Abstract
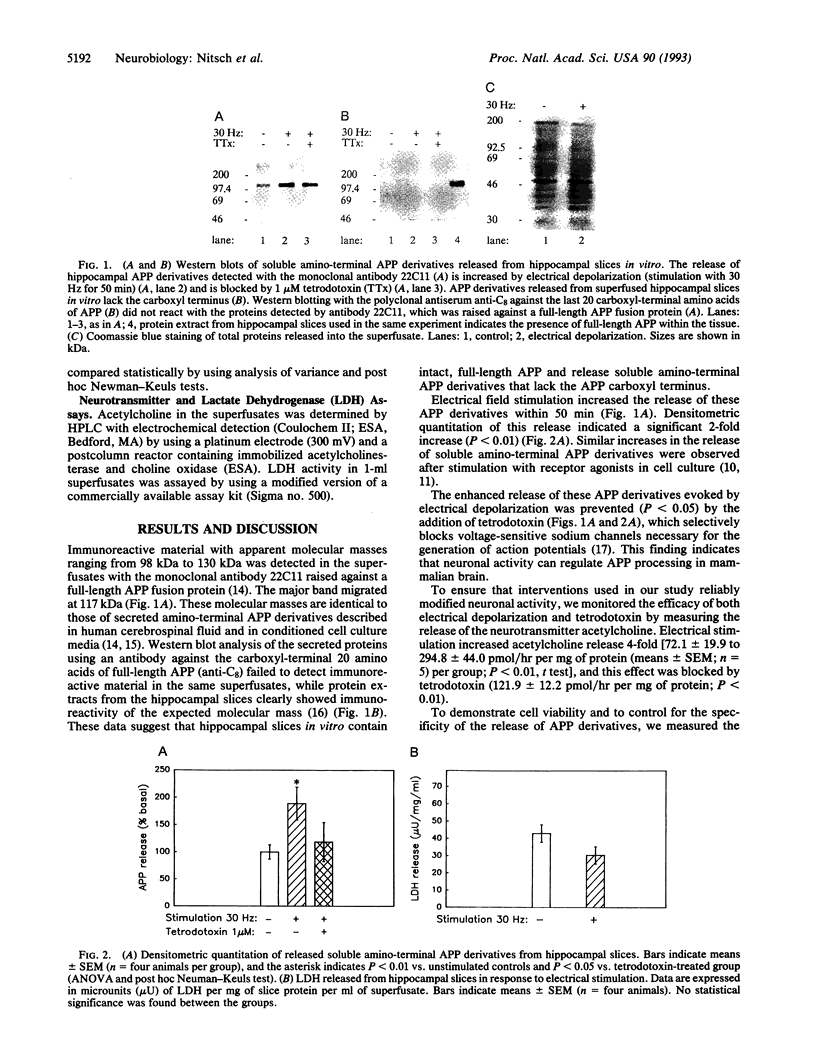
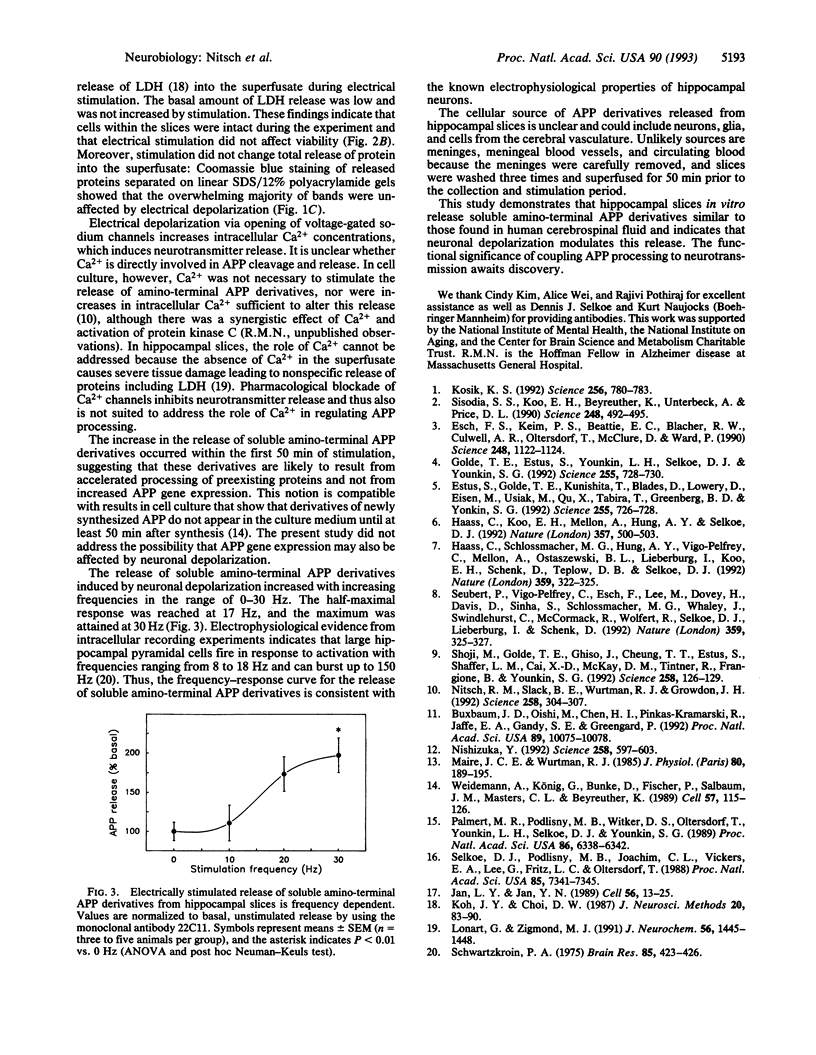
Proteolytic processing of the beta-amyloid protein precursor (APP) is regulated by cell-surface receptors. To determine whether neurotransmitter release in response to neuronal activation regulates APP processing in brain, we electrically depolarized superfused rat hippocampal slices and measured soluble APP derivatives released into the superfusate. Electrical depolarization caused a rapid increase in the release of both neurotransmitters and amino-terminal APP cleavage products. These derivatives lacked the APP carboxyl terminus and were similar to those found in both cell culture media and human cerebrospinal fluid. Superfusate proteins including lactate dehydrogenase were not changed by electrical depolarization. The release of amino-terminal APP derivatives increased with increasing stimulation frequencies from 0 to 30 Hz. The increased release was inhibited by the sodium-channel antagonist tetrodotoxin, suggesting that action-potential formation mediates the release of large amino-terminal APP derivatives. These results suggest that neuronal activity regulates APP processing in the mammalian brain.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buxbaum J. D., Oishi M., Chen H. I., Pinkas-Kramarski R., Jaffe E. A., Gandy S. E., Greengard P. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta/A4 amyloid protein precursor. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10075–10078. doi: 10.1073/pnas.89.21.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., McClure D., Ward P. J. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990 Jun 1;248(4959):1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Estus S., Golde T. E., Kunishita T., Blades D., Lowery D., Eisen M., Usiak M., Qu X. M., Tabira T., Greenberg B. D. Potentially amyloidogenic, carboxyl-terminal derivatives of the amyloid protein precursor. Science. 1992 Feb 7;255(5045):726–728. doi: 10.1126/science.1738846. [DOI] [PubMed] [Google Scholar]
- Golde T. E., Estus S., Younkin L. H., Selkoe D. J., Younkin S. G. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science. 1992 Feb 7;255(5045):728–730. doi: 10.1126/science.1738847. [DOI] [PubMed] [Google Scholar]
- Haass C., Koo E. H., Mellon A., Hung A. Y., Selkoe D. J. Targeting of cell-surface beta-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992 Jun 11;357(6378):500–503. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Voltage-sensitive ion channels. Cell. 1989 Jan 13;56(1):13–25. doi: 10.1016/0092-8674(89)90979-3. [DOI] [PubMed] [Google Scholar]
- Koh J. Y., Choi D. W. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987 May;20(1):83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- Kosik K. S. Alzheimer's disease: a cell biological perspective. Science. 1992 May 8;256(5058):780–783. doi: 10.1126/science.1589757. [DOI] [PubMed] [Google Scholar]
- Lonart G., Zigmond M. J. Incubation of tissue slices in the absence of Ca2+ and Mg2+ can cause nonspecific damage. J Neurochem. 1991 Apr;56(4):1445–1448. doi: 10.1111/j.1471-4159.1991.tb11445.x. [DOI] [PubMed] [Google Scholar]
- Maire J. C., Wurtman R. J. Effects of electrical stimulation and choline availability on the release and contents of acetylcholine and choline in superfused slices from rat striatum. J Physiol (Paris) 1985;80(3):189–195. [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992 Oct 23;258(5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Nitsch R. M., Slack B. E., Wurtman R. J., Growdon J. H. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science. 1992 Oct 9;258(5080):304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- Palmert M. R., Podlisny M. B., Witker D. S., Oltersdorf T., Younkin L. H., Selkoe D. J., Younkin S. G. The beta-amyloid protein precursor of Alzheimer disease has soluble derivatives found in human brain and cerebrospinal fluid. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6338–6342. doi: 10.1073/pnas.86.16.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin P. A. Characteristics of CA1 neurons recorded intracellularly in the hippocampal in vitro slice preparation. Brain Res. 1975 Mar 7;85(3):423–436. doi: 10.1016/0006-8993(75)90817-3. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Podlisny M. B., Joachim C. L., Vickers E. A., Lee G., Fritz L. C., Oltersdorf T. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Sisodia S. S., Koo E. H., Beyreuther K., Unterbeck A., Price D. L. Evidence that beta-amyloid protein in Alzheimer's disease is not derived by normal processing. Science. 1990 Apr 27;248(4954):492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]



