Abstract
Background
Leishmania major infection induces robust interleukin-12 (IL12) production in human dendritic cells (hDC), ultimately resulting in Th1-mediated immunity and clinical resolution. The surface of Leishmania parasites is covered in a dense glycocalyx consisting of primarily lipophosphoglycan (LPG) and other phosphoglycan-containing molecules (PGs), making these glycoconjugates the likely pathogen-associated molecular patterns (PAMPS) responsible for IL12 induction.
Methodology/Principal Findings
Here we explored the role of parasite glycoconjugates on the hDC IL12 response by generating L. major Friedlin V1 mutants defective in LPG alone, (FV1 lpg1-), or generally deficient for all PGs, (FV1 lpg2-). Infection with metacyclic, infective stage, L. major or purified LPG induced high levels of IL12B subunit gene transcripts in hDCs, which was abrogated with FV1 lpg1- infections. In contrast, hDC infections with FV1 lpg2- displayed increased IL12B expression, suggesting other PG-related/LPG2 dependent molecules may act to dampen the immune response. Global transcriptional profiling comparing WT, FV1 lpg1-, FV1 lpg2- infections revealed that FV1 lpg1- mutants entered hDCs in a silent fashion as indicated by repression of gene expression. Transcription factor binding site analysis suggests that LPG recognition by hDCs induces IL-12 in a signaling cascade resulting in Nuclear Factor κ B (NFκB) and Interferon Regulatory Factor (IRF) mediated transcription.
Conclusions/Significance
These data suggest that L. major LPG is a major PAMP recognized by hDC to induce IL12-mediated protective immunity and that there is a complex interplay between PG-baring Leishmania surface glycoconjugates that result in modulation of host cellular IL12.
Author Summary
Leishmaniasis is a group of parasitic diseases caused by intracellular protozoa belonging to the genus Leishmania, pathological manifestations ranging from self-healing cutaneous forms to severe visceral infections that result in death. These clinical outcomes are dictated by the Leishmania species initiating the infection and are influenced by early responses of host immune cells, which ultimately initiate an IL12 mediated immune response in resolving infections. Like the diseases themselves, the magnitude of IL12 induction in hDCs is Leishmania-species and strain specific, where species that elicit visceral disease do not induce IL12, while most cutaneous disease-causing L. major strains induce robust IL12 responses and confer life-long immunity. The molecular mechanisms that mediate the ability of these innate immune cells to discriminate between pathogens remain elusive and have been primarily investigated in murine model systems. Here we identified L. major LPG as a major PAMP that induces IL12 in hDCs. Elucidation of this critical component of human immunity to L. major has ramifications for leishmaniasis vaccine development.
Introduction
Leishmaniasis constitutes a group of vector-borne parasitic diseases that affects approximately 12 million people worldwide and results in diverse clinical pathologies [1]. The causative intracellular protozoa belonging to the genus Leishmania, generally dictate disease outcome in a distinct species-specific manner. Visceral leishmaniasis may result from infection with Leishmania donovani parasites that disseminate throughout the body, manifesting into fatal systemic disease if left untreated. In contrast, Leishmania major, which is a causative agent for cutaneous leishmaniasis, produces ulcerative lesions localized at the site of sand fly vector inoculation. In the majority of L. major patients, lesions heal within several months, conferring life-long acquired immunity [2]. Recovery of cutaneous leishmaniasis with a strong immune response can be attributed to early cellular activities that occur following initial entry of the parasites into host cells.
Leishmania parasites have evolved mechanisms to survive within host cells and mediate infectivity in sand fly vectors through the interaction of their cellular surface coat molecules. The Leishmania surface coat is densely packed with glycosylphosphatidylinositol (GPI)-anchored glycoconjugates, including lipophosphoglycan (LPG), proteophosphoglycans (PPGs), glycosylinositolphospholipids (GIPLs), and glycoprotein 63 (GP63) [3–5]. Together these molecules provide a protective barrier for parasites to persist within the host environment [6]. LPG is one of the most intensely studied Leishmania surface molecules, in both the sand fly vector and vertebrate hosts, playing a distinct role in modulating host immune function [7] and even vectorial capacity of various sand fly species [8]. LPG is polymorphic among Leishmania species and developmentally regulated [6]. One dominant feature of LPG, the phosphoglycan repeating unit [Gal-Man-P] (PG), contains species-, strain-, and stage-specific modifications usually on the Gal residues [9–13]. The number of PG repeat units almost doubles during metacyclogenesis [14] and LPG is dramatically down regulated in the amastigote stage [15]. Thus, the role of LPG in mammalian infections is limited to the initial period of invasion and establishment of infection by metacyclic promastigotes.
Protective immunity to cutaneous leishmaniasis requires a robust IL12 driven type 1 helper T-cell (Th1) mediated response that produces high levels of interferon-gamma (IFNG), which ultimately promotes anti-microbicidal production of nitric oxide (NO) and reactive oxygen species (ROS) that destroy invading pathogens [16,17]. Dendritic cells (DCs) and macrophages are among the major cell sources of IL12, whose bioactive secretion is dependent upon the covalent linkage between the p40 (IL12B) and p35 (IL12A) subunits [18]. The ability of Leishmania to selectively suppress IL12 production, as first established by using murine macrophages [19,20], occurs through the transcriptional inhibition of the IL12B promoter [21] and is one immune evasion strategy employed by parasites to establish infection. Phagocytosis of Leishmania parasites by murine DCs induces IL12, driving the differentiation of Th1 cells to elicit their effector function [22–27]. The precise role of different DC subsets during murine infection in vivo is discordant depending on the Leishmania strain utilized, the infection route, and the timing of analysis [28,29]. A role for DCs early in infection has been identified in vivo, however, as DCs carrying Leishmania antigen produce IL12 within 8 hours following infection [30]. The murine DC IL12 response can be altered depending on the biochemical composition of the parasite surface, as evidenced by a study demonstrating that infection with L. major LV39c5 lpg2 −, a mutant that lacks phosphoglycan (PG)-containing molecules and other LPG2-dependent metabolites [31], induced IL12B in bone marrow derived mouse DCs (BMDCs) co-stimulated with anti-CD40 and IFNG [32]. This effect along with the long-term persistence of these parasites likely account for why vaccination with these LV39c5 lpg2 − parasites protects mice against L. major wild type (WT) challenge [33].
Remarkably, hDCs exhibit a dynamic range in IL12 production in response to Leishmania infection that is largely dependent upon the nature of the infecting species or strain. L. donovani fails to elicit IL12, whereas a general induction of IL12 is observed during L. major infections [34]. However, IL12 production also varies across L. major strains. Strains LV39 and SD do not induce IL12, whereas Friedlin V1 (FV1), IR173, IR176, and CC-1 strains elicit high levels of IL12 [34,35]. These differences are not well-correlated with LPG structural polymorphisms, as L. major LV39cl5 bears a highly poly-galactosylated LPG [36], while L.major SD synthesizes an unsubstituted LPG similar to that of L. donovani [37]. Several groups have reported differences in lesion pathology following in vivo infection with these same L. major strains. For example, L. major FV1 infected C57BL/6 mice develop lesions that eventually heal over time, whereas mice infected with L. major SD produce non-healing lesions [38]. BALB/C IL4RA knockout mice are resistant to L. major IR173 strain but susceptible to L. major LV39 strain [39]. Moreover, while L. major FV1 strain infected BALB/C mice quickly develop lesions, L. major LV39c5, a clonal derivative of the LV39 strain, elicits slower lesion development. Hybrid crosses of L. major FV1 x LV39c5 segregate at a 1:1 ratio into “fast” or “slow” virulence progeny [40]. These differential host responses to variant intra-species strains of L. major have important implications for the parasite strain-specific factors that could dictate disease persistence versus healing and induction of immunity.
In this study, we focus on elucidating whether parasite surface molecules are associated with the robust cytokine response observed in hDCs using the ‘high-IL12 inducing’ L. major FV1 strain. We generated parasite mutants lacking LPG alone, as done previously with the ‘low-IL12 inducing’ L. major LV39c5, through inactivation of the LPG1 galactofuranosyl transferases required for LPG core synthesis. Mutants generally lacking in all PG-containing structures were generated through inactivation of the Golgi GDP-mannose nucleotide sugar transporter gene, LPG2 [31]. This approach is powerful for probing the role of LPG as it allowed us to assess the impact of LPG deficiency in the context of the parasite, rather than through exogenous and relatively artificial routes. A second advantage is that multiple mutants provided a means to discriminate between LPG effects and those of molecules that bear structures related to or shared with those found in LPG. Notably, the PG repeating units present on LPG also are abundant on secreted molecules, such as acid phosphatases and other PPGs, which can be anchored to the parasite surface through glycosylphosphatidylinositol (GPI). Inactivation of LPG1 results in a parasite lacking LPG alone but otherwise normal in GIPL and PPGs levels [41].
Our results demonstrate that hDC infection with the LPG-null L. major FV1 lpg1 − mutant resulted in significantly diminished IL12B mRNA, relative to FV1 WT parasites, indicating that LPG is essential for stimulating host IL12 production. However, the PG-null L. major FV1 lpg2 − mutant infected DCs exhibited an increase in IL12B expression, suggesting that PGs and/or other LPG2-dependent metabolites may suppress IL12 induction. These results suggest that L. major parasites balance stimulatory and inhibitory effects on the host cells to establish infection.
Materials and Methods
Ethics statement
The study protocol was approved by the University of Notre Dame Institutional Review Board in compliance with all applicable Federal regulations governing the protection of human subjects (Human Subjects Assurance #M1262). The research was deemed exempt under exemption #4. The samples were purchased from Central Indiana Regional Blood Center, Indianapolis, IN and no identifying information was provided.
Dendritic cell generation and infection
Monocytes were isolated from healthy human donor buffy coats (Central Indiana Regional Blood Center, Indianapolis, IN) by enriching for CD14+ cells using a magnetic bead separator (AutoMACs, Miltenyi Biotech systems, Germany). Monocytes from each donor were cultured in 6-well plates at a concentration of 106 cells/2ml of RPMI-complete media (10% heat-inactivated FBS, 2mM l-glutamine 100U/ml, 1% penicillin/streptomycin) and supplemented with recombinant human IL4 (40U/ml, Peprotech, NJ) and granulocyte-macrophage colony-stimulating factor, GMSCF (1000U/ml, Peprotech, NJ) on days 0, 3, and 6 to allow differentiation into immature DCs. Cells were harvested, washed one day before infection to remove any residual cytokines, and assessed for DC marker CD1A to confirm a homogenous population of immature DCs. All parasite strains were cultured at 26°C without CO2 in M199 medium containing 10% heat-inactivated FBS [42]. Metacyclic promastigotes were isolated according to previously described methods [43] and opsonized by treatment with 5% human serum for 30 min at 37°C. DCs were then infected at a concentration of 10 parasites per 1 DC in RPMI-complete media. As we previously demonstrated that the peak of IL12B expression occurs at 8 hours post L. major infection [44] and to avoid the complication that mutant parasites might be degraded at later time points as previously observed [31,41], samples were typically harvested at 8 hours post-infection. For kinetic analyses we focused on the early time points following infection (2, 4, 8, or 24 hours). Cytospins were prepared at the conclusion of each experiment and Diff-quick stained (Fischer Scientific, Pittsburgh, PA) for visual analysis by light microscopy. Uninfected and infected DCs (100 total) were counted to calculate the infection rate (% infected DCs) and the parasite indices (# parasites per 100 cells) for each infection sample. All parasite and human cell cultures tested negative for mycoplasma (PCR detection, Takara) and tested below the limits of detection for endotoxin (<0.25U/ml) (Limulus Amoeboctye Assay, Endosafe, Charleston, NC).
Generation of L. major FV1 lpg1 − and L. major FV1 lpg2 − mutants and complemented lines
Leishmania major strain Friedlin clone V1 (MHOM/IL/81/Friedlin) and L. donovani strain 1S (MHOM/SD/62/1S) were grown in M199 medium containing 10% heat-inactivated FBS [45]. Methods for electroporation of logarithmic phase promastigotes and plating on semisolid media to obtain clonal lines were as described previously [46].
L. major FV1 lpg1 − mutants were obtained by a gene disruption strategy, in which autonomous drug resistance cassettes were inserted within the LPG1 coding region [41]. The methods and constructs used were the same as in the prior study generating the L. major LV39c5 lpg1 − mutants [41]. In the first round, plasmid B2947 DNA was digested with restriction enzymes XhoI and HindIII to yield the LPG1::HYG targeting construct, conferring selective resistance gene to hygromycin B (hygromycin phosphotransferase). 10μg of DNA was used for electroporation and parasites were plated on semisolid medium containing 50μg/ml of hygromycin B. Clonal parasite lines were obtained at typical frequencies and screened for the presence of the expected heterozygous LPG1 and LPG1::HYG insertion by PCR (S1 Fig, S1 Table). Several clones were inoculated into susceptible BALB/C mice (107 stationary phase, footpad) and recovered after 1 month; such mouse passaged lines are designated as ‘M1’. These heterozygotes underwent a second round of transfection; electroporating 10μg of LPG1::PAC, conferring a selective resistance gene to puromycin (puromycin acetyltransferase), derived from BamHI digestion of plasmid B2949, and followed by plating parasites on semisolid media containing 50μg/ml hygromycin B and 30μg/ml puromycin. Clonal lines bearing disruptions in both LPG1 alleles, and lacking unmodified LPG1 (^LPG1::HYG/^LPG1::PAC), were identified by PCR analysis and confirmed by Western blot analysis and agglutination tests. Several clones were inoculated into susceptible BALB/C mice (107 stationary phase, footpad) and recovered after 1 month (M1). For simplicity, these lines are referred to as FV1 lpg1 −. To generate complemented ‘add back’ lines, several FV1 lpg1 − clonal lines were electroporated with the LPG1 expression plasmid pSNBR-LPG1::NEO (B3340), conferring an episomal selective resistance gene to the aminoglycoside antibiotic G418 via expression of the neomycin phosphotransferase gene NEO, and clonal lines were recovered by plating on semisolid media containing 50μg/ml HYG, 30μg puromycin, and 12μg/ml of G418. Successful transfection was established by PCR tests and restoration of LPG expression by western blot, and agglutination tests. Formally, the genotype of such lines is (^LPG1::HYG/^LPG1::PAC/+pSNBR-LPG1), which for simplicity is referred to as FV1 lpg1 − /+LPG1. Sibling clonal lines displayed similar phenotypes and one representative FV1 lpg1 − line (cl2.10, M1), and its complemented offspring (cl2.10 AB3, M1), designated FV1 lpg1 − /+LPG1 were used in the experiments.
L. major FV1 lpg2 − mutants were obtained by a gene replacement strategy; where the drug resistance gene ORFS replaced the LPG2 coding region. In the first round, plasmid B3950 was digested with XhoI I, yielding the LPG2::HYG targeting construct; 10μg was used for electroporation and cells were plated on semisolid medium containing 50μg/ml of hygromycin B. Clonal lines were obtained at typical frequencies and screened for the presence of the expected heterozygous LPG2 and LPG2::HYG insertion by PCR (S2 Fig, S1 Table). Several clones were inoculated into susceptible BALB/C mice (107 stationary phase, footpad, M1). These heterozygotes underwent a second round of transfection, electroporating 10μg of LPG2::SAT, conferring a selective resistance gene to nourseothricin (streptothricin acetyltransferase), derived from XhoI, HindIII digestion of plasmid B6598, followed by plating on semisolid media containing 50μg/ml hygromycin B and 100μg/ml nourseothricin. Clonal lines bearing disruptions in both LPG2 alleles and lacking unmodified LPG2 (ΔLPG2::HYG/ ΔLPG LPG2::SAT) were identified by PCR analysis, and confirmed by Western blot analysis and agglutination tests. For simplicity, these lines will be referred to as FV1 lpg2 −. To generate complemented ‘add back’ lines, several FV1 lpg2 − clonal lines were electroporated with the LPG2 expression plasmid pXG-LPG2::NEO (B4296) and clonal lines recovered by plating on semisolid media containing 50μg/ml HYG, 100μg/ml SAT, and 15μg/ml of G418. Successful transfection was established by PCR tests and restoration of LPG and the PPGs region expression by western blot, and agglutination tests. Formally, the genotype of such lines is (ΔLPG2::HYG/ ΔLPG LPG2::SAT/+pXG-LPG2), which for simplicity is referred to as FV1 lpg2 − /+LPG2. Sibling clonal lines displayed similar phenotypes and one representative FV1 lpg2 − line (cl6.1A, M1), and its complemented offspring (cl6.1A AB15, M1), designated FV1 lpg2 −/+LPG2 were used in the experiments.
Gene replacement plasmid generation
Plasmid B6598 was generated by a fusion PCR strategy. Briefly, the 5’LPG2 flanking sequence, 3’LPG2 flanking sequence, LPG2 ORF, and selected drug marker, SAT ORF were amplified by PCR and inserted into the pGEM-T-Easy vector by TA cloning according to manufacturer’s instruction (Promega, Madison, WI) and transformed into E. coli. Its structure was confirmed by DNA sequencing. The primers used for constructing B6598 are provided in S1 Table.
Western blot
For Western blot analysis of PG-containing molecules, parasites were grown to logarithmic phase and harvested for cell lysate preparation in 4X Lamelli buffer (50 mM Tris-HCl pH 6.8, 2% SDS, 10% Glycerol, 1% 2-mercaptoethanol, 12.5 mM EDTA, and 0.02% Bromophenol Blue). Samples were separated on 10–12% SDS-PAGE gels at a concentration of (3.5x106 cells/well) and transferred onto methanol activated nitrocellulose membrane for 3 hrs at 60V, 4°C. Ponceau staining was performed to assure macromolecule transfer prior to blocking in 5% milk overnight. Membranes were stained with primary mouse monoclonal anti-sera WIC79.3 antibody (1:1000), recognizing galactosylated Gal-Man-P repeats on LPG, and detected using a goat anti-mouse HRP conjugated secondary antibody (1:5000) (Invitrogen, Carlsbad, CA). Membranes were developed using West-Pico detection solution assay (Thermo Scientific, Rockford, IL) and an X-ray film developer.
LPG purification
LPG was isolated from 109 L. major FV1 metacyclic promastigotes as previously described, with minor modification [47,48]. Cellular membranes were disrupted by sonicating pelleted cells suspended in a cold chlorform:methanol:water (1:2:0.8) solution, centrifuged (5000rpm, 10min, 4°C), and the top de-lipidated layer containing the majority of GIPLs and phospholipids was removed. The remaining insoluble material was quick-dried under stream of N2 and further extracted with two rounds of 9% 1-butanol extraction to release LPG molecules into the top aqueous layer. Hydrophobic interaction chromatography was performed to purify LPG molecules from the Leishmania surface coat. Briefly, LPG-containing butanol extracts were pooled and added to a 20% Octyl-Sepharose column that was pre-equilibrated with (5% proponal, 1M ammonium acetate). A desalting gradient (5%-60%) was applied to the column to elute LPG fractions utilizing the fraction collector, (BioRad Fraction Collector, Model 2128). LPG was detected by thin layer chromatography (TLC) and quantified by phenol sulfuric assay. Sample fractions were spotted on silica containing TLC plate. Glycan determinants were visualized by spraying the plate with orcinol (0.5mg/ml in 95% ethanol), dried, and sprayed with 75% sulfuric acid. All LPG containing fractions were pooled and dried in speed-vacuum at room temperature. Lyophilized LPG was resuspended in water and quantified by a colorimetric phenol-sulfuric assay [49]. Purification of LPG molecules was confirmed by a standard Stains-All protocol. Briefly, 5–10μg of LPG was boiled in 2X Loading Dye and loaded onto 10% SDS PAGE gel, running at 140V (room temperature). Gels were fixed in 25% 2-propanol and stained with stains-all solution (Fluka Analytical, Switzerland) containing 10% formamide, followed by destaining with 40% ethanol. Bands were visualized under white light, based on the observation that LPG molecules give rise to a blue colored complex (wavelength– 649nm) [50]. WIC79.3 western blot analysis was utilized to confirm LPG purification. Lyophilized purified LPG was resuspended in serum free RPMI and a titration of LPG (0.5μg, 1μg, and 10μg) was used for the hDC infection assay.
Quantitative real-time polymerase chain reactions
Relative levels of human gene transcripts were determined by qRT-PCR. Total RNA from uninfected or Leishmania-infected DCs was isolated using an RNeasy kit (Qiagen, Valencia, CA) and 1μg of RNA per infection sample was used to generate cDNA using SuperScript III Synthesis (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. For analysis of IL12B, IL12A, IRF1, IRF8, TNF, IL10, IL1B, SOCS3, TNFAIP3, and HPRT (hypoxanthine-guanine phosphoribosyltransferase) mRNA expressions, qRT-PCRs were conducted utilizing SYBR Green PCR Master Mix (Applied Biosystems by Life Technologies, Carlsbad, CA) according to manufacturer’s protocol and detected with an ABI 7900HT Fast Real-Time PCR System (Applied Biosystems by Life Technologies, Carlsbad, CA). All human primer sequences were designed by Integrated Design Tools (IDT) and used at a concentration of 5μM per reaction (S2 Table). For select analysis of IL12B, IL12A, and GAPDH (glyceraldehydes 3-phosphoate dehydrogenase) mRNA expressions, PCR reactions were setup employing TaqMan pre-developed assay kits (Life Technologies, Foster City, CA) and determined using an ABI 7500 Real Time PCR System (Applied Biosystems, Foster City, CA). For each gene, relative numbers of mRNA copies were determined by the ΔΔCT method [42].
Microarray expression profiling
Total RNA was isolated 8 hours post-infection from four additional donors’ uninfected monocyte-derived DCs and DCs infected with L. major FV1 WT, FV1 lpg1 −, FV1 lpg1 −/+LPG1, FV1 lpg2 −, and FV1 lpg2 −/+LPG2 using RNeasy kits (Qiagen, Valencia, CA). RNA 6000 Nano kits (Agilent Technologies, Santa Clara, CA) were used to determine total RNA integrity on a Bioanalyzer 2100 instrument (Agilent Technologies, Santa Clara, CA). 25ng of high quality RNA was converted to double stranded cDNA using a TransPlex Complete Whole Transcriptome Amplification kit (Sigma-Aldrich, Saint Louis, MO). RNA degradation, double stranded cDNA purification, and cDNA precipitation was conducted following NimbleGen Gene Expression Array user’s guide protocols (Roche-NimbleGen, Madison, WI). A Nanodrop ND-2000 (Thermo Fisher Scientific, Waltham, MA) was used to determine total RNA and double stranded cDNA concentrations. Sample cDNAs were Cy3-labeled using NimbleGen Single Color Labeling Kit (Roche-NimbleGen, Madison, WI) per manufacturer's recommendations. Labeled cDNAs were hybridized to 12-plex NimbleGen Homo sapiens Expression Arrays (platform GPL16025), featuring 140,096 probes, representing 21,269 genes and transcripts, using Hybridization LS and Wash Buffer Kits (Roche-NimbleGen, Madison, WI) per manufacturer's recommendations. Image acquisition of arrays was performed using a NimbleGen MS 200 Microarray Scanner (Roche-NimbleGen, Madison, WI), at a 2 micron resolution. NimbleGen array image data were processed using NimbleScan version 2.5 (Roche-NimbleGen, Madison, WI) to extract intensity values for each gene. NimbleScan software automates the pre-processing of NimbleGen microarray image data, including identifying the location of each probe, extraction of intensity data from the image, background correction, and obtaining expression summary values for each gene using a probe-level summarization robust multi-array average method (RMA). Probes with intensity values greater than twice that of background were retained for downstream analysis. Log2 normalized expression ratios for each gene were calculated between infected samples and paired uninfected samples. Z-scores were calculated between infected and uninfected samples as previously described [51]. Briefly, Z-score = (log2(infected intensity value/inter-quartile mean of uninfected intensity values)Gi−average(log2(infected intensity value/ inter-quartile mean of uninfected intensity values)Gi…Gn) / standard deviation(log2(infected intensity value/ inter-quartile mean of uninfected intensity values)Gi…Gn). An absolute Z-score value of 1.96 may be inferred as significant (p<0.05) [51]. Complete array data generated in this study are accessible at the NCBI Gene Expression Omnibus database (accession GSE59766). Gene expression data of RMA normalized raw microarray probe hybridization fluorescence values, where at least one sample value was twice that of background resulted in 12,911 genes.
Functional enrichment analyses
Genes that displayed significant differential expression from FV1 WT, FV1 lpg1 −, or FV1 lpg2 − samples compared to uninfected samples on NimbleGen microarrays were fed into the Short Time-series Expression Miner (STEM) program [52,53]. Briefly, log2 ratio values for each of four donors were loaded into the program as repeated data, where FV1 WT data represented a “time point 1”, FV1 lpg1 − data represented “time point 2”, and FV1 lpg2 − data represented “time point 3”. The datasets were clustered using the STEM clustering method with minimum correlation values of 0.6. The genes from the resultant model expression profile containing IL12B were used for downstream enrichment analysis in the Web-based Gene Set Analysis Toolkit (WEBGESTALT) [54,55] with a simple list of 233 official gene symbols as input. KEGG Pathway enrichment was conducted on that list of genes with similar expression profiles to that of IL12B using the following parameters: protein-coding EntrezGene database as a reference set and a hypergeometric test with Benjamini and Hochberg multiple test adjustments. Pathways with an adjusted p-value < 0.01 and a minimum of three genes found were considered significant. The same list of gene symbols was input to The Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.7 Functional Annotation Tool [56] and transcription factor binding sites for each gene were identified using protein interaction enrichment. The annotations were cross-referenced to report the most common transcription factor binding sites found in the IL12B gene and genes with IL12B-like expression between DC samples infected with L. major FV1 LPG mutants.
Statistical analysis
All statistical tests were performed using Graph Pad Prism version 5.0 (Graph Pad Software, San Diego, CA). Statistical analysis was performed using Log2 transformed ΔΔCT values using a paired Student’s T-test. Differences were considered significant at p<0.05.
Results
Differential IL12 response in hDCs is L. major strain and developmental stage specific
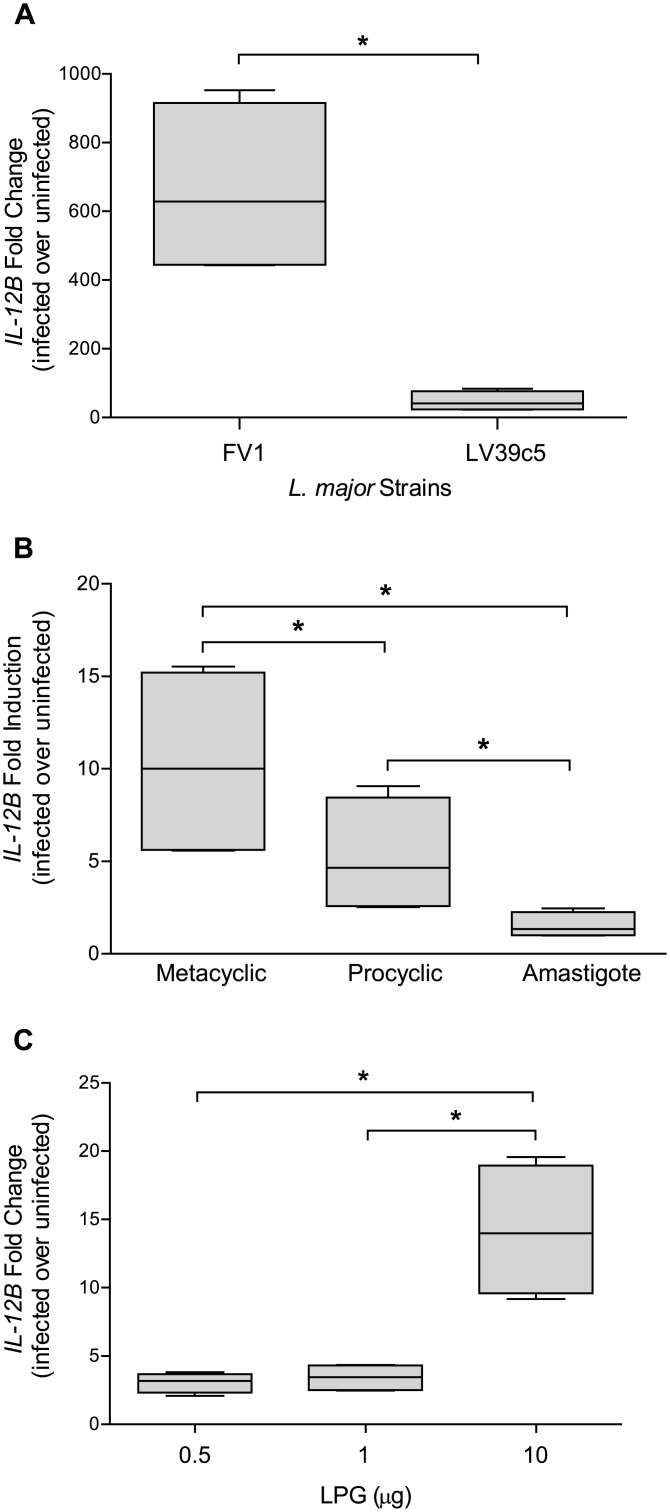
First, we confirmed that the IL12B mRNA expression in hDCs infected with L. major strains FV1 WT was greater than DCs infected with LV39c5 WT. We demonstrated that L. major FV1 induced approximately 15-fold greater amounts of IL12B than L. major LV39c5 (Fig 1A) at 8 hours post infection, the optimal time for peak IL12B mRNA expression following L. major infection [44]. These data support previous work that illustrated that the hDC IL12 response is strain-specific, and also that infection with L. major FV1 promotes a high induction of IL12 and that LV39c5 is similar to the LV39 strain tested previously [34]. We also confirmed that the increased IL12B expression observed during L. major FV1 WT infections were significantly associated with the infective metacyclic promastigote stage, whereas smaller effect was observed with the non-infective procyclic promastigote stage, and no response was elicited by amastigotes (Fig 1B). These data are consistent with prior studies indicating that IL12 induction depends on the life cycle stage of Leishmania parasites [57,58].
Fig 1. L. major strain FV1 metacyclic promastigotes and LPG stimulate IL12B expression.
(A) Human DCs were infected with L. major FV1 or L. major LV39c5 parasite strains (n = 4 donors). After 8 hours, RNA was extracted from infected hDCs for cDNA generation and analyzed for IL12B expression by qRT-PCR. All values were significantly greater than uninfected. (B) Human DCs were infected with L. major FV1 metacyclic promastigotes (metacyclic), procyclic promastigotes (procyclic) or amastigotes (n = 3 donors). After 8 hours, RNA was extracted from infected hDCs for cDNA generation and analyzed for IL12B expression by qRT-PCR. All values were significantly greater than uninfected, except for amastigote infections. (C) Human DCs were exposed to different concentrations of LPG (0.5, 1, and 10 μg), derived from L. major FV1 metacyclic promastigotes (n = 4 donors). After 8 hours, RNA was extracted from infected hDCs for cDNA generation and analyzed for IL12B expression by qRT-PCR. Fold change was calculated utilizing the ΔΔCT method and depicted as fold change over uninfected samples. Box plots display the median value (line), the interquartile range (box), and Tukey whiskers encompassing data within 1.5 fold of the interquartile range. *Statistical significance as compared to uninfected control, (p<0.05). All values were significantly greater than uninfected.
Purified LPG induces IL12B expression
To determine whether the enhanced IL12B production observed following infection with L. major FV1 metacyclic promastigotes was an LPG-dependent response, we first assessed the role of purified LPG on the hDC IL12B response. Human DCs were cultured in the presence of varying amounts of purified metacyclic L. major FV1 LPG for 8 hour and then assessed for IL12B expression. At lower concentrations (0.5, 1 μg), LPG induced a slight increase over uninfected samples, while at a higher concentration (10 μg) a significant 15-fold induction of IL12B mRNA was observed (Fig 1C), indicating that LPG alone is capable of stimulating IL12B production. Albeit to a lower level than what is observed with L. major LPG, purified L. donovani LPG induced a significant increase in IL-12B expression in 2 out of 3 donors (S5 Fig). Due to variation amongst the human donors, however, this difference was not statistically significant.
Generation of L. major FV1 LPG- and PG-null mutants and complemented lines
To probe the role of LPG and related PGs in host cell IL12 responses in the context of a Leishmania infection, we generated parasites lacking LPG alone (FV1 lpg1 −) or all PGs (FV1 lpg2 −) (Table 1). As L. major strain FV1 is disomic for chromosomes 25 and 34 bearing LPG1 and LPG2 respectively, two rounds of gene targeting were required to generate null mutants (S3A and S3B Fig). PCR tests confirmed the loss of LPG1 (S1B Fig) and LPG2 (S2B Fig) ORFs in the FV1 lpg1 − and FV1 lpg2 − mutants, respectively. Similarly, PCR tests confirmed the generation of the planned genetic alterations for the LPG1 disruption (FV1 lpg1 −) (S1C Fig) and the LPG2 replacement (FV1 lpg2 −) (S2C Fig). Complemented ‘add back’ lines were generated by introducing episomal constructs expressing the LPG1 or LPG2 genes into their respective null mutants (S3A and S3B Fig, bottom), which were confirmed by PCR and drug sensitivity tests. Western blot analysis with an anti-PG anti-sera (WIC79.3) showed that LPG expression alone was lost in the FV1 lpg1 − mutant (S3C Fig, lane 6) and restored in the complemented FV1 lpg1 − /+LPG1 line (S3C Fig, lanes 4 and 5). Similarly, Western blot analysis with WIC79.3 verified the absence of both PPGs and LPG in the FV1 lpg2 − mutant (S3C Fig, lane 2), and their restoration in the complemented FV1 lpg2 − /+LPG2 line (S3C Fig, lane 3).
Table 1. Formal names for L. major FV1 LPG and PG null mutants and add back lines.
| L. major strain | a Alleles | Loss of function |
|---|---|---|
| FV1 WT | LPG1/LPG1; LPG2/LPG2 | |
| FV1 lpg1 − | ^LPG1::HYG/^LPG1::PAC | LPG biosynthesis |
| FV1 lpg1 − /+LPG1 | ^LPG1::HYG/^LPG1::PAC + LPG1::NEO | |
| FV1 lpg2 − | ΔLPG2::HYG/ΔLPG2::SAT | PG biosynthesis |
| FV1 lpg2 − /+LPG2 | ΔLPG2::HYG/ΔLPG2::SAT+LPG2::NEO |
aA ^ denotes gene disruption and a Δ denotes gene replacement
L. major FV1 LPG required for robust IL12 responses in hDCs
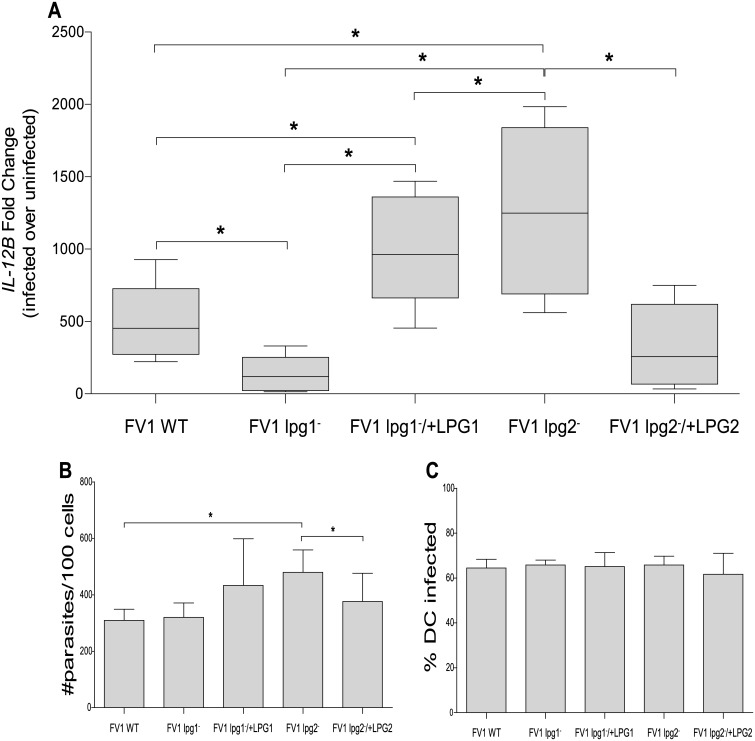
To explore the role of LPG on the IL12 response elicited from L. major infected hDCs, we quantified the relative amount of IL12B mRNA in hDCs after 8 hours of infection with FV1 WT, FV1 lpg1 −, and FV1 lpg1 − /+LPG1 parasites. Compared to FV1 WT, FV1 lpg1 − infected hDCs displayed a substantial decrease in IL12 expression (3.2 fold; Fig 2A) that was restored to levels approximately twice more than WT in the complemented FV1 lpg1 − /+LPG1 line, perhaps consistent with a slight elevation of LPG in this line (S3C Fig, lanes 4 and 5). Our results indicate LPG plays a key role in IL12 induction in hDCs, consistent with the stimulatory effect seen with purified LPG (Fig 1B).
Fig 2. L. major FV1 lpg1 − and FV1 lpg2 − modulate the IL12B response in hDCs.
Human DCs (n = 9 donors) were infected with L. major FV1 parasites: L. major FV1 (WT), LPG null mutant (FV1 lpg1 −), LPG add back (FV1 lpg1 −/+LPG1), PG null mutant (FV1 lpg2 −), or PG add back (FV1 lpg2 −/+LPG2). (A) At 8 hours post infection, IL12B expression was measured by qRT-PCR. Fold changes were calculated using the ΔΔCT method and are represented as fold change over uninfected samples. Box plots display the median value (line), the interquartile range (box), and Tukey whiskers encompassing data within 1.5 fold of the interquartile range. All values were significantly greater than uninfected. Aliquots from the infected hDC samples were prepared by Diff-Quick staining and visualized by light microscopy. (B) The parasite index (#parasites/100 cells) and (C) the percentage of infected cells (%DC infected) is displayed. Mean values of individual donors ± SD are presented. *Statistical significance (p<0.05).
Conversely, FV1 lpg2 − infected hDCs, relative to FV1 WT, displayed a significant increase in IL12B expression, that returned to comparable FV1 WT levels in the complemented FV1 lpg2 − /+LPG2 line (Fig 2A). This observation was unexpected as FV1 lpg2 − lacks LPG as well as other PGs, including PPGs (Fig 2C, lane 2). We considered the possibility that differences in infectivity between the WT and lpg2 − could contribute to this result as L. major Lv39c5 lpg1 − and lpg2 − mutants exhibit reduced survival in peritoneal macrophages [41,59]. While parasite survival was slightly elevated in FV1 lpg2 − infections, a comparable fraction of DCs were infected (Fig 2B and 2C), indicating, the differences observed in IL12 induction are likely not related to parasite survival in hDC under the conditions tested.
Thus, our studies showed that LPG is associated with increased IL12 production when tested biochemically (purified) or genetically (FV1 lpg1 −), while paradoxically lpg2- which also lacks LPG showed increased production. These data invoke the possibility LPG2-dependent molecules, such as phophoglycans including PPGs or other metabolites [60] may play a suppressive role on IL12 production. Alternatively, the loss of all LPG2-dependent structures may reveal another PAMP on the parasite surface that is able to induce IL12. Either scenario indicates a complex balance and interplay between parasite glycoconjugates and host cells.
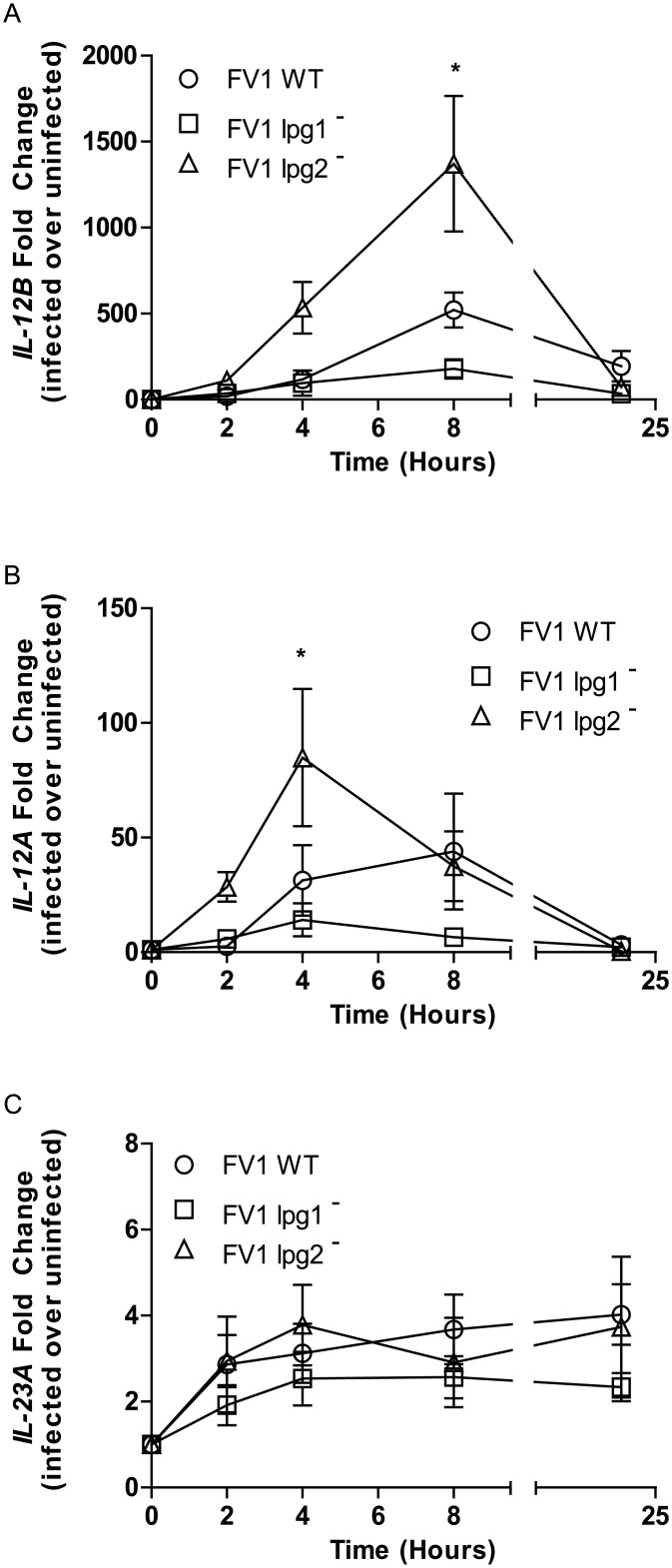
A kinetic analysis of these phenomena was conducted in DCs across four time points: 2, 4, 8, and 24 hours post-infection with FV1 WT and knockout mutants (Fig 3A). By 2 hours post-infection, FV1 lpg2 − mutant infected hDCs induced slightly more IL12B compared with FV1 WT infected DCs. Albeit at higher expression levels than FV1 WT, FV1 lpg2 − induced a similar kinetic IL12B mRNA response that declined by 24 hrs post infection. FV1 lpg1 −, on the other hand, induced little to no IL12B mRNA (Fig 3A). Similarly, FV1 lpg2 − induced a quicker and more robust IL12A response compared to FV1 lpg1 − and FV1 WT infections (Fig 3B). There were no differences between the WT and mutant strains for expression of the IL12 homolog IL23A (Fig 3C), suggesting that LPG and PGs regulate IL-12 production rather than IL-23.
Fig 3. Kinetic analysis of IL12 and IL23 expression modulation by L. major LPG and PG null mutants.
Human DCs (n = 3 donors) were infected with L. major FV1 parasites: L. major FV1 (WT), LPG null mutant (FV1 lpg1 −), LPG add back (FV1 lpg1 −/+LPG1), PG null mutant (FV1 lpg2 −), or PG add back (FV1 lpg2 −/+LPG2). At 2, 4, 8, and 24 hours post infection, IL12B (A), IL12A (B), and IL23A (C) expression was measured by qRT-PCR. Fold changes were calculated using the ΔΔCT method and are represented as fold change over uninfected samples. Mean values of individual donors ± SD are presented. Statistical significance p<0.05, ANOVA with Bonferroni multiple comparisons test.
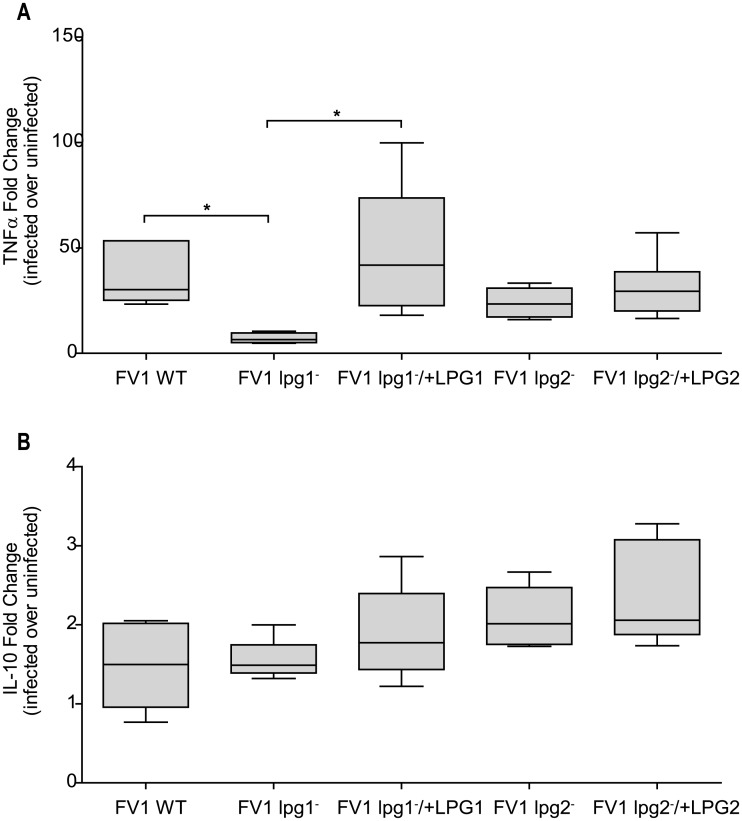
Human DC TNF expression is reduced during L. major FV1 lpg1 − infections
In addition to IL12, DCs are strong producers of other Th1 proinflammatory cytokines. TNF, for example, is significantly up-regulated in L. major infected hDCs [61]. We determined the relative fold induction of TNF in hDCs following infection with FV1 lpg1 − and FV1 lpg2 − mutants. We demonstrated that FV1 lpg1 − induces significantly less TNF mRNA compared to WT or FV1 lpg1 − /+LPG1 add back infections (Fig 4A), similar to the pattern of IL12B expression (Fig 2A). Infection with FV1 lpg2 −, however, was not statistically different compared to WT infection. The effect LPG has on both IL12 and TNF may contribute to the overall skewing of L. major towards a predominant Th1 response during cutaneous leishmaniasis.
Fig 4. Relative TNF and IL10 levels in L. major Friedlin V1 infected DCs.
Human DCs were infected with L. major FV1 parasites: L. major FV1 (WT), LPG null mutant (FV1 lpg1 −), LPG add back (FV1 lpg1 −/+LPG1), PG null mutant (FV1 lpg2 −), or PG add back (FV1 lpg2 −/+LPG2). At 8 hrs post infection, (A) TNF (n = 5 donors) and (B) IL10 (n = 5 donors) expression was measured by qRT-PCR. Fold changes were calculated using the ΔΔCT method and are represented as fold change over uninfected samples. Box plots display the median value (line), the interquartile range (box), and Tukey whiskers encompassing data within 1.5 fold of the interquartile range; data outside this range are presented as individual data points (open circles). *Statistical significance (p<0.05). All values were significantly greater than uninfected.
Down-regulation of IL12B in L. major FV1 lpg1 − infection is not dependent upon IL10 induction
IL10 is generally implicated as a powerful inhibitor of IL12 production [62], and neutralizing IL10 promotes the ability of L. major parasites to establish IL12 production [63]. Here we quantified the IL10 mRNA levels in hDCs infected with our mutant parasites to determine whether the failure of FV1 lpg1 − to elicit sustained host IL12 induction relative to FV1 WT is due to the over-expression of IL10. The IL10 expression elicited from hDCs infected with FV1 lpg1 − or FV1 lpg2 − did not differ from WT induced expression levels (Fig 4B), suggesting the mechanism by which these mutant parasites modulate IL12B expression is not dependent upon IL10.
Human microarray analysis reveals broader gene expression effects of LPG and PPGs
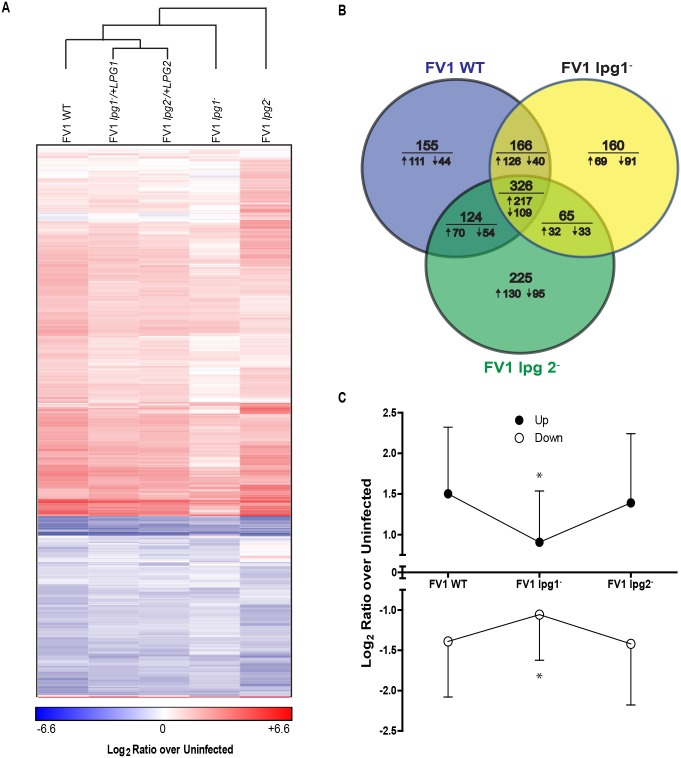
To further assess the influence of LPG and PPGs on host immunological responses, we infected additional DCs with L. major FV1 WT, mutants, and complemented strains, collecting mRNA at 8 hours post-infection. cDNA generated from these samples was hybridized to NimbleGen Homo sapiens Expression Microarrays. Expression of ten genes (IL12B, IL1B, IL8, TLR4, TLR2, FKBP4, SOCS3, SMOX, FCGR1A, and TNFAIP3) correlated significantly using qRT-PCR (p<0.000001, Spearman correlation coefficient = 0.784), validating the array values (S4 Fig). Gene transcript expression values were transformed to Z-scores and those genes that were significantly differentially expressed compared to uninfected cells (Z-score ≥ 1.96) were retained for downstream analysis. Hierarchical clustering of 730 genes that were expressed differently than FV1 WT infections in at least one mutant infection revealed that the complemented strains clustered more closely to the WT strains than their respective mutant strains (Fig 5A). Compared to uninfected cells, similar numbers of genes were regulated by infection with FV1 WT (771), FV1 lpg1 −(717) and FV1 lpg2 − (740) (Fig 5B). Infection with FV1 WT resulted in more genes being up-regulated than either mutant strain (FV1 WT—524; FV1 lpg1 -—444; and FV1 lpg2 −—449). Notably, the magnitude of regulation (either up or down) was less during infection with FV1 lpg1 − compared to either FV1 WT or lpg2 − (Fig 5A and 5C), suggesting that this strain enters hDC in a silent fashion.
Fig 5. Leishmania major human host dendritic cells gene expression profiles.
(A) Gene transcript expression heat map of in vitro infected monocyte-derived hDCs. The color scale is based on average log2 ratios of RMA-normalized microarray gene probe set values for variably infected host cells over uninfected cells. Only the genes that displayed significant differential expression by z-ratios, from both uninfected samples and between infections with FV1 WT and FV1 lpg1 − or FV1 lpg2 − mutants or their respective add back strains, were included in the map. Genes and sample types were clustered by city block distance metric using average linkage in GENE-E. (B) Venn diagrams with the number of host DC genes significantly differentially expressed from uninfected samples in FV1 WT, FV1 lpg1 −, and FV1 lpg2 − mutants as quantified by microarrays. Values below the horizontal line indicate the number of genes from the above total that were up- (↑) or down-regulated (↓) compared to uninfected samples. (C) Total average log2 ratios of up- and down-regulated genes significantly differentially expressed from uninfected samples in FV1 WT, FV1 lpg1 −, and FV1 lpg2 − mutants as quantified by microarrays, plus or minus standard deviation. *Significant difference of log2 ratio values (p<0.05) between FV1 lpg1 − infected DCs compared to FV1 WT and FV1 lpg2 − infected DCs by ANOVA with Bonferroni multiple comparisons test.
LPG regulates immune response and infectious disease pathways
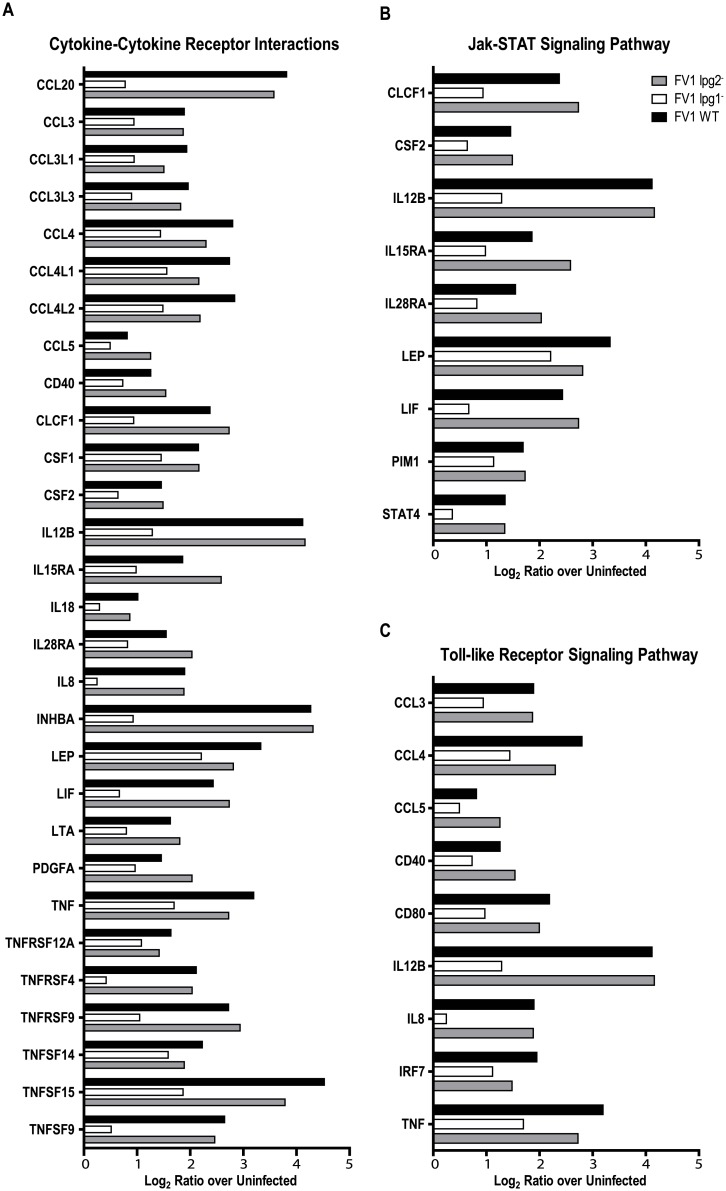
To assess the pathways involved in the regulation of IL12 by LPG, we utilized STEM and identified 233 genes that exhibited expression patterns similar to IL12B in response to infection with FV1 WT, FV1 lpg1 −, and FV1 lpg2 −. Overall lpg2 − resembled WT while lpg1 −differed (Fig 6). Pathway enrichment revealed 22 significantly enriched pathways, mostly belonging to the immune response or infectious disease categories (Table 2). The most striking observation was the enrichment of three pathways: Cytokine-Cytokine Receptor Interactions, JAK-STAT Signaling and Toll-like Signaling, in which all the genes were down-regulated by infection with FV1 lpg1 − compared to FV1 WT and FV1 lpg2 − (Fig 6). Although the lpg2- pathway genes did not reflect any significance in this initial analysis compared to WT, future analysis of enriched pathways by criteria other than IL12 expression could reveal significant pathways enriched by lpg2- infection.
Fig 6. Enriched immunologically relevant pathways for genes expressed in IL12B-like patterns.
Gene transcripts lists from L. major FV1 WT, FV1 lpg1 −, and FV1 lpg2 − mutant in vitro infected monocyte-derived hDCs microarray analysis, with log2 ratio over uninfected value patterns between samples that clustered with the IL12B, were analyzed to identify significantly enriched (Benjamini and Hochberg adjusted p<0.01) KEGG pathways. Three immunologically relevant pathways, which also contained IL12B, were enriched among that gene list: cytokine-cytokine receptor interactions (A), Jak-STAT signaling pathway (B), and toll-like receptor signaling pathway (C). All graphs display average log2 ratio over uninfected values for genes present in the expression datasets of FV1 WT, FV1 lpg1 −, and FV1 lpg2 − mutant infected samples which were members of the corresponding enriched pathways.
Table 2. Significantly enriched pathways for genes regulated similarly to IL12B by LPG.
| Pathway Name | # Genes | p value |
|---|---|---|
| Cytokine-cytokine receptor interaction | 29 | 2.31E-21 |
| Rheumatoid arthritis | 11 | 1.52E-08 |
| Toll-like receptor signaling pathway | 9 | 5.96E-06 |
| Chagas disease (American trypanosomiasis) | 8 | 5.22E-05 |
| Amoebiasis | 8 | 5.22E-05 |
| Jak-STAT signaling pathway | 9 | 8.16E-05 |
| Cytosolic DNA-sensing pathway | 6 | 8.16E-05 |
| NOD-like receptor signaling pathway | 6 | 8.16E-05 |
| Chemokine signaling pathway | 9 | 0.0003 |
| Focal adhesion | 9 | 0.0004 |
| ECM-receptor interaction | 6 | 0.0004 |
| Allograft rejection | 4 | 0.0011 |
| Type I diabetes mellitus | 4 | 0.0021 |
| Intestinal immune network for IgA production | 4 | 0.0032 |
| Toxoplasmosis | 6 | 0.0033 |
| Hematopoietic cell lineage | 5 | 0.0033 |
| Malaria | 4 | 0.0033 |
| Small cell lung cancer | 5 | 0.0033 |
| Pathogenic Escherichia coli infection | 4 | 0.0038 |
| Phagosome | 6 | 0.0061 |
| African trypanosomiasis | 3 | 0.0082 |
| RIG-I-like receptor signaling pathway | 4 | 0.0082 |
The most common transcription factor binding sites present in the promoters of genes regulated similarly to IL12B were identified using the DAVID functional annotation tool [56]. Not surprisingly, binding sites for transcription factor families known to regulate IL12B were identified, including, Octomer-binding transcription factor (OCT), Nuclear Factor Kappa B (NFκB), Interferon Regulatory Factor (IRF), cAMP Response Element Binding protein (CREB), and CCAAT/Enhancer Binding Protein families [64–68] (Table 3).
Table 3. Enriched Transcription Factor Binding sites in IL12B-like gene promoters.
| Transcription Factor | # IL12B-like Genes | % IL12B-like Genes |
|---|---|---|
| AREB6 | 130 | 75.14 |
| OCT1 | 128 | 73.99 |
| AML1 | 123 | 71.10 |
| CEBP | 122 | 70.52 |
| MEF2 | 113 | 65.32 |
| NKX25 | 95 | 54.91 |
| CDPCR3 | 80 | 46.24 |
| GR | 79 | 45.66 |
| NFκB | 78 | 45.09 |
| RSRFC4 | 75 | 43.35 |
| FOXO4 | 73 | 42.20 |
| HMX1 | 72 | 41.62 |
| ARNT | 71 | 41.04 |
| SOX9 | 71 | 41.04 |
| COMP1 | 70 | 40.46 |
| IRF7 | 70 | 40.46 |
| MEIS1 | 70 | 40.46 |
| CART1 | 68 | 39.31 |
| NRSF | 68 | 39.31 |
| ELK1 | 67 | 38.73 |
| RORA2 | 65 | 37.57 |
| ARP1 | 64 | 36.99 |
| CREBP1 | 63 | 36.42 |
| NFKAPPAB | 63 | 36.42 |
| MEIS1BHOXA9 | 62 | 35.84 |
| OCT | 62 | 35.84 |
| GFI1 | 61 | 35.26 |
| IRF2 | 61 | 35.26 |
| RORA1 | 61 | 35.26 |
| HNF3B | 60 | 34.68 |
| NKX22 | 60 | 34.68 |
| FOXO1 | 59 | 34.10 |
| HAND1E47 | 59 | 34.10 |
| HLF | 59 | 34.10 |
| P300 | 57 | 32.95 |
| POU6F1 | 47 | 27.17 |
| STAT5B | 44 | 25.43 |
Human DC IRF8 expression is reduced during L. major FV1 lpg1 − infection
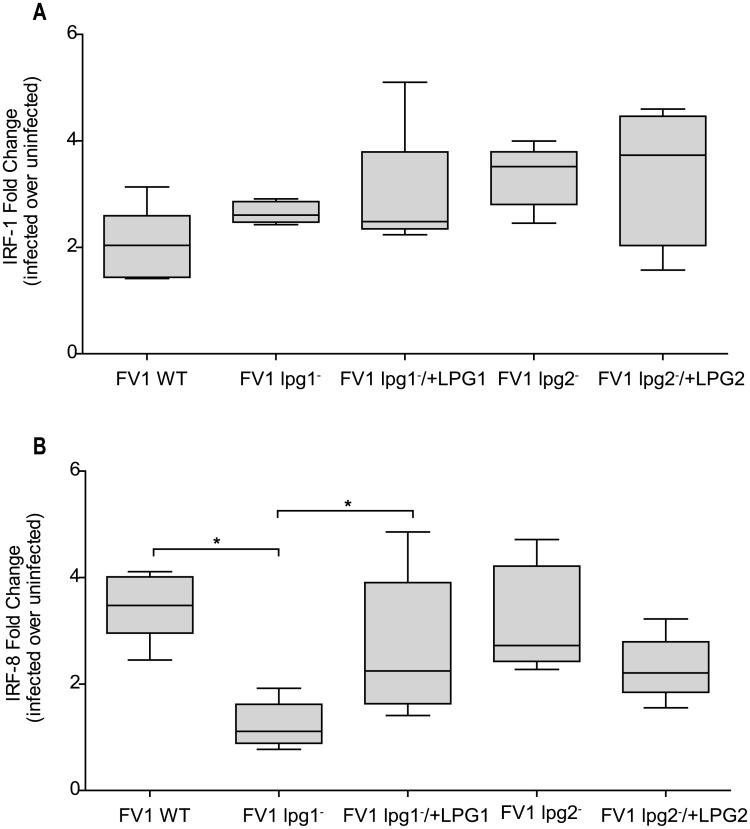
Production of IL12 relies on the nuclear translocation and cooperative binding of IRF-1 and IRF8 to IFNG-activated sequences (GAS) found within the IL12B promoter [18]. We previously demonstrated that L. major infection of hDC results in the early activation of NFκB transcription factors resulting in the transcriptional induction and nuclear translocation of IRF-1 and IRF-8 and, ultimately, IL12 production [42]. To delineate the effect of FV1 lpg1 − and/or FV1 lpg2 − on the upstream transcriptional features that regulate IL12B expression, we assessed IRF1 expression in hDCs and observed that infection with FV1 mutants up-regulated IRF1, but not significantly more compared to WT induced levels (Fig 7A). This result suggests that the different IL12B responses displayed during FV1 lpg1 − and FV1 lpg2 − DC infections are not influenced by IRF1 expression. IRF8 mRNA levels, however, were regulated by LPG. Infection with FV1 lpg1 − resulted in a reduction of IRF8 that is restored following infection with the FV1 lpg1 − add back strain (Fig 7B). Infection with FV1 lpg2 − did not significantly affect IRF8 expression.
Fig 7. The lpg1 − mutant affects IL12 associated gene regulator IRF8, and not IRF1.
Human DCs were infected with L. major FV1 parasites: L. major FV1 (WT), LPG null mutant (FV1 lpg1 −), LPG add back (FV1 lpg1 −/+LPG1), PG null mutant (FV1 lpg2 −), or PG add back (FV1 lpg2 −/+LPG2). At 8 hrs post infection, (A) IRF8 (n = 3 donors) and (B) IRF1 (n = 5 donors) expression was measured by qRT-PCR. Fold changes were calculated using the ΔΔCT method and are represented as fold change over uninfected samples. Box plots display the median value (line), the interquartile range (box), and Tukey whiskers encompassing data within 1.5 fold of the interquartile range. *Statistical significance (p<0.05). All values were significantly greater than uninfected.
Discussion
The major focus of this study was to investigate whether the enhanced IL12 immune response observed in L. major FV1 WT infected hDCs is dependent upon parasite LPG; as previous studies have implicated LPG plays a major role in modulating immune function in murine cells [31,69,70], as well as in human mononuclear cells [71–73]. First, we showed that, for this strain, infection with metacyclic promastigotes induces a high IL12B response (Fig 1B), compared to procyclic promastigotes and amastigotes, consistent with prior studies [57,58]. Additionally, we demonstrated that purified LPG stimulates an IL12B response in hDCs (Fig 1C). Similar studies utilizing purified L. major LPG from another strain have also highlighted the stimulatory effect LPG has on IL12 in human PBMCs [72].
To assess the role of surface molecules in situ, we employed genetic strategies to generate parasite mutants devoid of LPG (FV1 lpg1 −) or PG molecules and other LPG2-dependent metabolites (FV1 lpg2 −) in the L. major strain FV1 background (S3A Fig). Previous studies on the ‘low hDC IL12, L. major strain LV39c5 mutant parasites established several roles for LPG and PGs in regulating immune function [31–33,41,60]. For example, LV39c5 lpg2 − induces IL12 in mouse BMDCs co-stimulated with anti-CD40 or IFNG [32,33]. In the absence of co-stimulation, however, there was no significant difference between IL12 elicited from LV39c5 WT or LV39c5 lpg2 − parasites. We observed a similar result in our hDC assay where there was little difference in IL-12 induction between LV39c5 WT, LV39c5 lpg2 −, and LV39c5 lpg2 − /+LPG2 infections (S6 Fig). Compared to FV1 WT, LV39c5 WT does not induce the same robust levels of IL12B (Fig 1A, S6 Fig).
Here, we generated LPG1 and LPG2 knockout mutants in the ‘high hDC IL12’ L. major FV1 background strain, in order to directly assess the parasite-derived molecular factors that contribute to the robust hDC IL12 response elicited by this strain of L. major. Our data demonstrated that the FV1 lpg1 − mutant does not induce a high amount of IL12B transcript in hDCs as compared to FV1 WT (Figs 2A and 3A). Consistent with this observation, we showed that application of purified LPG was able to induce significant IL12 expression (S5 Fig), with both metacyclic L. major LPG which bears abundant PG side chain modifications, and L. donovani LPG, which is unmodified.
In contrast, and somewhat surprisingly given its similar lack of LPG, FV1 lpg2 − up-regulates the IL12B response (Figs 2A and 3A) relative to FV1 WT. While in macrophage and animal infections the lpg1- and lpg2- mutants are typically attenuated [41,59], in our studies the survival of the WT and two mutant parasites did not differ significantly in DC survival over the course of these studies (Fig 2B and 2C). One explanation for this finding is that in L.major strain FV1, LPG and other LPG2-dependent glycoconjugates play inverse roles in stimulating the IL12 response in human DCs. One candidate for such an inhibitory LPG2-dependent molecule are the proteophosphoglycans (PPGs), which remain intact in the lpg1 − mutant. Compared to LPG, little is known about the function of PPGs on host cell immune response, with evidence supporting roles as both an inhibitor or enhancer depending on the species and study [74–77]. PPGs vary structurally across species both in their PG and protein composition, and their large size and tendency to form polymeric aggregates renders their study more challenging [78]. Clearly, the development of mutants lacking only PPGs would be beneficial for future studies to directly assess the role these molecules have on the host cell response. Interestingly, amastigotes do not express significant amounts of the ‘pro-IL12’ LPG but do express high levels of PPG, which may further contribute to their inability to stimulate IL12 expression in hDCs. Importantly, the LPG2-dependent effect was also observed in the ‘low hDC IL12’ LV39 line, where ablation of LPG2 similarly resulted in increased IL12 production (S6 Fig)
Thus our data cause us to infer the presence of other LPG2-dependent PAMPs beyond LPG, with PPG as a possible candidate, and acting in an inhibitory fashion. The potential dominance of these inhibitory LPG2-dependent PAMPs provides an explanation for the conundrum that while all Leishmania species express LPG, despite that many do not induce IL12 [79]. Potentially, the strength of these suppressive LPG2-dependent PAMPs/processes may vary in different species and/or strains.
As it has been established that IRF1 and IRF8 are up-regulated in L. major infected hDCs and positively regulate IL12B gene expression [42], we assessed whether FV1 lpg1 − or FV1 lpg2 − affected the expression of IRF1 and IRF8. Interestingly, FV1 lpg1 − parasites caused a significant decrease in IRF8 expression compared to WT (Fig 5A), indicating that LPG may influence the induction of IL12B by targeting upstream IL12B associated transcription factors that mediate its expression. Although IRF1 and IRF8 are known to cooperatively regulate IL12B gene transcription [42,80], we report that the FV1 lpg1 − mutant does not affect IRF1 expression compared to WT at 8 hours post-infection (Fig 4B). The distinct expression phenotypes exhibited by IRF1 and IRF8 following infection with FV1 lpg1 − may be due to the difference in regulation of these two transcription factors. IRF1 is ubiquitously expressed, whereas IRF8 is preferentially expressed in immune cells and in response to activating signals. Furthermore, IRF1 and IRF8 can be differentially expressed in hDCs [81]. To bind target DNA sequences, IRF8 must bind to another transcription factor, compared to other IRF family members that can bind DNA sequences alone [82]. It is possible that infection with FV1 lpg1 − reduces the amount of IRF8, which in turn inhibits the capacity of other transcription factors, such as IRF1, to form heterodimeric complexes that bind the IL12B promoter. These data suggest that LPG and not other PGs, enhance the IL12B response by a common mechanism involving IRF8.
Like IL12, L. major induces TNF in both human macrophages and DCs [61]. We therefore evaluated the relationship between parasite derived PG-bearing molecules on TNF using our LPG and PG null mutants. Our results demonstrate that the lpg1 − mutant exhibits a significant decrease of TNF expression, similar to the reduction observed for IL12B (Fig 5A). Interestingly, the promoter regions for IL12B and TNF have similar transcription factor binding sequences, namely NFκB and ETS sites; the latter containing ISRE sequences that promote gene transcription upon IRF8 complex binding [83]. Therefore, it is possible that the reduction in TNF expression observed during FV1 lpg1 − infection (Fig 5A) may also be IRF8-specific. A murine study demonstrated that cholera toxin (CT) inhibits plasmacytoid dendritic cellular IL12 by blocking the ability of IRF8 to bind to the ISRE sequence within the IL12B promoter, while IRF1 phosphorylation and subsequent binding to its DNA target sequence remained unaffected [84]. It is feasible that a similar mechanism exists in L. major infected cells, whereby IRF8 is specifically targeted for induction downstream of parasite LPG binding, subsequently leading to the induction of IL12B and TNF. Altogether, our data indicates that L. major FV1 skews the hDC response in an LPG-dependent manner towards a Th1-like polarization characterized by an increase in IL12 and TNF production which may be regulated by a common mechanism involving IRF8. A recent study demonstrated that macrophage induction of IL12B is controlled at the level of IRF8, which is specifically targeted for activation downstream of TLR4 in concert with Notch signaling pathways [85]. Interestingly, TLR4 [86] and other TLRs [87–91] have been implicated in recognition of parasite LPG.
An alternative explanation for the lack of an IL12 signal observed in the FV1 lpg1 − infections may be a consequence of other functionally active PG-containing molecules, such as the PPGs which remain intact in the lpg1 − mutant. These PPGs could provide an inhibitory IL12 signal. This theory is supported by our results demonstrating that FV1 lpg2 −, which lacks both LPG and PPGs, induces higher levels of IL12 compared to WT (Fig 2A), suggesting that some PG-containing molecules actually inhibit IL12 responses. In addition, amastigotes, on which LPG expression is drastically down-regulated and high levels of other PG containing glycoconjugates are highly expressed [15], do not induce IL12 (Fig 1B). Compared to LPG, little is known about the function of PPGs on host cell immune response. Previous work illustrating the ability of PPGs to induce complement activation by triggering the mannose binding protein pathway [76] and their inability to elicit CD4+ T-cell response in murine bone marrow derived macrophages [74], concludes that PPGs may contribute to the chronic infections observed during L. mexicana infections. However, it has been demonstrated that L. major PPGs require IFNG priming to induce TNF and NO production in murine macrophages [77]. In human PBMCs, PPGs cause an induction of IL10 and to a lesser extent NO and IL12 [75]. Although these studies provide conflicting implications for PPGs role as either inhibitor or enhancer of immune response, it is difficult to compare studies because the repertoire of PPGs structure varies across species [78]. Additionally, the use of purified PPGs can be problematic because the amount of purified PPGs added is often higher than what is biologically present during an actual infection, therefore the development of mutants lacking only PPGs would be beneficial for future studies to directly assess the role these molecules have on the host cell response. We measured IL-10 mRNA levels in our mutant-infected DCs, because of the generally inhibitory effects of IL-10 on IL12 [62]. However, IL10 expression exhibited between FV1 lpg1 −, FV1 lpg2 −, and WT infected hDCs did not differ (Fig 5B), ruling out one theory that the decrease in IL12B expression observed during FV1 lpg1 − could be consequence IL10 overproduction. Another explanation for the induction of IL12 by FV1 lpg2 −, is the possibility that the absence of all surface and secreted PGs reveals a molecular pattern or some other molecule that induces IL12.
Our microarray analyses of FV1WT, FV1 lpg1 −, and FV1 lpg2 − infected hDCs revealed that FV1 lpg1 − enter hDC in a relatively silent fashion as indicated by the overall down-regulation of significantly expressed transcripts, (Fig 6), and the overall reduction in genes belonging to cytokine and TLR related gene pathways, (Fig 7). Altogether these data suggest that a lack of LPG molecules results in silent entry and that LPG is a major pattern recognized by pattern recognition receptors on DCs. As with the IL12 response, the absence of all PGs appears to either release some sort of repression or reveals a molecular pattern that compensates for the lack of LPG, highlighting the complexity of DC pattern recognition receptor interactions in controlling host responses to Leishmania infection. Future analyses focusing on FV1 lpg2- mutant infections may reveal pathways uniquely regulated by PGs.
This work adds to the growing set of genetically modified parasites (lpg1 −, lpg2 − in the L. major FV1 background) providing biologically relevant tools for assessing the role of parasite surface glycoconjugates on cellular function in human and mouse model systems, as well as, provides insight into the complex interplay of LPG and other PG molecules on the cellular immune response elicited following L. major infections by global gene expression analyses.
Supporting Information
(A) Schematic representation of WT (top) and lpg1 − alleles (bottom). Numbers represent primers used for PCR amplification. PCR analysis for one representative FV1 WT, FV1 lpg1 − (cl 2.10) and FV1 lpg1 − /+LPG1 (cl 2.10 AB3) is depicted. (B) Primers 1/2 (SMB1023/SMB1626) confirmed LPG1 disruption: WT (420bp), FV1 lpg1 − (3200bp) and FV1 lpg1 − /+LPG1 (420bp & 3200bp). (C) Primers 3/11 (SMB4183/SMB2566), and 12/6 (SMB4185/SMB4184) established the integration of the 5’ flanking (3300bp) and 3’ flanking (2600bp) sequences of the LPG1::HYG disruption cassette, respectively. Primers 3/7 (SMB4183/SMB2889), and 8/6 (SMB2888/SMB4184) established the integration of the 5’ flanking (3400bp) and 3’ flanking (2800bp) sequences, of the LPG1::PAC disruption cassette, respectively. Primers 9/10 (SMB2891/SMB2892) and 4/5 (SMB1568/SMB1569) confirmed the presence of the HYG (1080bp) and PAC (600bp) ORFs, respectively.
(EPS)
(A) Schematic representation of WT (top) and lpg2 − alleles (bottom). Numbers represent primers used for PCR amplification. PCR analysis for one representative FV1 WT, FV1 lpg2 − (cl 6.1A) and FV1 lpg2 − /+LPG2 (cl 6.1A AB15) is depicted. (B) Primers 13/14 (SMB1023/SMB1626) confirmed replacement of LPG2: WT (1000bp), FV1 lpg2 − (absent) and FV1 lpg2 − /+LPG2 (1000bp). (C) Primers 15/11 (SMB4124/SMB2566), and 19/20 (SMB2565/SMB4125) established the integration of the 5’ flanking (1300bp) and 3’ flanking (2200bp) sequences of the LPG2::HYG replacement cassette, respectively. Primers 15/17 (SMB4124/SMB3507), and 16/18 (SMB3506/SMB4417) established the integration of the 5’ flanking (1700bp) and 3’ flanking (2400bp) sequences, of the LPG2::SAT replacement cassette, respectively. Primers 9/10 (SMB2891/SMB2892) and 16/17 (SMB3506/SMB3507) confirmed the presence of the HYG (1080bp) and SAT (600bp) ORFs, respectively.
(EPS)
WT parasites underwent two rounds of electroporation as described in the methods to generate the FV null mutants. (A) For FV1 lpg1 −, WT parasites were transfected with ^LPG1::HYG and screened heterozygotes underwent a 2nd round of transfection with ^LPG1::PAC to yield FV1 lpg1 − null mutant. A third round of transfection with add back vector pXG-LPG1::NEO restored LPG1. (B) The FV1 lpg2 − was created utilizing a similar transfection strategy with targeting constructs, ΔLPG2::HYG, and ΔLPG2::SAT. The LPG2 was restored by electroporation with add back vector pSNBR-LPG2::NEO. (C) Western blot analysis with anti sera WIC79.3 indicated a loss of LPG in the FV1 lpg1 − mutant (lane 6) and loss of both PPGs and LPG in the FV1 lpg2 − mutant (lane 2). The FV1 lpg1 −/+LPG1 add backs (two clones are shown, lane 4,5) and the FV1 lpg2 −/+LPG2 add back (lane 3) exhibit restored levels of LPG and PPGs, comparable to WT parasites (lane 1).
(EPS)
Ten genes were selected for validation by qRT-PCR analysis (A)IL12B, (B) SOCS3, (C) TNFAIP3, (D) IL1B, (E) IL8, (F) TLR4, (G) TLR2, (H) FKBP4, (I) SMOX, and (J) FCGR1A. For each gene, fold change was calculated using fold changes were calculated using the ΔΔCT. Log2 ratios of RMA-normalized microarray gene probe set values for infected host cells over uninfected cells from the microarray (black bars) and fold changes from the qRT-PCR analysis (gray bars) are plotted and subjected to Pearson Correlation test (R value). Mean ± SEM is presented.
(EPS)
Human DCs were exposed to 1 μg LPG derived from L. major or L. donovani promastigotes (n = 3 donors). After 8 hours, RNA was extracted from infected hDCs for cDNA generation and analyzed for IL12B expression by qRT-PCR. Fold change was calculated utilizing the ΔΔCT method and depicted as fold change over uninfected samples. Mean ± SEM is presented.
(EPS)
Human DCs (n = 4 donors) were infected with L. major parasites: L. major FV1 WT (FV1), L. major LV39c5 WT (LV39c5), LV39c5 PG null (LV39c5 lpg2 −), and LV39c5 PG add back (LV39c5 lpg2 − /+LPG2). At 8 hrs post infection, IL12B expression was measured by qRT-PCR. Fold change was calculated utilizing the ΔΔCT method and depicted as fold change over uninfected samples. Box plots display the median value (line), the interquartile range (box), and Tukey whiskers encompassing data within 1.5 fold of the interquartile range. *Statistical significance as compared to uninfected control, (p<0.05). All values were significantly greater than uninfected.
(EPS)
(DOCX)
(DOCX)
Acknowledgments
The authors thank all members belonging to Stephen M. Beverley and Salvatore J. Turco’s laboratories for providing their expertise through the completion of the mutant parasites and LPG purifications, in their respective laboratories. We also thank Gwen Stayback, for her assistance with the parasite endotoxin and mycoplasma screening tests, and Wibke Ballhorn for her help with the hDC protocol. We are thankful to the University of Notre Dame Genomics and Bioinformatics Core Facility for their assistance in conducting microarray sample preparation, hybridizations, and initial analysis.
Data Availability
Complete array data generated in this study are accessible at the NCBI Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/, accession #GSE59766).
Funding Statement
This work was supported by National Institutes of of Allergy and Infectious Diseases grants R01AI056242 (MAM) and NIH RO1AI31078 (SMB, SJT). MAF was a fellow of the Chemistry-Biochemistry-Biology Interface (CBBI) Program at the University of Notre Dame, supported by training grant T32GM075762 from the National Institute of General Medical Sciences. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, et al. (2012) Leishmaniasis worldwide and global estimates of its incidence. PloS one 7: e35671 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Piscopo TV, Mallia Azzopardi C (2007) Leishmaniasis. Postgrad Med J 83: 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turco SJ (2003) Trypanosomatid surface and secreted carbohydrates. Molecular Medical Parasitology. pp. 225–240. [Google Scholar]
- 4. Cummings R, Turco S (2009) Parasitic Infections In: Varki A CR, Esko JD, editor. Essentials of Glycobiology. 2nd ed: Cold Spring Harbor Laboratory Press; pp. 553–565. [PubMed] [Google Scholar]
- 5. Turco SJ, Spath GF, Beverley SM (2001) Is lipophosphoglycan a virulence factor? A surprising diversity between Leishmania species. Trends Parasitol 17: 223–226. [DOI] [PubMed] [Google Scholar]
- 6. Turco SJ, Descoteaux A (1992) The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol 46: 65–94. [DOI] [PubMed] [Google Scholar]
- 7. Franco LH, Beverley SM, Zamboni DS (2012) Innate Immune Activation and Subversion of Mammalian Functions by Leishmania Lipophosphoglycan. Journal of Parasitology Research 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Svarovska A, Ant TH, Seblova V, Jecna L, Beverley SM, et al. (2010) Leishmania major glycosylation mutants require phosphoglycans (lpg2-) but not lipophosphoglycan (lpg1-) for survival in permissive sand fly vectors. PLoS Negl Trop Dis 4: e580 10.1371/journal.pntd.0000580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McConville MJ, Turco SJ, Ferguson MA, Sacks DL (1992) Developmental modification of lipophosphoglycan during the differentiation of Leishmania major promastigotes to an infectious stage. Embo J 11: 3593–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dobson DE, Kamhawi S, Lawyer P, Turco SJ, Beverley SM, et al. (2010) Leishmania major survival in selective Phlebotomus papatasi sand fly vector requires a specific SCG-encoded lipophosphoglycan galactosylation pattern. PLoS pathogens 6: e1001185 10.1371/journal.ppat.1001185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dobson DE, Mengeling BJ, Cilmi S, Hickerson S, Turco SJ, et al. (2003) Identification of genes encoding arabinosyltransferases (SCA) mediating developmental modifications of lipophosphoglycan required for sand fly transmission of leishmania major. The Journal of biological chemistry 278: 28840–28848. [DOI] [PubMed] [Google Scholar]
- 12. Pimenta PF, Turco SJ, McConville MJ, Lawyer PG, Perkins PV, et al. (1992) Stage-specific adhesion of Leishmania promastigotes to the sandfly midgut. Science 256: 1812–1815. [DOI] [PubMed] [Google Scholar]
- 13. Glaser TA, Moody SF, Handman E, Bacic A, Spithill TW (1991) An antigenically distinct lipophosphoglycan on amastigotes of Leishmania major. Mol Biochem Parasitol 45: 337–344. [DOI] [PubMed] [Google Scholar]
- 14. Sacks DL, Brodin TN, Turco SJ (1990) Developmental modification of the lipophosphoglycan from Leishmania major promastigotes during metacyclogenesis. Mol Biochem Parasitol 42: 225–233. [DOI] [PubMed] [Google Scholar]
- 15. Moody SF, Handman E, McConville MJ, Bacic A (1993) The structure of Leishmania major amastigote lipophosphoglycan. J Biol Chem 268: 18457–18466. [PubMed] [Google Scholar]
- 16. Mattner F, Di Padova K, Alber G (1997) Interleukin-12 is indispensable for protective immunity against Leishmania major. Infect Immun 65: 4378–4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, et al. (1995) Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol 154: 5071–5079. [PubMed] [Google Scholar]
- 18. Trinchieri G (2003) Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 3: 133–146. [DOI] [PubMed] [Google Scholar]
- 19. Reiner SL, Zheng S, Wang ZE, Stowring L, Locksley RM (1994) Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J Exp Med 179: 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carrera L, Gazzinelli RT, Badolato R, Hieny S, Muller W, et al. (1996) Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J Exp Med 183: 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jayakumar A, Widenmaier R, Ma X, McDowell MA (2008) Transcriptional inhibition of interleukin-12 promoter activity in Leishmania spp.-infected macrophages. J Parasitol 94: 84–93. 10.1645/GE-1153.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gorak PM, Engwerda CR, Kaye PM (1998) Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur J Immunol 28: 687–695. [DOI] [PubMed] [Google Scholar]
- 23. von Stebut E, Belkaid Y, Jakob T, Sacks DL, Udey MC (1998) Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J Exp Med 188: 1547–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leon B, Lopez-Bravo M, Ardavin C (2007) Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 26: 519–531. [DOI] [PubMed] [Google Scholar]
- 25. Konecny P, Stagg AJ, Jebbari H, English N, Davidson RN, et al. (1999) Murine dendritic cells internalize Leishmania major promastigotes, produce IL-12 p40 and stimulate primary T cell proliferation in vitro. Eur J Immunol 29: 1803–1811. [DOI] [PubMed] [Google Scholar]
- 26. von Stebut E, Belkaid Y, Nguyen BV, Cushing M, Sacks DL, et al. (2000) Leishmania major-infected murine langerhans cell-like dendritic cells from susceptible mice release IL-12 after infection and vaccinate against experimental cutaneous Leishmaniasis. Eur J Immunol 30: 3498–3506. [DOI] [PubMed] [Google Scholar]
- 27. Blank C, Fuchs H, Rappersberger K, Rollinghoff M, Moll H (1993) Parasitism of epidermal Langerhans cells in experimental cutaneous leishmaniasis with Leishmania major. J Infect Dis 167: 418–425. [DOI] [PubMed] [Google Scholar]
- 28. Ashok D, Acha-Orbea H (2014) Timing is everything: dendritic cell subsets in murine Leishmania infection. Trends Parasitol 30: 499–507. 10.1016/j.pt.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 29. Tacchini-Cottier F, Weinkopff T, Launois P (2012) Does T Helper Differentiation Correlate with Resistance or Susceptibility to Infection with L. major? Some Insights From the Murine Model. Frontiers in immunology 3: 32 10.3389/fimmu.2012.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Misslitz AC, Bonhagen K, Harbecke D, Lippuner C, Kamradt T, et al. (2004) Two waves of antigen-containing dendritic cells in vivo in experimental Leishmania major infection. Eur J Immunol 34: 715–725. [DOI] [PubMed] [Google Scholar]
- 31. Spath GF, Garraway LA, Turco SJ, Beverley SM (2003) The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts. Proc Natl Acad Sci U S A 100: 9536–9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu D, Kebaier C, Pakpour N, Capul AA, Beverley SM, et al. (2009) Leishmania major phosphoglycans influence the host early immune response by modulating dendritic cell functions. Infection and immunity 77: 3272–3283. 10.1128/IAI.01447-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Uzonna JE, Spath GF, Beverley SM, Scott P (2004) Vaccination with phosphoglycan-deficient Leishmania major protects highly susceptible mice from virulent challenge without inducing a strong Th1 response. J Immunol 172: 3793–3797. [DOI] [PubMed] [Google Scholar]
- 34. McDowell MA, Marovich M, Lira R, Braun M, Sacks D (2002) Leishmania priming of human dendritic cells for CD40 ligand-induced interleukin-12p70 secretion is strain and species dependent. Infection and immunity 70: 3994–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marovich MA, McDowell MA, Thomas EK, Nutman TB (2000) IL-12p70 production by Leishmania major-harboring human dendritic cells is a CD40/CD40 ligand-dependent process. J Immunol 164: 5858–5865. [DOI] [PubMed] [Google Scholar]
- 36. Dobson DE, Mengeling BJ, Cilmi S, Hickerson S, Turco SJ, et al. (2003) Identification of genes encoding arabinosyltransferases (SCA) mediating developmental modifications of lipophosphoglycan (LPG) required for sand fly transmission of Leishmania major. J Biol Chem 15: 15. [DOI] [PubMed] [Google Scholar]
- 37. McConville MJ, Schnur LF, Jaffe C, Schneider P (1995) Structure of Leishmania lipophosphoglycan: inter- and intra-specific polymorphism in Old World species. Biochem J 310: 807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anderson CF, Mendez S, Sacks DL (2005) Nonhealing infection despite Th1 polarization produced by a strain of Leishmania major in C57BL/6 mice. Journal of immunology 174: 2934–2941. [DOI] [PubMed] [Google Scholar]
- 39. Noben-Trauth N, Paul WE, Sacks DL (1999) IL-4- and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania major parasite substrains. J Immunol 162: 6132–6140. [PubMed] [Google Scholar]
- 40. Akopyants NS, Kimblin N, Secundino N, Patrick R, Peters N, et al. (2009) Demonstration of genetic exchange during cyclical development of Leishmania in the sand fly vector. Science 324: 265–268. 10.1126/science.1169464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spath GF, Epstein L, Leader B, Singer SM, Avila HA, et al. (2000) Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc Natl Acad Sci U S A 97: 9258–9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jayakumar A, Donovan MJ, Tripathi V, Ramalho-Ortigao M, McDowell MA (2008) Leishmania major infection activates NF-kappaB and interferon regulatory factors 1 and 8 in human dendritic cells. Infection and immunity 76: 2138–2148. 10.1128/IAI.01252-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Spath GF, Beverley SM (2001) A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Experimental parasitology 99: 97–103. [DOI] [PubMed] [Google Scholar]
- 44. Favila MA, Geraci NS, Zeng E, Harker B, Condon D, et al. (2014) Human dendritic cells exhibit a pronounced type I IFN signature following Leishmania major infection that is required for IL-12 induction. J Immunol 192: 5863–5872. 10.4049/jimmunol.1203230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kapler GM, Coburn CM, Beverley SM (1990) Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol 10: 1084–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Robinson KA, Beverley SM (2003) Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Molecular and biochemical parasitology 128: 217–228. [DOI] [PubMed] [Google Scholar]
- 47. Turco SJ, Wilkerson MA, Clawson DR (1984) Expression of an unusual acidic glycoconjugate in Leishmania donovani. The Journal of biological chemistry 259: 3883–3889. [PubMed] [Google Scholar]
- 48. McConville MJ, Bacic A, Mitchell GF, Handman E (1987) Lipophosphoglycan of Leishmania major that vaccinates against cutaneous leishmaniasis contains an alkylglycerophosphoinositol lipid anchor. Proceedings of the National Academy of Sciences of the United States of America 84: 8941–8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F (1951) A colorimetric method for the determination of sugars. Nature 168: 167. [DOI] [PubMed] [Google Scholar]
- 50. Bahr V, Stierhof YD, Ilg T, Demar M, Quinten M, et al. (1993) Expression of lipophosphoglycan, high-molecular weight phosphoglycan and glycoprotein 63 in promastigotes and amastigotes of Leishmania mexicana. Mol Biochem Parasitol 58: 107–121. [DOI] [PubMed] [Google Scholar]
- 51. Cheadle C, Vawter MP, Freed WJ, Becker KG (2003) Analysis of microarray data using Z score transformation. The Journal of molecular diagnostics: JMD 5: 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ernst J, Bar-Joseph Z (2006) STEM: a tool for the analysis of short time series gene expression data. BMC bioinformatics 7: 191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ernst J, Nau GJ, Bar-Joseph Z (2005) Clustering short time series gene expression data. Bioinformatics 21 Suppl 1: i159–168. [DOI] [PubMed] [Google Scholar]
- 54. Wang J, Duncan D, Shi Z, Zhang B (2013) WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res 41: W77–83. 10.1093/nar/gkt439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang B, Kirov S, Snoddy J (2005) WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res 33: W741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huang da W, Sherman BT, Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37: 1–13. 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sartori A, Oliveira MA, Scott P, Trinchieri G (1997) Metacyclogenesis modulates the ability of Leishmania promastigotes to induce IL-12 production in human mononuclear cells. J Immunol 159: 2849–2857. [PubMed] [Google Scholar]
- 58. Sartori A, Scott P, Trinchieri G (1996) Leishmania major metacyclogenesis modulates ability to induce IL-12. Ann N Y Acad Sci 795: 400–402. [DOI] [PubMed] [Google Scholar]
- 59. Spath GF, Lye LF, Segawa H, Sacks DL, Turco SJ, et al. (2003) Persistence without pathology in phosphoglycan-deficient Leishmania major. Science 301: 1241–1243. [DOI] [PubMed] [Google Scholar]
- 60. Capul AA, Hickerson S, Barron T, Turco SJ, Beverley SM (2007) Comparisons of mutants lacking the Golgi UDP-galactose or GDP-mannose transporters establish that phosphoglycans are important for promastigote but not amastigote virulence in Leishmania major. Infection and immunity 75: 4629–4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chaussabel D, Semnani RT, McDowell MA, Sacks D, Sher A, et al. (2003) Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood 102: 672–681. [DOI] [PubMed] [Google Scholar]
- 62. O'Garra A, Murphy KM (2009) From IL-10 to IL-12: how pathogens and their products stimulate APCs to induce T(H)1 development. Nature immunology 10: 929–932. 10.1038/ni0909-929 [DOI] [PubMed] [Google Scholar]
- 63. D'Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, et al. (1993) Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med 178: 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Koshiba R, Yanai H, Matsuda A, Goto A, Nakajima A, et al. (2013) Regulation of cooperative function of the Il12b enhancer and promoter by the interferon regulatory factors 3 and 5. Biochem Biophys Res Commun 430: 95–100. 10.1016/j.bbrc.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 65. Liu J, Cao S, Herman LM, Ma X (2003) Differential regulation of interleukin (IL)-12 p35 and p40 gene expression and interferon (IFN)-gamma-primed IL-12 production by IFN regulatory factor 1. J Exp Med 198: 1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Plevy SE, Gemberling JH, Hsu S, Dorner AJ, Smale ST (1997) Multiple control elements mediate activation of the murine and human interleukin 12 p40 promoters: evidence of functional synergy between C/EBP and Rel proteins. Mol Cell Biol 17: 4572–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sun HJ, Xu X, Wang XL, Wei L, Li F, et al. (2006) Transcription factors Ets2 and Sp1 act synergistically with histone acetyltransferase p300 in activating human interleukin-12 p40 promoter. Acta biochimica et biophysica Sinica 38: 194–200. [DOI] [PubMed] [Google Scholar]
- 68. Wang IM, Contursi C, Masumi A, Ma X, Trinchieri G, et al. (2000) An IFN-gamma-inducible transcription factor, IFN consensus sequence binding protein (ICSBP), stimulates IL-12 p40 expression in macrophages. J Immunol 165: 271–279. [DOI] [PubMed] [Google Scholar]
- 69. Lodge R, Descoteaux A (2005) Modulation of phagolysosome biogenesis by the lipophosphoglycan of Leishmania. Clin Immunol 114: 256–265. [DOI] [PubMed] [Google Scholar]
- 70. Ponte-Sucre A, Heise D, Moll H (2001) Leishmania major lipophosphoglycan modulates the phenotype and inhibits migration of murine Langerhans cells. Immunology 104: 462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hatzigeorgiou DE, Geng J, Zhu B, Zhang Y, Liu K, et al. (1996) Lipophosphoglycan from Leishmania suppresses agonist-induced interleukin 1 beta gene expression in human monocytes via a unique promoter sequence. Proceedings of the National Academy of Sciences of the United States of America 93: 14708–14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kavoosi G, Ardestani SK, Kariminia A, Abolhassani M, Turco SJ (2006) Leishmania major: Reactive oxygen species and interferon gamma induction by soluble lipophosphoglycan of stationary phase promastigotes. Experimental parasitology 114: 323–328. [DOI] [PubMed] [Google Scholar]
- 73. Becker I, Salaiza N, Aguirre M, Delgado J, Carrillo-Carrasco N, et al. (2003) Leishmania lipophosphoglycan (LPG) activates NK cells through toll-like receptor-2. Mol Biochem Parasitol 130: 65–74. [DOI] [PubMed] [Google Scholar]
- 74. Aebischer T, Harbecke D, Ilg T (1999) Proteophosphoglycan, a major secreted product of intracellular Leishmania mexicana amastigotes, is a poor B-cell antigen and does not elicit a specific conventional CD4+ T-cell response. Infection and immunity 67: 5379–5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kavoosi G, Ardestani SK, Kariminia A, Zeinali M, Alimohammadian MH (2008) Leishmania major: effects of proteophosphoglycan on reactive oxygen species, IL-12, IFN-gamma and IL-10 production in healthy individuals. Experimental parasitology 120: 62–66. 10.1016/j.exppara.2008.04.022 [DOI] [PubMed] [Google Scholar]
- 76. Peters C, Kawakami M, Kaul M, Ilg T, Overath P, et al. (1997) Secreted proteophosphoglycan of Leishmania mexicana amastigotes activates complement by triggering the mannan binding lectin pathway. European journal of immunology 27: 2666–2672. [DOI] [PubMed] [Google Scholar]
- 77. Piani A, Ilg T, Elefanty AG, Curtis J, Handman E (1999) Leishmania major proteophosphoglycan is expressed by amastigotes and has an immunomodulatory effect on macrophage function. Microbes and infection / Institut Pasteur 1: 589–599. [DOI] [PubMed] [Google Scholar]
- 78. Novozhilova NM, Bovin NV (2010) Structure, functions, and biosynthesis of glycoconjugates of Leishmania spp. cell surface. Biochemistry (Mosc) 75: 686–694. [DOI] [PubMed] [Google Scholar]
- 79. McDowell MA, Marovich M, Lira R, Braun M, Sacks D (2002) Leishmania priming of human dendritic cells for CD40 Ligand-induced interleukin-12p70 secretion is strain and species dependent. Infect Immun 70: 3994–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Masumi A, Tamaoki S, Wang IM, Ozato K, Komuro K (2002) IRF-8/ICSBP and IRF-1 cooperatively stimulate mouse IL-12 promoter activity in macrophages. FEBS Lett 531: 348–353. [DOI] [PubMed] [Google Scholar]
- 81. Lehtonen A, Veckman V, Nikula T, Lahesmaa R, Kinnunen L, et al. (2005) Differential expression of IFN regulatory factor 4 gene in human monocyte-derived dendritic cells and macrophages. Journal of immunology 175: 6570–6579. [DOI] [PubMed] [Google Scholar]
- 82. Dror N, Alter-Koltunoff M, Azriel A, Amariglio N, Jacob-Hirsch J, et al. (2007) Identification of IRF-8 and IRF-1 target genes in activated macrophages. Molecular immunology 44: 338–346. [DOI] [PubMed] [Google Scholar]
- 83. Vila-del Sol V, Punzon C, Fresno M (2008) IFN-gamma-induced TNF-alpha expression is regulated by interferon regulatory factors 1 and 8 in mouse macrophages. Journal of immunology 181: 4461–4470. [DOI] [PubMed] [Google Scholar]
- 84. la Sala A, He J, Laricchia-Robbio L, Gorini S, Iwasaki A, et al. (2009) Cholera toxin inhibits IL-12 production and CD8alpha+ dendritic cell differentiation by cAMP-mediated inhibition of IRF8 function. The Journal of experimental medicine 206: 1227–1235. 10.1084/jem.20080912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Xu H, Zhu J, Smith S, Foldi J, Zhao B, et al. (2012) Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nature immunology 13: 642–650. 10.1038/ni.2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kropf P, Freudenberg MA, Modolell M, Price HP, Herath S, et al. (2004) Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infect Immun 72: 1920–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. de Veer MJ, Curtis JM, Baldwin TM, DiDonato JA, Sexton A, et al. (2003) MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur J Immunol 33: 2822–2831. [DOI] [PubMed] [Google Scholar]
- 88. Flandin JF, Chano F, Descoteaux A (2006) RNA interference reveals a role for TLR2 and TLR3 in the recognition of Leishmania donovani promastigotes by interferon-gamma-primed macrophages. Eur J Immunol 36: 411–420. [DOI] [PubMed] [Google Scholar]
- 89. Ives A, Ronet C, Prevel F, Ruzzante G, Fuertes-Marraco S, et al. (2011) Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science 331: 775–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kavoosi G, Ardestani SK, Kariminia A (2009) The involvement of TLR2 in cytokine and reactive oxygen species (ROS) production by PBMCs in response to Leishmania major phosphoglycans (PGs). Parasitology 136: 1193–1199. 10.1017/S0031182009990473 [DOI] [PubMed] [Google Scholar]
- 91. Tuon FF, Fernandes ER, Pagliari C, Duarte MI, Amato VS (2010) The expression of TLR9 in human cutaneous leishmaniasis is associated with granuloma. Parasite immunology 32: 769–772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Schematic representation of WT (top) and lpg1 − alleles (bottom). Numbers represent primers used for PCR amplification. PCR analysis for one representative FV1 WT, FV1 lpg1 − (cl 2.10) and FV1 lpg1 − /+LPG1 (cl 2.10 AB3) is depicted. (B) Primers 1/2 (SMB1023/SMB1626) confirmed LPG1 disruption: WT (420bp), FV1 lpg1 − (3200bp) and FV1 lpg1 − /+LPG1 (420bp & 3200bp). (C) Primers 3/11 (SMB4183/SMB2566), and 12/6 (SMB4185/SMB4184) established the integration of the 5’ flanking (3300bp) and 3’ flanking (2600bp) sequences of the LPG1::HYG disruption cassette, respectively. Primers 3/7 (SMB4183/SMB2889), and 8/6 (SMB2888/SMB4184) established the integration of the 5’ flanking (3400bp) and 3’ flanking (2800bp) sequences, of the LPG1::PAC disruption cassette, respectively. Primers 9/10 (SMB2891/SMB2892) and 4/5 (SMB1568/SMB1569) confirmed the presence of the HYG (1080bp) and PAC (600bp) ORFs, respectively.
(EPS)
(A) Schematic representation of WT (top) and lpg2 − alleles (bottom). Numbers represent primers used for PCR amplification. PCR analysis for one representative FV1 WT, FV1 lpg2 − (cl 6.1A) and FV1 lpg2 − /+LPG2 (cl 6.1A AB15) is depicted. (B) Primers 13/14 (SMB1023/SMB1626) confirmed replacement of LPG2: WT (1000bp), FV1 lpg2 − (absent) and FV1 lpg2 − /+LPG2 (1000bp). (C) Primers 15/11 (SMB4124/SMB2566), and 19/20 (SMB2565/SMB4125) established the integration of the 5’ flanking (1300bp) and 3’ flanking (2200bp) sequences of the LPG2::HYG replacement cassette, respectively. Primers 15/17 (SMB4124/SMB3507), and 16/18 (SMB3506/SMB4417) established the integration of the 5’ flanking (1700bp) and 3’ flanking (2400bp) sequences, of the LPG2::SAT replacement cassette, respectively. Primers 9/10 (SMB2891/SMB2892) and 16/17 (SMB3506/SMB3507) confirmed the presence of the HYG (1080bp) and SAT (600bp) ORFs, respectively.
(EPS)
WT parasites underwent two rounds of electroporation as described in the methods to generate the FV null mutants. (A) For FV1 lpg1 −, WT parasites were transfected with ^LPG1::HYG and screened heterozygotes underwent a 2nd round of transfection with ^LPG1::PAC to yield FV1 lpg1 − null mutant. A third round of transfection with add back vector pXG-LPG1::NEO restored LPG1. (B) The FV1 lpg2 − was created utilizing a similar transfection strategy with targeting constructs, ΔLPG2::HYG, and ΔLPG2::SAT. The LPG2 was restored by electroporation with add back vector pSNBR-LPG2::NEO. (C) Western blot analysis with anti sera WIC79.3 indicated a loss of LPG in the FV1 lpg1 − mutant (lane 6) and loss of both PPGs and LPG in the FV1 lpg2 − mutant (lane 2). The FV1 lpg1 −/+LPG1 add backs (two clones are shown, lane 4,5) and the FV1 lpg2 −/+LPG2 add back (lane 3) exhibit restored levels of LPG and PPGs, comparable to WT parasites (lane 1).
(EPS)
Ten genes were selected for validation by qRT-PCR analysis (A)IL12B, (B) SOCS3, (C) TNFAIP3, (D) IL1B, (E) IL8, (F) TLR4, (G) TLR2, (H) FKBP4, (I) SMOX, and (J) FCGR1A. For each gene, fold change was calculated using fold changes were calculated using the ΔΔCT. Log2 ratios of RMA-normalized microarray gene probe set values for infected host cells over uninfected cells from the microarray (black bars) and fold changes from the qRT-PCR analysis (gray bars) are plotted and subjected to Pearson Correlation test (R value). Mean ± SEM is presented.
(EPS)
Human DCs were exposed to 1 μg LPG derived from L. major or L. donovani promastigotes (n = 3 donors). After 8 hours, RNA was extracted from infected hDCs for cDNA generation and analyzed for IL12B expression by qRT-PCR. Fold change was calculated utilizing the ΔΔCT method and depicted as fold change over uninfected samples. Mean ± SEM is presented.
(EPS)
Human DCs (n = 4 donors) were infected with L. major parasites: L. major FV1 WT (FV1), L. major LV39c5 WT (LV39c5), LV39c5 PG null (LV39c5 lpg2 −), and LV39c5 PG add back (LV39c5 lpg2 − /+LPG2). At 8 hrs post infection, IL12B expression was measured by qRT-PCR. Fold change was calculated utilizing the ΔΔCT method and depicted as fold change over uninfected samples. Box plots display the median value (line), the interquartile range (box), and Tukey whiskers encompassing data within 1.5 fold of the interquartile range. *Statistical significance as compared to uninfected control, (p<0.05). All values were significantly greater than uninfected.
(EPS)
(DOCX)
(DOCX)
Data Availability Statement
Complete array data generated in this study are accessible at the NCBI Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/, accession #GSE59766).