Abstract
Hyperglycemia is the primary factor responsible for the microvascular, and to a lesser extent macrovascular, complications of diabetes. Despite this well-established relationship, approximately half of all type 2 diabetic patients in the US have a hemoglobin A1c (HbA1c) ≥7.0%. This is associated in part with the side effects, i.e., weight gain and hypoglycemia, of currently available antidiabetic agents and in part with the failure to utilize medications that reverse the basic pathophysiological defects present in patients with type 2 diabetes. The kidney has been shown to play a central role in the development of hyperglycemia by excessive production of glucose throughout the sleeping hours and enhanced reabsorption of filtered glucose by the renal tubules secondary to an increase in the threshold at which glucose spills into the urine. Recently, a new class of antidiabetic agents, the sodium-glucose cotransporter 2 (SGLT2) inhibitors, has been developed and approved for the treatment of patients with type 2 diabetes. In this review, we examine their mechanism of action, efficacy, safety, and place in the therapeutic armamentarium. Since the SGLT2 inhibitors have a unique mode of action that differs from all other oral and injectable antidiabetic agents, they can be used at all stages of the disease and in combination with all other antidiabetic medications.
Keywords: type 2 diabetes, kidney, sodium-glucose cotransport, SGLT2 inhibition
hyperglycemia is the sine qua non of type 2 diabetes mellitus (T2DM) and is the principal risk factor for the development of diabetic microvascular complications, i.e., retinopathy, nephropathy, and neuropathy. T2DM patients also manifest multiple other metabolic abnormalities including obesity, hypertension, and dyslipidemia, which complicate management of the diabetic patient and predispose to the development of atherosclerotic cardiovascular complications. The United Kingdom Prospective Diabetes Study (UKPDS) (95) and the Diabetes Control and Complications Trial (DCCT) (24) have demonstrated that for every 1% decrease in hemoglobin A1c (HbA1c) there is a 37% reduction in the risk of microvascular complications. However, despite the irrefutable evidence for the importance of maintaining optimal glycemic control (HbA1c <6.5–7%), approximately half of the people with diabetes in the United States and worldwide fail to achieve this target of glycemic control and manifest a HbA1c >7% (5a, 37). Weight gain and hypoglycemia, which are encountered with many current antihyperglycemic therapies, are major obstacles that preclude the achievement of the treatment goal in many T2DM patients.
Although lowering the HbA1c decreases the risk of microvascular complications, many clinical trials have demonstrated that tight glycemic control fails to produce a significant decrease in macrovascular events, which are the major cause of death in T2DM patients (4, 5, 25). Conversely, lowering blood pressure and correcting lipid abnormalities result in robust reductions in macrovascular risk (83). Thus antihyperglycemic agents that also promote weight loss, lower blood pressure, and improve the lipid profile are more likely to have a greater impact on diabetic morbidity and mortality than agents that only lower the plasma glucose concentration.
Inhibitors of renal sodium-glucose cotransport are a novel class of drugs that recently have been approved for the treatment of T2DM (1). In addition to lowering the plasma glucose concentration, members of this class also produce weight loss, lower the blood pressure, and are lipid neutral. In addition to lowering the HbA1c, sodium-glucose cotransporter 2 (SGLT2) inhibitors have the potential to reduce cardiovascular risk independent of lowering the plasma glucose concentration. In the present review, we examine the mechanism of action, clinical efficacy, and metabolic effects of SGLT2 inhibitors, as well as their place in the management of T2DM.
Renal Handling of Glucose
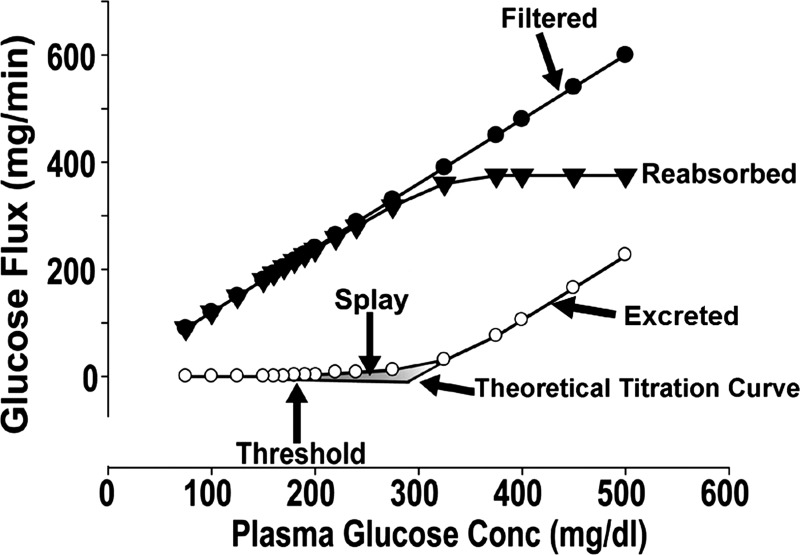
The kidney filters ∼180 liters of plasma each day. Thus, in normal glucose-tolerant (NGT) individuals with a mean day-long plasma glucose concentration of 100 mg/dl, ∼180 g of glucose is filtered every day. All of this glucose is reabsorbed in the proximal tubule, and no glucose is excreted in the urine. Two glucose transporters are responsible for renal glucose reabsorption (102). Both transporters are located in proximal tubule and couple glucose reabsorption to sodium reabsorption (99, 102). SGLT2 is located in the early (S1) proximal tubule and is responsible for the reabsorption of 80–90% of filtered glucose. SGLT1 is located in the more distal part of the proximal tubule (S2/S3) and is responsible for the reabsorption of the remaining 10–20% of filtered glucose (99, 102). The SGLT1 transporter also is expressed in the gut, heart, and lungs (99, 102). In the gut, it transports glucose and galactose and is responsible for the absorption of the majority of ingested glucose, as well as galactose. SGLT2 is highly localized to the kidney, thereby minimizing the potential for off-target effects. The maximum glucose transport capacity (Tm) of the proximal tubule, on average, is ∼375 mg/min and is slightly higher in men than in women (27).
In NGT individuals with a mean day-long plasma glucose concentration of ∼100 mg/dl, the filtered glucose load (∼125 mg/min or 180 g/day) is less than the maximal renal glucose transport capacity. Thus all of the filtered glucose is reabsorbed and returned to the circulation. However, as the plasma glucose concentration increases, as occurs in poorly controlled diabetic individuals, the filtered glucose load may exceed 375 mg/min, and all of the filtered glucose in excess of the Tm is excreted in the urine (1, 102). The plasma glucose concentration at which the filtered glucose load reaches the Tm (375 mg/min) is called the threshold (Fig. 1). The theoretical plasma glucose threshold that corresponds to the Tm (375 mg/min) is ∼300 mg/dl. However, in healthy individuals, the plasma glucose threshold at which glucosuria begins is ∼180 mg/dl. This difference between the “theoretical threshold” and the “actual threshold” is due to the splay, which represents the nonlinear transition in the reabsorption and excretion curves as the Tm for glucose is approached. This “rounding” of the curves has been explained by heterogeneity in the Tm of individual nephrons and/or by glomerulotubular imbalance (1, 102).
Fig. 1.
Kinetics of renal glucose handling.
Studies in experimental animals have demonstrated that a chronic increase in plasma glucose concentration, as occurs in diabetes, is associated with upregulation of SGLT2 expression in the kidney (42). Consistent with this observation, poorly controlled patients with T2DM (27) and type 1 diabetes mellitus (T1DM) (61) have an increase in the renal Tm for glucose. Moreover, lowering the plasma glucose concentration with insulin has been shown to return the elevated Tm to normal (27), indicating that the chronically elevated plasma glucose concentration is the signal that stimulates the increase in Tm. It has been postulated that the increase in Tm in response to hyperglycemia developed during human evolution to prevent the loss of energy (glucose) during periods of fasting dictated by a hunter-gatherer existence. Today, access to food is not a problem, and ∼60% of the US population is overweight/obese; thus a mechanism that previously aided survival now has become maladaptive. For both type 2 and type 1 diabetic individuals it would be advantageous for the diabetic kidney to excrete the excess filtered glucose load in an attempt to restore normoglycemia. Unfortunately, the elevated Tm for glucose “minimizes” glucosuria and hyperglycemia. Consequently, the increased Tm for glucose contributes to the maintenance of hyperglycemia, and the kidney should be viewed as an organ that contributes to the pathogenesis of hyperglycemia in T2DM (19).
Because sodium and glucose are cotransported in renal proximal tubular cells, it is not surprising that type 2 diabetic patients have an increase in total body sodium content and ∼60–70% develop hypertension (28). Consistent with this, a two- to threefold increase in sodium excretion has been demonstrated after acute SGLT2 blockade in diabetic animals. However, renal sodium excretion returned to normal within 2 wk, demonstrating the importance of tubuloglomerular feedback in the long-term regulation of salt and water balance (93).
Pharmacological Inhibition of Renal Glucose Reabsorption
Phlorizin was the first renal sodium-glucose cotransport inhibitor to be identified (17, 98). It is a natural compound isolated from the bark of apple trees when they bloom in the spring. Structurally, phlorizin is comprised of a glucose ring, which binds to SGLT via an oxygen atom (O-glucoside) to two phenol rings. Phlorizin is a nonspecific inhibitor of both SGLT1 and SGLT2 in the proximal tubule. When administered intravenously to healthy individuals phlorizin induces glucosuria, similar to that observed in individuals with familial renal glucosuria (98), while in diabetic subjects it causes marked glucosuria and normalizes plasma glucose levels. Low bioavailability (∼15%) after oral administration due to gastrointestinal breakdown and inhibition of SGLT1 in the gastrointestinal tract have negated its clinical usefulness in human subjects with diabetes (26).
Substitution of the O-link (between the glucose and phenol moieties) with a C-link provides greater resistance to beta-glucosidase and hence greater bioavailability after oral administration, while substitutions in the phenol rings generate compounds with higher selectivity for SGLT2 compared with SGLT1 and a longer circulating half-life. These structural changes reduce the gastrointestinal side effects observed with phlorizin and make it suitable for single daily dosing. Three members of the SGLT2 inhibitor class [dapagliflozin (Farxiga), canagliflozin (Invokana), and empagliflozin (Jardiance)] have been approved in the US, Europe, and other countries. Nonglucoside inhibitors with even greater SGLT2 selectivity have been developed (53) but have yet to be introduced clinically.
Clinical Efficacy
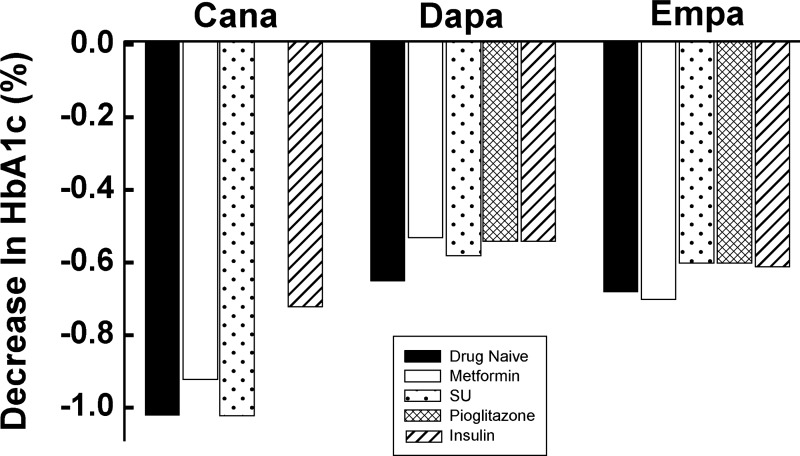
All three approved SGLT2 inhibitors (dapagliflozin, canagliflozin, empagliflozin) produce a dose-dependent glucosuria in healthy individuals and in T2DM patients. In healthy individuals, the maximal amount of glucosuria was 62 g/day with 20 mg of dapagliflozin (48), 74 g/day with 100 mg of empagliflozin (33, 82), and 70 g/day with 400 mg of canagliflozin (87). Further increases in the drug dose (up to 500 mg of dapagliflozin and 800 mg of canagliflozin) did not cause any further increase in the amount of glucose excreted in the urine. Similarly, in short-term studies (up to 2 wk), all three SGLT2 inhibitors caused a dose-dependent increase in urinary glucose excretion (UGE) in T2DM patients and a dose-dependent decrease in both fasting and postprandial plasma glucose concentrations (48, 49, 87). With more prolonged treatment (>12 wk) in T2DM patients, SGLT2 inhibitors produced a dose-dependent decrease in HbA1c as monotherapy (2) and in combination with other antidiabetic agents including insulin (7, 31, 35, 39, 76, 84, 90, 101) (Fig. 2).
Fig. 2.
Effect of background therapy on hemoglobin A1c (HbA1c) reduction in type 2 diabetic patients treated with canagliflozin (Cana), dapagliflozin (Dapa), and empagliflozin (Empa). SU, sulfonylurea. Drawn from data in Refs. 2, 7, 29, 31, 35, 39, 76, 84, 90, and 101.
All three approved SGLT2 inhibitors effectively lower the HbA1c in drug-naive T2DM patients. In 24-wk studies, the placebo-subtracted decrease in HbA1c in T2DM patients with baseline HbA1c ∼8.0% was −0.54% and −0.66% for 5 and 10 mg of dapagliflozin (n = 134) (29), −0.91% and −1.17% for 100 and 300 mg of canagliflozin (n = 392) (88), and −0.74% and −0.86% for 10 and 25 mg of empagliflozin (n = 448) (75), respectively.
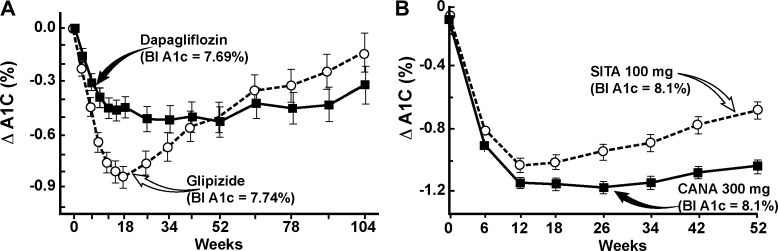
SGLT2 inhibitors also significantly lower the HbA1c in poorly controlled T2DM patients on other antidiabetic agents. Importantly, the efficacy of SGLT2 in lowering the plasma glucose concentration and HbA1c was independent of the background antidiabetic therapy. Since metformin is the most commonly used antidiabetic agent worldwide, many studies have examined the efficacy of SGLT2 in poorly controlled T2DM patients on metformin monotherapy or metformin plus other antidiabetic agents, e.g., sulfonylureas and thiazolidinediones (TZDs). In a 24-wk study with 409 poorly controlled (baseline HbA1c ∼8%) metformin-treated T2DM patients, dapagliflozin (5 and 10 mg) caused −0.4% and −0.54% placebo-subtracted reduction in T2DM (29). Dapagliflozin has been compared to a sulfonylurea as add-on therapy in metformin-treated T2DM patients (62), with both groups demonstrating a similar decrease in HbA1c (−0.52%) over 52 wk (Fig. 3). However, the time course of decline in the 2-yr groups was quite different. Thus after 6 mo the HbA1c began to rise progressively in the sulfonylurea-treated group, while it remained stable in the dapagliflozin-treated group, and this trend persisted for up to 2 yr (63).
Fig. 3.
A: time course of effect of dapagliflozin (n = 400) vs. glipizide on the decrement in A1c (ΔA1c) in metformin-treated type 2 diabetic patients. Drawn from data in Refs. 22 and 62. B: time course of effect of canagliflozin (CANA; n = 377) vs. sitagliptin (SITA; n = 378) in poorly controlled type 2 diabetic patients treated with metformin + sulfonylurea. Redrawn from Ref. 84 with permission.
Likewise, canagliflozin produced a significant reduction in HbA1c in poorly controlled T2DM patients on metformin therapy that was comparable to the decrease in HbA1c caused by sulfonylurea (16) and DPP-4 inhibitor (51). In a 52-wk study with 1,450 T2DM patients 100 and 300 mg of canagliflozin caused a −0.82% and −0.93% reduction in HbA1c compared with a −0.81% reduction with sulfonylurea. Similarly, 100 and 300 mg of canagliflozin caused a −0.73% and −0.88% reduction in HbA1c over 52 wk in metformin-treated subjects compared with −0.73% reduction with sitagliptin (5 mg). The baseline HbA1c was ∼8.0% in both studies.
Because SGLT2 inhibitors lower the plasma glucose concentration independent of insulin action, they effectively reduce glucose levels in insulin-treated patients. In 71 insulin-treated (≥50 U/day) T2DM patients who also were receiving an insulin sensitizer (metformin and/or TZD), dapagliflozin (5 and 10 mg/day) reduced the HbA1c (placebo subtracted) at 12 wk by 0.70% and 0.78%, respectively (P < 0.01 vs. placebo) despite a 50% reduction in insulin dose (100). In 800 insulin-treated (∼70–80 U/day) type 2 diabetic patients (101) addition of dapagliflozin (2.5, 5, and 10 mg/day) to insulin produced a dose-dependent decline in HbA1c (−0.40%, −0.49%, and −0.57%, respectively) vs. placebo over 48 wk (101). One hundred and three hundred milligrams of canagliflozin caused −0.62% and −0.73% placebo-subtracted reduction in HbA1c, respectively, in 2,072 insulin-treated T2DM patients over 52 wk (64), while empagliflozin (10 and 25 mg) caused −0.46% and −0.62% reduction in HbA1c (placebo subtracted), respectively, in insulin-treated T2DM patients (77). Similar reductions in HbA1c have been reported with the addition of empagliflozin to metformin/sulfonylurea (39)- and pioglitazone(10)-treated T2DM subjects.
Durability of SGLT2 Inhibitors
Studies that have examined the reduction in HbA1c for up to 2 yr have demonstrated that SGLT2 inhibitors cause a more durable reduction in HbA1c compared with sulfonylureas (62, 63) and DPP-4 inhibitors (51, 84) (Fig. 3). In a head-to-head comparison between dapagliflozin vs. glipizide in 814 poorly controlled T2DM patients on metformin, the reduction from baseline in HbA1c by dapagliflozin and glipizide was identical at 1 yr (−0.52%). However, after 2 yr the reduction in HbA1c caused by glipizide was attenuated (−0.18%) compared with dapagliflozin (−0.32%), and this trend continued at 4 yr (22). Similarly, in a 2-yr study, 1,450 poorly controlled, metformin-treated T2DM patients (HbA1c = 7.8%) were randomized to receive 100 and 300 mg of canagliflozin or glimepiride. The maximal reduction in HbA1c with canagliflozin and glimepiride occurred at 12 and 18 mo, respectively. Thereafter, the rate of HbA1c increase over time with both doses of canagliflozin was 0.16% per year compared with 0.37% in glimepiride-treated subjects, and at year 2 the reduction in HbA1c with both doses of canagliflozin (−0.58% and −0.60%) was significantly greater than that with glimepiride (−0.38%) (52). Thus currently available data suggest that SGLT2 inhibitors produce a more durable reduction in HbA1c compared with sulfonylureas and DPP-4 inhibitors over a 2- to 4-yr treatment period.
Mechanism of Glucosuria
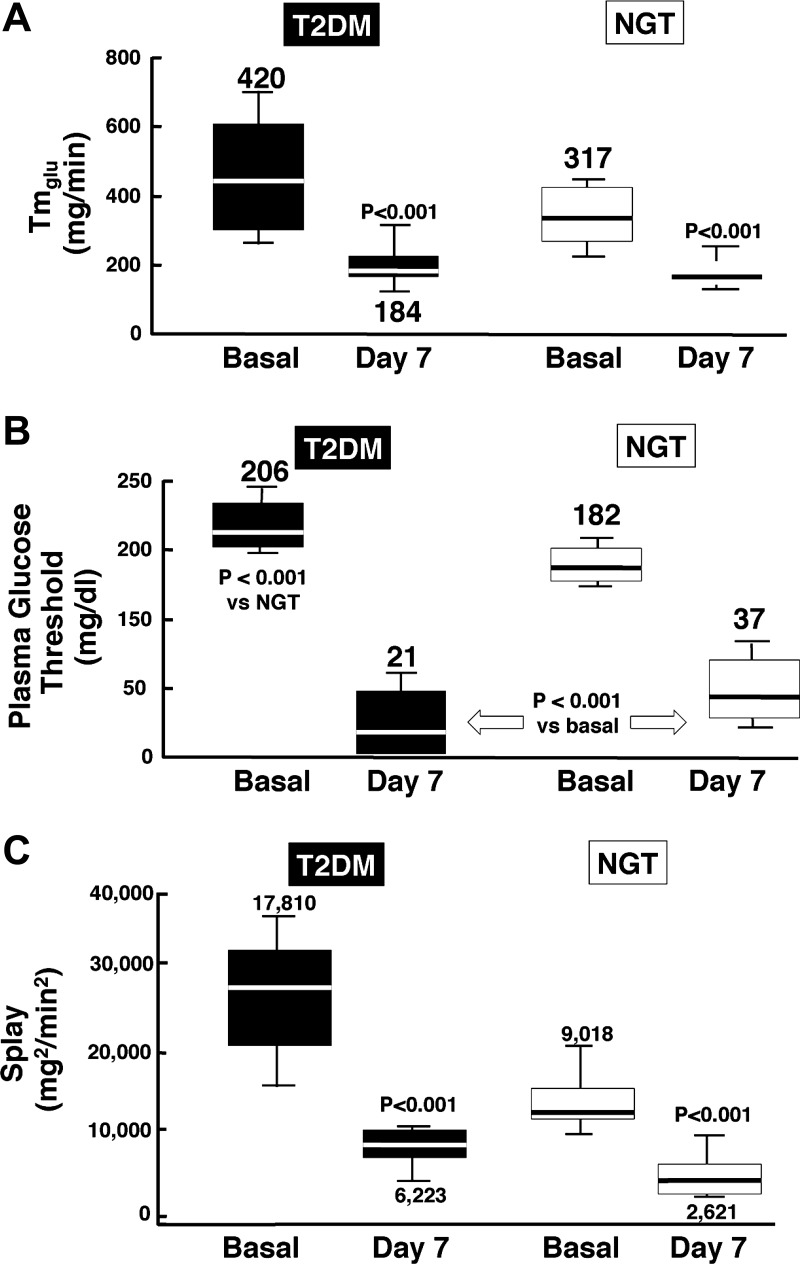
Glucosuria can be induced by lowering the Tm, by reducing the plasma glucose concentration threshold for glucosuria, by increasing the splay, or by a combination of the three. A study in rodents with sergliflozin demonstrated that SGLT2 inhibition markedly reduced the Tm without significant change in the glucose threshold or splay (43). In contrast, in humans dapagliflozin produced a decrease in the Tm as well as a marked reduction in both the threshold and splay in diabetic and nondiabetic individuals (20) (Fig. 4). Using the stepped hyperglycemic clamp, we demonstrated that dapagliflozin decreased Tm from 420 to 184 mg/min (20). Since in the presence of dapagliflozin the Tm is greater than the rate of filtered glucose in many T2DM patients and occurs at a plasma glucose concentration of ∼150 mg/dl [i.e., well above the fasting plasma glucose (FPG) concentration in NGT subjects and in many T2DM subjects], the decrease in Tm alone cannot be sufficient to explain the glucosuria produced by dapagliflozin. Moreover, dapagliflozin reduced the renal glucose splay both in healthy individuals and in T2DM patients (Fig. 4). Despite the decrease in the splay, dapagliflozin reduced the threshold of plasma glucose concentration from ∼180 mg/dl to ∼40 mg/dl (Fig. 4). Thus the decrease in the renal glucose threshold for glucosuria explains the urinary glucose loss during the fasting state in healthy individuals with FPG concentrations in the 80–90 mg/dl range.
Fig. 4.
Effect of dapagliflozin on the renal maximum glucose transport capacity (Tmglu; A), threshold (B), and splay (C) for glucose in healthy normal-glucose-tolerant (NGT) and type 2 diabetic (T2DM) subjects. From Ref. 20 with permission.
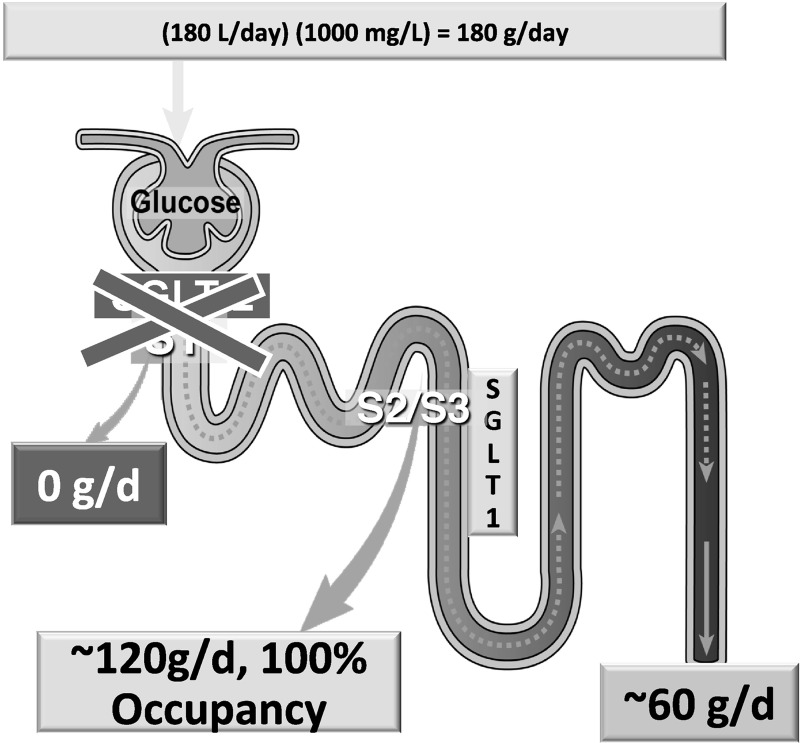
Although SGLT2 is responsible for the reabsorption of ∼80–90% of the filtered glucose load (∼150 g/24 h in NGT individuals), the increase in UGE (60–70 g/day) observed in NGT individuals with maximal dosages of SGLT2 inhibitors represents the inhibition of <50% of the filtered glucose load (20, 33, 48, 82). This can be explained by the anatomical location of SGLT1 and SGLT2 in the proximal tubule (99, 102) and the glucose transport capacities of SGLT1 and SGLT2 (3). Because of their proximal location in the S1 segment of the proximal tubule, the filtered glucose first encounters SGLT2, which reabsorbs the majority (80–90%) of glucose in the glomerular filtrate. Therefore, only a small amount of glucose reaches the distal part (S2/S3) of the proximal tubule where SGLT1 is located. Consequently, under physiological conditions, SGLT1 operates at submaximal transport capacity (∼20%) (Fig. 3). However, under conditions of complete inhibition of SGLT2, all of the filtered glucose reaches the S2/S3 segment of the proximal tubule, and SGLT1 can now use its full capacity (∼100–200 g/day) to reabsorb glucose. Thus only the fraction of filtered glucose that escapes SGLT1 will be excreted in the urine (Fig. 5) (3).
Fig. 5.
Renal tubular reabsorption of glucose in healthy NGT subjects with a glomerular filtration rate (GFR) of 180 liters/day and a mean day-long plasma glucose concentration of 100 mg/dl. Impact of complete SGLT2 inhibition on glucose reabsorption by SGLT1 and glucose excretion. Adapted from Ref. 3 with permission.
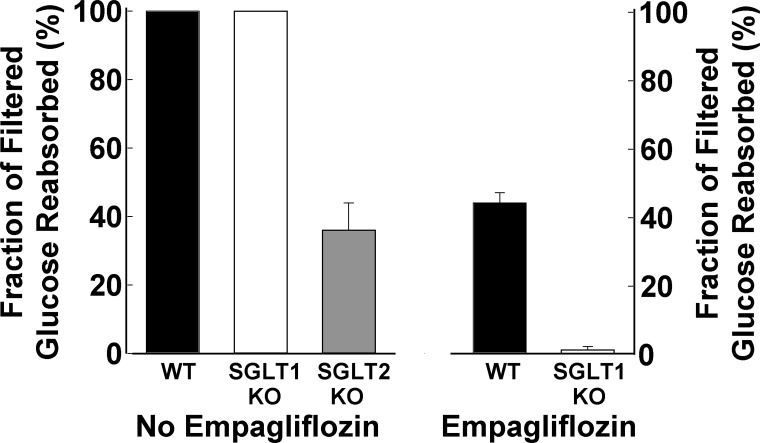
Consistent with the preceding scenario, SGLT1 knockout (KO) mice do not manifest significant glucosuria, documenting that, under nondiabetic conditions, SGLT2 alone is capable of reabsorbing essentially all of the filtered glucose in mice (32, 69). However, when SGLT1 KO mice were treated with empagliflozin, virtually all of the filtered glucose was excreted in the urine. These results indicate that the difference between the amount of filtered and excreted glucose during SGLT2 inhibition in normal mice is reabsorbed by SGLT1 (74) (Fig. 6). Further support for this scenario comes from the results of Powell et al. (69). In SGLT2 KO mice ∼30% of the filtered glucose load appears in the urine. When the SGLT2 KO mouse was bred with the SGLT1 KO mouse, UGE increased threefold compared with that observed in the SGLT2 KO mouse (747 vs. 224 mg/day). In mice lacking only SGLT1, the amount of glucose excreted in the urine was small (<15 mg/day). These results in mice provide conclusive evidence that, in the absence of SGLT2, SGLT1 is capable of reabsorbing ∼30% of the filtered glucose load. In humans there are no data about SGLT1 expression following chronic SGLT2 inhibition. However, in the SGLT2 KO mouse there is an ∼30% decrease in SGLT1 expression. Studies with the isolated, perfused tubule also support an important role for SGLT1 in renal glucose absorption. In the proximal (S1) and distal (S3) parts of the rabbit proximal tubule the glucose transport capacity was reported to be 12.9 ± 1.1 and 7.0 ± 0.5 pmol·min−1·mm−2, respectively (8). Immunohistochemical studies have documented the absence of SGLT2 in the distal part of the proximal tubule. Thus the distal S3 segment of the rabbit proximal tubule that presumably contains only SGLT1 can reabsorb 38% (7.9/12.9) of the filtered glucose load. These observations are consistent with our estimate in humans (3) that the SGLT2 transporter is capable of reabsorbing up to 30–40% of the filtered glucose load under conditions when SGLT2 is inhibited.
Fig. 6.
Left: renal tubular glucose reabsorption in wild-type, SGLT1 knockout (KO), and SGLT2 KO mice. Right: renal tubular reabsorption in WT mice and SGLT1 KO mice treated with empagliflozin. From Ref. 74 with permission.
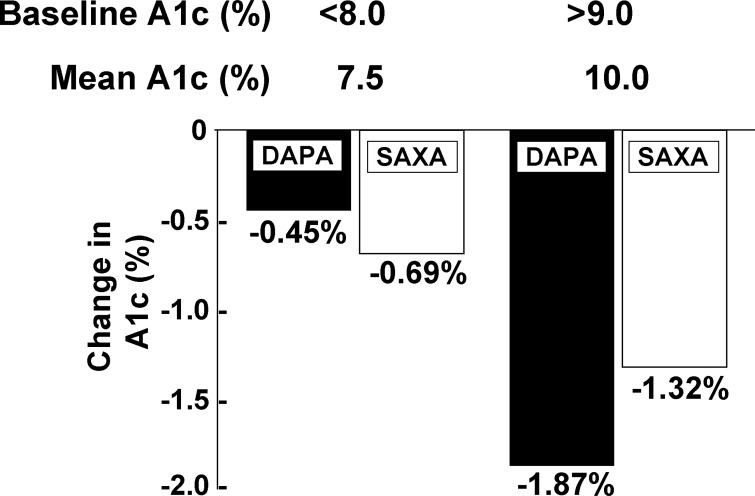
When SGLT2 is inhibited, a constant amount of glucose will be reabsorbed by SGLT1 and only the amount of glucose that bypasses SGLT1 will be excreted in the urine. Thus the fraction of filtered glucose excreted will increase progressively with the increase in the plasma glucose concentration. Furthermore, the amount of glucose removed from the body by SGLT2 inhibition will increase progressively with increasing plasma glucose concentration, as we have demonstrated (20). One would predict that the efficacy of SGLT2 inhibitors in lowering the HbA1c would markedly increase with increasing baseline HbA1c, and clinical studies have confirmed this prediction (21, 78). In initial exploratory studies, subjects with high initial HbA1c experienced a marked decrease in HbA1c compared with those with lower HbA1c. This point is clearly demonstrated in a clinical study comparing the efficacy of an SGLT2 inhibitor vs. a DPP-4 inhibitor. In poorly controlled, metformin-treated T2DM patients with an HbA1c <8.0%, the decrease in HbA1c (−0.45%) was slightly lower than that observed with saxagliptin (−0.69%) (Fig. 7). However, in T2DM subjects with an HbA1c >9.0% (mean HbA1c = 10.0%) dapagliflozin produced a −1.87% decrease in HbA1c compared with a −1.32% decrease with saxagliptin (78). Stated otherwise, a 2.5% increase in baseline HbA1c from 7.5% to 10% caused a more than fourfold increase in the efficacy of the SGLT2 inhibitor, while a similar difference in baseline HbA1c caused a less than twofold increase in the HbA1c reduction produced by the DPP-4 inhibitor. These results are explained by the greater amount of glucose removed from the body by the SGLT2 inhibitor at higher plasma glucose concentrations and have important clinical relevance (3). It follows that the SGLT2 inhibitor will have a greater advantage in lowering the HbA1c over other antidiabetic therapies in subjects with a high initial HbA1c (e.g., HbA1c >9.0%). To further put this into perspective, in the study by Ferrannini et al. (29), dapagliflozin reduced the HbA1c by 2.7%, from 10.0% to 7.3% in poorly controlled T2DM patients. Thus combination therapy with dapagliflozin plus another antidiabetic agent can be expected to get the majority of poorly controlled T2DM patients to goal (HbA1c = 6.5–7.0%). This represents a novel approach to the treatment of nonketotic, poorly controlled T2DM patients and is deserving of future study.
Fig. 7.
Influence of baseline HbA1c on the efficacy of dapagliflozin (DAPA) and saxagliptin (SAXA). From Ref. 78 with permission.
Dual SGLT1/SGLT2 Inhibitors
Because SGLT1 is present in the kidney and the gut (99, 102), there is concern that inhibition of SGLT1 could be associated with gastrointestinal side effects. Thus pharmaceutical companies have focused on developing compounds with high selectivity for SGLT2 over SGLT1. However, because under conditions of SGLT2 inhibition SGLT1 assumes a much greater role in renal glucose reabsorption, there has been renewed interest in a combined SGLT2/SGLT1 inhibitor. Thus an SGLT2 inhibitor that can partially inhibit SGLT1 would be anticipated to produce significantly greater glucosuria than a highly specific SGLT2 inhibitor. Moreover, because SGLT1 is responsible for absorption of ingested glucose, inhibition of SGLT1 in the gut would ameliorate the rise in postprandial plasma glucose concentration. Furthermore, inhibition of intestinal glucose reabsorption will result in a greater amount of glucose reaching the distal gut where the L cells are located and stimulate glucagon-like peptide 1 (GLP-1) secretion (70). However, since SGLT1 is the sensor in the L cells for GLP-1 secretion, there is concern that SGLT1 inhibition could impair GLP-1 secretion and, subsequently, insulin release. However, studies in both humans (70) and rodents (106) have demonstrated that fermentation products of glucose, i.e., short-chain fatty acids, are potent stimulators of GLP-1 secretion and overcome any effect of SGLT1 inhibition on GLP-1 secretion by the L cells. Because plasma GLP-1 levels increase during SGLT1 inhibition, this suggests that combination therapy with a DPP-4 inhibitor plus a dual SGLT1/SGLT2 inhibitor will have the potential to activate the incretin axis. In one study (106), the dual SGLT1/SGLT2 inhibitor LX4211 caused a small increase in plasma GLP-1 concentration after a glucose load in mice. When LX4211 was combined with sitagliptin, however, a robust increase in plasma GLP-1 was observed following the glucose load. Finally, SGLT1 protein has been immunolocalized in cardiac capillaries (rather than in previously assumed myocyte sarcolemma) (99). Therefore, clinical development of an SGLT1 inhibitor will require safety studies to exclude a deleterious effect on the myocardium.
SGLT2 Inhibitors and Renal Function
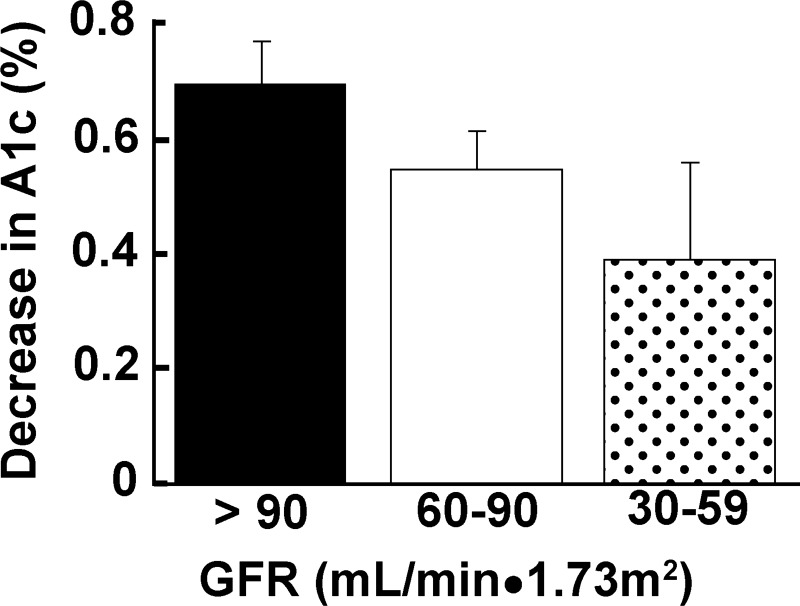
Studies with dapagliflozin, canagliflozin, and empagliflozin have demonstrated that SGLT2 inhibition had no clinically significant deleterious effect on renal function (18, 20). Although a small decline in estimated glomerular filtration rate (eGFR; ∼4–5 ml·min−1·1.73 m−2) has been observed in some studies, the eGFR tended to return to baseline values over time and returned completely to baseline when the SGLT2 inhibitor was discontinued (71). The small decline in eGFR is secondary to the mild reduction in intravascular volume that occurs secondary to inhibition of the sodium-glucose cotransporter and resultant natriuresis (50). The ability of SGLT2 inhibitors to decrease the plasma glucose concentration is strongly related to the level of renal function. With declining GFR, the filtered glucose load is reduced and impairs the drug's glucose-lowering efficacy. With a GFR = 60–90 ml/min, the glucosuria induced by dapagliflozin (15) was decreased by ∼40%, while the HbA1c declined by only ∼22% (Fig. 8). In subjects with moderately impaired renal function (GFR = 30–59 ml/min), the glucosuria produced by dapagliflozin (56) and all other SGLT2 inhibitors (46, 103) is reduced by ∼80% and the decrease in FPG and HbA1c is quite modest (4 mg/dl and −0.11%, respectively). In subjects with eGFR < 30 ml·min−1·1.73 m−2, the glucosuric effect of the SGLT2 inhibitors is severely impaired and the reduction in HbA1c (0.1–0.2%) is clinically insignificant (56). The impaired glucosuric effect with declining eGFR is due to the progressive reduction in the filtered glucose load and the tubular damage that parallels the glomerular dysfunction. It is noteworthy that, even though the glucose-lowering efficacy of the SGLT2 inhibitors declines with falling eGFR, the blood pressure lowering and weight loss effect of the SGLT2 inhibitors is largely retained (56).
Fig. 8.
Impact of reduced renal function on the glucose-lowering efficacy (A1c) of dapagliflozin [adapted from Bristol Myers Squibb NDA (Ref. 15)].
Metabolic Impact of SGLT2 Inhibition
Chronic hyperglycemia causes insulin resistance and inhibits insulin secretion, i.e., glucotoxicity (79). Studies in diabetic animals have demonstrated that normalization of plasma glucose levels with phlorizin reverses insulin resistance by enhancing GLUT4 translocation and corrects the defects in first- and second-phase insulin secretion (41, 80, 81). Similarly, in T2DM humans, we demonstrated that reduction in FPG concentration by 35 mg/dl with dapagliflozin (10 mg/day for 2 wk) increased both insulin sensitivity and insulin secretion (58, 59). Since the primary action of dapagliflozin is on the kidney and SGLT2 inhibitors have no direct effect on skeletal muscle, the increase in muscle insulin sensitivity reflects reversal of glucotoxicity. The improvement in insulin sensitivity was associated with decreased glucose oxidation and increased lipid oxidation (unpublished results). Similar results have been reported by others (30). Because increased intramyocellular fat plays a major role in skeletal muscle insulin resistance (and beta cell failure) (9), we speculate that increased fat oxidation following SGLT2 inhibition reduces intramyocellular fat content and contributes to enhanced insulin sensitivity.
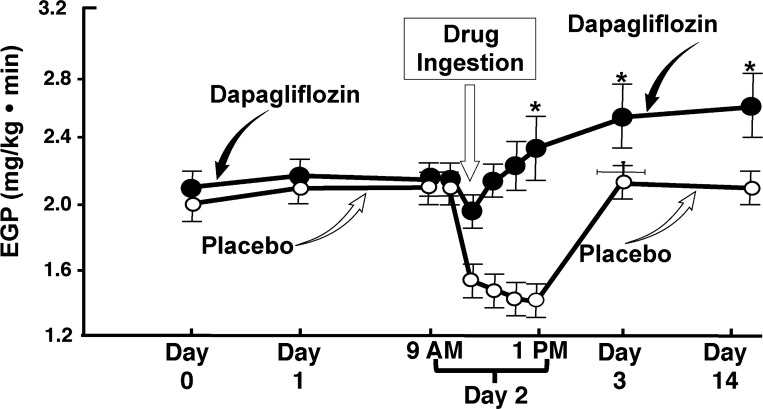
Because SGLT2 inhibition lowers the FPG concentration and since the FPG strongly correlates with the basal rate of endogenous (hepatic) glucose production (HGP), we anticipated that SGLT2 inhibition would reduce HGP. Surprisingly, dapagliflozin caused a “paradoxical” increase in basal rate of HGP within 60 min after administration and the increase in HGP persisted for the 2 wk of dapagliflozin administration (Fig. 9) (58). Quantitatively, the amount of glucose added to the circulation by the increase in HGP offset by ∼50% the amount of glucose excreted in the urine by SGLT2 inhibition. The increase in HGP was associated with a small decrease in plasma insulin concentration and a large increase in plasma glucagon concentration, both of which stimulate HGP (30, 58). These observations suggest that addition of an incretin-based agent, i.e., DPP-4 inhibitor or especially a GLP-1 receptor agonist, which simultaneously stimulates insulin and inhibits glucagon secretion, could block the rise in HGP and produce an additive or even a synergistic decrease in plasma glucose concentration and HbA1c. However, recent studies (21, 78) have demonstrated that addition of DPP-4 inhibitor to SGLT2 inhibitor failed to produce an additive reduction in plasma glucose concentration despite prevention of the increase in plasma glucagon (34). In subjects with baseline HbA1c >8.5–9.0%, the decrease in HbA1c in subjects treated with DPP-4 inhibitor plus SGLT2 inhibitor was not significantly greater than that caused by the SGLT2 inhibitor alone (78). Although HGP was not measured in these studies, these findings suggest that 1) the DPP-4 inhibitor failed to prevent the increase in HGP caused by SGLT2 inhibitor and a more potent inhibitor of HGP, i.e., GLP-1 receptor agonist, is required, and 2) signals other than the increase in plasma glucagon play a key role in mediating the increase in HGP in response to glucosuria. With respect to the latter, the “paradoxical” rise in HGP following dapagliflozin therapy in our studies occurred before the increase in plasma glucagon (58). The rapidity of rise in HGP suggests that activation of renal nerves may play an important role in mediating the increase in HGP. Finally, although we believe that the liver is the organ primarily responsible for the increase in HGP following dapagliflozin administration, the kidney cannot be excluded as a contributor.
Fig. 9.
Effect of dapagliflozin on endogenous (primarily reflects hepatic) glucose production (EGP) in type 2 diabetic subjects. First 2 symbols on the curves represent the basal rate of EGP. Dapagliflozin or placebo was administered at 9 AM on day 1. From Ref. 58 with permission.
Lowering the plasma glucose concentration with an SGLT2 inhibitor also improves beta cell function in T2DM patients. Two weeks of dapagliflozin treatment caused a twofold increase in beta cell function (59). Similarly, 4 wk of empagliflozin treatment enhanced beta cell function in T2DM patients (30). These results demonstrate that glucotoxicity plays an important role in the development of beta cell dysfunction (30, 59), as well as insulin resistance.
Hemodynamic Impact of SGLT2 Inhibition
SGLT2 inhibitors block sodium, as well as glucose, reabsorption in the proximal tubule. The inhibition of sodium reabsorption in the proximal tubule during the first 3–4 days of treatment with an SGLT2 inhibitor results in negative sodium balance and a decrease in the extracellular volume (50). In T2DM patients, SGLT2 inhibition with dapagliflozin was associated with a 7% decrease in plasma volume that was maintained after 8 wk (50). The decrease in the extracellular volume most likely is responsible for the reduction in blood pressure (5–6/1–2 mmHg) that is observed within the first 1–2 wk after initiation of therapy with an SGLT2 inhibitor (67).
Inhibition of sodium reabsorption in the proximal tubule by SGLT2 inhibitors also affects intrarenal hemodynamics. Studies in hyperglycemic diabetic rodents have demonstrated increased sodium, with glucose, reabsorption in the proximal tubule (66). This results in decreased sodium delivery to the juxtaglomerular apparatus, a perceived reduction in effective circulating volume, and afferent renal arteriolar vasodilation leading to an elevation in intraglomerular pressure and increase in GFR (hyperfiltration), which play an important role in the development of diabetic nephropathy (66). By inhibiting sodium, along with glucose, transport in the proximal tubule and increasing sodium delivery to the juxtaglomerular apparatus, SGLT2 inhibitors cause afferent renal arteriolar vasoconstriction, decreased intraglomerular pressure, and reduction in GFR hyperfiltration. Studies in diabetic mice have demonstrated that chronic SGLT2 inhibition with T-1095 decreased HbA1c and stopped the progression of diabetic nephropathy with prevention of proteinuria and normalization of glomerular mesangial area (6). Consistent with the preceding scenario, hyperfiltration and increased kidney size in type 1 diabetic patients can be reversed by 6 wk of intensive insulin therapy that normalizes the plasma glucose concentration (94). A recent study demonstrated that 8 wk of treatment with empagliflozin also reversed hyperfiltration and decreased intraglomerular pressure in poorly controlled type 1 diabetic patients due to afferent renal arteriolar vasoconstriction (18). Importantly, no active comparator group was included in this study. Therefore, it is difficult to determine whether the reversal of the hyperfiltration was due to reduction in plasma glucose concentration, increase in sodium delivery to the juxtaglomerular apparatus, or some combination of the two. Interestingly, empagliflozin had no significant effect on GFR in T1DM without hyperfiltration. Thus whether SGLT2 inhibitors exert a renoprotective action independent of their glucose-lowering effect remains to be determined.
Weight Loss
The majority of T2DM patients are overweight or obese, and many of the currently available antidiabetic medications, e.g., sulfonylureas, TZDs, and insulin, are associated with weight gain. The urinary loss of 60–80 g/day of glucose (4 cal/g) equates to 240–320 cal/day or ∼2–3 lb/mo. Consistent with this, weight loss has been observed in diabetic subjects treated with SGLT2 inhibitors in all clinical studies (7, 16, 29, 31, 35, 39, 51, 62–64, 75–77, 84, 88, 90, 100, 101). However, body weight tends to level off within 6 mo after the start of SGLT2 inhibitor therapy (7, 16, 29, 31, 35, 39, 51, 62–64, 75–77, 84, 88, 90, 100, 101), suggesting a compensatory increase in food intake. Indeed, studies in experimental animals have demonstrated an increase in food intake following treatment with SGLT2 inhibitors (23). This raises the intriguing possibility that combination therapy with an SGLT2 inhibitor plus a GLP-1 receptor agonist or other appetite suppressant drug could produce an additive, even synergistic, effect to promote weight loss.
Effect of SGLT2 Inhibitors on Plasma Lipid Profile
In phase III trials, canagliflozin produced a small, but significant, increase (∼8%) in plasma HDL cholesterol concentration and a significant decrease (∼5%) in plasma triglyceride concentration in drug-naive subjects and in T2DM patients poorly controlled with metformin (11, 89). Canagliflozin also caused a small, but significant, increase (∼5%) in plasma LDL cholesterol levels, which was independent of background statin therapy (11, 89). Although the increase in plasma HDL and decrease in plasma triglyceride concentrations could be explained by the weight loss (9), the etiology of the increase in LDL cholesterol is unknown. Although the increase in LDL cholesterol is small, its impact on cardiovascular events is unknown and awaits the completion of CANVAS, which includes 4,300 high-risk T2DM subjects and is due to be read out in 2017 (64). Similar effects on the plasma lipid profile have been observed with dapagliflozin and empagliflozin, and long-term cardiovascular safety studies with empagliflozin (EMPA-REG) and dapagliflozin (DECLARE) are ongoing (73).
Effect on blood pressure.
The salt and water deficit that occurs during the first several days of SGLT2 treatment and the weight loss that occurs with more long-term therapy (50) contribute to the decrease in blood pressure. In all clinical trials with SGLT2 inhibitors, reduction in systolic/diastolic blood pressure of 5–6/1–2 mmHg has been a consistent finding (67). Local inhibition of the renin-angiotensin system secondary to enhanced sodium delivery to the juxtaglomerular apparatus (93, 96) can provide an alternative explanation for the decrease in blood pressure. In T2DM patients treated with PF04791729, the blood pressure reduction was similar to that produced with thiazide diuretics. Uric acid reabsorption is coupled to sodium reabsorption in the proximal tubule. SGLT2 inhibitors block sodium reabsorption, and with it uric acid reabsorption, in the proximal tubule. This leads to an increase in uric acid excretion and resultant decrease in serum uric acid concentration of 0.8–1.0 mg/dl (100).
Safety
Because of their high selectivity of these inhibitors for SGLT2 over SGLT1, significant inhibition of gut glucose/galactose transport does not occur and gastrointestinal side effects have not been observed. The major side effect observed with SGLT2 inhibitors is mycotic vaginal infections in women, occurring in ∼1.5–2% of placebo-treated subjects and 7–8% of women treated with an SGLT2 inhibitor (7, 16, 29, 31, 35, 39, 51, 62–64, 73–75, 88, 90, 100, 101). A small increase in balanitis has been observed in men, primarily in men who are uncircumcised (7, 16, 29, 31, 35, 39, 51, 62–64, 73–75, 88, 90, 100, 101). Although the package inserts for dapagliflozin, canagliflozin, and empagliflozin state that the incidence of urinary tract infections (UTIs) is increased in patients treated with an SGLT2 inhibitor, this is not substantiated by the results of clinical trials (7, 16, 29, 31, 35, 39, 51, 62–64, 73–75, 88, 90, 100, 101) or by the information provided in the package inserts. Diabetic patients in poor control are prone to develop UTIs (45). In one study in which midstream urine was collected, treatment with an SGLT2 inhibitor did not increase the prevalence of urinary bacteriuria (40).
An increased incidence of volume-related side effects (patient-reported symptoms) has been demonstrated in diabetic patients receiving treatment with SGLT2 inhibitors, especially elderly individuals and individuals treated with diuretics (97). However, documentation of orthostatic hypotension by direct blood pressure measurement was not employed in these studies. In diabetic patients treated with dapagliflozin for 12–24 wk, a small increase in urine volume and sodium excretion was observed during the initial 2–3 days of treatment (55), and this was accompanied by a small rise in hematocrit (1–2 volume %) and plasma urea nitrogen-to-creatinine ratio. Plasma concentrations of Na+, K+, Cl−, and Ca2+ remained unchanged after dapagliflozin treatment (55, 97). An increase in plasma K+ concentration rarely has been reported with canagliflozin (60), but it is unclear whether this is related to the SGLT2 inhibitor per se or to the unrecognized presence of the hyporeninemic hypoaldosteronemia syndrome (54). Hyperkalemia has not been reported with other SGLT2 inhibitors. Changes in plasma Na+, Cl−, Ca2+, and HCO3− concentrations have not been observed with the SGLT2 inhibitors.
As previously reviewed, SGLT2 inhibitors do not adversely affect renal function or cause/exacerbate albuminuria in either T2DM or T1DM patients (18, 20). To the contrary, a decrease in proteinuria has been reported with SGLT2 inhibitors in experimental animals (46, 97) and humans (71, 104), and the decrease was independent of lowering the plasma glucose concentration. Importantly, subjects with mutations in the SGLT2 gene maintain normal kidney function without proteinuria despite excreting large amounts of glucose (>50–100 g/24 h) over a lifetime (85). Moreover, some of these individuals with familial renal glucosuria have had renal biopsies that revealed completely normal renal histology (105).
Although hypoglycemia is a potential concern with all antidiabetic agents, no increase in the incidence of hypoglycemia has been observed with SGLT2 inhibitors when used as monotherapy or with agents other than sulfonylureas or insulin, even when given to normoglycemic individuals (105). This can be explained by an increase in glucagon secretion (58) and the stimulation of HGP.
An increase in the incidence of bladder and breast cancer was observed in the dapagliflozin clinical trials, but the total number of cases was small. Of note, SGLT2 is not expressed in either breast or bladder tissues, and carcinogenic studies in multiple animal species did not detect any preneoplastic or neoplastic activity. Breast, and particularly bladder, cancer are known to take many years to develop. In the clinical trials dapagliflozin exposure was short (<1 yr). Therefore, it seems unlikely that the small increase in the incidence of these two tumors is related to the SGLT2 inhibitor. Since frequent testing for UTIs was performed in these clinical trials, this could have introduced detection bias for bladder cancer by leading to the discovery of microscopic hematuria. Furthermore, 7 of the 10 cases of bladder cancer had hematuria at the time of entry into the study. Nonetheless, because of this potential carcinogenic signal, the producer (AstraZeneca) of dapagliflozin has committed to a long-term surveillance study. No increase in the incidence of bladder cancer has been observed with either canagliflozin or empagliflozin.
Recent reports have demonstrated the development of ketoacidosis in diabetic patients treated with SGLT2 inhibitors (27a, 68, 91). Studies by Ferrannini et al. (30), as well as by Merovci et al. (58), have shown that type 2 diabetic subjects treated with SGLT2 inhibitors demonstrate a decrease in glucose oxidation and a reciprocal increase in fat oxidation. We interpret these changes in substrate oxidation as follows. In T2DM individuals muscle tissue is severely resistant to insulin (19). Consequently, plasma glucose concentration rises to a level that is sufficient to augment glucose entry into the cell by the mass action effect of hyperglycemia (12). Treatment with SGLT2 inhibitors induces glucosuria, causing an acute reduction in plasma glucose concentration and decreased glucose entry into muscle both in postabsorptive and insulin-stimulated states. To meet the energy demand of the cell, myocytes and hepatocytes switch to fat as an alternate source of energy. Increased fat oxidation results in increased production of acetyl-CoA, which either can be oxidized in the Krebs cycle or converted to ketones (acetoacetic and β-hydroxybutyric acid). The plasma glucagon-to-insulin ratio perfusing the liver is a key determinant of the fate of acetyl-CoA (57). Glucagon stimulates the expression of hydroxyl-methylglutaryl-CoA synthase (HMGS), the rate-limiting step for the conversion of acetyl-CoA to ketones, while insulin suppresses its expression. The increase in fasting plasma glucagon concentration observed in T2DM individuals treated with SGLT2 inhibitors (13, 30, 58) would be expected to increase HMGS activity, causing an increase in ketone production. Clinical reports documenting the development of ketoacidosis following the initiation of SGLT2 inhibitor therapy in diabetic patients (27a, 68, 91) have a number of features in common. Many cases involved type 1 diabetic patients in whom the insulin dose was reduced when SGLT2 inhibitor therapy was initiated. Since SGLT2 inhibitors increase glucagon secretion (13, 30, 58), it is not surprising that insulin dose reduction might result in ketoacidosis. Another common feature in many cases was an associated medical or surgical condition resulting in moderate to severe stress. Release of catecholamines predisposes to the development of ketoacidosis both by inhibiting insulin secretion and by stimulating ketone production (44).
The Place of SGLT2 Inhibitors in the Management of T2DM Individuals
The American Diabetes Association/European Association for the Study of Diabetes recommends metformin as the first-line therapy in individuals with new-onset type 2 diabetes. However, because metformin does not affect beta cell function, metformin-treated individuals experience a progressive increase in HbA1c after an initial good response (14, 95). SGLT2 inhibitors provide a therapeutic option in metformin-failing diabetic individuals or in individuals who cannot tolerate metformin because of adverse gastrointestinal side effects. Moreover, because of their unique mechanism of action on the kidney, the SGLT2 inhibitors effectively can be used in combination with all other antihyperglycemic agents including insulin. Furthermore, they promote weight loss, reduce blood pressure, and have an advantage over other antidiabetic agents in subjects with very high HbA1c, e.g., HbA1c >9%. This latter group of patients often is treated with insulin to correct the metabolic decompensation, i.e., glucotoxicity and lipotoxicity. In this group of individuals metformin alone will not lower the HbA1c to the treatment goal (<6.5–7.0%). In a study of new-onset T2DM patients initiation of therapy with metformin plus dapagliflozin produced an additive decrease in HbA1c vs. either therapy alone, and more subjects (∼60%) with the combination therapy achieved the target glycemic goal (HbA1c <7.0%) than with either therapy alone (36). Because SGLT2 inhibitors are effective in lowering the HbA1c at all stages of diabetes, they can be added in subjects who are inadequately controlled with multiple oral agents, GLP-1 receptor agonists, and/or insulin therapy. Because of their potential to produce an additive or even synergistic effect to reduce the HbA1c and promote weight loss, combination therapy with an SGLT2 inhibitor and GLP-1 receptor agonist may be an especially effective intervention. Finally, SGLT2 inhibitors also can be used in combination with basal insulin therapy to improve glycemic control while promoting weight loss and reducing the dose of insulin without provoking hypoglycemia (101).
Finally, the efficacy of the SGLT2 inhibitors can accessed quickly by measuring 1) the decline in FPG concentration in the morning following the first administration of the SGLT2 inhibitor or 2) the fractional excretion of glucose (Uglu·PCr/UCr·Pglu) following the first dose administration. A fractional excretion >30–40% indicates a good therapeutic response even in those with a reduced GFR.
GRANTS
R. A. DeFronzo (5R01 DK-240923), L. Norton (1 K01 DK-098314-01A1), and M. A. Abdul-Ghani (R01 DK-097554-01) receive National Institute of Diabetes and Digestive and Kidney Diseases grant support. Part of R. A. DeFronzo's salary is supported by the South Texas Veterans Health Care System.
DISCLOSURES
R. A. DeFronzo is a member of the Advisory Board of Janssen, Boehringer Ingelheim, AstraZeneca, Novo Nordisk, Intarcia and Lexicon. R. A. DeFronzo is a member of the Speaker Bureau of Novo Nordisk and AstraZeneca. R. A. DeFronzo has grant support from AstraZeneca, Janssen, and Boehringer Ingelheim. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors received no direct compensation related to the development of this manuscript.
AUTHOR CONTRIBUTIONS
Author contributions: M.A.A.-G. and R.A.D. conception and design of research; M.A.A.-G., L.N., and R.A.D. analyzed data; M.A.A.-G., L.N., and R.A.D. interpreted results of experiments; M.A.A.-G. and R.A.D. prepared figures; M.A.A.-G. and R.A.D. drafted manuscript; M.A.A.-G. and R.A.D. edited and revised manuscript; M.A.A.-G., L.N., and R.A.D. approved final version of manuscript.
REFERENCES
- 1.Abdul-Ghani MA, Norton L, DeFronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev 32: 515–531, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Abdul-Ghani MA, DeFronzo RA. Dapagliflozin for the treatment of type 2 diabetes. Expert Opin Pharmacother 14: 1695–1703, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdul-Ghani M, DeFronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30–50% of filtered glucose load in humans. Diabetes 62: 3324–3328, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358: 2545–2559, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358: 2560–2572, 2008. [DOI] [PubMed] [Google Scholar]
- 5a.American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care 38, Suppl 1: S4–S41, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Arakawa K, Ishihara T, Oku A, Nawano M, Ueta K, Kitamura K, Matsumoto M, Saito A. Improved diabetic syndrome in C57BL/KsJ-db/db mice by oral administration of the Na+-glucose cotransporter inhibitor T-1095. Br J Pharmacol 132: 578–586, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 375: 2223–2233, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Barfuss DW, Schafer JA. Differences in active and passive glucose transport along the proximal nephron. Am J Physiol Renal Fluid Electrolyte Physiol 241: F322–F332, 1981. [DOI] [PubMed] [Google Scholar]
- 9.Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab 89: 463–478, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Blevins T. Combination therapy for patients with uncontrolled type 2 diabetes mellitus: adding empagliflozin to pioglitazone or pioglitazone plus metformin. Expert Opin Drug Saf 14: 789–793, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Bode B, Stenlöf K, Harris S, Sullivan D, Fung A, Usiskin K, Meininger G. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55–80 years with type 2 diabetes. Diabetes Obes Metab 17: 294–303, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Bonadonna RC, Del Prato S, Bonora E, Saccomani MP, Gulli G, Natali A, Frascerra S, Pecori N, Ferrannini E, Bier D, Cobelli C, DeFronzo RA. Roles of glucose transport and glucose phosphorylation in muscle insulin resistance of NIDDM. Diabetes 45: 915–925, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thévenet J, Beaucamps C, Delalleau N, Popescu I, Malaisse WJ, Sener A, Deprez B, Abderrahmani A, Staels B, Pattou F. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 21: 512–517, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Brown JB, Conner C, Nichols GA. Secondary failure of metformin monotherapy in clinical practice. Diabetes Care 33: 501–506, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bristol Myers-Squibb, AstraZeneca. U.S. Food and Drug Administration Endocrinologic and Metabolic Advisory Committee Background Document: Dapagliflozin, BMS-512148, NDA 202293. Princeton, NJ: Bristol-Myers Squibb; Accessed at www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/ucm262993.htm, 2011. [Google Scholar]
- 16.Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, Balis DA, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 382: 941–950, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Chassis H, Jolliffe N, Smith H. The action of phlorizin on the excretion of glucose, xylose, sucrose, creatinine, and urea by man. J Clin Invest 12: 1083–1089, 1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587–597, 2014. [DOI] [PubMed] [Google Scholar]
- 19.DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58: 773–795, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeFronzo RA, Hompesch M, Kasichayanula S, Liu X, Hong Y, Pfister M, Morrow LA, Leslie BR, Boulton DW, Ching A, LaCreta FP, Griffen SC. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 36: 3169–3176, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Lewin A, Patel S, Liu D, Kaste R, Woerle HJ, Broedl UC. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care 38: 384–393, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Del Prato S, Nauck M, Durán-Garcia S, Maffei L, Rohwedder K, Theuerkauf A, Parikh S. Long-term glycaemic response and tolerability of dapagliflozin versus a sulphonylurea as add-on therapy to metformin in type 2 diabetes patients: 4-year data. Diabetes Obes Metab 38: 384–393, 2015. [DOI] [PubMed] [Google Scholar]
- 23.Devenny JJ, Godonis HE, Harvey SJ, Rooney S, Cullen MJ, Pelleymounter MA. Weight loss induced by chronic dapagliflozin treatment is attenuated by compensatory hyperphagia in diet-induced obese (DIO) rats. Obesity (Silver Spring) 20: 1645–1652, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329: 977–986, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD, VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360: 129–139, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev 21: 31–38, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Farber SJ, Berger EY, Earle DP. Effect of diabetes and insulin of the maximum capacity of the renal tubules to reabsorb glucose. J Clin Invest 30: 125–129, 1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.FDA. FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. FDA Drug Safety Communication (http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm). [Google Scholar]
- 28.Felicetta JV, Sowers JR. Systemic hypertension in diabetes mellitus. Am J Cardiol 61: 34H–40H, 1988. [DOI] [PubMed] [Google Scholar]
- 29.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 33: 2217–2224, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle HJ. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 124: 499–508, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forst T, Guthrie R, Goldenberg R, Yee J, Vijapurkar U, Meininger G, Stein P. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab 16: 467–477, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorboulev V, Schurmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, Veyhl-Wichmann M, Srinivasan A, Balen D, Breljak D, Rexhepaj R, Parker HE, Gribble FM, Reimann F, Lang F, Wiese S, Sabolic I, Sendtner M, Koepsell H. Na+-d-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61: 187–196, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grempler R, Thomas L, Eckhardt M, Himmelsbach F, Sauer A, Sharp DE, Bakker RA, Mark M, Klein T, Eickelmann P. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab 14: 83–90, 2012. [DOI] [PubMed] [Google Scholar]
- 34.Hansen L, Iqbal N, Ekholm E, Cook W, Hirshberg B. Postprandial dynamics of plasma glucose, insulin, and glucagon in patients with type 2 diabetes treated with saxagliptin plus dapagliflozin add-on to metformin therapy. Endocr Pract 20: 1187–1197, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Häring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Woerle HJ, Broedl UC, EMPA-REG METSU Trial Investigators . Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 36: 3396–3404, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henry RR, Murray AV, Marmolejo MH, Hennicken D, Ptaszynska A, List JF. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract 66: 446–456, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care 31: 81–86, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Jabbour SA, Hardy E, Sugg J, Parikh S, Study 10 Group. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24-week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care 37: 740–750, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Urinary tract infections in patients with diabetes treated with dapagliflozin. J Diabetes Complications 27: 473–478, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Kahn BB, Shulman GI, DeFronzo RA, Cushman SW, Rossetti L. Normalization of blood glucose in diabetic rats with phlorizin treatment reverses insulin-resistant glucose transport in adipose cells without restoring glucose transporter gene expression. J Clin Invest 87: 561–570, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamran M, Peterson RG, Dominguez JH. Overexpression of GLUT2 gene in renal proximal tubules of diabetic Zucker rats. J Am Soc Nephrol 8: 943–948, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Katsuno K, Fujimori Y, Takemura Y, Hiratochi M, Itoh F, Komatsu Y, Fujikura H, Isaji M. Sergliflozin, a novel selective inhibitor of low-affinity sodium glucose cotransporter (SGLT2), validates the critical role of SGLT2 in renal glucose reabsorption and modulates plasma glucose level. J Pharmacol Exp Ther 320: 323–330, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Kitabchi AE, Umpierrez GE, Murphy MB. Diabetic ketoacidosis and hyperosmolar state. In: International Textbook of Diabetes Mellitus (4th ed), edited by DeFronzo RA, Ferrannini E, Zimmet P, Alberti KG. Chichester, UK: Wiley, 2015, p. 799–814. [Google Scholar]
- 45.Knapp S. Diabetes and infection: is there a link?—A mini-review. Gerontology 59: 99–104, 2013. [DOI] [PubMed] [Google Scholar]
- 46.Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 85: 962–971, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kojima N, Williams JM, Takahashi T, Miyata N, Roman RJ. Effects of a new SGLT2 inhibitor, luseogliflozin, on diabetic nephropathy in T2DN rats. J Pharmacol Exp Ther 345: 464–472, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Komoroski B, Vachharajani N, Boulton D, Kornhauser D, Geraldes M, Li L, Pfister M. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther 85: 520–526, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther 85: 513–519, 2009. [DOI] [PubMed] [Google Scholar]
- 50.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 15: 853–862, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lavalle-Gonzalez FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 56: 2582–2592, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leiter LA, Yoon KH, Arias P, Langslet G, Xie J, Balis DA, Millington D, Vercruysse F, Canovatchel W, Meininger G. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care 38: 355–364, 2015. [DOI] [PubMed] [Google Scholar]
- 53.Li AR, Zhang J, Greenberg J, Lee T, Liu J. Discovery of non-glucoside SGLT2 inhibitors. Bioorg Med Chem Lett 21: 2472–2475, 2011. [DOI] [PubMed] [Google Scholar]
- 54.Liamis G, Liberopoulos E, Barkas F, Elisaf M. Diabetes mellitus and electrolyte disorders. World J Clin Cases 2: 488–496, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 32: 650–657, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Macha S, Mattheus M, Halabi A, Pinnetti S, Woerle HJ, Broedl UC. Pharmacokinetics, pharmacodynamics and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in subjects with renal impairment. Diabetes Obes Metab 16: 215–222, 2014. [DOI] [PubMed] [Google Scholar]
- 57.McGarry J, Wright PH, Foster DW. Hormonal control of ketogenesis. Rapid activation of hepatic ketogenic capacity in fed rats by anti-insulin serum and glucagon. J Clin Invest 55: 1202–1209, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, Xiong J, Perez Z, Norton L, Abdul-Ghani MA, DeFronzo RA. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 124: 509–514, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Merovci A, Mari A, Solis C, Xiong J, Daniele G, Chavez A, Tripathy D, Urban McCarthy S, Abdul-Ghani M, DeFronzo RA. Dapagliflozin lowers plasma glucose concentration and improves beta cell function. J Clin Endocrinol Metab 100: 1927–1932, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mikhail N. Safety of canagliflozin in patients with type 2 diabetes. Curr Drug Saf 9: 127–132, 2014. [DOI] [PubMed] [Google Scholar]
- 61.Mogensen KE. Maximum tubular reabsorption capacity for glucose and renal hemodynamics during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand J Lab Clin Invest 28: 101–109, 1971. [DOI] [PubMed] [Google Scholar]
- 62.Nauck MA, Del Prato S, Meier JJ, Durán-García S, Rohwedder K, Elze M, Parikh SJ. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care 34: 2015–2022, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nauck MA, Del Prato S, Durán-García S, Rohwedder K, Langkilde AM, Sugg J, Parikh SJ. Durability of glycaemic efficacy over 2 years with dapagliflozin versus glipizide as add-on therapies in patients whose type 2 diabetes mellitus is inadequately controlled with metformin. Diabetes Obes Metab 16: 1111–1120, 2014. [DOI] [PubMed] [Google Scholar]
- 64.Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Stein P, Desai M, Shaw W, Jiang J, Vercruysse F, Meininger G, Matthews D. Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS)—a randomized placebo-controlled trial. Am Heart J 166: 217–223, 2013. [DOI] [PubMed] [Google Scholar]
- 65.Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Ways K, Desai M, Shaw W, Capuano G, Alba M, Jiang J, Vercruysse F, Meininger G, Matthews D. Efficacy and safety of canagliflozin, an inhibitor of sodium-glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care 38: 403–411, 2015. [DOI] [PubMed] [Google Scholar]
- 66.Nelson RG, Bennett PH, Beck GJ, Tan M, Knowler WC, Mitch WE, Hirschman GH, Myers BD. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. N Engl J Med 335: 1636–1642, 1996. [DOI] [PubMed] [Google Scholar]
- 67.Oliva RV, Bakris GL. Blood pressure effects of sodium-glucose co-transport 2 (SGLT2) inhibitors. J Am Soc Hypertens 8: 330–339, 2014. [DOI] [PubMed] [Google Scholar]
- 68.Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 38: 1687–1693, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Powell DR, DaCosta CM, Gay J, Ding ZM, Smith M, Greer J, Doree D, Jeter-Jones S, Mseeh F, Rodriguez LA, Harris A, Buhring L, Platt KA, Vogel P, Brommage R, Shadoan MK, Sands AT, Zambrowicz B. Improved glycemic control in mice lacking Sglt1 and Sglt2. Am J Physiol Endocrinol Metab 304: E117–E130, 2013. [DOI] [PubMed] [Google Scholar]
- 70.Powell DR, Smith M, Greer J, Harris A, Zhao S, DaCosta C, Mseeh F, Shadoan MK, Sands A, Zambrowicz B, Ding ZM. LX4211 increases serum glucagon-like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)-mediated absorption of intestinal glucose. J Pharmacol Exp Ther 345: 250–259, 2013. [DOI] [PubMed] [Google Scholar]
- 71.Ptaszynska A, Johnsson KM, Parikh SJ, de Bruin TW, Apanovitch AM, List JF. Safety profile of dapagliflozin for type 2 diabetes: pooled analysis of clinical studies for overall safety and rare events. Drug Saf 37: 815–829, 2014. [DOI] [PubMed] [Google Scholar]
- 73.Ridderstråle M, Svaerd R, Zeller C, Kim G, Woerle HJ, Broedl UC, EMPA-REG H2H-SU trial investigators . Rationale, design and baseline characteristics of a 4-year (208-week) phase III trial of empagliflozin, an SGLT2 inhibitor, versus glimepiride as add-on to metformin in patients with type 2 diabetes mellitus with insufficient glycemic control. Cardiovasc Diabetol 12: 129–138, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, Thomson SC, Koepsell H, Vallon V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol 306: F188–F193, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, Broedl UC, EMPA-REG MONO trial investigators . Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 1: 208–19, 2013. [DOI] [PubMed] [Google Scholar]
- 76.Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA1c, body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care 35: 1473–1478, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosenstock J, Jelaska A, Frappin G, Salsali A, Kim G, Woerle HJ, Broedl UC, EMPA-REG MDI Trial Investigators . Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care 37: 1815–1823, 2014. [DOI] [PubMed] [Google Scholar]
- 78.Rosenstock J, Hansen L, Zee P, Li Y, Cook W, Hirshberg B, Iqbal N. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care 38: 376–383, 2015. [DOI] [PubMed] [Google Scholar]
- 79.Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care 13: 610–630, 1990. [DOI] [PubMed] [Google Scholar]
- 80.Rossetti L, Shulman GI, Zawalich W, DeFronzo RA. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest 80: 1037–1044, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 79: 1510–1515, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sarashina A, Koiwai K, Seman LJ, Yamamura N, Taniguchi A, Negishi T, Sesoko S, Woerle HJ, Dugi KA. Safety, tolerability, pharmacokinetics and pharmacodynamics of single doses of empagliflozin, a sodium-glucose co-transporter inhibitor (SGLT2), in Japanese healthy volunteers. Drug Metab Pharmacokinet 28: 213–219, 2013. [DOI] [PubMed] [Google Scholar]
- 83.Sattar N. Revisiting the links between glycaemia, diabetes and cardiovascular disease. Diabetologia 56: 686–695, 2013. [DOI] [PubMed] [Google Scholar]
- 84.Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, Kawaguchi M, Canovatchel W, Meininger G. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care 36: 2508–2515, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sessa A, Cioffi A, Conte F, Castelli L, Dei Poli M. Familial renal glycosuria. Nephron 20: 235–236, 1978. [DOI] [PubMed] [Google Scholar]
- 87.Sha S, Devineni D, Ghosh A, Polidori D, Chien S, Wexler D, Shalayda K, Demarest K, Rothenberg P. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab 13: 669–672, 2011. [DOI] [PubMed] [Google Scholar]
- 88.Stenlöf K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 15: 372–382, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stenlöf K, Cefalu WT, Kim KA, Jodar E, Alba M, Edwards R, Tong C, Canovatchel W, Meininger G. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: findings from the 52-week CANTATA-M study. Curr Med Res Opin 30: 163–175, 2014. [DOI] [PubMed] [Google Scholar]
- 90.Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab 13: 928–938, 2011. [DOI] [PubMed] [Google Scholar]
- 91.Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 100: 2849–2852, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol 302: R75–R83, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thomson SC, Vallon V, Blantz RC. Kidney function in early diabetes: the tubular hypothesis of glomerular filtration. Am J Physiol Renal Physiol 286: F8–F15, 2004. [DOI] [PubMed] [Google Scholar]
- 94.Tuttle KR, Bruton JL, Perusek MC, Lancaster JL, Kopp DT, DeFronzo RA. Effect of strict glycemic control on renal hemodynamic response to amino acids and renal enlargement in insulin-dependent diabetes mellitus. N Engl J Med 324: 1626–1632, 1991. [DOI] [PubMed] [Google Scholar]
- 95.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352: 837–853, 1998. [PubMed] [Google Scholar]
- 96.Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol 10: 2569–2576, 1999. [DOI] [PubMed] [Google Scholar]
- 97.Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, Koepsell H, Thomson SC, Rieg T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 306: F194–F204, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vick H, Diedrich DF, Baumann K. Reevaluation of renal tubular glucose transport inhibition by phlorizin analogs. Am J Physiol 224: 552–557, 1973. [DOI] [PubMed] [Google Scholar]
- 99.Vrhovac I, Balen Eror D, Klessen D, Burger C, Breljak D, Kraus O, Radovic N, Jadrijevic S, Aleksic I, Walles T, Sauvant C, Sabolic I, Koepsell H. Localizations of Na+-d-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflügers Arch 467: 1881–1898, 2015. [DOI] [PubMed] [Google Scholar]
- 100.Wilding JP, Norwood P, T'joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care 32: 1656–1662, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, Parikh S, Dapagliflozin 006 Study Group. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med 156: 405–415, 2012. [DOI] [PubMed] [Google Scholar]
- 102.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 91: 733–794, 2011. [DOI] [PubMed] [Google Scholar]
- 103.Yale JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, Figueroa K, Wajs E, Usiskin K, Meininger G. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 15: 463–473, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamout H, Perkovic V, Davies M, Woo V, de Zeeuw D, Mayer C, Vijapurkar U, Kline I, Usiskin K, Meinger G, Bakris G. Efficacy and safety of canagliflozin in patients with type 2 diabetes and stage 3 nephropathy. Am J Nephrol 40: 64–74, 2014. [DOI] [PubMed] [Google Scholar]
- 105.Yu L, Lv JC, Zhou XJ, Zhu L, Hou P, Zhang H. Abnormal expression and dysfunction of novel SGLT2 mutations identified in familial renal glucosuria patients. Hum Genet 129: 335–344, 2011. [DOI] [PubMed] [Google Scholar]
- 106.Zambrowicz B, Ding ZM, Ogbaa I, Frazier K, Banks P, Turnage A, Freiman J, Smith M, Ruff D, Sands A, Powell D. Effects of LX4211, a dual SGLT1/SGLT2 inhibitor, plus sitagliptin on postprandial active GLP-1 and glycemic control in type 2 diabetes. Clin Ther 35: 273–285, 2013. [DOI] [PubMed] [Google Scholar]