Overview
Francisella tularensis is a gram-negative, facultative, intracellular bacterium that survives in mammals, arthropods, and amoebae; however, macrophages are considered the key cells in pathogenesis of tularemia in mammals. Understanding intracellular trafficking of F. tularensis within various host cells is indispensable to our understanding of bacterial ecology, intracellular adaptation to various hosts’ microenvironments, and subversion of host cell defenses. Within mammalian and arthropod-derived cells, F. tularensis transiently resides within an acidic vacuole prior to escaping to the cytosol, where the bacteria replicate. In contrast, F. tularensis resides and replicates within non-acidified, membrane-bound vacuoles within the trophozoites of amoebae. The Francisella pathogenicity island (FPI) genes encode a type VI Secretion System (T6SS), which is indispensable for phagosomal escape of F. tularensis within mammalian and arthropod cells and for intravacuolar growth within amoeba. In this review, we discuss the divergent F. tularensis intracellular lifestyle in different hosts and its role in pathogenic evolution and intracellular proliferation within diverse hosts.
Introduction
Nonpathogenic bacteria are taken up by host cells into vacuoles or phagosomes that are processed through the endocytic pathway, through which the vacuoles mature and fuse to the lysosomes, in which the bacteria are degraded. To avoid this fate within phagocytic cells, intracellular pathogens have evolved different strategies to survive and evade phagosome–lysosome fusion [1]. Understanding the mechanisms by which pathogens manipulate vesicle trafficking in different hosts is extremely important for understanding the ability of various pathogens to cause disease and is essential for designing novel and effective strategies for prevention and therapeutic intervention.
Francisella tularensis is a gram-negative, highly infectious, facultative intracellular bacterium that causes the zoonotic disease tularemia. The genus Francisella contains five species: F. tularensis, F. philomiragia, F. hispaniensis, F. noatunensis, and F. novicida [2,3]. Two subspecies of F. tularensis, tularensis (Type A) and holarctica (Type B), cause most cases of the illness in humans. F. novicida causes disease only in immunocompromised persons, but is highly virulent in mice [3]. However, it is important to note that in comparison to F. tularensis subsp. tularensis and F. tularensis subsp. holarctica, F. novicida elicits a different immune response in the host [2].
Humans acquire infection by several routes, including direct contact with infected animals, ingestion of water or food contaminated by infected animals, exposure to infected arthropod vectors, or by inhalation of infective aerosols, resulting in pneumonic, oropharyngeal, glandular, ulceroglandular, or oculoglandular tularemia [4]. Considering the ease of dissemination and high infectivity, F. tularensis subsp. tularensis and F. tularensis subsp. holarctica have been classified by the Centers for Disease Control and Prevention (CDC) as Tier 1 select agents.
The existence of Francisella in the environment is divided into two cycles: the terrestrial cycle and aquatic cycle [5]. Small rodents, hares, and arthropods play a major role in the terrestrial cycle, while rodents associated with water are important in the water cycle [4,5]. Organisms such as ticks, flies, and mosquitoes are considered vectors of tularemia transmission to mammals [6]. Although it causes disease in various animal species, no animal has been identified as a main reservoir of this pathogen. F. tularensis subsp. holarctica and F. novicida have a strong association with freshwater environments, free-living amoeba, and biofilms [7,8]. Since mosquito larvae can feed on aquatic protozoa, they may be infected with F. tularensis during development in their natural aquatic environment [7]. The effect of wars, natural disasters, climate change, and global warming will probably have an impact on increased incidences of tularemia [9].
The Role of T6SS in Intracellular Replication
Understanding the virulence factors of Francisella is indispensable for elucidating various aspects of tularemia pathogenesis. Many studies have been focused on a genomic region called the Francisella pathogenicity island (FPI). The FPI is duplicated in F. tularensis subsp. tularensis and F. tularensis subsp. holarctica strains, but is a single copy in F. novicida. It has been shown that many of the FPI genes are essential for phagosome biogenesis and escape of the bacterium into the cytosol, which is the crucial event in the intracellular life cycle of Francisella [10].
The FPI genes encode a type VI Secretion System (T6SS), since the FPI-encoded proteins IglA, IglB, VgrG, PdpB, and DotU show similarity to structural components of T6SS of other bacteria [10,11]. The existence of the FPI-encoded secretion system is supported by finding that IglI and VgrG proteins are secreted by F. novicida to the cytosol of macrophages [10]. A lipoprotein that anchors parts of the secretion apertures to the outer membrane is commonly encoded by a T6SS gene cluster [12]. It has been recently shown that the FPI-encoded protein IglE is a lipoprotein that localizes to the outer membrane and interacts with other FPI proteins, resulting in a channel formation [13]. The T6SS assembles a phage tail-like injectisome for translocation of effector proteins across the two bacterial membranes and host cell membrane, and into the cytosol of the host cell [14]. This secretion system is involved in F. tularensis phagosomal escape and intracellular growth. However, the Francisella FPI system seems to be different from all other T6SS described so far, since no homolog of ClpV has been identified and the Francisella VgrG is significantly smaller than VgrG homologs from other bacteria [15,16].
The most investigated FPI protein, IglC, and the global regulator MglA (encoded outside the FPI) are essential for the ability of Francisella to escape from the Francisella-containing phagosome (FCP) into the cytoplasm and for pathogenesis of tularemia in vivo [17]. The FPI genes iglA, iglB, iglD, pdpA, vgrG, and iglI are also required for intracellular growth and virulence of F. tularensis [10,18]. Although the mechanism of escape of Francisella from the phagosome is not known yet, many of the proteins encoded by the FPI are indispensable for this process.
Intracellular Life of F. tularensis in Macrophages
F. tularensis survives and replicates within various cells, but macrophages are considered the important cells in developing tularemia [19]. Francisella enters into macrophages by looping phagocytosis and binding to surface receptors, depending on the opsonization state [20]. Upon entry into macrophages, F. tularensis recruits ''lipid rafts'' (cholesterol-rich lipid domains) with caveolin-1 on the host cell membrane [21]. Cholesterol and caveolin-1 are incorporated into the FCP membrane during the initial phase of biogenesis of the FCP [22].
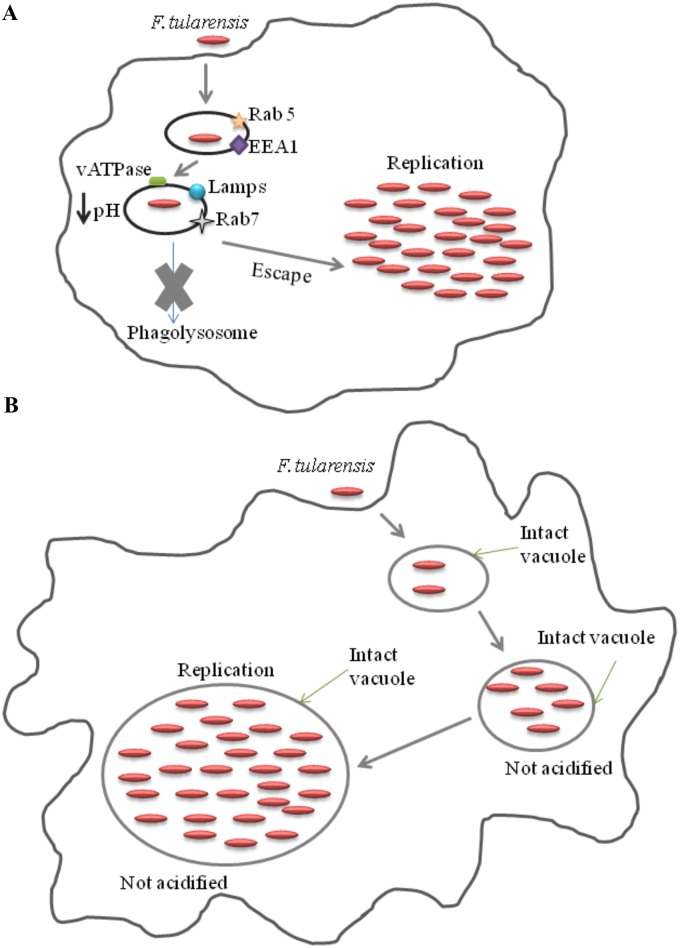
Following uptake of Francisella by macrophages, the bacteria reside within the FCP, which matures into an early endosome characterized by Rab-5 and early endosome antigen 1 (EEA1) [23]. The FCP matures into a late endosomal stage characterized by the late endosomal markers Rab-7, mannose-6-phosphate, and lysosomal associated proteins (LAMPs), but the FCP does not mature into a phagolysosome [17,24,25]. The FCP is acidified through acquisition of the vATPase proton pump [23,25]. This is followed by escape of the bacteria to the cytosol within 30 to 60 minutes of entry [17,25]. The short time spent in the FCP is a dynamic step in the infection during which Francisella actively evades host antimicrobial defenses, including reactive oxygen species and antimicrobial peptides [26]. Interestingly, inhibition of the acidification by a specific inhibitor of the vATPase proton pump, bafilomycin A, does not block, but delays, bacterial escape to the cytosol (Fig 1A) [25]. It appears that acidification of the FCP enables rapid phagosomal membrane disruption and bacterial egress in the macrophage cytosol, where they replicate (Fig 2A) [23]. It seems that a decrease in pH stimulates Francisella to express or secrete some unique, unknown factor to disrupt the phagosomal membrane, but the mechanisms involved are still to be determined. Late stages of intracellular proliferation are accompanied with or followed by death of infected macrophages, mediated by a versatile apoptotic pathway able to switch from pyroptosis to a mitochondrial-intrinsic apoptotic pathway [27]. It is followed by egress of intracellular bacteria [28].
Fig 1. Trafficking of Francisella within macrophages (A) and amoebae cells (B).
(A) Upon phagocytosis by macrophages, bacteria reside in the FCP that interact with early (EEA1 and Rab 5) and late (Lamps and Rab 7) endocytic compartments. The FCP is rapidly acidified by the vATPase proton pump, resulting in bacterial escape into the cytosol, where it replicates. (B) After phagocytosis by amoebae, bacteria are localized in intact vacuoles. Bacteria reside in the vacuoles and replicate.
Fig 2. F. tularensis escapes from the phagosome and replicates in the cytosol of human macrophages (A) and Drosophila melanogaster-derived S2 cells (B), but resides and replicates in vacuoles within amoeba (C).
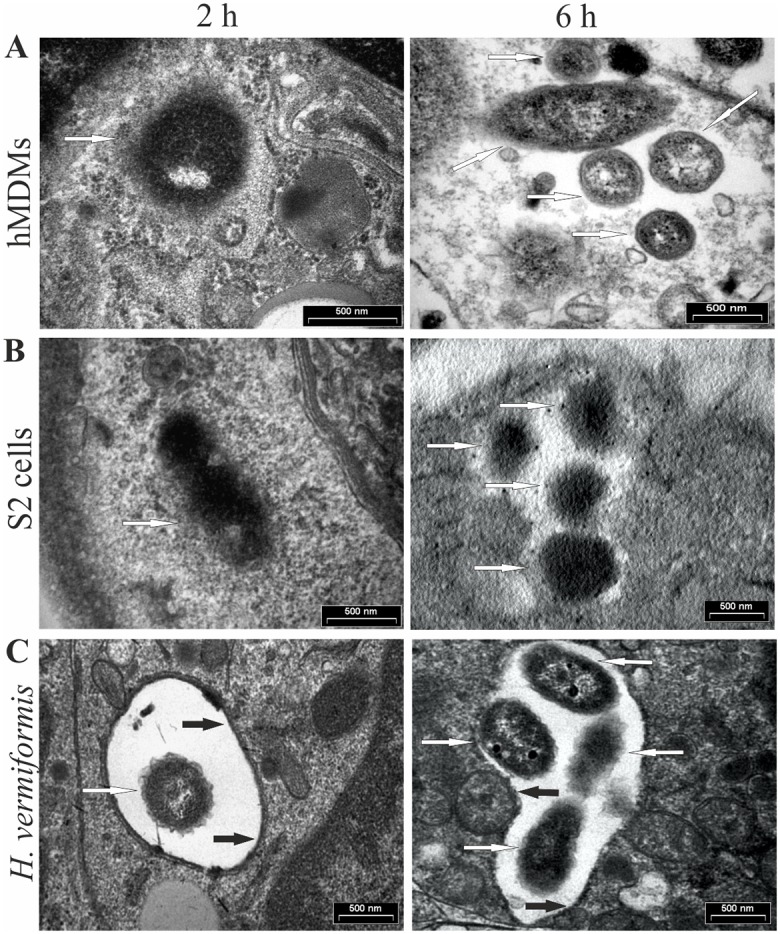
Representative electron micrographs of hMDMs, D. melanogaster, and Hartmannella vermiformis infected with F. tularensis at 2 and 6 h after infection. The white arrows indicate bacteria, while black arrows indicate intact vacuolar membrane.
Intracellular Life of F. tularensis in Arthropod-Derived Cells
Vector-borne transmission of tularemia to mammalian hosts has an important role in pathogenesis of the disease [29]. However, little is known about the interaction of F. tularensis with the arthropod vectors at the molecular level. Drosophila melanogaster and D. melanogaster-derived S2 cells have been used for studying F. tularensis infection [30]. It has been shown that F. tularensis infects and kills adult Drosophila flies in a dose-dependent manner [31]. Similar to human macrophages, within arthropod cells, F. tularensis transiently resides within an acidified phagosome that matures to a late endosomal stage, followed by rapid bacterial escape into the cytosol, where the bacteria proliferate (Fig 2B) [32]. The intracellular lifestyle of F. tularensis within human macrophages and arthropod-derived cells is very similar in terms of the virulence factors involved. Studies using a mosquito cell line, SualB, derived from Anopheles gambie, have shown that the FPI-encoded IglA, IglB, IglC, IglD, PdpA, and PdpB are necessary for efficient intracellular replication of F. novicida within this insect’s cells [33]. However, the FPI proteins PdpC and PdpD are not required for replication within the SualB cell line [33]. In addition, out of 394 mutants of F. novicida identified to be defective for proliferation within S2 cells, only 135 (including the FPI genes) are also defective for replication within human macrophages [30]. F. novicida virulence factors have also been studied in adult flies compared to mice [34]. Among 249 F. tularensis genes important for virulence in the mice model, only 49 genes were important in adult D. melanogaster, including 14 of the FPI genes [34]. The FPI genes that are not required for virulence in the adult fly are pdpC, pdpE, pdpD, and anmK [34], which is consistent with findings in the mosquito cell line [33]. Therefore, although F. tularensis uses similar molecular mechanisms of pathogenesis within arthropod and mammalian cells, some distinct virulence factors are differentially utilized for bacterial proliferation in the two evolutionarily distinct hosts [30].
Intravacuolar Proliferation of F. tularensis in Amoeba
Recent studies have shown that Francisella enters and multiplies within Acanthamoeba castellanii [35], Hartmannella vermiformis [36], and Dictyostelium discoideum cells (our unpublished results). In addition, F. tularensis subsp. holarctica is found within cysts of A. castellanii, suggesting a potential for long-term bacterial survival in nature [37]. Studies have also shown intravacuolar replication of F. novicida within H. vermiformis (Figs 1B and 2C), which is a major difference from the cytosolic proliferation of this bacterium within mammalian and arthropod-derived cells. Interestingly, the FPI-encoded protein IglC and the MglA global regulator, which are essential for phagosomal escape and intramacrophage growth of F. tularensis, are also important for intravacuolar replication of F. tularensis within amoeba cells [36,38].
In addition, imaging studies using the lysosomotropic agent LysoTracker Red DND-99, which concentrates in acidified vesicles and compartments, have shown that FCP did not acquire this dye at any time point during the infection of amoebae with F. novicida [36]. In addition, F. novicida blocks lysosomal fusion to the FCP within A. castellanii [37]. Therefore, F. tularensis escapes from acidified vacuoles in human and arthropod-derived cells, but replicates within non-acidified vacuoles in H. vermiformis [36]. Although the FPI protein IglC plays an important role in intravacuolar proliferation of F. tularensis within protozoa, it is not sufficient to trigger bacterial escape into the cytosol. It is unclear how IglC contributes to phagosomal escape in human and arthropod-derived cells but is also required for intravacuolar growth of F. tularensis within amoeba. It is also unclear why F. tularensis within amoeba has a different intracellular cycle compared to arthropod and mammalian cells.
Conclusions and Future Directions
The ability to invade and replicate in a variety of host cells appears to be a major feature of the ecology and epidemiology of F. tularensis. Within both mammalian and arthropod-derived cells, the FCP transiently matures to an acidified late endosome, followed by rapid bacterial escape into the host cell cytosol where the replication occurs. However, within amoeba cells, the bacterium resides and replicates within non-acidified, membrane-bound vacuoles. It is possible that the virulence of Francisella for mammalian hosts may be higher after intra-amoebal growth. Many virulence factors of F. tularensis have been discovered and investigated, including the FPI proteins, which play a crucial role in patho-adaptation of the bacteria to different hosts. At the moment, some studies are focused on the FPI genes that encode the T6SS in Francisella. Future studies should elucidate the role of T6SS translocated proteins in disruption of the phagosome and intracellular replication of F. tularensis. The relevance of bacterial escape into the cytosol should be investigated from various perspectives and cells models. It is evident that the short transition of the bacterium in vacuoles within mammalian and arthropod cells plays an important role in pathogenesis of tularemia. The differences between the amoebal vacuoles and the mammalian and arthropod vacuoles where the bacterium permanently or shortly resides, respectively, have yet to be discovered. It is intriguing how IglC is required for phagosomal escape in mammalian and arthropod-derived cells but is indispensable for intravacuolar growth within amoeba. It is possible that the long-term evolution of F. tularensis within amoeba has facilitated its intravacuolar adaptation in the aquatic environment for long-term survival and for transmission to arthropod and mammalian hosts.
Funding Statement
This work is supported by a Grant of University of Rijeka (Grant No. 811.10.1111). YAK is supported by Public Health Service Award 1R01AI120244 and R21AI116517 from the National Institute of Health and by the Commonwealth of Kentucky Research Challenge Trust Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ray K, Marteyn B, Sansonetti PJ, Tang CM. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nature reviews. 2009;7(5):333–40. 10.1038/nrmicro2112 [DOI] [PubMed] [Google Scholar]
- 2. Kingry LC, Petersen JM. Comparative review of Francisella tularensis and Francisella novicida . Frontiers in cellular and infection microbiology. 2014;4:35 10.3389/fcimb.2014.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sjodin A, Svensson K, Ohrman C, Ahlinder J, Lindgren P, Duodu S, et al. Genome characterisation of the genus Francisella reveals insight into similar evolutionary paths in pathogens of mammals and fish. BMC genomics. 2012;13:268 10.1186/1471-2164-13-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ellis J, Oyston PC, Green M, Titball RW. Tularemia. Clinical microbiology reviews. 2002;15(4):631–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gurcan S. Epidemiology of tularemia. Balkan medical journal. 2014;31(1):3–10. 10.5152/balkanmedj.2014.13117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petersen JM, Mead PS, Schriefer ME. Francisella tularensis: an arthropod-borne pathogen. Veterinary research. 2009;40(2):7 10.1051/vetres:2008045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lundstrom JO, Andersson AC, Backman S, Schafer ML, Forsman M, Thelaus J. Transstadial transmission of Francisella tularensis holarctica in mosquitoes, Sweden. Emerging infectious diseases. 2011;17(5):794–9. 10.3201/eid1705.100426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Hoek ML. Biofilms: an advancement in our understanding of Francisella species. Virulence. 2013;4(8):833–46. 10.4161/viru.27023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ryden P, Sjostedt A, Johansson A. Effects of climate change on tularaemia disease activity in Sweden. Global health action; 2009;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barker JR, Chong A, Wehrly TD, Yu JJ, Rodriguez SA, Liu J, et al. The Francisella tularensis pathogenicity island encodes a secretion system that is required for phagosome escape and virulence. Molecular microbiology. 2009;74(6):1459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Bruin OM, Duplantis BN, Ludu JS, Hare RF, Nix EB, Schmerk CL, et al. The biochemical properties of the Francisella pathogenicity island (FPI)-encoded proteins IglA, IglB, IglC, PdpB and DotU suggest roles in type VI secretion. Microbiology (Reading, England). 2011;157(Pt 12):3483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Filloux A, Hachani A, Bleves S. The bacterial type VI secretion machine: yet another player for protein transport across membranes. Microbiology (Reading, England). 2008;154(Pt 6):1570–83. [DOI] [PubMed] [Google Scholar]
- 13. Nguyen JQ, Gilley RP, Zogaj X, Rodriguez SA, Klose KE. Lipidation of the FPI protein IglE contributes to Francisella tularensis ssp. novicida intramacrophage replication and virulence. Pathogens and disease. 2014;72(1):10–8. 10.1111/2049-632X.12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hood RD, Singh P, Hsu F, Guvener T, Carl MA, Trinidad RR, et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell host & microbe. 2010;7(1):25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mougous JD, Cuff ME, Raunser S, Shen A, Zhou M, Gifford CA, et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science (New York, NY. 2006;312(5779):1526–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pukatzki S, Ma AT, Revel AT, Sturtevant D, Mekalanos JJ. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(39):15508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Santic M, Molmeret M, Klose KE, Jones S, Kwaik YA. The Francisella tularensis pathogenicity island protein IglC and its regulator MglA are essential for modulating phagosome biogenesis and subsequent bacterial escape into the cytoplasm. Cellular microbiology. 2005;7(7):969–79. [DOI] [PubMed] [Google Scholar]
- 18. de Bruin OM, Ludu JS, Nano FE. The Francisella pathogenicity island protein IglA localizes to the bacterial cytoplasm and is needed for intracellular growth. BMC microbiology. 2007;7:1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Llewellyn AC, Jones CL, Napier BA, Bina JE, Weiss DS. Macrophage replication screen identifies a novel Francisella hydroperoxide resistance protein involved in virulence. PloS one. 2011;6(9):e24201 10.1371/journal.pone.0024201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clemens DL, Lee BY, Horwitz MA. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infection and immunity. 2004;72(6):3204–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moreau GB, Mann BJ. Adherence and uptake of Francisella into host cells. Virulence. 2013;4(8):826–32. 10.4161/viru.25629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tamilselvam B, Daefler S. Francisella targets cholesterol-rich host cell membrane domains for entry into macrophages. J Immunol. 2008;180(12):8262–71. [DOI] [PubMed] [Google Scholar]
- 23. Chong A, Wehrly TD, Nair V, Fischer ER, Barker JR, Klose KE, et al. The early phagosomal stage of Francisella tularensis determines optimal phagosomal escape and Francisella pathogenicity island protein expression. Infection and immunity. 2008;76(12):5488–99. 10.1128/IAI.00682-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Celli J, Zahrt TC. Mechanisms of Francisella tularensis intracellular pathogenesis. Cold Spring Harbor perspectives in medicine. 2013;3(4):a010314 10.1101/cshperspect.a010314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Santic M, Asare R, Skrobonja I, Jones S, Abu Kwaik Y. Acquisition of the vacuolar ATPase proton pump and phagosome acidification are essential for escape of Francisella tularensis into the macrophage cytosol. Infection and immunity. 2008;76(6):2671–7. 10.1128/IAI.00185-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jones CL, Napier BA, Sampson TR, Llewellyn AC, Schroeder MR, Weiss DS. Subversion of host recognition and defense systems by Francisella spp. Microbiol Mol Biol Rev. 2012;76(2):383–404. 10.1128/MMBR.05027-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pierini R, Juruj C, Perret M, Jones CL, Mangeot P, Weiss DS, et al. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell death and differentiation. 2012;19(10):1709–21. 10.1038/cdd.2012.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Asare R, Kwaik YA. Exploitation of host cell biology and evasion of immunity by francisella tularensis . Frontiers in microbiology. 2011;1:145 10.3389/fmicb.2010.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Keim P, Johansson A, Wagner DM. Molecular epidemiology, evolution, and ecology of Francisella . Annals of the New York Academy of Sciences. 2007;1105:30–66. [DOI] [PubMed] [Google Scholar]
- 30. Asare R, Akimana C, Jones S, Abu Kwaik Y. Molecular bases of proliferation of Francisella tularensis in arthropod vectors. Environmental microbiology. 2010;12(9):2587–612. 10.1111/j.1462-2920.2010.02230.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vonkavaara M, Telepnev MV, Ryden P, Sjostedt A, Stoven S. Drosophila melanogaster as a model for elucidating the pathogenicity of Francisella tularensis . Cellular microbiology. 2008;10(6):1327–38. 10.1111/j.1462-5822.2008.01129.x [DOI] [PubMed] [Google Scholar]
- 32. Santic M, Akimana C, Asare R, Kouokam JC, Atay S, Kwaik YA. Intracellular fate of Francisella tularensis within arthropod-derived cells. Environmental microbiology. 2009;11(6):1473–81. 10.1111/j.1462-2920.2009.01875.x [DOI] [PubMed] [Google Scholar]
- 33. Read A, Vogl SJ, Hueffer K, Gallagher LA, Happ GM. Francisella genes required for replication in mosquito cells. Journal of medical entomology. 2008;45(6):1108–16. [DOI] [PubMed] [Google Scholar]
- 34. Ahlund MK, Ryden P, Sjostedt A, Stoven S. Directed screen of Francisella novicida virulence determinants using Drosophila melanogaster . Infection and immunity. 2010;78(7):3118–28. 10.1128/IAI.00146-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abd H, Johansson T, Golovliov I, Sandstrom G, Forsman M. Survival and growth of Francisella tularensis in Acanthamoeba castellanii . Applied and environmental microbiology. 2003;69(1):600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Santic M, Ozanic M, Semic V, Pavokovic G, Mrvcic V, Kwaik YA. Intra-Vacuolar Proliferation of F. Novicida within H. Vermiformis . Frontiers in microbiology. 2011;2:78 10.3389/fmicb.2011.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. El-Etr SH, Margolis JJ, Monack D, Robison RA, Cohen M, Moore E, et al. Francisella tularensis type A strains cause the rapid encystment of Acanthamoeba castellanii and survive in amoebal cysts for three weeks postinfection. Applied and environmental microbiology. 2009;75(23):7488–500. 10.1128/AEM.01829-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lauriano CM, Barker JR, Yoon SS, Nano FE, Arulanandam BP, Hassett DJ, et al. MglA regulates transcription of virulence factors necessary for Francisella tularensis intraamoebae and intramacrophage survival. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(12):4246–9. [DOI] [PMC free article] [PubMed] [Google Scholar]