Abstract
Background
Evidence suggests the norepinephrine system represents an important treatment target for alcohol dependence (AD) and the α1-blocker prazosin may reduce alcohol drinking in rodents and alcoholic patients. The α1-blocker doxazosin demonstrates a more favorable pharmacokinetic profile than prazosin but has never been studied for AD.
Methods
A double-blind placebo-controlled randomized clinical trial was conducted in AD individuals seeking outpatient treatment. Doxazosin or matched placebo was titrated to 16-mg/day (or maximum tolerable dose). Drinks per week (DPW) and heavy drinking days (HDD) per week were primary outcomes. Family history density of alcoholism (FHDA), severity of AD, and gender were a priori moderators.
Results
Forty-one AD individuals were randomized, 30 (doxazosin = 15) completed the treatment phase, and 28 (doxazosin = 14) also completed the follow-up. There were no significant differences between groups on DPW and HDD per week. With FHDA as a moderator, there were significant FHDA x medication interactions for both DPW [pcorrected = .001, d = 1.18] and HDD [pcorrected = .00009, d = 1.30]. Post-hoc analyses revealed that doxazosin significantly reduced alcohol drinking in AD patients with high FHDA and by contrast increased drinking in those with low FHDA.
Conclusions
Doxazosin may be effective selectively in AD patients with high FHDA. This study provides preliminary evidence for personalized medicine using α1-blockade to treat AD. However, confirmatory studies are required.
Keywords: doxazosin, α1-blockade, alcoholism, craving, family history density of alcoholism, clinical trial
INTRODUCTION
Alcohol dependence (AD) afflicts about 10% of the United States population causing serious morbidity and mortality. Only a few medications are approved for AD, however, their therapeutic effects are modest or limited to certain subgroups [for review, see: (Leggio et al., 2009)]. Thus, developing new medications for AD remains a priority.
Evidence suggests an important role of the norepinephrine system in AD [for review, see: (Koob, 2008)], pointing to the norepinephrine system as a potentially important pharmacological target. Norepinephrine innervates key limbic areas for arousal, reinforcement, and stress – processes involved in developing and maintaining AD (Koob, 2008). Elevated epinephrine (Ehrenreich et al., 1997) and norepinephrine (Patkar et al., 2004) plasma levels have been found in abstinent alcoholic patients. Hyperexcitability, a key feature in the predisposition and development of AD, is associated with increased adrenergic activation (Koob, 2008). Enhanced acoustic startle response is a proxy for hyperexcitability, and brain noradrenergic-α1 mechanisms mediate enhanced acoustic startle response (Stevens et al., 1994), which is characteristic in alcohol preferring (P line) animals (Chester et al., 2004) and AD patients (Krystal et al., 1997). In animals, norepinephrine depletion attenuates ethanol self-administration (Amit et al., 1977) and alcohol withdrawal symptoms (Trzaskowska et al., 1986); α1-receptor antagonism reduces the locomotor hyperactivity produced by alcohol withdrawal (Trzaskowska et al., 1986).
In summary, animal and human studies demonstrate that α1-blockade may represent a therapeutic approach for AD. Recently, promising results have been obtained with the noradrenergic-α1-blocker prazosin, approved by the Food and Drug Administration (FDA) for hypertension and benign prostatic hyperplasia. Prazosin reduced alcohol self-administration and was more potent in ethanol-dependent rats than in non-dependent, suggesting that prazosin blocks dependence-induced increases in responding to alcohol (Walker et al., 2008). Subsequently, both acute and chronic prazosin treatment demonstrated decreased ethanol consumption in alcohol P rats (Rasmussen et al., 2009). Prazosin also blocked yohimbine- and footshock-induced reinstatement of alcohol seeking (Le et al., 2011).
Based on this preclinical evidence, a 6-week pilot randomized clinical trial (RCT) was performed (Simpson et al., 2009); 24 AD individuals were treated with placebo or prazosin 16-mg, divided over three daily doses. During the last 3 weeks of the study, the prazosin group compared to placebo, had a statistically significant reduction in drinking days per week, and a trend in reduction in drinks per week (Simpson et al., 2009). No significant medication effect on craving was found. Though frequent non-serious adverse events (AEs) such as dizziness, lack of energy, drowsiness, and one case of syncope were reported, there were no serious AEs (Simpson et al., 2009). In a more recent study with detoxified abstinent AD individuals (n = 17), prazosin (16mg/day), compared to placebo, decreased both stress- and cue-induced alcohol craving measured via guided imagery exposures to stress, alcohol cue, and neutral-relaxing/control conditions (Fox et al., 2012).
The noradrenergic-α1-blocker doxazosin is also FDA-approved for hypertension and benign prostatic hyperplasia. Doxazosin and prazosin share the same piperazine ring structure, and have a non-specific action on all three α1-subtypes, i.e., α1A, α1B and α1D (Gross et al., 1989). However, doxazosin has a more manageable dosing and safety profile than prazosin. Specifically, doxazosin is long-acting [half-life (t1/2) approximately 22 hours)] and dosed only once daily, thus facilitating adherence (Kirby et al., 1998). Doxazosin is also less likely to produce hypotension, because of the slower onset of action and long t1/2. Furthermore, unlike other α1-blockers (i.e., prazosin), doxazosin can be taken at any time of day, with or without food, properties that further promote patient adherence (Kirby et al., 1998). Thus, in clinical practice, doxazosin is often preferred to treat hypertension or benign prostatic hyperplasia, over short-acting α1-blockers, such as prazosin (Akduman and Crawford, 2001). Therefore given its favorable pharmacokinetics, a proof-of-concept RCT was conducted to test the hypothesis that doxazosin may represent a safe and effective medication for the treatment of AD. Specifically, the primary aim of the study was that doxazosin, compared to placebo, may significantly reduce alcohol consumption. Secondary aims were that doxazosin may significantly reduce alcohol craving, anxiety and stress levels. Finally, we hypothesized that family history of alcoholism, severity of AD and gender may moderate doxazosin’s response on alcohol consumption.
METHODS AND MATERIALS
Study Design
A 10-week between-subject double-blind placebo-controlled RCT with doxazosin was conducted at the Brown University Center for Alcohol and Addiction Studies (ClinicalTrials.gov: NCT01437046). The Brown University Institutional Review Board reviewed and approved the study. Patients were individuals seeking outpatient treatment for AD. For inclusion/exclusion criteria, see Supplement (Table 1S).
Study Drug
Doxazosin or matched placebo were prepared as opaque capsules by a compounding pharmacy and inserted into blister packs. Consistent with the recommended titration, doxazosin was titrated up to 16-mg daily (or maximum tolerable dose) during the first 4 weeks (Supplement: Table 2S). A 1-week downward titration for safety reasons was also planned. The choice to test 16-mg daily, the highest dose used in hypertension, was made on the basis of the prazosin alcohol trial (Simpson et al., 2009), where: 1) prazosin was used at the highest dose for hypertension (16-mg/day); and 2) participants had a statistically significant reduction in alcohol consumption during the last 3 weeks, when prazosin was administered at the full dose. Study medication adherence was assessed by self-report and pill count. Additionally, capsules contained 25-mg riboflavin as a marker of adherence through urine sample (Del Boca et al., 1996).
Medical Management (MM)
At each medication visit, participants also received a Medical Management session. Medical Management is an intervention with demonstrated efficacy as a behavioral platform for the treatment of AD (Anton et al., 2006). The Medical Management sessions provide personalized education regarding alcohol. It is structured to help the participant develop and implement a plan to stop/reduce drinking, motivate participants for medication adherence and assess AEs and concomitant medication use. The Medical Management approach was based on the COMBINE study (Anton et al., 2006), and revised and adapted for this study. For example, special emphasis in the assessment of AEs was given to side-effects already described for doxazosin; particular attention was given not only to medication adherence per se but also to the compliance to the study medication titration during the first four weeks of the trial. Special attention was given to those concomitant medications with known possible interaction with doxazosin.
Study Procedures
The study consisted of four phases: telephone pre-screening, in-person screening, 10-week treatment, and 2-week follow-up. Potential participants, recruited via advertisements in public transportation and mass media, and referrals from other clinics, were phone screened. Those meeting initial pre-screening criteria came for an in-person screen in which they provided written informed consent. Screening (Week 00 visit) included psychological assessments, medical history, physical, ECG, and blood/urine labs (e.g., liver and kidney function tests, CBC, urine drug and pregnancy tests). Breath alcohol concentration was measured, vital signs were taken, and recent alcohol consumption was collected using the Timeline Follow-back (TLFB) (Sobell et al., 1988). At Week 01 visit (Day 01), eligible participants were randomized to doxazosin or placebo. A brief telephone assessment occurred at days 2–4 to assess possible AEs. Then, participants were assessed in person at Weeks 2, 3, 4, 6, 8 and 10 for in-person visits, during which medical assessments, questionnaires, study medication and Medical Management sessions were provided. A brief telephone assessment occurred at Weeks 5, 7 and 9 to address possible AEs. At Week 10, a downward titration dose of doxazosin/placebo was administered. Subsequently, two weeks after Week 10 (thus, ~1 week after the last study medication dose) a brief in-person follow-up visit took place to assess general health status.
Study Outcomes and Assessments
Drinking Outcomes
Primary outcomes were drinks per week (DPW) and heavy drinking days (HDD) per week, as assessed by the TLFB.
Craving, Anxiety and Stress
It has been suggested that the role of prazosin in AD may be mediated by its effects on stress-related anxiety (Rasmussen et al., 2009; Simpson et al., 2009; Walker et al., 2008), therefore secondary outcomes were stress, anxiety and craving. Alcohol craving was assessed by the Obsessive Compulsive Drinking Scale (OCDS), including the total, obsessive (ODS) and compulsive (CDS) scores (Anton et al., 1995). Anxiety and stress were assessed by using the Hamilton Anxiety Scale (HAMA) (Hamilton, 1959), the Perceived Stress Scale (PSS) (Cohen et al., 1983) and the Anxiety-Tension Subscale of the Profile of Mood States (POMS-TA) (Pollock et al., 1979).
Moderators
Analyses of family history of alcoholism, severity of AD and gender as potential moderators of a medication effect were planned a priori. Consistent with the literature (Koob, 2008; Trzaskowska et al., 1986; Walker et al., 2008), α1-blockade represents a mechanism of action more likely to be effective in patients with more biologically-based AD and/or higher severity of dependence. For example, prazosin’s ability to reduce ethanol self-administration was more potent in ethanol-dependent rats than in non-dependent rats (Walker et al., 2008). Consistent with previous reports (Capone et al., 2011; Rohsenow et al., 2007), we used the Family Tree Questionnaire (Mann et al., 1985) to calculate the family history density of alcoholism (FHDA) among first degree relatives; FHDA was dichotomized at .50 (median split) into low or high FHDA. We used the Alcohol Dependence Scale (ADS) (Skinner and Allen, 1982) as a direct measure of severity of dependence; ADS was dichotomized into low or high ADS based on the median of 10.5. Finally, we assessed gender as it has been associated with severity of AD (Rohsenow et al., 2007).
Adverse Events (AEs)
Adverse Events were assessed at each visit using the SAFTEE (Levine and Schooler, 1986), revised and adapted for this study. Clinical assessments (e.g., blood pressure [BP]) were used to identify other AEs.
Statistical Analysis
Distributional characteristics of outcome measures were examined to evaluate similarity to the normal distribution. Drinks per week had a skewness and kurtosis slightly in excess of two; consequently, the data were transformed using a square root transformation. The Mixed Model procedure, which accommodates cases with missing data, was used to assess medication effects on outcomes with time nested under subjects. The baseline value of each particular dependent measure was added as a covariate. Additionally, as the two groups differed in racial make-up (Table 1), race was added as a covariate, to ensure that any differences on outcome variables between groups were not accounted for by race difference. Chi-square (χ2) tests were conducted to assess if demographic characteristics, medication adherence, or AEs differed between doxazosin and placebo groups. Moderator analyses for FHDA and ADS were conducted using a median split [consistent with (Rhemtulla et al., 2012), who confirmed the conventional wisdom that, with just two to four categories, continuous methodology is generally not recommended] while gender was already a dichotomous variable. Consistent with recent recommendations (Falk et al., 2010), the previous data showing a prazosin effect at the target dose (Simpson et al., 2009) and the doxazosin titration scheduled (Supplement: Table 2S), a grace period was applied for the first 4 weeks of medication to account for titration to peak pharmacological effect; therefore, except for the baseline comparisons, medication adherence and AE analyses, all other analyses were conducted for the target dose (16-mg) period only. Standard errors (SE) were reported for Mixed Model analyses; standard deviations (SD) were reported for t-tests. All participants with at least one valid outcome data point were included in the analyses (mITT). SPSS version 21 (NY, U.S.) was used to conduct the analyses.
Table 1.
Participant Characteristics at baseline [M ± (SD) or percentage (%)]
| Doxazosin (n = 20) | Placebo (n = 21) | |
|---|---|---|
| Age | 42.1 (10.2) | 42.1 (7.5) |
| Women (%) | 30 | 29 |
| Hispanic/Latino (%) | 10 | 5 |
| Race (%): | ||
| American/Alaskan Indian | 0 | 5 |
| African American | 25 | 43 |
| White* | 65 | 33 |
| Other | 0 | 5 |
| More than one race | 10 | 14 |
| Drinking Per Week (DPW) | 69.0 (33.1) | 75.6 (71.2) |
| Heavy Drinking Days (HDD) | 5.3 (1.6) | 5.1 (1.8) |
| Obsessive Compulsive Drinking Scale (OCDS) score | 18.3 (6.4) | 14.8 (7.6) |
| Obsessive Drinking Scale (ODS) sub-score | 7.7 (3.7) | 6.0 (4.5) |
| Compulsive Drinking Scale (CDS) sub-score | 10.6 (3.2) | 8.9 (3.7) |
| Family History Density of Alcoholism (FHDA) (%) | 52 | 38 |
| Cigarette Smokers (%) | 70 | 71 |
| Urine drug screen positive for THC (%) | 10 | 29 |
| Systolic Blood Pressure Supine | 126 (21) | 124 (11) |
| Diastolic Blood Pressure Supine | 81 (13) | 79 (10) |
| Systolic Blood Pressure Standing | 124 (16) | 124 (12) |
| Diastolic Blood Pressure Standing | 83 (13) | 83 (9) |
| Systolic Blood Pressure (BP)Δ | 2.5 (12.9) | −0.3 (12.1) |
χ2 [(1, N = 41) = 4.11, p = .04]; no other significant baseline differences between groups were found [p’s > .05]
RESULTS
Participant Characteristics
Of 197 individuals pre-screened by phone, 52 signed the informed consent and were screened in-person; 11 were ineligible, while 41 were eligible and randomized (doxazosin, n = 20: placebo, n = 21). Thirty participants (doxazosin = 15) completed the treatment phase and 28 (doxazosin = 14) completed the follow-up (Supplement: Figure 1S). Participants’ baseline characteristics are shown in Table 1.
Drinking Outcomes
There were no significant differences between groups in DPW [F1,36 = 0.43, p > .05] and HDD [F1,35 = 1.03, p > .05], although there was a small reduction in DPW and HDD in the doxazosin group compared to placebo (effect sizes d =.23 and .35, respectively). There was no significant time or medication by time interaction for either of the two outcomes (p’s > 0.05). (Please see, Table 5S for results for all terms in the model for these two DVs.)
Craving, Anxiety and Stress
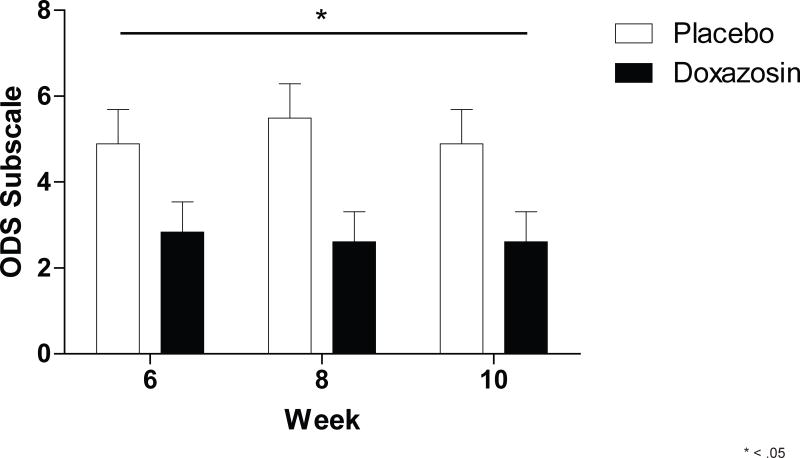
There was a significant main effect for medication on the ODS subscale [F1,33 = 4.92, p =.034] (Figure 1). There were no significant medication effects on total OCDS [F1,35 = 2.55, p > .05], CDS [F1,36 = 0.87, p > .05], HAMA [F1,36 = 0.46, p > .05], PSS [F1,36 = 0.46, p > .05] or POMS-TA [F1,34 = 0.34, p > .05] scales. There were no significant main effects for time or medication by time interaction for these outcomes [p’s > .05]. (Please see, Tables 6S and 7S for results for all terms in the model for these DVs.)
Figure 1.
Significant effect for doxazosin on the obsessive craving (ODS) subscale.
Moderators
Analyses for the 3 potential moderators (FHDA, ADS and gender) of the 2 primary drinking outcomes were conducted using an alpha corrected by a factor of 6 (3 x 2).
There were main effects of FHDA on DPW [F1,35 = 9.48, pcorrected = .024] and HDD [F1,34 = 13.38, pcorrected = .005], as well as significant FHDA x medication interactions for both DPW [F1,35 = 17.35, pcorrected = .001] and HDD [F1,34 = 25.29, pcorrected = .00009]. Post-hoc analyses showed a significant medication effect for high FHDA in the expected direction (i.e., reduction of drinking) for DPW [t31 = 3.47, pcorrected = .003; Figure 2A] and HDD [t31 = 3.84, pcorrected = .0004; Figure 2B]. Large effect sizes were reported for both DPW (d = 1.18) and HDD (d = 1.30) with high FHDA as moderator (Table 2). Notably, there was a significant reverse medication effect for low FHDA on DPW [t29 = 2.59, pcorrected = .04; Figure 2A] and trend for a reverse medication effect for low FHDA on HDD [t29 = 2.40, pcorrected = .08; Figure 2B]. FHDA was not related to differences in age, gender, race, ethnicity, or baseline DPW or HDD. There were no significant effects for ADS or gender on the two main outcomes (data not shown).
Figure 2.
(A) Significant effect for doxazosin on Drinks per Week (DPW), Family History Density for Alcoholism (FHDA) x medication interaction; (B) Significant effect for doxazosin on Heavy Drinking Days (HDD) per week, FHDA x medication interaction. Horizontal lines indicate significant interactions, and brackets indicate post-hoc analyses with significant findings.
Table 2.
Family History Density of Alcoholism (FHDA) as moderator of the primary drinking outcomes [M ± (SE)]
| Drinks Per Week (DPW) | Heavy Drinking Days (HDD) | ||||||
|---|---|---|---|---|---|---|---|
| High FHDA | 4.2 (0.5) | 2.3 (0.3) | |||||
| FHDA | Low FHDA | 1.8 (0.6) | 0.7 (0.4) | ||||
| pcorrected | .024 | .005 | |||||
| Doxazosin | Placebo | Doxazosin | Placebo | ||||
| High FHDA | 2.3 (0.7) | 6.1 (0.8) | 1.1 (0.5) | 3.6 (0.5) | |||
| FHDA X Medication | Low FHDA | 3.3 (0.7) | 0.2 (0.9) | 1.7 (0.4) | 0.0 (0.6) | ||
| pcorrected | .001 | .00009 | |||||
| High FHDA X Medication effect size (d) | 1.18 | 1.30 | |||||
Exploratory Analyses
Based on the above results for FHDA, additional analyses were performed to investigate the extent to which effects in secondary outcomes were also moderated by FHDA. Given the exploratory nature of these analyses, results are presented with uncorrected alpha levels (Table 3). There were significant main effects of FHDA on craving scales [total OCDS: F1,34 = 6.25, p = .017; ODS: F1,35 = 6.97, p = .012; CDS F1,34 = 5.45, p = .026], anxiety [HAMA: F1,34 = 5.13, p = .030], and mood [POMS-TA: F1,32 = 7.44 p = .010], but not PSS [p > .05]. There were significant FHDA by medication interactions for craving [total OCDS: F1,35 = 10.06, p = .003; ODS: F1,35 = 5.23, p = .028; CDS: F1,35 = 11.08, p = .002] and anxiety [HAMA: F1,32 = 9.76, p = .004].
Table 3.
Family History Density of Alcoholism (FHDA) as moderator of the secondary aims [M ± (SE)]
| Total OCDS | ODS | CDS | HAMA | POMS-TA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FHDA | ||||||||||
| High FHDA | 11.1 (1.3) | 5.3 (.6) | 6.8 (.6) | 2.4 (.3) | 5.5 (.6) | |||||
| Low FHDA | 3.4 (1.4) | 1.3 (.7) | 4.5 (.6) | .4 (.3) | 2.8 (.6) | |||||
| p | < .001 | < .001 | .009 | < .001 | .004 | |||||
| FHDA X Medication | Doxasozin | Placebo | Doxasozin | Placebo | Doxasozin | Placebo | Doxasozin | Placebo | Doxasozin | Placebo |
|
|
|
|
|
|
||||||
| High FHDA | 5.5 (1.7) | 16.8 (1.8) | 3.5 (0.8) | 7.2 (0.9) | 3.8 (0.8) | 9.9 (0.8) | 1.8 (0.4) | 3.1 (0.4) | 7.0 (0.8) | 3.9 (0.8) |
| Low FHDA | 5.8 (1.7) | 1.0 (2.1) | 1.3 (0.8) | 1.3 (0.9) | 5.6 (0.8) | 3.3 (0.8) | 1.0 (0.4) | 0.0 (0.5) | 2.8 (0.8) | 2.8 (1.0) |
| p | < .001 | .010 | < .001 | .004 | .078 | |||||
Obsessive Compulsive Drinking Scale: total score (OCDS), and obsessive (ODS) and compulsive (CDS) subscales; Hamilton Anxiety Scale (HAMA); Anxiety-Tension Subscale of the Profile of Mood States (POMS-TA).
Study Medication Adherence
Percent days of medication adherence were as follows: doxazosin = 85.7 (24.0) percent days adherent and placebo = 75.6 (32.7) percent days adherent, [t39 = 1.13, p =.27]. The two groups did not differ on medication adherence [p > .05].
Drug titration
There were no statistical differences between doxazosin and placebo groups during the drug titration phase and maximum tolerable dose. Of the 28 who completed the study, 24 participants reached 16-mg (12 doxazosin; 12 placebo), and four completed the study at a lower dose (maximum tolerable dose of doxazosin: one remained at 4-mg and one reached 16-mg for a week then returned to 8-mg; maximum tolerable dose of placebo: one remained at 4-mg and one at 2-mg). Of the 13 who did not complete the study, six participants reached 16-mg (three received doxazosin; three received placebo) and seven never reached 16-mg (three received doxazosin, however, one completed the 10-week study at 4-mg but did not return for follow-up; four received placebo).
Adverse Events (AEs)
Four AEs were more frequent in the doxazosin group vs. placebo: dizziness, depression or other mood disturbance, trouble urinating [p’s < .05] and headache [p < .01] (Supplement: Table 3S). Particular attention was also given to BP, given doxazosin’s expected hypotensive effects, assessed as systolic and diastolic blood pressure changes (BPΔ; average supine minus average standing reading). No baseline differences were observed between the two groups (Table 1). No significant differences in BPΔ were observed between the two groups during treatment (Supplement: Table 4S).
DISCUSSION
This study provides preliminary yet promising results on the potential role of doxazosin in the treatment of AD. Specifically, findings from this relative small sample do not support a role for doxazosin in reducing alcohol use in a general AD population; however doxazosin may be effective in reducing drinking and craving in AD patients with high FHDA. While this trial was developed based only on the previous preclinical and clinical literature related to prazosin in AD, a subsequent preclinical study (O’Neil et al., 2013) showed that doxazosin decreased voluntary alcohol consumption in P rats without affecting total fluid intake, locomotor activity or alcohol clearance. The positive effects of doxazosin in P rats are consistent with our clinical findings where doxazosin significantly reduced drinking only in patients with high FHDA. In fact, the P rat line is a well-characterized model of excessive voluntary alcohol drinking and these lines are based on repeated generations of selective breeding for alcohol preference (Li et al., 1979). An additional preclinical observation consistent with the present clinical findings is that, in another set of experiments, prazosin was more potent in ethanol-dependent rats than in non-dependent rats (Walker et al., 2008).
Results also suggest for those patients with low FHDA, not only did doxazosin not reduce drinking, it appears there was a trend towards increased drinking. This is interesting because it further supports the potential selectivity of doxazosin, by suggesting not only a sub-type of AD patients (with high FHDA) for whom doxazosin could reduce drinking, but also another sub-type for whom the use of doxazosin would not be recommended (patients with low FHDA). Furthermore, the opposite effects of high vs. low FHDA in moderating doxazosin’s effect suggest that, when pooled together, the two effects may cancel each other out, thus the lack of doxazosin’s effects on drinking outcomes in the whole general sample.
It is also important to consider the potential confounding by the placebo effect observed in this study as lower alcohol use was reported by placebo-treated subjects with low FHDA. In the high FHDA group, placebo clearly was not associated with reduced alcohol drinking like it did in the low FHDA group, but doxazosin did. It is possible that doxazosin’s effect was exhibited in the high FHDA group because they expressed adequate drinking to resolve the response, whereas drinking in the low FHDA group was so profoundly suppressed by the placebo effect that the interpretation was confounded. It could even be plausibly hypothesized that the low FHDA group exhibited a differentially potent placebo effect due to motivating conditions of the clinical trial, that doxazosin treatment could have actually diminished the impact of these conditions and allowed expression of moderate drinking, perhaps through an anxiolytic effect, a potential explanation consistent with what already observed in non-dependent rats where a low dose of prazosin actually increased alcohol self-administration (Walker et al., 2008).
Although this study does not provide definitive answers on the potential biobehavioral mechanism(s) of action, some hypotheses are possible. The effect of doxazosin in reducing alcohol craving in patients with high FHDA, in the absence of effects on anxiety and stress, could suggest one potential pathway. Notably, these outpatients were exposed to alcohol cues in their “real-word” life and these cues might have frequently elicited craving, against which doxazosin may have facilitated its effect in those with high FHDA. Taking into account the consistent doxazosin suppression of alcohol craving reflected in the obsessive drinking score, and the potential confounding by placebo effect on alcohol consumption discussed above, it is possible to speculate that doxazosin treatment may be effective only in subjects that are expressing high voluntary alcohol drinking, a potential explanation consistent with previous rodents research showing that other drugs working on the noradrenergic system suppress alcohol drinking in high alcohol-drinking but not in low alcohol-drinking rats (Moorman and Aston-Jones, 2009). While drawing conclusive findings in this domain is beyond the scope of this research, human laboratory studies assessing doxazosin’s effects on craving may be helpful to better investigate this putative mechanism of action.
Another potential mechanism of action is via influence on stress-induced anxiety, and stress-induced relapse/reinstatement of alcohol seeking, which are mediated, at least partially, by the norepinephrine system (Koob, 2008). However, the present study provides no support for this putative mechanism of action in humans. It is conceivable that an effect of doxazosin on anxiety and/or stress could be detected in a different study design with abstinent AD patients who start doxazosin after an inpatient detoxification phase. Supporting evidence for this includes the fact that brain α1-adrenergic mechanisms mediate enhanced acoustic startle response (Stevens et al., 1994), which is characteristic of abstinent alcoholic individuals (Krystal et al., 1997) and outbred rats experiencing prolonged abstinence following long-term chronic daily ethanol consumption and withdrawal (Rasmussen et al., 2009).
An open question remains with what may be the potential causes underlying differential doxazosin response by family history of alcoholism. We examined if high FHDA was a proxy of another more direct biomarker but found that FHDA was not related to baseline differences, such as age, gender, race, ethnicity, or baseline alcohol use. One may speculate that FHDA was a proxy of a potential genetic biomarker that might predict doxazosin response, an intriguing hypothesis that could not be tested in this study, given that genetic sampling was not conducted. Consistent with the growing literature suggesting an important role of pharmacogenetics in AD (Heilig et al., 2011; Leggio and Schwandt, 2014), future studies will need to investigate potential biological (genetic) markers, such as, for example, genetic variants that have been associated with the response to doxazosin in hypertensive patients (Lynch et al., 2008). These objective biomarkers might allow the identification of more precise and replicable subtypes of AD patients more likely to respond to doxazosin. This future line of inquiry will be important not only because family history of alcoholism is typically self-reported and there is not enough evidence to suggest it as a reliable predictor of response, but also because its implementation in clinical practice may be challenging due to the need to standardize the way how family history of alcoholism is defined, operationalized and assessed.
Given recent findings suggesting a role of doxazosin in PTSD (De Jong et al., 2010) and cocaine abuse (Newton et al., 2012; Shorter et al., 2013), future research exploring the role of doxazosin in the common comorbidities between AD and both PTSD and cocaine use is worth considering. In this regard, another important consideration is that while this preliminary study aimed at testing the maximum tolerable dose of doxazosin, i.e., up to 16-mg/day, future studies may also be designed as dose-ranging studies, especially considering that lower doses of doxazosin have been used in PTSD (De Jong et al., 2010) and cocaine abuse (Newton et al., 2012; Shorter et al., 2013).
This study provides important information on the safety of doxazosin specifically in the context of alcohol misuse. While this was not a medication-alcohol interaction study in a controlled inpatient setting, these findings support the safety of doxazosin-alcohol interaction in a “real-word” clinical outpatient setting. This study also presents questions on safety- and efficacy-related differences between doxazosin and prazosin in AD. Within the general safety profile of this class of drugs, doxazosin has been designated as a safer medication compared to prazosin (Akduman and Crawford, 2001). The slower onset of action of doxazosin and its relatively long t1/2 decreases the likelihood of first-dose postural hypotension compared to prazosin (Kirby et al., 1998). In the context of AD patients, Simpson and colleagues (Simpson et al., 2009) reported one case of clinically-significant hypotension out of 24 enrolled patients, while in our study (n = 41), no patients presented with clinically relevant hypotension. This is also consistent with the observation that, while effective for lowering blood pressure in hypertensive patients, doxazosin has no significant effect on blood pressure in normotensive patients, thus further decreasing the risk of hypotension (Akduman and Crawford, 2001). In summary, the lack of severe AEs or other safety concerns, together with the low number of dropouts (whose rates were similar in the doxazosin and placebo groups), indicate that safety and tolerability of doxazosin in this trial was fair and make it an acceptable medication for AD patients.
In terms of efficacy, in contrast to the prazosin study (Simpson et al., 2009), we did not find a main doxazosin effect in the general sample. One possible explanation for this inconsistency is that the prazosin trial overestimated the medication’s effect, a potential Type I error in small RCTs. Another possibility is that, unlike prazosin, doxazosin only works in patients with significant family history for alcoholism. However, it is unknown if prazosin’s effects might be even stronger in patients with high FHDA. Only a 3-arm RCT (doxazosin vs. prazosin vs. placebo) would be able to address safety- and efficacy-related differences between the two medications.
Although we did not collect central spinal fluid to measure doxazosin concentrations, there is evidence that doxazosin crosses the blood-brain barrier. Preclinical studies demonstrate central nervous system (CNS)-actions of doxazosin, administered peripherally (McLeod and Cairncross, 1995). In humans, somnolence is a dose-dependent side effect of doxazosin and a pilot study reported that doxazosin reduced PTSD symptoms (De Jong et al., 2010). In our trial, three of the four side-effects more common in the doxazosin group were CNS-related (dizziness, headache, and mood disturbances), further supporting doxazosin’s central effects. This is consistent with the fact that doxazosin works on all subtypes, α1A, α1B, and α1D (Gross et al., 1989). Blockade of the α1B subtypes (located in the brain) by doxazosin contributes to the central side-effects thus demonstrating indirectly its actions in the CNS (Akduman and Crawford, 2001).
Strengths of this study include that this is the first clinical study of the efficacy of doxazosin in AD, and the enrollment of outpatient treatment-seeking AD patients, a population more closely representing the “real-word” in terms of clinical practice. Limitations include the lack of actual genetic testing, for which future pharmacogenetic work is warranted; the use of riboflavin and UV light for adherence measurement (Herron et al., 2013), thus highlighting the need for better ways to assess adherence in future studies [for review, see: (Gurvich et al., 2013)] ; and the small sample. Notably, although the small sample did not allow for describing family history of alcoholism as a tripartite categorization (Rohsenow et al., 2007), we calculated family history based on first-degree relatives, an approach consistent with the COMBINE study (the largest pharmacotherapy RCT in the alcoholism field) (Anton et al., 2006) and took into account the “density” of family history, consistent with previous recommendations (Capone et al., 2011; Rohsenow et al., 2007).
Importantly, this initial clinical study provides a platform for future studies both in terms of safety and power estimation for efficacy, and suggests that a follow-up RCT may be designed by a priori enrollment of AD patients with high FHDA. Considering an RCT with 80% power to detect an effect size on DPW and HDD, 20 participants with high FHDA per cell would need to be retained. On the other hand, if participants were not screened on FHDA, >400 participants per cell would need to be retained.
In conclusion, this RCT provides preliminary evidence for a role of doxazosin in the treatment of AD, albeit limited to patients with high FHDA. Future studies are warranted to identify possible biomarkers of doxazosin’s response in AD patients in order to best identify potential AD patients who are responders or non-responders.
Supplementary Material
Study Flow-chart.
Acknowledgments
This study was funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), Grant R21AA019994 (PIs: Drs Leggio and Dr. Kenna). Dr. Leggio’s current work is supported by the NIAAA Division of Intramural Clinical and Biological Research and the National Institute on Drug Abuse (NIDA) Intramural Research Program. Dr. Haass-Koffler’s work is supported by the 5T32AA007459-28 training grant from NIAAA.
The authors would like to thank Samuel Fricchione (Brown University) for providing technical support and Dr. Melanie Schwandt (NIAAA) for reviewing and commenting on the manuscript.
Footnotes
CONFLICT OF INTEREST
Dr. Kenna has received consultant fees from CT Laboratories. Dr. Swift has received travel and honorarium from D&A Pharma, Lundbeck and consultant fees from CT Laboratories. The other authors report no biomedical financial interests or potential conflicts of interest.
AUTHORS CONTRIBUTION
GAK and LL were responsible for the study concept and design. CLHK, SME, and MBB contributed to the acquisition of the data. WHZ performed the statistical analyses. GAK, CLHK, RMS, and LL assisted with data analysis and interpretation of findings. LL drafted the manuscript. GAK, CLHK, and RMS provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
References
- Akduman B, Crawford ED. Terazosin, doxazosin, and prazosin: current clinical experience. Urology. 2001;58:49–54. doi: 10.1016/s0090-4295(01)01302-4. [DOI] [PubMed] [Google Scholar]
- Amit Z, Brown ZW, Levitan DE, Ogren SO. Noradrenergic mediation of the positive reinforcing properties of ethanol: I. Suppression of ethanol consumption in laboratory rats following dopamine-beta-hydroxylase inhibition. Archives internationales de pharmacodynamie et de therapie. 1977;230:65–75. [PubMed] [Google Scholar]
- Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcoholism, clinical and experimental research. 1995;19:92–99. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Capone C, Kahler CW, Swift RM, O’Malley SS. Does family history of alcoholism moderate naltrexone’s effects on alcohol use? Journal of studies on alcohol and drugs. 2011;72:135–140. doi: 10.15288/jsad.2011.72.135. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Froehlich JC. Acoustic startle reactivity during acute alcohol withdrawal in rats that differ in genetic predisposition toward alcohol drinking: effect of stimulus characteristics. Alcohol Clin Exp Res. 2004;28:677–687. doi: 10.1097/01.alc.0000125345.19665.09. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- De Jong J, Wauben P, Huijbrechts I, Oolders H, Haffmans J. Doxazosin treatment for posttraumatic stress disorder. Journal of clinical psychopharmacology. 2010;30:84–85. doi: 10.1097/JCP.0b013e3181c827ae. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Schuck J, Stender N, Pilz J, Gefeller O, Schilling L, Poser W, Kaw S. Endocrine and hemodynamic effects of stress versus systemic CRF in alcoholics during early and medium term abstinence. Alcohol Clin Exp Res. 1997;21:1285–1293. [PubMed] [Google Scholar]
- Falk D, Wang XQ, Liu L, Fertig J, Mattson M, Ryan M, Johnson B, Stout R, Litten RZ. Percentage of subjects with no heavy drinking days: evaluation as an efficacy endpoint for alcohol clinical trials. Alcohol Clin Exp Res. 2010;34:2022–2034. doi: 10.1111/j.1530-0277.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, Morgan PT, Sinha R. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcoholism, clinical and experimental research. 2012;36:351–360. doi: 10.1111/j.1530-0277.2011.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross G, Hanft G, Mehdorn HM. Demonstration of alpha 1A- and alpha 1B-adrenoceptor binding sites in human brain tissue. Eur J Pharmacol. 1989;169:325–328. doi: 10.1016/0014-2999(89)90032-0. [DOI] [PubMed] [Google Scholar]
- Gurvich EM, Kenna GA, Leggio L. Use of novel technology-based techniques to improve alcohol-related outcomes in clinical trials. Alcohol and alcoholism. 2013;48:712–719. doi: 10.1093/alcalc/agt134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O’Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nature reviews Neuroscience. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron AJ, Mariani JJ, Pavlicova M, Parrinello CM, Bold KW, Levin FR, Nunes EV, Sullivan MA, Raby WN, Bisaga A. Assessment of riboflavin as a tracer substance: comparison of a qualitative to a quantitative method of riboflavin measurement. Drug and alcohol dependence. 2013;128:77–82. doi: 10.1016/j.drugalcdep.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby RS, Chapple CR, Sethia K, Flannigan M, Milroy EJ, Abrams P. Morning vs evening dosing with doxazosin in benign prostatic hyperplasia: efficacy and safety. Prostate cancer and prostatic diseases. 1998;1:163–171. doi: 10.1038/sj.pcan.4500220. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Grillon C, Cooney N, Casal L, Morgan CA, 3rd, Southwick SM, Davis M, Charney DS. Evidence of acoustic startle hyperreflexia in recently detoxified early onset male alcoholics: modulation by yohimbine and m-chlorophenylpiperazine (mCPP) Psychopharmacology (Berl) 1997;131:207–215. doi: 10.1007/s002130050285. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology. 2011;218:89–99. doi: 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Kenna GA, Fenton M, Bonenfant E, Swift RM. Typologies of alcohol dependence. From Jellinek to genetics and beyond. Neuropsychology review. 2009;19:115–129. doi: 10.1007/s11065-008-9080-z. [DOI] [PubMed] [Google Scholar]
- Leggio L, Schwandt ML. Topiramate for alcoholism treatment: novel pharmacogenetic evidence for the journey to personalized medicine? The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2014;17:1541–1544. doi: 10.1017/S1461145714000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacology bulletin. 1986;22:343–381. [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Waller MB. Progress toward a voluntary oral consumption model of alcoholism. Drug and alcohol dependence. 1979;4:45–60. doi: 10.1016/0376-8716(79)90040-1. [DOI] [PubMed] [Google Scholar]
- Lynch AI, Boerwinkle E, Davis BR, Ford CE, Eckfeldt JH, Leiendecker-Foster C, Arnett DK. Pharmacogenetic association of the NPPA T2238C genetic variant with cardiovascular disease outcomes in patients with hypertension. JAMA : the journal of the American Medical Association. 2008;299:296–307. doi: 10.1001/jama.299.3.296. [DOI] [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend. 1985;15:61–67. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- McLeod SD, Cairncross KD. Preliminary evidence of a synergistic alpha 1- and beta 1-adrenoceptor regulation of rat pineal hydroxyindole-O-methyltransferase. General and comparative endocrinology. 1995;97:283–288. doi: 10.1006/gcen.1995.1028. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol--preferring Sprague--Dawley rats. Alcohol. 2009;43:379–386. doi: 10.1016/j.alcohol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, 2nd, Brown G, Kosten TR, Mahoney JJ, 3rd, Haile CN. Noradrenergic alpha(1) receptor antagonist treatment attenuates positive subjective effects of cocaine in humans: a randomized trial. PloS one. 2012;7:e30854. doi: 10.1371/journal.pone.0030854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil ML, Beckwith LE, Kincaid CL, Rasmussen DD. The alpha1-adrenergic receptor antagonist, doxazosin, reduces alcohol drinking in alcohol-preferring (P) Rats. Alcoholism, clinical and experimental research. 2013;37:202–212. doi: 10.1111/j.1530-0277.2012.01884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar AA, Marsden CA, Naik PC, Kendall DA, Gopalakrishnan R, Vergare MJ, Weinstein SP. Differences in peripheral noradrenergic function among actively drinking and abstinent alcohol-dependent individuals. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2004;13:225–235. doi: 10.1080/10550490490459898. [DOI] [PubMed] [Google Scholar]
- Pollock V, Cho DW, Reker D, Volavka J. Profile of Mood States: the factors and their physiological correlates. The Journal of nervous and mental disease. 1979;167:612–614. doi: 10.1097/00005053-197910000-00004. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC. The alpha1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2009;33:264–272. doi: 10.1111/j.1530-0277.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhemtulla M, Brosseau-Liard PE, Savalei V. When can categorical variables be treated as continuous? A comparison of robust continuous and categorical SEM estimation methods under suboptimal conditions. Psychol Methods. 2012;17:354–373. doi: 10.1037/a0029315. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Miranda R, Jr, McGeary JE, Monti PM. Family history and antisocial traits moderate naltrexone’s effects on heavy drinking in alcoholics. Exp Clin Psychopharmacol. 2007;15:272–281. doi: 10.1037/1064-1297.15.3.272. [DOI] [PubMed] [Google Scholar]
- Shorter D, Lindsay JA, Kosten TR. The alpha-1 adrenergic antagonist doxazosin for treatment of cocaine dependence: A pilot study. Drug and alcohol dependence. 2013;131:66–70. doi: 10.1016/j.drugalcdep.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, Gross CA, Hart KL, Raskind M. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res. 2009;33:255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. Journal of abnormal psychology. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Stevens DR, McCarley RW, Greene RW. The mechanism of noradrenergic alpha 1 excitatory modulation of pontine reticular formation neurons. J Neurosci. 1994;14:6481–6487. doi: 10.1523/JNEUROSCI.14-11-06481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzaskowska E, Pucilowski O, Dyr W, Kostowski W, Hauptmann M. Suppression of ethanol tolerance and dependence in rats treated with DSP-4, a noradrenergic neurotoxin. Drug Alcohol Depend. 1986;18:349–353. doi: 10.1016/0376-8716(86)90098-0. [DOI] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Flow-chart.