Abstract
Study Objectives:
To analyze predictors of excessive daytime sleepiness (EDS) and to analyze how changes within risk factors over time predict incident EDS in women.
Design:
Population-based prospective study.
Setting:
General population of the City of Uppsala, Sweden.
Participants:
From a random, general population sample of 7,051 women from the Sleep and HEalth in women (“SHE”) cohort, 4,322 women without EDS at baseline were followed up after 10 y.
Interventions:
N/A.
Measurements and Results:
At baseline and follow-up, women answered a questionnaire on sleeping habits, somatic disease, obesity, insomnia, anxiety and depression, lifestyle, and social factors. The risk of incident EDS was analyzed from changes over time in risk factors using logistic regression modeling. Of the women, EDS developed in 7.9%. Incident: insomnia (adjusted odds ratio = 5.01; 95% confidence interval 3.63–6.92), anxiety and/or depression (3.34; 2.22–5.02), somatic disease (1.73; 1.17–2.55), obesity (1.91; 1.14–2.57), snoring (1.91; 1.17–3.10) and smoking (4.31; 1.95–9.54) were all independent risk factors for the development of EDS. In addition, persistent: insomnia (4.44; 2.97–6.65) and anxiety and/or depression (4.91; 3.17–7.62) increased the risk of developing EDS. Apart from incident: snoring and obesity, similar results were obtained when only including women without somatic disease in the analyses.
Conclusion:
Insomnia, anxiety and/or depression, and smoking were the most important factors for predicting incident excessive daytime sleepiness (EDS) and, in addition, somatic disease, obesity, and snoring predicted EDS. It is important not only to treat these conditions but also to inform women of the importance of a healthy lifestyle in order to prevent and reduce EDS in women.
Citation:
Theorell-Haglöw J, Åkerstedt T, Schwarz J, Lindberg E. Predictors for development of excessive daytime sleepiness in women: a population-based 10-year follow-up. SLEEP 2015;38(12):1995–2003.
Keywords: risk factors, daytime sleepiness, longitudinal, women, population-based
INTRODUCTION
Excessive daytime sleepiness (EDS) has an estimated prevalence of 2.5% to 18.5% in the general population1–7 and it is more common in women than men.8,9 We have previously reported a prevalence of 16.1% for EDS in a population-based group of women.7 Insomnia, poor general health and psychiatric disorders are all related to daytime sleepiness7,10–14 and, in addition, EDS is associated with an increased risk of cardiovascular disease and mortality9 as well as having negative effects on daily activities.15 Finding the factors that predict the development of EDS is therefore of great importance to the general population.
Cross-sectional studies have related not only insomnia but also anxiety, depression, and somatic disease and obesity to EDS1–4,7,10–13,16,17 and two studies have analyzed the longitudinal relationships between risk factors and EDS.14,18 Hasler et al.18 showed that symptoms of insomnia and anxiety at baseline were associated with the subsequent occurrence of EDS, whereas Fernandez-Mendoza et al.14 reported that, apart from depression, weight gain also increases the risk of EDS over time. However, there is still a lack of longitudinal studies analyzing the way changes in several potential risk factors over time are related to development of EDS later.
The aims of this population-based study in women were therefore to analyze the incidence of EDS and factors relating to the development of EDS in a general population of women and to analyze how change in risk factors over time can predict incident EDS.
METHODS
Participants and Setting
This longitudinal population-based study (Sleep and HEalth in women, “SHE”)7 started in 2000, when a questionnaire on sleeping habits and somatic disorders was sent to women aged ≥ 20 y, randomly selected from the population registry of the city of Uppsala, Sweden. The response rate was 71.6% (n = 7,051). In 2010, a follow-up questionnaire was sent to all the women who had answered the baseline questionnaire and were still alive.19 Of the original study population, 8.5% were lost at follow-up due to death (n = 461), emigration (n = 130), and unknown addresses (n = 5). The follow-up questionnaire was therefore sent to 6,455 women (91.6% of the initial study population) and completed by 5,193 (response rate 80.5%). In the current study, women with missing information on EDS in either the baseline or the follow-up questionnaire were excluded (n = 164). In addition, women with EDS at baseline were also excluded in order to analyze new cases of EDS (n = 707). The final study population therefore comprised a total of 4,322 women. The study was approved by the Ethics Committee at the Medical Faculty at Uppsala University and all the participants gave their informed consent before participating.
A comparison between responders and nonresponders still alive showed that at follow-up, the nonresponders were older (mean ages 45.0 ± 16.0 versus 43.3 ± 15.3 y, P < 0.001), somewhat more obese (mean body mass index [BMI] 24.5 ± 4.3 versus 24.0 ± 4.1, P = 0.0007) and more often smokers (21.9 % versus 15.9 %, P < 0.0001) compared with the responders. The responders had somewhat longer baseline sleep duration (mean sleep duration 7.0 versus 6.9 h, P = 0.03), but the prevalence of EDS (14.0 versus 15.0%, P = 0.28) and habitual snoring (7.0 versus 7.8 %, P = 0.31) did not differ between the groups. However, the nonresponders were less physically active at baseline, as 16.3% reported a high level of physical activity and 23.8% reported a low level of physical activity as compared to 21.1% and 15.7%, respectively, of the responders (P < 0.001).
Questionnaires
The follow-up questionnaire included mostly the same questions as the baseline questionnaire, which has previously been described in detail.7 In short, the follow-up questionnaire comprised questions on sleeping habits, snoring habits, insomnia, occupational status, shift work, civil status, physical activity, smoking, obesity, somatic disease, medication, and anxiety and depression.
Both at baseline and at follow-up, daytime sleepiness was assessed by asking the participants to state how severe their problems were regarding daytime sleepiness using the question “How severe are your problems when it comes to feeling sleepy during the day?” The responses were given on a five-point scale: 1 = no problems, 2 = small problems, 3 = moderate problems, 4 = severe problems, and 5 = very severe problems where a score of 4 to 5 was regarded as having EDS.20,21
The questions on the symptoms of insomnia and chronic insomnia were adopted from the Uppsala Sleep Inventory (USI),22 which has previously been used in several epidemiological studies.20,23,24 Having insomnia was characterized as scoring 4–5 on at least one of the questions on difficulty initiating sleep, difficulty maintaining sleep, or early morning awakening. As in the questions on EDS, the women rated their difficulties on a five-point scale where 1 indicated ‘no problems’ and 5 ‘very severe problems’20.
Snoring habits were assessed using the question: “How often do you snore loudly and disturbingly?” The response options for this question were ‘never’ (1), ‘seldom’ (2), ‘sometimes’ (3), ‘often’ (4) and ‘very often’ (5). Based on their response to this question, the participants were categorized into two groups: non-snorers (scores 1–3) and snorers (scores 4–5).
The women further indicated their current height and weight in both questionnaires and BMI was calculated and rounded off to one decimal point. BMI was categorized as normal weight/ overweight (18–27.9 kg/m2) and obese (≥ 28 kg/m2). Women with a BMI < 18 kg/m2 were not included in the analyses.
The participants' physical activity was analyzed using four questions adopted from a questionnaire used in a large population-based study of the correlation between physical activity and mortality.25 The participants were categorized as having a high or medium/low level of activity. Six questions assessed smoking habits and the women were categorized as smokers or nonsmokers.
The women were asked if they had any somatic disease(s) that required regular medical attention20 and they were also asked to specify any regular medication. In addition, anxiety and depression was assessed using the Hospital Anxiety and Depression (HAD) scale and the total score (both the anxiety and the depression score) was dichotomized with a cutoff at score 10 for either anxiety or depression, as this limit can be used as an indication of anxiety and/or depression.26
The participants were asked at both time points to indicate how many months they had worked nights and shifts over the past 10 y. A cutoff point of 60 mo was chosen when analyzing working nights and shifts. Analyses were made of working shifts and working nights as separate variables but also as a combined variable. Because there were no significant differences in the results for the separate variables compared with the combined variable the combined variable was used in the statistical analyses.
All variables were also grouped into “change variables” where the reference was no symptom at either baseline or follow-up (“No-No”) and the other groups were: increase in variable between time points (“No-Yes”/”Increased”/”Incident”), decrease between time points (“Yes-No”/”Decreased”/”Remitted”), and having answered yes to a symptom or variable at both time points (“Yes-Yes”/”Persistent”/”Habitual”).
Statistical Analyses
Statistical analyses were performed using Stata 13 (Stata Corporation, College Station, TX, USA). Univariate analyses for variables at both time points and also developments within risk factors between baseline and follow-up (changes between groups) with incident EDS as the outcome variable were conducted. The variables were added to multiple logistic regression models in order to predict incident EDS from the changes within potential risk factors over time. Analyses were performed with adjustment for age only and with adjustment for all potential predictors of incident EDS (fully adjusted model). In addition, because EDS is closely correlated with anxiety and depression and because insomnia could arguably be a mediator of several of the other variables, the analyses were also performed (1) without entering the variable of anxiety and/or depression into the model and (2) without entering the variable of insomnia into the model. Analyses were also performed in the group of women without any reported somatic disease at either baseline or follow-up (n = 3,066) in order to analyze data in healthy women alone. For some of the possible risk factors for incident EDS, a reverse relationship was plausible and analyses of reverse causality were therefore performed for these variables. The results of the logistic regression models are presented as adjusted odds ratios (ORs) with 95% confidence intervals (95% CI). A value of P < 0.05 was considered significant.
RESULTS
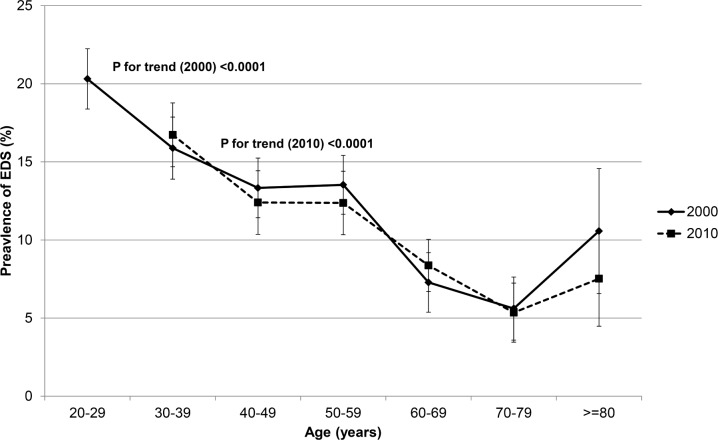
The incidence of EDS over the 10-y period was 7.9%. There was negative linearity between the prevalence of EDS and age at both time points showing that younger women reported more EDS (Figure 1). Univariate analysis revealed that insomnia, anxiety/depression, regular medication, and smoking at baseline or follow-up were all associated with incident EDS. In addition, somatic disease, snoring, obesity, physical activity, and full-time work at follow-up were related to incident EDS, as was marital status and having children at home at baseline (Table 1).
Figure 1.
Prevalence of EDS (with 95% confidence interval) by age at baseline (2000) and follow-up (2010).
Table 1.
Description of the women at baseline (2000) and follow-up (2010).
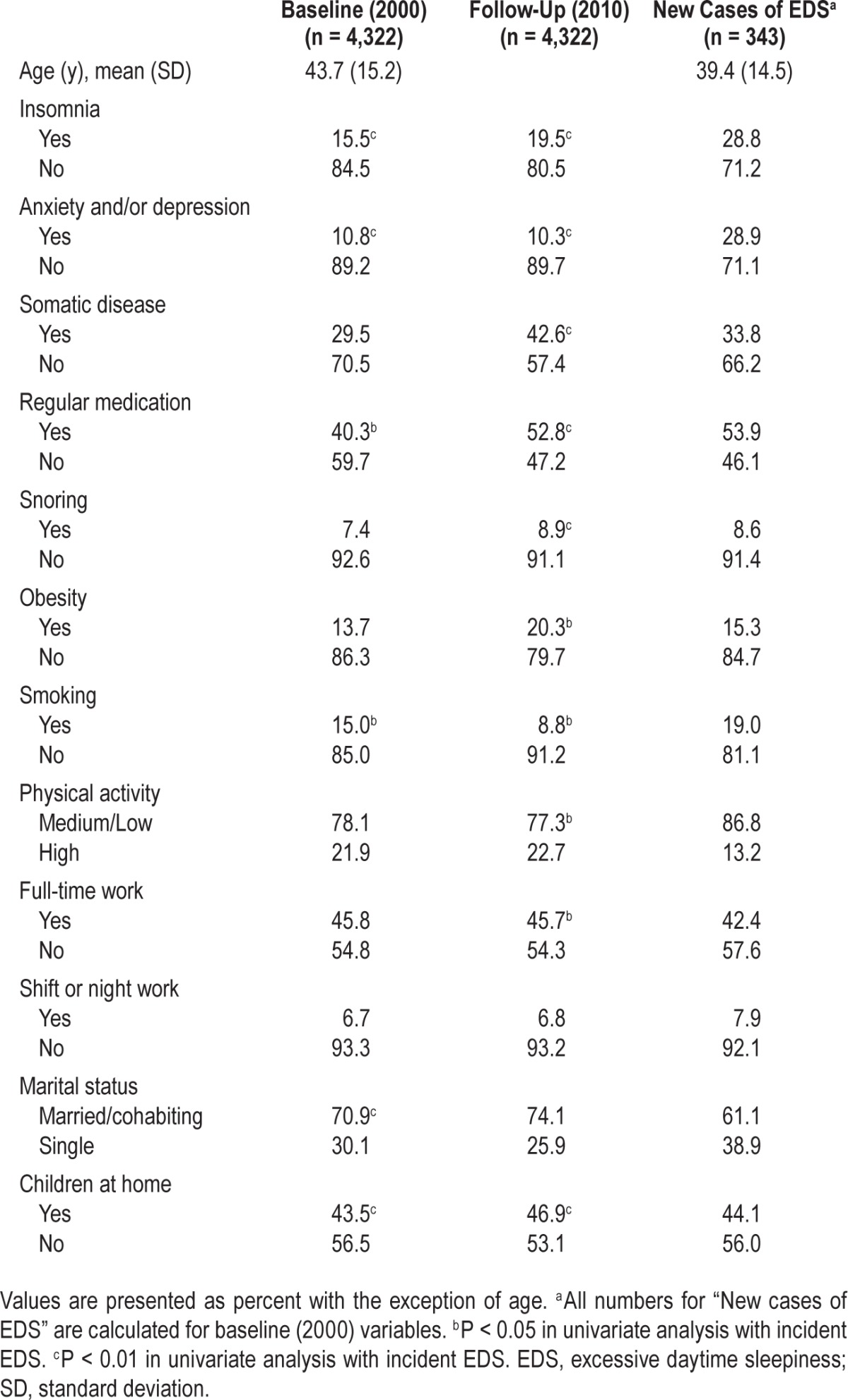
In the group of women with incident EDS, there was a higher prevalence of insomnia, anxiety/depression, regular medication, and a low/medium level of physical activity compared with the baseline group. There was also a somewhat higher prevalence of snoring, obesity, smoking, and shift/night work and fewer women in this group were cohabiting compared with the baseline group (Table 1).
Change over Time in Potential Predictors of EDS
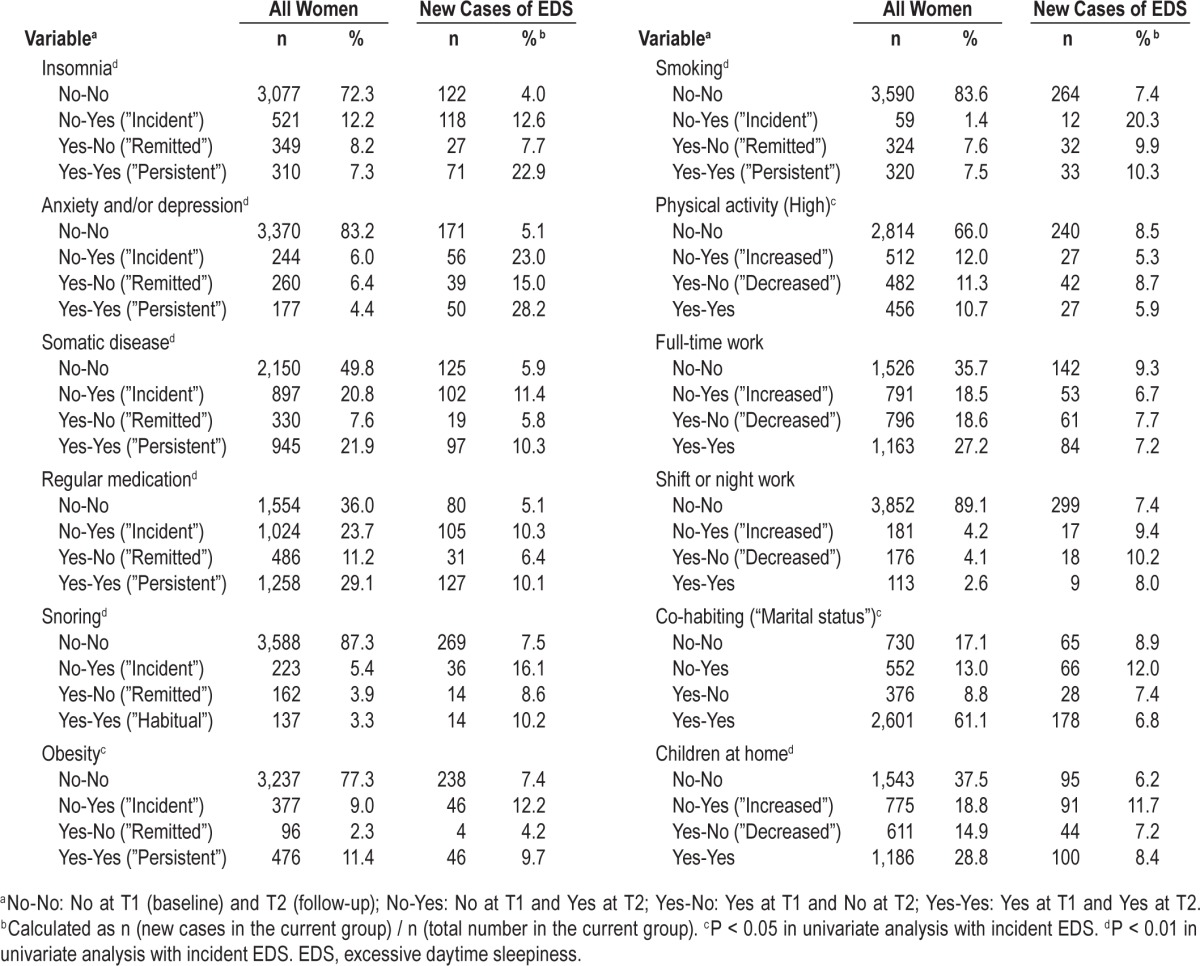
Compared with all other women, there was more persistent insomnia and also more incident and persistent anxiety and/or depression in women with incident EDS. In addition, in this group, there were more incident and persistent snorers, more incident obesity, more incident and persistent smokers and more shift workers or night workers (both new and persistent) compared with the whole group. Furthermore, in the women with incident EDS there were fewer with increased or persistent high physical activity, fewer women who had become full-time workers or were full-time workers at both time points, and also fewer women cohabiting at both time points (Table 2).
Table 2.
Changes within variables during the 10-year follow-up.
Prediction of Incident EDS from Changes within Risk Factors over Time
Changes within variables over time affected the development of EDS. Incident and persistent: insomnia, anxiety and/ or depression, somatic disease, regular medication, smoking and obesity, as well as incident snoring, were all risk factors for developing EDS in the age adjusted analysis, whereas increased and persistent high physical activity levels and starting full-time work reduced the risk. In addition, women with remitted insomnia or anxiety/depression still ran an increased risk of EDS, although lower than for women with incident or persistent insomnia or anxiety/depression. When adjusting for confounders, all these variables, apart from remitted insomnia and persistent somatic disease, regular medication, obesity or smoking, remained as risk factors for incident EDS. In addition, in the fully adjusted model, the reduced risk of EDS with increased physical activity or with persistent high physical activity did not reach statistical significance (Table 3).
Table 3.
Prediction of new cases of excessive daytime sleepiness from changes within variables over time: results from multivariate logistic regression analyses.
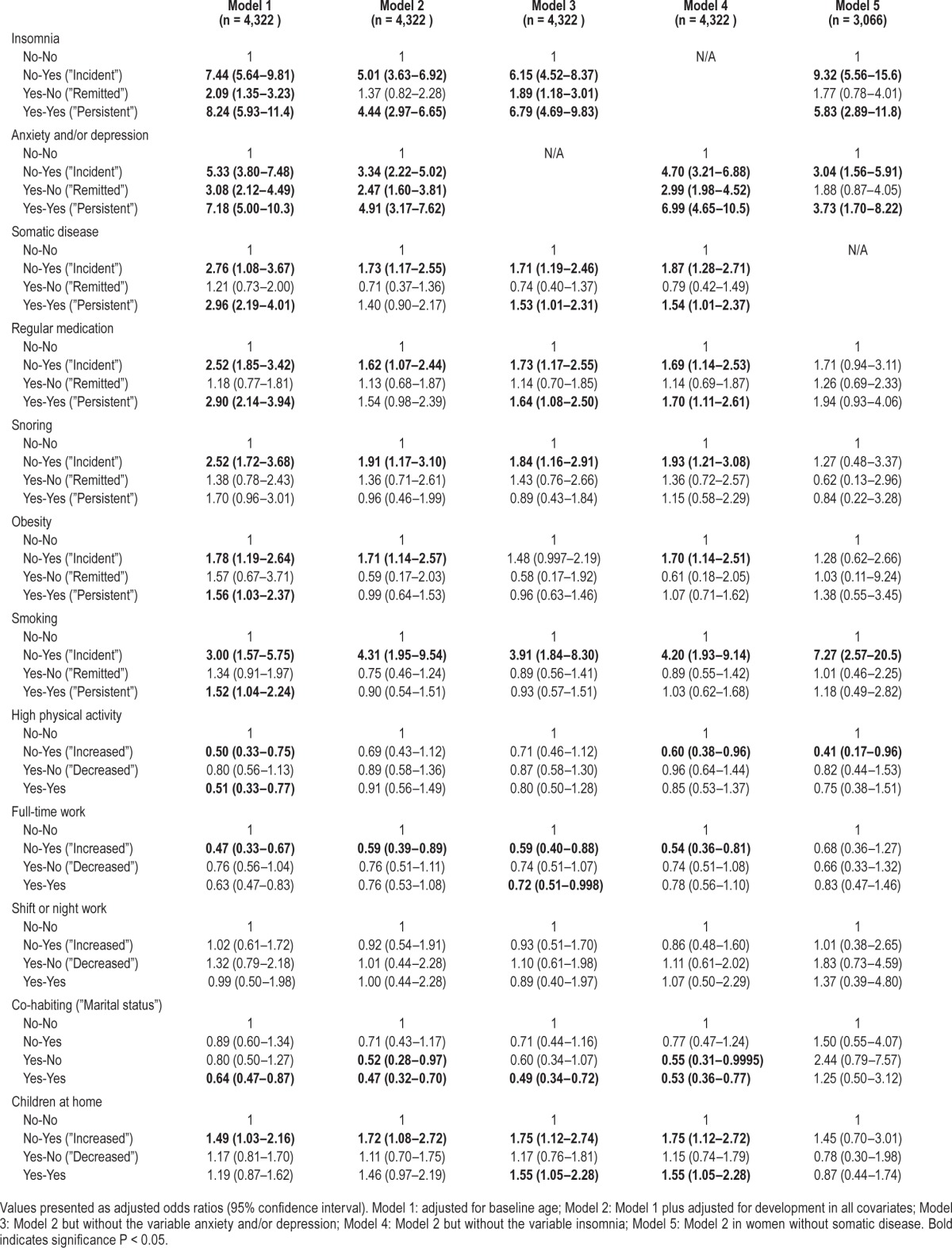
Moreover, without entering anxiety and/or depression into the model, incident, remitted and persistent insomnia were the most important risk factors for EDS. In this model, becoming a full-time worker or having full-time work at both time points reduced the risk of EDS, as did co-habiting at both time points. The results were also similar in analyses in which the variable of insomnia was not included, but, in this context, going from living to not living with someone was also a risk factor for incident EDS. Having children at home at follow-up but not at baseline and children living at home at both time points increased the risk of EDS in the whole group of women (Table 3).
Because the current study was based on questionnaire data, a true knowledge of menopausal state could not be obtained. However, age was obtained and based on previous analysis of this female population27 at least 94% of the women who were 46 y of age were classified as being premenopausal, whereas at least 93% of the women 53 y of age were considered post-menopausal. Therefore, analysis in the whole group of women was performed dichotomized at 50 y at baseline but also 40 y at baseline (i.e., 50 y at follow-up). Age-stratified results are presented in the supplemental material (Table S1).
Risk Factors in Women without Somatic Disease
As in the whole group of women, women without somatic disease ran an increased risk of the development of EDS if they had incident or persistent insomnia or anxiety/depression, as well as incident smoking, after adjusting for confounders. In contrast, increased physical activity reduced the risk of EDS in this group, whereas there was no significant relationship between obesity, full-time work, marital status, or having children at home and incident EDS (Table 3).
Bidirectional Relationships
For several of the variables presenting as risk factors for incident EDS, a bidirectional relationship was plausible. In the whole population, incident EDS increased the risk of incident insomnia (4.85; 3.52–6.69), persistent insomnia (3.60; 2.00–6.49), incident anxiety and/or depression (2.02; 1.20–3.40), incident somatic disease (1.79; 1.21–2.65), incident obesity (1.56; 1.04–2.34) and incident smoking (4.27; 1.92–9.47). In addition, in the whole population, incident EDS was associated with a reduced chance of becoming a full-time worker (0.53; 0.34–0.81).
As in the whole population, incident EDS in healthy women was a risk factor for incident insomnia (8.31; 4.96–13.9), persistent insomnia (4.95; 1.73–14.2), incident anxiety and/or depression (2.38; 1.07–5.31), and incident smoking (8.74; 2.95–26.0). Furthermore, incident EDS reduced the chance of a remission in anxiety and/or depression (0.17; 0.04–0.65), increased the risk of persistent anxiety and/or depression (6.04; 1.54–23.7), and reduced the chance of a change to high physical activity (0.43; 0.19–0.99).
DISCUSSION
This 10-y follow-up study showed that 7.9% of the women developed EDS. In the whole group of women, incident and persistent: insomnia, anxiety and/or depression, somatic disease, as well as incident: snoring, obesity, smoking and having children at home at follow-up but not at baseline predicted the development of EDS. Taking on full-time work and co-habiting at both time points both reduced the risk. In women without somatic disease, incident and persistent: insomnia and anxiety/depression, as well as incident smoking, emerged as risk factors for EDS. In this group increased physical activity reduced the risk, whereas no significant relationships were seen for snoring, obesity, marital status, or children at home.
The incidence of EDS in the current study was on par with previously published results14 and, as expected (as women became 10 y older), the prevalence of EDS decreased.7,28,29 Some studies have shown a U-shaped relationship between age and EDS,14 a result not seen in the current study. One possible explanation for the discrepancy between the current study and the one by Fernandez-Mendoza et al.14 is that the incidence of EDS is more likely to increase in elderly men, as Fernandez-Mendoza et al. showed that male sex was a risk factor for incident EDS and also demonstrated a U-shaped relationship.14
Role of Insomnia and Anxiety and/or Depression
Incident and persistent: insomnia, as well as anxiety and/or depression, were the most prominent predictors of the development of EDS after adjusting for confounders in the current study. A remission in anxiety and/or depression also increased the risk of EDS, but the risk was lower than that for women with incident or persistent anxiety and/or depression indicating that anxiety and depression have long-term effects on EDS, even if the symptoms themselves are no longer present. Moreover, without entering anxiety and/or depression and insomnia respectively into the model, the ORs for predicting EDS remained largely the same in comparison with the fully adjusted model. This indicates that, although anxiety and/or depression and insomnia may correlate with other predictors, these correlations do not influence the prediction of incident EDS to any great extent. In addition, insomnia and anxiety and/or depression were also strong predictors of incident EDS in somatically healthy women.
Several previous studies have shown associations between insomnia and daytime sleepiness10,30–32 and also between anxiety/depression and daytime sleepiness.8,13,33,34 In addition, in longitudinal studies in both young adults18 and in the general population,14 baseline symptoms of insomnia but also psychological disorder such as anxiety were associated with EDS. Therefore, treatment of insomnia and also treatment of anxiety and depressive symptoms could significantly reduce EDS in women.
Somatic Disease, Obesity, Physical Activity, and Snoring
The current study shows that both incident and persistent somatic disease are risk factors for EDS and somatic diseases (diabetes, asthma/allergy, cardiac diseases, hypertension) have previously been related to daytime sleepiness both cross-sectionally11,35–37 and longitudinally.14 In addition, we showed that obesity is a risk factor for incident EDS. Obesity measured as weight or BMI, but also changes in weight or BMI have previously been associated with an increased risk of EDS, and it has also been argued that obesity per se adds to EDS.7,14,16,17,38,39 One factor that could affect the relationship between obesity and EDS is physical activity, and Basta et al.17 showed that physical inactivity was a risk factor for EDS. In the current study, obesity was a risk factor also after controlling for physical activity, thereby indicating that incident obesity per se increases the risk of developing EDS. In addition, increased physical activity only reduced the risk of developing EDS in healthy women, which may indicate that having a somatic disease impedes the effect of increased physical activity. Furthermore, a result was seen for an increase in activity to high physical activity, which may be difficult for people with a somatic disease to achieve. Previous studies have indicated that daytime sleepiness increases with lower levels of physical activity,40 but, to our knowledge, there are no previous studies primarily assessing the reverse.
As in previous studies,41 there was an association between snoring and daytime sleepiness in the current population and becoming a snorer increased the risk of developing EDS, although not in healthy women. Snoring-related daytime sleepiness may be explained by snoring-induced vibrations in the pharynx that induce an inflammatory state with elevated cytokine levels,42 previously associated with daytime sleepiness.43 However, snoring may also be a proxy for some other condition, such as obstructive sleep apnea. In the longitudinal study by Fernandez-Mendoza et al.,14 snoring was also associated with incident EDS, especially in those with milder levels of sleep apnea leading to the conclusion that these individuals are likely to progress to more severe sleep apnea.
Lifestyle Factors
Becoming a smoker predicted the development of EDS both in the whole population and in healthy women, where the relationship was even stronger. Few studies have analyzed the effect of smoking on EDS, although there are studies showing that smokers are more prone to sleepiness.14,16,44 In addition, both the Sleep Heart Health Study and the National Health and Nutrition Examination Survey show that cigarette smoking is independently associated with disturbances in sleep architecture, including a longer latency to sleep onset45,46 and a shift toward lighter stages of sleep,45 which could explain the relationship with daytime sleepiness. Furthermore, although not related to incident EDS in previous longitudinal studies,14 cigarette smoking has been related to the evolution of poor sleep into insomnia.47 Taken as a whole, this information indicates that cigarette smoking may alter sleep and make individuals more prone to the development of EDS.
Becoming a full-time worker reduced the risk of EDS over the 10-y period, which may indicate that women become more alert when they start full-time work. However, it may also indicate that reduced EDS make women able to work full-time. When analyzing the reverse causality, there was no indication that reduced EDS was related to full-time work (adjusted OR = 0.85; 95% CI 0.61–1.20), thereby indicating that becoming a full-time worker can reduce the risk of EDS. Working shifts or nights did not increase the risk of EDS in this female population. Arguably, this could be due to the fact that both working shifts and working nights were combined. However, entering the variables separately into the models did not change the results. Studies of full-time work and EDS are sparse. Breslau et al.48 showed, in a young population, that working full-time was associated with daytime sleepiness. Several studies have reported on the risk of EDS when working shift and/or nights both in young populations48 and in populations with a wider age range.32 The discrepancies between previous studies and ours regarding both full-time work and night work or shift work may be due to differences in the selection and size of the population, but also to differences in the assessment of occupational status and shift work/night work.
Cohabiting at both time points but also going from cohabiting to not cohabiting reduced the risk of EDS, which could indicate that marital status has a different effect on EDS in different women. It could also be argued that the correlation between not cohabiting at follow-up but at baseline and the lower risk of incident EDS is due to women in this group being older and caring for a sick spouse at baseline but not at follow-up, thereby becoming more alert over the study period. However, age in this group did not differ significantly compared with the other groups within the variable of “marital status”. Few previous studies have reported on association between marital status and EDS. Nonetheless, Breslau et al.48 showed that marriage reduces the risk of EDS in young adults.
In the whole group of women, children living at home at follow-up but not at baseline was a factor leading to increased risk of development of EDS, whereas there was no increased risk in healthy women. This indicates that somatic disease in women modifies the effect of children living at home on the risk of EDS. We have previously shown in a cross-sectional study of this female population that having children did not correlate with having EDS7 and, moreover, in a cross-sectional analysis of the women at follow-up in the current study, there was no relationship between children at home and having EDS (1.22; 0.89–2.04).
Bidirectional Relationships
Analyses of reverse causality showed that, in the whole group of women, incident EDS increased the risk of incident and persistent insomnia, as well as incident anxiety/depression, somatic disease, obesity, and smoking. In addition, incident EDS reduced the chance of becoming a full-time worker. As in the whole population, incident EDS in healthy women was a risk factor for incident and persistent insomnia and also for incident anxiety/depression and smoking. Furthermore, in otherwise healthy women, incident EDS reduced the chance of a remission in anxiety/depression and increased the risk of persistent anxiety/depression. A remission of EDS did not increase the chance of changing to a high physical activity level, but incident EDS did reduce the chance of changing to a high physical activity level, indicating that the relationship is likely to be oriented toward an increase in physical activity, reducing EDS.
Although we saw several bidirectional relationships, it is also important to remember that several of the risk factors that were examined, such as anxiety and depression, as well as insomnia symptoms, have a waxing and waning natural course,49 whereas other risk factors such as obesity are more persistent. These facts may have had an effect on the reverse causality analyses. Nonetheless, it seems important not only to treat insomnia, anxiety/depression, somatic disease, and obesity and to inform women about the hazards of smoking, as they all increase the risk of EDS, but also to treat EDS in women with and without these issues, as they may have a negative effect on prognosis.
The current study is a large population-based longitudinal study with a good response rate. However, there are limitations when interpreting the results. First, all the data are self-reported and, second, EDS was assessed using a five-point scale instead of using a more common measurement, such as the Epworth Sleepiness Scale (ESS).50 Nonetheless, the five-point scale has been compared with the ESS and has been found to correlate relatively well with the ESS.51 Third, there is a close relationship between sleepiness and fatigue, which could make it difficult to differentiate between the two. By assessing subjective EDS, there may have been an overlap with fatigue, as we did not include a question on sleep attacks that would have assessed sleep propensity more effectively. However, from baseline data in this female cohort, we know that the question used produces an overlap with fatigue but also assesses EDS as a separate entity.7 Fourth, the definition of insomnia used in the current study could potentially include participants with a wide range of symptoms from poor sleep to chronic insomnia and insomnia syndrome. This may have had an effect on the results, as insomnia has a stronger relationship with fatigue than with EDS. In addition, because of the subjective nature of data collection a full knowledge of concurrent sleep disorders may not have been attained. Last, it should also be noted that the study was conducted in a sample of urban women in a country with a fairly high economic status, which may interfere with the generalizability of the results.
To summarize, the current longitudinal study shows that insomnia, anxiety, and depression and smoking are the most important factors for predicting incident EDS, but also that incident somatic disease, obesity and snoring predict the development of EDS. There are also several bidirectional relationships. It is therefore important not only to treat the symptoms of insomnia, anxiety/depression, somatic disease, and obesity and to inform women about a healthy lifestyle in order to reduce EDS but also to treat EDS in women with and without these issues, as they may have a negative effect on prognosis.
DISCLOSURE STATEMENT
This was not an industry supported study. Funding from the study was provided by The Swedish Heart Lung Foundation and the Uppsala County Association against Heart and Lung Diseases. The authors have indicated no financial conflicts of interest. The work was performed at the Department of Medical Sciences, Respiratory, Allergy and Sleep Research, Uppsala University, Sweden.
REFERENCES
- 1.Kaneita Y, Ohida T, Uchiyama M, et al. Excessive daytime sleepiness among the Japanese general population. J Epidemiol. 2005;15:1–8. doi: 10.2188/jea.15.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hara C, Lopes Rocha F, Lima-Costa MFF. Prevalence of excessive daytime sleepiness and associated factors in a Brazilian community: the Bambuí study. Sleep Med. 2004;5:31–6. doi: 10.1016/j.sleep.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Joo S, Baik I, Yi H, Jung K, Kim J, Shin C. Prevalence of excessive daytime sleepiness and associated factors in the adult population of Korea. Sleep Med. 2009;10:182–8. doi: 10.1016/j.sleep.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Pereira EC, Schmitt AC, Cardoso MR, et al. Prevalence of excessive daytime sleepiness and associated factors in women aged 35-49 years from the “Pindamonhangaba Health Project” (PROSAPIN) Rev Assoc Med Bras. 2012;58:447–52. [PubMed] [Google Scholar]
- 5.Ford ES, Cunningham TJ, Giles WH, Croft JB. Trends in insomnia and excessive daytime sleepiness among US adults from 2002 to 2012. Sleep Med. 2015;16:372–8. doi: 10.1016/j.sleep.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohayon MM. Determining the level of sleepiness in the American population and its correlates. J Psychiatr Res. 2012;46:422–7. doi: 10.1016/j.jpsychires.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Theorell-Haglow J, Lindberg E, Janson C. What are the important risk factors for daytime sleepiness and fatigue in women? Sleep. 2006;29:751–7. doi: 10.1093/sleep/29.6.751. [DOI] [PubMed] [Google Scholar]
- 8.Lindberg E, Janson C, Gislason T, Björnsson E, Hetta J, Boman G. Sleep disturbances in a young adult population: can gender differences be explained by differences in psychological status? Sleep. 1997;20:381–7. doi: 10.1093/sleep/20.6.381. [DOI] [PubMed] [Google Scholar]
- 9.Rockwood K, Davies HS, Merry HR, MacKnight C, MacDowell I. Sleep disturbances and mortality: results from the Canadian study of health and aging. J Am Geriatr Soc. 2001;49:639–41. doi: 10.1046/j.1532-5415.2001.49125.x. [DOI] [PubMed] [Google Scholar]
- 10.Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. II. Sleep. 1999;22:354–8. [PubMed] [Google Scholar]
- 11.Asplund R, Åberg H. Daytime sleepiness in 40-64 year-old women in relation to somatic health and medical treatment. Scand J Prim Health Care. 1998;16:112–6. doi: 10.1080/028134398750003278. [DOI] [PubMed] [Google Scholar]
- 12.Asplund R. Sleep and cardiac diseases amongst elderly people. J Intern Med. 1994;236:65–71. doi: 10.1111/j.1365-2796.1994.tb01121.x. [DOI] [PubMed] [Google Scholar]
- 13.Ohayon MM, Caulet M, Philip P, Guilleminault C, Priest RG. How sleep and mental disorders are related to complaints of daytime sleepiness. Arch Intern Med. 1997;157:2645–52. [PubMed] [Google Scholar]
- 14.Fernandez-Mendoza J, Vgontzas AN, Kritikou I, Calhoun SL, Liao D, Bixler EO. Natural history of excessive daytime sleepiness: role of obesity, weight loss, depression, and sleep propensity. Sleep. 2015;38:351–60. doi: 10.5665/sleep.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johns M, Hocking B. Daytime sleepiness and sleep habits of Australian workers. Sleep. 1997;20:844–9. doi: 10.1093/sleep/20.10.844. [DOI] [PubMed] [Google Scholar]
- 16.Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90:4510–5. doi: 10.1210/jc.2005-0035. [DOI] [PubMed] [Google Scholar]
- 17.Basta M, Lin HM, Pejovic S, Sarrigiannidis A, Bixler E, Vgontzas AN. Lack of regular exercise, depression, and degree of apnea are predictors of excessive daytime sleepiness in patients with sleep apnea: sex differences. J Clin Sleep Med. 2008;4:19–25. [PMC free article] [PubMed] [Google Scholar]
- 18.Hasler G, Buysse DJ, Gamma A, et al. Excessive daytime sleepiness in young adults: a 20-year prospective community study. J Clin Psychiatry. 2005;66:521–9. doi: 10.4088/jcp.v66n0416. [DOI] [PubMed] [Google Scholar]
- 19.Theorell-Haglow J, Berglund L, Janson C, Lindberg E. Sleep duration and central obesity in women - differences between short sleepers and long sleepers. Sleep Med. 2012;13:1079–85. doi: 10.1016/j.sleep.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Janson C, Lindberg E, Gislason T, Elmasry A, Boman G. Insomnia in men - A 10-year prospective poulation based study. Sleep. 2001;24:425–30. doi: 10.1093/sleep/24.4.425. [DOI] [PubMed] [Google Scholar]
- 21.Lindberg E, Janson C, Svardsudd K, Gislason T, Hetta J, Boman G. Increased mortality among sleepy snorers: a prospective population based study. Thorax. 1998;53:631–7. doi: 10.1136/thx.53.8.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hetta J, Ågren H, Hambert G, Liljengren G, Roos B. Prevalence of sleep disturbances and related symptoms in a middle-aged Swedish population. In: Koella W, Ruther E, Schultz H, editors. Sleep ‘84. Stuttgart: Gustav Fischer Verlag; 1985. pp. 373–6. [Google Scholar]
- 23.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28:2762–7. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 24.Janson C, Norbäck D, Omenaas E, et al. Insomnia is more common among subjects living in damp buildings. Occup Environ Med. 2005;62:113–8. doi: 10.1136/oem.2003.011379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lissner L, Bengtsson C, Björkelund C, Wedel H. Physical activity levels and changes in relation to longevity. A prospective study of Swedish women. Am J Epidemiol. 1996;143:54–62. doi: 10.1093/oxfordjournals.aje.a008657. [DOI] [PubMed] [Google Scholar]
- 26.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 27.Svensson M, Lindberg E, Naessen T, Janson C. Risk factors associated with snoring in women with special emphasis on body mass index: a population-based study. Chest. 2006;129:933–41. doi: 10.1378/chest.129.4.933. [DOI] [PubMed] [Google Scholar]
- 28.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults. Results of the 2003 National Sleep Foudation Sleep in America I Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Janson C, Gislason T, De Backer W, et al. Daytime sleepiness, snoring and gastro-oesophagal reflux amongst young adults in three European countries. J Intern Med. 1995;237:277–85. doi: 10.1111/j.1365-2796.1995.tb01176.x. [DOI] [PubMed] [Google Scholar]
- 30.Sauter C, Asenbaum S, Popovic R, et al. Excessive daytime sleepiness in patients suffering from different levels of obstructive sleep apnoea syndrome. J Sleep Res. 2000;9:293–301. doi: 10.1046/j.1365-2869.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- 31.Kim K, Uchiyama M, Okawa M, Liu X, Ogihara R. An epidemiological study of insomnia among the Japanese general population. Sleep. 2000;23:41–7. [PubMed] [Google Scholar]
- 32.Wilsmore BR, Grunstein RR, Fransen M, Woodward M, Norton R, Ameratunga S. Sleep habits, insomnia, and daytime sleepiness in a large and healthy community-based sample of New Zealanders. J Clin Sleep Med. 2013;9:559–66. doi: 10.5664/jcsm.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voderholzer U, Al-Shajlawi A, Weske G, Feige B, Riemann D. Are there gender differences in objective and subjective sleep measures? A study of insomniacs and healthy controls. Depress Anxiety. 2003;17:162–72. doi: 10.1002/da.10101. [DOI] [PubMed] [Google Scholar]
- 34.Silverstein B. Gender difference in the prevalence of clinical depression: the role played by depression associated with somatic symptoms. Am J Psychiatry. 1999;156:480–2. doi: 10.1176/ajp.156.3.480. [DOI] [PubMed] [Google Scholar]
- 35.Janson C, De Backer W, Gislason T, et al. Increased prevalence of sleep disturbances and daytime sleepiness in subjects with bronchial asthma: a population study of young adults in three European countries. Eur Respir J. 1996;9:2132–8. doi: 10.1183/09031936.96.09102132. [DOI] [PubMed] [Google Scholar]
- 36.de Weerd A, de Haas S, Otte A, et al. Subjective sleep disturbance in patients with partial epilepsy: a questionnaire-based study on prevalence and impact on quality of life. Epilepsia. 2004;45:1397–404. doi: 10.1111/j.0013-9580.2004.46703.x. [DOI] [PubMed] [Google Scholar]
- 37.Scott B, Borgman A, Engler H, Johnels B, Aquilonius SM. Gender differences in Parkinson's disease symptom profile. Acta Neurol Scand. 2000;102:37–43. doi: 10.1034/j.1600-0404.2000.102001037.x. [DOI] [PubMed] [Google Scholar]
- 38.Dixon JB, Dixon ME, Anderson ML, Schachter L, O'Brien PE. Daytime sleepiness in the obese: not as simple as obstructive sleep apnea. Obesity (Silver Spring) 2007;15:2504–11. doi: 10.1038/oby.2007.297. [DOI] [PubMed] [Google Scholar]
- 39.Panossian LA, Veasey SC. Daytime sleepiness in obesity: mechanisms beyond obstructive sleep apnea--a review. Sleep. 2012;35:605–15. doi: 10.5665/sleep.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McClain JJ, Lewin DS, Laposky AD, Kahle L, Berrigan D. Associations between physical activity, sedentary time, sleep duration and daytime sleepiness in US adults. Prev Med. 2014;66:68–73. doi: 10.1016/j.ypmed.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Svensson M, Franklin KA, Theorell-Haglow J, Lindberg E. Daytime sleepiness relates to snoring independent of the apnea-hypopnea index in women from the general population. Chest. 2008;134:919–24. doi: 10.1378/chest.08-0847. [DOI] [PubMed] [Google Scholar]
- 42.Puig F, Rico F, Almendros I, Montserrat JM, Navajas D, Farre R. Vibration enhances interleukin-8 release in a cell model of snoring-induced airway inflammation. Sleep. 2005;28:1312–6. doi: 10.1093/sleep/28.10.1312. [DOI] [PubMed] [Google Scholar]
- 43.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 44.Phillips BA, Danner FJ. Cigarette smoking and sleep disturbance. Arch Intern Med. 1995;155:734–7. [PubMed] [Google Scholar]
- 45.Zhang L, Samet J, Caffo B, Punjabi NM. Cigarette smoking and nocturnal sleep architecture. Am J Epidemiol. 2006;164:529–37. doi: 10.1093/aje/kwj231. [DOI] [PubMed] [Google Scholar]
- 46.McNamara JP, Wang J, Holiday DB, et al. Sleep disturbances associated with cigarette smoking. Psychol Health Med. 2014;19:410–9. doi: 10.1080/13548506.2013.832782. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez-Mendoza J, Vgontzas AN, Bixler EO, et al. Clinical and polysomnographic predictors of the natural history of poor sleep in the general population. Sleep. 2012;35:689–97. doi: 10.5665/sleep.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Breslau N, Roth T, Rosenthal L, Andreski P. Daytime sleepiness: an epidemiological study of young adults. Am J Public Health. 1997;87:1649–53. doi: 10.2105/ajph.87.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morin CM, Belanger L, LeBlanc M, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447–53. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 50.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 51.Theorell-Haglöw J, Janson C, Lindberg E. Assessing daytime sleepiness in women: a comparison of different methods. Lungeforum. 2005;15:13. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.