Abstract
The sensing of viral DNA is an essential step of cellular immune response to infections with DNA viruses. These human pathogens are spread worldwide, triggering a wide range of virus-induced diseases, and are associated with high levels of morbidity and mortality. Despite similarities between DNA molecules, mammalian cells have the remarkable ability to distinguish viral DNA from their own DNA. This detection is carried out by specialized antiviral proteins, called DNA sensors. These sensors bind to foreign DNA to activate downstream immune signaling pathways and alert neighboring cells by eliciting the expression of antiviral cytokines. The sensing of viral DNA was shown to occur both in the cytoplasm and nucleus of infected cells, disproving the notion that sensing occurred by simple spatial separation of viral and host DNA. A number of omic approaches, in particular mass spectrometry-based proteomic methods, have significantly contributed to the constantly evolving field of viral DNA sensing. Here, we review the impact of omic methods on the identification of viral DNA sensors, as well as on the characterization of mechanisms involved in host defense or viral immune evasion.
Introduction
The ability of mammalian cells to recognize pathogenic nucleic acids has long been established as an essential step in the initiation of intrinsic and innate immune responses. Viral pathogens can be diverse in terms of their DNA or RNA genomes and subcellular sites of viral replication. During infection, the viral nucleic acids, which are deposited in the nucleus or cytoplasm of infected cells, act as potent stimulators of immune response pathways that induce the expression of a variety of interferons and inflammatory cytokines (Figure 1). Detection of nucleic acids is part of the inbuilt cellular patrol achieved by proteins known as pattern recognition receptors (PRRs). These receptors can detect pathogen-associated molecular patterns (PAMPs), which, in addition to nucleic acids, can include proteins, lipids, glycans, and glycolipids1; 2; 3. Remarkably, mammalian cells can distinguish self from non-self despite similarities between these PAMPs and equivalent cellular molecules.
Figure 1. Localization-dependent viral DNA sensing.
A. Model of nuclear viral DNA sensing in response to infection with nuclear-replicating viruses (e.g., HSV-1) in non-immune cells (e.g., fibroblasts or natural host dermal cells). The virus particle fuses to the host membrane (1). The capsid is assisted by microtubules to move through the cytoplasm and then dock at the nuclear pore (2). The viral genome is then released into the nucleus (3), where it is sensed by a nuclear viral DNA sensor (4). This culminates in the expression of Type I IFN (5). B. Model of cytoplasmic viral DNA sensing as it occurs in immune cells, such as macrophages (1-4a) or dendritic cells (4b). In macrophages, the HSV-1 capsid is broken down in the cytoplasm before reaching the nucleus (2), triggering leakage of viral DNA in the cytoplasm (3), and leading to the detection of viral DNA by cytoplasmic DNA sensors (4a) and induction of Type I IFN (5). In dendritic cells, the DNA of viruses that are endocytosed are sensed by TLR9 in the endosomal compartment (4b). This sensing event results in the expression of Type I IFN (5). Continuous arrows represent signaling; dashed arrows represent movement.
Given their essential roles in cellular defense, significant effort has been placed on the discovery of PRRs and the characterization of the mechanisms involved in nucleic acid detection and subsequent pathway-specific induction of immune response. The discovery of Toll-like receptors was the initial driving force for studying PRRs. A wealth of important information was accumulated with regard to mechanisms of viral RNA detection, in part made possible by the identification of differences between viral and cellular RNA. For example, the Rig-I-like receptors (RLR) sense pathogenic RNA by recognizing several PAMPs, which include 5′triphosphate RNA, processing signatures by RNaseL, and properties related to RNA length and structure (reviewed in 4; 5). In contrast to the growing understanding of RNA sensors, the knowledge regarding DNA sensors is not yet as mature.
In order for a protein to be classified as a viral DNA sensor, the protein must satisfy two requirements: 1) It must physically interact with viral DNA, and 2) It must subsequently stimulate an innate immune program, such as the expression of interferons (IFN α, β, γ, λ) and/or chemokines and proinflammatory cytokines (e.g. IL-1β, IL-18, IL-6, TNF, CXCL10, and CCL5). The endosomal, membrane-bound Toll-like receptor 9 (TLR9) was the first identified DNA sensor6. TLR9 recognizes unmethylated cytosine-phosphate-guanine (CpG) nucleotide sequences, which are abundant in many DNA viruses and bacterial pathogens, but occur infrequently in the human genome7; 8; 9; 10. However, immune responses to a variety of DNA stimuli were induced in the absence of TLR9, predicting the presence of other DNA sensors11; 12; 13; 14. Additionally, cells were able to respond to adenine-thymine (AT)-rich DNA even following chemical inhibition of TLR915. As foreign and mammalian DNA have similar features, including AT-rich regions, these findings suggest that distinguishing self from non-self may be more challenging for DNA sensors than for their RNA sensing counterparts. How then can cells recognize foreign DNA? For most viral DNA sensors, this still remains an open question. Given the lack of a clear difference between host and viral DNA, the recognition of foreign DNA was thought for many years to rely primarily on compartmentalization, i.e. the spatial separation between virus and host DNA. The first cytoplasmic viral DNA sensor, the DNA-dependent activator of IFN-regulatory factors (DAI), was only discovered within the last decade16, and since then, the characterization of cytoplasmic sensors has been the focus of most DNA sensing studies. These studies have been the main contributors to expanding the field of DNA sensing, leading to the discovery of several cytoplasmic viral DNA sensors, including Absent in melanoma 2 (AIM2), cyclic-GMP-AMP synthase (cGAS), DNA-protein kinase (DNA-PK), DEAD box polypeptide- 41 and -60 (DDX41 and DDX60), DEAH box helicase -9 and -36 (DHX9 and DHX36), Interferon inducible protein 16 (IFI16), and RNA polymerase III (RNA Pol III) (Table 1). Two other proteins, Mre1117 and Lrrfip118, have also been described as cytosolic sensors of exogenous DNA, but the roles of these proteins in sensing in response to DNA virus infections have not yet been characterized. However, this sole understanding of cytoplasmic sensing brought a conceptual challenge. The majority of DNA viruses are known to replicate their genomes in host cell nuclei as a part of their natural viral life cycle (Figure 1A). These viruses include wide-spread human pathogens, such as human cytomegalovirus (HCMV), herpes simplex virus 1 (HSV-1), adenovirus (AdV), hepatitis B (HBV), and human papilloma virus (HPV). In certain immune cells, such as macrophages and plasmocytoid dendritic cells (pDCs), the cellular cytoplasmic environment can cause the leakage of viral DNA, for example by proteasomal-mediated degradation or proteolysis of viral capsid proteins. As a result, the viral DNA is partly exposed in the cytoplasm19 or in endosomes20; 21, allowing for sensing in these subcellular compartments (Figure 1B). However, in many natural cellular hosts of these viruses (non-immune cells), the viral DNA is protected by capsid proteins during its transit through the cytoplasm, prior to its deposition in the nucleus (Figure 1A). Therefore, it became evident that mammalian cells must have some means to also recognize the presence of viral DNA in the nucleus, and that proteins capable of nuclear sensing should exist. Indeed, in recent years, this emerging field of DNA sensing was further expanded by the identification of a nuclear DNA sensor, the interferon inducible protein IFI1622; 23; 24; 25. More recently, another interferon inducible protein, IFIX, was also shown to act as a sensor and bind nuclear HSV-1 DNA in infected cells26. Overall, the development and application of omic technologies have had a significant impact on this field27, aiding the discovery and characterization of both cytoplasmic and nuclear DNA sensors. Here, we provide an overview of the field of DNA sensing, with an emphasis on the contribution of proteomic approaches to the discovery and characterization of viral DNA sensors. For a broader view of microbial immune sensing, we also point the readers to additional valuable reviews28; 29; 30. In this review, we discuss omic workflows that have been used for studying virus-host interactions in the context of DNA sensing, including both DNA-protein and protein-protein interactions. We next elaborate on the mechanisms by which these interactions induce immune responses. We go on to review the current knowledge regarding the mechanisms that viruses have evolved to block immune response. We also emphasize the role of post-translational modifications (PTMs) of either host or viral proteins that contribute to host defense or viral immune evasion. Lastly, we provide a perspective for both the future of proteomics in studying viral DNA sensors and important standing questions regarding the intracellular recognition of viral nucleic acids.
Table 1. Overview of viral DNA sensors.
Viral DNA sensors (PRR) are listed, together with the viruses shown to activate their sensing functions. Indicated are the viral substrates recognized by the different DNA sensors, the subcellular compartment in which sensing occurs, and the pathways through which the sensors have been shown to signal immune responses. The methods used to first identify the protein and/or show evidence of DNA binding are included. Note: additional non DNA pathogenic substrates are not included in this table.
| PRR | Viral Substrates | Substrate | Sensing Location | Pathway | Immune response | Methods | Refs |
|---|---|---|---|---|---|---|---|
| AIM2 | CMV, AdV, VACV, HBV, HPV16 | dsDNA | Cytoplasm | Inflammasome | IL-1β, IL-18 | α-Screen; DNA beads AP-MS | 31; 42; 43; 86; 87; 88 |
| cGAS | HSV-1, MAV/VACVa, AdV | dsDNA | Cytoplasm | STING | IFNα , IFNβ | Biochemical fractionation-MS | 37; 121; 122 |
| DAI | HSV-1, CMV | dsDNA | Cytoplasm | STING? RIP-3 | IFNβ, necrosisb | FRET; IP-WB | 16; 78; 123 |
| DDX41 | HSV-1, AdV | dsDNA | Cytoplasm | STING | IFNα, IFNβ | MS; bioinformatics screen | 39; 74 |
| DDX60 | HSV-1 | dsDNA | Cytoplasm | RIG-I | IFNβ | DNA microarray, gel shift assay | 47 |
| DHX9 | HSV | Class B CpG | Cytoplasm | MyD88 | TNFα, IL-6 | CpG beads AP-MS | 35 |
| DHX36 | HSV | Class A CpG | Cytoplasm | MyD88 | IFNα | CpG beads AP-MS | 35 |
| DNA-PK | HSV-1, HSV-2G, VACV, | dsDNA | Cytoplasm | STING | IFNλ1, IFNβ, IL-6 | DNA beads AP-MS | 33; 34; 110 |
| IFI16 | HSV-1, CMV, KSHV, VACV70mer, HIV | dsDNA, ssDNA | Nucleus Cytoplasm | STING Inflammasome | IFNβ, CXCL10, CCL5, CCL20, IL-6, IL-1β, IL-18 | DNA beads AP-MS; α-Screen | 22; 23; 24; 32; 70; 92 |
| IFIX | VACV70mer, HSV-1 | dsDNA | Cytoplasm Nucleus* | n.d. | IFNβ | AP-MS; dsDNA array | 26 |
| RNA Pol III | HSV-1, EBV, AdV | AT-rich dsDNA | Cytoplasm | RIG-I | IFNβ | chromatography fractionation; MS | 15; 36 |
| TLR9 | HSV-1+2, CMV, AdV, EBV | Unmethylated CpG motif | Endosome | MyD88 | IFNα, IFNβ | Luciferase reporter; SPR; bioinformatic modeling | 9; 12; 44; 45; 124; 125; 126; 127 |
modified vaccinia virus (MAV/VACV);
programmed necrosis through the RIP-3 pathway.
IFIX was shown to bind HSV-1 DNA, a nuclear replicating virus, suggestive of another possible nuclear sensor, which remains to be demonstrated.
A. DNA-protein interactions during viral DNA sensing
Proteomic methods employed for discovering viral DNA sensors
Technological advances in methods that integrate mass spectrometry-based proteomics within studies of DNA-protein interactions have contributed significantly to the field of viral DNA sensing (Table 1). One commonly used approach involves affinity purification (AP) in conjunction with analysis by mass spectrometry (MS). The isolation of proteins that interact with DNA can be performed using beads conjugated to dsDNA (or ssDNA) or by spiking the sample with biotinylated DNA and performing the AP with streptavidin beads. The isolated proteins are usually subjected to proteolytic digestion (in-solution or in-gel following SDS-PAGE separation) and identified by MS (Figure 2). In fact, five of the twelve viral DNA sensors (Table 1) were identified using this type of proteomic approach, including AIM231, IFI1632, DNA-PK33; 34, and the DEAH/RNA Helicases DHX9 and DHX3635. Additionally, two other DNA sensors, RNA Pol III36 and cGAS37, were characterized using a modified proteomic approach, by coupling biochemical fractionation with MS. One example of an elegant integration of OMIC technologies came from the laboratory of Yong-Jun Liu, where the RNA helicases DHX9 and DHX36 were discovered as DNA sensors35, and an RNA sensing complex consisting of 3 DExD/Hbox helicases was identified by AP-MS/MS38. These findings led them to expand these studies using a bioinformatic approach to find additional DExD/Hbox helicases that may have nucleic acid sensing capability39. By coupling MS data with bioinformatic tools, they identified DDX41, which was further experimentally validated as a new viral DNA sensor39. Most recently, the interferon inducible protein X (IFIX) was discovered as a viral DNA sensor by using an integrative proteomic, virology, and molecular biology approach26. Thus, nine out of 12 viral DNA sensors were identified through approaches integrating mass spectrometry. Collectively, these studies emphasize the value of omic approaches for identifying previously unknown viral DNA sensors. One technical challenge to consider when performing AP-MS experiments is distinguishing DNA-protein from protein-protein interactions. Comparisons done in the presence or absence of Dnase I or Benzonase treatment can help prioritize the proteins identified from AP-MS for follow-up studies. Several techniques, such as in vitro electromobility shift assay (EMSA)40 and the α-screen41, described below, can help determine if the protein binds directly to DNA or if it interacts with DNA indirectly through another protein.
Figure 2. Proteomic workflows for identifying DNA-protein and protein-protein interactions.
DNA-coated beads or antibody-coated beads are placed in the cell lysate from uninfected or virally infected cells to capture interactions. Affinity purification is usually followed by enzymatic digestion, and the resulting peptides are analyzed by mass spectrometry. These analyses lead to the identification of proteins that interact with either DNA or protein. Protein interaction networks can be constructed to gain insights into complexes and modulated cellular pathways. These studies can be expanded by mapping PTMs that can contribute to the formation of interactions. Affinity purification using antibody-coupled beads can also be used to detect the DNA that associates to the proteins of interest, e.g. by PCR.
AIM2 was reported as a DNA sensor by multiple groups around the same time31; 42; 43. In addition to the AP-MS approach described above31, AIM2 was also identified by other groups using an α-screen43. The α-screen allows DNA-protein or protein-protein interactions to be studied using basic donor-acceptor principles, where microbeads are coated with the molecules of interest and placed in the cell lysate. For example, if photosensitive donor beads come in close proximity to the chemiluminescent acceptor beads, the interaction is detected spectrophotometrically in a microplate reader (as reviewed in 41). Both MS-based and α-screen approaches were also valuable in identifying IFI16, the other AIM2-like receptor (ALR), as a sensor and to validate its interaction with DNA32.
Only three viral DNA sensors were found independently of the use of mass spectrometry, TLR9, DAI, and DDX60. Of note, two of these (TLR9 and DAI) were the first two viral DNA sensors to be reported, suggesting that advances in MS-based proteomics and the resulting increase in the popularity of these methods have helped to expand this field with the discovery of additional viral DNA sensors. TLR9 was initially shown to recognize CpG motifs in bacterial DNA6. Later, surface plasmon resonance (SPR) was used to demonstrate a direct interaction between TLR9 and CpG DNA44, and, more recently, a bioinformatics approach was employed to model the 3D structure of the TLR9-CpG complex45. It should be noted that current technology now allows bridging SPR with protein identification by MS46. Several years after the discovery of TLR9, DAI, the first cytosolic DNA sensor, was described16. DAI, previously known as DLM-1 or ZBP-1, was predicted to act as a sensor given its identification in a DNA chip screen and its upregulation upon IFNβ or B-DNA stimulation16. Indeed, its requirement for IFN response to transfected vaccinia virus (VACV) DNA was observed in L929 fibroblasts16. Furthermore, the co-localization of DAI with B-DNA was demonstrated by Fluorescence Resonance Energy Transfer (FRET), and their interaction was confirmed by isolation assays using biotin-labeled B-DNA and streptavidin beads, followed by western blotting (AP-WB)16. DDX60 was identified using a similar approach. A DNA microarray was used to identify potential antiviral genes, followed by a gel shift assay to confirm DDX60 binding to nucleic acids and IFNβ induction following herpes simplex 1 (HSV-1) infection 47. Altogether, these findings portray the evolution of DNA-protein interaction technology. The characterization of the first viral DNA sensors (TLR9, DAI, and DDX60) was predicated on knowing the identity of the protein itself. Conversely, the identities of the other eight viral DNA sensors were found without a priori knowledge of their identity, which underscores the value of mass spectrometry-based approaches in the growing field of DNA sensing.
Structural insights into DNA-protein interactions
While several DNA sensors, TLR9, DHX9, DHX36 and RNA Pol III, were shown to have preference for binding to GC or AT content6; 9; 15; 35; 44, other sensors, such as AIM248, IFI1648, IFIX26 and cGAS49, are able to effectively bind DNA in a sequence-independent manner. This is an interesting difference, exemplifying distinct host defense strategies to recognize viral invasion. The ability to bind viral DNA in a sequence-independent manner can point to broad-spectrum antiviral factors that are efficient against numerous pathogens. Structural studies have contributed not only to elucidating how these DNA sensors bind to DNA, but also to how signaling events are mediated through changes in protein conformation. Here, we highlight the significance of these structural studies for characterizing AIM2, IFI16, and cGAS, and the downstream IFNβ effector protein Interferon regulatory factor 3 (IRF3). The AIM2-like receptors AIM2 and IFI16 are members of the PYHIN family. This family is termed PYHIN due to the two domains that characterize these proteins (Figure 3); the pyrin (PY) domain that mediates homotypic interactions with other pyrin domain-containing proteins, and one or two HIN domains that bind DNA. The HIN domains of AIM2 and IFI16 have been shown to bind DNA in a sequence independent manner (48 and reviewed in50). The positively charged residues of the HIN200 domain bind DNA via electrostatic attraction to the negative charge of the sugar-phosphate backbone of DNA, as determined by x-ray crystallography and molecular replacement48. For both AIM2 and IFI16, the DNA binding interface is formed by two oligonucleotide/oligosaccharide binding (OB) folds within each HIN domain48. In agreement with this, the recently reported DNA sensor, IFIX, which also contains a HIN domain, was shown to bind DNA in a sequence-independent manner26. Due to the sequence similarities between these PYHIN proteins, it is likely that IFIX also binds DNA via an OB fold electrostatic mechanism. The studies by Jin et al. also predicted the likely mechanism for AIM2 in sequestering inflammasome components, whereby the pyrin domain is autoinhibited by the HIN domain until its release through HIN domain binding to DNA48; 51. This may be a common auto-inhibition mechanism for other PYHIN proteins.
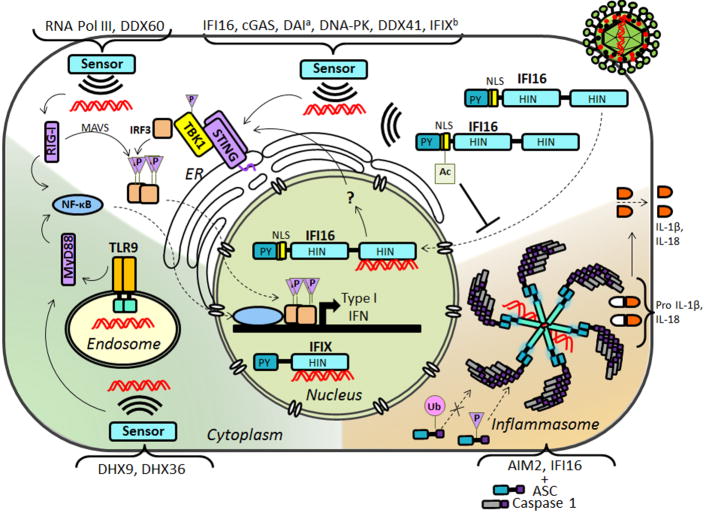
Figure 3. Viral DNA sensing pathways and associated PTMs.
The main signaling pathways of viral DNA sensors are depicted. The nuclear DNA sensor IFI16 and the cytoplasmic DNA sensors cGAS, DDX41, IFI16 and DNA-PK signal through STING. aDAI was also shown to activate TBK1 and IRF3 prior to the discovery of STING. bIFIX was shown to induce IFN following VACV, but the downstream signaling pathway has not been yet investigated. IFIX was also reported to bind HSV-1 DNA, a nuclear replicating virus. RNA Pol III and DDX60 signal through RIG-I, which further signals through MAVS. DHX9 and DHX36 signal through MyD88. AIM2 and IFI16 interact with ASC and Caspase 1 to form inflammasomes, which lead to the proteolytic cleavage of proinflammatory cytokines Il-1β and IL-18. Continuous arrows represent signaling; dashed arrows represent movement.
The crystal structure of cGAS also revealed why this sensor can bind DNA in a sequence independent manner, as cGAS associates with a span of 7 nucleotides by binding the sugar-phosphate strands in the minor groove49. Two arginine residues further stabilize the cGAS-DNA interaction by their insertion into the minor groove49. Additionally, another study showed the requirement of a 2:2 stoichiometry of cGAS-DNA52, demonstrating the requirement of a key lysine residue (mouse K335 ; human K347) for dimer formation and interaction with DNA, both of which are important for IFNβ induction via IRF3 activation52. cGAS was also observed in an autoinhibited state until it bound to its ligand, which caused a conformational change in its activation loop52. Intriguingly, this conformational change only occurred upon cGAS binding to B-form DNA, and was not observed upon binding to A-form RNA. The authors reasoned that the lack of conformational change in cGAS in complex with dsRNA could explain why cGAS-dependent RNA sensing had not been observed52. However, in another study, cGAS was observed to play an antiviral role during infection with RNA viruses53, suggesting a possible interplay between the DNA and RNA sensing pathways.
A common mechanistic feature of DNA sensing activity described above is protein autoinhibition prior to the conformational change induced by the protein-DNA interaction. These conformation changes induce dimer or oligomer formation, which is required for downstream IFN signaling. IRF3, a crucial component in Stimulator of interferon genes (STING)-dependent IFN activation (Figure 3 and section below), has also been shown to be autoinhibited, though the mechanism of release is different. The N- and C-terminal domains of IRF3 interact to hide a hydrophobic surface implicated in IRF3 oligomerization and subsequent immune response54, with phosphorylation likely serving to release the autoinhibited state54. It remains to be determined how many DNA sensors and pathway-associated proteins are self-controlled in a similar manner. Structural studies are expected to continue to significantly contribute to understanding the mechanisms involved in DNA sensing.
B. Protein-protein interactions involved in host immune response
Upon binding to viral DNA, the sensors rely on the formation of cascades of protein-protein interactions that mediate downstream immune signaling. In this section, we review the main pathways through which DNA sensors are known to signal (Figure 3).
Protein interactions within the STING-dependent DNA sensing pathway
Currently, several identified viral DNA sensors (IFI16, cGAS, DDX41, DNAPK, and likely DAI and IFIX) have been shown to stimulate an immune response that signals through the stimulator of interferon genes (STING) (Table 1 and Figure 3). Although the signaling events upstream of STING are still being elucidated, the downstream signaling cascade starts to be well understood. The adaptor protein STING is an endoplasmic reticulum resident protein55. Upon stimulation, STING forms a dimer and recruits TANK binding kinase 1 (TBK1) and IRF355; 56; 57, triggering a signaling cascade mediated by several phosphorylation events (Figure 3). Specifically, STING binding stimulates the autophosphorylation of TBK1 at Ser17258; 59. TBK1 then phosphorylates IRF3 on a serine/threonine cluster, which enables IRF3 homodimer formation and interaction with CREB-binding protein and p30060; 61. Two different potential sites for IRF3 phosphorylation, site 1 (Ser385 or Ser386) and site 2 (localized between Ser396 and Ser405), have been reported, yet the necessity of phosphorylation at both sites and the order of the phosphorylation events have been debated62; 63; 64; 65; 66. Panne and colleagues used constitutively activated TBK1 in vitro to demonstrate that phosphorylation at site 2 relieves IRF3 autoinhibition, which allows phosphorylation at site 1 and IRF3 dimerization and activation67. However, Bergstroem et al. performed MS/MS studies on IRF3 in human embryonic kidney (HEK)293 cells infected with Sendai virus, and in vitro studies with TBK1, which indicated that phosphorylation at site 1 precedes that of site 2 68. In addition these studies identified constitutive phosphorylations at Ser173 and Ser175, as well as a new site of phosphorylation by TBK1 during viral infection at Thr39068. While more studies are needed to fully characterize the temporality of these events, the importance of phosphorylation is well established for the nuclear localization of the IRF3 complex, where it binds to type I IFN promoters to activate transcription62; 69. Type I IFNs then work in an autocrine and paracrine manner to elicit innate immune response and the expression of antiviral cytokines.
Several studies have indicated that IFI16 triggers immune signaling through the STING-dependent pathway. Interestingly, IFI16 is the one sensor shown to sense foreign DNA in both the cytoplasm and the nucleus23; 24; 25; 32, and published reports have associated both of these signaling events with the STING pathway. The dual localization of IFI16 seems to be cell type-dependent, expanding its range of sensing. Indeed, IFI16 has been reported to induce the expression of antiviral cytokines in response to nuclear-localized DNA during HSV-1, HCMV, and KSHV infections, and to cytoplasmic-localized VACV DNA and proviral DNA from human immunodeficiency virus (HIV) (Table 1).
IFI16 sensing ability was first identified in the cytoplasm, in cells transfected with VACV DNA32. A co-IP assay demonstrated an interaction between IFI16 and the adaptor protein STING, suggesting that IFI16 cytoplasmic sensing signals through this pathway32. Later, a study integrating AP-MS with mutagenesis, microscopy, and cytokine assays, demonstrated that the localization of IFI16 can be modulated by its acetylation23 (Figure 3). Targeted mass spectrometry determined that, in several different cell types, endogenous IFI16 is acetylated at residues Lys99 and Lys128 within its multipartite nuclear localization signal (NLS). The acetylation was shown to prevent the nuclear import of IFI16, leading to its accumulation in the cytoplasm and allowing for sensing in response to transfected VACV23. Furthermore, cytoplasmic sensing by IFI16 has been observed following HSV-1 infection in macrophages, where the viral capsid is broken down prior to the docking of the viral capsid at the nuclear pore19. This process was shown to involve the ubiquitination and proteasomal degradation of the HSV-1 capsid protein Vp519. More recently, similar IFI16 cytoplasmic sensing in primary macrophages has been reported in response to ssDNA from HIV proviral DNA70.
While a subset of IFI16 is localized to the cytoplasm in certain cells types, its predominant localization is nuclear. Indeed, IFI16 was identified as the first known protein able to detect viral DNA in the nucleus. Importantly, using NLS deletion mutants, IFI16 nuclear localization was demonstrated to be required for the efficient binding to viral DNA and for eliciting IFN-β response following HSV-1 infection23. In agreement, another study showed that IFI16 was retained in the nucleus during the early stages of HSV-1 infection24. This was also the case for the infection with another nuclear-replicating virus, HCMV, as IFI16 was observed to remain nuclear early in infection and to be required for triggering antiviral cytokine expression following its binding to HCMV DNA25. Interestingly, although IFI16 binds viral DNA in the nucleus, these studies have shown that following both HSV-1 and HCMV infections, the immune signaling occurs through the STING-dependent pathway24; 25. Therefore, a question that remains to be addressed is how the signals are transmitted between IFI16 and STING. A yet-to-be identified protein or a small molecule may interact with IFI16 to facilitate signaling.
Another protein in the same PYHIN family as IFI16, the interferon inducible protein IFIX, was also recently found to act as a DNA sensor26. Similar to IFI16, IFIX may also function both in the cytoplasm and nucleus. In response to transfection with VACV 70mers, IFIX was observed to co-localize with cytoplasmic viral DNA and to aid the induction of IFNβ, thereby satisfying both requirements for a DNA sensor26. Interestingly, IFIX retained its predominant nuclear localization during early HSV-1 infection and was shown by chromatin immunoprecipitation to bind HSV-1 DNA in infected cells26. Its antiviral function was further strengthened by the demonstration that IFIX knockdown triggers an increase in HSV-1 titer. As HSV-1 is a nuclear replicating virus in non-immune cells, this study points to IFIX as the second DNA sensor that associates with nuclear viral DNA. Due to the similarities in the domain structures, localization, and functions of IFIX and IFI16, it is possible that the induction of IFNβ by IFIX involves the STING-dependent pathway, although this remains to be investigated.
Using a multi-disciplinary approach that worked backwards from STING activation, cGAS was also recently identified as a potent cytoplasmic DNA sensor71. After establishing that the activator of STING was not protein, DNA or RNA, a mass spectrometry approach was utilized to discover that it was a small molecule, the cyclin guanosine monophosphate-adenosine monophosphate (cGAMP). The cyclic GMP-AMP synthase cGAS was then shown to bind DNA, to catalyze the synthesis of cGAMP in a DNA-dependent manner, and to be required for IFN induction37; 71; 72. Additionally, cGAS knockdown led to reduced levels of IRF3 dimers in response to HSV-1. Collectively, these studies established cGAS as a STING-dependent viral DNA sensor where the interactions of cGAS in this immune program are cGAS with DNA, cGAS with its product cGAMP, and cGAMP with STING. Whether cGAS and IFI16 have interdependent sensing functions remains to be established. However, a recent study demonstrated that IFI16, cGAS, and STING are all necessary for eliciting cytoplasmic sensing in macrophages in response to Listeria monocytogenes infection, and that the resulting IFNβ expression was independent of the presence of cyclic-di-AMP, but rather was dependent on Listeria DNA73. Interestingly, this study indicated that IFI16 was necessary for sustained IFN induction and that, although acting through the same STING-dependent pathway in response to Listeria DNA, IFI16 and cGAS do not seem to be redundant73. Therefore, interconnected functions between different DNA sensors, such as IFI16 and cGAS may exist. Indeed, another study indicated that following HIV-1 infection IFI16 signals through a pathway dependent on cGAS and STING70. In terms of viral DNA sensing, it is not yet understood whether IFI16 and cGAS function through parallel or within the same pathway(s), and whether the coordination of their functions is also expanded to the nucleus.
The DNA sensor DDX41 was found to bind directly to STING and to induce IFNβ in response to either transfected DNA or HSV-1 infection39. The Asp-Glu-Ala-Asp (DEAD) domain of DDX41 was shown to act as the DNA binding domain, and the shRNA-mediated knockdown of either DDX41 or STING led to severe reductions in IFN expression39. DDX41 has also been shown to play a role in sensing AdV in the mouse macrophage-like cell line RAW264.774. In this study, the immune response was tested by detecting levels of activated IRF3 (phosphorylation level at pSer388), which were reduced when DDX41, STING, or TBK1 were knocked down74. Although direct interactions were not tested, this study indicated a correlation between DDX41 and AdV DNA sensing.
DNA-dependent protein kinase (DNA-PK) is a complex made up of the Ku70/Ku80 heterodimer and the catalytic subunit of the kinase-DNA-PKcs and is best known for its role in DNA repair75. Recently, this complex was identified as a cytoplasmic viral DNA sensor that activates IFNλ1 in response to HIV33. Although the transcriptional regulation of type III IFNs has not been elucidated in response to many viruses, IFNλ1 was shown to be regulated by IRF7, NF-κB, and IRF3 in HepC infected hepatocytes76, which suggests the possible involvement of STING-TBK1. Additionally, Ferguson et. al. showed that the induction of IFNβ in response to mouse VACV (mAV) or HSV-1 was reduced following knockdown of prkdc (gene for DNA-PKcs) 34. The immune response to mAV was abolished in the absence of IRF3, which implicated the STING pathway34.
There is also evidence that DAI-mediated IFN induction may also occur via the STING-dependent pathway, whereby an interaction between DAI, IRF3, and TBK1 has been observed16; the latter two proteins are a requirement for STING-dependent IFN activation (Figure 3). The induction of IFN expression in response to viral DNA is known to be dependent on STING in the cells in which DAI was shown to function (i.e., mouse fibroblasts)77, further supporting its likely signaling through a STING pathway. However, after the identification of DAI, several other viral DNA sensors (described above) have been discovered and shown to be more potent activators of IFN response. Therefore, it remains to be determined whether the function of DAI in this response is redundant. Recent evidence suggests the involvement of DAI in another antiviral response, through pathway the receptor interacting protein 3 (RIP-3)-dependent necrosis pathway78 (see section below).
Interactions that lead to the formation of the inflammasome
In addition to the STING-dependent pathway, selected DNA sensors have been shown to trigger immune response through the formation of an inflammasome. The inflammasome (reviewed in 79; 80) is a complex with important roles in defense against foreign pathogens, triggering immune response through the maturation and subsequent release of proinflammatory cytokines such as IL-1β, and IL-18. Cytokine maturation is carried out through the proteolytic activity of caspase 1, which is recruited to the inflammasome in its immature form (procaspase 1) via its C-terminal caspase-recruitment domain (CARD). Currently, in the context of DNA sensing, only the ALRs (i.e., AIM2 and IFI16) have been shown to elicit the formation of inflammasome macromolecular assemblies22; 31; 42; 43. These inflammasomes are composed of the DNA sensor (AIM2 or IFI16), caspase 1, and the apoptosis-associated speck-like protein containing a CARD (ASC). The N-terminal pyrin domain of ASC mediates homotypic pyrin domain interactions with AIM2 or IFI16, while its CARD binds procaspase 1 to trigger the formation of the heterotetrameric caspase 1. Furthermore, the PTM status of ASC modulates inflammasome formation, either by inhibiting or promoting protein interactions. Hara et al. reported that phosphorylation of ASC Tyr146 (Tyr144 in mouse) is needed for speck aggregate formation and subsequent cytokine signaling of IL-1881. Indeed, small molecule inhibition or knockdown of Syk and Jnk kinases reduced inflammasome formation upon exposure of cells to foreign DNA (i.e., poly (dA:dT))81. Martin et al. found that IκB kinase α (IKKα) downregulates inflammasome formation through its interaction with ASC82. ASC is phosphorylated by IKKα at residues Ser193 and Ser16, which triggers the segregation of ASC-IKKα to the perinucleus. Another phosphorylation event, the IKKi phosphorylation of Ser58 on ASC, leads to the disassembly of the ASC-IKKα association, reestablishing the formation of the inflammasome82. Protein kinase R (PKR) was also shown to regulate inflammasome formation in response to poly (dA:dT)83. PKR was found to interact with several inflammasome components, including AIM2, NLRP3 and ASC83. PKR knockdown in macrophages or the chemical inhibition of PKR autophosphorylation showed diminished AIM2 inflammasome formation, although no phosphorylations were detected on inflammasome components83. Interestingly, the requirement of PKR in inflammasome activation has recently been challenged, as He et al. found PKR to be dispensable for inflammasome activity84. As these studies were done in a similar system (i.e., macrophages from Pkr−/− mice) and both used induction with poly(dA:dT), it remains to be determined whether other differences in the experimental workflows or inflammasome formation readouts triggered this discrepancy in results. Further studies will help resolve this issue. In addition to the role of phosphorylation in inflammasome regulation, ubiquitination was also shown to regulate ASC aggregation. Inhibition of deubiquitinase (DUB) activity was reported to impede ASC aggregation, although this occurs to a lesser degree in AIM2 inflammasomes responding to cytoplasmic DNA than NLRP3 inflammasomes responding to lipopolysaccharide, a component of the outer membrane of bacteria85.
Interesting is the seemingly cell-type and, in some cases, virus-type specific inflammasome responses. These differences may reflect the differential expression of inflammasome components in different cell types, the subcellular localization of the viral dsDNA, the ability of host cells to induce immune response through diverse mechanisms, and the ability of certain viruses to suppress immune responses by specifically blocking inflammasome formation. For example, to date, AIM2 inflammasome formation has been demonstrated only in macrophages, dendritic cells, and keratinocytes, whereas IFI16 inflammasomes have been shown in fibroblasts and endothelial cells. In macrophages and dendritic cells, AIM2 inflammasomes formed in response to VACV and mouse cytomegalovirus (MCMV), but not in response to HSV-186. AIM2 was also shown to play a role in sensing adenovirus (AdV) in RAW264.7 mouse macrophages; however, AIM2 inflammasome formation was not evaluated. These results showed an AIM2- and DDX41-dependent decrease in phosphorylated IRF374, suggesting the involvement of the STING-dependent pathway. In a clinical study, AIM2 was found to sense hepatitis B (HBV) DNA in human glomerular mesangial cells, where IL-18 levels were decreased in response to transfected Hep-B DNA when AIM2 was knocked down87. Finally, in keratinocytes, AIM2 inflammasome formation was seen in response to human papilloma virus (HPV-16) DNA88. The selection of the cell type in which the studies are performed is critical, as the ability of different cells to trigger immune responses can vary substantially. The studies mentioned above used cells that are permissive to infection. It remains unknown if AIM2 senses these viruses in cell types other than those listed above. Remarkably, AIM2 senses MCMV but not HSV-1 even though both are herpesviruses with similar modes of infection and replication. Because the capsids of both CMV and HSV-1 are broken down in the cytoplasm of macrophages19, triggering the release of viral DNA in the cytosol, the selectivity of AIM2 in sensing these viruses seems remarkable. Since cytoplasmic IFI16 was shown to sense cytoplasmic HSV-1 DNA in macrophages independent of inflammasome assembly, this differential specificity of AIM2 and IFI16 may represent a tactic through which a detrimental overactive immune response is avoided. This hypothesis is in agreement with the study by Reinholz et al that showed that, although the AIM2 inflammasome is triggered in response to HPV-16 in keratinocytes (as evidenced by IL-1β secretion without increase in IFNβ), AIM2 knockdown triggers a decrease in IL-1β levels, while IFNβ levels are still observed to increase88. It will be interesting to see how this mystery unfolds in forthcoming studies.
Interestingly, while several groups have shown that IFI16 signals through a STING-dependent pathway following infections of fibroblasts with HSV-124 and HCMV25, one group observed the formation of IFI16 inflammasomes in endothelial cells and human fibroblasts in response to the herpesviruses Kaposi Sarcoma-associated herpesvirus (KSHV) and HSV-1, respectively22; 89; 90. By fluorescent in situ hybridization and immunofluorescence microscopy, IFI16 was shown to co-localize with KSHV DNA and ASC in nuclei of infected cells22. In addition, this study from the Chandran laboratory reported interactions between IFI16, ASC, and mature caspase 1 by immunoaffinity purification, and showed an IFI16-dependent increase in IL-1β in response to KSHV infection22. Sensing via IFI16 inflammasomes was also observed during KSHV and EBV latent infection90; 91, a persistent form of infection in which viral particles are not being produced, yet the viral genome is still present. In addition, a study from the same lab reported that IFI16 can form inflammasomes in human foreskin fibroblasts (HFF) early during HSV-1 infection; however, due to the proteasomal degradation of IFI16 dependent on the viral protein ICP0 and the entrapment of caspase 1 in actin clusters, HSV-1 infection inhibits IFI16 inflammasomes by 8 hours post infection89. Interestingly, in contrast to these observations following HSV-1 infection in HFFs, inflammasome formation was not detected following HCMV infection in these cells92. These distinct responses of IFI16 to different herpesviruses remain to be further characterized.
Alternative immune response pathways employed in viral DNA sensing
In addition to the STING-dependent and the inflammasome pathways described above, viral DNA sensors have been observed to signal through several alternative immune pathways. For example, DAI was shown to function both through the activation of IRF3 and NFκB16; 93. Each of these pathways uses a set of kinases to signal and activate the relevant transcription factors. DAI has been demonstrated to have two receptor-interacting protein (RIP) homotypic interaction motifs (RHIMs), which mediate recruitment of RIP-1 and -394; 95; 96. In addition RIP-3 phosphorylation activity has been shown to be essential for NFκB signaling via the activation of the IKK complex, suggesting that RIP-3 is an effector of DAI signaling94; 97; 98.
Two DNA sensors, RNA Pol III and DDX60, were reported to signal through the RNA-sensing RIG-I-like receptors (Figure 3). RIG-I is a PRR that recognizes short 5′ triphosphate uncapped RNA and signals through MAVS to induce type I IFN. RNA Pol III was shown to synthesize 5′ triphosphate from AT-rich viral DNA, which is subsequently sensed by RIG-I in HEK293 cells transfected with poly dA-dT or during AdV, HSV-1 or EBV infection36. DDX60 was demonstrated to associate with RIG-I, MDA5 and LGP2 in HEK293FTs in the absence of RNA, and to directly bind dsDNA47. A reduction in IFNβ expression in HSV-1 infected HeLa cells was seen upon DDX60 knockdown47. However, the DDX60-dependent IFN induction following HSV-1 infection was observed as moderate at 19 hours post infection (hpi) and only significant at 26 hpi 47. As the initial IFN response to HSV-1 is usually documented at ∼6hpi, these observations suggest that DDX60, while not critical for the initial induction, it may be important for amplifying the IFN response. The exact mechanisms behind this RLR-dependent DNA sensing by DDX60 remain to be understood, as does the apparent redundancy of RNA pol III sensing99; however these findings provide further evidence for the divergence of DNA sensing pathways100.
Three DNA sensors, DHX9, DHX36, and TLR9, signal through MyD88 (Figure 3). MyD88 leads to the activation of IRF7 and NF-κB101. In plasmocytoid dendritic cells (pDCs) DHX-9 and -36 were shown to bind class B and A CpG, respectively, and to associate with the TIR domain of MyD8835. Additionally, siRNA-mediated knockdown of DHX-9 and -36 in HSV-1 infected pDCs led to a decrease in TNFα and IFNα35. TLR9 signaling through MyD88 during viral infection is well understood and relies on downstream IRAK1-dependent IRF7 activation (reviewed in 28). Interestingly, pDC sensing of HSV-1 DNA was observed to occur in both TLR9-dependent and -independent manners12. Viral DNA sensing by TLR9 has been shown only in pDCs, probably because TLR9 expression is limited to immune cells.
Altogether, the immune signaling pathways described above highlight the relevance of protein-protein interactions in antiviral response against DNA viruses. The use of mass spectrometry-based proteomic approaches within these studies promises to continue to lead to insights into the dynamic nature of these interactions and their regulation by PTMs. The observations that the expression patterns of DNA sensors and their subsequent signaling pathways are both diverse and, in some cases, redundant, underscore the strength of this branch of innate immunity. This is especially important in light of the fact that most viruses have evolved effective means to evade cellular immune detection.
C. Protein-protein interactions involved in viral immune evasion
The efficacy of immune response is influenced by the dynamic balance between host defense and the ability of viruses to block these host defense mechanisms. In fact, viral immune evasion mechanisms are not only detrimental from a clinical perspective, but the lack of understanding of these evasion mechanisms can also impede basic research. Without the knowledge of how viruses block host immune responses, the mechanisms for how DNA sensors trigger immune signaling are challenging to decipher. In this section we review viral proteins that impede viral DNA sensing pathways and proteins.
One mechanism by which viruses block immune signaling is through direct sequestration of anti-viral host factors. AP-MS has proven invaluable for identifying unknown host factors that are targeted by viral proteins27. These studies were instrumental in elucidating mechanisms of viral evasion during DNA sensing and promotion of a pro-viral replicative environment (Figure 4). For example, an AP-MS study determined that IFI16 is targeted by the viral tegument protein pUL83 during infection with HCMV102. Later on, a multidisciplinary study, integrating mass spectrometry and domain mapping, demonstrated that pUL83 targets specifically the pyrin domain of IFI16 to block its ability to oligomerize92 (Figure 4). This inhibition by pUL83 was shown to impede the ability of IFI16 to stimulate STING/TBK1/IRF3-dependent IFN and proinflammatory cytokine production in human fibroblasts92. Interestingly, pUL83 was shown to also inhibit the oligomerization of the IFIX pyrin domain, suggesting that IFIX may also have antiviral functions inhibited by this viral protein during HCMV infection; more studies are needed to investigate this possibility. Additionally, this AP-MS study also identified numerous phosphorylation sites on pUL83. Interestingly, phosphorylation at Ser364 by a host kinase was shown to lead to a partial rescue of IFI16 oligomerization92. This may reflect a host response to release a subset of IFI16 for signaling. Therefore, omic approaches can provide valuable insights into the dynamic virus-host interplay, whereby both have evolved strategies to foster their survival.
Figure 4. Mechanisms involved in viral immune evasion.
Several viral proteins known to inhibit DNA sensing are depicted. The HCMV pUL83 binds to the IFI16 pyrin (PY) domain, inhibiting IFI16 oligomerization and the transmission of immune signals through the STING-dependent pathway. Phosphorylation of pUL83 seems to rescue IFI16 oligomerization. The HSV-1 protein ICP0 is thought to be partly involved in the degradation of IFI16 following infection, thereby preventing IFN induction. Other viral proteins inhibit the STING/IRF IFN induction pathway directly. HSV-1 ICP34.5 prevents TBK-1 phosphorylation and activation. The VZV proteins ORF62 and ORF61 prevent IRF3 from being phosphorylated by TBK1 and targeted for degradation, respectively. Finally, ICP0 causes IRF3 to be segregated in the nucleus and prevents it from up-regulating IFN production.
In addition to the role of HCMV pUL83 in inhibiting pyrin domain oligomerization and IFI16 immune signaling, the recruitment of IFI16 by pUL83 was also shown to play a role in regulating viral transcription102. Specifically, pUL83 targets IFI16 to the HCMV major immediate-early promoter, and this recruitment seems to be necessary for the stimulation of viral transcription102. More recently, the ability of IFI16 to modulate viral transcription has been expanded. One study demonstrated that, during HCMV infection, IFI16 bound to Sp1 binding regions to inhibit sp1-like transcription factors, leading to a decrease in virus replication103. Another study showed that, during HSV-1 infection, IFI16 is connected to heterochromatin marks on the viral genome, and associated with the transcriptional repression of HSV-1 genomes104. Furthermore, IFI16 knockdown led to an increase in transcriptionally active histone marks on HSV-1 DNA104. Altogether, these studies indicate that IFI16 can act as an antiviral factor both through stimulation of an immune response upon sensing of viral DNA and through its transcriptional regulation functions. Both of these antiviral functions seem to be targeted by viral proteins for inhibition of host defense.
Another mechanism by which viruses block immune signaling is through degradation of anti-viral host factors. Orzalli et. al. has demonstrated that the HSV-1 immediate early protein ICP0 can abrogate nuclear IFI16 expression and sensing24 (Figure 4). During HSV-1 infection, IFI16 was observed to be rapidly targeted for proteasomal degradation, which was shown to be in part dependent on the E3 ubiquitin ligase activity of ICP024 provided by its RING finger domain105. While these studies imply the involvement of IFI16 ubiquitination, the sites of modification remain to be determined. Furthermore, the sole involvement of ICP0 or the requirement of other viral factors for IFI16 degradation is still being debated106. The ectopic expression of ICP0 alone, in the absence of infection, was not sufficient to degrade IFI16; however, infection with an ICP0 null virus in conjunction with the expression of the HCMV immediate-early protein IE1 was able to largely recapitulate IFI16 degradation106. Indeed, another study used an ICP0 mutant virus that lacks E3 ligase activity to show that ICP0 is necessary, but not sufficient, for the degradation of IFI16107. The mechanism involved in IFI16 degradation requires further investigation, but, collectively, these studies suggest that IFI16 degradation during infection is dependent on the function of herpesvirus immediate-early proteins. Nevertheless, it is interesting that related herpesviruses, such as HSV-1 and HCMV, have evolved different means for blocking IFI16 antiviral functions. While HSV-1 adopts a destructive approach, HCMV recruits IFI16, which possibly better suits its longer infection life cycle. Altogether, this knowledge regarding viral immune evasion mechanism is critical for gaining a better understanding of the mechanisms involved in sensing of viral DNA. As IFI16 is able to trigger an immune response following infection with the ICP0 mutant virus, a proteomic study took advantage of this knowledge to characterize IFI16 protein interactions in the context of HSV-1 infection107. Interestingly, by using AP-MS followed by validation studies, IFI16 was shown to interact with components of cellular promylocytic leukemia (PML) nuclear bodies during infection with the HSV-1 ICP0 mutant virus. By similar means, another study demonstrated that IFIX also associates with PML nuclear bodies; however, this association was already detected in uninfected cells26. It remains to be determined whether this interaction also occurs during infection. The interaction of these sensors with PML nuclear bodies is intriguing, given that PML bodies are known to localize to herpesvirus replication compartments108. It is tempting to speculate that these associations are playing a role in recruiting these sensors to viral DNA to facilitate immune response. Another possibility is that these interactions have antiviral functions through other means, such as transcriptional repression of the viral genome or initiation of apoptosis, as PML bodies have been shown to possess both functions109.
Viral proteins have been shown to inhibit other viral DNA sensors following diverse infections. For example, the C16 protein of VACV binds the Ku70/80 complex, thereby impeding the ability of the Ku complex to bind DNA110. This disruption in DNA binding leads to a decrease in antiviral cytokines110. Other viral proteins counter immune response by directly inhibiting the downstream signaling factors, IRF3 and/or TBK1 (Figure 4). These viral proteins include the Varicella-zoster herpesvirus (VZV) immediate early proteins encoded by ORFs 61 and 62, and the HSV-1 proteins ICP0 and ICP34.5. In VZV- infected HEK293 cells, the E3 ligase ORF61 triggers the specific ubiquitination and degradation of active, phosphorylated IRF-3111. In human embryonic lung fibroblasts, ORF62 was shown to inhibit phosphorylation of IRF3 by TBK1112. While the specific mechanism remains to be fully understood, it was demonstrated that ORF62 did not bind to or cause the degradation of either IRF3 or TBK112. The HSV-1 protein ICP34.5 was also reported to interact with TBK1, which inhibited its interaction and phosphorylation of IRF3113. An alternative HSV-1 mechanism for inhibiting IRF3 involves ICP0. In this case ICP0 does not disrupt IRF3 phosphorylation or dimerization, but instead sequesters activated IRF3 in complex with its cofactors CBP/p300 to nuclear foci away from host chromatin114. Other HSV-1 proteins have been shown to interfere with IRF3 and/or IFN responses (as reviewed in 115), highlighting the elaborate mechanisms used by viruses to suppress host immune responses. Interestingly, the ability of viruses to block immune responses was also recently connected to the establishment of a latent infection. During infection with the murine gammaherpesvirus 68 (MHV68), the inhibition of the STING-dependent pathway by the viral tegument ORF64 deubiquitinase was shown to be important for the ability of the virus to establish a latent reservoir116.
Closing Remarks
The exciting and relatively young field of DNA sensing has significantly expanded the understanding of mammalian immune response to virus infections. Here, we reviewed the current knowledge of viral DNA sensors, including the viruses these sensors detect and the activated cellular pathways associated with host defenses. A number of viral DNA sensors have been identified, and, importantly, recent studies have demonstrated that these defense factors can recognize viral DNA in both the cytoplasm and nuclei of infected cells. As expected for an emerging field in which many mechanisms are still to be fully elucidated, some interesting, but seemingly, paradoxical findings have been described. For example, IFI16 and cGAS have both been shown to act as cytoplasmic viral DNA sensors and to be required for IFN expression in macrophages infected with HIV. However, an involvement for cGAS in nuclear sensing has not been observed. It is also not yet known if IFI16 and cGAS work together in the same signaling cascade or if their activities are independent of one another within parallel pathways. Given the knowledge of a number of viral DNA sensors, and the possible future identification of additional defense factors, it becomes increasingly important to understand their possible coordinated or redundant functions. Also remaining to be further examined is how these immune signaling pathways are regulated since, in certain cell types, multiple pathways have been shown to be active. For example, AIM2 inflammasomes have been observed in response to MCMV in macrophages, but not in response to another herpesvirus, HSV-186. Conversely, IFI16 has been shown to mediate IFN expression in response to HSV-1 in the same cell type19. The opposite regulation of PYHIN proteins has also been observed in keratinocytes infected with HPV-16, where AIM2 inflammasomes were formed, while IFI16 was downregulated; however, in the absence of AIM2, IFI16-dependent IFN response was upregulated88. Thus, there may be a coordinated regulation of these sensing pathways that prevents the cell from producing a detrimental overactivation of immune responses. This is relevant, as overactivation of the immune system is known to contribute to the development of autoimmune diseases117. The mechanisms involved in regulating nucleic acid sensors in this context have been the focus of recent work118; 119; 120. In this review, we have also summarized the contribution of omic technologies to the identification of viral DNA sensors and to characterizing the mechanisms involved in host defense or in viral immune evasion. The implementation of modern omic technologies is expected to continue to enhance the knowledge of viral DNA sensing. Specifically, the use of quantitative mass spectrometry-based proteomics promises to provide a better understanding of the dynamic nature of protein interactions during infection. Furthermore, there is still limited information regarding the impact of posttranslational modification on the activation or inhibition of protein interactions and downstream signaling pathways. While phosphorylation, acetylation, and ubiquitination have been reported to modulate DNA sensing pathways, other PTMs are likely to play critical roles in immune response. Therefore, future omic studies can continue to significantly contribute to this important and still emerging field of DNA sensing.
Table 2. PTMs involved in regulating DNA sensing pathways.
Overview of the types and sites of posttranslational modifications shown to have roles in regulating DNA sensors and/or downstream pathway components. The function of each of the modifications, as well as the protein responsible for the modification, are indicated when known.
| Protein | Modification | Site | Function | Modifying protein | Refs |
|---|---|---|---|---|---|
| TBK1 | Phosphorylation | Ser172 | Activation | TBK-1 | 58; 59 |
| Ubiquitination | Lys48 | Degradation | DTX4 | 119 | |
| IRF3 | Phosphorylation | Ser385 or Ser386 Ser396 – Ser405 | Removal of autoinhibition, Dimerization and activation | TBK-1 | 60; 61; 62; 63; 64; 65; 66; 67; 68 |
| Ser173/Ser175 | Constitutive | 68 | |||
| Thr39 | n/a (occurs during infection) | 68 | |||
| Ubiquitination | - | Degradation | ORF61 (VZV) | 111 | |
| IFI16 | Acetylation | Lys99 Lys128 |
Cytoplasmic localization | p300 | 23 |
| ASC | Phosphorylation | Tyr146 | Aggregation | - | 81 |
| Ubiquitination | - | Inhibits aggregation | - | 85 | |
| RIP3 | Phosphorylation | - | Promotes IKK complex activation | RIP3 (autophosphorylation upon DAI binding) | 94 |
| Vp5 (HSV-1) | Ubiquitination | - | Capsid degradation | - | 19 |
| pUL83 (HCMV) | Phosphorylation | Ser364 | Prevents pul83 mediated IFI16 signaling inhibition | - | 92 |
| DDX41 | Ubiquitination | Lys9 Lys115 |
Degradation | Trim21 | 120 |
Acknowledgments
This work was supported by NIH grants R21AI102187 and R21HD073044 to IMC. We thank Todd Greco and Benjamin Diner, two members of our lab, for careful review of this manuscript.
References
- 1.Krutzik SR, Sieling PA, Modlin RL. The role of Toll-like receptors in host defense against microbial infection. Current Opinion in Immunology. 2001;13:104–108. doi: 10.1016/s0952-7915(00)00189-8. [DOI] [PubMed] [Google Scholar]
- 2.Brown GD, Gordon S. Immune recognition of fungal β-glucans. Cellular Microbiology. 2005;7:471–479. doi: 10.1111/j.1462-5822.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 3.Maizels R, Hewitson J. Immune Recognition of Parasite Glycans. In: Kosma P, Müller-Loennies S, editors. In Anticarbohydrate Antibodies. Springer; Vienna: 2012. pp. 161–180. [Google Scholar]
- 4.Yoneyama M, Fujita T. Function of RIG-I-like Receptors in Antiviral Innate Immunity. Journal of Biological Chemistry. 2007;282:15315–15318. doi: 10.1074/jbc.R700007200. [DOI] [PubMed] [Google Scholar]
- 5.Loo YM, Gale M., Jr Immune Signaling by RIG-I-like Receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 7.Bird AP. DNA methylation and the frequency of CpG in animal DNA. Nucleic Acids Research. 1980;8:1499–1504. doi: 10.1093/nar/8.7.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 9.Takeshita F, Leifer CA, Gursel I, Ishii KJ, Takeshita S, Gursel M, Klinman DM. Cutting Edge: Role of Toll-Like Receptor 9 in CpG DNA-Induced Activation of Human Cells. The Journal of Immunology. 2001;167:3555–3558. doi: 10.4049/jimmunol.167.7.3555. [DOI] [PubMed] [Google Scholar]
- 10.Hoelzer K, Shackelton LA, Parrish CR. Presence and role of cytosine methylation in DNA viruses of animals. Nucleic Acids Research. 2008;36:2825–2837. doi: 10.1093/nar/gkn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malmgaard L, Melchjorsen J, Bowie AG, Mogensen SC, Paludan SR. Viral Activation of Macrophages through TLR-Dependent and -Independent Pathways. The Journal of Immunology. 2004;173:6890–6898. doi: 10.4049/jimmunol.173.11.6890. [DOI] [PubMed] [Google Scholar]
- 12.Hochrein H, Schlatter B, O'Keeffe M, Wagner C, Schmitz F, Schiemann M, Bauer S, Suter M, Wagner H. Herpes simplex virus type-1 induces IFN-α production via Toll-like receptor 9-dependent and -independent pathways. Proc Natl Acad Sci U S A. 2004;101:11416–11421. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stetson DB, Medzhitov R. Recognition of Cytosolic DNA Activates an IRF3-Dependent Innate Immune Response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Ishii KJ, Coban C, Kato H, Takahashi K, Torii Y, Takeshita F, Ludwig H, Sutter G, Suzuki K, Hemmi H, Sato S, Yamamoto M, Uematsu S, Kawai T, Takeuchi O, Akira S. Erratum: A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:427–427. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 15.Ablasser A, Bauernfeind F, Hartmann G, Latz E, Fitzgerald KA, Hornung V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol. 2009;10:1065–1072. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Taniguchi T. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 17.Kondo T, Kobayashi J, Saitoh T, Maruyama K, Ishii KJ, Barber GN, Komatsu K, Akira S, Kawai T. DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proceedings of the National Academy of Sciences. 2013;110:2969–2974. doi: 10.1073/pnas.1222694110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang P, An H, Liu X, Wen M, Zheng Y, Rui Y, Cao X. The cytosolic nucleic acid sensor LRRFIP1 mediates the production of type I interferon via a [beta]-catenin-dependent pathway. Nat Immunol. 2010;11:487–494. doi: 10.1038/ni.1876. [DOI] [PubMed] [Google Scholar]
- 19.Horan KA, Hansen K, Jakobsen MR, Holm CK, Søby S, Unterholzner L, Thompson M, West JA, Iversen MB, Rasmussen SB, Ellermann-Eriksen S, Kurt-Jones E, Landolfo S, Damania B, Melchjorsen J, Bowie AG, Fitzgerald KA, Paludan SR. Proteasomal Degradation of Herpes Simplex Virus Capsids in Macrophages Releases DNA to the Cytosol for Recognition by DNA Sensors. The Journal of Immunology. 2013;190:2311–2319. doi: 10.4049/jimmunol.1202749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like Receptor 9–mediated Recognition of Herpes Simplex Virus-2 by Plasmacytoid Dendritic Cells. The Journal of Experimental Medicine. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor. 2004;9:103. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- 22.Kerur N, Veettil MV, Sharma-Walia N, Bottero V, Sadagopan S, Otageri P, Chandran B. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe. 2011;9:363–75. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T, Diner BA, Chen J, Cristea IM. Acetylation modulates cellular distribution and DNA sensing ability of interferon-inducible protein IFI16. Proc Natl Acad Sci U S A. 2012;109:10558–63. doi: 10.1073/pnas.1203447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orzalli MH, DeLuca NA, Knipe DM. Nuclear IFI16 induction of IRF-3 signaling during herpesviral infection and degradation of IFI16 by the viral ICP0 protein. Proc Natl Acad Sci U S A. 2012;109:E3008–17. doi: 10.1073/pnas.1211302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li T, Chen J, Cristea IM. Human cytomegalovirus tegument protein pUL83 inhibits IFI16-mediated DNA sensing for immune evasion. Cell Host Microbe. 2013;14:591–9. doi: 10.1016/j.chom.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diner BA, Li T, Greco TM, Crow MS, Fuesler JA, Wang J, Cristea IM. The functional interactome of PYHIN immune regulators reveals IFIX is a sensor of viral DNA. Mol Syst Biol. 2015;11:787. doi: 10.15252/msb.20145808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greco TM, Diner BA, Cristea IM. The Impact of Mass Spectrometry–Based Proteomics on Fundamental Discoveries in Virology. Annual Review of Virology. 2014;1:581–604. doi: 10.1146/annurev-virology-031413-085527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akira S, Uematsu S, Takeuchi O. Pathogen Recognition and Innate Immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Rathinam VAK, Fitzgerald KA. Innate immune sensing of DNA viruses. Virology. 2011;411:153–162. doi: 10.1016/j.virol.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gürtler C, Bowie AG. Innate immune detection of microbial nucleic acids. Trends in Microbiology. 2013;21:413–420. doi: 10.1016/j.tim.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 32.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, Fitzgerald KA, Paludan SR, Bowie AG. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Brann TW, Zhou M, Yang J, Oguariri RM, Lidie KB, Imamichi H, Huang DW, Lempicki RA, Baseler MW, Veenstra TD, Young HA, Lane HC, Imamichi T. Cutting Edge: Ku70 Is a Novel Cytosolic DNA Sensor That Induces Type III Rather Than Type I IFN. The Journal of Immunology. 2011;186:4541–4545. doi: 10.4049/jimmunol.1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferguson BJ, Mansur DS, Peters NE, Ren H, Smith GL. In: DNA-PK is a DNA sensor for IRF-3-dependent innate immunity. Medzhitov R, editor. 2012. p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim T, Pazhoor S, Bao M, Zhang Z, Hanabuchi S, Facchinetti V, Bover L, Plumas J, Chaperot L, Qin J, Liu YJ. Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proceedings of the National Academy of Sciences. 2010;107:15181–15186. doi: 10.1073/pnas.1006539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiu YH, MacMillan JB, Chen ZJ. RNA Polymerase III Detects Cytosolic DNA and Induces Type I Interferons through the RIG-I Pathway. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP Synthase Is a Cytosolic DNA Sensor That Activates the Type I Interferon Pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, Kim T, Bao M, Facchinetti V, Jung Sung Y, Ghaffari Amir A, Qin J, Cheng G, Liu YJ. DDX1, DDX21, and DHX36 Helicases Form a Complex with the Adaptor Molecule TRIF to Sense dsRNA in Dendritic Cells. Immunity. 2011;34:866–878. doi: 10.1016/j.immuni.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z, Yuan B, Bao M, Lu N, Kim T, Liu YJ. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol. 2011;12:959–965. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hellman LM, Fried MG. Electrophoretic mobility shift assay (EMSA) for detecting protein-nucleic acid interactions. Nat Protoc. 2007;2:1849–61. doi: 10.1038/nprot.2007.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taouji S, Dahan S, Bossé R, Chevet E. Current Screens Based on the AlphaScreen™ Technology for Deciphering Cell Signalling Pathways. Current Genomics. 2009;10:93–101. doi: 10.2174/138920209787847041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–13. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–8. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cornélie S, Hoebeke J, Schacht AM, Bertin B, Vicogne J, Capron M, Riveau G. Direct Evidence that Toll-like Receptor 9 (TLR9) Functionally Binds Plasmid DNA by Specific Cytosine-phosphate-guanine Motif Recognition. Journal of Biological Chemistry. 2004;279:15124–15129. doi: 10.1074/jbc.M313406200. [DOI] [PubMed] [Google Scholar]
- 45.Zhou W, Li Y, Pan X, Gao Y, Li B, Qiu Z, Liang L, Zhou H, Yue J. Toll-like receptor 9 interaction with CpG ODN - An in silico analysis approach. Theoretical Biology and Medical Modelling. 2013;10:18. doi: 10.1186/1742-4682-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Mol N. Surface Plasmon Resonance for Proteomics. In: Zanders ED, editor. Chemical Genomics and Proteomics. Vol. 800. Humana Press; 2012. pp. 33–53. [DOI] [PubMed] [Google Scholar]
- 47.Miyashita M, Oshiumi H, Matsumoto M, Seya T. DDX60, a DEXD/H Box Helicase, Is a Novel Antiviral Factor Promoting RIG-I-Like Receptor-Mediated Signaling. Molecular and Cellular Biology. 2011;31:3802–3819. doi: 10.1128/MCB.01368-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin T, Perry A, Jiang J, Smith P, Curry JA, Unterholzner L, Jiang Z, Horvath G, Rathinam VA, Johnstone RW, Hornung V, Latz E, Bowie AG, Fitzgerald KA, Xiao TS. Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity. 2012;36:561–71. doi: 10.1016/j.immuni.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Civril F, Deimling T, de Oliveira Mann CC, Ablasser A, Moldt M, Witte G, Hornung V, Hopfner KP. Structural mechanism of cytosolic DNA sensing by cGAS. Nature. 2013;498:332–337. doi: 10.1038/nature12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaw N, Liu ZJ. Role of the HIN Domain in Regulation of Innate Immune Responses. Molecular and Cellular Biology. 2014;34:2–15. doi: 10.1128/MCB.00857-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin T, Perry A, Smith P, Jiang J, Xiao TS. Structure of the absent in melanoma 2 (AIM2) pyrin domain provides insights into the mechanisms of AIM2 autoinhibition and inflammasome assembly. J Biol Chem. 2013;288:13225–35. doi: 10.1074/jbc.M113.468033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X, Wu J, Du F, Xu H, Sun L, Chen Z, Brautigam Chad A, Zhang X, Chen Zhijian J. The Cytosolic DNA Sensor cGAS Forms an Oligomeric Complex with DNA and Undergoes Switch-like Conformational Changes in the Activation Loop. Cell Reports. 6:421–430. doi: 10.1016/j.celrep.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schoggins JW, MacDuff DA, Imanaka N, Gainey MD, Shrestha B, Eitson JL, Mar KB, Richardson RB, Ratushny AV, Litvak V, Dabelic R, Manicassamy B, Aitchison JD, Aderem A, Elliott RM, Garcia-Sastre A, Racaniello V, Snijder EJ, Yokoyama WM, Diamond MS, Virgin HW, Rice CM. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505:691–695. doi: 10.1038/nature12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qin BY, Liu C, Lam SS, Srinath H, Delston R, Correia JJ, Derynck R, Lin K. Crystal structure of IRF-3 reveals mechanism of autoinhibition and virus-induced phosphoactivation. Nat Struct Mol Biol. 2003;10:913–921. doi: 10.1038/nsb1002. [DOI] [PubMed] [Google Scholar]
- 55.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F, Lei C, He X, Zhang L, Tien P, Shu HB. The Adaptor Protein MITA Links Virus-Sensing Receptors to IRF3 Transcription Factor Activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 57.Saitoh T, Fujita N, Hayashi T, Takahara K, Satoh T, Lee H, Matsunaga K, Kageyama S, Omori H, Noda T, Yamamoto N, Kawai T, Ishii K, Takeuchi O, Yoshimori T, Akira S. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proceedings of the National Academy of Sciences. 2009;106:20842–20846. doi: 10.1073/pnas.0911267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishii KJ, Kawagoe T, Koyama S, Matsui K, Kumar H, Kawai T, Uematsu S, Takeuchi O, Takeshita F, Coban C, Akira S. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–U6. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki T, Oshiumi H, Miyashita M, Aly HH, Matsumoto M, Seya T. Cell Type-Specific Subcellular Localization of Phospho-TBK1 in Response to Cytoplasmic Viral DNA. PLoS ONE. 2013;8:e83639. doi: 10.1371/journal.pone.0083639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hiscott John, P P, Genin Pierre, Nguyen Hannah, Heylbroeck Christophe, Mamane Yael, Algarte Michele, Lin Rongtuan. Triggering the Interferon Response: The Role of IRF-3 Transcription Factor. Journal of Interferon & Cytokine Research. 1999;19(1):1–13. doi: 10.1089/107999099314360. [DOI] [PubMed] [Google Scholar]
- 61.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKK[epsi] and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 62.Lin RT, Heylbroeck C, Pitha PM, Hiscott J. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Molecular and Cellular Biology. 1998;18:2986–2996. doi: 10.1128/mcb.18.5.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin RT, Mamane Y, Hiscott J. Structural and functional analysis of interferon regulatory factor 3: Localization of the transactivation and autoinhibitory domains. Molecular and Cellular Biology. 1999;19:2465–2474. doi: 10.1128/mcb.19.4.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qin BY, Liu C, Lam SS, Srinath H, Delston R, Correia JJ, Derynck R, Lin K. Crystal structure of IRF-3 reveals mechanism of autoinhibition and virus-induced phosphoactivation. Nature Structural Biology. 2003;10:913–921. doi: 10.1038/nsb1002. [DOI] [PubMed] [Google Scholar]
- 65.Qin BY, Liu C, Srinath H, Lam SS, Correia JJ, Derynck R, Lin K. Crystal structure of IRF-3 in complex with CBP. Structure. 2005;13:1269–1277. doi: 10.1016/j.str.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 66.Yoneyama M, Suhara W, Fukuhara Y, Fukuda M, Nishida E, Fujita T. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. Embo Journal. 1998;17:1087–1095. doi: 10.1093/emboj/17.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Panne D, McWhirter SM, Maniatis T, Harrison SC. Interferon regulatory factor 3 is regulated by a dual phosphorylation-dependent switch. Journal of Biological Chemistry. 2007;282:22816–22822. doi: 10.1074/jbc.M703019200. [DOI] [PubMed] [Google Scholar]
- 68.Bergstroem B, Johnsen IB, Nguyen TT, Hagen L, Slupphaug G, Thommesen L, Anthonsen MW. Identification of a Novel in Vivo Virus-targeted Phosphorylation Site in Interferon Regulatory Factor-3 (IRF3) Journal of Biological Chemistry. 2010;285:24904–24914. doi: 10.1074/jbc.M109.084822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wathelet MG, Lin CH, Parekh BS, Ronco LV, Howley PM, Maniatis T. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Molecular Cell. 1998;1:507–518. doi: 10.1016/s1097-2765(00)80051-9. [DOI] [PubMed] [Google Scholar]
- 70.Jakobsen MR, Bak RO, Andersen A, Berg RK, Jensen SB, Jin T, Laustsen A, Hansen K, Østergaard L, Fitzgerald KA, Xiao TS, Mikkelsen JG, Mogensen TH, Paludan SR. IFI16 senses DNA forms of the lentiviral replication cycle and controls HIV-1 replication. Proceedings of the National Academy of Sciences. 2013;110:E4571–E4580. doi: 10.1073/pnas.1311669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. Cyclic GMP-AMP Is an Endogenous Second Messenger in Innate Immune Signaling by Cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Rohl I, Hopfner KP, Ludwig J, Hornung V. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498:380–4. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hansen K, Prabakaran T, Laustsen A, Jørgensen SE, Rahbæk SH, Jensen SB, Nielsen R, Leber JH, Decker T, Horan KA, Jakobsen MR, Paludan SR. Listeria monocytogenes induces IFNβ expression through an IFI16-, cGAS- and STING-dependent pathway. 2014:33. doi: 10.15252/embj.201488029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stein SC, Falck-Pedersen E. Sensing Adenovirus Infection: Activation of Interferon Regulatory Factor 3 in RAW 264.7 Cells. Journal of Virology. 2012;86:4527–4537. doi: 10.1128/JVI.07071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith GC, Jackson SP. The DNA-dependent protein kinase. Genes Dev. 1999;13:916–34. doi: 10.1101/gad.13.8.916. [DOI] [PubMed] [Google Scholar]
- 76.Lee HC, Narayanan S, Park SJ, Seong SY, Hahn YS. Transcriptional Regulation of IFN-λ Genes in Hepatitis C Virus-infected Hepatocytes via Irf-3·Irf-7·nf-κb Complex. Journal of Biological Chemistry. 2014;289:5310–5319. doi: 10.1074/jbc.M113.536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Upton Jason W, Kaiser William J, Mocarski Edward S. DAI/ZBP1/DLM-1 Complexes with RIP3 to Mediate Virus-Induced Programmed Necrosis that Is Targeted by Murine Cytomegalovirus vIRA. Cell Host & Microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunological Reviews. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hara H, Tsuchiya K, Kawamura I, Fang R, Hernandez-Cuellar E, Shen Y, Mizuguchi J, Schweighoffer E, Tybulewicz V, Mitsuyama M. Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat Immunol. 2013;14:1247–1255. doi: 10.1038/ni.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martin BN, Wang C, Willette-Brown J, Herjan T, Gulen MF, Zhou H, Bulek K, Franchi L, Sato T, Alnemri ES, Narla G, Zhong XP, Thomas J, Klinman D, Fitzgerald KA, Karin M, Nunez G, Dubyak G, Hu Y, Li X. IKK alpha negatively regulates ASC-dependent inflammasome activation. Nature Communications. 2014;5 doi: 10.1038/ncomms5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, Zou Y, Erlandsson-Harris H, Yang H, Ting JPY, Wang H, Andersson U, Antoine DJ, Chavan SS, Hotamisligil GS, Tracey KJ. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He Y, Franchi L, Núñez G. The protein kinase PKR is critical for LPS-induced iNOS production but dispensable for inflammasome activation in macrophages. European Journal of Immunology. 2013;43:1147–1152. doi: 10.1002/eji.201243187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lopez-Castejon G, Luheshi NM, Compan V, High S, Whitehead RC, Flitsch S, Kirov A, Prudovsky I, Swanton E, Brough D. Deubiquitinases Regulate the Activity of Caspase-1 and Interleukin-1β Secretion via Assembly of the Inflammasome. Journal of Biological Chemistry. 2013;288:2721–2733. doi: 10.1074/jbc.M112.422238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhen J, Zhang L, Pan J, Ma S, Yu X, Li X, Chen S, Du W. AIM2 Mediates Inflammation-Associated Renal Damage in Hepatitis B Virus-Associated Glomerulonephritis by Regulating Caspase-1, IL-1β, and IL-18. Mediators of Inflammation. 2014;2014:9. doi: 10.1155/2014/190860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reinholz M, Kawakami Y, Salzer S, Kreuter A, Dombrowski Y, Koglin S, Kresse S, Ruzicka T, Schauber J. HPV16 activates the AIM2 inflammasome in keratinocytes. Arch Dermatol Res. 2013 doi: 10.1007/s00403-013-1375-0. [DOI] [PubMed] [Google Scholar]
- 89.Johnson KE, Chikoti L, Chandran B. Herpes Simplex Virus 1 Infection Induces Activation and Subsequent Inhibition of the IFI16 and NLRP3 Inflammasomes. J Virol. 2013;87:5005–5018. doi: 10.1128/JVI.00082-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Singh VV, Kerur N, Bottero V, Dutta S, Chakraborty S, Ansari MA, Paudel N, Chikoti L, Chandran B. Kaposi's Sarcoma-Associated Herpesvirus Latency in Endothelial and B Cells Activates Gamma Interferon-Inducible Protein 16-Mediated Inflammasomes. Journal of Virology. 2013;87:4417–4431. doi: 10.1128/JVI.03282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ansari MA, Singh VV, Dutta S, Veettil MV, Dutta D, Chikoti L, Lu J, Everly D, Chandran B. Constitutive Interferon-Inducible Protein 16-Inflammasome Activation during Epstein-Barr Virus Latency I, II, and III in B and Epithelial Cells. Journal of Virology. 2013;87:8606–8623. doi: 10.1128/JVI.00805-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li T, Chen J, Cristea Ileana M. Human Cytomegalovirus Tegument Protein pUL83 Inhibits IFI16-Mediated DNA Sensing for Immune Evasion. Cell Host Microbe. 2013;14:591–599. doi: 10.1016/j.chom.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaiser WJ, Upton JW, Mocarski ES. Journal of immunology. Vol. 181. Baltimore, Md: 2008. Receptor-Interacting Protein Homotypic Interaction Motif-Dependent Control of nf-κb Activation via the DNA-Dependent Activator of IFN Regulatory Factors; pp. 6427–6434. 1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rebsamen M, Heinz LX, Meylan E, Michallet MC, Schroder K, Hofmann K, Vazquez J, Benedict CA, Tschopp J. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate nf-κb. EMBO Reports. 2009;10:916–922. doi: 10.1038/embor.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem. 2002;277:9505–11. doi: 10.1074/jbc.M109488200. [DOI] [PubMed] [Google Scholar]
- 96.Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, Tschopp J. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–7. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 97.Rebsamen M, Tschopp J. Viruses under the control of RIP kinases. Cell Cycle. 2010;9:438–439. doi: 10.4161/cc.9.3.10776. [DOI] [PubMed] [Google Scholar]
- 98.Devin A, Cook A, Lin Y, Rodriguez Y, Kelliher M, Liu Zg. The Distinct Roles of TRAF2 and RIP in IKK Activation by TNF-R1: TRAF2 Recruits IKK to TNF-R1 while RIP Mediates IKK Activation. Immunity. 2000;12:419–429. doi: 10.1016/s1074-7613(00)80194-6. [DOI] [PubMed] [Google Scholar]
- 99.Melchjorsen J, Rintahaka J, Søby S, Horan KA, Poltajainen A, Østergaard L, Paludan SR, Matikainen S. Early Innate Recognition of Herpes Simplex Virus in Human Primary Macrophages Is Mediated via the MDA5/MAVS-Dependent and MDA5/MAVS/RNA Polymerase III-Independent Pathways. Journal of Virology. 2010;84:11350–11358. doi: 10.1128/JVI.01106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Choi MK, Wang Z, Ban T, Yanai H, Lu Y, Koshiba R, Nakaima Y, Hangai S, Savitsky D, Nakasato M, Negishi H, Takeuchi O, Honda K, Akira S, Tamura T, Taniguchi T. A selective contribution of the RIG-I-like receptor pathway to type I interferon responses activated bycytosolic DNA. Proceedings of the National Academy of Sciences. 2009;106:17870–17875. doi: 10.1073/pnas.0909545106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, Taya C, Taniguchi T. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–40. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 102.Cristea IM, Moorman NJ, Terhune SS, Cuevas CD, O'Keefe ES, Rout MP, Chait BT, Shenk T. Human cytomegalovirus pUL83 stimulates activity of the viral immediate-early promoter through its interaction with the cellular IFI16 protein. J Virol. 2010;84:7803–14. doi: 10.1128/JVI.00139-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gariano GR, Dell'Oste V, Bronzini M, Gatti D, Luganini A, De Andrea M, Gribaudo G, Gariglio M, Landolfo S. The Intracellular DNA Sensor IFI16 Gene Acts as Restriction Factor for Human Cytomegalovirus Replication. PLoS Pathog. 2012;8:e1002498. doi: 10.1371/journal.ppat.1002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Orzalli MH, Conwell SE, Berrios C, DeCaprio JA, Knipe DM. Nuclear interferon-inducible protein 16 promotes silencing of herpesviral and transfected DNA. Proceedings of the National Academy of Sciences. 2013;110:E4492–E4501. doi: 10.1073/pnas.1316194110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boutell C, Sadis S, Everett RD. Herpes Simplex Virus Type 1 Immediate-Early Protein ICP0 and Its Isolated RING Finger Domain Act as Ubiquitin E3 Ligases In Vitro. Journal of Virology. 2002;76:841–850. doi: 10.1128/JVI.76.2.841-850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cuchet-Lourenço D, Anderson G, Sloan E, Orr A, Everett RD. The Viral Ubiquitin Ligase ICP0 Is neither Sufficient nor Necessary for Degradation of the Cellular DNA Sensor IFI16 during Herpes Simplex Virus 1 Infection. Journal of Virology. 2013;87:13422–13432. doi: 10.1128/JVI.02474-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Diner BA, Lum KK, Javitt A, Cristea IM. Interactions of the antiviral factor IFI16 mediate immune signaling and herpes simplex virus-1 immunosuppression. Molecular and Cellular Proteomics. 2015 doi: 10.1074/mcp.M114.047068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Burkham J, Coen DM, Weller SK. ND10 Protein PML Is Recruited to Herpes Simplex Virus Type 1 Prereplicative Sites and Replication Compartments in the Presence of Viral DNA Polymerase. Journal of Virology. 1998;72:10100–10107. doi: 10.1128/jvi.72.12.10100-10107.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Geoffroy MC, CA M. Role of promyelocytic leukemia protein in host antiviral defense. J Interferon Cytokine Res. 2011;31:145–158. doi: 10.1089/jir.2010.0111. [DOI] [PubMed] [Google Scholar]
- 110.Peters NE, Ferguson BJ, Mazzon M, Fahy AS, Krysztofinska E, Arribas-Bosacoma R, Pearl LH, Ren H, Smith GL. A Mechanism for the Inhibition of DNA-PK-Mediated DNA Sensing by a Virus. PLoS Pathog. 2013;9:e1003649. doi: 10.1371/journal.ppat.1003649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhu HF, Zheng CF, Xing JJ, Wang S, Li SP, Lin RT, Mossman KL. Varicella-Zoster Virus Immediate-Early Protein ORF61 Abrogates the IRF3-Mediated Innate Immune Response through Degradation of Activated IRF3. Journal of Virology. 2011;85:11079–11089. doi: 10.1128/JVI.05098-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sen N, Sommer M, Che X, White K, Ruyechan WT, Arvin AM. Varicella-Zoster Virus Immediate-Early Protein 62 Blocks Interferon Regulatory Factor 3 (IRF3) Phosphorylation at Key Serine Residues: a Novel Mechanism of IRF3 Inhibition among Herpesviruses. Journal of Virology. 2010;84:9240–9253. doi: 10.1128/JVI.01147-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Verpooten D, Ma Y, Hou S, Yan Z, He B. Control of TANK-binding Kinase 1-mediated Signaling by the γ134.5 Protein of Herpes Simplex Virus 1. Journal of Biological Chemistry. 2009;284:1097–1105. doi: 10.1074/jbc.M805905200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Melroe GT, Silva L, Schaffer PA, Knipe DM. Recruitment of activated IRF-3 and CBP/p300 to herpes simplex virus ICP0 nuclear foci: Potential role in blocking IFN-β induction. Virology. 2007;360:305–321. doi: 10.1016/j.virol.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mossman KL, P P. Mechanisms Employed by Herpes Simplex Virus 1 to Inhibit the Interferon Response. Journal of Interferon & Cytokine Research. 2009;29(9):599–608. doi: 10.1089/jir.2009.0074. [DOI] [PubMed] [Google Scholar]
- 116.Sun C, Schattgen SA, Pisitkun P, Jorgensen JP, Hilterbrand AT, Wang LJ, West JA, Hansen K, Horan KA, Jakobsen MR, O'Hare P, Adler H, Sun R, Ploegh HL, Damania B, Upton JW, Fitzgerald KA, Paludan SR. Evasion of Innate Cytosolic DNA Sensing by a Gammaherpesvirus Facilitates Establishment of Latent Infection. The Journal of Immunology. 2015;194:1819–1831. doi: 10.4049/jimmunol.1402495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Munz C, Lunemann JD, Getts MT, Miller SD. Antiviral immune responses: triggers of or triggered by autoimmunity? Nat Rev Immunol. 2009;9:246–58. doi: 10.1038/nri2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cui J, Zhu L, Xia X, Wang HY, Legras X, Hong J, Ji J, Shen P, Zheng S, Chen ZJ, Wang RF. NLRC5 Negatively Regulates the NF-?B and Type I Interferon Signaling Pathways. Cell. 2010;141:483–496. doi: 10.1016/j.cell.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cui J, Li Y, Zhu L, Liu D, Songyang Z, Wang HY, Wang RF. NLRP4 negatively regulates type I interferon signaling by targeting the kinase TBK1 for degradation via the ubiquitin ligase DTX4. Nat Immunol. 2012;13:387–395. doi: 10.1038/ni.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang ZQ, Bao MS, Lu N, Weng LY, Yuan B, Liu YJ. The E3 ubiquitin ligase TRIM21 negatively regulates the innate immune response to intracellular double-stranded DNA. Nature Immunology. 2013;14:172–178. doi: 10.1038/ni.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lam E, Stein S, Falck-Pedersen E. Adenovirus detection by the cGAS/STING/TBK1 DNA sensing cascade. Journal of Virology. 2013 doi: 10.1128/JVI.02702-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dai P, Wang W, Cao H, Avogadri F, Dai L, Drexler I, Joyce JA, Li XD, Chen Z, Merghoub T, Shuman S, Deng L. Modified Vaccinia Virus Ankara Triggers Type I IFN Production in Murine Conventional Dendritic Cells via a cGAS/STING-Mediated Cytosolic DNA-Sensing Pathway. PLoS Pathog. 2014;10:e1003989. doi: 10.1371/journal.ppat.1003989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.DeFilippis VR, Alvarado D, Sali T, Rothenburg S, Früh K. Human Cytomegalovirus Induces the Interferon Response via the DNA Sensor ZBP1. Journal of Virology. 2010;84:585–598. doi: 10.1128/JVI.01748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proceedings of the National Academy of Sciences. 2006;103:17343–17348. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhu J, Huang X, Yang Y. Innate Immune Response to Adenoviral Vectors Is Mediated by both Toll-Like Receptor-Dependent and -Independent Pathways. Journal of Virology. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rasmussen SB, Jensen SB, Nielsen C, Quartin E, Kato H, Chen ZJ, Silverman RH, Akira S, Paludan SR. Herpes simplex virus infection is sensed by both Toll-like receptors and retinoic acid-inducible gene- like receptors, which synergize to induce type I interferon production. Journal of General Virology. 2009;90:74–78. doi: 10.1099/vir.0.005389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]