis in a myocardial infarction model (28). The induction of angiogenesis by Sirt1 may be mediated by p53. p53 acts as an antiangiogenetic factor because it inhibits the activity of hypoxia-inducible factor-1, which regulates hypoxic adaptation genes such as VEGF, and Sirt1 deacetylates p53 and reduces its transcriptional activity in cardiomyocytes (6). Supporting this speculation, endothelial-cell-specific ablation of Sirt1 induces impairment of angiogenesis and diastolic dysfunction in the heart through downregulation of VEGF receptors FLT 1 and FLK 1 (70). Sirt3 also appears to be involved in angiogenesis in the heart. In fact, Sirt3 induces bone marrow cell-induced VEGF expression and angiogenesis post-myocardial infarction (151). However, less is known regarding the involvement of Sirt3 in angiogenesis. In addition, thus far, there have been no reports of a role for other sirtuin family proteins.
Ca2+ homeostasis is also closely involved in the pathogenesis of heart failure. Sarco(endo)plasmic reticulum Ca2+-ATPase 2a-regulated Ca2+ uptake into the sarcoplasmic reticulum is decreased in the failing heart (7). Resveratrol improves sarco(endo)plasmic reticulum Ca2+-ATPase 2a promoter activity under high-glucose conditions in a Sirt1-dependent manner. However, the role of sirtuins in Ca2+ regulation in cardiomyocytes remains unclear.
Sirtuins and I/R Injury
I/R is a major cause of myocardial injury and a life-threatening event in the clinical setting. Ischemia or hypoxia in the heart induces a rapid decrease in ATP content in cardiomyocytes. To survive under these conditions, cardiomyocytes switch their metabolism from fatty acid oxidation (aerobic mechanism) to glycolysis (anaerobic). Prolonged ischemia induces cytochrome-c release from mitochondria and triggers apoptosis in cardiomyocytes. Mitochondrial permeability transition pore (mPTP) opening is followed by increases in Ca2+ overload, depletion of ATP, and decreases in the intracellular pH, eventually causing necrosis in cardiomyocytes (71). Although rapid reperfusion supplies oxygen and fuel, including glucose and fatty acids, and washes out toxic substances derived from necrotic cells, the rapid recovery of the oxygen supply and extracellular pH causes further ROS production and Ca2+ overload, leading to reperfusion injury such as necrosis and apoptosis (24). If cardiomyocytes survive after reperfusion, both oxidative stress and endoplasmic reticulum stress can induce cellular malfunction. Thus I/R induces irreversible damage to cardiac muscle and leads to cardiac remodeling and heart failure characterized by dilation of the ventricle and reduced contractility.
In the consideration of the favorable effects of Sirt1 in energy metabolism and stress resistance in the heart, Sirt1 can be expected to have a beneficial effect on I/R injury. Indeed, alteration of the expression level of Sirt1 in cardiomyocytes has been demonstrated to affect myocardial injury and cardiac function after I/R. After I/R, expression of Sirt1 is decreased in the heart, and cardiac-specific deletion of Sirt1 increases apoptosis and myocardial injury during I/R (39). Conversely, overexpression of cardiac-specific Sirt1 decreases apoptosis and infarct size and improves functional recovery after I/R by upregulating cardioprotective molecules, including MnSOD, Trx1, and Bcl-xL, and downregulating proapoptotic molecules, including Bax (Fig. 1) (39). NF-κB, isocitrate dehydrogenase, glyceraldehyde-3-phosphate dehydrogenase, and endothelial NO synthase have been proposed as functional targets of IPC-induced Sirt1 (88). On the other hand, resveratrol-induced Sirt1 activation attenuates cardiac I/R injury via a reduction in p38 and JNK phosphorylation and an increase in ERK phosphorylation, which are regulated by Akt/apoptosis signal-regulating kinase 1 signaling (13).
Whereas Sirt1 is upregulated by aging, oxidative stress, and heart failure, it is downregulated by I/R (39), suggesting that regulation of Sirt1 is stimulus specific. The precise mechanism by which a given stress upregulates/downregulates Sirt1 and controls the function of specific substrates is not well understood. Sirt1 deacetylase activity is regulated in an NAD+-dependent manner. Administration of NAM mononucleotide increases NAD+ in the heart and attenuates myocardial I/R injury, accompanied by a decrease in acetylation of FoxO1. However, this effect is not observed in Sirt1 knockout mice (147). The protective effects of IPC and CR, which upregulate Nampt, the rate-limiting enzyme for NAD+ synthesis, are abolished by Sirt1 knockout, suggesting that the Nampt-Sirt1 axis plays a pivotal role in mediating the protective effects of IPC and CR in I/R injury (147). Prolonged CR enhances myocardial ischemic tolerance by increasing nuclear Sirt1 in a nitric oxide-dependent manner in middle-aged mice (122). In addition, several compounds, such as Sildenafil (phosphodiesterase-5 inhibitor) and Curcumin (a strong natural antioxidant), exhibit cardioprotective effects against I/R injury via activation of Sirt1 (120).
Except for those examining Sirt1, there are presently only a few studies regarding the role of sirtuins in I/R. Sirt2 has been shown to be upregulated by anoxia-reoxygenation injury. On the other hand, downregulation of Sirt2 upregulates 14-3-3-ζ and changes the subcellular localization of Bcl-2-associated death promoter from mitochondrial to cytosolic, leading to a cardioprotective phenotype (67). A recent study demonstrated that Sirt3 plays a protective role in I/R. In an isolated perfused heart model, adult Sirt3 heterozygous knockout mice exhibit less cardiac functional recovery and a larger infarct size than wild-type mice during I/R, accompanied by an increase in mitochondrial protein acetylation and a decrease in the activity of mitochondrial complex I and MnSOD (Fig. 2) (106). Unlike the other sirtuins, Sirt4 does not have NAD+-dependent deacetylase activity but rather functions as a histone ADP-ribosyltransferase (69). Sirt4 has been shown to decrease apoptosis in H9C2 cells under hypoxic conditions by suppressing mitochondrial Bax translocation (60), but it has not been investigated in the context of I/R. Sirt6 has also been investigated in the context of hypoxia. Cardiomyocytes from transgenic mice with overexpression of Sirt6 exhibit resistance to hypoxia, accompanied by activation of AMPK-α, an increase in B-cell lymphoma 2 (Bcl2), inhibition of NF-κB, and decreases in ROS production and phospho-Akt (p-Akt) (Fig. 3) (72). Although Sirt5 and Sirt7 also have protective roles in the heart (137), their roles during I/R remain to be elucidated.
Involvement of Sirtuins and Oxidative Stress
Oxidative stress, which is the presence of ROS in excess of the antioxidant capacity, induces myocardial damage. The mitochondrial ETC is the one of the major sources of ROS in the heart because cardiomyocytes have a large number of mitochondria and electrons continuously leak from the ETC. Endogenous antioxidants, such as SOD, catalase, and glutathione peroxidase (GPx), cooperatively detoxify ROS to H2O. Superoxide anion (O2−) is dismutated by SOD to produce hydrogen peroxide (H2O2), which is detoxified to H2O by catalase or GPx. However, O2− can also be a source of hydroxyl radical and peroxynitrite, highly reactive ROS (121). Under pathological conditions such as heart failure, mitochondrial ROS production in cardiomyocytes increases to a level that exceeds the antioxidant capacity, leading to oxidative stress. An increase in ROS production causes oxidation of mitochondrial proteins and induces mitochondrial dysfunction, further exacerbating oxidative stress in a process known as ROS-induced ROS production (43).
Increasing lines of evidence suggest that oxidative stress plays a crucial role in the pathogenesis of cardiovascular disease. MnSOD-deficient mice exhibit dilated ventricles with endocardial fibrosis and die 10 days after birth (57). Heterozygous deletion of MnSOD in mice causes a decrease in ejection fraction and progression of cardiac remodeling (63). Overexpression of catalase suppresses doxorubicin-induced cardiotoxicity in mice (49), and other antioxidants, such as GPx and peroxiredoxin-3, prevent the progression of cardiac remodeling and heart failure after myocardial infarction by suppressing mitochondrial oxidative stress (78).
Sirt1 deacetylates FoxO1 at K242, K245, and K262 and induces its nuclear translocation, thereby activating transcription of MnSOD in HEK293 cells (20). In addition, Sirt1 was reported to upregulate MnSOD via hypoxia-inducible factor-2α and FoxO4 (22). Several lines of evidence suggest that Sirt1 plays a protective role against oxidative stress in cardiomyocytes and the heart (5). Cardiac-specific overexpression of Sirt1 induces an increase in protein expression of catalase after exposure to paraquat (5). Overexpression of Sirt1 also upregulates MnSOD and Trx1 and attenuates oxidative stress during cardiac I/R (39). However, it should be noted that a high level of Sirt1 expression in the heart conversely induces oxidative stress through dysregulation of mitochondrial biogenesis and function at baseline in mice (5). Thus it appears that the cardiac effect of Sirt1 against oxidative stress is dose dependent. Furthermore, the activity of Sirt1 is controlled by the ratio of NAD+ to NADH, an intracellular redox-sensitive mechanism, in skeletal muscle (29). An intimate relationship between Sirt1 and oxidative stress may affect cell survival in some pathological situations.
Sirt3 also increases antioxidant capacity by upregulating MnSOD through FoxO3a in the heart (124) (Fig. 2). In addition, Sirt3 increases the ROS-scavenging activity of MnSOD by deacetylating it at K53/K68 and K122 in mouse embryonic fibroblasts (110, 133). Other antioxidants are regulated by Sirt3 as well, probably in an indirect manner. Sirt3 is thought to increase NADPH production by deacetylating and activating isocitrate dehydrogenase 2 and glutamate dehydrogenase, which in turn enhances the conversion of oxidized glutathione to reduced glutathione (64). Sirt3 inhibits ROS-induced Ras-PI3K-Akt signaling by suppressing mitochondrial ROS production (124).
Recently, the biological function of Sirt2 in oxidative stress was reported. Oxidative stress, such as due to increased hydrogen peroxide levels, increases expression of Sirt2 in NIH3T3 cells, and Sirt2 lowers the ROS level by upregulating MnSOD through deacetylation and activation of FoxO3. A cell-permeable peptide-1-SIRT2 protein attenuates lipopolysaccharide- and H2O2-induced cell death in a murine macrophage-like cell line. Likewise, peptide-1-SIRT2 decreases cellular levels of ROS, accompanied by elevation of MnSOD, catalase, and GPx expression. The Sirt2-mediated deacetylation and activation of glucose-6-phosphate dehydrogenase stimulates the pentose phosphate pathway to supply cytosolic NADPH to counteract oxidative damage and protect mouse erythrocytes (142). On the other hand, a study using a cDNA microarray demonstrated that Sirt2 knockdown in primary HUVECs causes global gene expression changes in response to oxidative stress, most of which are not altered by Sirt1 knockdown. Importantly, pharmacological blockade of Sirt2 attenuates oxidative stress-induced endothelial cell injury (62). Thus the effects of Sirt2 are thought to depend on the type of cell and stimulation. Thus far, no intensive study regarding Sirt2 and oxidative stress in the heart has been reported.
Sirt5 is known to be downregulated by oxidative stress in cardiomyocytes and to act as a safeguard against ROS by reducing ROS-induced cell death (61). However, it is still unclear whether and how Sirt5 regulates the ROS level in the heart. Sirt6 physically interacts with PARP1 and increases PARP1 poly-ADP-ribosylase activity via mono-ADP-ribosylation of PARP1 at K521, thereby enhancing DNA double-strand break repair under conditions of oxidative stress (74). Sirt7-deficient cardiomyocytes exhibit increases in apoptosis under basal conditions and a significant increase in sensitivity to oxidative stress (137). These findings suggest that Sirt5, Sirt6, and Sirt7 play protective roles against ROS-induced injury, consistent with their beneficial effects under other stress conditions. Less is known, however, regarding the role of Sirt4 in oxidative stress.
NADPH oxidase (Nox) proteins, which are dedicated producers of O2− and/or H2O2, were recently recognized to be major sources of ROS in cells. Thus far, seven members of the Nox family of proteins (Nox1-5 and dual oxidases 1 and 2) have been identified. Nox isoforms are thought to be widely involved in myocardial damage/death, cardiac hypertrophy, cell proliferation/differentiation, and fibrosis (68). Recent studies suggest an intimate link between sirtuins and Nox. Nox1 inhibits acetylation of p53 at K382. This same lysine is deacetylated by Sirt1, resulting in impairment of the transcriptional activity of p53 and inhibition of apoptosis in cancer cells, suggesting that inhibition of p53 transcriptional activity by Nox1 is Sirt1 dependent (109). Resveratrol protects against high glucose-induced increases in expression of p47phox, a cytosolic component of Nox, and sirtinol, a Sirt1 inhibitor, aggravates the increase in p47phox protein expression induced by high glucose in rat aortas and cultured bovine aortic endothelial cells. Inhibition of Sirt1 significantly increases Nox activity, mRNA expression of the Nox subunits, p22phox and Nox4, and vascular superoxide production, all of which are attenuated by resveratrol. In addition, Sirt1 inhibition-induced endothelial dysfunction is inhibited by treatment with apocynin or SOD in rat aortas (150). These data suggest that the protective effect of Sirt1 against oxidative stress in the heart may be partly due to suppression of Nox4 activity, since Nox4 is a major Nox isoform in cardiomyocytes (2). Furthermore, the activities of sirtuins are NAD+ dependent and Nox isoforms use NADH or NADPH as a substrate for the generation of ROS. This raises the exciting possibility that Nox isoforms may regulate sirtuin activity by changing the balance of NAD+/NADH and NADP+/NADPH or vice versa. However, whether sirtuins and Nox isoforms affect each other in an NAD+/NADH− or NADP+/NADPH-dependent manner in the heart has not yet been elucidated.
Role of Sirtuins in Cell Survival and Aging in the Heart
Several lines of evidence suggest that the balance between survival/growth and death in cardiomyocytes plays a pivotal role in cardiac function in cardiovascular disease (86). Sirt1 knockout mice (Sirt1−/−) exhibit abnormal development of the heart and prenatal lethality, suggesting that Sirt1 performs important functions in cell growth and death during development (17).
Sirt1 is predominantly expressed in cardiomyocytes. Inhibition of Sirt1 by NAM, sirtinol, or expression of dominant-negative Sirt1 induces nuclear fragmentation and cleavage of caspase-3 (6). Sirt1 has been reported to deacetylate p53 and FoxO, thereby regulating transcription and apoptosis in mouse and human cells (17, 65, 83, 138). In the heart, Sirt1 positively regulates expression of the antiapoptotic protein Bcl-xL, whereas it negatively regulates proapoptotic proteins Bax and cleaved caspase-3 through activation of FoxO (39). Importantly, inhibition of the activity of Sirt1, but not other HDACs, increases the acetylation and transcriptional activity of p53 in cardiomyocytes, suggesting that Sirt1 plays a more critical role in regulating p53 than other classes of HDACs in cardiomyocytes. These data strongly suggest that Sirt1 exerts protective effects in the heart. The deacetylase activity of Sirt1 critically depends on the nuclear NAD+ content, which is consumed when DNA strand breaks activate PARP. Thus decreases in Sirt1 activity induce cardiomyocyte death in some physiological and pathological conditions, such as aging, myocardial I/R, or exposure to genotoxic reagents that cause DNA damage and reduce the nuclear ratio of NAD+ to NAM (153).
Sirt3 deacetylates Ku70 to sequester Bcl2-associated X protein away from mitochondria, thus inhibiting apoptosis (Fig. 2) (126). Likewise, Sirt4, Sirt5, Sirt6, and Sirt7 also exhibit antiapoptotic effects in the heart (60, 61, 72, 137).
The mPTP is found in the mitochondrial inner membrane and passes small molecules nonselectively in response to stimuli. mPTP opening is a hallmark of mitochondrial dysfunction and causes depletion of ATP and release of proapoptotic factors, eventually leading to apoptosis and necrosis of cardiomyocytes (117). mPTP opening is closely related to apoptosis/necrosis and the aging process. A recent study showed that Sirt3, a mitochondrial sirtuin protein, deacetylates cyclophilin D, a component of the mPTP, and inhibits mPTP opening, thereby reducing oxidative stress and slowing down cardiac aging in the heart (Fig. 2). Thus regulation of the mPTP by Sirt3 is an important pathway for the cardioprotective effect of Sirt3.
Aging phenotypes are determined by cooperation of diverse intrinsic and extrinsic factors that are closely related to the function of organs and the risk of disease. Generally, aging affects the expression of INK4/ARF family senescence factors (53). Aging hearts are characterized by increased myocyte volume, proliferation of myocyte nuclei, increases in apoptosis and necrosis, and interstitial fibrosis (48). In addition, cellular protective mechanisms, such as induction of antioxidants and heat shock proteins, are attenuated in aging hearts (128). Importantly, the heart is one of the most prominent organs that exhibit aging-induced mitochondrial DNA deletions and mitochondrial dysfunction (47). Therapeutic intervention to retard aging should decrease accumulation of senescent myocytes and cell death, leading to prevention of adult heart diseases.
A Sir2-dependent mechanism is thought to be involved in regulating the longevity of organisms, from yeast to primates (18, 59). However, some studies have demonstrated that the molecular mechanisms mediating longevity in lower organisms may be different from those in higher organisms. Sir2-deficient nonreplicating yeast cells exhibit greater stress resistance in extremely long-lived yeast mutants, indicating that Sir2 increases replicative life span but inhibits chronological life span under extreme stress conditions in yeast (25).
In mice on high-calorie diets, stimulation of Sirt1 by the sirtuin-activating compound resveratrol is capable of prolonging life span (11). Moderate upregulation of Sirt1 attenuates age-dependent cardiac phenotypes, whereas animals with a high level of Sirt1 (12.5-fold overexpression of Sirt1) develop heart failure spontaneously (5). This may be due to the consumption of NAD+ by Sirt1. Because NAD+ is necessary for the mitochondrial ETC and ATP production, a decrease in NAD+ could cause a deficiency in ATP content and cellular malfunction, thereby leading to cell death. PGC-1α, a key regulator of fatty acid oxidation, is decreased in aging hearts, and Sirt1 regulates PGC-1α expression (118), indicating that Sirt1 may exert an antiaging effect by improving energy metabolism.
As a mechanism mediating life span extension, the hormesis hypothesis, in which accumulated stress resistance conferred by chronic nonlethal stress leads to life span extension, is currently well accepted. This hypothesis allows for speculation that stimulation of Sirt1 by low-grade stress, such as CR or repetitive ischemia, may partly mediate the beneficial effect of preconditioning.
Sirtuins and Autophagy
Autophagy is a mechanism of degradation of cytosolic proteins and organelles involving sequestration into double-membrane vesicles (autophagosomes) and subsequent fusion of the autophagosomes with lysosomes for degradation. Autophagy is activated in response to several kinds of stress and promotes cell survival by removing damaged organelles and preventing activation of the apoptotic machinery. In the heart, autophagy acts as a mechanism for quality control of proteins and is implicated in several important pathophysiologies, including cardiac hypertrophy (21), cardiomyopathies (95), heart failure (90), and ischemic heart disease (33). Autophagy allows for adaptation to nutrient and oxygen deprivation during myocardial ischemia and mediates cell death during reperfusion injury (77). The genes encoding proteins involved in autophagosome formation are regulated by the FoxO family of transcription factors, especially FoxO1 and FoxO3 (73). Sirt1 deacetylates FoxO1, FoxO3, and FoxO4 and regulates FoxO-dependent gene transcription either positively or negatively, leading to stress resistance and organismal longevity. For example, Sirt1 enhances FoxO3's ability to induce resistance to oxidative stress and cell cycle arrest, whereas it inhibits FoxO3's ability to induce cell death (14). Sirt1 deacetylates and suppresses the activity of FoxO3a and other mammalian forkhead factors, thereby reducing FoxO-dependent apoptosis (83).
Studies using pharmacological manipulation of Sirt1 support the existence of a link between Sirt1 and autophagy. Resveratrol induces autophagy in cancer cell lines in a Sirt1-dependent manner (82). Resveratrol also exhibits protective effects against apoptosis induced by rotenone and enhances degradation of α-synucleins via autophagy (143). Prolonged administration of Longevinex, a resveratrol derivative, increases autophagy in rat hearts after I/R, accompanied by upregulation of Sirt1 and Sirt3 and nuclear translocation of FoxO1, FoxO3a, and FoxO4. Manipulation of Nampt, a key molecule for the replenishment of the NAD+ pool, modulates autophagy in a rat cerebral artery occlusion model and in the presence of oxygen and glucose deprivation in cultured cortical neurons in a Sirt1-dependent manner (141). The findings that Sirt1 coimmunoprecipitates with Atg proteins provide direct evidence that Sirt1 is involved in regulation of the autophagic machinery. Sirt1 directly regulates autophagy by interacting with and deacetylating autophagy-related proteins such as Atg5, Atg7, and Atg8 in MEFs (56). As described above, Sirt1 also promotes the expression of autophagy-related proteins via deacetylation of transcription factors, such as FoxO family proteins (40).
The role of Sirt1 in autophagy in the heart was recently described. Downregulation of Nampt impairs autophagic flux in cardiomyocytes, raising the possibility that Nampt regulates autophagy via the NAD+-dependent Sirt1 pathway (38). A more recent study demonstrated that the absence of glucose increased Sirt1-mediated deacetylation, activation, and nuclear translocation of FoxO1 in cardiomyocytes. Sirt1-induced deacetylation of FoxO1 and upregulation of Rab7, a small GTPase mediating late autophagosome-lysosome fusion, are essential for glucose starvation-induced autophagy in cardiomyocytes and play a pivotal role in preserving cardiac function during starvation (Fig. 1) (35). These lines of evidence suggest that Sirt1 is a key regulator of autophagy in the heart and that, in general, enhanced autophagy could be part of a protection mechanism under stress conditions. The Sirt1-FoxO axis modulates autophagy in a cell type-dependent manner in response to multiple stress signals (91, 145). Thus the role of Sirt1 in autophagy in noncardiomyocytes of the heart should be examined in the future. Moreover, other transcription factors that are deacetylated by Sirt1 may also be potential targets in autophagy regulation, as in the recent findings that nuclear p53 induces autophagy whereas cytoplasmic p53 suppresses autophagy (Fig. 1) (134).
Recent and increasing lines of evidence have demonstrated an intimate link between Sirt2 and autophagy as well. Overexpression of Sirt2 suppresses lysosome-mediated autophagic turnover by inhibiting aggresome formation in neuronal cells. Sirt2 knockdown increases basal autophagy and causes mitotic catastrophe malfunction (45). However, the role of Sirt2 in regulating autophagy in the heart remains to be elucidated.
Several studies using gain- or loss-of-function of Sirt3 and Sirt6 indicate that Sirt3 and Sirt6 are also involved in autophagy regulation. Sirt3 overexpression enhances autophagy and postpones primary HUVEC apoptosis during long-term oxide LDL treatment (66). Under basal conditions, Sirt3-knockout mouse embryonic fibroblast cells exhibit increased autophagic flux compared with wild-type cells that are not inhibited by JNK inhibitor, indicating that Sirt3-related autophagy is independent of JNK activity (58). Sirt6 overexpression induced autophagy via attenuation of IGF-Akt-mammalian target of rapamycin signaling, and Sirt6 attenuates cigarette smoke extract-induced human bronchial epithelial cell senescence via induction of autophagy (129). However, there is no report of direct evidence of a link between Sirt3 or Sirt6 and autophagy in the heart. As for the other sirtuin family proteins, to date little is known regarding the roles of Sirt4, Sirt5, and Sirt7 in autophagy. Interestingly, however, inhibitors of other types of HDACs, namely, class I and II HDACs, are known to induce autophagy (144).
Sirtuin-Activating Compounds
Sirtuins are considered attractive drug targets because of their obvious role in mediating benefits not only in life span extension related to caloric restriction but also in several diseases, including cancer, inflammation, metabolic disorder, and cardiovascular disease. Some substances, such as flavones, stilbenes, and anthocyanidins, can directly stimulate the enzymatic activity of Sirt1 in vitro through an allosteric mechanism (37). Most of the activators are polyphenolic, with planar multiphenyl rings bearing hydroxyl groups. Resveratrol (3,5,4′-trihydroxystilbene), originally identified in 1940 as a phenolic substance in the white hellebore, is well known as a natural potent activator of Sirt1 (37). It can also be found in red wine and the Japanese knotweed, Polygonum sachalinense (12). To date, more than 3,500 Sirt1-activating compounds (STACs) have been synthesized and analyzed (55). Small molecule Sirt1 activators include first-generation molecules such as resveratrol and polyphenol (37), second-generation STACs such as imidazothiazoles (80), and third-generation STACs such as benzimidazoles and urea-based scaffolds (41). Many studies have shown that STACs mimic the effect of Sirt1 activation upon gene expression in mammals, and these effects were found to be Sirt1 dependent (81). Modulation of sirtuin activity was assessed by the Fluor de Lys fluorescent biochemical assay, the validity of which has been questioned (99). However, several mechanisms of direct regulation of Sirt1 by STACs have recently been proposed in renewed experiments. One is a model of direct interaction of the compound with enzyme-bound substrates (31). Another is an assisted-allosteric activation in which STACs bind to a substrate-induced exosite on Sirt1 and, in turn, stabilize substrate binding and subsequent deacetylation (41).
Of the numerous STACs analyzed, resveratrol is still one of the most potent Sirt1 activators. However, resveratrol has many off-target effects unrelated to sirtuins, such as antioxidant effects, and negatively regulates proinflammatory cytokines and growth factors (99, 100). Resveratrol also activates AMPK through competitive inhibition of cAMP-degrading phosphodiesterases (100). Although it remains unclear whether the beneficial effects of resveratrol in the heart are solely mediated by Sirt1, based on numerous reports, resveratrol does appear to be beneficial to the treatment of cardiovascular disease. Resveratrol scavenges free radicals and chelates copper, thereby reducing oxidation of LDL particles, a contributing factor to the development of coronary heart disease (12). Resveratrol exerts diverse effects on the vasculature by increasing expression of endothelial and inducible NO synthase and inhibiting vascular smooth muscle cell proliferation and expression of vascular cell adhesion molecules (VCAMs) (19). SRT1720, which is structurally unrelated to resveratrol, stimulates 750% Sirt1 activity at a concentration of 10 μM and binds Sirt1 at the same site as resveratrol (amino acids 183–225 in the NH2-terminus) (80). SRT1720 reduces myocardial injury in ischemic hearts in mice (136). SRT1720 also reduces whole body fat mass and raises serum HDL (81). Recently, several clinical trials on STACs have been reported. Longevinex, a modified resveratrol, specifically improves endothelial function in subjects with metabolic syndrome (27). SRT2104, a newly developed compound for attenuating inflammatory colitis, was demonstrated to improve lipid profiles in otherwise healthy cigarette smokers (139). SRT2104 was also associated with improvement in lipid profiles in a phase-II, randomized, placebo-controlled, double-blind, multidose study of subjects with type 2 diabetes (10). Although there are some STACs, such as Longevinex, that stimulate not only Sirt1 but also Sirt3 (85), almost all sirtuin activators analyzed thus far have only been reported to affect Sirt1. While increased Sirt1 activity is beneficial in cardiovascular disease, sirtuin inhibitors can potentially be useful as therapeutic agents for cancer. Small-molecule inhibitors of Sirt2 are available and exhibit neuroprotective properties in a cellular model of Parkinson's disease (98).
As described above, abundant data indicate that multiple diseases of aging can be prevented or reversed by small molecules in rodents and primates. Sirt1 activators have the most potential to be agents for treatment of cardiovascular disease. However, despite recent progress, many questions regarding the precise mechanism of direct activation of Sirt1 by STACs, the optimal dose and timing for application to humans, and the existence of endogenous activators that bind to the same site as STACs remain. Thus further investigation of STACs will be needed in the future.
Conclusions
In general, stimulation of longevity mechanisms is thought to be beneficial in the context of age-associated diseases. Sirtuins have been shown to be intimately involved in cell survival and longevity. Increasing lines of evidence suggest that Sirt1 protects the heart from several stresses, including I/R injury, hypertrophic stimuli, and oxidative injury. Stimulation of Sirt1 activity leads to preservation of energy production and mitochondrial function, antiapoptotic effects, scavenging of ROS, and regulation of autophagy in the heart. Sirt3 also appears to be a potential target for cardiovascular disease treatment, mainly because of its protective effects upon mitochondrial function. Although Sirts4-7 also have beneficial effects in cardiovascular disease, Sirt2 appears to have the opposite effect in the heart (Table). So far, Sirt1 and Sirt3 have been well investigated and documented, and activating compounds for these proteins have therapeutic potential for cardiovascular diseases such as metabolic disease, ischemic heart disease, and heart failure. However, the balance between the dosage of the longevity mechanism and the pathogenesis of the cardiovascular disease must be carefully evaluated to obtain the beneficial effects but eliminate the undesirable side effects of sirtuins.
Table 1.
Diverse roles of sirtuins in the heart
| Roles of Sirtuins in the Heart | |
|---|---|
| Energy metabolism | • Sirt1: FFA↑ • Sirt3: ATP production↑ |
| • Sirt2, Sirt4, Sirt5, Sirt6, and Sirt7: unknown | |
| Cardiac hypertrophy | • Sirt1: antihypertrophic (high level of Sirt1: hypertrophic) • Sirt3: antihypertrophic |
| • Sirt6: antihypertrophic • Sirt2, Sirt4, Sirt5, and Sirt7: unknown | |
| Heart failure | • Sirt1: protective (high level of Sirt1: detrimental) • Sirt3: protective |
| • Sirt2, Sirt4, Sirt5, Sirt6, and Sirt7: unknown | |
| Ischemia-reperfusion injury | • Sirt1: protective • Sirt2: detrimental • Sirt3: protective • Sirt4: protective (hypoxia) |
| • Sirt5: protective (hypoxia) • Sirt5 and Sirt7: unknown | |
| Oxidative stress | • Sirt1: oxidative stress↓ (high level of Sirt1: oxidative stress↑) |
| • Sirt2: oxidative stress ↓ or ↑ • Sirt3: oxidative stress↓ | |
| • Sirt5, Sirt6, and Sirt7: protective against oxidative stress • Sirt4: unknown | |
| Cell death | • Sirt1, Sirt3, Sirt4, Sirt5, Sirt6, and Sirt7: negative regulator |
| • Sirt2: positive regulator | |
| Autophagy | • Sirt1: autophagy↑ • Sirt2: autophagy↑ • Sirt3 and Sirt6: autophagy↑ |
| • Sirt4, Sirt5, and Sirt7: unknown |
Sirtuins are closely involved in energy metabolism, cardiac hypertrophy, heart failure, ischemia-reperfusion injury, oxidative stress, cell death, and autophagy. silent mating-type information regulation (Sirt) 1 and Sirt3 have been well investigated and documented. Overall, Sirt1 and Sirt3-7 are thought to have beneficial effects in cardiovascular disease, whereas Sirt2 appears to be detrimental. Upregulation of Sirt1 may have detrimental effects in the heart in response to pressure overload. FFA, free fatty acid.
An important unresolved question in the field of sirtuin research in the heart is whether sirtuins act through direct modification of histones and, if so, at what regions of the genome. Most of the observations relating acetyl-transferring enzymes to cardiac phenotype have been made without an understanding of the totality of molecular targets. Further investigations are needed to address the molecular mechanisms mediating the actions of sirtuins in the heart and elucidate their functional significance.
GRANTS
This work was supported in part by National Institutes of Health Grants HL067724, HL091469, HL102738, HL112330, and AG023039 (to J. Sadoshima), the Fondation Leducq Transatlantic Networks of Excellence (to J. Sadoshima), and an American Heart Association postdoctoral fellowship (to S. Matsushima).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.M. and J.S. conception and design of research; S.M. prepared figures; S.M. and J.S. drafted manuscript; S.M. and J.S. edited and revised manuscript; S.M. and J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Christopher Brady and Daniela Zablocki for critical reading of the manuscript.
REFERENCES
- 1.Abdelmohsen K, Pullmann R Jr, Lal A, Kim HH, Galban S, Yang X, Blethrow JD, Walker M, Shubert J, Gillespie DA, Furneaux H, Gorospe M. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell 25: 543–557, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106: 1253–1264, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ago T, Liu T, Zhai P, Chen W, Li H, Molkentin JD, Vatner SF, Sadoshima J. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell 133: 978–993, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA 105: 14447–14452, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 100: 1512–1521, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Alcendor RR, Kirshenbaum LA, Imai S, Vatner SF, Sadoshima J. Silent information regulator 2alpha, a longevity factor and class III histone deacetylase, is an essential endogenous apoptosis inhibitor in cardiac myocytes. Circ Res 95: 971–980, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res 72: 463–469, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134: 317–328, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res 98: 15–24, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Baksi A, Kraydashenko O, Zalevkaya A, Stets R, Elliott P, Haddad J, Hoffmann E, Vlasuk GP, Jacobson EW. A phase II, randomized, placebo-controlled, double-blind, multi-dose study of SRT2104, a SIRT1 activator, in subjects with type 2 diabetes. Br J Clin Pharmacol 78: 69–77, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444: 337–342, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5: 493–506, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Becatti M, Taddei N, Cecchi C, Nassi N, Nassi PA, Fiorillo C. SIRT1 modulates MAPK pathways in ischemic-reperfused cardiomyocytes. Cell Mol Life Sci 69: 2245–2260, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303: 2011–2015, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Buler M, Aatsinki SM, Izzi V, Uusimaa J, Hakkola J. SIRT5 is under the control of PGC-1alpha and AMPK and is involved in regulation of mitochondrial energy metabolism. FASEB J 28: 3225–3237, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell 123: 437–448, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci USA 100: 10794–10799, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305: 390–392, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci 67: 811–820, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci USA 101: 10042–10047, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dammrich J, Pfeifer U. Cardiac hypertrophy in rats after supravalvular aortic constriction. II. Inhibition of cellular autophagy in hypertrophying cardiomyocytes. Virchows Arch B Cell Pathol Incl Mol Pathol 43: 287–307, 1983. [DOI] [PubMed] [Google Scholar]
- 22.Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science 324: 1289–1293, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Dominy JE Jr, Lee Y, Jedrychowski MP, Chim H, Jurczak MJ, Camporez JP, Ruan HB, Feldman J, Pierce K, Mostoslavsky R, Denu JM, Clish CB, Yang X, Shulman GI, Gygi SP, Puigserver P. The deacetylase Sirt6 activates the acetyltransferase GCN5 and suppresses hepatic gluconeogenesis. Mol Cell 48: 900–913, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med 17: 1391–1401, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, McGrew K, Longo VD. Sir2 blocks extreme life-span extension. Cell 123: 655–667, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature 460: 587–591, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujitaka K, Otani H, Jo F, Jo H, Nomura E, Iwasaki M, Nishikawa M, Iwasaka T, Das DK. Modified resveratrol Longevinex improves endothelial function in adults with metabolic syndrome receiving standard treatment. Nutr Res 31: 842–847, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Fukuda S, Kaga S, Zhan L, Bagchi D, Das DK, Bertelli A, Maulik N. Resveratrol ameliorates myocardial damage by inducing vascular endothelial growth factor-angiogenesis and tyrosine kinase receptor Flk-1. Cell Biochem Biophys 44: 43–49, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell 12: 51–62, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466: 1105–1109, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gertz M, Nguyen GT, Fischer F, Suenkel B, Schlicker C, Franzel B, Tomaschewski J, Aladini F, Becker C, Wolters D, Steegborn C. A molecular mechanism for direct sirtuin activation by resveratrol. PloS One 7: e49761, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, Wolberger C, Prolla TA, Weindruch R, Alt FW, Guarente L. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 126: 941–954, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem 281: 29776–29787, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Hamamori Y, Schneider MD. HATs off to Hop: recruitment of a class I histone deacetylase incriminates a novel transcriptional pathway that opposes cardiac hypertrophy. J Clin Invest 112: 824–826, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ Res 107: 1470–1482, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464: 121–125, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191–196, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res 105: 481–491, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 122: 2170–2182, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci 120: 2479–2487, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, ESY, Lamming DW, Pentelute BL, Schuman ER, Stevens LA, Ling AJ, Armour SM, Michan S, Zhao H, Jiang Y, Sweitzer SM, Blum CA, Disch JS, Ng PY, Howitz KT, Rolo AP, Hamuro Y, Moss J, Perni RB, Ellis JL, Vlasuk GP, Sinclair DA. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 339: 1216–1219, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hung LM, Su MJ, Chen JK. Resveratrol protects myocardial ischemia-reperfusion injury through both NO-dependent and NO-independent mechanisms. Free Radic Biol Med 36: 774–781, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, Utsumi H, Hamasaki N, Takeshita A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res 88: 529–535, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403: 795–800, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Inoue T, Nakayama Y, Li Y, Matsumori H, Takahashi H, Kojima H, Wanibuchi H, Katoh M, Oshimura M. SIRT2 knockdown increases basal autophagy and prevents postslippage death by abnormally prolonging the mitotic arrest that is induced by microtubule inhibitors. FEBS J 281: 2623–2637, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Jiang H, Khan S, Wang Y, Charron G, He B, Sebastian C, Du J, Kim R, Ge E, Mostoslavsky R, Hang HC, Hao Q, Lin H. SIRT6 regulates TNF-alpha secretion through hydrolysis of long-chain fatty acyl lysine. Nature 496: 110–113, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Judge S, Leeuwenburgh C. Cardiac mitochondrial bioenergetics, oxidative stress, and aging. Am J Physiol Cell Physiol 292: C1983–C1992, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Kajstura J, Cheng W, Sarangarajan R, Li P, Li B, Nitahara JA, Chapnick S, Reiss K, Olivetti G, Anversa P. Necrotic and apoptotic myocyte cell death in the aging heart of Fischer 344 rats. Am J Physiol Heart Circ Physiol 271: H1215–H1228, 1996. [DOI] [PubMed] [Google Scholar]
- 49.Kang YJ, Chen Y, Epstein PN. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. J Biol Chem 271: 12610–12616, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Kim HS, Xiao C, Wang RH, Lahusen T, Xu X, Vassilopoulos A, Vazquez-Ortiz G, Jeong WI, Park O, Ki SH, Gao B, Deng CX. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab 12: 224–236, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature 451: 583–586, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Kirsh O, Seeler JS, Pichler A, Gast A, Muller S, Miska E, Mathieu M, Harel-Bellan A, Kouzarides T, Melchior F, Dejean A. The SUMO E3 ligase RanBP2 promotes modification of the HDAC4 deacetylase. EMBO J 21: 2682–2691, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114: 1299–1307, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krishnan J, Danzer C, Simka T, Ukropec J, Walter KM, Kumpf S, Mirtschink P, Ukropcova B, Gasperikova D, Pedrazzini T, Krek W. Dietary obesity-associated Hif1alpha activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes Dev 26: 259–270, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins–novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov 7: 841–853, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci USA 105: 3374–3379, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet 11: 376–381, 1995. [DOI] [PubMed] [Google Scholar]
- 58.Liang Q, Benavides GA, Vassilopoulos A, Gius D, Darley-Usmar V, Zhang J. Bioenergetic and autophagic control by Sirt3 in response to nutrient deprivation in mouse embryonic fibroblasts. Biochem J 454: 249–257, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 418: 344–348, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Liu B, Che W, Xue J, Zheng C, Tang K, Zhang J, Wen J, Xu Y. SIRT4 prevents hypoxia-induced apoptosis in H9c2 cardiomyoblast cells. Cell Physiol Biochem 32: 655–662, 2013. [DOI] [PubMed] [Google Scholar]
- 61.Liu B, Che W, Zheng C, Liu W, Wen J, Fu H, Tang K, Zhang J, Xu Y. SIRT5: a safeguard against oxidative stress-induced apoptosis in cardiomyocytes. Cell Physiol Biochem 32: 1050–1059, 2013. [DOI] [PubMed] [Google Scholar]
- 62.Liu J, Wu X, Wang X, Zhang Y, Bu P, Zhang Q, Jiang F. Global gene expression profiling reveals functional importance of Sirt2 in endothelial cells under oxidative stress. Int J Mol Sci 14: 5633–5649, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loch T, Vakhrusheva O, Piotrowska I, Ziolkowski W, Ebelt H, Braun T, Bober E. Different extent of cardiac malfunction and resistance to oxidative stress in heterozygous and homozygous manganese-dependent superoxide dismutase-mutant mice. Cardiovasc Res 82: 448–457, 2009. [DOI] [PubMed] [Google Scholar]
- 64.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV Jr, Weissman S, Verdin E, Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 27: 8807–8814, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107: 137–148, 2001. [DOI] [PubMed] [Google Scholar]
- 66.Luo X, Yang Z, Zheng S, Cao Y, Wu Y. Sirt3 activation attenuated oxidized low-density lipoprotein-induced human umbilical vein endothelial cells' apoptosis by sustaining autophagy. Cell Biol Int. 2014 May 5. doi: 10.1002/cbin.10291 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 67.Lynn EG, McLeod CJ, Gordon JP, Bao J, Sack MN. SIRT2 is a negative regulator of anoxia-reoxygenation tolerance via regulation of 14-3-3 zeta and BAD in H9c2 cells. FEBS Lett 582: 2857–2862, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maejima Y, Kuroda J, Matsushima S, Ago T, Sadoshima J. Regulation of myocardial growth and death by NADPH oxidase. J Mol Cell Cardiol 50: 408–416, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mahlknecht U, Voelter-Mahlknecht S. Fluorescence in situ hybridization and chromosomal organization of the sirtuin 4 gene (Sirt4) in the mouse. Biochem Biophys Res Commun 382: 685–690, 2009. [DOI] [PubMed] [Google Scholar]
- 70.Maizel J, Xavier S, Chen J, Lin CH, Vasko R, Goligorsky MS. Sirtuin 1 ablation in endothelial cells is associated with impaired angiogenesis and diastolic dysfunction. Am J Physiol Heart Circ Physiol 307: H1691–H1704, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol 146: 3–15, 1995. [PMC free article] [PubMed] [Google Scholar]
- 72.Maksin-Matveev A, Kanfi Y, Hochhauser E, Isak A, Cohen HY, Shainberg A. Sirtuin 6 protects the heart from hypoxic damage. Exp Cell Res 330: 81–90, 2015. [DOI] [PubMed] [Google Scholar]
- 73.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6: 458–471, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Mao Z, Hine C, Tian X, Van Meter M, Au M, Vaidya A, Seluanov A, Gorbunova V. SIRT6 promotes DNA repair under stress by activating PARP1. Science 332: 1443–1446, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Masri S, Rigor P, Cervantes M, Ceglia N, Sebastian C, Xiao C, Roqueta-Rivera M, Deng C, Osborne TF, Mostoslavsky R, Baldi P, Sassone-Corsi P. Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell 158: 659–672, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Masri S, Sassone-Corsi P. Sirtuins and the circadian clock: bridging chromatin and metabolism. Sci Signal 7: re6, 2014. [DOI] [PubMed] [Google Scholar]
- 77.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 100: 914–922, 2007. [DOI] [PubMed] [Google Scholar]
- 78.Matsushima S, Ide T, Yamato M, Matsusaka H, Hattori F, Ikeuchi M, Kubota T, Sunagawa K, Hasegawa Y, Kurihara T, Oikawa S, Kinugawa S, Tsutsui H. Overexpression of mitochondrial peroxiredoxin-3 prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation 113: 1779–1786, 2006. [DOI] [PubMed] [Google Scholar]
- 79.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104: 14855–14860, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450: 712–716, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin YK, Canto C, Scheibye-Knudsen M, Krawczyk M, Irusta PM, Martin-Montalvo A, Hubbard BP, Zhang Y, Lehrmann E, White AA, Price NL, Swindell WR, Pearson KJ, Becker KG, Bohr VA, Gorospe M, Egan JM, Talan MI, Auwerx J, Westphal CH, Ellis JL, Ungvari Z, Vlasuk GP, Elliott PJ, Sinclair DA, de Cabo R. SRT1720 improves survival and healthspan of obese mice. Sci Rep 1: 70, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morselli E, Marino G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, Cabrera S, Benit P, Rustin P, Criollo A, Kepp O, Galluzzi L, Shen S, Malik SA, Maiuri MC, Horio Y, Lopez-Otin C, Andersen JS, Tavernarakis N, Madeo F, Kroemer G. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol 192: 615–629, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell 116: 551–563, 2004. [DOI] [PubMed] [Google Scholar]
- 84.Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab 2: 105–117, 2005. [DOI] [PubMed] [Google Scholar]
- 85.Mukherjee S, Ray D, Lekli I, Bak I, Tosaki A, Das DK. Effects of Longevinex (modified resveratrol) on cardioprotection and its mechanisms of action. Can J Physiol Pharmacol 88: 1017–1025, 2010. [DOI] [PubMed] [Google Scholar]
- 86.Nadal-Ginard B, Kajstura J, Anversa P, Leri A. A matter of life and death: cardiac myocyte apoptosis and regeneration. J Clin Invest 111: 1457–1459, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nadtochiy SM, Redman E, Rahman I, Brookes PS. Lysine deacetylation in ischaemic preconditioning: the role of SIRT1. Cardiovasc Res 89: 643–649, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nadtochiy SM, Yao H, McBurney MW, Gu W, Guarente L, Rahman I, Brookes PS. SIRT1-mediated acute cardioprotection. Am J Physiol Heart Circ Physiol 301: H1506–H1512, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134: 329–340, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 13: 619–624, 2007. [DOI] [PubMed] [Google Scholar]
- 91.Nasrin N, Kaushik VK, Fortier E, Wall D, Pearson KJ, de Cabo R, Bordone L. JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PloS One 4: e8414, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science 306: 2105–2108, 2004. [DOI] [PubMed] [Google Scholar]
- 93.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem 280: 16456–16460, 2005. [DOI] [PubMed] [Google Scholar]
- 94.Neubauer S. The failing heart–an engine out of fuel. N Engl J Med 356: 1140–1151, 2007. [DOI] [PubMed] [Google Scholar]
- 95.Nishino I, Fu J, Tanji K, Yamada T, Shimojo S, Koori T, Mora M, Riggs JE, Oh SJ, Koga Y, Sue CM, Yamamoto A, Murakami N, Shanske S, Byrne E, Bonilla E, Nonaka I, DiMauro S, Hirano M. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease). Nature 406: 906–910, 2000. [DOI] [PubMed] [Google Scholar]
- 96.Ohsawa S, Miura M. Caspase-mediated changes in Sir2alpha during apoptosis. FEBS Lett 580: 5875–5879, 2006. [DOI] [PubMed] [Google Scholar]
- 97.Oka S, Alcendor R, Zhai P, Park JY, Shao D, Cho J, Yamamoto T, Tian B, Sadoshima J. PPARalpha-Sirt1 complex mediates cardiac hypertrophy and failure through suppression of the ERR transcriptional pathway. Cell Metab 14: 598–611, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson's disease. Science 317: 516–519, 2007. [DOI] [PubMed] [Google Scholar]
- 99.Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem 285: 8340–8351, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148: 421–433, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 81: 1161–1172, 1990. [DOI] [PubMed] [Google Scholar]
- 102.Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J Biol Chem 280: 43121–43130, 2005. [DOI] [PubMed] [Google Scholar]
- 103.Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pillai JB, Samant S, Ravindra PV, Isbatan A, Gupta MP. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem 285: 3133–3144, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Planavila A, Dominguez E, Navarro M, Vinciguerra M, Iglesias R, Giralt M, Lope-Piedrafita S, Ruberte J, Villarroya F. Dilated cardiomyopathy and mitochondrial dysfunction in Sirt1-deficient mice: a role for Sirt1-Mef2 in adult heart. J Mol Cell Cardiol 53: 521–531, 2012. [DOI] [PubMed] [Google Scholar]
- 105.Planavila A, Iglesias R, Giralt M, Villarroya F. Sirt1 acts in association with PPARalpha to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovasc Res 90: 276–284, 2011. [DOI] [PubMed] [Google Scholar]
- 106.Porter GA, Urciuoli WR, Brookes PS, Nadtochiy SM. SIRT3 deficiency exacerbates ischemia-reperfusion injury: implication for aged hearts. Am J Physiol Heart Circ Physiol 306: H1602–H1609, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev 21: 2644–2658, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Potthoff MJ, Wu H, Arnold MA, Shelton JM, Backs J, McAnally J, Richardson JA, Bassel-Duby R, Olson EN. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J Clin Invest 117: 2459–2467, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Puca R, Nardinocchi L, Starace G, Rechavi G, Sacchi A, Givol D, D'Orazi G. Nox1 is involved in p53 deacetylation and suppression of its transcriptional activity and apoptosis. Free Radic Biol Med 48: 1338–1346, 2010. [DOI] [PubMed] [Google Scholar]
- 110.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12: 662–667, 2010. [DOI] [PubMed] [Google Scholar]
- 111.Rajamohan SB, Pillai VB, Gupta M, Sundaresan NR, Birukov KG, Samant S, Hottiger MO, Gupta MP. SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly(ADP-ribose) polymerase 1. Mol Cell Biol 29: 4116–4129, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ramakrishnan G, Davaakhuu G, Kaplun L, Chung WC, Rana A, Atfi A, Miele L, Tzivion G. Sirt2 deacetylase is a novel AKT binding partner critical for AKT activation by insulin. J Biol Chem 289: 6054–6066, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324: 651–654, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rimbaud S, Ruiz M, Piquereau J, Mateo P, Fortin D, Veksler V, Garnier A, Ventura-Clapier R. Resveratrol improves survival, hemodynamics and energetics in a rat model of hypertension leading to heart failure. PloS One 6: e26391, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rine J, Strathern JN, Hicks JB, Herskowitz I. A suppressor of mating-type locus mutations in Saccharomyces cerevisiae: evidence for and identification of cryptic mating-type loci. Genetics 93: 877–901, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434: 113–118, 2005. [DOI] [PubMed] [Google Scholar]
- 117.Sadoshima J. Sirt3 targets mPTP and prevents aging in the heart. Aging (Albany NY) 3: 12–13, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sakamoto J, Miura T, Shimamoto K, Horio Y. Predominant expression of Sir2alpha, an NAD-dependent histone deacetylase, in the embryonic mouse heart and brain. FEBS Lett 556: 281–286, 2004. [DOI] [PubMed] [Google Scholar]
- 119.Scher MB, Vaquero A, Reinberg D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev 21: 920–928, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shalwala M, Zhu SG, Das A, Salloum FN, Xi L, Kukreja RC. Sirtuin 1 (SIRT1) activation mediates sildenafil induced delayed cardioprotection against ischemia-reperfusion injury in mice. PloS One 9: e86977, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shao D, Oka S, Brady CD, Haendeler J, Eaton P, Sadoshima J. Redox modification of cell signaling in the cardiovascular system. J Mol Cell Cardiol 52: 550–558, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol 295: H2348–H2355, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 115: 2108–2118, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 119: 2758–2771, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sundaresan NR, Pillai VB, Wolfgeher D, Samant S, Vasudevan P, Parekh V, Raghuraman H, Cunningham JM, Gupta M, Gupta MP. The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Sci Signal 4: ra46, 2011. [DOI] [PubMed] [Google Scholar]
- 126.Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol 28: 6384–6401, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sundaresan NR, Vasudevan P, Zhong L, Kim G, Samant S, Parekh V, Pillai VB, Ravindra PV, Gupta M, Jeevanandam V, Cunningham JM, Deng CX, Lombard DB, Mostoslavsky R, Gupta MP. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat Med 18: 1643–1650, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Takahashi T, Schunkert H, Isoyama S, Wei JY, Nadal-Ginard B, Grossman W, Izumo S. Age-related differences in the expression of proto-oncogene and contractile protein genes in response to pressure overload in the rat myocardium. J Clin Invest 89: 939–946, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Takasaka N, Araya J, Hara H, Ito S, Kobayashi K, Kurita Y, Wakui H, Yoshii Y, Yumino Y, Fujii S, Minagawa S, Tsurushige C, Kojima J, Numata T, Shimizu K, Kawaishi M, Kaneko Y, Kamiya N, Hirano J, Odaka M, Morikawa T, Nishimura SL, Nakayama K, Kuwano K. Autophagy induction by SIRT6 through attenuation of insulin-like growth factor signaling is involved in the regulation of human bronchial epithelial cell senescence. J Immunol 192: 958–968, 2014. [DOI] [PubMed] [Google Scholar]
- 130.Tanno M, Kuno A, Horio Y, Miura T. Emerging beneficial roles of sirtuins in heart failure. Basic Res Cardiol 107: 273, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tanno M, Kuno A, Yano T, Miura T, Hisahara S, Ishikawa S, Shimamoto K, Horio Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem 285: 8375–8382, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem 282: 6823–6832, 2007. [DOI] [PubMed] [Google Scholar]
- 133.Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, Olivier AK, Spitz DR, Gius D. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell 40: 893–904, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tasdemir E, Maiuri MC, Galluzzi L, Vitale I, Djavaheri-Mergny M, D'Amelio M, Criollo A, Morselli E, Zhu C, Harper F, Nannmark U, Samara C, Pinton P, Vicencio JM, Carnuccio R, Moll UM, Madeo F, Paterlini-Brechot P, Rizzuto R, Szabadkai G, Pierron G, Blomgren K, Tavernarakis N, Codogno P, Cecconi F, Kroemer G. Regulation of autophagy by cytoplasmic p53. Nat Cell Biol 10: 676–687, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thandapilly SJ, Wojciechowski P, Behbahani J, Louis XL, Yu L, Juric D, Kopilas MA, Anderson HD, Netticadan T. Resveratrol prevents the development of pathological cardiac hypertrophy and contractile dysfunction in the SHR without lowering blood pressure. Am J Hypertens 23: 192–196, 2010. [DOI] [PubMed] [Google Scholar]
- 136.Tong C, Morrison A, Mattison S, Qian S, Bryniarski M, Rankin B, Wang J, Thomas DP, Li J. Impaired SIRT1 nucleocytoplasmic shuttling in the senescent heart during ischemic stress. FASEB J 27: 4332–4342, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res 102: 703–710, 2008. [DOI] [PubMed] [Google Scholar]
- 138.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107: 149–159, 2001. [DOI] [PubMed] [Google Scholar]
- 139.Venkatasubramanian S, Noh RM, Daga S, Langrish JP, Joshi NV, Mills NL, Hoffmann E, Jacobson EW, Vlasuk GP, Waterhouse BR, Lang NN, Newby DE. Cardiovascular effects of a novel SIRT1 activator, SRT2104, in otherwise healthy cigarette smokers. J Am Heart Assoc 2: e000042, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang C, Chen L, Hou X, Li Z, Kabra N, Ma Y, Nemoto S, Finkel T, Gu W, Cress WD, Chen J. Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nat Cell Biol 8: 1025–1031, 2006. [DOI] [PubMed] [Google Scholar]
- 141.Wang P, Guan YF, Du H, Zhai QW, Su DF, Miao CY. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy 8: 77–87, 2012. [DOI] [PubMed] [Google Scholar]
- 142.Wang YP, Zhou LS, Zhao YZ, Wang SW, Chen LL, Liu LX, Ling ZQ, Hu FJ, Sun YP, Zhang JY, Yang C, Yang Y, Xiong Y, Guan KL, Ye D. Regulation of G6PD acetylation by SIRT2 and KAT9 modulates NADPH homeostasis and cell survival during oxidative stress. EMBO J 33: 1304–1320, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson's disease. Neurosignals 19: 163–174, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xie M, Kong Y, Tan W, May H, Battiprolu PK, Pedrozo Z, Wang ZV, Morales C, Luo X, Cho G, Jiang N, Jessen ME, Warner JJ, Lavandero S, Gillette TG, Turer AT, Hill JA. Histone deacetylase inhibition blunts ischemia/reperfusion injury by inducing cardiomyocyte autophagy. Circulation 129: 1139–1151, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu P, Das M, Reilly J, Davis RJ. JNK regulates FoxO-dependent autophagy in neurons. Genes Dev 25: 310–322, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA 105: 13421–13426, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PloS One 9: e98972, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang Y, Fu W, Chen J, Olashaw N, Zhang X, Nicosia SV, Bhalla K, Bai W. SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat Cell Biol 9: 1253–1262, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yoshizawa T, Karim MF, Sato Y, Senokuchi T, Miyata K, Fukuda T, Go C, Tasaki M, Uchimura K, Kadomatsu T, Tian Z, Smolka C, Sawa T, Takeya M, Tomizawa K, Ando Y, Araki E, Akaike T, Braun T, Oike Y, Bober E, Yamagata K. SIRT7 controls hepatic lipid metabolism by regulating the ubiquitin-proteasome pathway. Cell Metab 19: 712–721, 2014. [DOI] [PubMed] [Google Scholar]
- 150.Zarzuelo MJ, Lopez-Sepulveda R, Sanchez M, Romero M, Gomez-Guzman M, Ungvary Z, Perez-Vizcaino F, Jimenez R, Duarte J. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol 85: 1288–1296, 2013. [DOI] [PubMed] [Google Scholar]
- 151.Zeng H, Li L, Chen JX. Loss of sirt3 limits bone marrow cell-mediated angiogenesis and cardiac repair in post-myocardial infarction. PloS One 9: e107011, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110: 479–488, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang J. Are poly(ADP-ribosyl)ation by PARP-1 and deacetylation by Sir2 linked? Bioessays 25: 808–814, 2003. [DOI] [PubMed] [Google Scholar]
- 154.Zhang Q, Wang SY, Fleuriel C, Leprince D, Rocheleau JV, Piston DW, Goodman RH. Metabolic regulation of SIRT1 transcription via a HIC1:CtBP corepressor complex. Proc Natl Acad Sci USA 104: 829–833, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 155.Zhao W, Kruse JP, Tang Y, Jung SY, Qin J, Gu W. Negative regulation of the deacetylase SIRT1 by DBC1. Nature 451: 587–590, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]