SUMMARY
Articular cartilage degeneration is hallmark of osteoarthritis (OA). Low-grade chronic inflammation in the joint can promote OA progression. Emerging evidence indicates that bioenergy sensors couple metabolism with inflammation to switch physiological and clinical phenotypes. Changes in cellular bioenergy metabolism can reprogram inflammatory responses, and inflammation can disturb cellular energy balance and increase cell stress. AMP-activated protein kinase (AMPK) and sirtuin 1 (SIRT1) are two critical bioenergy sensors that regulate energy balance at both cellular and whole-body levels. Dysregulation of AMPK and SIRT1 has been implicated in diverse human diseases and aging. This review reveals recent findings on the role of AMPK and SIRT1 in joint tissue homeostasis and OA, with a focus on how AMPK and SIRT1 in articular chondrocytes modulate intracellular energy metabolism during stress responses (e.g., inflammatory responses) and how these changes dictate specific effector functions, and discusses translational significance of AMPK and SIRT1 as new therapeutic targets for OA.
Keywords: Inflammation, Energy metabolism, Cartilage homeostasis, AMPK, SIRT1
Introduction
Osteoarthritis (OA) is the most common form of arthritis. Aging, obesity and biomechanical injury increase the risk of developing OA1. The central characteristic of OA is progressive degeneration of cartilage, which leads to permanent functional joint failure and disability1. However, OA is a disorder of the whole synovial joint organ1. Other joint tissues such as synovium and subchondral bone are also affected in OA1–4. Increasing evidence indicates that low-grade local joint inflammation (synovitis), induced by endogenous molecular products derived from cellular stress and extra-cellular matrix disruption acting through innate inflammatory network, can influence integrity and function of articular cartilage and promote OA progression5,6. Low-grade systemic inflammation resulted from metabolic disturbance could also contribute to OA progression7. Mounting an inflammatory response is an energy-intensive process8,9. The concept of interplay between cellular bioenergetics/metabolism and inflammation has been emerged8–10. Pro-inflammatory mediators alter cellular energy metabolism. Disturbances in the maintenance of cellular energy balance trigger cell stress and induce inflammation8–10. Effective regulation of cellular energy metabolism is critical for tissue homeostasis11. AMP-activated protein kinase (AMPK) and sirtuin 1 (SIRT1) are two critical energy sensors that regulate energy balance at both cellular and whole-body levels11. The aim of this review is to discuss recent advancement in understanding the role of AMPK and SIRT1 in joint tissue homeostasis and OA, and their potential to be used as therapeutic targets in OA.
Function of AMPK and SIRT1 in articular cartilage
Articular cartilage is an avascular and hypoxic connective tissue12. Chondrocytes embedded in the articular cartilage have to cope with a nutritionally challenging environment in which metabolic demands may change due to alterations in biomechanical demands, local inflammatory mediators, aging and other factors12. Glucose transport and glycolysis, and less so mitochondrial oxidative phosphorylation (OXPHOS), provide the primary sources of metabolic energy in articular chondrocytes12,13.
AMPK in chondrocytes
AMPK, a serine/threonine kinase, is a master regulator of cellular energy balance, whose notable conservation in evolution supports its fundamental role in cell biology14,15. AMPK exists in essentially all eukaryotic cells in the form of heterotrimeric complexes comprising catalytic α-subunits and regulatory β- and γ-subunits. Each subunit has 2–3 isoforms (α1, α2, β1, β2, γ1, γ2, γ3) encoded by different genes14,15. Importantly, phosphorylation of a conserved threonine within the catalytic domain of both the α1 and α2 subunits (which are 90% homologous in their catalytic cores) is crucial for AMPK activity14,15. Articular chondrocytes express α1, α2, β1, β2 and γ1 isoforms of AMPK subunits, and α1 appears to be the predominantly expressed and functionally active AMPK α isoform16. AMPK is activated by metabolic stress (e.g., nutrient deprivation, hypoxia, heat shock, and exercise/muscle contraction)14,15. Once activated, AMPK responds by phosphorylating multiple downstream targets, which allow inhibition of pathways that consume ATP such as fatty acid, phospholipid, and protein biosynthesis, and activation of pathways that generate ATP such as glucose uptake and fatty acid oxidation14,15. In this manner, AMPK allows cells to adjust to changes in energy demand14,15. AMPK can also be activated by pharmacological compounds such as the nucleotide mimetic AICAR (5-aminoimidazole-4-caboxamide 1-b-D-ribofuranoside), and A-769662 which is a selective and direct AMPK activator14,15.
We discovered that phosphorylation of AMPKα Thr172 (indication of AMPK activity) is constitutively present in normal articular chondrocytes/cartilage, but is decreased in human knee OA chondrocytes/cartilage16, in mouse knee OA cartilage17, and in aged mouse knee cartilage17. In addition, phosphorylation of AMPKα is decreased, correlated with increased catabolic responses in normal chondrocytes challenged with either inflammatory cytokines IL-1β and TNFα or biomechanical injury16,17. Moreover, AMPK pharmacological activators are able to reverse these effects16,17. These data suggest that sustained AMPK activity in articular chondrocytes could be critical for cartilage matrix homeostasis.
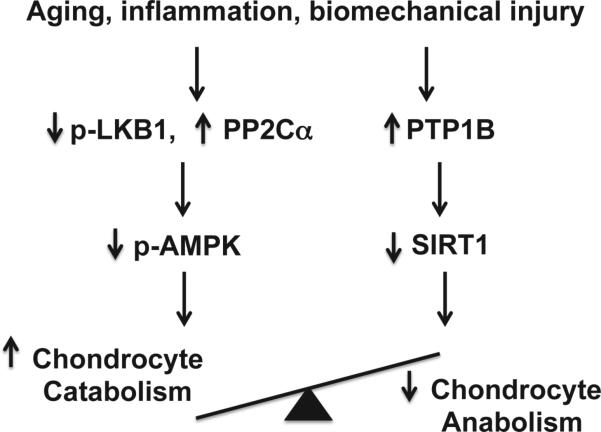
Regulation of AMPK activity involves phosphorylation by upstream kinases. The liver protein kinase B1 (LKB1) is thought to be the primary upstream kinase that phosphorylates AMPKα Thr17214,15. This is true in articular chondrocytes, as phosphorylation of AMPKα is nearly completely inhibited in LKB1 knockdown chondrocytes17. In addition, chondrocyte catabolic responses to IL-1β and TNFα are significantly enhanced in LKB1 knockdown chondrocytes17. Interestingly, concomitant reduction of phosphorylation of both LKB1 and AMPKα is observed in primary human knee OA chondrocytes, in mouse knee OA cartilage, in aged mouse knee cartilage, and in chondrocytes challenged with mechanical injury17, indicating that dysregulation of LKB1 in aged and OA cartilage may play a major role in suppression of AMPK activation. AMPK activity, on the other hand, can be negatively regulated through de-phosphorylation by protein phosphatases such as protein phosphatase 2A (PP2A) and PP2Cα14,15. We found that phosphorylation of AMPKα (Thr172) was increased, while catabolic responses were decreased in the PP2Ca knockdown chondrocytes stimulated with IL-1β and TNFα (unpublished observation). Thus, chondrocytes with reduced AMPK activity are more susceptible to inflammation-induced catabolic responses (Fig. 1).
Fig. 1.
Impaired chondrocyte AMPK and SIRT1 activity disrupts cartilage matrix homeostasis. Aging, inflammation and biomechanical injury in the joint can decrease phosphorylation of AMPKα Thr172 (indicating AMPK activity) and expression of SIRT1 in articular chondrocytes, likely through down-regulation of activity of the major upstream AMPK kinase LKB1 and up-regulation of protein phosphatase PP2Cα, and PTB1B. As a result, the balance between chondrocyte catabolic and anabolic function is disrupted. Overactive chondrocyte catabolic responses ultimately lead to cartilage matrix degradation.
SIRT1 in chondrocytes
SIRT1, another evolutionary conserved energy sensor, is a member of sirtuins that are nicotinamide adenine dinucleotide (NAD+)-dependent lysine deacetylases, acting on a wide range of protein substrates11,18,19. Expression of SIRT1 is decreased in both human and mouse knee OA cartilage, as well as in aged mouse knee cartilages20–22. Inhibition of SIRT1 in chondrocytes leads to increased apoptosis and enhanced pro-catabolic responses to IL-1β and TNFα23–25. Moreover, SIRT1 promotes cartilage-specific gene expression26, protects chondrocytes from radiation-induced senescence27, and inhibits apoptosis in chondrocytes23,28,29. SIRT1 enhances human OA chondrocyte survival by repressing protein tyrosine phosphatase 1B (PTP1B), a potent pro-apoptotic protein23. Furthermore, adult heterozygous Sirt1 knockout (KO) mice and mice with a Sirt1 point mutation exhibit increased OA progression29,30, and cartilage-specific Sirt1 KO mice develop accelerated OA progression22. Clearly, SIRT1 has a chondroprotective role.
AMPK and SIRT1 in chondrocyte stress resistance
Activation of AMPK is shown to stimulate the functional activity of SIRT1 by increasing the intracellular concentrations of NAD+ in several different cell types11,31. SIRT1 deacetylates LKB1, which subsequently increase LKB1 activity, leading to AMPK activation11,31. The same scenario was seen in articular chondrocytes as well (unpublished observation). This positive feedback loop between SIRT1 and AMPK could potentiate the function of AMPK, and effectively control cellular energy balance11,31. Importantly, AMPK and SIRT1 not only regulate cellular energy metabolism, but also coordinate several housekeeping mechanisms to increase cell stress resistance via certain downstream mediators.
Regulation of mitochondrial biogenesis and function
AMPK phosphorylates PGC-1α (peroxisome proliferator-activated receptor γ co-activator 1α) protein that subsequently allows SIRT1 to deacetylate and activate PGC-1α11,31. PGC-1α, a transcriptional co-activator, is a master regulator of mitochondrial biogenesis and function11,31. The primary function of mitochondria is to produce ATP through the process of OXPHOS, transduced by the respiratory complexes (I–IV) and the ATP synthase (complex V)13,32. Mitochondrial function is known to decline with aging33. As cells age, the efficacy of the mitochondrial respiratory chain tends to diminish, thus increasing electron leakage that leads to increases in reactive oxygen species (ROS) production and oxidative damage, and reduced ATP generation33. Mitochondrial function is impaired in OA chondrocytes, reflected by decreased numbers of mitochondria and activity of respiratory complexes I, II and III13,32,34. Although the majority of the ATP in chondrocytes is made by glycolysis rather than by OXPHOS, ATP levels per chondrocyte are reduced despite glycolysis is increased in OA chondrocytes35, which not only contributes to decreased mitochondrial bioenergetic reserve36–38, but also adversely affects cellular redox balance39–42, and chondrocyte homeostatic functions dependent on physiological generation of low levels of ROS41,42.
Mitochondrial dysfunction is implicated in onset and progression of cartilage degradation. It increases responsiveness of chondrocytes to pro-inflammatory cytokines, leading to increased matrix catabolism43,44. Mitochondrial biogenesis is important for maintenance of mitochondrial function. We recently found that expression of PGC-1α is decreased in both mouse knee OA cartilage and in aged mouse knee cartilage45. In addition, we observed that mitochondrial biogenesis capacity and function are significantly reduced in advanced human knee OA chondrocytes, indicated by deceased mitochondrial DNA content and mitochondrial mass, and reduced oxygen consumption rate and intracellular ATP level, all of which were correlated with concomitant reduction of phosphorylation of AMPKα, expression of SIRT1 and PGC-1α, and increased acetylation of PGC-1α (unpublished observation). Moreover, the established impairments in mitochondrial biogenesis and function in advanced human knee OA chondrocytes can be reversed by either AMPK pharmacologic activation or overexpression of SIRT1 or PGC-1α (unpublished observation).
Inhibition of oxidative stress and inflammatory responses
FOXO3a, a transcription factor that belongs to the forkhead box O (FOXO) family, is another downstream target of AMPK and SIRT111,31. As for PGC-1α, AMPK directly phosphorylates FOXO3a, and SIRT1 deacetylates and activates FOXO3a11,31. PGC-1α and FOXO3a are closely related. FOXO3a is a direct transcriptional regulator of PGC-1α, and PGC-1α itself can augment the transcriptional activity of FOXO3a46. Both PGC-1α and FOXO3a have been shown to limit cellular oxidative stress by up-regulating antioxidant enzymes, including manganese superoxide dismutase (MnSOD or SOD2) and catalase46,47. In this light, reduction of SOD2 expression has been linked to chondrocyte mitochondrial dysfunction48 and OA progression49. Similar to PGC-1α, expression of FOXO3a is reduced in both mouse knee OA cartilage and in aged mouse knee cartilage45. AMPK pharmacologic activation enhances expression of PGC-1α and FOXO3a, as well as SOD2 and catalase, and inhibits oxidative stress in articular chondrocytes45.
Mitochondria dysfunction leads to elevated levels of ROS, which promotes cartilage degradation directly by cleaving collagen and aggrecan and indirectly by activating matrix metalloproteinases (MMPs)50,51. Additionally, ROS act indirectly by modulating redox-sensitive NF-κB and other signaling pathways that increase chondrocyte catabolic activity41,42,52. NF-κB plays a major role in innate immune responses. The regulation of innate immunity and energy metabolism is connected together through an antagonistic crosstalk between NF-κB and AMPK and SIRT1 signaling pathways11,53,54. Many studies have demonstrated that activities of AMPK and SIRT1 have anti-inflammatory effects in diverse types of cells and tissues11,31. Activation of AMPK inhibits NF-κB activation via SIRT1, which deacetylates p65 NF-κB subunit, ultimately primes p65 for proteasome degradation11,31. Studies have shown that activation of AMPK or SIRT1 inhibits pro-inflammatory responses to IL-1β and TNFα via attenuation of NF-κB activation in articular chondrocytes16,24,25,55–57. In addition, PGC-1α and FOXO3a, at least in part, mediate AMPK to inhibit NF-κB activation and inflamma-tory cytokine-induced catabolic responses in chondrocytes45. Inflammatory cytokines have negative impact on both phosphorylation of AMPKα and SIRT1 expression in chondrocytes16,26. IL-1β and TNFα de-phosphorylate AMPKα partially by inducing increased expression of PP2Cα (unpublished observation). TNFα also reduces SIRT1 activity in chondrocytes by inducing cathepsin B-mediated cleavage of SIRT158. In addition, activation of NF-κB can down-regulate SIRT1 activity through induction of expression of miR-34a, which targets the 3′ UTR of SIRT1 and inhibits the expression of SIRT159. Chondrocytes with reduced activity of AMPK or SIRT1 have increased responsiveness to in-flammatory cytokines16,25. These findings suggest that chronic low-grade inflammation in OA and aging joint could be associated with dysregulation of AMPK and SIRT1 signaling, which reduces chondrocyte resistance to inflammatory stress and further provokes inflammatory responses.
Modulation of endoplasmic reticulum (ER) stress
The ER is a sub-cellular organelle where all secretory and integral membrane proteins are folded and post-translationally modified. The ER is also a site of calcium storage and lipid biosynthesis. Protein folding in the oxidizing environment of the ER is an energy-requiring process60,61. Stresses that compromise the ER homeostasis such as perturbations in calcium homeostasis, energy stores, redox state, and metabolic and inflammatory challenges result in the accumulation of misfolded proteins and activation of a stress response termed the unfolded protein response (UPR)60,61. Several adaptive signaling pathways have evolved to restore an efficient protein-folding environment through the induction of chaperones, degradation of misfolded proteins and attenuation of protein translation60,61. Inositol-requiring kinase 1 (IRE1), ER eukaryotic translation initiation factor 2 (eIF2a) kinase (PERK), and activating transcription factor 6 (ATF6) are the three branches of UPR signaling cascade, which are triggered by disassociation of the chaperon GRP78 upon ER stress60,61. When ER stress is too severe or chronic, or the UPR is unable to resolve the protein-folding defects, cells are led to apoptosis through induction of C/EBP homologous protein (CHOP)60,61.
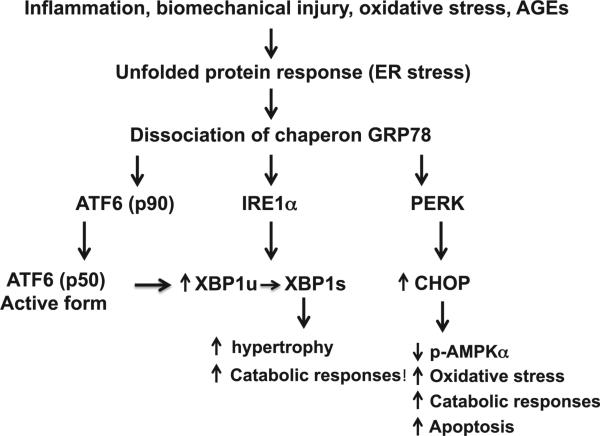
The UPR is activated in OA articular chondrocytes (Fig. 2), evidenced by increased expression of GRP78 and CHOP, as well as generation of alternatively spliced and transcriptionally activated X-box protein 1 (XBP1) in OA cartilage62,63. ATF6 upregulates XBP1 expression in OA chondrocytes by promoting direct binding to XBP1 promoter64, and increased XBP1s expression accelerates chondrocyte hypertrophy65. XBP1 expression is also increased by IL-1β in chondrocytes62. Inhibition of XBP1 expression in chondrocytes via siRNA attenuates nitric oxide and MMP-3 release induced by IL-1β62. Several factors implicated in OA pathogenesis including biomechanical injury, IL-1β, nitric oxide and advanced glycation end products (AGEs) upregulate expression of GRP78 and CHOP in cultured articular chondrocytes62,63,66–69. CHOP potentiates the capacity of IL-1β to induce catabolic responses, superoxide generation and apoptosis in chondrocytes, and does so by inhibiting AMPK activity62. Moreover, CHOP-mediated apoptosis contributes to the progression of cartilage degeneration in mice70. Moreover, pharmacologic AMPK activation blunts CHOP expression and catabolic responses induced by IL-1β and biomechanical injury17,62. These data indicate that AMPK modulates UPR, and activation of AMPK alleviates ER stress in chondrocytes.
Fig. 2.
The UPR in articular chondrocytes in OA. ER stress in OA chondrocytes caused by certain inflammatory mediators, biomechanical injury, nitric oxide and AGEs leads to activation of the UPR signaling cascades triggered by dissociation of the chaperon GRP78 from the ER transmembrane proteins PERK, IRE1α, and ATF6. The active XBP1 spliced form (XBP1s), generated by IRE1a through alternatively splicing mRNA of unspliced XBP1 (XBP1u), promotes chondrocyte hypertrophy and increases catabolic responses. The cleaved active form of ATF6 (p50) can induce XBP1 expression through direct binding to XBP1 promoter. Increased CHOP expression potentiates IL-1β to decrease phosphorylation of AMPKα and induce catabolic responses, superoxide generation and apoptosis.
Regulation of autophagy
Autophagy is a cellular housekeeping and protein quality control mechanism, which can remove damaged or defective proteins and organelles, e.g., damaged mitochondria11,71. AMPK controls autophagy through mammalian target of rapamycin (mTOR) and Unc-51-like kinase 1 (ULK1) signaling71. mTOR, a conserved Ser/Thr protein kinase, is a potent inhibitor of autophagy. ULK1 is a critical kinase that governs the cascade of events triggering autophagy. AMPK can inhibit activity of mTOR complex (mTORC1) either by directly phosphorylating Raptor, a regulatory component of mTORC1, or by phosphorylating tuberous sclerosis protein 2 (TSC2), which subsequently suppresses mTOR activity71. AMPK stimulates autophagy by dissociating mTORC1 from the ULK1 complex via the phosphorylation of the Raptor component, as well as by directly binding to the ULK1 complex and phosphorylating ULK171. In addition, AMPK can also enhance the later steps in autophagosome formation through SIRT1 by deacetylating several autophagy-related proteins (e.g., Atg5, Atg7 and Atg8)11. Chondrocyte auto-phagy is known to be a constitutive homeostatic mechanism in articular cartilage72, which can be promoted by AMPK signaling73,74 through mTOR suppression. As for both phosphorylation of AMPKα and SIRT1 expression, chondrocyte autophagy is reduced with a linked increase in apoptosis in human knee OA, mouse knee OA and aged mouse knee cartilages75. Suppressed autophagy also is observed in cartilage ex vivo in response to mechanical injury76. Inhibition of autophagy in chondrocyte exacerbated IL-1β–induced OA-like gene expression changes and apoptotic signals, while activation of autophagy inhibited them, possibly through modulation of ROS in chondrocytes in vitro77. Cartilage-specific genetic deletion of mTOR78 and pharmacologic inhibition of mTOR signaling by rapamycin79 upregulates autophagy and reduces the severity of experimental OA in vivo.
Function of AMPK and SIRT1 in synovium and bone
The function of bioenergy sensors in synovium is merely studied yet. One study using fibroblast-like synoviocytes (FLS) of rheumatoid arthritis (RA) showed that SIRT1 expression is decreased by HMGB1, but is recovered by pre-treatment with resveratrol, a SIRT1 activator80. Interestingly, Cilostazol, a drug used to treat intermittent claudication that has been shown to activate AMPK, prevents HMGB1-induced angiogenesis via SIRT1 in RA FLS in vitro and exhibits anti-angiogenic effect in collagen-induced arthritis (CIA) mouse model in vivo80. Simvastatin, a cholesterol-lowering drug that is also known to activate AMPK, inhibits cysteine-rich angiogenic inducer 61 (CYR61) and CCL20 in RA FLS via up-regulation of SIRT1/FOXO3a signaling81. These results implicate an anti-inflammatory role of synovial activation of AMPK and SIRT1. Whether activation of AMPK and SIRT1 limits synovial inflammation in OA needs to be investigated.
Both AMPK and SIRT1 are involved in regulation of bone metabolism. Activation of AMPK promotes osteoblast differentiation, bone formation and bone mass, and inhibits osteoclast differentiation in vitro82–91. Mice deficient in AMPKα1 or AMPKα2 exhibit reduced bone mass compared with WT mice83. AMPKα1 deficient mice have an elevated rate of bone remodeling, whereas AMPKα2 deficient mice largely have elevated bone resorption83. Osteoclast- or osteoblast-specific SIRT1 conditional KO mice have decreased bone mass caused by increased resorption and reduced bone formation90. Wnt/β-catenin is known to be indispensable for osteoblast generation. SIRT1 is shown to regulate differentiation of mesenchymal stem cells (MSCs) by deacetylating β-catenin92. MSCs isolated from MSC-specific SIRT1 KO mice have reduced differentiation towards osteoblasts and chondrocytes in vitro92. Activation of AMPK or SIRT1 decreases adipocyte and promotes osteoblast formation during differentiation of MSCs93–95, suggesting a critical role of AMPK and SIRT1 in regulation of the lineage commitment of MSCs. Osteoprogenitor-specific SIRT1 KO mice display lower cortical thickness in femora and vertebrae because of reduced bone formation at the endocortical surface, associated with decreased Wnt/b-catenin signaling88. Decreased expression of SIRT1 is found in human OA subchondral bone osteoblasts89. Inhibition of SIRT1 in osteoblasts leads to abnormal sclerostin expression that decreases Wnt/β-catenin activity89, which can be inhibited by resveratrol89. Obviously, AMPK and SIRT1 play an important role in bone homeostasis. Abnormal AMPK and SIRT1 levels in osteoblasts and osteoclasts may contribute to subchondral bone pathological changes in OA.
Clinical relevance and translation
Dysregulation of AMPK and SIRT1 is linked to diabetes, atherosclerosis, cardiovascular disease, cancer, neurodegenerative diseases11,33, as well as OA based on recent findings, all of which are age-related diseases. Studies have revealed that responsiveness of AMPK activation declines during the aging process11, and low-grade inflammation present in aging tissues may be at least in part responsible for suppressing AMPK signaling11. The prevalence of OA increases with aging further supports the concept that a dysfunction of AMPK is involved in the disease process.
Nutritional factors can affect AMPK signaling. Caloric restriction is known to stimulate AMPK activity, whereas nutritional overload seems to impair AMPK activity, which can induce insulin resistance in many tissues11. The metabolic disturbance can cause low-grade inflammation leading to development of metabolic syndrome such as obesity and diabetes11, which are often associated with OA7. Diet rich in n-3 long chain polyunsaturated fatty acids (PUFAs) is considered as a nutritional tool to prevent insulin resistance associated to type 2 diabetes and obesity96. This is probably at least in part owing to the ability of n-3 PUFAs to stimulate AMPK activity97–100. Exercise is considered as one of the most cost effective approaches to provide health benefits101. Evidence supports benefits of various types of exercise for improving pain and function in knee OA102. Studies in both animals and humans demonstrate that skeletal muscle contraction and exercise activate AMPK in an intensity and time-dependent manner101,103, and increased AMPK activation promotes adaptation to muscle endurance exercise101,103. As such, activation of AMPK provides molecular basis of the benefits of exercise, and supports clinical recommendation of exercise for the management of OA102.
A number of drugs already in the clinic for arthritis and other conditions (e.g., sodium salicylate, high dose aspirin, methotrexate, metformin), and a variety of natural plant products either present in traditional medicine or derived from food (e.g., berberine, resveratrol, curcumin, quercetin) appealed to have “nutraceutical” properties are able to activate AMPK104,105. However, the majority of them were in use for their respective purposes before they were known to be activators of AMPK104,105. Interestingly, a recent study on a randomized placebo-controlled small trial of methotrexate in symptomatic knee OA showed significant improvement in physical function associated with reduced pain and synovitis106. In addition, a randomized double-blind placebo-controlled small trial of curcuminoid (closely related to curcumin) in treatment of knee OA also showed significant improvements in pain and physical function107. Moreover, several randomized placebo-controlled trials of avocado–soybean unsaponifiables (ASU) on both knee and hip OA demonstrated significant efficacy in improving the symptoms of OA and reducing progression of joint space narrowing108–110. ASU is a natural vegetable extract made from avocado and soybean oils used as a dietary supplement. The carotenoids such as β-carotene and lutein present in avocado, and a key isoflavone genistein present in soybean are capable of activating AMPK111–113. The beneficial effects of the agents tested in the OA clinical trails aforementioned are conceivably in part due to AMPK activation, which warrant further investigation.
Conclusion
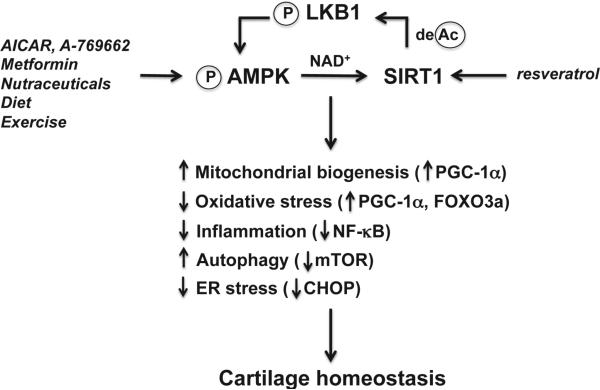
Disturbances in the maintenance of energy balance provoke diseases and jeopardize healthy aging11. Reduced capacity of AMPK and SIRT1 activation in articular joint tissues could limit energy availability for cellular maintenance, trigger significant cell stress by inducing mitochondrial dysfunction, oxidative stress and inflammation, ultimately, compromise cell survival and tissue function. These bioenergy sensors can couple metabolism with inflammation to switch physiologic and clinical phenotypes. Given the fact that activation of AMPK and SIRT1 in articular chondrocytes regulates energy balance and coordinates several housekeeping mechanisms to increase cell stress resistance and maintain quality control (Fig. 3), targeted activation of AMPK and SIRT1 appeals to be an attractive therapeutic strategy for OA, which could be potentially achieved by pharmacologics, lifestyle measures (diet and exercise) and nutraceuticals.
Fig. 3.
AMPK and SIRT1 activity in regulation of cartilage homeostasis by increasing chondrocyte stress resistance. Pharmacological activators (e.g., AICAR, A-769662, and metformin), possibly some natural plant products (nutraceuticals), caloric restriction and exercise, activate AMPK in articular chondrocytes. As a result, intracellular NAD+ level is increased, leading to activation of SIRT1. Resveratrol, a natural plant product, also activates SIRT1. In turn, SIRT1 activates LKB1, the major upstream kinase of AMPK, through deacetylation of LKB1. Activation of AMPK and SIRT1 coordinates several housekeeping mechanisms to increase chondrocyte stress resistance by increasing mitochondrial biogenesis and function via PGC-1α; inhibiting oxidative stress and via PGC-1α and FOXO3a; attenuating inflammatory responses via inhibition of NF-κB activation, alleviating ER stress via limiting excessive CHOP; and promoting autophagy via suppression of mTOR, ultimately leading to cartilage homeostasis.
Acknowledgment
Dr R Liu-Bryan's research is supported by U.S. Department of Veterans Affairs grant 1I01BX002234, National Institutes of Health grant AR1067966 and an Innovative Science Grant 6045 from the Arthritis Foundation.
Footnotes
Author contribution
Dr Liu-Bryan is the sole contributor to this review.
Conflict of interest
None.
References
- 1.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–35. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 3.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–57. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–7. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 5.Liu-Bryan R, Terkeltaub R. The growing array of innate in-flammatory ignition switches in osteoarthritis. Arthritis Rheum. 2012;64:2055–8. doi: 10.1002/art.34492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu-Bryan R. Synovium and the innate inflammatory network in osteoarthritis progression. Curr Rheumatol Rep. 2013;15:323. doi: 10.1007/s11926-013-0323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sellam J, Berenbaum F. Is osteoarthritis a metabolic disease? Jt Bone Spine. Dec. 2013;80(6):568–73. doi: 10.1016/j.jbspin.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 8.O'Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–55. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 9.Liu TF, Brown CM, El Gazzar M, McPhail L, Millet P, Rao A, et al. Fueling the flame: bioenergy couples metabolism and inflammation. J Leukoc Biol. 2012;92:499–507. doi: 10.1189/jlb.0212078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fullerton MD, Steinberg GR, Schertzer JD. Immunometabolism of AMPK in insulin resistance and atherosclerosis. Mol Cell Endocrinol. 2013;366:224–34. doi: 10.1016/j.mce.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11:230–41. doi: 10.1016/j.arr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Mobasheri A, Vannucci SJ, Bondy CA, Carter SD, Innes JF, Arteaga MF, et al. Glucose transport and metabolism in chondrocytes: a key to understanding chondrogenesis, skeletal development and cartilage degradation in osteoarthritis. Histol Histopathol. 2002;17:1239–67. doi: 10.14670/HH-17.1239. [DOI] [PubMed] [Google Scholar]
- 13.Blanco FJ, Lopez-Armada MJ, Maneiro E. Mitochondrial dysfunction in osteoarthritis. Mitochondrion. 2004;4:715–28. doi: 10.1016/j.mito.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89:1025–78. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 15.Witczak CA, Sharoff CG, Goodyear LJ. AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell Mol Life Sci. 2008;65:3737–55. doi: 10.1007/s00018-008-8244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terkeltaub R, Yang B, Lotz M, Liu-Bryan R. Chondrocyte AMP-activated protein kinase activity suppresses matrix degradation responses to inflammatory cytokines IL-1β and TNFα. Arthritis Rheum. 2011;63:1928–37. doi: 10.1002/art.30333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petursson F, Husa M, June R, Lotz M, Terkeltaub R, Liu-Bryan R. Linked decreases in liver kinase B1 and AMP-activated protein kinase activity modulate matrix catabolic responses to biomechanical injury in chondrocytes. Arthritis Res Ther. 2013;24(15):R77. doi: 10.1186/ar4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X. SIRT1 and energy metabolism. Acta Biochim Biophys Sin (Shanghai) 2013;45:51–60. doi: 10.1093/abbs/gms108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab. 2014;25:138–45. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dvir-Ginzberg M, Steinmeyer J. Towards elucidating the role of SirT1 in osteoarthritis. Front Biosci. 2013;18:343–55. doi: 10.2741/4105. [DOI] [PubMed] [Google Scholar]
- 21.Fujita N, Matsushita T, Ishida K, Kubo S, Matsumoto T, Takayama K, et al. Potential involvement of SIRT1 in the pathogenesis of osteoarthritis through the modulation of chondrocyte gene expressions. J Orthop Res. 2011;29:511–5. doi: 10.1002/jor.21284. [DOI] [PubMed] [Google Scholar]
- 22.Matsuzaki T, Matsushita T, Takayama K, Matsumoto T, Nishida K, Kuroda R, et al. Disruption of Sirt1 in chondrocytes causes accelerated progression of osteoarthritis under mechanical stress and during ageing in mice. Ann Rheum Dis. 2014;73:1397–404. doi: 10.1136/annrheumdis-2012-202620. [DOI] [PubMed] [Google Scholar]
- 23.Gagarina V, Gabay O, Dvir-Ginzberg M, Lee EJ, Brady JK, Quon MJ, et al. SirT1 enhances survival of human osteoarthritic chondrocytes by repressing protein tyrosine phosphatase 1B and activating the insulin-like growth factor receptor pathway. Arthritis Rheum. 2010;62:1383–92. doi: 10.1002/art.27369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon MH, Jeong JK, Lee YJ, Seol JW, Jackson CJ, Park SY. SIRT1, a class III histone deacetylase, regulates TNF-α-induced inflammation in human chondrocytes. Osteoarthritis Cartilage. 2013;21:470–80. doi: 10.1016/j.joca.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 25.Matsushita T, Sasaki H, Takayama K, Ishida K, Matsumoto T, Kubo S, et al. The overexpression of SIRT1 inhibited osteoarthritic gene expression changes induced by interleukin-1β in human chondrocytes. J Orthop Res. 2013;31:531–7. doi: 10.1002/jor.22268. [DOI] [PubMed] [Google Scholar]
- 26.Dvir-Ginzberg M, Gagarina V, Lee EJ, Hall DJ. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamid phosphoribosyltransferase. J Biol Chem. 2008;283:36300–10. doi: 10.1074/jbc.M803196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong EH, Lee SJ, Kim JS, Lee KH, Um HD, Kim JH, et al. Ionizing radiation induces cellular senescence of articular chondrocytes via negative regulation of SIRT1 by p38 kinase. J Biol Chem. 2010;285:1283–95. doi: 10.1074/jbc.M109.058628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takayama K, Ishida K, Matsushita T, Fujita N, Hayashi S, Sasaki K, et al. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Rheum. 2009;60:2731–40. doi: 10.1002/art.24864. [DOI] [PubMed] [Google Scholar]
- 29.Gabay O, Oppenhiemer H, Meir H, Zaal K, Sanchez C, Dvir-Ginzberg M. Increased apoptotic chondrocytes in articular cartilage from adult heterozygous SirT1 mice. Ann Rheum Dis. 2012;71:613–6. doi: 10.1136/ard.2011.200504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabay O, Sanchez C, Dvir-Ginzberg M, Gagarina V, Zaal KJ, Song Y, et al. Sirtuin 1 enzymatic activity is required for cartilage homeostasis in vivo in a mouse model. Arthritis Rheum. 2013;65:159–66. doi: 10.1002/art.37750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, et al. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298:E751–60. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011;7:161–9. doi: 10.1038/nrrheum.2010.213. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maneiro E, Martín MA, de Andres MC, Lopez-Armada MJ, Fernandez-Sueiro JL, del Hoyo P, et al. Mitochondrial respiratory activity is altered in osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48:700–8. doi: 10.1002/art.10837. [DOI] [PubMed] [Google Scholar]
- 35.Johnson K, Svensson CI, Etten DV, Ghosh SS, Murphy AN, Powell HC, et al. Mediation of spontaneous knee osteoarthritis by progressive chondrocyte ATP depletion in Hartley guinea pigs. Arthritis Rheum. 2004;50:1216–25. doi: 10.1002/art.20149. [DOI] [PubMed] [Google Scholar]
- 36.Terkeltaub R, Johnson K, Murphy A, Ghosh S. Invited review: the mitochondrion in osteoarthritis. Mitochondrion. 2002;1:301–19. doi: 10.1016/s1567-7249(01)00037-x. [DOI] [PubMed] [Google Scholar]
- 37.Lotz M, Loeser RF. Effects of aging on articular cartilage homeostasis. Bone. 2012;51:241–8. doi: 10.1016/j.bone.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee RB, Urban JP. Evidence for a negative Pasteur effect in articular cartilage. Biochem J. 1997;321:95–102. doi: 10.1042/bj3210095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee RB, Urban JP. Functional replacement of oxygen by other oxidants in articular cartilage. Arthritis Rheum. 2002;46:3190–200. doi: 10.1002/art.10686. [DOI] [PubMed] [Google Scholar]
- 40.Martin JA, Martini A, Molinari A, Morgan W, Ramalingam W, Buckwalter JA, et al. Mitochondrial electron transport and glycolysis are coupled in articular cartilage. Osteoarthritis Cartilage. 2012;20:323–9. doi: 10.1016/j.joca.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henrotin Y, Kurz B, Aigner T. Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthritis Cartilage. 2005;13:643–54. doi: 10.1016/j.joca.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteo-arthritis Cartilage. 2003;11:747–55. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- 43.Cillero-Pastor B, Rego-Perez I, Oreiro N, Fernandez-Lopez C, Blanco FJ. Mitochondrial respiratory chain dysfunction modulates metalloproteases-1, -3 and -13 in human normal chondrocytes in culture. BMC Musculoskelet Disord. 2013;14:235. doi: 10.1186/1471-2474-14-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaamonde-García C, Riveiro-Naveira RR, Valc arcel-Ares MN, Hermida-Carballo L, Blanco FJ, Lopez-Armada MJ. Mitochondrial dysfunction increases inflammatory responsiveness to cytokines in normal human chondrocytes. Arthritis Rheum. 2012;64:2927–36. doi: 10.1002/art.34508. [DOI] [PubMed] [Google Scholar]
- 45.Zhao X, Petursson F, Viollet B, Lotz M, Terkeltaub R, Liu-Bryan R. Peroxisome proliferator-activated receptor γ coactivator 1α and FoxO3A mediate chondroprotection by AMP-activated protein kinase. Arthritis Rheumatol. 2014;66:3073–82. doi: 10.1002/art.38791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olmos Y, Valle I, Borniquel S, Tierrez A, Soria E, Lamas S, et al. Mutual dependence of Foxo3a and PGC-1alpha in the induction of oxidative stress genes. J Biol Chem. 2009;284:14476–84. doi: 10.1074/jbc.M807397200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang C, Li Ji L. Role of PGC-1alpha signaling in skeletal muscle health and disease. Ann N Y Acad Sci. 2012;1271:110–7. doi: 10.1111/j.1749-6632.2012.06738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gavriilidis C, Miwa S, von Zglinicki T, Taylor RW, Young DA. Mitochondrial dysfunction in osteoarthritis is associated with down-regulation of superoxide dismutase 2. Arthritis Rheum. 2013;65:378–87. doi: 10.1002/art.37782. [DOI] [PubMed] [Google Scholar]
- 49.Scott JL, Gabrielides C, Davidson RK, Swingler TE, Clark IM, Wallis GA, et al. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann Rheum Dis. 2010;69:1502–10. doi: 10.1136/ard.2009.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metal-loproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–9. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petersen SV, Oury TD, Ostergaard L, Valnickova Z, Wegrzyn J, Thogersen IB, et al. Extracellular superoxide dismutase (ECSOD) binds to type I collagen and protects against oxidative fragmentation. J Biol Chem. 2004;279:13705–10. doi: 10.1074/jbc.M310217200. [DOI] [PubMed] [Google Scholar]
- 52.Loeser RF. Molecular mechanisms of cartilage destruction in osteoarthritis. J Musculoskelet Neuronal Interact. 2008;8:303–6. [PubMed] [Google Scholar]
- 53.Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on health span and lifespan. J Mol Med. 2011;89:667–76. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25:1939–48. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Lei M, Wang JG, Xiao DM, Fan M, Wang DP, Xiong JY, et al. Resveratrol inhibits interleukin 1β-mediated inducible nitric oxide synthase expression in articular chondrocytes by activating SIRT1 and thereby suppressing nuclear factor-κB activity. Eur J Pharmacol. 2012;674:73–9. doi: 10.1016/j.ejphar.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 56.Csaki C, Mobasheri A, Shakibaei M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther. 2009;11:R165. doi: 10.1186/ar2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shakibaei M, Csaki C, Nebrich S, Mobasheri A. Resveratrol suppresses interleukin-1beta-induced inflammatory signaling and apoptosis in human articular chondrocytes: potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem Pharmacol. 2008;76:1426–39. doi: 10.1016/j.bcp.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 58.Dvir-Ginzberg M, Gagarina V, Lee EJ, Booth R, Gabay O, Hall DJ. Tumor necrosis actor α-mediated cleavage and inactivation of SirT1 in human osteoarthritic chondrocytes. Arthritis Rheum. 2011;63:2363–73. doi: 10.1002/art.30279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamakuchi M. MicroRNA regulation of SIRT1. Front Physiol. 2012;3:68. doi: 10.3389/fphys.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006;66:S102–9. doi: 10.1212/01.wnl.0000192306.98198.ec. [DOI] [PubMed] [Google Scholar]
- 61.Dufey E, Sepúlveda D, Rojas-Rivera D, Hetz C. Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 1. An overview. Am J Physiol Cell Physiol. 2014;307:C582–94. doi: 10.1152/ajpcell.00258.2014. [DOI] [PubMed] [Google Scholar]
- 62.Husa M, Petursson F, Lotz M, Terkeltaub R, Liu-Bryan R. C/EBP homologous protein drives pro-catabolic responses in chondrocytes. Arthritis Res Ther. 2013;15(6):R218. doi: 10.1186/ar4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takada K, Hirose J, Senba K, Yamabe S, Oike Y, Gotoh T, et al. Enhanced apoptotic and reduced protective response in chondrocytes following endoplasmic reticulum stress in osteoarthritic cartilage. Int J Exp Pathol. 2011;92:232–42. doi: 10.1111/j.1365-2613.2010.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo FJ, Xiong Z, Lu X, Ye M, Han X, Jiang R. ATF6 upregulates XBP1S and inhibits ER stress-mediated apoptosis in osteo-arthritis cartilage. Cell Signal. 2014;26:332–42. doi: 10.1016/j.cellsig.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 65.Liu Y, Zhou J, Zhao W, Li X, Jiang R, Liu C, et al. XBP1S associates with RUNX2 and regulates chondrocyte hypertrophy. J Biol Chem. 2012;287:34500–13. doi: 10.1074/jbc.M112.385922. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Oliver BL, Cronin CG, Zhang-Benoit Y, Goldring MB, Tanzer ML. Divergent stressresponses to IL-1beta, nitric oxide, and tunicamycin by chondrocytes. J Cell Physiol. 2005;204:45–50. doi: 10.1002/jcp.20261. [DOI] [PubMed] [Google Scholar]
- 67.Takada K, Hirose J, Yamabe S, Uehara Y, Mizuta H. Endoplasmic reticulum stress mediates nitric oxide-induced chondrocyte apoptosis. Biomed Rep. 2013;1:315–9. doi: 10.3892/br.2013.52. E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamabe S, Hirose J, Uehara Y, Okada T, Okamoto N, Oka K, et al. Intracellular accumulation of advanced glycation end products induces apoptosis via endoplasmic reticulum stress in chondrocytes. FEBS J. 2013;280:1617–29. doi: 10.1111/febs.12170. [DOI] [PubMed] [Google Scholar]
- 69.Rasheed Z, Haqqi TM. Endoplasmic reticulum stress induces the expression of COX-2 through activation of eIF2a, p38-MAPK and NF-κB in advanced glycation end products stimulated human chondrocytes. Biochim Biophys Acta. 2012;1823:2179–89. doi: 10.1016/j.bbamcr.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uehara Y, Hirose J, Yamabe S, Okamoto N, Okada T, Oyadomari S, et al. Endoplasmic reticulum stress-induced apoptosis contributes to articular cartilage degeneration via C/EBP homologous protein. Osteoarthritis Cartilage. 2014;22:1007–17. doi: 10.1016/j.joca.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 71.Alers S, Löffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lotz M, Caramés B. Autophagy: a new therapeutic target in cartilage injury and osteoarthritis. J Am Acad Orthop Surg. Apr. 2012;20(4):261–2. doi: 10.5435/JAAOS-20-04-261. [DOI] [PubMed] [Google Scholar]
- 73.Srinivas V, Bohensky J, Shapiro IM. Autophagy: a new phase in the maturation of growth plate chondrocytes is regulated by HIF, mTOR and AMP kinase. Cells Tissues Organs. 2009;189:88–92. doi: 10.1159/000151428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bohensky J, Leshinsky S, Srinivas V, Shapiro IM. Chondrocyte autophagy is stimulated by HIF-1 dependent AMPK activation and mTOR suppression. Pediatr Nephrol. 2010;4:633–42. doi: 10.1007/s00467-009-1310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Auto-phagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caramés B, Taniguchi N, Seino D, Blanco FJ, D'Lima D, Lotz M. Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection. Arthritis Rheum. 2012;64:1182–92. doi: 10.1002/art.33444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sasaki H, Takayama K, Matsushita T, Ishida K, Kubo S, Matsumoto T, et al. Autophagy modulates osteoarthritis-related gene expression in human chondrocytes. Arthritis Rheum. 2012;64:1920–8. doi: 10.1002/art.34323. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y, Vasheghani F, Li YH, Blati M, Simeone K, Fahmi H, et al. Cartilage-specific deletion of mTOR upregulates auto-phagy and protects mice from osteoarthritis. Ann Rheum Dis. 2014 Mar 20; doi: 10.1136/annrheumdis-2013-204599. http://dx.doi.org/10.1136/annrheumdis-2013-204599 (Epub ahead of print) [DOI] [PubMed]
- 79.Caramés B, Hasegawa A, Taniguchi N, Miyaki S, Blanco FJ, Lotz M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis. 2012;71:575–81. doi: 10.1136/annrheumdis-2011-200557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim HY, Park SY, Lee SW, Lee HR, Lee WS, Rhim BY, et al. Inhibition of HMGB1-induced angiogenesis by cilostazol via SIRT1 activation in synovial fibroblasts from rheumatoid arthritis. PLoS One. Aug 15. 2014;9(8):e104743. doi: 10.1371/journal.pone.0104743. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Kok SH, Lin LD, Hou KL, Hong CY, Chang CC, Hsiao M, et al. Simvastatin inhibits cysteine-rich protein 61 expression in rheumatoid arthritis synovial fibroblasts through the regulation of sirtuin-1/FoxO3a signaling. Arthritis Rheum. 2013;65:639–49. doi: 10.1002/art.37807. [DOI] [PubMed] [Google Scholar]
- 82.Indo Y, Takeshita S, Ishii KA, Hoshii T, Aburatani H, Hirao A, et al. Metabolic regulation of osteoclast differentiation and function. J Bone Miner Res. 2013;28:2392–9. doi: 10.1002/jbmr.1976. [DOI] [PubMed] [Google Scholar]
- 83.Kang H, Viollet B, Wu D. Genetic deletion of catalytic subunits of AMP-activated protein kinase increases osteoclasts and reduces bone mass in young adult mice. J Biol Chem. 2013;288:12187–96. doi: 10.1074/jbc.M112.430389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jeyabalan J, Shah M, Viollet B, Chenu C. AMP-activated protein kinase pathway and bone metabolism. J Endocrinol. 2012;212:277–90. doi: 10.1530/JOE-11-0306. [DOI] [PubMed] [Google Scholar]
- 85.Lee YS, Kim YS, Lee SY, Kim GH, Kim BJ, Lee SH, et al. AMP kinase acts as a negative regulator of RANKL in the differentiation of osteoclasts. Bone. 2010;47:926–37. doi: 10.1016/j.bone.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 86.Shah M, Kola B, Bataveljic A, Arnett TR, Viollet B, Saxon L, et al. AMP-activated protein kinase (AMPK) activation regulates in vitro bone formation and bone mass. Bone. 2010;47:309–19. doi: 10.1016/j.bone.2010.04.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quinn JM, Tam S, Sims NA, Saleh H, McGregor NE, Poulton IJ, et al. Germline deletion of AMP-activated protein kinase beta subunits reduces bone mass without altering osteoclast differentiation or function. FASEB J. 2010;24:275–85. doi: 10.1096/fj.09-137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iyer S, Han L, Bartell SM, Kim HN, Gubrij I, de Cabo R, et al. Sirtuin1 (Sirt1) promotes cortical bone formation by preventing β-catenin sequestration by FoxO transcription factors in osteoblast progenitors. J Biol Chem. 2014;289:24069–78. doi: 10.1074/jbc.M114.561803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abed É , Couchourel D, Delalandre A, Duval N, Pelletier JP, Martel-Pelletier J, et al. Low sirtuin 1 levels in human osteoarthritis subchondral osteoblasts lead to abnormal sclerostin expression which decreases Wnt/β-catenin activity. Bone. 2014;59:28–36. doi: 10.1016/j.bone.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 90.Edwards JR, Perrien DS, Fleming N, Nyman JS, Ono K, Connelly L, et al. Silent information regulator (Sir)T1 inhibits NF-κB signaling to maintain normal skeletal remodeling. J Bone Miner Res. 2013;28:960–9. doi: 10.1002/jbmr.1824. [DOI] [PubMed] [Google Scholar]
- 91.Cohen-Kfir E, Artsi H, Levin A, Abramowitz E, Bajayo A, Gurt I, et al. Sirt1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin, a bone formation inhibitor. Endocrinology. 2011;152:4514–24. doi: 10.1210/en.2011-1128. [DOI] [PubMed] [Google Scholar]
- 92.Simic P, Zainabadi K, Bell E, Sykes DB, Saez B, Lotinun S, et al. SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating β-catenin. EMBO Mol Med. 2013;5:430–40. doi: 10.1002/emmm.201201606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen H, Liu X, Chen H, Cao J, Zhang L, Hu X, et al. Role of SIRT1 and AMPK in mesenchymal stem cells differentiation. Ageing Res Rev. 2014;13:55–64. doi: 10.1016/j.arr.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 94.Kim EK, Lim S, Park JM, Seo JK, Kim JH, Kim KT, et al. Human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by AMP-activated protein kinase. J Cell Physiol. 2012;227:1680–7. doi: 10.1002/jcp.22892. [DOI] [PubMed] [Google Scholar]
- 95.Bäckesjö CM, Li Y, Lindgren U, Haldosén LA. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. Cells Tissues Organs. 2009;189:93–7. doi: 10.1159/000151744. [DOI] [PubMed] [Google Scholar]
- 96.Fedor D, Kelley DS. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2009;12:138–46. doi: 10.1097/MCO.0b013e3283218299. [DOI] [PubMed] [Google Scholar]
- 97.Jelenik T, Rossmeisl M, Kuda O, Jilkova ZM, Medrikova D, Kus V, et al. AMP-activated protein kinase α2 subunit is required for the preservation of hepatic insulin sensitivity by n-3 polyunsaturated fatty acids. Diabetes. 2010;59:2737–46. doi: 10.2337/db09-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Suchankova G, Tekle M, Saha AK, Ruderman NB, Clarke SD, Gettys TW. Dietary polyunsaturated fatty acids enhance hepatic AMP-activated protein kinase activity in rats. Biochem Biophys Res Commun. 2005;326:851–8. doi: 10.1016/j.bbrc.2004.11.114. [DOI] [PubMed] [Google Scholar]
- 99.Rossmeisl M, Flachs P, Brauner P, Sponarova J, Matejkova O, Prazak T, et al. Role of energy charge and AMP-activated protein kinase in adipocytes in the control of body fat stores. Int J Obes Relat Metab Disord. 2004;28:S38–44. doi: 10.1038/sj.ijo.0802855. [DOI] [PubMed] [Google Scholar]
- 100.Gabler NK, Radcliffe JS, Spencer JD, Webel DM, Spurlock ME. Feeding long-chain n-3 polyunsaturated fatty acids during gestation increases intestinal glucose absorption potentially via the acute activation of AMPK. J Nutr Biochem. 2009;20:17–25. doi: 10.1016/j.jnutbio.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 101.Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J. 2009;418:261–75. doi: 10.1042/BJ20082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bennell KL, Dobson F, Hinman RS. Exercise in osteoarthritis: moving from prescription to adherence. Best Pract Res Clin Rheumatol. 2014;28:93–117. doi: 10.1016/j.berh.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 103.Friedrichsen M, Mortensen B, Pehmøller C, Birk JB, Wojtaszewski JF. Exercise-induced AMPK activity in skeletal muscle: role in glucose uptake and insulin sensitivity. Mol Cell Endocrinol. 2013;366:204–14. doi: 10.1016/j.mce.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 104.Hardie DG, Ross FA, Hawley SA. AMP-activated protein kinase: a target for drugs both ancient and modern. Chem Biol. 2012;19:1222–36. doi: 10.1016/j.chembiol.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hwang JT, Kwon DY, Yoon SH. AMP-activated protein kinase: a potential target for the diseases prevention by natural occurring polyphenols. N Biotechnol. 2009;26:17–22. doi: 10.1016/j.nbt.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 106.Abou-Raya A, Abou-Raya S, Khadrawe T. Methotrexate in the treatment of symptomatic knee osteoarthritis: randomised placebo-controlled trial. Ann Rheum Dis. 2014 Mar 27; doi: 10.1136/annrheumdis-2013-204856. http:// dx.doi.org/10.1136/annrheumdis-2013-204856 (Epub ahead of print) [DOI] [PubMed]
- 107.Panahi Y, Rahimnia AR, Sharafi M, Alishiri G, Saburi A, Sahebkar A. Curcuminoid treatment for knee osteoarthritis: a randomized double-blind placebo-controlled trial. Phytother Res. 2014 May 22; doi: 10.1002/ptr.5174. http://dx.doi.org/10.1002/ptr. 5174 (Epub ahead of print) [DOI] [PubMed]
- 108.Maheu E, Cadet C, Marty M, Moyse D, Kerloch I, Coste P, et al. Randomised, controlled trial of avocado-soybean unsaponi-fiable (Piascledine) effect on structure modification in hip osteoarthritis: the ERADIAS study. Ann Rheum Dis. 2014;73:376–84. doi: 10.1136/annrheumdis-2012-202485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Christensen R, Bartels EM, Astrup A, Bliddal H. Symptomatic efficacy of avocado-soybean unsaponifiables (ASU) in osteo-arthritis (OA) patients: a meta-analysis of randomized controlled trials. Osteoarthritis Cartilage. 2008;16:399–408. doi: 10.1016/j.joca.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 110.Maheu E, Mazières B, Valat JP, Loyau G, Le Löet X, Bourgeois P, et al. Symptomatic efficacy of avocado/soybean unsaponifiables in the treatment of osteoarthritis of the knee and hip: a prospective, randomized, double-blind, placebo-controlled, multicenter clinical trial with a six-month treatment period and a two-month followup demonstrating a persistent effect. Arthritis Rheum. 1998;41:81–91. doi: 10.1002/1529-0131(199801)41:1<81::AID-ART11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 111.Yamagata K, Tanaka N, Matsufuji H, Chino M. β-carotene reverses the IL-1β-mediated reduction in paraoxonase-1 expression via induction of the CaMKKII pathway in human endothelial cells. Microvasc Res. 2012;84(3):297–305. doi: 10.1016/j.mvr.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 112.Tang L, Zhang Y, Jiang Y, Willard L, Ortiz E, Wark L, et al. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp Biol Med (Maywood) 2011;236:1051–63. doi: 10.1258/ebm.2011.010400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arunkumar E, Anuradha CV. Genistein promotes insulin action through adenosine monophosphate-activated protein kinase activation and p70 ribosomal protein S6 kinase 1 inhibition in the skeletal muscle of mice fed a high energy diet. Nutr Res. 2012;32:617–25. doi: 10.1016/j.nutres.2012.06.002. [DOI] [PubMed] [Google Scholar]