Abstract
Endotoxin causes gastrointestinal motility disorder. Aim of this study is to clarify inhibitory mechanisms of lipopolysaccharide (LPS) on smooth muscle contraction in rat ileum. Ileal tissues were isolated from control rat or from LPS-induced peritonitis model rat. Treatment with LPS inhibited carbachol (CCh)-mediated contraction in a time-dependent manner. Cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) genes were also upregulated, but iNOS expression was preceded by a rising of COX-2. All subtypes of prostaglandin E2 (PGE2) receptors (EP1-EP4) were expressed in ileum, and PGE2 and selective EP2 or EP4 agonist inhibited CCh-mediated contraction. Selective iNOS inhibitor did not reverse LPS-induced inhibition of contraction by CCh at 1 and 2 hr, but reduced the inhibitory action at 4 hr after the LPS treatment. COX-2 inhibitor reversed the inhibitory action by LPS in all exposure time. Finally, in ileal tissues isolated from peritonitis model rat, iNOS expression was upregulated only at 4 hr after LPS administration, resulting in enhanced inhibitory action of LPS against CCh-induced contraction. In conclusion, LPS induces COX-2 to produce PGE2, which initially activates EP2 and/or EP4 on smooth muscle cells to inhibit the contractility in early phase of LPS exposure. Moreover, in late phase of LPS treatment, iNOS is expressed to produce NO, which in turn inhibited the contraction by CCh. The inhibitory cascade is similar in the ileum isolated from peritonitis model rat, indicating time-dependent changes of inhibitory action by LPS on intestinal motility in peritonitis.
Keywords: ileum, inducible nitric oxide synthase, lipopolysaccharide, macrophage, prostaglandin E2
Intestinal motility is physiologically regulated by cell-to-cell communication between smooth muscle cells, myenteric plexus neuron and interstitial cells of Cajal (ICC) [21]. In addition to this network, muscularis resident macrophages are normally present in the same layer [9, 14]. Recent works accumulated evidence that the muscularis resident macrophages affect the gastrointestinal (GI) network to modulate GI motility in pathophysiological situation. For example, colonic obstruction activates the muscularis resident macrophages to impair ICC functions, resulting in induced abnormal pacemaking ability [25, 32]. Resident macrophages and infiltrated monocytes-derived macrophages induce nitric oxide (NO) synthase (NOS) to produce NO, which in turn inhibits GI motility in postoperative ileus [1, 10, 28]. It has been also reported that intestinal muscularis resident macrophages play an important role to induce motility disorder in peritonitis induced by lipopolysaccharide (LPS) [7, 30].
We previously focused on the inhibitory mechanism of LPS on GI motility in the ileum and found that LPS selectively activated muscularis resident macrophages to induce cyclooxygenase-2 (COX-2) and inducible NOS (iNOS) genes, resulting in motility disorder via NO production [9, 27]. In the myenteric plexus region, interestingly, LPS initially induces COX-2 to produce PGE2, which in turn induces iNOS via activation of prostaglandin (PG) E2 receptors on intestinal macrophages in an autocrine and/or a paracrine manner [26]. However, it is still unclear whether released PGE2 may directly modulate smooth muscle contractility in small intestine when LPS is exposed to the ileum.
PGE2 receptors (EPs) have characteristic seven-hydrophobic transmembrane segment structure of G-protein coupled receptors, which are classified into 4 subtypes (EP1–EP4). It has been reported that small intestine mainly expresses EP2 and EP4 whereas expressions of EP1 and EP3 are minor [24]. However, each EP exhibits differences in signal transduction, tissue localization and animal species. PGE2 contracts longitudinal smooth muscles in mice ileum possibly via EP1 and EP3 [19, 20]. On the other hand, PGE2 inhibits ileal contractility in rat [22]. Thus, it is necessary to examine the effects of PGE2 on smooth muscle contractility in each tissue of each animal species.
Objective of this study is to clarify detailed inhibitory mechanisms of LPS on contractility of ileal smooth muscle in rat, which will lead to a better understanding of mechanism of intestinal motility disorder in peritonitis.
MATERIALS AND METHODS
Animal: Male Sprague-Dawley rats (250–300 g; Charles River Japan, Yokohama, Japan) were cared for in strict compliance with the Guide to Animal Use and Care published by the University of Tokyo. The Institutional Review Board of the Graduate School of Agricultural and Life Sciences of the University of Tokyo approved this study. The rats were maintained under a constant 12 hr light-dark cycle at environmental temperature of 20–25°C.
Measurement of muscle contractile ability: The smooth muscle layer was isolated from the terminal ileum of rat and set in organ bath filled with modified physiological salt solution (PSS; containing NaCl 136.9 mM, KCl 5.4 mM, MgCl2 1.0 mM, CaCl2 1.5 mM, NaHCO3 23.8 mM and glucose 5.5 mM) which was aerated with 95% O2–5% CO2 to adjust pH to 7.4 at 37°C. Muscle contraction of longitudinal ileal tissue was isometrically measured under a resting tension of 10 mN. Isometric force in the presence of papaverine (100 µM) and maintained level of carbachol (CCh)-induced contraction at 10 min was taken as 0% or 100%, respectively. In some experiments, absolute force was calculated by mN/mg wet weight of each tissue. In all control experiments, we added the same amount of dimethyl sulfoxide (DMSO) as vehicle.
RT-PCR analysis: Total RNA was extracted from the muscle layer of the terminal ileum using Trizol (Molecular Research Center, Inc., Cincinnati, OH, U.S.A.) and analyzed by semi-quantitative RT-PCR as described previously [9]. Briefly, the purified total RNA was reverse transcribed using ReverTra Ace in random 9-mer oligonucleotide primers (TaKaRa Bio, Otsu, Japan) at 30°C for 10 min, 42°C for 1 hr and 99°C for 5 min. Amplification was performed by a PCR Thermal Cycler MP (TaKaRa Bio) using 30–35 cycles, each consisting of 98°C for 10 sec, 52–58°C for 30 sec and 72°C for 1 min. The sequences of the primer were as follows: AAG RGA GTG YTG TTC CAG GT (rat iNOS forward), CCA CCA GCT TCT TCA AMG TG (rat iNOS reverse), CTG TAT CCC GCC CTG CTG GTG (rat COX-2 forward), ACT TGC GTT GAT GGT GGC TGT CTT (rat COX-2 reverse), ATG GTC TTC TTT GGC CTG TG (rat EP1 forward), GTT CTC TCG GAA ACG TCG AG (rat EP1 reverse), GAA CGC TAC CTC GCC ATC GG (rat EP2 forward), CGA AGG TGA TGG TCA TAA ATG GC (rat EP2 reverse), CTT TGC CTC CGC CTT CGC C (rat EP3 forward), CTT AGC AGC AGA TAA ACC CAG G (rat EP3 reverse), CAT CTT ACT CAT CGC CAC CTC TC (rat EP4 forward), GTT AGG TCT GGC AGG TAT AGG AGG (rat EP4 reverse), TAC CAG CCG GGG GAC CAC (rat GAPDH forward) and CGA GCT GAC AGA GTA GTA (rat GAPDH reverse). The expected product sizes were 184, 282, 387, 421, 363, 393 and 308 base pairs, respectively. The PCR products were electrophoresed on 2% agarose gels containing 0.01% ethidium bromide. Separated bands were detected using an ultraviolet -transilluminator equipped with FAS III gel scanner (FAS III, Toyobo, Tokyo, Japan). The bands were measured using NIH Image software (Image J, Ver. 1.38x), and the levels of PCR products were normalized to those of GAPDH for quantification.
Animal model of peritonitis: Peritonitis model was made by intraperitoneal administration of LPS as previously reported [6, 17]. Briefly, animals were injected intraperitoneally with LPS (15 mg/kg body weight) or vehicle (phosphate buffered saline). The dose of LPS was conformed to LD50 in strict compliance with the Guide to Animal Use and Care published by The University of Tokyo.
Statistical analyses: Results are expressed as means ± SEM. The data were statistically analyzed using the unpaired Student’s t test for comparisons between 2 groups and by one-way analysis of variance (ANOVA) followed by Dunnett’s test for comparisons among 3 or more groups. A value of P<0.05 was taken as significant.
RESULTS
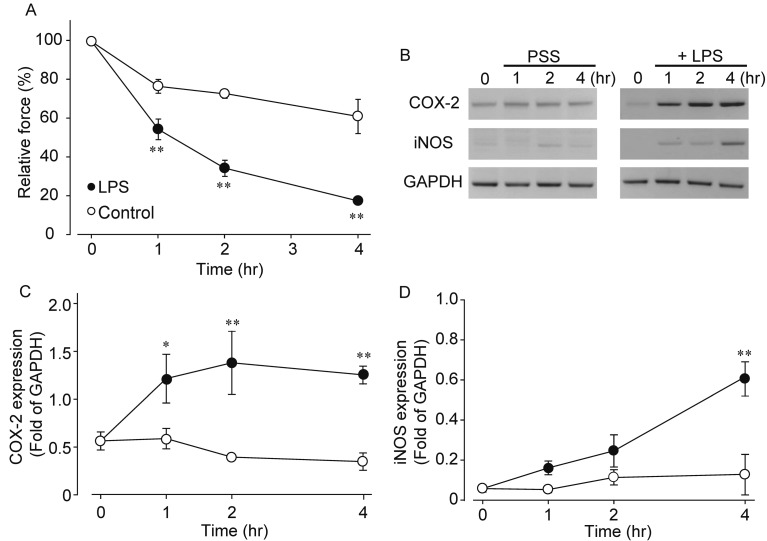
Effects of LPS on muscle contraction and expression of COX-2 and iNOS genes in isolated rat ileum: CCh (1 µM) induced phasic contraction followed by sustained contraction. Pre-treatment with LPS (100 µg/ml) inhibited CCh-induced contraction in both phases, and we quantified the inhibitory action at sustained contraction 10 min after application of CCh (Fig. 1A). Results indicated that LPS time-dependently inhibited the CCh-induced contraction (control 1 hr; 77.0 ± 8.4%, control 2 hr; 73.1 ± 5.7%, control 4 hr; 61.5 ± 24.4%, LPS 1hr; 54.8 ± 12.2%, LPS 2 hr; 34.8 ± 9.8% and LPS 4 hr; 18.0 ± 4.6%, n=5–9). It has been reported that LPS induces the expression of COX-2 and iNOS genes in rat ileum [9]. Therefore, we measured time-dependent changes in mRNA expression of both genes by LPS stimulation (Fig. 1B–1D). Expression of COX-2 mRNA was increased at 1 hr after treatment with LPS and then maintained steady level. On the other hand, iNOS gene tended to increase at 1–2 hr after treatment with LPS, but was insignificant; however, after 4 hr treatment with LPS, iNOS gene was significantly upregulated. Thus, iNOS expression was preceded by a rising of COX-2 gene.
Fig. 1.
Effects of LPS on CCh-induced contraction and time-dependent changes in COX-2 and iNOS gene expression by LPS. A: Inhibitory action of LPS on CCh-induced contraction. The sustained contraction 10 min after application of CCh (1 µM) was quantified. LPS (100 µg/ml) was pre-treated 0 to 4 hr before application of CCh. B-D: Effects of LPS on mRNA expression of COX-2 and iNOS. Typical results were shown in B from 4-8 experiments. Quantified results for COX-2 (C) and iNOS (D) were shown. Each symbol showed mean ± SEM. * or **P<0.05 or P<0.01, significantly different from control, respectively (n=5–9). Control means non-treated ileal tissue. The ileal tissue was incubated in the modified physiological salt solution (PSS) for 1–4 hr.
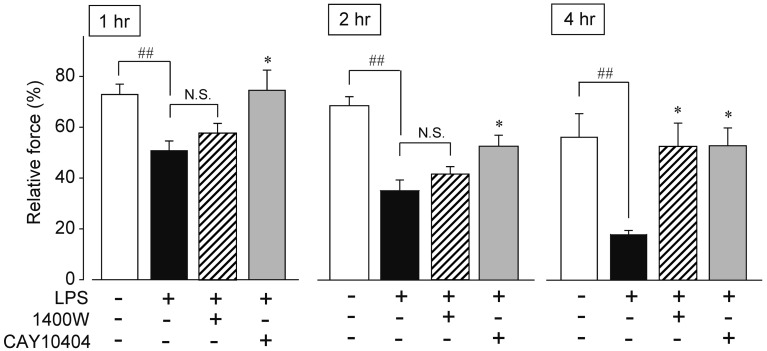
Effects of 1400W and CAY10404 on LPS-induced inhibitory action against the CCh-induced contraction in isolated rat ileum: Results of Fig. 1 indicated that COX-2-mediated PGs and iNOS-mediated NO may be related to inhibitory action of LPS on smooth muscle contraction. So, we next examined the effects of iNOS inhibitor, 1400 W, and COX-2 inhibitor, CAY10404, on the LPS-induced inhibitory action at 1, 2 and 4 hr after the LPS treatment (Fig. 2). In this study, we selected maximum concentration of CAY10404 and 1400 W to obtain maximum inhibitory action as previously reported [18, 26]. At 1 hr after the LPS (100 µM) treatment, CCh-mediated contraction was inhibited about 35% of control (control; 77.0 ± 3.8%, LPS 1 hr; 50.8 ± 3.9, n=8, respectively). 1400 W (10 µM) did not recover the inhibitory action. The inhibitory action of LPS was increased to 50% 2 hr after the LPS treatment (control; 67.8 ± 3.5%, LPS 2 hr; 34.8 ± 4.1, n=6 or 8, respectively). 1400 W tended to recover the LPS-induced inhibitory action against CCh-mediated contraction, but it is not significantly different. At 4 hr after the LPS treatment, the LPS-induced inhibitory action was further increased to 70% of control (control; 55.7 ± 9.2%, LPS 4 hr; 18.0 ± 1.7, n=6 or 5, respectively). 1400 W almost completely reduced the inhibitory action of LPS. On the other hand, COX-2 inhibitor, CAY-10404 (10 µM), completely abolished the LPS-induced inhibition of CCh-mediated contraction in all exposure time of LPS. Similar results were also obtained by non-selective COX inhibitor, indomethacin (10 µM) (n=6–8, data not shown).
Fig. 2.
Effects of iNOS inhibitor, 1400 W, and COX-2 inhibitor, CAY10404, on the LPS-induced inhibition of CCh-induced contraction. 1400 W (10 µM) or CAY10404 (10 µM) was simultaneously added with LPS (100 µM). Each column showed mean ± SEM. ##P<0.01 significantly different from control. *P<0.05 significantly different from LPS (n=4–10). N.S.; not significantly different.
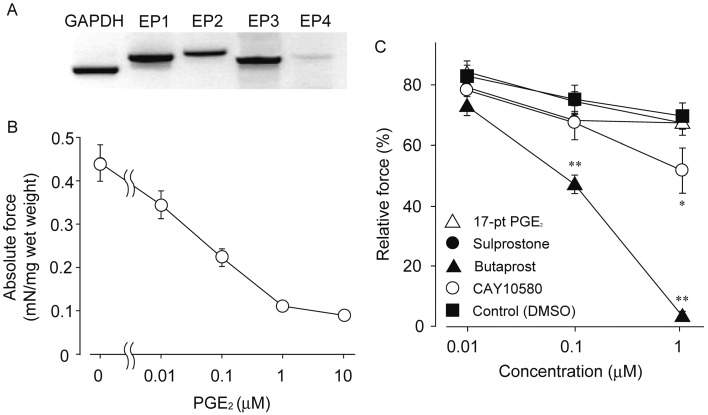
Expression of EPs and effects of PGE2 and EPs agonists on CCh-induced contraction in isolated rat ileum: Figure 3A showed mRNA expression of EPs in rat ileal smooth muscle tissue. All EPs were expressed at mRNA level, but expression level of EP4 was lower than other EPs. We next examined effect of PGE2 on CCh-mediated contraction. PGE2 (0.01–10 µM) inhibited the CCh-mediated contraction in a concentration-dependent manner as shown in Fig. 3B. PGE2 did not show any contractile response when it was applied to sustained phase of the CCh-induced contraction. We further investigated effects of several EPs agonists on the CCh-induced contraction (Fig. 3C). Relatively selective agonist for EP2, butaprost (0.1 and 1 µM), significantly inhibited the contraction (1 µM butaprost; 3.4 ± 3.5%). EP4 selective agonist, CAY10580 (1 µM), also induced a weak inhibitory action against the CCh-mediated contraction (1 µM CAY10580; 51.7 ± 16.9%). On the other hand, relatively selective agonist for EP1 and EP3, 17-phenyl trinor PGE2 (17-pt PGE2; 1 µM) and sulprostone (1 µM), had no effects on the CCh-mediated contraction (Fig. 3C). In all control experiments, administration of only vehicle (0.01% DMSO) had no effect.
Fig. 3.
Expression of EPs in intestinal smooth muscle in rat and effects of PGE2 and EPs agonists on the CCh-induced contraction. A showed mRNA expression of EPs in intestinal smooth muscle of rat. B: Inhibitory effect of PGE2 on the CCh (1 µM)-induced contraction. Each symbol showed mean ± SEM (n=8 each). C: Inhibitory effects of EPs agonists on the CCh-induced contraction. Each symbol showed mean ± SEM (n=10–14). * or **P<0.05 or P<0.01, significantly different from control, respectively. 17-pt PGE2; 17-phenyl trinor PGE2
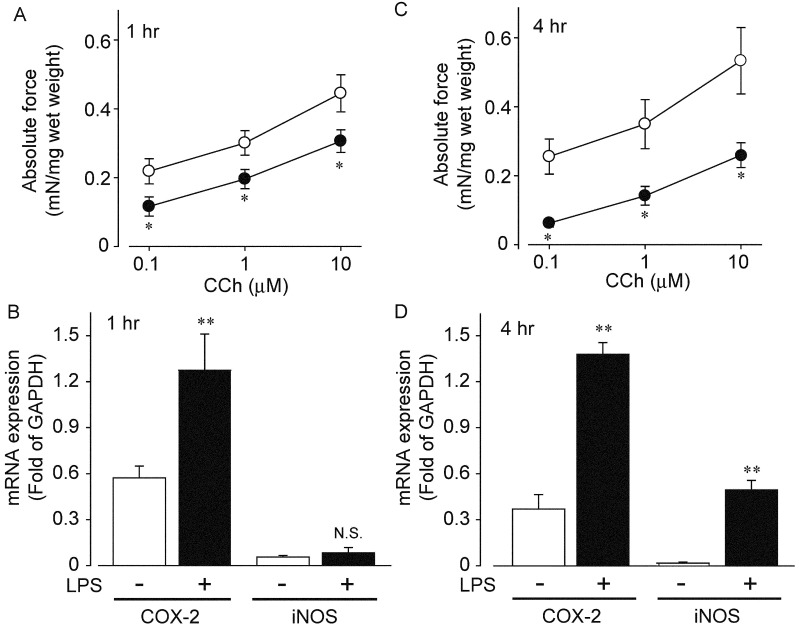
Changes in CCh-mediated contraction, and mRNA expression of COX-2 and iNOS in ileal smooth muscles isolated from peritonitis model rat: Finally, we investigated changes in CCh-mediated contraction in ileal smooth muscle tissue isolated from peritonitis model rat induced by LPS treatment. We isolated ileum from the model rat 1 or 4 hr after intraperitoneal administration of LPS (15 mg/kg) and made the preparations. CCh-mediated contraction was significantly inhibited at all concentrations of CCh as shown in Fig. 4A. Absolute force induced by CCh was decreased by approximately 35% of control (control CCh 1 µM; 0.30 ± 0.09 mN/mg and peritonitis 1 hr CCh 1 µM; 0.19 ± 0.06 mN/mg, n=5 and 8, respectively) at 1 hr after LPS treatment. The absolute force was further decreased by approximately 60% of control 4 hr after intraperitoneal administration of LPS (control CCh 1 µM; 0.34 ± 0.16 mN/mg and peritonitis 1 hr CCh 1 µM; 0.14 ± 0.06 mN/mg, n=5 and 8, respectively, Fig. 4C).
Fig. 4.
Changes in CCh-induced contraction and mRNA expression of COX-2 and iNOS in the ileum isolated from wild type or peritonitis model of rat treated with LPS. LPS (15 mg/kg) was intraperitoneally injected. Each muscle strip was made 1 or 4 hr after the LPS administration. A and C showed change in CCh-induced contraction in the ileum isolated from control (open circle) or peritonitis model (closed circle) of rat. Each symbol showed mean ± SEM (n=5–9). B and D indicated changes in mRNA expression of COX-2 and iNOS in the ileum isolated from wild type or peritonitis model of rat. Each column showed mean ± SEM. * or **P<0.05 or P<0.01, significantly different from control, respectively.
We further investigated changes in mRNA expression of both COX-2 and iNOS in the ileal tissues prepared from the peritonitis model rat. COX-2 mRNA was significantly increased 1 hr after intraperitoneal administration of LPS (Fig. 4B), and the expression level was maintained at 4 hr after the administration (Fig. 4D). In contrast, mRNA expression of iNOS was not increased at 1hr, but significantly upregulated at 4 hr after the administration (Fig. 4B and 4D).
DISCUSSION
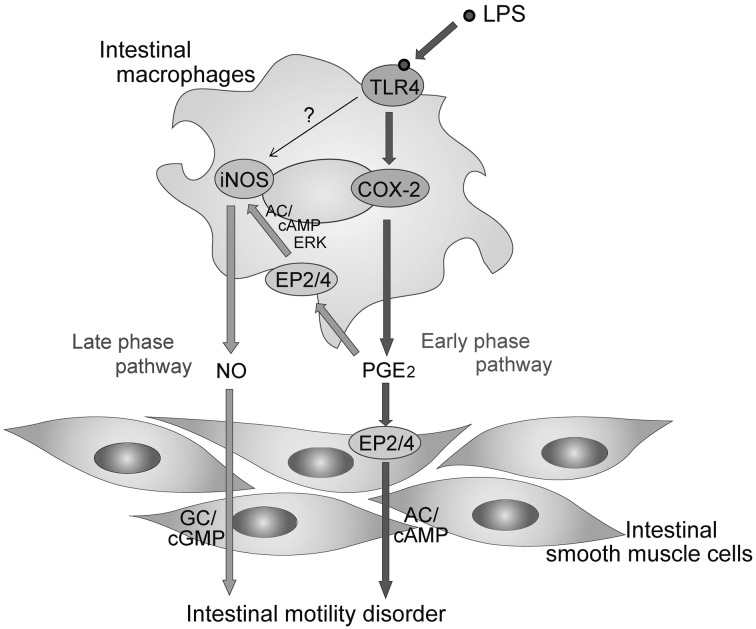
Intestinal resident macrophages and monocyte derived infiltrated macrophages have been known to play an important role in intestinal dysmotility in postoperative ileus [11, 29, 31] and peritonitis [6, 7]. Series of experiments by using iNOS null mice revealed that motility disorder is caused by NO through iNOS induction in postoperative ileus [29]. In a similar fashion, inflammatory events in postoperative ileus are reduced in COX-2 null mice, indicating pathogenic importance of PGs through COX-2 activation in postoperative ileus [23]. We previously elucidated interaction between COX-2 and iNOS in small intestine [9, 26, 27]. LPS directly activates resident macrophages to produce PGE2 that subsequently stimulates EP2/EP4 on resident macrophages in an autocrine and/or paracrine manner. The activated macrophages by PGE2 induce iNOS gene to produce NO, resulting in impaired intestinal motility through guanylate cyclase (GC)/cGMP signaling [12] (Fig. 5; Late phase pathway). The induction of iNOS by PGE2 may be regulated through adenylate cyclase (AC)/cAMP signaling and ERK activation [26]. In this study, COX-2 inhibitor, CAY10404, completely reversed LPS-induced inhibition of CCh-mediated contraction 4 hr after the LPS treatment. As ileal muscle tissues were simultaneously treated with CAY10404 and LPS, the result may reflect regulation of iNOS gene by COX-2/PGE2 signaling as described previously [9, 26].
Fig. 5.
Possible hypothesis; two signaling pathways of LPS to induce motility disorder in intestinal smooth muscles. LPS stimulates TLR4 receptor to induce COX-2. Released PGE2 stimulates EP2/ EP4 on smooth muscle cells via AC/cAMP signaling to lead motility disorder (Early phase pathway). In addition, prolonged release of PGE2 stimulates EP2/EP4 receptors on intestinal macrophages to induce iNOS via AC/cAMP/ERK signal. Inducible NOS produces NO, resulting in the inhibition of intestinal motility via GC/cGMP signaling (Late phase pathway) [9, 26]. In small intestinal muscle layer, direct induction of iNOS gene via activation of TLR4 may be minor pathway. TLR4; Toll-like receptor 4. To confirm this hypothesis, it will be required to measure time-dependent changes in NO and PGE2 when LPS is applied to rat ileum.
On the other hand, it is still unclear whether produced PGE2 by LPS could directly affect smooth muscle cells to inhibit contraction in the ileum. There are several reports about effects of PGE2 on intestinal motility. PGE2 induced contraction and relaxation of the longitudinal and the circular muscle layers, respectively [2, 3], whereas PGE2 was observed to contract the circular muscles in some reports [2, 15]. In this study, PGE2 inhibited CCh-mediated contraction of ileal tissues, supporting an idea that PGE2 may induce muscle relaxation at least in longitudinal smooth muscle tissues without mucosa in rat ileum. In addition, several reports support that LPS decreases contractility via PGE2 production in small intestine [9, 19, 20, 26].
It has been reported that intestinal motility is also decreased by PGI2 in addition to PGE2 [13]. PGI2 content is increased in the rat ileal tissue stimulated with LPS [9], indicating a possibility that PGI2 may also induce dysmotility in the LPS-treated ileum. However, as half-life (t1/2) of PGI2 is much faster than PGE2 (t1/2 of PGI2; 4 min and t1/2 of PGE2; 26 hr) [8], and produced content of PGI2 was much less than PGE2 [9], suggesting the less involvement of PGI2 for the LPS-induced intestinal motility disorder. Taken together, this study proposes that LPS can induce COX-2 gene to produce PGE2, which in turn inhibited intestinal motility.
We further examined effects of EPs agonists on the CCh-mediated contraction to identify EPs subtype to induce muscle relaxation. Results indicated that relatively selective agonist for EP2, butaprost, significantly inhibited the CCh-mediated contraction. EP4 agonist, CAY10580, also induced a weak inhibitory action, indicating that stimulation of EP2 and/or EP4 may inhibit the CCh-induced contraction. As shown in Fig. 3A, mRNA expression of EP4 was less than that of EP2, leading to possibility that activation of EP2 may mainly induce muscle relaxation rather than that of EP4. In fact, it has been reported that EP4 is mainly expressed in mucosal layer of rat intestine to regulate mucus secretion and sodium absorption [5, 16]. Here, we showed that LPS-induced inhibition of contraction by CCh may be mediated through activation of EP2 and/or EP4 in smooth muscle cells 1–2 hr after the LPS-treatment, because selective iNOS inhibitor, 1400 W, did not reduce the inhibitory action. Whereas, 1400 W significantly reduced the LPS-induced inhibitory action at 4 hr after the treatment with LPS, indicating NO-mediated action via iNOS expression. In accordance with these results, mRNA expression of iNOS was significantly upregulated at 4 hr after the LPS treatment. It is well known that EP2 and EP4 coupled with Gs stimulate AC/cAMP signaling [4, 16], indicating that accumulated cAMP may induce motility disorder in intestinal smooth muscle cells [12]. Thus, LPS exposure initially induced intestinal motility disorder via direct activation of EP2/EP4 on smooth muscle cells indicated as “early phase pathway” in Fig. 5. The early phase pathway via direct activation of EP2/EP4 on smooth muscle cells may disappear at the late phase of LPS exposure, because the inhibitory action of CAY-10404 was the same with that of 1400 W at 4 hr after treatment with LPS as shown in Fig. 2. We could not clarify the reason why PGE2/cAMP signaling in smooth muscle cells disappears at the late phase of LPS exposure in rat ileum. Further examination will be required to clarify this point.
In the isolated ileal tissues from peritonitis model rat induced by LPS, CCh-induced contraction was inhibited in a time-dependent manner as shown in Fig. 4A and 4C. One hour after intraperitoneal administration of LPS, the expression of COX-2 but not iNOS gene was significantly upregulated in the ileal smooth muscle tissue. On the other hand, 4 hr after the administration of LPS, both genes were upregulated. These results indicate that similar early and late phase pathways obtained in ex vivo experiments as shown in Fig. 5 may induce intestinal muscle relaxation in peritonitis. Further investigation will be necessary to clarify it.
In conclusion, LPS induces relaxation of intestinal smooth muscles via early and late phase signaling pathways. COX-2-mediated PGE2 directly causes the relaxation via EP2/EP4 on smooth muscle cells as early phase pathway. Prolonged production of PGE2 further stimulates macrophages to induce iNOS gene, which in turn inhibits muscle contraction via NO as late phase pathway. These signalings will occur in disorder of intestinal motility in peritonitis in vivo.
Acknowledgments
This work was supported by Grant-in-Aid for Scientific Research from the Japanese Ministry of Education to MH and HO. No conflicts of interest, financial or otherwise, are declared by the authors. HO, TM and MH designed the research; HO and MS performed experiments; MSI, NK, TM, HO and MH discussed the research study; SM and MH wrote the paper.
REFERENCES
- 1.Bauer A. J., Boeckxstaens G. E.2004. Mechanisms of postoperative ileus. Neurogastroenterol. Motil. 16 (Suppl. 2: 54–60. doi: 10.1111/j.1743-3150.2004.00558.x [DOI] [PubMed] [Google Scholar]
- 2.Bennett A., Eley K. G., Stockley H. L.1975. The effects of prostaglandins on guinea-pig isolated intestine and their possible contribution to muscle activity and tone. Br. J. Pharmacol. 54: 197–204. doi: 10.1111/j.1476-5381.1975.tb06929.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Botella A., Delvaux M., Fioramonti J., Frexinos J., Bueno L.1993. Stimulatory (EP1 and EP3) and inhibitory (EP2) prostaglandin E2 receptors in isolated ileal smooth muscle cells. Eur. J. Pharmacol. 237: 131–137. doi: 10.1016/0014-2999(93)90102-N [DOI] [PubMed] [Google Scholar]
- 4.Breyer R. M., Bagdassarian C. K., Myers S. A., Breyer M. D.2001. Prostanoid receptors: subtypes and signaling. Annu. Rev. Pharmacol. Toxicol. 41: 661–690. doi: 10.1146/annurev.pharmtox.41.1.661 [DOI] [PubMed] [Google Scholar]
- 5.Ding M., Kinoshita Y., Kishi K., Nakata H., Hassan S., Kawanami C., Sugimoto Y., Katsuyama M., Negishi M., Narumiya S., Ichikawa A., Chiba T.1997. Distribution of prostaglandin E receptors in the rat gastrointestinal tract. Prostaglandins 53: 199–216. doi: 10.1016/S0090-6980(97)00015-4 [DOI] [PubMed] [Google Scholar]
- 6.Eskandari M. K., Kalff J. C., Billiar T. R., Lee K. K., Bauer A. J.1997. Lipopolysaccharide activates the muscularis macrophage network and suppresses circular smooth muscle activity. Am. J. Physiol. 273: G727–G734. [DOI] [PubMed] [Google Scholar]
- 7.Eskandari M. K., Kalff J. C., Billiar T. R., Lee K. K., Bauer A. J.1999. LPS-induced muscularis macrophage nitric oxide suppresses rat jejunal circular muscle activity. Am. J. Physiol. 277: G478–G486. [DOI] [PubMed] [Google Scholar]
- 8.Gryglewski R. J.2008. Prostacyclin among prostanoids. Pharmacol. Rep. 60: 3–11. [PubMed] [Google Scholar]
- 9.Hori M., Kita M., Torihashi S., Miyamoto S., Won K. J., Sato K., Ozaki H., Karaki H.2001. Upregulation of iNOS by COX-2 in muscularis resident macrophage of rat intestine stimulated with LPS. Am. J. Physiol. Gastrointest. Liver Physiol. 280: G930–G938. [DOI] [PubMed] [Google Scholar]
- 10.Kalff J. C., Carlos T. M., Schraut W. H., Billiar T. R., Simmons R. L., Bauer A. J.1999. Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology 117: 378–387. doi: 10.1053/gast.1999.0029900378 [DOI] [PubMed] [Google Scholar]
- 11.Kalff J. C., Schraut W. H., Billiar T. R., Simmons R. L., Bauer A. J.2000. Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology 118: 316–327. doi: 10.1016/S0016-5085(00)70214-9 [DOI] [PubMed] [Google Scholar]
- 12.Karaki H., Ozaki H., Hori M., Mitsui-Saito M., Amano K., Harada K., Miyamoto S., Nakazawa H., Won K. J., Sato K.1997. Calcium movements, distribution, and functions in smooth muscle. Pharmacol. Rev. 49: 157–230. [PubMed] [Google Scholar]
- 13.Lawrence R. A., Jones R. L., Wilson N. H.1992. Characterization of receptors involved in the direct and indirect actions of prostaglandins E and I on the guinea-pig ileum. Br. J. Pharmacol. 105: 271–278. doi: 10.1111/j.1476-5381.1992.tb14245.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikkelsen H. B., Thuneberg L., Rumessen J. J., Thorball N.1985. Macrophage-like cells in the muscularis externa of mouse small intestine. Anat. Rec. 213: 77–86. doi: 10.1002/ar.1092130111 [DOI] [PubMed] [Google Scholar]
- 15.Mishima K., Kuriyama H.1976. Effects of prostaglandins on electrical and mechanical activities of the guinea pig stomach. Jpn. J. Physiol. 26: 537–548. doi: 10.2170/jjphysiol.26.537 [DOI] [PubMed] [Google Scholar]
- 16.Narumiya S., Sugimoto Y., Ushikubi F.1999. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 79: 1193–1226. [DOI] [PubMed] [Google Scholar]
- 17.Ohata S., Moriyama C., Yamashita A., Nishida T., Kusumoto C., Mochida S., Minami Y., Nakada J., Shomori K., Inagaki Y., Ohta Y., Matsura T.2010. Polaprezinc Protects Mice against Endotoxin Shock. J. Clin. Biochem. Nutr. 46: 234–243. doi: 10.3164/jcbn.09-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ok S. H., Han J. Y., Sung H. J., Yang S. M., Park J., Kwon S. C., Choi M. J., Sohn J. T.2013. Ropivacaine-induced contraction is attenuated by both endothelial nitric oxide and voltage-dependent potassium channels in isolated rat aortae.Biomed. Res. Int. 2013: 565271. . doi: 10.1155/2013/565271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rebollar E., Arruebo M. P., Plaza M. A., Murillo M. D.2002. Effect of lipopolysaccharide on rabbit small intestine muscle contractility in vitro: role of prostaglandins. Neurogastroenterol. Motil. 14: 633–642. doi: 10.1046/j.1365-2982.2002.00364.x [DOI] [PubMed] [Google Scholar]
- 20.Rebollar E., Guerrero-Lindner E., Arruebo M. P., Plaza M. A., Murillo M. D.2003. Role of prostaglandins in lipopolysaccharide effects on K+-induced contractions in rabbit small intestine. Acta Physiol. Scand. 179: 299–307. doi: 10.1046/j.0001-6772.2003.01189.x [DOI] [PubMed] [Google Scholar]
- 21.Sanders K. M., Koh S. D., Ro S., Ward S. M.2012. Regulation of gastrointestinal motility–insights from smooth muscle biology. Nat. Rev. Gastroenterol. Hepatol. 9: 633–645. doi: 10.1038/nrgastro.2012.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato K., Torihashi S., Hori M., Nasu T., Ozaki H.2007. Phagocytotic activation of muscularis resident macrophages inhibits smooth muscle contraction in rat ileum. J. Vet. Med. Sci. 69: 1053–1060. doi: 10.1292/jvms.69.1053 [DOI] [PubMed] [Google Scholar]
- 23.Schwarz N. T., Kalff J. C., Turler A., Engel B. M., Watkins S. C., Billiar T. R., Bauer A. J.2001. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology 121: 1354–1371. doi: 10.1053/gast.2001.29605 [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto Y., Narumiya S.2007. Prostaglandin E receptors. J. Biol. Chem. 282: 11613–11617. doi: 10.1074/jbc.R600038200 [DOI] [PubMed] [Google Scholar]
- 25.Suzuki T., Won K. J., Horiguchi K., Kinoshita K., Hori M., Torihashi S., Momotani E., Itoh K., Hirayama K., Ward S. M., Sanders K. M., Ozaki H.2004. Muscularis inflammation and the loss of interstitial cells of Cajal in the endothelin ETB receptor null rat. Am. J. Physiol. Gastrointest. Liver Physiol. 287: G638–G646. doi: 10.1152/ajpgi.00077.2004 [DOI] [PubMed] [Google Scholar]
- 26.Tajima T., Murata T., Aritake K., Urade Y., Michishita M., Matsuoka T., Narumiya S., Ozaki H., Hori M.2012. EP2 and EP4 receptors on muscularis resident macrophages mediate LPS-induced intestinal dysmotility via iNOS upregulation through cAMP/ERK signals. Am. J. Physiol. Gastrointest. Liver Physiol. 302: G524–G534. doi: 10.1152/ajpgi.00264.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torihashi S., Ozaki H., Hori M., Kita M., Ohota S., Karaki H.2000. Resident macrophages activated by lipopolysaccharide suppress muscle tension and initiate inflammatory response in the gastrointestinal muscle layer. Histochem. Cell Biol. 113: 73–80. doi: 10.1007/s004180050009 [DOI] [PubMed] [Google Scholar]
- 28.Tsuchida Y., Hatao F., Fujisawa M., Murata T., Kaminishi M., Seto Y., Hori M., Ozaki H.2011. Neuronal stimulation with 5-hydroxytryptamine 4 receptor induces anti-inflammatory actions via α7nACh receptors on muscularis macrophages associated with postoperative ileus. Gut 60: 638–647. doi: 10.1136/gut.2010.227546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Türler A., Kalff J. C., Moore B. A., Hoffman R. A., Billiar T. R., Simmons R. L., Bauer A. J.2006. Leukocyte-derived inducible nitric oxide synthase mediates murine postoperative ileus. Ann. Surg. 244: 220–229. doi: 10.1097/01.sla.0000229963.37544.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unno N., Wang H., Menconi M. J., Tytgat S. H., Larkin V., Smith M., Morin M. J., Chavez A., Hodin R. A., Fink M. P.1997. Inhibition of inducible nitric oxide synthase ameliorates endotoxin-induced gut mucosal barrier dysfunction in rats. Gastroenterology 113: 1246–1257. doi: 10.1053/gast.1997.v113.pm9322519 [DOI] [PubMed] [Google Scholar]
- 31.Wehner S., Behrendt F. F., Lyutenski B. N., Lysson M., Bauer A. J., Hirner A., Kalff J. C.2007. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut 56: 176–185. doi: 10.1136/gut.2005.089615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Won K. J., Suzuki T., Hori M., Ozaki H.2006. Motility disorder in experimentally obstructed intestine: relationship between muscularis inflammation and disruption of the ICC network. Neurogastroenterol. Motil. 18: 53–61. doi: 10.1111/j.1365-2982.2005.00718.x [DOI] [PubMed] [Google Scholar]