Abstract
Although dipeptidyl peptidase 4 (DPP4) is an adipokine known to positively correlate with adiposity, the effects of pharmacological DPP4 inhibition on body composition have not been fully understood. This study was aimed to assess the effects of DPP4 inhibitors on adiposity for the first time in the established obese mice model. The weight loss effects of multiple DPP4 inhibitors were compared after a 4 week treatment in diet-induced obese mice. In addition, a 2 week study was performed to explore and compare the acute effects of evogliptin, a novel DPP4 inhibitor, and exenatide, a glucagon-like peptide-1 (GLP-1) analogue, on whole body composition, energy consumption, various plasma adipokines and gene expression in white adipose tissue (WAT). After the 4 week treatment, weight loss and blood glucose reductions were consistently observed with multiple DPP4 inhibitors. Moreover, after 2-week treatment, evogliptin dose-dependently reduced whole body fat mass while increasing the proportion of smaller adipocytes. However, insulin sensitivity or plasma lipid levels were not significantly altered. In addition to increased active GLP-1 levels by plasma DPP4 inhibition, evogliptin also enhanced basal metabolic rate without reduction in caloric intake, in contrast to exenatide; this finding suggested evogliptin's effects may be mediated by pathways other than via GLP-1. Evogliptin treatment also differentially increased Ppargc1a expression, a key metabolic regulator, in WAT, but not in skeletal muscle and brown adipose tissue. The increased expression of the downstream mitochondrial gene, Cox4i1, was also suggestive of the potential metabolic alteration in WAT by DPP4 inhibitors. We are the first to demonstrate that pharmacological DPP4 inhibition by evogliptin directly causes fat loss in established obese mice. In contradistinction to exenatide, the fat-loss effect of DPP4 inhibitor is partly attributed to enhanced energy expenditure along with metabolic changes in WAT. These results provide insight into the regulation of energy storage in WAT caused by DPP4 inhibition.
Introduction
Obesity is closely related to insulin resistance and type 2 diabetes because adipose tissue, which secretes various adipokines with autocrine and paracrine action, is central to the control of energy storage and whole body insulin sensitivity [1]. Recently, dipeptidyl peptidase 4 (DPP4; also known as CD26, EC3.4.14.5) was identified as a novel adipokine potentially linking obesity to metabolic syndrome [2]. DPP4 has gained considerable interest as an antidiabetic target for lowering blood glucose levels; inhibition of DPP4 enzyme activity increases endogenous intact glucagon-like peptide-1 (GLP-1), thereby stimulating insulin secretion which subsequently lowers blood glucose. Therefore, multiple DPP4 inhibitors have been developed for treating type 2 diabetes [3–4]. Although these DPP4 inhibitors have been reportedly neutral with regards to their impact on body weight in humans [3, 5], their effects on body composition have not yet been reported. Animal studies have revealed conflicting results on the effect of DPP4 inhibitors on body weight. In one study, DPP4 inhibitor reduced fat pad weight and adipocyte size in weight gaining mice [6], whereas other studies showed no changes in body weight [7–10]. Meanwhile, there are no reports, to our knowledge, investigating the effects of DPP4 inhibitors on the weight loss and whole body composition in established obese mice.
GLP-1 receptors also exist on the surface of adipose tissue and their activation can cause adipogenesis [11] but enhance lipolysis [12]. GLP-1 analogues have shown to reduce body weight by restricting caloric intake [13–15]. In contrast to GLP-1 analogues and genetic deficiency of DPP4 [16], DPP4 inhibitors do not affect food consumption [8, 17], suggesting its effects in energy homeostasis may be governed by different mechanisms of action. Increased GLP-1 action by pharmacological DPP4 inhibition partly accounts for reduced adiposity in weight-gaining mice through improving lipid metabolism [18]. Due to the limited secretion of GLP-1, plasma levels of endogenous GLP-1 enhanced by inhibiting DPP4 are also limited, yet a much higher plasma level of exenatide, a GLP-1 analogue, is needed for body fat loss than for glucose control. Thus it has been unclear whether the effect of DPP4 inhibitors on reducing adiposity can be fully explained by increased circulating GLP-1 levels.
There is very limited information about the direct effects of DPP4 inhibitors on the adipokine action of DPP4 [2], independent of GLP-1 action. DPP4 is highly expressed in kidneys and also exists in various types of adipose tissue such as visceral and to a lesser extent, subcutaneous fat tissue [2, 18]. Adipocytic DPP4 expression increases according to adipocyte differentiation [19] with a positive correlation between plasma DPP4 and body weight [20–21]. This evidence provides a potential strong linkage between DPP4 and adiposity. Although DPP4-deficient mice with at least 6-fold higher ambient plasma GLP-1 levels had less body weight and fat mass due to the increased energy expenditure and reduced food intake when fed on a high-fat diet [16], GLP-1 dependent or independent effects on adiposity have not been dissected yet. Fukuda and colleagues thereafter hypothesized that energy dissipation in muscle can be the potential underlying mechanism of reduced weight gain by DPP4 inhibition after a 10-week long-term treatment [22]. However, because observed effects on fat tissue in long-term studies can be confounded by multiple factors by way of both direct and indirect actions, our understanding of the direct effects of DPP4 inhibitors on fat tissue has remained nebulous.
Herein we investigated the direct effects of our novel DPP4 inhibitor, evogliptin [17, 23–24], on body composition and adiposity in an established obese mice model. Additionally, the effects of evogliptin were compared with those of exenatide, a GLP-1 analogue.
Materials and Methods
Materials
Evogliptin ((R)-4-[(R)-3-amino-4-(2, 4, 5-trifluorophenyl)butanoyl]-3-(t-butoxymethyl) piperazin-2-one, purity > 97.0%) L-tartrate salt was synthesized in house [24]. Sitagliptin phosphate and vildagliptin were purchased from Jiacheng-Chem (Hangzhou, China). Saxagliptin hydrate and linagliptin were products of APIchem (Shanghai, China) and Nanjing Chemlin (Nanjing, China), respectively. Gly-Pro-7-amido-4-methylcoumarin (Gly-Pro-AMC) as a substrate for the DPP4 enzyme and exenatide were obtained from Merck (Frankfurt, Germany) and American Peptide (Sunnyvale, CA), respectively. All other chemicals unless otherwise specified, were purchased from Sigma-Aldrich (St. Louis, MO).
Ethics statement
All experiments were performed in compliance with Korean legislation under the Laboratory Animal Act 2009 and were approved by the Institutional Animal Care and Use Committee of Dong-A ST Research Institute. Male C57BL/6J mice at age of 6 weeks were obtained from Dae Han Biolink (Eumseong, Korea). Mice were maintained at 23 ± 2°C on a 12:12-h light-dark cycle (lights on 0700 –1900h) and housed 2 animals per cage. Mice were allowed free access to food and water.
High-fat diet-induced obese (HF-DIO) mice studies
Seven-week-old mice were fed a high-fat diet (60% fat and 20% carbohydrates [kcal 100 g-1], D12492, Research Diets, New Brunswick, NJ) ad libitum. After 14 weeks on a high-fat diet, the mice were housed two animals per cage and were individually acclimated in metabolic cages for 1 or 2 weeks. As a lean control, age-matched male C57BL/6J mice were kept on a normal chow diet. Each repeated dosing study is summarized in Table 1.
Table 1. Study design.
| Duration of treatment | Dosing route/interval | Drug | Daily dose | Outcome measures | ||
|---|---|---|---|---|---|---|
| Study 1 | ||||||
| 4 weeks | Twice daily by oral gavage | Evogliptin | 60 mg kg-1 | body weight, plasma GLP-1, | ||
| Sitagliptin | 300 mg kg-1 | plasma insulin, blood glucose | ||||
| Vildagliptin | 200 mg kg-1 | |||||
| Saxagliptin | 60 mg kg-1 | |||||
| Linagliptin | 10 mg kg-1 | |||||
| Study 2 | ||||||
| 2 weeks | Once daily s.c. inj. | Exenatide | 30 μg kg-1 | body composition, plasma DPP4 activity, | ||
| Drug-diet mixture | Evogliptin | 0.027% | 20 mg kg-1 | food consumption, | ||
| 0.081% | 60 mg kg-1 | plasma TG, histology of WAT | ||||
| 0.27% | 200 mg kg-1 | |||||
| Study 3 | ||||||
| 2 weeks | Once daily s.c. inj. | Exenatide | 30 μg kg-1 | body composition, energy expenditure, | ||
| Drug-diet mixture | Evogliptin | 0.081% | 60 mg kg-1 | plasma insulin, adipokines, | ||
| gene expression, rectal temperature | ||||||
Study 1
HF-DIO mice were allocated to each group (8 animals/group) according to body weight and blood glucose levels. We selected preliminary doses of evogliptin, sitagliptin, vildagliptin, saxagliptin, and linagliptin in HF-DIO mice based on the doses used elsewhere [17, 25–27, 10]. To confirm their pharmacodynamic effects, plasma DPP4 inhibition was assessed 12 h after single oral administration of each drug. Due to the observed short half lives of sitagliptin and evogliptin in mice (unpublished data), all the drugs were administered twice daily by oral gavage and given for 4 weeks using 0.5% (w/v) methylcellulose as a vehicle. Body weight and diet consumption were measured weekly. Before the last administration, fed blood glucose levels were measured from the tail vein blood using a glucometer (AccuChek Active, Roche Diagnostics, Mannheim, Germany). Twelve hours after the last administration, mice were euthanized in the non-fasted state and fed GLP-1 and insulin levels were determined. Blood for determining active GLP-1 was collected in heparinized tubes with sitagliptin (final 1 mM) to minimize the degradation of GLP-1.
Study 2
HF-DIO mice were allocated to each group (8 animals/group) according to body weight and whole body fat mass. To determine the acute effects on body composition, treatment period was shortened to 2 weeks. In this study, evogliptin at three doses targeting 20, 60, and 200 mg kg-1 day-1 for determining dose response and exenatide at the moderate dose were compared. Considering the half-life (2–3 h) of exenatide in mice and the minimum effective dose of 1 μg kg-1 based on oral glucose tolerance test in mice after single subcutaneous injection of exenatide (unpublished data), we designated 30 μg kg-1 day-1 as the moderate dose. To reduce the stress caused by animal handling, evogliptin was given to mice as a drug-diet admixture (0.027, 0.081, or 0.27% (w/w)) and exenatide was subcutaneously given to mice once daily. Mice which did not receive exenatide received subcutaneous injections of saline. Control and exenatide-treated mice were fed on a repelleted diet without evogliptin. Diet consumption and body weight were measured daily for 2 weeks. Body composition was measured before and after treatment. After euthanasia in the non-fasted state, plasma was obtained for determining plasma DPP4 activity and triglycerides levels. Because epididymal fat mass was highly correlated with whole body fat mass (unpublished data; Pearson’s correlation coefficient = 0.949, P<0.0001, n = 60), epididymal white adipose fat pad (WAT) was isolated and immediately frozen for histology.
Study 3
HF-DIO mice were allocated to each group (8 animals/group) according to body weight and whole body fat mass. To explore the underlying mechanisms of fat loss, HF-DIO mice received either daily subcutaneous injections of 30 μg kg-1 day-1 of exenatide or saline with a high-fat diet with/without 0.081% (w/w) evogliptin. Two weeks after treatment, rectal temperature was determined and indirect calorimetry was performed. Body composition was measured before and after treatment. After euthanasia in the non-fasted state, plasma was obtained for determining plasma insulin and adipokine panel. Given that soleus muscle mass directly reflects metabolic change [28] and interscapular fat is representative of brown adipose tissue (BAT) and widely used to investigate the metabolic effects on BAT [29–30], soleus muscle, interscapular BAT, and epididymal WAT were isolated and immediately frozen for evaluation of gene expression.
Body composition analysis
Body composition was measured under conscious state using a Minispec LF90II NMR spectrometer (Bruker Optics, Ettlingen, Germany) before and after treatment. Lean body mass was obtained by subtracting fat mass from body weight.
Histological evaluation of adipose tissue
Epididymal adipose tissues were removed, prepared for 4 μm-thick microtome sections, and then mounted for hematoxylin-eosin staining. Images were obtained using an Axioskop2 Plus microscope (Carl Zeiss, Gottingen, Germany), and adipocyte area was measured using ImageJ (http://rsbweb.nih.gov/ij). After processing background of images, the cellular boundary line was automatically detected and manually compared to the original image. Then the area of each cell in the same area was automatically determined except for cells appearing partly at the edge of image frame. Three sections per animal were analyzed.
Determination of plasma parameters
Plasma DPP4 activity was measured using Gly-Pro-AMC as previously described [15]. Plasma triglycerides were determined using an automatic analyzer (Reflotron® Plus System, Roche, Mannheim, Germany). Plasma insulin was measured using a Milliplex Mouse Adipokine Panel assay service (Millipore, St. Charles, MO). Plasma active GLP-1 and leptin levels were measured using ELISA kits from LINCO Research (St. Charles, MO). An ELISA kit for high molecular weight adiponectin from Shibayaki (Shibukawa, Japan) was used for detecting biologically active form of adiponectin.
Indirect calorimetry
After the 2-week treatment, mice were transferred to a new cage to evaluate metabolic rate and accommodated under free access to food and water. Data were obtained for 21 h using a computer-controlled open-circuit indirect calorimetry system (Oxylet system; Panlab Harvard Apparatus, Barcelona, Spain) with airflow of 0.35~0.37 l min-1, and metabolic parameters such as oxygen consumption, carbon dioxide production and mean energy expenditure were automatically assessed according to manufacturer’s instructions. Respiratory quotient (Rq) was computed by dividing carbon dioxide production by oxygen consumption.
RT-PCR
Information of primers and probes used is listed in S1 Table. For mouse peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Ppargc1a) and hypoxia inducible factor 1-alpha (Hif1a), SYBR Green I Master Mix was used in a LightCycler 480 (Roche Applied Science, Indianapolis, IN). TaqMan probe method was used for mouse uncoupling protein 1 (Ucp1) and cytochrome c oxidase subunit 4 isoform 1 (Cox4i1). Data analysis was performed using standard curve method. Relative gene expression was presented after normalization by mouse ribosomal protein S3 (Rps3) expression (when fold difference = 2-ΔCt, an average fold change of < 2 and a maximal variability of < 6-fold between samples) which was validated as described elsewhere [31].
Statistical analysis
All data was expressed as means ± SE. Except for ANCOVA, statistical analyses were performed using SigmaStat® 2.0 (SPSS, Chicago, IL). Comparisons with baseline values and comparisons between two groups were analyzed using Student’s paired t-test and Student’s t-test, respectively. For comparisons of data involving more than three groups at the same time point, one-way analysis of variance (ANOVA) was used. For comparisons of data at different time points from the same animals among more than three groups, we used repeated measures (RM) two-way ANOVA. When ANOVA indicated a significant difference, the differences were evaluated further using Dunnett’s multiple comparison test. The adipocyte size distribution was analyzed using the Mann—Whitney rank sum test between two groups due to data skewness. P values < 0.05 were considered significant. One-way analysis of covariance (ANCOVA) was performed using SigmaPlot® 13.0 (Systat Software, Inc., San José, CA).
Results
Weight-loss effect of DPP4 inhibitors in HF-DIO mice
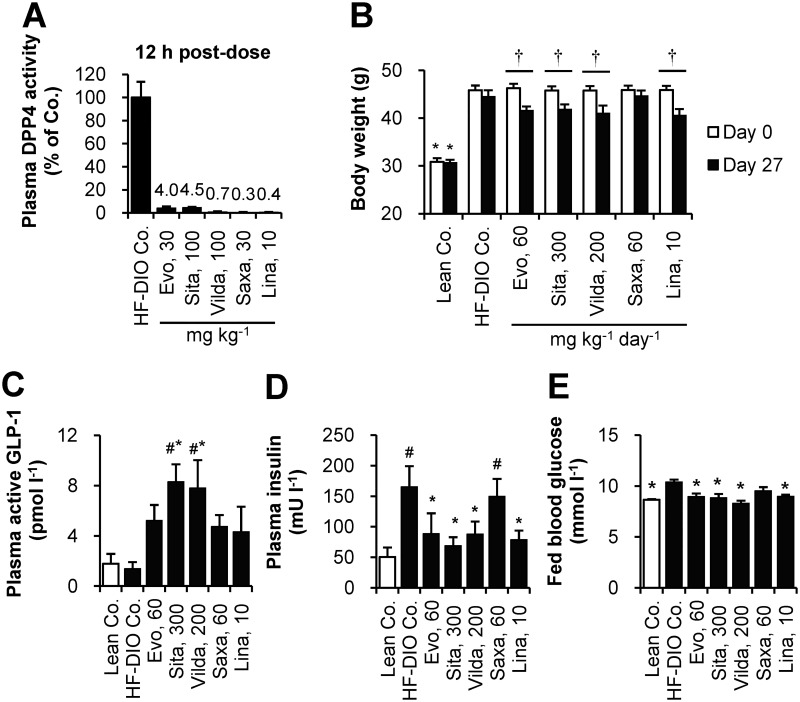
For dose selection, plasma DPP4 activity was assessed 12 h after single oral administration of each drug at a preselected dose in HF-DIO mice. All the DPP4 inhibitors showed over 90% inhibition of plasma DPP4 activity at the doses tested (Fig 1A). Given that DPP4 inhibition over 80% was considered clinically meaningful [32], those tested doses were selected for the next study phase except for sitagliptin whose dose was increased from 100 mg kg-1 to 150 mg kg-1 twice daily for full efficacy based on a reported target daily dose of 280 mg kg-1 [25].
Fig 1. Body weight-loss effect of DPP4 inhibitors in established obese mice after 4-week treatment.
(A) To select each dosage for twice daily administration, plasma DPP4 inhibition was assessed 12 h after a single oral administration in HF-DIO mice. (B-E) After 4 week-administration of evogliptin, sitagliptin, vildagliptin, saxagliptin, or linagliptin twice daily by oral gavage in HF-DIO mice, (B) body weight, (C) fed plasma levels of active GLP-1, (D) insulin, and (E) fed blood glucose levels were determined (n = 5/group). #, P < 0.05 vs. lean control; *, P < 0.05 vs. HF-DIO control by one-way ANOVA; †, P < 0.05 vs. the baseline by RM two-way ANOVA.
After four weeks of treatment to HF-DIO mice, body weight-loss effects were observed and statistically equivalent among four DPP4 inhibitors except for saxagliptin (Fig 1B). Cumulative diet consumption did not differ among treatment groups (data not shown). As expected, plasma DPP4 inhibition caused an increase of active GLP-1 levels, with the highest levels observed in sitagliptin or vildagliptin treatment groups at the doses tested (Fig 1C). However, basal insulin levels similarly declined in evogliptin, sitagliptin, vildagliptin and linagliptin-treated mice, indicative of improved insulin sensitivity. These results supported the significant reduction in blood glucose levels by four DPP4 inhibitors (Fig 1E). The changes in basal insulin and blood glucose were concordant with body weight loss across all drugs except saxagliptin whose effects on weight were not significant despite similar plasma DPP4 inhibition and increase of active GLP-1 levels.
Acute effect of evogliptin on whole body composition in HF-DIO mice
Next, we compared treatment response to evogliptin and exenatide to examine whether the observed weight loss was directly due to DPP4 inhibition. To confirm a dose response, evogliptin, as a drug-diet mixture, was given to mice at three doses including lower and higher dosages than the dose of evogliptin (60 mg kg-1 day-1) which was tested in the 4-week study. Mean administered doses were 21.0±1.3, 62.5±2.0, and 202.7±6.2 mg kg-1 day-1 for 0.027, 0.081, and 0.27% (w/w), respectively.
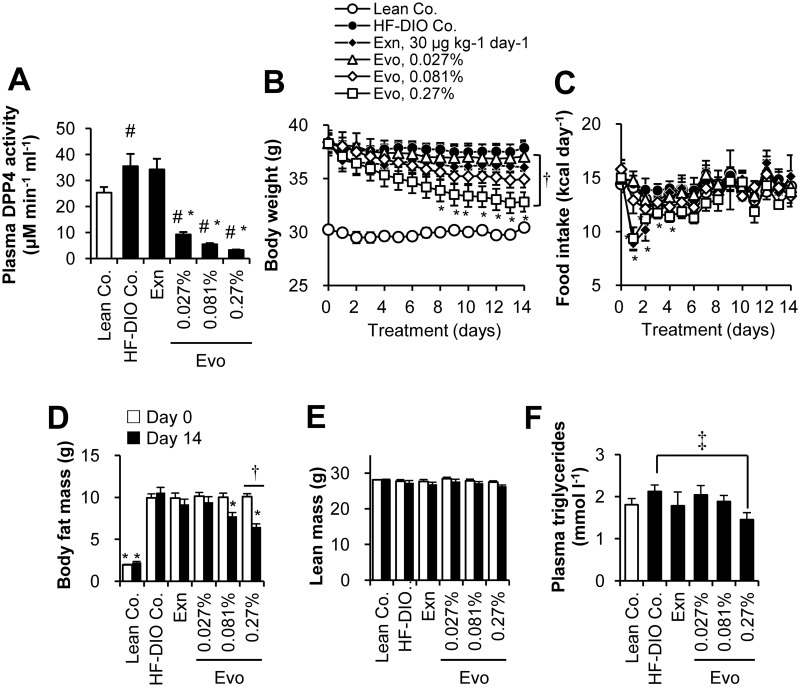
After 2-week treatment, evogliptin caused dose-dependent inhibition of plasma DPP4 activity (74.0%, 84.5%, and 90.7% at doses of 0.027%, 0.081%, and 0.27%, respectively)(Fig 2A). Evogliptin treatment at 0.27% continuously decreased body weight with a significant reduction of 13.3% vs. HF-DIO control (Fig 2B; P < 0.05 by RM two-way ANOVA). However, evogliptin at 0.081% and exenatide treatments did not show significant weight reductions (-7.74% and -4.77% vs. HF-DIO control, respectively). Furthermore, a return to a lean mouse phenotype was not observed with any of the treatments.
Fig 2. Two-week treatment of evogliptin decreased whole body fat mass in obese mice.
After HF-DIO mice were treated with exenatide (30 μg kg-1, once daily, s.c.) or evogliptin at 0.027%, 0.081%, or 0.27% (w/w) for 2 weeks, (A) plasma DPP4 activity, (B) body weight, (C) energy intake, (D) whole body fat mass, (E) lean body mass, and (F) plasma triglycerides levels were determined (n = 8/group). #, P < 0.05 vs. lean control and *, P < 0.05 vs. HF-DIO control by one-way ANOVA; †, P < 0.05 vs. the baseline by RM two-way ANOVA; ‡, P < 0.05 vs. HF-DIO control by Student’s t-test.
Daily energy intake acutely decreased on day 1 by 0.27% evogliptin and exenatide alone and then recovered 3–5 days afterwards (Fig 2C). However the pattern of energy intake of the same animals was unchanged and neither was cumulative diet consumption (data not shown). The body weight in 0.081% and 0.27% evogliptin-treated mice continuously declined throughout the treatment period regardless of energy intake; meanwhile, weight-loss by exenatide treatment was maintained after a rapid drop on the second day of administration, implying the effects of either drug were driven by different pharmacodynamics.
As for whole body fat mass, evogliptin treatment dose-dependently lowered fat mass with the greatest reduction achieved at the dose of 0.27% (-38.5% vs. HF-DIO Co., P < 0.05 by RM two-way ANOVA)(Fig 2D, S2 Table). Although there was no statistically significant difference in fat-loss trend (P > 0.05 by RM two-way ANOVA), evogliptin 0.081% treatment exhibited a greater reduction in fat mass than exenatide (-26.6% vs -13.0%, respectively) when compared to HF-DIO control after 2 week-treatment (P = 0.002 and P = 0.147 by one-way ANOVA, respectively). Lean body mass was not significantly altered by any treatments (P = 0.389)(Fig 2E). Plasma triglycerides levels were lower only at 0.27% evogliptin-treated mice relative to HF-DIO Co. (P = 0.012 by Student’s t-test)(Fig 2F). These results indicated an acute fat-loss effect due to DPP4 inhibition.
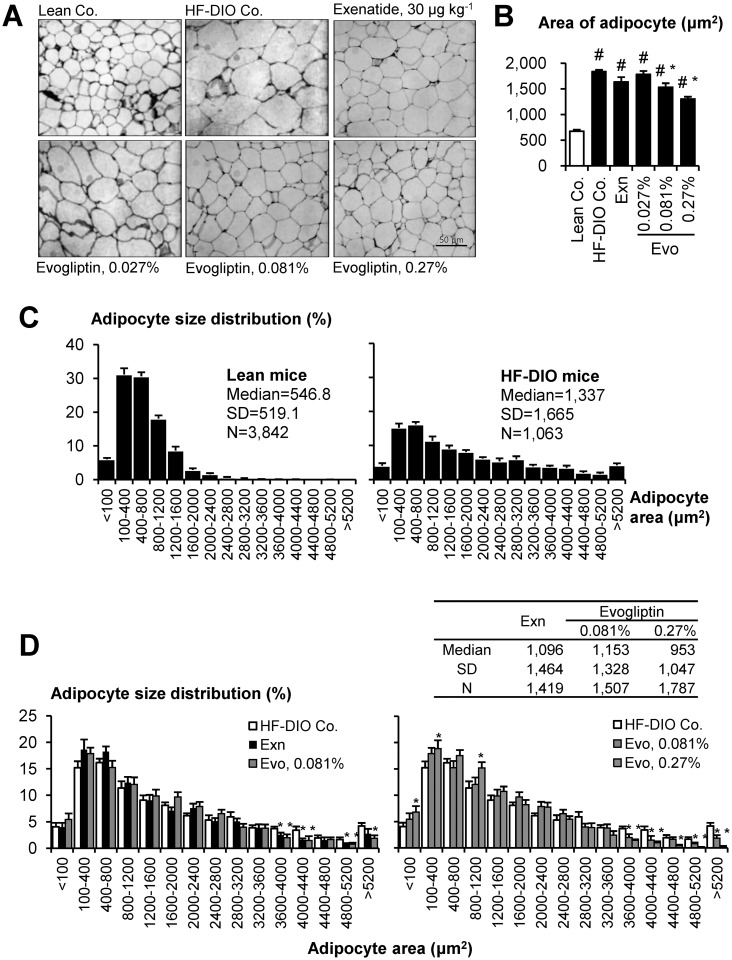
Acute effects on adiposity in HF-DIO mice
We histologically analyzed adipocyte size distribution in epididymal fat section. HF-DIO mice typically exhibited prominent adipocyte hypertrophy (Fig 3A and 3B) and the size distribution was significantly right-shifted in HF-DIO mice compared to that in lean mice (P < 0.05, Mann—Whitney rank sum test), indicating an increased proportion of large adipocytes (Fig 3C). Evogliptin treatment dose-dependently reduced mean adipocyte size and left-shifted its size distribution (Fig 3D). It was a noteworthy finding that the percentage of large size adipocytes > 3 600 μm2 decreased significantly in a dose-dependent manner. Intriguingly, when the 2-week evogliptin treatment significantly reduced whole body fat mass at 0.081%, mean adipocyte size and the median value of adipocyte size distribution was significantly improved compared to that of the HF-DIO control (P < 0.05, Mann—Whitney rank sum test). The adipocyte size distribution was comparable between exenatide- and 0.081% evogliptin-treated groups. Compared to exenatide, evogliptin treatment at 0.27% showed a similar reduction in initial diet consumption but was superior in amelioration of adiposity to exenatide (P < 0.05, Mann—Whitney rank sum test), which also suggested an additional fat-loss inducing mechanism of action by evogliptin treatment.
Fig 3. Evogliptin treatment reduced adipocyte size and distribution.
HF-DIO mice were treated with exenatide (30 μg kg-1, once daily, s.c.) or evogliptin at 0.027%, 0.081%, or 0.27% (w/w) for 2 weeks. The effects on adipocytes from epididymal fat tissues were histologically determined (three sections/animals, eight animals/group). (A) Representative images (original magnification, ×200), (B) mean adipocyte areas and (C-D) percentile distribution of adipocyte size were presented. #, P < 0.05 vs. lean control and *, P < 0.05 vs. HF-DIO control by one-way ANOVA.
Evogliptin increased energy expenditure unlike exenatide
The effects on glucose metabolism were compared at the end of 2-week treatment. Both evogliptin at 0.081% and exenatide did not significantly improve the insulin sensitivity (11–13%, P > 0.09 by One-way ANOVA) (S1A and S1B Fig). Notably, while fat-loss effects by both treatments were comparable, reduction of 6 h-fasted blood glucose was doubled in exenatide-treated mice compared to 0.081% evogliptin (-29.5% vs. -11.9%, respectively; P < 0.05)(S1C Fig). Endogenous active GLP-1 levels were not altered by exenatide treatment (S1D Fig).
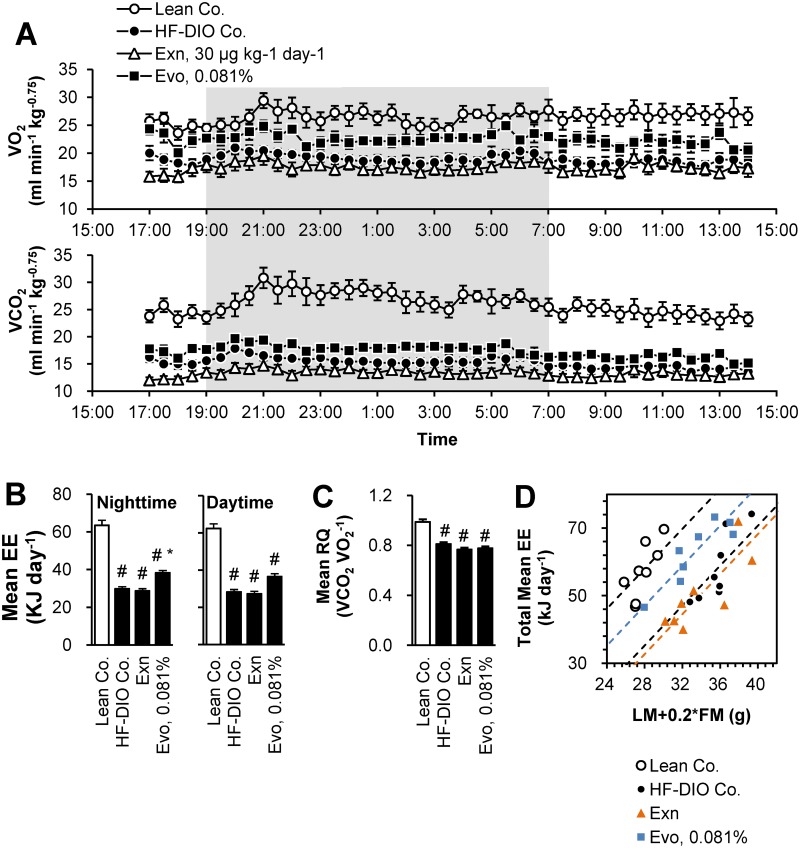
Because restricted energy intake was not a cause of fat-loss by 0.081% evogliptin, we examined the acute effects on energy expenditure. In Study 3, when the changes in body composition of Study 2 were recapitulated (S2 Fig), 0.081% evogliptin treatment enhanced oxygen consumption, carbon dioxide production (Fig 4A) and energy expenditure especially at nighttime (Fig 4B). Meanwhile, exenatide treatment had no effect on energy expenditure. Neither evogliptin nor exenatide treatment altered the respiratory quotient (Fig 4C). Because energy expenditure can be altered by both lean body mass and fat mass, mean total energy expenditure was examined according to body composition (S3 Fig). When the effect of treatment on energy expenditure was comprehensively analyzed by ANCOVA using [lean mass+0.2×fat mass] as a covariate as previously described [33], the equal slope assumption passed indicating no interactions between each treatment and covariates. Interestingly, adjusted means of energy expenditure were greater in evogliptin-treated mice than HF-DIO control or exenatide-treated mice. Moreover, in this equal slope model (R2 = 0.717, adjusted R2 = 0.675), the y-intercept of evogliptin treatment was greater than those of HF-DIO control and exenatide (-35.8 vs. -47.6 and -50.2, respectively; P < 0.001, ANCOVA) (Fig 4D), indicative of higher energy expenditure in evogliptin treated-mice. However, there were no differences in core body temperature (S4 Fig). This result suggested that part of evogliptin-induced fat loss may be attributed to the enhanced metabolic rate which may not be mediated by increased active GLP-1.
Fig 4. Evogliptin increased energy expenditure unlike exenatide.
HF-DIO mice were treated with exenatide (30 μg kg-1, once daily, s.c.) or evogliptin at 0.081% (w/w) for 2 weeks. Metabolic parameters were measured using an indirect calorimetry system; (A) Oxygen consumption (VO2) and carbon dioxide production (VCO2), (B) mean energy expenditure (EE), (C) respiratory quotient (Rq), and (D) a plot of individual data of total mean EE vs. (lean mass + 0.2 × fat mass) as a covariate (n = 8/group). #, P < 0.05 vs. lean control and *, P < 0.05 vs. HF-DIO control by one-way ANOVA.
Effects on plasma parameters
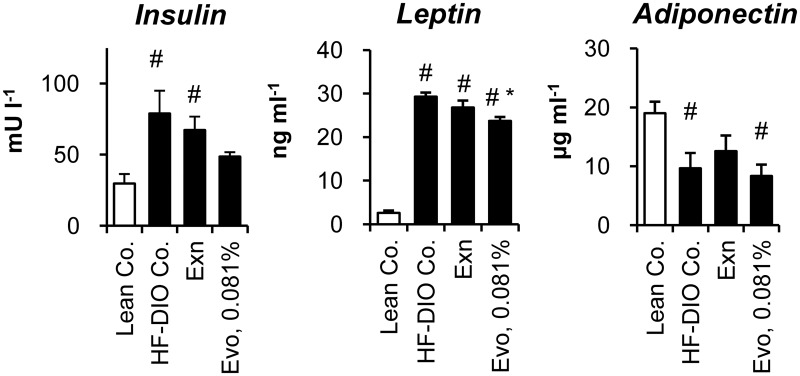
Adipose tissue is an endocrine organ secreting various cytokines that regulate whole body metabolism, as well as a highly active metabolic organ for storing surplus energy. Therefore, we evaluated the effects of each treatment on the plasma levels of insulin and five cytokines. Increased plasma levels of insulin and leptin denoted insulin resistance and leptin resistance in HF-DIO mice (Fig 5). Both exenatide and 0.081% evogliptin alone did not significantly improve hyperinsulinemia in HF-DIO mice (P = 0.535 and P = 0.071 by One-way ANOVA, respectively), which corroborated the results of the insulin tolerance test. Meanwhile, only 0.081% evogliptin treatment led to a significant reduction of plasma leptin. The physiologically active form of adiponectin stayed lower in obese mice than lean mice, while neither exenatide (P = 0.690) nor 0.081% evogliptin altered adiponectin levels.
Fig 5. Effects on plasma insulin and adipokines.
After 2-week treatment of exenatide (30 μg kg-1, once daily, s.c.) or evogliptin at 0.081% (w/w) in HF-DIO mice, fed plasma concentrations of (A) insulin, (B) leptin and (D) high molecular weight (HMW) adiponectin were assessed (n = 8/group). #, P < 0.05 vs. lean control and *, P < 0.05 vs. HF-DIO control by one-way ANOVA.
Plasma levels of plasminogen activator inhibitor-1, another adipokine, and C-reactive protein, as an inflammatory indicator, were higher in obese mice than lean mice, yet these levels were not significantly altered by any treatments (S5 Fig). Plasma levels of tumor necrosis factor α and monocyte chemoattractant protein-1 simultaneously analyzed using a Milliplex kit were under the minimum detection limit of 2.5 pg ml-1 and 7.8 pg ml-1, respectively (data not shown).
Taken together, these results suggested that reduced adiposity after 2-week treatment of evogliptin was not attributed to alteration of adipokines levels or inflammation.
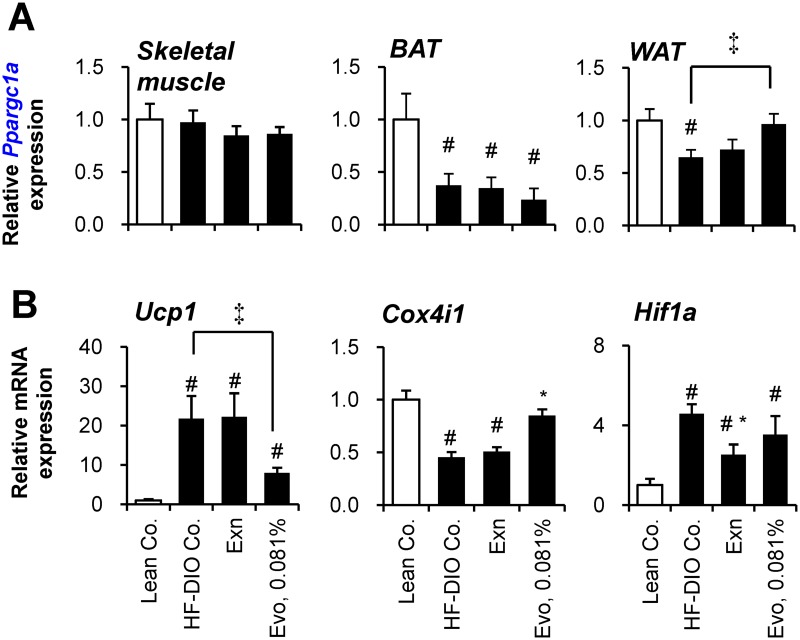
Evogliptin increased Ppargc1a gene expression in WAT
Increased energy expenditure can be explained by enhanced metabolic rate in skeletal muscle [22] or adipose tissues [34]. Given that PGC1α (Ppargc1a) is a key regulator involved in lipid metabolism in various peripheral tissues including muscle [35–36] and adipocyte [37–38], we presumed that evogliptin regulates PGC1α expression in its target organ.
To determine the target organ of evogliptin, we evaluated the gene expression levels of Ppargc1a in skeletal muscle, BAT and WAT with regard to energy homeostasis. Ppargc1a expression in skeletal muscle was not altered by a high-fat diet or any treatments (Fig 6A). In BAT, Ppargc1a expression was significantly decreased by a high-fat diet, but not affected by any treatments. However, in WAT, evogliptin treatment significantly restored Ppargc1a gene expression which was normally suppressed by feeding a high-fat diet, suggesting evogliptin’s influence on metabolic changes in WAT.
Fig 6. Effect of evogliptin treatment on the gene expression in muscle and adipose tissues.
After 2–week administration of exenatide (30 μg kg-1, once daily, s.c.) or evogliptin at 0.081% (w/w) in HF-DIO mice, (A) PGC-1α (Ppargc1a) gene expression in soleus muscle and two types of fat tissues (interscapular fat and epididymal fat for brown adipose tissue and white adipose tissue, respectively), and gene expression levels of (B) UCP1 (Ucp1), (C) COXIV (Cox4i1) and HIF-1α (Hif1a) were assessed in white adipose tissue by realtime qPCR method (n = 8/group). #, P < 0.05 vs. lean control and *, P < 0.05 vs. HF-DIO control by one-way ANOVA; ‡, P < 0.05 by Student’s t-test.
Given that PGC1α stimulates thermogenesis via inducing UCP1 expression, gene expression of UCP1 (Ucp1) was first examined. Unexpectedly, evogliptin treatment reduced gene expression of Ucp1 in WAT (Fig 6B), suggesting an alternative pathway may be responsible. Because PGC1α enhances metabolism through up-regulating mitochondrial biogenesis in adipose tissue, the effect of evogliptin on the expression levels of a putative mitochondrial gene, Cox4i1, was also examined. High-fat feeding reduced mitochondrial Cox4i1 gene expression, but evogliptin treatment, unlike exenatide, restored gene expression of Cox4i1. Conversely, exenatide, unlike evogliptin, reduced Hif1a gene expression enhanced by a high-fat diet. Evogliptin showed a differential reduction in adipocyte gene expression of Pparg2 contrary to Srebp1c and Dpp4 (S6 Fig). Given that PPARγ2 is a typical adipogenic transcription factor, we examined whether evogliptin directly inhibited the differentiation of human adipocytes. In this assay, evogliptin neither induced adipogenesis per se nor antagonized the adipocyte differentiation induced by rosiglitazone, a typical PPARγ agonist, along with no direct activation of human and mouse PPARα/γ (S6 Fig).
These results indicated that, compared to exenatide, 0.081% evogliptin treatment differentially influenced the lipid metabolism in WAT which may serve the potential target for evogliptin.
Discussion
Although various gliptins are known to be neutral on body weight in type 2 diabetic patients, the effect on body fat mass has not been fully elucidated in humans and animals yet. To the best of our knowledge, this is the first report on the body weight-loss effect through pharmacologically inhibiting DPP4 activity in established obese mice. We also demonstrated that our novel DPP4 inhibitor, evogliptin, in contrast to exenatide, directly reduced whole body fat mass by enhanced energy expenditure at a clinically relevant dose achieving over 80% plasma DPP4 inhibition for 24 hours. Our findings proposed the possibility that the target organ of evogliptin treatment may be WAT.
An imbalance between energy intake and expenditure causes weight gain or loss. Exenatide produced an anorexic effect at three- to ten-fold higher doses than that for the glucose-lowering effect [6, 39]. On the other hand, the maximum increase in endogenous intact GLP-1 levels enhanced through inhibiting DPP4 activity was limited due to restricted secretion. While chronic treatment with sitagliptin led to peak active GLP-1 levels of around 50 pM after a meal challenge which rapidly declined within 2–3 h in HF-DIO mice [40], evogliptin 0.081% led to mean ambient GLP-1 levels of 10.3±1.01 pM (3.7 fold greater compared to HF-DIO control). This evidence supports the notion that the endogenous GLP-1 levels increased by DPP4 inhibition are insufficient to produce the anorexic effect of GLP-1 signaling.
In this study, the glucose-lowering effect of exenatide at 30 μg kg-1 was superior to 0.081% evogliptin, suggesting that the fat-loss effect of evogliptin may not be a result of improvement in glucose metabolism. Among adipokines, 2-week evogliptin treatment significantly lowered only plasma leptin levels. Leptin acts as a negative regulator of energy intake on hypothalamus [41] and plasma leptin levels were lowered by 0.081% evogliptin at the end of treatment. Furthermore, evogliptin at 0.081% did not alter daily diet consumption, but enhanced energy expenditure. These results cannot be fully explained by the improvement of leptin resistance [42], although we did not determine levels of leptin receptors. Thus we concluded that reduction in plasma leptin may be a result of reduced adiposity rather than direct causality with DPP4 inhibition.
We suggested that one possible mechanism in evogliptin-induced fat loss is increased energy dissipation. While it is clear that exenatide decreases food intake by increasing satiety and anorexic effect, the effect of exenatide on energy expenditure was controversial. It was reported that exenatide reduced physical activity, oxygen consumption, and energy expenditure in rodents [43–44]. While some clinical researchers have suggested the possibility of increased energy expenditure [45–47], a recent clinical study, which investigated energy expenditure in obese subjects after exenatide administration, disproved this theory [48]. Goldsmith et al. reported that sitagliptin induced energy expenditure after 10 week-treatment in diabetic mice, which was partially sustained even in GLP-1 receptor-deficient mice [49]. Therefore, this data supports the notion that increased energy expenditure by evogliptin may be partly GLP-1 independent.
In humans, vildagliptin tended to increase energy expenditure and augmented lipid mobilization and fatty acid oxidation after 7-day treatment, suggesting a potential contribution of GLP-1-mediated activation of the sympathetic nervous system [50]. Although several studies reported that DPP4 inhibitors prevented weight gain [6, 18, 22, 51–52], the underlying mechanisms remained not fully understood. Teneligliptin also was reported to increased energy expenditure in weight-gaining mice [22]. The proposed mechanism therein was an increase of energy dissipation in skeletal muscle. Enhanced energy expenditure can be explained by increased metabolic rate and/or thermogenesis in adipose tissues and skeletal muscle [36, 53]. Herein evogliptin treatment did not induce thermogenesis but increased metabolic rate. We discovered that the key metabolic factor was altered in WAT along with alteration of its downstream target gene, but not in muscle or BAT. Fat tissue accounts for a large proportion of body mass (15–30% in healthy subjects and more than 40% in obese subjects) [54]. Moreover, the evidence that reduced gene expression of AMPKα, PGC1α and PPARα was associated with decreased oxidative capacity in WAT [55] and that chronic activation of adipocyte AMP-activated protein kinase (AMPK) increased energy dissipation through enhancing fatty acid oxidation in adipose tissue [56], supports the hypothesis that metabolic change in WAT can contribute to energy expenditure. Nevertheless, further studies are needed to explain how evogliptin up-regulates Ppargc1a expression in adipose tissue and whether evogliptin affects PGC1α activation state.
PGC-1α regulates thermogenesis and mitochondrial biogenesis through increasing UCPs expression and augmenting mitochondrial DNA replication in adipocytes [57]. Unexpectedly, evogliptin caused a significant decrease of Ucp1 expression in WAT, but this result was partly in line with the unaffected rectal temperature in the evogliptin-treated mice. On the other hand, one of mitochondrial components, Cox4i1 expression, which correlates with increased Ppargc1a expression and was not seen with exenatide, was increased by evogliptin treatment.
The 2-week evogliptin treatment significantly ameliorated adipocyte size distribution. Adipocyte size can reflect the metabolic function of adipocytes [58]. It is yet unknown which absolute range of adipocytes sizes is metabolically harmful and no feasible method exists to directly compare adipocyte size from different studies due to discrepancies in sample preparation methods [59]. However, larger adipocytes are known to be metabolically more harmful than smaller ones [58]. In our data, the area of greater than 99% of the adipocytes from lean mice were <2,400 μm2. Interestingly, evogliptin treatment significantly reduced the portion of large adipocytes (>3 600 μm2), implying the metabolic improvement.
Our study has some limitations. The maximum fat-loss effect of evogliptin has not yet been verified. We previously reported the weight-loss effect of cannabinoid (CB) 1 receptor antagonist (SR 141716A also known as rimonabant which had an approval for anti-obesity drug) in a similar mice model [60]. Rimonabant achieved maximum efficacy 2 to 3 weeks post-dose according to the doses tested. However, the appropriate time for maximum efficacy can differ based on the mode of action involved in the fat-loss effects. We speculate that the fat-loss effect of evogliptin along with improvement in glucose and lipid metabolism, could be amplified with longer-term treatment. Additionally, we used a high-fat diet containing relatively lower carbohydrate, supplying 60% calories from fat. In mice fed on a high-fat diet with lower caloric contribution from fat (45%) with increased sucrose, the contribution of glucose metabolism to fat storage may be more significant.
In conclusion, our findings suggest the beneficial effects of evogliptin at a clinically relevant dose, on whole body composition, particularly whole body fat loss in an established obese mice model. This study verified fat loss by DPP4 inhibition which is likely mediated by increased energy expenditure and alteration in white adipose tissue metabolism. Our findings provide new insight into the role of DPP4 inhibitors in adiposity.
Supporting Information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We would like to thank Mr. Young E. Lee, M.D., M.B.A. (UC Irvine Medical Center, CA, USA) for his careful proofreading and language help during the revision of the present paper.
Abbreviations
- AMC
7-amido-4-methylcoumarin
- AMPK
AMP-activated protein kinase
- ANCOVA
analysis of covariance
- ANOVA
analysis of variance
- AUC
the area under the time-blood glucose levels curve
- BAT
brown adipose tissue
- COX4 (Cox4il)
cytochrome c oxidase subunit 4 isoform 1
- DPP4 (Dpp4)
dipeptidyl peptidase 4
- GLP-1
glucagon-like peptide-1
- EE
energy expenditure
- HIF-1α
hypoxia-inducible factor -1α
- HF
high-fat diet
- HF-DIO
high-fat diet-induced obese
- PPARγ2
peroxisome proliferator-activated γ2
- PGC1α (Ppargc1a)
peroxisome proliferator-activated receptor-γ coactivator
- Rq
respiratory quotient
- SREBP1c (Srebp1c)
sterol response element-binding protein 1c
- UCP1 (Ucp1)
uncoupling protein 1
- Rsp3
ribosomal protein S3
- VCO2
carbon dioxide production VO2, oxygen consumption
- WAT
white adipose tissue
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was funded by Dong-A ST Co., Ltd. The funder provided support in the form of salaries for all authors [YNC, THK, MKK, CYS, IHJ, YSS, and MHS], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1. Guillet C, Masgrau A, Walrand S, Boirie Y. Impaired protein metabolism: interlinks between obesity, insulin resistance and inflammation. Obes Rev. 2012; 13 Suppl 2: 51–57. 10.1111/j.1467-789X.2012.01037.x [DOI] [PubMed] [Google Scholar]
- 2. Lamers D, Famulla S, Wronkowitz N, Hartwig S, Lehr S, Ouwens DM, et al. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 2011; 60(7): 1917–1925. 10.2337/db10-1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scheen AJ. A review of gliptins in 2011. Expert Opin Pharmacother. 2012; 13(1): 81–99. 10.1517/14656566.2012.642866 [DOI] [PubMed] [Google Scholar]
- 4. Cahn A, Raz I. Emerging gliptins for type 2 diabetes. Expert Opin Emerg Drugs. 2013; 18(2): 245–258. 10.1517/14728214.2013.807796 [DOI] [PubMed] [Google Scholar]
- 5. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007; 298(2): 194–206. [DOI] [PubMed] [Google Scholar]
- 6. Lamont BJ, Drucker DJ. Differential antidiabetic efficacy of incretin agonists versus DPP-4 inhibition in high fat fed mice. Diabetes. 2008; 57(1): 190–198. [DOI] [PubMed] [Google Scholar]
- 7. Hamamoto S, Kanda Y, Shimoda M, Tatsumi F, Kohara K, Tawaramoto K, et al. Vildagliptin preserves the mass and function of pancreatic beta cells via the developmental regulation and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes. Diabetes Obes Metab. 2013; 15(2): 153–163. 10.1111/dom.12005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thomas L, Tadayyon M, Mark M. Chronic treatment with the dipeptidyl peptidase-4 inhibitor BI 1356 [(R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-yl methyl)-3,7-dihydro-purine-2,6-dione] increases basal glucagon-like peptide-1 and improves glycemic control in diabetic rodent models. J Pharmacol Exp Ther. 2009; 328(2): 556–563. 10.1124/jpet.108.143966 [DOI] [PubMed] [Google Scholar]
- 9. Flock G, Baggio LL, Longuet C, Drucker DJ. Incretin receptors for glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide are essential for the sustained metabolic actions of vildagliptin in mice. Diabetes. 2007; 56(12): 3006–3013. [DOI] [PubMed] [Google Scholar]
- 10. Kern M, Klöting N, Niessen HG, Thomas L, Stiller D, Mark M, et al. Linagliptin improves insulin sensitivity and hepatic steatosis in diet-induced obesity. PLoS One. 2012; 7(6): e38744 10.1371/journal.pone.0038744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Challa TD, Beaton N, Arnold M, Rudofsky G, Langhans W, Wolfrum C. Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J Biol Chem. 2012; 287(9): 6421–6430. 10.1074/jbc.M111.310342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vendrell J, El Bekay R, Peral B, Garcia-Fuentes E, Megia A, Macias-Gonzalez M, et al. Study of the potential association of adipose tissue GLP-1 receptor with obesity and insulin resistance. Endocrinology. 2011; 152(11): 4072–4079. 10.1210/en.2011-1070 [DOI] [PubMed] [Google Scholar]
- 13. Ostenson CG, Matthaei S, Reaney M, Krarup T, Guerci B, Kiljanski J, et al. Treatment outcomes after initiation of exenatide twice daily or insulin in clinical practice: 12-month results from CHOICE in six European countries. Diabetes Metab Syndr Obes. 2013; 6: 171–185. 10.2147/DMSO.S41827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flint A, Kapitza C, Zdravkovic M. The once-daily human GLP-1 analogue liraglutide impacts appetite and energy intake in patients with type 2 diabetes after short-term treatment. Diabetes Obes Metab. 2013; 15(10): 958–962. 10.1111/dom.12108 [DOI] [PubMed] [Google Scholar]
- 15. Elfers CT, Simmons JH, Roth CL. Glucagon-like peptide-1 agonist exendin-4 leads to reduction of weight and caloric intake in a rat model of hypothalamic obesity. Horm Res Paediatr. 2012; 78(1): 47–53. 10.1159/000338464 [DOI] [PubMed] [Google Scholar]
- 16. Conarello SL, Li Z, Ronan J, Roy RS, Zhu L, Jiang G, et al. Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci U S A. 2003; 100(11):6825–6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim MK, Chae YN, Kim HD, Yang EK, Cho EJ, Choi SH, et al. DA-1229, a novel and potent DPP4 inhibitor, improves insulin resistance and delays the onset of diabetes. Life Sci. 2012; 90(1–2): 21–29. 10.1016/j.lfs.2011.10.007 [DOI] [PubMed] [Google Scholar]
- 18. Kos K, Baker AR, Jernas M, Harte AL, Clapham JC, O'Hare JP, et al. DPP-IV inhibition enhances the antilipolytic action of NPY in human adipose tissue. Diabetes Obes Metab. 2009; 11(4): 285–92. 10.1111/j.1463-1326.2008.00909.x [DOI] [PubMed] [Google Scholar]
- 19. Rosmaninho-Salgado J, Marques AP, Estrada M, Santana M, Cortez V, Grouzmann E, et al. Dipeptidyl-peptidase-IV by cleaving neuropeptide Y induces lipid accumulation and PPAR-gamma expression. Peptides. 2012; 37(1): 49–54. 10.1016/j.peptides.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 20. Kirino Y, Kamimoto T, Sato Y, Kawazoe K, Minakuchi K, Nakahori Y. Increased plasma dipeptidyl peptidase IV (DPP IV) activity and decreased DPP IV activity of visceral but not subcutaneous adipose tissue in impaired glucose tolerance rats induced by high-fat or high-sucrose diet. Biol Pharm Bull. 2009; 32(3): 463–467. [DOI] [PubMed] [Google Scholar]
- 21. Kirino Y, Sato Y, Kamimoto T, Kawazoe K, Minakuchi K. Altered dipeptidyl peptidase-4 activity during the progression of hyperinsulinemic obesity and islet atrophy in spontaneously late-stage type 2 diabetic rats. Am J Physiol Endocrinol Metab. 2011; 300(2): E372–E379. 10.1152/ajpendo.00319.2010 [DOI] [PubMed] [Google Scholar]
- 22. Fukuda-Tsuru S, Kakimoto T, Utsumi H, Kiuchi S, Ishii S. The novel dipeptidyl peptidase-4 inhibitor teneligliptin prevents high-fat diet-induced obesity accompanied with increased energy expenditure in mice. Eur J Pharmacol. 2014; 723: 207–215. 10.1016/j.ejphar.2013.11.030 [DOI] [PubMed] [Google Scholar]
- 23. Cho JM, Jang HW, Cheon H, Jeong YT, Kim DH, Lim YM, et al. A novel dipeptidyl peptidase IV inhibitor DA-1229 ameliorates streptozotocin-induced diabetes by increasing beta-cell replication and neogenesis. Diabetes Res Clin Pract. 2011; 91: 72–79. 10.1016/j.diabres.2010.10.012 [DOI] [PubMed] [Google Scholar]
- 24. Kim HJ, Kwak WY, Min JP, Lee JY, Yoon TH, Kim HD, et al. Discovery of DA-1229: a potent, long acting dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. Bioorg Med Chem Lett. 2011; 21: 3809–3812. 10.1016/j.bmcl.2011.04.029 [DOI] [PubMed] [Google Scholar]
- 25. Mu J, Petrov A, Eiermann GJ, Woods J, Zhou YP, Li Z, et al. Inhibition of DPP-4 with sitagliptin improves glycemic control and restores islet cell mass and function in a rodent model of type 2 diabetes. Eur J Pharmacol. 2009; 623:148–154. 10.1016/j.ejphar.2009.09.027 [DOI] [PubMed] [Google Scholar]
- 26. Wu YJ, Guo X, Li CJ, Li DQ, Zhang J, Yang Y, et al. Dipeptidyl peptidase-4 inhibitor, vildagliptin, inhibits pancreatic beta cell apoptosis in association with its effects suppressing endoplasmic reticulum stress in db/db mice. Metabolism. 2015; 64(2): 226–235. 10.1016/j.metabol.2014.08.006 [DOI] [PubMed] [Google Scholar]
- 27. Vyas AK, Aerni-Flessner LB, Payne MA, Kovacs A, Jay PY, Hruz PW. Saxagliptin Improves Glucose Tolerance but not Survival in a Murine Model of Dilated Cardiomyopathy. Cardiovasc Endocrinol. 2012; 1(14): 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cannavino J, Brocca L, Sandri M, Bottinelli R, Pellegrino MA. PGC1-α over-expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice. J Physiol. 2014; 592(Pt 20): 4575–4589. 10.1113/jphysiol.2014.275545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yo K, Yu YM, Zhao G, Bonab AA, Aikawa N, Tompkins RG, et al. Brown adipose tissue and its modulation by a mitochondria-targeted peptide in rat burn injury-induced hypermetabolism. Am J Physiol Endocrinol Metab. 2013; 304(4): E331–E341. 10.1152/ajpendo.00098.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014; 10(1): 24–36. 10.1038/nrendo.2013.204 [DOI] [PubMed] [Google Scholar]
- 31. Dheda K, Huggett JF, Bustin SA, Johnson MA, Rook G, Zumla A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques. 2004; 37(1): 112–114, 116,, 118–119. [DOI] [PubMed] [Google Scholar]
- 32. Bergman AJ, Stevens C, Zhou Y, Yi B, Laethem M, De Smet M, et al. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther. 2006; 28(1):55–72. [DOI] [PubMed] [Google Scholar]
- 33. Even PC, Nadkarni NA. Indirect calorimetry in laboratory mice and rats: principles, practical considerations, interpretation and perspectives. Am J Physiol Regul Integr Comp Physiol. 2012; 303(5): R459–R476. 10.1152/ajpregu.00137.2012 [DOI] [PubMed] [Google Scholar]
- 34. Wendel AA, Purushotham A, Liu LF, Belury MA. Conjugated linoleic acid induces uncoupling protein 1 in white adipose tissue of ob/ob mice. Lipids. 2009; 44(11): 975–982. 10.1007/s11745-009-3348-9 [DOI] [PubMed] [Google Scholar]
- 35. Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003; 100(14): 8466–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002; 418(6899): 797–801. [DOI] [PubMed] [Google Scholar]
- 37. Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Adv Physiol Educ. 2006; 30(4): 145–151. [DOI] [PubMed] [Google Scholar]
- 38. Bogacka I, Xie H, Bray GA, Smith SR. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005; 54(5): 1392–1399. [DOI] [PubMed] [Google Scholar]
- 39. Talsania T, Anini Y, Sinus S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology. 2005; 146(9): 3748–3756. [DOI] [PubMed] [Google Scholar]
- 40. Zambrowicz B, Ding ZM, Ogbaa I, Frazier K, Banks P, Turnage A, et al. Effects of LX4211, a dual SGLT1/SGLT2 inhibitor, plus sitagliptin on postprandial active GLP-1 and glycemic control in type 2 diabetes. Clin Ther. 2013; 35(3): 273–285.e7. 10.1016/j.clinthera.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 41. Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995; 269(5223): 546–549. [DOI] [PubMed] [Google Scholar]
- 42. Ceddia RB. Direct metabolic regulation in skeletal muscle and fat tissue by leptin: implications for glucose and fatty acids homeostasis. Int J Obes (Lond). 2005; 29(10):1175–1183. [DOI] [PubMed] [Google Scholar]
- 43. Mack CM, Moore CX, Jodka CM, Bhavsar S, Wilson JK, Hoyt JA, et al. Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int J Obes (Lond). 2006; 30(9): 1332–1340. [DOI] [PubMed] [Google Scholar]
- 44. Baggio LL, Huang Q, Brown TJ, Drucker DJ. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology. 2004; 127(2): 546–558. [DOI] [PubMed] [Google Scholar]
- 45. Horowitz M, Flint A, Jones KL, Hindsberger C, Rasmussen MF, Kapitza C, et al. Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res Clin Pract. 2012; 97(2): 258–266. 10.1016/j.diabres.2012.02.016 [DOI] [PubMed] [Google Scholar]
- 46. Flint A, Raben A, Rehfeld JF, Holst JJ, Astrup A. The effect of glucagon-like peptide-1 on energy expenditure and substrate metabolism in humans. Int J Obes Relat Metab Disord. 2000; 24(3): 288–298. [DOI] [PubMed] [Google Scholar]
- 47. Pannacciulli N, Bunt JC, Koska J, Bogardus C, Krakoff J. Higher fasting plasma concentrations of glucagon-like peptide 1 are associated with higher resting energy expenditure and fat oxidation rates in humans. Am J Clin Nutr. 2006; 84(3): 556–560. [DOI] [PubMed] [Google Scholar]
- 48. Bradley DP, Kulstad R, Racine N, Shenker Y, Meredith M, Schoeller DA. Alterations in energy balance following exenatide administration. Appl Physiol Nutr Metab. 2012; 37: 893–899. 10.1139/h2012-068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goldsmith F, Keenan MJ, Raggio AM, Ye X, Hao Z, Durham H, et al. Induction of Energy Expenditure by Sitagliptin Is Dependent on GLP-1 Receptor. PLoS One. 2015; 10(5): e0126177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boschmann M, Engeli S, Dobberstein K, Budziarek P, Strauss A, Boehnke J, et al. Dipeptidyl-peptidase-IV inhibition augments postprandial lipid mobilization and oxidation in type 2 diabetic patients. J Clin Endocrinol Metab. 2009; 94(3):846–852. 10.1210/jc.2008-1400 [DOI] [PubMed] [Google Scholar]
- 51. Dobrian AD, Ma Q, Lindsay JW, Leone KA, Ma K, Coben J, et al. Dipeptidyl peptidase IV inhibitor sitagliptin reduces local inflammation in adipose tissue and in pancreatic islets of obese mice. Am J Physiol Endocrinol Metab. 2011; 300(2):E410–E421. 10.1152/ajpendo.00463.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Souza-Mello V, Gregório BM, Cardoso-de-Lemos FS, de Carvalho L, Aguila MB, Mandarim-de-Lacerda CA. Comparative effects of telmisartan, sitagliptin and metformin alone or in combination on obesity, insulin resistance, and liver and pancreas remodelling in C57BL/6 mice fed on a very high-fat diet. Clin Sci (Lond). 2010; 119(6):239–250. 10.1042/CS20100061 [DOI] [PubMed] [Google Scholar]
- 53. Sawada T, Miyoshi H, Shimada K, Suzuki A, Okamatsu-Ogura Y, Perfield JW 2nd, et al. Perilipin overexpression in white adipose tissue induces a brown fat-like phenotype. PLoS One. 2010; 5(11): e14006 10.1371/journal.pone.0014006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Abernathy RP, Black DR. Healthy body weights: an alternative perspective. Am J Clin Nutr. 1996; 63(3 Suppl):448S–451S. [DOI] [PubMed] [Google Scholar]
- 55. Gaidhu MP, Anthony NM, Patel P, Hawke TJ, Ceddia RB. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am J Physiol Cell Physiol. 2010; 298(4):C961–971. 10.1152/ajpcell.00547.2009 [DOI] [PubMed] [Google Scholar]
- 56. Gaidhu MP, Fediuc S, Anthony NM, So M, Mirpourian M, Perry RL, et al. Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J Lipid Res. 2009; 50(4):704–715. 10.1194/jlr.M800480-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006; 116(3): 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007; 92(3): 1023–1033. [DOI] [PubMed] [Google Scholar]
- 59. Verhoef SPM, van Dijk P, Westerterp KR. Relative shrinkage of adipocytes by paraffin in proportion to plastic embedding in human adipose tissue before and after weight loss. Obes Res Clin Pract. 2013; 7: e8–13. 10.1016/j.orcp.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 60. Son MH, Kim HD, Chae YN, Kim MK, Shin CY, Ahn GJ, et al. Peripherally acting CB1-receptor antagonist: the relative importance of central and peripheral CB1 receptors in adiposity control. Int J Obes (Lond). 2010; 34(3): 547–556. 10.1038/ijo.2009.253 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.