Abstract
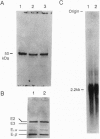
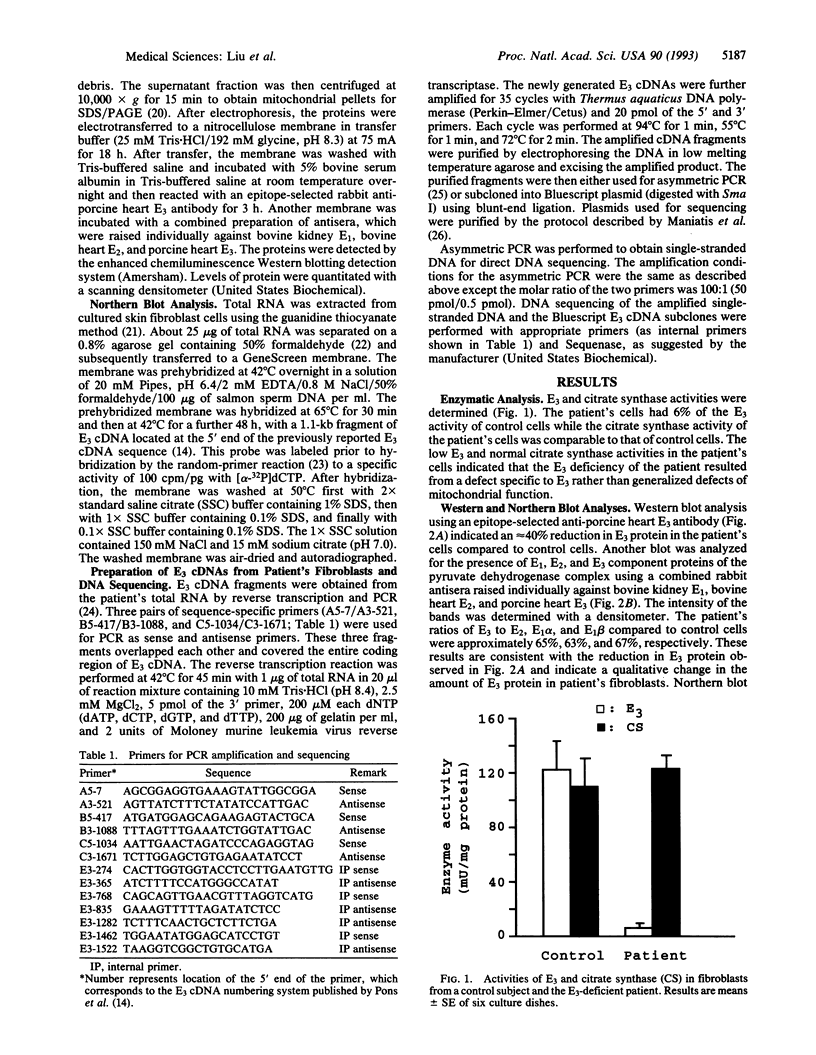
The molecular basis of dihydrolipoamide dehydrogenase (E3; dihydrolipoamide:NAD+ oxidoreductase, EC 1.8.1.4) deficiency in an E3-deficient patient was studied. Fibroblasts cultured from the patient contained only approximately 6% of the E3 activity of cells from a normal subject. Western and Northern blot analyses indicated that, compared to control cells, the patient's cells had a reduced amount of protein but normal amounts of E3 mRNA. Direct sequencing of E3 cDNA derived from the patient's RNA as well as each of the subclones of the cDNA revealed that the patient had two substitution mutations in the E3 coding region. One mutation changed a single nucleotide from A to G, resulting in substitution of Glu (GAA) for Lys-37 (AAA). The other point mutation was a nucleotide change from C to T, resulting in the substitution of Leu (CTG) for Pro-453 (CCG). These mutations appear to be significant in that they alter the active site and possibly the binding of FAD.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benen J. A., Van Berkel W. J., Van Dongen W. M., Müller F., De Kok A. Molecular cloning and sequence determination of the lpd gene encoding lipoamide dehydrogenase from Pseudomonas fluorescens. J Gen Microbiol. 1989 Jul;135(7):1787–1797. doi: 10.1099/00221287-135-7-1787. [DOI] [PubMed] [Google Scholar]
- Browning K. S., Uhlinger D. J., Reed L. J. Nucleotide sequence for yeast dihydrolipoamide dehydrogenase. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1831–1834. doi: 10.1073/pnas.85.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns G., Brown T., Hatter K., Sokatch J. R. Sequence analysis of the lpdV gene for lipoamide dehydrogenase of branched-chain-oxoacid dehydrogenase of Pseudomonas putida. Eur J Biochem. 1989 Jan 15;179(1):61–69. doi: 10.1111/j.1432-1033.1989.tb14521.x. [DOI] [PubMed] [Google Scholar]
- Carothers D. J., Pons G., Patel M. S. Dihydrolipoamide dehydrogenase: functional similarities and divergent evolution of the pyridine nucleotide-disulfide oxidoreductases. Arch Biochem Biophys. 1989 Feb 1;268(2):409–425. doi: 10.1016/0003-9861(89)90309-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth J. C., Perry T. L., Blass J. P., Hansen S., Urquhart N. Lactic acidosis in three sibs due to defects in both pyruvate dehydrogenase and alpha-ketoglutarate dehydrogenase complexes. Pediatrics. 1976 Oct;58(4):564–572. [PubMed] [Google Scholar]
- Hemilä H., Palva A., Paulin L., Arvidson S., Palva I. Secretory S complex of Bacillus subtilis: sequence analysis and identity to pyruvate dehydrogenase. J Bacteriol. 1990 Sep;172(9):5052–5063. doi: 10.1128/jb.172.9.5052-5063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L., Hu C. W., Packman S., Patel M. S. Deficiency of the pyruvate dehydrogenase component in pyruvate dehydrogenase complex-deficient human fibroblasts. Immunological identification. J Clin Invest. 1986 Sep;78(3):844–847. doi: 10.1172/JCI112651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentoft J. E., Shoham M., Hurst D., Patel M. S. A structural model for human dihydrolipoamide dehydrogenase. Proteins. 1992 Sep;14(1):88–101. doi: 10.1002/prot.340140110. [DOI] [PubMed] [Google Scholar]
- Kikuchi G., Hiraga K. The mitochondrial glycine cleavage system. Unique features of the glycine decarboxylation. Mol Cell Biochem. 1982 Jun 25;45(3):137–149. doi: 10.1007/BF00230082. [DOI] [PubMed] [Google Scholar]
- Kim H., Liu T. C., Patel M. S. Expression of cDNA sequences encoding mature and precursor forms of human dihydrolipoamide dehydrogenase in Escherichia coli. Differences in kinetic mechanisms. J Biol Chem. 1991 May 25;266(15):9367–9373. [PubMed] [Google Scholar]
- Kuhara T., Shinka T., Inoue Y., Matsumoto M., Yoshino M., Sakaguchi Y., Matsumoto I. Studies of urinary organic acid profiles of a patient with dihydrolipoyl dehydrogenase deficiency. Clin Chim Acta. 1983 Sep 30;133(2):133–140. doi: 10.1016/0009-8981(83)90398-4. [DOI] [PubMed] [Google Scholar]
- Kuroda Y., Kline J. J., Sweetman L., Nyhan W. L., Groshong T. D. Abnormal pyruvate and alpha-ketoglutarate dehydrogenase complexes in a patient with lactic acidemia. Pediatr Res. 1979 Aug;13(8):928–931. doi: 10.1203/00006450-197908000-00011. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Matalon R., Stumpf D. A., Michals K., Hart R. D., Parks J. K., Goodman S. I. Lipoamide dehydrogenase deficiency with primary lactic acidosis: favorable response to treatment with oral lipoic acid. J Pediatr. 1984 Jan;104(1):65–69. doi: 10.1016/s0022-3476(84)80591-0. [DOI] [PubMed] [Google Scholar]
- Mattevi A., Obmolova G., Sokatch J. R., Betzel C., Hol W. G. The refined crystal structure of Pseudomonas putida lipoamide dehydrogenase complexed with NAD+ at 2.45 A resolution. Proteins. 1992 Aug;13(4):336–351. doi: 10.1002/prot.340130406. [DOI] [PubMed] [Google Scholar]
- Mattevi A., Schierbeek A. J., Hol W. G. Refined crystal structure of lipoamide dehydrogenase from Azotobacter vinelandii at 2.2 A resolution. A comparison with the structure of glutathione reductase. J Mol Biol. 1991 Aug 20;220(4):975–994. doi: 10.1016/0022-2836(91)90367-f. [DOI] [PubMed] [Google Scholar]
- Matuda S., Kitano A., Sakaguchi Y., Yoshino M., Saheki T. Pyruvate dehydrogenase subcomplex with lipoamide dehydrogenase deficiency in a patient with lactic acidosis and branched chain ketoaciduria. Clin Chim Acta. 1984 Jun 27;140(1):59–64. doi: 10.1016/0009-8981(84)90151-7. [DOI] [PubMed] [Google Scholar]
- Otulakowski G., Nyhan W., Sweetman L., Robinson B. H. Immunoextraction of lipoamide dehydrogenase from cultured skin fibroblasts in patients with combined alpha-ketoacid dehydrogenase deficiency. Clin Chim Acta. 1985 Oct 31;152(1-2):27–36. doi: 10.1016/0009-8981(85)90172-x. [DOI] [PubMed] [Google Scholar]
- Otulakowski G., Robinson B. H. Isolation and sequence determination of cDNA clones for porcine and human lipoamide dehydrogenase. Homology to other disulfide oxidoreductases. J Biol Chem. 1987 Dec 25;262(36):17313–17318. [PubMed] [Google Scholar]
- Otulakowski G., Robinson B. H., Willard H. F. Gene for lipoamide dehydrogenase maps to human chromosome 7. Somat Cell Mol Genet. 1988 Jul;14(4):411–414. doi: 10.1007/BF01534650. [DOI] [PubMed] [Google Scholar]
- Palmer J. A., Hatter K., Sokatch J. R. Cloning and sequence analysis of the LPD-glc structural gene of Pseudomonas putida. J Bacteriol. 1991 May;173(10):3109–3116. doi: 10.1128/jb.173.10.3109-3116.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. A., Madhusudhan K. T., Hatter K., Sokatch J. R. Cloning, sequence and transcriptional analysis of the structural gene for LPD-3, the third lipoamide dehydrogenase of Pseudomonas putida. Eur J Biochem. 1991 Dec 5;202(2):231–240. doi: 10.1111/j.1432-1033.1991.tb16367.x. [DOI] [PubMed] [Google Scholar]
- Pons G., Raefsky-Estrin C., Carothers D. J., Pepin R. A., Javed A. A., Jesse B. W., Ganapathi M. K., Samols D., Patel M. S. Cloning and cDNA sequence of the dihydrolipoamide dehydrogenase component human alpha-ketoacid dehydrogenase complexes. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1422–1426. doi: 10.1073/pnas.85.5.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. H., Sherwood W. G., Kahler S., O'Flynn M. E., Nadler H. Lipoamide dehydrogenase deficiency. N Engl J Med. 1981 Jan 1;304(1):53–54. doi: 10.1056/NEJM198101013040116. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Taylor J., Kahler S. G., Kirkman H. N. Lactic acidemia, neurologic deterioration and carbohydrate dependence in a girl with dihydrolipoyl dehydrogenase deficiency. Eur J Pediatr. 1981 Mar;136(1):35–39. doi: 10.1007/BF00441708. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Taylor J., Sherwood W. G. Deficiency of dihydrolipoyl dehydrogenase (a component of the pyruvate and alpha-ketoglutarate dehydrogenase complexes): a cause of congenital chronic lactic acidosis in infancy. Pediatr Res. 1977 Dec;11(12):1198–1202. doi: 10.1203/00006450-197712000-00006. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sakaguchi Y., Yoshino M., Aramaki S., Yoshida I., Yamashita F., Kuhara T., Matsumoto I., Hayashi T. Dihydrolipoyl dehydrogenase deficiency: a therapeutic trial with branched-chain amino acid restriction. Eur J Pediatr. 1986 Sep;145(4):271–274. doi: 10.1007/BF00439399. [DOI] [PubMed] [Google Scholar]
- Schulz G. E., Schirmer R. H., Pai E. F. FAD-binding site of glutathione reductase. J Mol Biol. 1982 Sep 15;160(2):287–308. doi: 10.1016/0022-2836(82)90177-2. [DOI] [PubMed] [Google Scholar]
- Stephens P. E., Lewis H. M., Darlison M. G., Guest J. R. Nucleotide sequence of the lipoamide dehydrogenase gene of Escherichia coli K12. Eur J Biochem. 1983 Oct 3;135(3):519–527. doi: 10.1111/j.1432-1033.1983.tb07683.x. [DOI] [PubMed] [Google Scholar]
- Westphal A. H., de Kok A. Lipoamide dehydrogenase from Azotobacter vinelandii. Molecular cloning, organization and sequence analysis of the gene. Eur J Biochem. 1988 Mar 1;172(2):299–305. doi: 10.1111/j.1432-1033.1988.tb13887.x. [DOI] [PubMed] [Google Scholar]
- Yoshida I., Sweetman L., Kulovich S., Nyhan W. L., Robinson B. H. Effect of lipoic acid in a patient with defective activity of pyruvate dehydrogenase, 2-oxoglutarate dehydrogenase, and branched-chain keto acid dehydrogenase. Pediatr Res. 1990 Jan;27(1):75–79. doi: 10.1203/00006450-199001000-00020. [DOI] [PubMed] [Google Scholar]