Mitochondrial respiration and protein mass are higher in female cerebral arteries. Mitochondrial depolarization by diazoxide only affected mitochondrial respiration in female arteries, but mitochondrial respiration increased dramatically in both sexes after nitric oxide synthase inhibition. New insights regarding the mechanisms of sex-related differences in health, and perhaps disease, are provided.
Keywords: ATP production, mitochondria, mitochondrial respiration, mitoKATP channels, oxidative phosphorylation, voltage-dependent anion channel, nitric oxide
Abstract
Mitochondrial respiration has never been directly examined in intact cerebral arteries. We tested the hypothesis that mitochondrial energetics of large cerebral arteries ex vivo are sex dependent. The Seahorse XFe24 analyzer was used to examine mitochondrial respiration in isolated cerebral arteries from adult male and female Sprague-Dawley rats. We examined the role of nitric oxide (NO) on mitochondrial respiration under basal conditions, using Nω-nitro-l-arginine methyl ester, and following pharmacological challenge using diazoxide (DZ), and also determined levels of mitochondrial and nonmitochondrial proteins using Western blot, and vascular diameter responses to DZ. The components of mitochondrial respiration including basal respiration, ATP production, proton leak, maximal respiration, and spare respiratory capacity were elevated in females compared with males, but increased in both male and female arteries in the presence of the NOS inhibitor. Although acute DZ treatment had little effect on mitochondrial respiration of male arteries, it decreased the respiration in female arteries. Levels of mitochondrial proteins in Complexes I–V and the voltage-dependent anion channel protein were elevated in female compared with male cerebral arteries. The DZ-induced vasodilation was greater in females than in males. Our findings show that substantial sex differences in mitochondrial respiratory dynamics exist in large cerebral arteries and may provide the mechanistic basis for observations that the female cerebral vasculature is more adaptable after injury.
NEW & NOTEWORTHY
Mitochondrial respiration and protein mass are higher in female cerebral arteries. Mitochondrial depolarization by diazoxide only affected mitochondrial respiration in female arteries, but mitochondrial respiration increased dramatically in both sexes after nitric oxide synthase inhibition. New insights regarding the mechanisms of sex-related differences in health, and perhaps disease, are provided.
mitochondria are dual-membrane, dynamic organelles that provide energy for ATP-dependent processes via oxidative phosphorylation (10). The inner mitochondrial membrane contains the respiratory chain complexes (Complex I–IV) and the ATP synthase, Complex V. Under physiological conditions the electron transport chain produces a proton gradient across the inner mitochondrial membrane leading to ATP production (1). Individual respiratory chain proteins are encoded by either nuclear or mitochondrial genomes (11, 20, 51). The outer membrane contains the voltage-dependent anion channel (VDAC) and the proteins comprising this channel are nuclear encoded (35). Intricate signaling as well as coordinating and transport mechanisms, which are not fully elucidated, ensure the proper composition and functioning of essential elements of the electron transport chain.
We and other laboratories have shown that mitochondria play an important role in physiological and pathophysiological functions of the cerebral circulation (9, 49). Under normal conditions, the mitochondria-dependent vasodilation of large cerebral arteries by diazoxide (DZ), which opens the ATP-sensitive potassium (mitoKATP) channels located on the inner mitochondrial membrane (23, 28), has endothelial and vascular smooth muscle (VSM) components that contribute to the overall, integrated vascular response in male rats (12, 33, 58). Diseases such as insulin resistance and experimental stroke affect the expression of mitochondrial proteins as well as mitochondria-dependent vascular responses (32, 49). In male rats and neonatal pigs, mitoKATP channel activation prior to transient global ischemia or transient middle cerebral artery occlusion reduces brain infarct volume, preserves the normal responsiveness of large cerebral arteries to vasoactive stimuli, and protects the blood-brain barrier (15, 31, 34, 42).
Mitochondrial function has not been sufficiently examined in female cerebral arteries. Previous investigations have shown that estrogen enhances the expression of mitochondrial proteins and suppresses mitochondria-derived oxidative stress while increasing the synthesis of substances such as nitric oxide (NO), which might affect mitochondrial respiratory rate in cerebral vascular cells (2, 18, 22, 24). The suggestion that estrogen increases efficiency of mitochondrial respiration appears to be based on evaluations of mitochondrial protein levels of cerebral vascular cells rather than by direct measurements. Thus there has been no specific research concerning whether mitochondrial respiration is different in male and female cerebral arteries (39). Furthermore, whether mitochondrial function is affected by exogenous agents which depolarize mitochondria, such as DZ, or by inhibition of nitric oxide synthase (NOS) is also not known.
We have examined, for the first time, whether the mitochondrial energetics of large cerebral arteries are sex dependent and regulated via mitochondrial membrane potential and NO. Using large cerebral arteries, ex vivo, from male and female rats, we determined 1) the mitochondrial oxygen consumption rate (OCR) under basal conditions and after pharmacological challenge using a mitochondria-specific drug, DZ, in the presence and absence of the NOS inhibitor, Nω-nitro-l-arginine methyl ester (l-NAME); 2) the levels of mitochondrial and nonmitochondrial proteins relevant to mitochondrial function using Western blots; and 3) the vascular responses of isolated, pressurized cerebral arteries to DZ.
MATERIALS AND METHODS
Animals.
Male and female, 8- to 10-wk-old Sprague-Dawley rats (SD) from Charles River Laboratories (Wilmington, MA) (n = 38 and 40, respectively) were randomly assigned to the experimental protocols. Animals were housed and cared for according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Tulane University in compliance with NIH guidelines. Animal care was provided by the Department of Comparative Medicine. Standard food and water were given ad libitum. Under 5% isoflurane (VetOne, Boise, ID)-induced anesthesia, rats were decapitated and brains were removed and transferred to 4°C, oxygenated (20% O2, 5% CO2, 75% N2) Ca2+ Krebs solution (mmol/l: 110 NaCl, 5.0 KCl, 2.5 CaCl2, 1.0 MgSO4, 1.0 KH2PO4, 5.0 glucose, and 24.0 NaHCO3) at 7.4 pH. This mode of anesthesia was chosen to minimize animal discomfort as recommended by the IACUC. The anterior, middle cerebral, and basilar arteries were isolated, removed, and cleaned for the experiments described below.
Electron microscopy.
Rats were euthanized with anesthesia and perfused with a PBS solution containing 2% of glutaraldehyde and 3% of formaldehyde. Arteries were removed and kept in the perfusion solution for 1 h and postfixed in 1% osmium tetroxide and embedded in Spurr's resin. Ultrathin sections (80–90 nm) were mounted on formvar-coated copper grids (200 mesh), air dried, and stained with uranyl acetate and lead citrate (at 7 min and 7 min, respectively). The sections were put on grids and viewed at a magnification of 11,000× using a FEI Tecnai BioTwin 120 keV TEM with a digital imaging setup (Wake Forest University Health Sciences, Winston-Salem, NC).
Measurement of mitochondrial function.
Arteries were transferred into an XF24 islet capture microplate (no. 101122-100, Seahorse Bioscience). The Seahorse Bioscience XFe24 extracellular flux analyzer measured mitochondrial OCR, an indicator of mitochondrial respiration (8, 25, 29). The Seahorse XFe24 analyzer uses oxygen and hydrogen ion sensitive fluorophores for repeated measurements of oxygen and proton concentrations in the assay medium surrounding the arteries. The assay cartridge plate (no. 100867-100, Seahorse Bioscience) was hydrated overnight using an XF calibration solution (no. 100867-000, Seahorse Bioscience) at 37°C in a non-carbon dioxide incubator. Seahorse XF Assay medium (no. 102365-100, Seahorse Bioscience), containing 5.0 mmol/l glucose and 2.0 mmol/l pyruvate at pH 7.4 and 37°C, was used for the experiments. Isolated cerebral arteries were placed in the bottom of the islet plate wells and covered with a screen to minimize movement during the assay. Wells were filled with 525 μl XF assay medium and maintained at 37°C in a non-CO2 incubator for 20 min and then the islet plate was inserted into the instrument. Our assay protocol included three cycles for baseline measurements, and then five cycles for each treatment (Fig. 1). For the assay, plate wells were loaded with a final concentration of 250 μmol/l DZ or an equivalent amount of vehicle (DMSO), and then exposed sequentially to (in μmol/l) 2 oligomycin, 1 carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP), or 1.5 antimycin plus 1.5 rotenone in the presence and absence of the nonselective NOS inhibitor l-NAME at 100 μmol/l (Fig. 1). We had four groups for both males and females: 1) DMSO vehicle (Vehicle-only), 2) DZ only (DZ-only), 3) DMSO plus l-NAME (Vehicle + l-NAME), and 4) DZ plus l-NAME (DZ + l-NAME). We performed the following mathematical calculations using the raw OCR values after normalization for vascular protein mass: nonmitochondrial respiration = the minimum value of the five OCR measurements after antimycin and rotenone injection; basal respiration = the values for OCR measurements prior to the first injections minus nonmitochondrial respiration; proton leak = OCR measurements after oligomycin injection prior to FCCP injection minus nonmitochondrial respiration; ATP production = basal respiration minus proton leak; maximal respiration = OCR values after FCCP injection prior to antimycin/rotenone injection minus nonmitochondrial respiration; spare respiratory capacity = maximal respiration minus basal respiration.
Fig. 1.

Seahorse experimental design. Determinations of the various parameters of oxygen consumption rate (OCR), expressed as pM·min−1·μg protein−1, are shown schematically. The Roman numerals show five different measurement cycles, and the vertical arrows demonstrate the injection of diazoxide/DMSO/l-NAME, oligomycin, FCCP, antimycin, and rotenone. The gray boxes represent the different parameters that were calculated to characterize mitochondrial bioenergetics in cerebral arteries: basal respiration, ATP production, proton leak, maximal respiration, nonmitochondrial respiration, and spare capacity.
The protein concentration of each well was determined after each Seahorse experiment. The arteries from all islet plate wells were transferred into individual Eppendorf tubes containing 4°C NP40 lysis buffer (Invitrogen, Frederick, MD) with the inhibitors proteinase and phosphatase, both at 5 μl/ml (Sigma Aldrich, St. Louis, MO). Arteries were homogenized and centrifuged at 1,000 g for 10 min and the supernatant was used for the Bradford protein assay (Thermo Scientific, Rockford, IL) according to manufacturer's instructions: the BCA reagent was diluted to 50:1, reagent A:B. We pipetted duplicates of the samples and BSA Standards with the following concentrations (mg/ml): 0, 25, 125, 250, 500, 750, 1,000, 1,500, and 2,000 into a 96-well plate. The BCA reagents were added to the wells containing either samples or standards. Afterward the plate was incubated at 37°C for 30 min, and an uQuant (BioTek) spectrophotometer at 540 nm was used to read the absorbance of the samples and standards. The standard curve and interpolation was used to calculate the concentration of the artery samples. For mitochondrial function, we expressed OCR data in picomoles per minute per microgram.
Western blot.
For Western blot analysis on isolated cerebral arteries, proteins were harvested as described above and separated by a 4–20% SDS-PAGE gradient gel and transferred onto a PVDF membrane (Bio-Rad, Hercules, CA). The arteries were not used for Seahorse experiments. Membranes were blocked with casein blocking buffer (no. 92740200, Li-cor, Lincoln, NE) for 1 h at room temperature, then washed 3 times with Tris-buffered saline and 1% Tween-20 (TBST) (Sigma Aldrich, St. Louis, MO). The membranes were incubated overnight at 4°C with the primary antibodies in casein blocking buffer for the following mitochondrial proteins: anti-Complex I MT-ND1 at 1:500 dilution (36 kDa; no. Ab181848, Abcam, Cambridge, MA); anti-Complex II Fp subunit I at 1:1,000 dilution (70 kDa; no. 459200, Invitrogen, Frederick, MD); anti-Complex III Subunit I core at 1:1,000 dilution (53 kDa; no. 459140, Invitrogen); anti-OxPhos Complex IV Subunit I at 1:500 dilution (57 kDa; no. Ab14705, Abcam); ATP synthase Complex V subunit alpha at 1:500 dilution (50 kDa; no. 459240, Invitrogen); anti-VDAC at 1:1,000 dilution, detects the endogenous levels of total VDAC (32 kDa; no. 4866S, Cell Signaling Technology, Danvers, MA); and for nonmitochondrial proteins: anti-manganese superoxide dismutase (MnSOD) aa114-220 at 1:5,000 (25 kDa; no. 611581, BD Transduction Laboratories, San Jose, CA); total endothelial NOS (eNOS) at 1:500 dilution (140 kDa; no. 610297, BD Transduction Laboratories, San Jose, CA) and its Ser1176 phosphorylated form (peNOS) at 1:500 dilution (140 kDa; no. 9571, Cell Signaling Technology, Danvers, MA); total neuronal NOS (nNOS) at 1:1,000 dilution (150 kDa; no. 610309, BD Transduction Laboratories, San Jose, CA) and its Ser1417 phosphorylated form (pnNOS) at 1:1,000 dilution (160 kDa; no. ab5583, Abcam); and the loading control β-actin at 1:5,000 dilution (42 kDa; no. A5441, Sigma Aldrich, St. Louis, MO). The membranes were washed 3 times and incubated with their respective horseradish peroxidase-conjugated secondary anti-goat-rabbit IgG at 1:2,500 dilution (no. 7074S, Cell Signaling Technology, Danvers, MA) or mouse IgG at 1:5,000 (no. 7076P2, Cell Signaling Technology) in 1% BSA-TBST for 1 h at room temperature. Chemiluminescence (LumiGLO, Gaithersburg, MD) and autoradiography were used to visualize the final reaction. The optical density of each band was quantified and normalized to β-actin immunoband intensity using ImageJ Software (% intensity).
Isolated, pressurized artery technique.
The middle cerebral arteries (MCAs) were isolated in 4°C Ca2+ Krebs solution and then cannulated on both ends using two glass micropipettes filled with Ca2+ Krebs solution (33, 49). Intraluminal pressure and temperature (70 mmHg and 37°C, respectively) were maintained while changes in arteriolar diameter were continuously recorded using a video microscope (Nikon Eclipse TS100, Sony CCD camera, VDA-10 Living Systems Instrumentation Video Dimension Analyzer, LabView 2.0, and HP computer). The arteries developed spontaneous myogenic tone in response to 70 mmHg intraluminal pressure. Vascular responses to 100, 200, and 250 μmol/l of DZ (Sigma Aldrich) were determined on MCAs with intact endothelium in the presence and absence of 100 μmol/l of l-NAME.
Data analysis and statistics.
All data were expressed as means ± SE and analyzed using one-way analysis of variance for repeated measures (ANOVA) and the Tukey post hoc test. A P < 0.05 was considered statistically significant. The abbreviation n indicates the number of independent measurements for each protocol.
RESULTS
Cerebral arteries are well supplied with mitochondria in both endothelium and VSM in adult cerebral arteries of both sexes (Fig. 2). Oxygen consumption rates for arteries during the various stages of the Seahorse protocol are presented in Figs. 3 and 4. In general, OCR values for Vehicle-only treated arteries were higher in females than in males (Figs. 3A and 4) and were increased in both groups in the presence of l-NAME (Figs. 3, B and C, and 4). DZ-only treatment reduced the OCR of female arteries to male values, but it had little or no effect on male arteries (Figs. 3, B and C, and 4). Mitochondrial proteins (Fig. 5) and NOS (Fig. 6) were higher in female compared with male arteries and arterial dilation to DZ was greater in female arteries (Fig. 7).
Fig. 2.
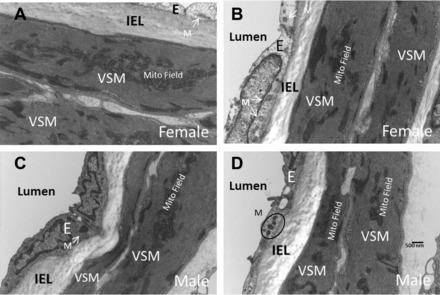
Electron microscopy of male and female cerebral arteries. Representative sections show heavy investment of mitochondria in cerebral vascular endothelium and vascular smooth muscle (VSM) in female (A and B) and male (C and D) rats. Mitochondria are located singly or in groups across the endothelial cells. Mitochondria in VSM are much larger and of different configurations than in endothelial cells. Typically, mitochondria in VSM are particularly numerous and are characteristically present in clusters or fields. Our methods did not allow quantification and comparison of mitochondrial numbers or volume in female and male arteries. Nonetheless, the basic features of mitochondrial morphology and location are similar in arteries from male and female rats. Magnification was 11,000× in each section. E, endothelium; IEL, internal elastic lamina; M, mitochondrion or mitochondria.
Fig. 3.
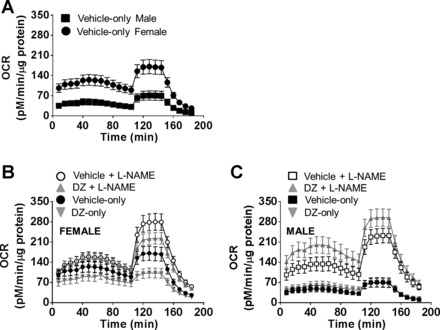
Mitochondrial respiration profiles of cerebral arteries from male and female rats. Mitochondrial OCR in cerebral arteries is greater in female compared with male cerebral arteries (A) and affected by l-NAME and diazoxide (DZ) (B and C). Statistical analyses of the different components of OCR are presented.
Fig. 4.
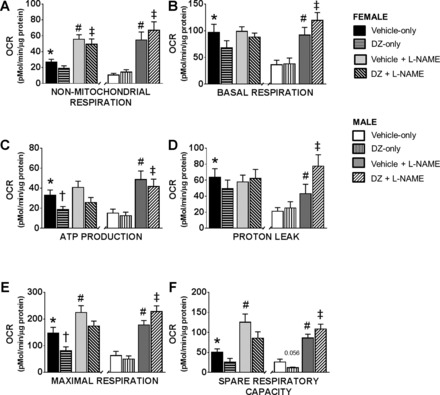
Components of OCR in male and female cerebral arteries. All mitochondrial bioenergetic parameters, including nonmitochondrial respiration (A), basal respiration (B), ATP production (C), proton leak (D), maximal respiration (E), and spare respiratory capacity (F) were significantly increased in female compared with male cerebral arteries. Furthermore, DZ caused a significant decrease in ATP production and maximal respiration in female but not male arteries. While NOS inhibition resulted in a greater increase in the respiration in male compared with female arteries, final values were similar. Data are expressed as means ± SE. Sample sizes are the same as in Fig. 3. *P < 0.05 (Vehicle-only, female vs. male), †P < 0.05 (DZ-only vs. Vehicle-only), #P < 0.05 (Vehicle in the absence of l-NAME vs. in the presence of l-NAME), ‡P < 0.05 (DZ treated in the absence of l-NAME vs. in the presence of l-NAME).
Fig. 5.
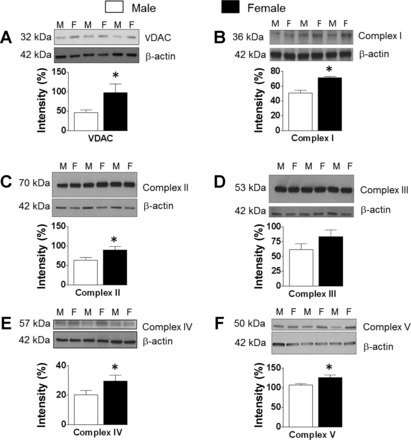
Mitochondrial protein expression is higher in female than male cerebral arteries. Representative Western blots and summary data: 32-kDa VDAC (A), 36-kDa Complex I (B), 70-kDa Complex II (C), 53-kDa Complex III (D), 57-kDa Complex IV (E), and 50-kDa Complex V (F). Histograms showing increased protein levels in female arteries compared with male. Data are expressed as means ± SE. *P < 0.05 (female vs. male, n = 10–14 samples in each group).
Fig. 6.
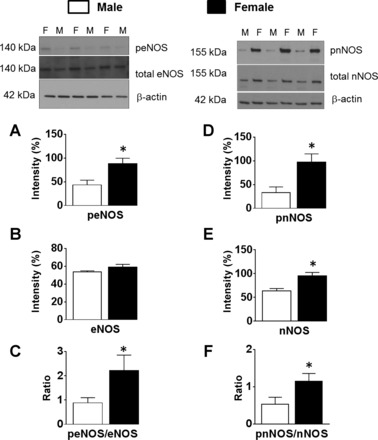
Phosphorylations of endothelial nitric oxide synthase (eNOS) and neuronal NOS (nNOS) are higher in female compared with male cerebral arteries. Representative Western blots and summary data: 140-kDa phosphorylated eNOS (peNOS) (A) and total eNOS (B), phosphorylated eNOS/total eNOS ratio (C), 155-kDa phosphorylated nNOS (pnNOS) (D), total nNOS (E), and phosphorylated nNOS/total nNOS ratio (F). Data are expressed as means ± SE. *P < 0.05 (female vs. male, n = 10–14 samples in each group).
Fig. 7.

Dilator responses of male and female cerebral arteries to DZ. A: DZ caused a dose-dependent dilation which was significantly greater in female compared with male to the highest dose (n = 5 measurements in each group, respectively). B: treatment with l-NAME (n = 5 measurements in each group) decreased the DZ-induced dilation in all groups. C: original recordings of diameter measurement. Data are expressed as means ± SE. *P < 0.05, female vs. male; #l-NAME vs. l-NAME + DZ.
Fine structure of cerebral arteries.
Electron microscopy demonstrated that both endothelium and VSM of adult rats of both sexes are heavily invested with mitochondria (Fig. 2). Mitochondria are located singly or in small groupings across the endothelial cells, while in VSM, mitochondria are much larger in size and of different configurations. A characteristic feature in VSM from cerebral arteries is the clustering or grouping of large numbers of mitochondria which form extensive mitochondrial “fields” in both sexes. Higher magnification of the mitochondrial clusters or fields shows the close association of mitochondria and sarcoplasmic reticula (images not shown). Our techniques did not allow us to quantify and compare the numbers or total volume of mitochondria in cerebral arteries of male and female rats.
OCR in the vehicle-only group.
Oxygen consumption rate is much higher in female compared with male arteries and increased in both groups with the administration of l-NAME (Fig. 3). Calculated components and statistical comparisons of OCR are presented in Fig. 4. Nonmitochondrial respiration was significantly greater in female (26.7 ± 3.8 pM·min−1·μg protein−1) than in male arteries (10.6 ± 2.2 pM·min−1·μg protein−1) in Vehicle-only groups (Fig. 4A). Inhibition of NOS (Vehicle + l-NAME) resulted in significantly higher nonmitochondrial respiration in both female and male groups (55.66 ± 5.7 and 54.6 ± 10.2 pM·min−1·μg protein−1, respectively) compared with the measurements in Vehicle-only groups. In addition, l-NAME eliminated the difference between female and male groups that was present in Vehicle-only groups. Basal respiration was significantly higher in female arteries compared with male arteries (96.9 ± 15.2 and 36.3 ± 8.5 pM·min−1·μg protein−1, respectively). Administration of l-NAME increased basal respiration in male (91.8 ± 14.4 pM·min−1·μg protein−1) but not in female (98.7 ± 8.8 pM·min−1·μg protein−1) arteries (Fig. 4B). ATP production was greater in female (33 ± 5.3 pM·min−1·μg protein−1) compared with male arteries (15.1 ± 4 pM·min−1·μg protein−1) in the Vehicle-only groups (Fig. 4C). Addition of l-NAME increased ATP production in male but not in female arteries, but values in Vehicle + l-NAME treated arteries were not significantly different for females and males (41 ± 6.1 and 48.6 ± 8.6 pM·min−1·μg protein−1, respectively). In the Vehicle-only groups we found significantly higher proton leak values (Fig. 4D) in female (63.6 ± 10.5 pM·min−1·μg protein−1) compared with male arteries (21.2 ± 4.6 pM·min−1·μg protein−1). However, in the presence of l-NAME the proton leak increased in male but not in female arteries (43.2 ± 11.7 and 57.8 ± 8.5 pM·min−1·μg protein−1, respectively). The maximal respiration rate (147.2 ± 21.6 vs. 62.8 ± 16 pM·min−1·μg protein−1) (Fig. 4E) and the spare respiratory capacity were significantly greater in female compared with male arteries in Vehicle-only groups (50.4 ± 8.4 vs. 26 ± 7.3 pM·min−1·μg protein−1, respectively) (Fig. 4F). In the presence of l-NAME maximal respiration rate (224.3 ± 25.8 vs. 177.7 ± 16.7 pM·min−1·μg protein−1) and spare respiratory capacity (125.6 ± 20.2 vs. 85.9 ± 9.7 pM·min−1·μg protein−1) were significantly increased in both female and male arteries compared with the Vehicle-only groups (Fig. 4, E and F).
OCR in the DZ-only group.
ATP production (18.6 ± 3.2 pM·min−1·μg protein−1; P < 0.05) and maximal respiration (78.4 ± 13.7 pM·min−1·μg protein−1; P < 0.05) were reduced from Vehicle-only values in female cerebral arteries by the administration of DZ (DZ-only group) (Fig. 4, C and E). Although there was a tendency for DZ to decrease nonmitochondrial respiration, basal respiration, spare respiratory capacity, and proton leak, these differences were not statistically significant in female arteries (Fig. 4, A, B, D, and F). In contrast, administration of DZ-only to male arteries did not significantly decrease the nonmitochondrial respiration, basal respiration, maximal respiration, ATP production, spare respiratory capacity, or proton leak compared with Vehicle-only treated arteries (Fig. 4, A–E). Only spare respiratory capacity (Fig. 4F) showed a tendency to decrease upon DZ administration in the male arteries (P = 0.0566). Treatment with DZ + l-NAME resulted in a significant increase in all mitochondrial respiratory parameters in the male arteries compared with DZ-only (nonmitochondrial respiration: 66.9 ± 10.7 vs. 14.3 ± 3.1 pM·min−1·μg protein−1; basal respiration: 119.3 ± 14.8 vs. 37.9 ± 11 pM·min−1·μg protein−1; ATP production: 41.8 ± 7.4 vs. 12.6 ± 3.5 pM·min−1·μg protein−1; proton leak: 77.5 ± 14 vs. 25.3 ± 7.8 pM·min−1·μg protein−1; maximal respiration: 227.9 ± 21.6 vs. 49.47 ± 12.06 pM·min−1·μg protein−1; spare respiratory capacity: 108.5 ± 12 vs. 11.6 ± 1.6 pM·min−1·μg protein−1, respectively). NOS inhibition caused a significant increase only in the nonmitochondrial respiration in the female DZ + l-NAME group (49.5 ± 6.6 pM·min−1·μg protein−1) compared with the DZ-only treated group (19.3 ± 3.2 pM·min−1·μg protein−1).
Mitochondrial protein levels.
All mitochondrial protein levels (Fig. 5, A–F) except Complex III protein (84 ± 11.4%; P = 0.08), were significantly increased in the female arteries, including the components of the respiratory chain: Complex I (71.6 ± 1.6%), Complex II (89.8 ± 9%), Complex IV (29.7 ± 3.8%), Complex V (126.2 ± 6.4%), and the VDAC (97.8 ± 21.9%) when compared with male arteries (Complex I: 50.9 ± 3.8%, Complex II: 63.7 ± 6.8%, Complex IV: 20.2 ± 3%, Complex V: 107.2 ± 3.4%, VDAC: 46.6 ± 6.4%) (Fig. 5, A–F). Levels of MnSOD were similar in the female (240.7 ± 60%) and male (210.9 ± 47%) arteries (data not shown).
Nonmitochondrial protein levels.
Levels of phosphorylated eNOS and the phosphorylated eNOS/total eNOS ratio were significantly higher in female (88.2 ± 11.6 and 2.2 ± 0.6%, respectively) compared with male arteries (43.7 ± 10 and 0.88 ± 0.2%, respectively) (Fig. 6, A and C). However, values for total eNOS were similar in the female compared with the male group (59.1 ± 3.2 and 53.7 ± 1.3%, respectively) (Fig. 6B).
Neuronal NOS levels were elevated in the female (95.8 ± 6.8) compared with male (63.8 ± 5%) arteries (Fig. 6E). In addition, levels of phosphorylated nNOS were significantly higher in female (97.7 ± 17.2%) compared with male (33.4 ± 12%) arteries (Fig. 6D). Furthermore, the phosphorylated nNOS/total nNOS ratio was significantly higher in female (1.2 ± 0.2%) compared with male arteries (0.5 ± 0.2%) (Fig. 6F).
Cerebral vasoreactivity to DZ.
There was no difference between the baseline diameters of female (266 ± 9 μm) and male (282 ± 16 μm) MCAs although the male MCAs tended to develop greater myogenic tone (52 ± 2.3%, P > 0.09) compared with females (38.6 ± 6.6%). Diazoxide-induced responses to 100 and 200 μmol/l were similar in both male (36.2 ± 7.5 and 40.9 ± 7.3, respectively) and female (35 ± 7.5 μm and 50.4 ± 8.4 μm, respectively) MCAs, whereas the response to 250 μmol/l was significantly increased in female (54 ± 6.5 μm) compared with male (30.1 ± 1.2 μm) arteries (Fig. 7, A and C).
Inhibition of NOS increased the myogenic tone in both female and male groups but was greater in female (36.8 ± 5.2 μm) compared with male (23 ± 7 μm) arteries (Fig. 7B). Cotreatment of l-NAME with DZ to endothelium-intact arteries resulted in similar reduced vasodilation to 100, 200, and 250 DZ μmol/l of DZ in both groups (male: 20.5 ± 8.3 μm, 17.1 ± 9.8 μm, and 17.5 ± 7.9 μm, respectively; female: 18.4 ± 7.3 μm, 30.7 ± 9.9 μm, 31.3 ± 9.4 μm, respectively) (Fig. 7, B and C).
DISCUSSION
This study demonstrates, for the first time, distinct sex-specific differences in the dynamics of mitochondrial respiration in freshly harvested cerebral arteries from adult rats as well as in levels of mitochondrial proteins and arterial dilator responses to DZ. There are many novel findings from our experiments. First, overall patterns as well as specific parameters of mitochondrial OCR were significantly greater in adult female than in male cerebral arteries. Second, the enhanced mitochondrial respiration in female cerebral arteries correlated with a greater level of mitochondrial proteins. Third, mitochondrial respiration after DZ administration was reduced in adult female but not in male arteries. Fourth, when comparing differences in baseline values of OCR, inhibition of NOS resulted in a greater effect on mitochondrial respiration in adult male than in female arteries. Fifth, dilator responses to DZ were greater in female arteries. The combination of evidence from many different perspectives and methods shows that there is considerable sex-related diversity in mitochondrial mass, function, and dynamics in rat cerebral arteries.
We adapted the Seahorse XFe24 analyzer technology to allow the determination of mitochondrial OCR in freshly isolated cerebral arteries for the first time (55). Although cerebral capillaries have been reported to have a high content of mitochondria because of transport mechanisms associated with the blood-brain barrier (38), we are unaware of a direct electron microscopy examination of mitochondria in endothelium and VSM of resistance arteries in male and female rats. While an extensive analysis is beyond the scope of the present study, electron microscopy showed that the endothelium and VSM heavily invested with mitochondria in male and female cerebral arteries. In particular, large clusters or fields of mitochondria are present in VSM while smaller, usually more isolated mitochondria are present in endothelium. Networks of interconnected mitochondria have been reported in cultured cerebral vascular endothelial cells (9), but it is unclear whether mitochondrial syncytia occur in native cerebral vascular endothelium. Previously, in male cerebral arteries, we showed that the sarcoplasmic reticula, the cellular structures involved in calcium release and sequestration, are in close proximity to mitochondria in VSM and evidence supports the contention that the mitochondria-sarcoplasmic reticula interactions promote relaxation of VSM (9). Although untested, it appears that the similar mitochondrial morphology of female arteries would likewise support mitochondrial interactions with sarcoplasmic reticula in promoting relaxation in VSM.
Previous studies, based largely upon expression of mitochondrial proteins, have suggested differences in mitochondrial respiration between male and female arteries and in cerebral vascular endothelial cells treated with estrogen, but did not actually measure OCR (39). In our current study when we measured OCR, we found significantly increased basal respiration in isolated female compared with male cerebral arteries and significantly increased nonmitochondrial respiration. Thus elevated mitochondrial OCR in female arteries under basal conditions, as well as OCR following manipulation of the electron transport chain with oligomycin, FCCP, antimycin, and rotenone, are positively associated with increased mitochondrial mass. The majority of the mitochondrial complex proteins are encoded by nuclear DNA and only 13 of the proteins are mitochondrial DNA (mtDNA) encoded (11, 20, 52). Therefore, we determined the expression of the components of the mtDNA encoded Complex I, Complex III, and Complex IV as well as the nuclear DNA encoded Complex II and Complex V subunits, and VDAC protein. All of the mitochondrial protein levels, except the Complex III subunit (P = 0.08), were significantly higher in female compared with male cerebral arteries from adult rats, corresponding to previous reports in the literature (50). Increases in Complex proteins encoded by nuclear DNA as well as mtDNA indicate the operation of integrating mechanisms to ensure optimal function of increased mitochondrial mass in female arteries. These data might indicate the enhanced capability of the cell to survive during an energetic crisis (14, 29).
Reactive oxygen species (ROS) such as superoxide anion (O2−) are produced as a by-product of oxidative phosphorylation under normal conditions and are important signaling agents for maintenance of cell function (51, 54). Under physiological conditions, the major sites of O2− production are Complex I and III, although Complex II has also been reported to contribute to the basal ROS levels (21, 51, 54, 59). Complex I primarily releases O2− to the mitochondrial matrix (36, 37, 41) while Complex III apparently releases O2− to both the mitochondrial matrix and intermembrane space (59). Superoxide anion is rapidly converted to hydrogen-peroxide (H2O2) by MnSOD and can exit the matrix via aquaporin-like channels and contribute to higher cytosolic ROS levels after passing through the VDAC in the outer mitochondrial membrane (26, 27, 52). We found significantly higher Complex I, II, III, and VDAC protein levels as well as higher OCR in female cerebral arteries and would therefore expect greater ROS availability in female arteries. Nonetheless, it has been reported that oxidative stress is lower in female compared with male arteries, probably due to higher levels of ROS scavengers, greater proton leak, and higher NO production. We found no difference in the expression level of the MnSOD protein between male and female arteries. Our results are similar to others who have reported a lack of substantial sex-dependent differences in MnSOD protein expression (46, 53). However, this enzyme has an extremely high catalytic efficiency and therefore reactions between MnSOD and O2− are diffusion rather than rate limited. Alternatively, under certain conditions, estrogen activation of the MAP kinase and the NF-κB pathway (6, 7) might reduce cellular ROS levels by increased production and/or activity of MnSOD (43, 45, 57).
Increased OCR in female arteries corresponded with enhanced ATP production as well as a higher proton leak. Proton leak, via mitochondrial anion carrier proteins such as adenine nucleotide translocase, occurs normally during oxidative phosphorylation and thus protons can return to the matrix independently of ATP synthase. Administration of oligomycin, which inhibits ATP synthase, allowed us to estimate the magnitude of proton leak. Prior findings indicated that increased proton leak is inversely linked to ROS release by mitochondria; therefore, increased proton leak provides an important mechanism to decrease the level of total cellular ROS derived from mitochondria.
We have shown significantly increased phosphorylated eNOS and nNOS levels in female compared with male arteries, and these findings are similar to reports by other laboratories (16, 17, 30, 44). To examine the role of NO in the mitochondrial respiration, we measured OCR in the presence of the NOS inhibitor, l-NAME. Similar to others, (40, 48, 56) we found that NOS inhibition caused an increase in the OCR in both groups, which was significant in males as well as in females. However, compared with OCR values in untreated arteries, the magnitude of the increase in males was greater than females after l-NAME treatment. This result shows that the dynamics of NO inhibition of mitochondrial respiration are different in male and female groups. Despite the increased NOS protein expression in females, the inhibitory effect of NO is lower in females compared with males on a percentage basis, a finding consistent with the results of others (16, 39, 50). We propose that under physiological conditions the bioavailable NO has a greater inhibitory effect on the mitochondrial respiratory chain Complex proteins in males. Superoxide anion, produced normally by mitochondria, can also react with the NO produced by NOS under physiological conditions and form peroxynitrite (ONOO−). Peroxynitrite can interact with heme proteins and thereby inhibit Complex IV and decrease the activity of Complex I via S-nitrosation mediation (13). Bolanos et al. (3, 5) have shown that ONOO− irreversibly inhibits Complex IV via an intracellular, time-dependent process without immediate effect. However, exogenous ONOO− applied on isolated brain mitochondria resulted in an irreversible loss of Complexes II and III (4). Furthermore, several studies have reported that isolated heart mitochondria and cultured oligodendrocytes treated with ONOO− and NO donors, respectively, caused a loss of Complex II activity (19, 40, 48, 56). Thus NO and O2− can singly or together alter mitochondrial respiration. On the other hand, our findings may represent the suppression of mitochondrial respiration by endogenous factors, including NO and ONOO− in male arteries under baseline conditions, and thus a greater capacity to increase OCR following l-NAME administration.
We have shown that mitoKATP channels have an important role in pharmacological preconditioning as well as in changes in vascular tone (9–10, 32–33, 49). In our current study we found a significantly greater arterial dilation to DZ, at the concentration used to determine OCR, in the female compared with male arteries. In accordance with our vasoreactivity measurements, we found a greater effect of DZ on OCR values in the cerebral arteries of females compared with males under physiological conditions. Male MCAs developed a greater myogenic tone compared with female MCAs, but l-NAME given alone caused vasoconstriction which was greater in female compared with male, consistent with the literature (16–18, 50). We also found similar vascular responses to DZ in females and males in the presence of l-NAME. When we explored the underlying mechanism of DZ in the presence of l-NAME application, we found a greater percent increase in OCR in males compared with females. However, the overall values of OCR in DZ + l-NAME-treated arteries were similar. This information further supports our recent finding that endothelium plays a key role in diazoxide-induced vasodilation in MCAs and suggests that its effect may depend on the energetic condition of the mitochondria and the available substrate, as reported by others (47). In addition, the effects of DZ on mitochondria, which could affect OCR, involve activation of the PI-3 kinase-Akt pathway, elevated production of ROS, uncoupling of the respiratory chain, increased intracellular levels of calcium, and augmented production of NO and prostaglandins. Whether these factors or other, yet unrecognized, factors caused the dramatic DZ-induced decrease in OCR in female arteries to values observed in male arteries, as well as the different degrees of dilation to DZ in male and female arteries, is unknown. Furthermore, we speculate that effects of mitoKATP channel opening on mitochondrial respiration might be dependent upon basal OCR.
There were a few limitations of our study. We did not separate the female rats based upon the stage of estrus cycle, which could have affected cerebral vascular protein levels, and therefore we may have missed minor variations in the amount of mitochondrial proteins as well as levels of mitochondrial respiration. For example, we did not see a significantly increased total eNOS protein expression in female compared with male arteries which is similar to work by Duckles and Krause (16) Nevertheless, our experiments provide a baseline for future studies exploring the effect of estrogen using ovariectomized rats with placebo and estradiol treatment. On the other hand, studying mitochondrial respiration ex vivo using freshly harvested cerebral arteries most closely mirrors the in vivo situation, and most previous studies of sex-dependent differences in cerebral arteries have not considered estrus cycle status. In addition, we cannot say with certainty whether mitochondrial dynamics of large cerebral arteries correspond to smaller arteries, capillaries, or veins. Last, regional and tissue-specific differences in mitochondrial dynamics can be examined in future studies.
Our overall findings have advanced knowledge and have further defined sex-specific differences in mitochondrial function using a novel approach combined with traditional methods on freshly isolated cerebral arteries. Our results provide new insights regarding the underlying mechanisms of sex-related differences in health, and perhaps, in disease.
GRANTS
This work was supported by National Institutes of Health Grants (D. W. Busija: HL-077731 and HL-093554), Louisiana Board of Regents Support Fund-Research Competitiveness Subprogram [P. V. G. Katakam: LEQSF-(2014-17)-RD-A-11], American Heart Association National Center NRCP Scientist Development Grant (P. V. G. Katakam: 14SDG20490359), and American Heart Association Postdoctoral Fellowship Grant (I. Rutkai: 15POST23040005). This research was supported in whole or in part by the Louisiana Board of Regents Endowed Chairs for Eminent Scholars program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: I.R. and D.W.B. conception and design of research; I.R. and S.D. performed experiments; I.R. analyzed data; I.R., P.V.G.K., and D.W.B. interpreted results of experiments; I.R. prepared figures; I.R. drafted manuscript; I.R., S.D., P.V.G.K., and D.W.B. edited and revised manuscript; I.R., S.D., P.V.G.K., and D.W.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank N. Busija for editorial assistance. We thank K. A. Walter for technical help. We also thank K. Grant of the Cellular Imaging Shared Resource at Wake Forest Univ. Health Sciences for assistance with electron microscopy.
REFERENCES
- 1.Acin-Perez R, Enriquez JA. The function of the respiratory supercomplexes: the plasticity model. Biochim Biophys Acta 1837: 444–450, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Arnold S, Victor MB, Beyer C. Estrogen and the regulation of mitochondrial structure and function in the brain. J Steroid Biochem Mol Biol 131: 2–9, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Bolanos JP, Almeida A, Stewart V, Peuchen S, Land JM, Clark JB, Heales SJ. Nitric oxide-mediated mitochondrial damage in the brain: mechanisms and implications for neurodegenerative diseases. J Neurochem 68: 2227–2240, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Bolanos JP, Heales SJ, Land JM, Clark JB. Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary culture. J Neurochem 64: 1965–1972, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Bolanos JP, Peuchen S, Heales SJ, Land JM, Clark JB. Nitric oxide-mediated inhibition of the mitochondrial respiratory chain in cultured astrocytes. J Neurochem 63: 910–916, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Borras C, Gambini J, Gomez-Cabrera MC, Sastre J, Pallardo FV, Mann GE, Vina J. 17Beta-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2[MAPK]/NFkappaB cascade. Aging cell 4: 113–118, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Borras C, Sastre J, Garcia-Sala D, Lloret A, Pallardo FV, Vina J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Rad Biol Med 34: 546–552, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J 435: 297–312, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busija DW, Katakam PV. Mitochondrial mechanisms in cerebral vascular control: shared signaling pathways with preconditioning. J Vasc Res 51: 175–189, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busija DW, Lacza Z, Rajapakse N, Shimizu K, Kis B, Bari F, Domoki F, Horiguchi T. Targeting mitochondrial ATP-sensitive potassium channels—a novel approach to neuroprotection. Brain Res Rev 46: 282–294, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Chaban Y, Boekema EJ, Dudkina NV. Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilisation. Biochim Biophys Acta 1837: 418–426, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Cheranov SY, Jaggar JH. Mitochondrial modulation of Ca2+ sparks and transient KCa currents in smooth muscle cells of rat cerebral arteries. J Physiol 556: 755–771, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahm CC, Moore K, Murphy MP. Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite: implications for the interaction of nitric oxide with mitochondria. J Biol Chem 281: 10056–10065, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Demarest TG, McCarthy MM. Sex differences in mitochondrial (dys)function: Implications for neuroprotection. J Bioenerg Biomembr 47: 173–188, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domoki F, Perciaccante JV, Veltkamp R, Bari F, Busija DW. Mitochondrial potassium channel opener diazoxide preserves neuronal-vascular function after cerebral ischemia in newborn pigs. Stroke 30: 2713–2718; discussion 2718–2719, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Duckles SP, Krause DN. Cerebrovascular effects of oestrogen: multiplicity of action. Clin Exper Pharmacol Physiol 34: 801–808, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Duckles SP, Krause DN. Mechanisms of cerebrovascular protection: oestrogen, inflammation and mitochondria. Acta Physiol 203: 149–154, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duckles SP, Miller VM. Hormonal modulation of endothelial NO production. Pflügers Arch 459: 841–851, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erusalimsky JD, Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol 27: 2524–2531, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Falkenberg M, Larsson NG, Gustafsson CM. DNA replication and transcription in mammalian mitochondria. Annu Rev Biochem 76: 679–699, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Felty Q, Roy D. Estrogen, mitochondria, and growth of cancer and non-cancer cells. J Carcinogenesis 4: 1, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Florian M, Lu Y, Angle M, Magder S. Estrogen induced changes in Akt-dependent activation of endothelial nitric oxide synthase and vasodilation. Steroids 69: 637–645, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Foster DB, Ho AS, Rucker J, Garlid AO, Chen L, Sidor A, Garlid KD, O'Rourke B. Mitochondrial ROMK channel is a molecular component of mitoK(ATP). Circ Res 111: 446–454, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geary GG, Krause DN, Duckles SP. Gonadal hormones affect diameter of male rat cerebral arteries through endothelium-dependent mechanisms. Am J Physiol Heart Circ Physiol 279: H610–H618, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Gerencser AA, Chinopoulos C, Birket MJ, Jastroch M, Vitelli C, Nicholls DG, Brand MD. Quantitative measurement of mitochondrial membrane potential in cultured cells: calcium-induced de- and hyperpolarization of neuronal mitochondria. J Physiol 590: 2845–2871, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grisolia S, Rivas J, Wallace R, Mendelson J. Inhibition of proteolysis of cytosol proteins by lysosomal proteases and of mitochondria of rat liver by antibiotics. Biochem Biophys Res Commun 77: 367–373, 1977. [DOI] [PubMed] [Google Scholar]
- 27.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem 278: 5557–5563, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Hanley PJ, Mickel M, Loffler M, Brandt U, Daut J. K(ATP) channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol 542: 735–741, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill BG, Benavides GA, Lancaster JR, Ballinger S, Dell'Italia L, Jianhua Z, Darley-Usmar VM. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol Chem 393: 1485–1512, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho KJ, Liao JK. Nonnuclear actions of estrogen. Arterioscler Thromb Vasc Biol 22: 1952–1961, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Horiguchi T, Kis B, Rajapakse N, Shimizu K, Busija DW. Opening of mitochondrial ATP-sensitive potassium channels is a trigger of 3-nitropropionic acid-induced tolerance to transient focal cerebral ischemia in rats. Stroke 34: 1015–1020, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Katakam PV, Gordon AO, Sure VN, Rutkai I, Busija DW. Diversity of mitochondria-dependent dilator mechanisms in vascular smooth muscle of cerebral arteries from normal and insulin-resistant rats. Am J Physiol Heart Circ Physiol 307: H493–H503, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katakam PV, Wappler EA, Katz PS, Rutkai I, Institoris A, Domoki F, Gaspar T, Grovenburg SM, Snipes JA, Busija DW. Depolarization of mitochondria in endothelial cells promotes cerebral artery vasodilation by activation of nitric oxide synthase. Arterioscler Thromb Vasc Biol 33: 752–759, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kis B, Nagy K, Snipes JA, Rajapakse NC, Horiguchi T, Grover GJ, Busija DW. The mitochondrial K(ATP) channel opener BMS-191095 induces neuronal preconditioning. Neuroreport 15: 345–349, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Lemasters JJ, Holmuhamedov E. Voltage-dependent anion channel (VDAC) as mitochondrial governator–thinking outside the box. Biochim Biophys Acta 1762: 181–190, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Lenaz G, Fato R, Baracca A, Genova ML. Mitochondrial quinone reductases: complex I. Methods Enzymol 382: 3–20, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Lenaz G, Fato R, Genova ML, Bergamini C, Bianchi C, Biondi A. Mitochondrial Complex I: structural and functional aspects. Biochim Biophys Acta 1757: 1406–1420, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Mancardi GL, Tabaton M, Liwnicz BH. Endothelial mitochondrial content of cerebral cortical capillaries in Alzheimer's disease. An ultrastructural quantitative study. Eur Neurol 24: 49–52, 1985. [DOI] [PubMed] [Google Scholar]
- 39.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev 60: 210–241, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitrovic B, Ignarro LJ, Montestruque S, Smoll A, Merrill JE. Nitric oxide as a potential pathological mechanism in demyelination: its differential effects on primary glial cells in vitro. Neuroscience 61: 575–585, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem 279: 49064–49073, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Nagy K, Kis B, Rajapakse NC, Bari F, Busija DW. Diazoxide preconditioning protects against neuronal cell death by attenuation of oxidative stress upon glutamate stimulation. J Neurosci Res 76: 697–704, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Nobes CD, Brown GC, Olive PN, Brand MD. Non-ohmic proton conductance of the mitochondrial inner membrane in hepatocytes. J Biol Chem 265: 12903–12909, 1990. [PubMed] [Google Scholar]
- 44.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 286: R233–R249, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Porter RK, Brand MD. Causes of differences in respiration rate of hepatocytes from mammals of different body mass. Am J Physiol Regul Integr Comp Physiol 269: R1213–R1224, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Razmara A, Duckles SP, Krause DN, Procaccio V. Estrogen suppresses brain mitochondrial oxidative stress in female and male rats. Brain Res 1176: 71–81, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riess ML, Camara AK, Heinen A, Eells JT, Henry MM, Stowe DF. KATP channel openers have opposite effects on mitochondrial respiration under different energetic conditions. J Cardiovasc Pharmacol 51: 483–491, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem 269: 26066–26075, 1994. [PubMed] [Google Scholar]
- 49.Rutkai I, Katakam PV, Dutta S, Busija DW. Sustained mitochondrial functioning in cerebral arteries after transient ischemic stress in the rat: a potential target for therapies. Am J Physiol Heart Circ Physiol 307: H958–H966, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stirone C, Duckles SP, Krause DN, Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol 68: 959–965, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol 552: 335–344, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turrens JF. Superoxide production by the mitochondrial respiratory chain. Biosci Reports 17: 3–8, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Valle A, Guevara R, Garcia-Palmer FJ, Roca P, Oliver J. Sexual dimorphism in liver mitochondrial oxidative capacity is conserved under caloric restriction conditions. Am J Physiol Cell Physiol 293: C1302–C1308, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Velarde MC. Mitochondrial and sex steroid hormone crosstalk during aging. Longevity Healthspan 3: 2, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallace KB, Starkov AA. Mitochondrial targets of drug toxicity. Annu Rev Pharmacol Toxicol 40: 353–388, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Widlansky ME, Gutterman DD. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxidants Redox Signal 15: 1517–1530, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, Chomicz S, Ferrick DA. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol 292: C125–C136, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Xi Q, Cheranov SY, Jaggar JH. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Circ Res 97: 354–362, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol 292: H2023–H2031, 2007. [DOI] [PubMed] [Google Scholar]


