ABSTRACT
Bacterial spores, despite being metabolically dormant, possess the remarkable capacity to detect nutrients and other molecules in their environment through a biochemical sensory apparatus that can trigger spore germination, allowing the return to vegetative growth within minutes of exposure of germinants. We demonstrate here that bacterial spores of multiple species retain memory of transient exposures to germinant stimuli that can result in altered responses to subsequent exposure. The magnitude and decay of these memory effects depend on the pulse duration as well as on the separation time, incubation temperature, and pH values between the pulses. Spores of Bacillus species germinate in response to nutrients that interact with germinant receptors (GRs) in the spore’s inner membrane, with different nutrient types acting on different receptors. In our experiments, B. subtilis spores display memory when the first and second germinant pulses target different receptors, suggesting that some components of spore memory are downstream of GRs. Furthermore, nonnutrient germinants, which do not require GRs, exhibit memory either alone or in combination with nutrient germinants, and memory of nonnutrient stimulation is found to be more persistent than that induced by GR-dependent stimuli. Spores of B. cereus and Clostridium difficile also exhibit germination memory, suggesting that memory may be a general property of bacterial spores. These observations along with experiments involving strains with mutations in various germination proteins suggest a model in which memory is stored primarily in the metastable states of SpoVA proteins, which comprise a channel for release of dipicolinic acid, a major early event in spore germination.
IMPORTANCE
Cellular memory is defined as a sustained response to a transient environmental stimulus, and yet its generation and storage have not been described in bacterial spores. We demonstrate here that bacterial spores of multiple species retain memory of transient exposures to germinant stimuli that can result in altered responses to subsequent exposure. Memory was induced by activation of germinant receptors (GRs) or by GR-independent germinants and was accessed by both GR-dependent and GR-independent germinants. Analysis of effects on memory of exposure to GR-dependent and GR-independent germinants as well as in spores lacking various germination proteins suggests a model in which memory is stored primarily in metastable states of SpoVA proteins which comprise a channel for release of spore dipicolinic acid. Spore memory can also significantly reduce the concentration of nutrient germinants necessary to trigger germination, and this may be used to respond to low levels of nutrient germinants.
INTRODUCTION
Cells can sense and respond to external stimuli, and when a cellular response to a transient stimulus is sustained, biological memory is formed. Biological memory is a natural phenomenon found in many organisms, including mammalian immunological memory for antigens (1), plant vernalization memory for cold (2), bacterial clustered regularly interspaced short palindromic repeat (CRISPR)-Cas immune systems to respond to phage infection (3), and bacterial chemotactic response to ligand concentration (4). Spores of bacteria of Bacillus species are metabolically dormant and highly resistant and can survive for years in that state (5–7). However, these spores monitor their environment, and when conditions, in particular, the appearance of nutrients, are favorable, they can resume vegetative life through spore germination followed by outgrowth (7). One issue concerning memory in bacterial spores is whether brief exposure of spores to germinants affects germination when spores are reexposed briefly to the same or different germinants, even if the first germinant exposure ended many minutes or hours earlier.
The germination of spores of members of the orders Bacillales and Clostridiales has significant research interest for two major reasons: (i) there are novel regulatory systems that allow spores to remain in a dormant, resistant state for years (7) and yet allow return to vegetative growth in minutes, and (ii) spores of some species are vectors of food spoilage and foodborne disease, as well as of a number of serious human diseases (8, 9). However, spores are very resistant to antibiotic treatments, radiation, heat, and chemicals, making them difficult to kill. While spores cause deleterious effects only after they germinate, they also lose their resistance properties in this process and become relatively easy to kill (8, 9). Consequently, understanding of spore germination may help in either preventing or accelerating spore germination in order to destroy the much less resistant germinated spores.
Spore germination involves both a complex signal transduction pathway and biophysical events that have been studied best in spores of Bacillus subtilis (7, 10). Dormant spores of Bacillus species contain a number of specific germinant receptors (GRs) present in their inner membrane (IM), and when appropriate nutrient germinants are present, these activate specific GRs, most probably by direct binding, and trigger spore germination. Spores of B. subtilis contain three functional GRs termed GerA, GerB, and GerK. GerA responds to l-alanine or l-valine, and GerB and GerK are both required for germination with a mixture of l-asparagine, d-glucose, d-fructose, and K+ (AGFK). Normally, l-asparagine alone does not trigger B. subtilis spore germination. However, a mutant form of GerB, GerB*, is activated by l-asparagine alone to trigger germination of gerB* spores (11). After nutrient germinant exposure, the GR’s germination signal is transduced and amplified in some manner, perhaps by the GerD germination protein. This activates downstream components of the germination apparatus, in particular, the SpoVA proteins that likely make up a crucial channel in the spore IM, and this leads to subsequent germination events. In addition to GR-dependent germinants, there are also GR-independent germinants, including very high hydrostatic pressure, a 1:1 chelate of Ca2+ and pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) (CaDPA), and cationic surfactants such as dodecylamine (7, 10).
The earliest measurable event after germinant-GR binding is termed the commitment step, in which even if nutrient germinants are removed or their further binding to GRs is inhibited, committed spores continue through germination (7, 10, 12, 13). The cascade of events after the commitment step generally includes (i) changes in the permeability of the spore IM; (ii) release of monovalent cations and the spore core’s huge depot of CaDPA, the latter almost certainly via the SpoVA protein channel noted above; (iii) CaDPA’s replacement by water, resulting in the elevation of core water content; and (iv) hydrolysis of the spore’s peptidoglycan cortex by either of two redundant cortex-lytic enzymes (CLEs), CwlJ and SleB, allowing the swelling and further hydration of the spore core. Spores that are not committed to germination carry out no germination events (7).
Recent work has indicated that spores given a brief exposure to a nutrient germinant that activates GerA (or GerB*) GRs are at least partially and reversibly activated for germination (14), because these spores respond more readily to a 2nd short exposure of the same germinant. This phenomenon appears to be spore memory, since the response to the 1st germinant pulse decreases when the interval between the 1st and 2nd germinant pulses increases. However, how this memory is formed and stored and how it decays and is accessed are unknown. Do spores that are exposed to a germinant pulse, for example, one that activates a particular GR, also show memory when reexposed to a 2nd pulse affecting either a GR-dependent or a GR-independent germinant? The answer to this question may help to determine if the spore’s memory is stored in activated GRs or in downstream germination components. From this study, we report results of new memory experiments performed by monitoring the dynamic germination of hundreds of individual spores of B. subtilis using differential interference contrast (DIC) microscopy. These spores were given a short pulse of various germinants and incubated in buffer at different pHs and temperatures and for different times, followed by a 2nd short pulse of various germinants. The results of this work indicate that spore memory can be generated and accessed by several types of germination proteins but is stored primarily in SpoVA proteins that allow CaDPA release and give insight into the mechanisms involved in this memory.
RESULTS
Spore memory can be formed by stimulation of different GRs.
Previous work showed that wild-type B. subtilis spores could acquire memory upon l-valine triggering germination via the GerA GR (14). This was confirmed in the current work examining the germination of multiple individual PS832 wild-type spores, as there was ~4-fold more germination in the 2nd l-valine pulse than in the 1st one (Fig. 1A). Similar results were obtained using spores of another wild-type strain, PS533 (see Fig. S1B in the supplemental material).
FIG 1 .
Spore memory can be generated and accessed by stimulation of different B. subtilis GRs. (A) Spores of B. subtilis (wild-type) PS832 were given two 5-min 3.5 mM l-valine (L-val) or 100 mM AGFK germinant pulses separated by 25 min. (B) PS3415 (high GerB*) spores were given two 1-min 2 mM l-valine or 0.8 mM l-asparagine (L-asn) germinant pulses. Percent germination induced by each pulse, calculated as described in Materials and Methods and the legend to Fig. S1 in supplemental material, is indicated in parentheses for each combination. Gray bars above the horizontal axis indicate pulse durations, and vertical arrows indicate the times when pulses were terminated.
The results given above indicated that GerA stimulation could generate memory that could heighten this GR’s response to subsequent l-valine stimulation. Two obvious issues are whether spore memory is also generated by stimulation of GerB and GerK GRs and whether memory acquired by one GR can heighten germination via a different GR. Both of these responses were indeed seen with PS832 spores (Fig. 1A) and PS533 spores (see Fig. S1B in the supplemental material). Not only did a 1st AGFK pulse stimulate germination following a 2nd AGFK pulse, but this was also seen with consecutive pulses of l-valine and AGFK in either order. In all cases, the extent of germination in the 2nd pulse was up to ~4-fold higher than in the 1st pulse, although the average values of the kinetic parameters of germination of multiple individual spores following germinant pulses were very similar for the various pulse sequences (see Table S1A and Fig. S1C and D).
To further examine the generality of the observations on spore memory made with wild-type spores, the effects of pulses of l-valine and l-asparagine on the germination of PS3415 spores (high GerB* levels) that germinate rapidly with l-asparagine alone were also examined. As found with PS3415 spores given two l-asparagine pulses (14), there was ~2-fold more germination in the 2nd pulse with l-asparagine than in the 1st one (Fig. 1B). In addition, when pulses of either l-valine or l-asparagine were used, there was ~2-fold more germination in the 2nd pulse independently of the identity of the germinants in the two pulses (Fig. 1B). As found with wild-type spores, the average values of the kinetic parameters of the germination of multiple individual PS3415 spores were essentially the same for spores germinating after both pulses and with both l-asparagine and l-valine (see Table S2 in the supplemental material). PS3415 spores have an ~12-fold-elevated GerB* level but a normal level of GerA (15). Since memory was observed with the PS3415 spores as well as with the wild-type spores, which have much lower levels of the various GRs, including GerA and GerB, this suggests that GR levels alone do not have essential roles in acquiring and accessing spore memory.
Longer incubation times between germinant pulses increased spore memory loss.
If the increased germination in the 2nd germinant pulse noted above were due to spore memory, this memory might decay with time. Indeed, PS3415 spores given a 1st l-asparagine pulse exhibited less germination after the 2nd l-asparagine pulse as the interval between pulses increased (Fig. 2B; see also Fig. S2A in the supplemental material), as shown previously (14) (note that the memory of the 1st l-asparagine pulse decreased almost to zero after 12 h). This experiment was repeated using two l-valine pulses (Fig. 2A). Again, with increased intervals between the two pulses, the germination following the 2nd pulse decreased. However, memory of the 1st l-valine pulse was lost more rapidly than memory of the 1st l-asparagine pulse (Fig. 2B). Notably, in this experiment and others (see below), the ratio of the germination in the 2nd pulse to that in the 1st one ultimately decreased to below 1.0, perhaps because spores that germinated in the 1st pulse had higher levels of proteins that recognize germinants than spores that germinated in the 2nd pulse.
FIG 2 .
Effects of time and temperature during incubation between pulses on PS3415 (high GerB*). (A) Spores were given two 1-min pulses of 2 mM l-valine at 37°C separated by 15, 20, or 45 min. Spores were also germinated with 2 mM l-valine for 2 min (■), followed by germinant removal and further incubation at 37°C. (B) Spores were given two 1-min pulses of 2 mM l-valine or 0.8 mM l-asparagine at 37°C separated by various times, and the ratio of the percentage of germination induced by the 2nd pulse to the percentage of germination induced by the 1st pulse was calculated as described in Materials and Methods and the legend to Fig. S1 in the supplemental material. (C) Spores were exposed to a 1st 1-min pulse of 2 mM l-valine, the germinant was removed, and the spores were incubated at 45, 37, or 15°C for 45 min and given a 2nd 1-min pulse of 2 mM l-valine, followed by germinant removal and further incubation at 37°C until 70 min. Spores were also germinated with 2 mM l-valine for 2 min (■), followed by germinant removal and further incubation. (D) Spores were given two 1-min 2 mM l-valine or 0.8 mM l-asparagine pulses and incubated at different temperatures following the 1st pulse, and the ratio of the percentage of germination after the 2nd pulse to that after the 1st pulse was calculated. All curves shown are from data determined with ~300 individual spores. Gray bars indicate pulse durations, and vertical arrows indicate the times of termination of the 2nd pulses.
Further indication that spore memory can decay was obtained from analysis of PS3415 spores germinating with a 2-min pulse of l-valine (Fig. 2A). Notably, the percentage of spores germinated in this 2-min pulse was significantly higher than that seen with two 1-min pulses separated by 15 min, indicating that after exposure to the 1st l-valine pulse, spore memory decays during the incubation prior to the 2nd pulse. In contrast, with a 2-min pulse, many spores that had acquired memory in the 1st min but that had not yet become committed to germinate became committed to germinate in the 2nd min. Similar results were obtained when levels of spore germination after a 2-min l-asparagine pulse were compared to the sum of the germination levels following two 1-min pulses separated by 15 min (see Fig. S2A in the supplemental material).
Elevated temperature between germinant pulses decreased spore memory.
In addition to the time interval between two germinant pulses, another factor that might affect spore memory retention is the temperature between pulses. To test this possibility, we used PS3415 spores, since they acquired significant memory after very short germinant pulses, minimizing memory loss during the germinant pulse itself. After exposure of PS3415 spores to l-valine in the 1st pulse and germinant removal, spores were incubated for 45 min at different temperatures (Fig. 2C). Strikingly, spores incubated at 45°C between germinant pulses lost all memory of the 1st pulse, while spores incubated at 37°C retained significant memory. Notably, the spores incubated at 15°C after the 1st pulse retained the most memory, and the sum of the germination percentages in the two pulses given these spores was almost identical to the percentage of germinated spores given a 2-min pulse (Fig. 2C). A comparable experiment examining the effects of incubation temperature between two l-asparagine pulses on levels of spore germination after the 2nd pulse gave results that were relatively similar to those seen with l-valine pulses (Fig. 2D; see also Fig. S2B in the supplemental material).
Spore memory is dependent on the incubation pH between germinant pulses.
Since pH represents another variable affecting spore germination, it seemed possible that incubation pH following a germinant pulse could also influence memory. After exposure to the 1st germinant pulse at pH 7.4 and germinant removal, wild-type spores were incubated in buffer with different pH values, and in the 2nd pulse spores were again incubated at pH 7.4 (see Table S1B and Fig. S3 in the supplemental material). The results showed that the percentages of germination after a 1st pulse with l-valine or AGFK were identical when postpulse incubation was at pH 6.0 to 8.4, indicating that postcommitment germination events were not appreciably affected over this pH range, as expected (16). However, the percentages of spore germination after the 2nd pulse changed depending on the incubation pH between pulses. When the 2nd pulse was l-valine, the germination percentages after this pulse were similar when incubation pH between pulses was 6.0 or 7.4 but were much lower with incubation at pH 8.4, while when the 2nd pulse was AGFK, incubation pH between pulses at 8.4 gave the most germination after the 2nd pulse.
Spore memory enhances sensitivity to low nutrient germinant concentrations.
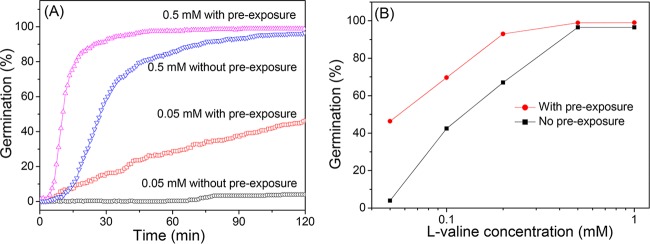
An issue that is obvious from the results given above is that of whether memory observed in nutrient germination affects the concentrations of nutrient germinants necessary to trigger spore germination in a second germinant exposure. To address this issue, we first measured the germination of PS832 spores with various concentrations of l-valine alone (Fig. 3A). We also gave PS832 spores an initial 5-min 10 mM l-valine pulse, removed the l-valine, and incubated for 20 min to allow completion of germination (~20%), followed by a second exposure to various concentrations of l-valine (Fig. 3B and data not shown). Notably, preexposure to the 5-min pulse of 10 mM l-valine increased the percentage of spore germination with 0.05 mM l-valine in 120 min from 4% to 46% (Fig. 3; see also Table S1C and Fig. S4 in the supplemental material). Similarly, spores preexposed to 10 mM l-valine and then given 0.5 mM l-valine germinated much faster than spores without preexposure, as lag time (Tlag) values were reduced ~3-fold (Fig. 3B; see also Table S1C and Fig. S4). These results indicate that memory created by the initial germinant pulse significantly reduced the concentrations of nutrient germinants necessary to trigger spore germination. Thus, memory enhances spore sensitivity to nutrient germinants.
FIG 3 .
Effect of spore memory on spores’ responses to various germinant concentrations. (A) Germination of spores with or without an initial 10 mM l-valine pulse. B. subtilis PS832 spores were germinated as described in Materials and Methods with 0.05 or 0.5 mM l-valine added at time zero and with or without a prior 5-min pulse of 10 mM l-valine. After the 5-min pulse giving ~20% germination, the l-valine was washed out and the spores were incubated in buffer for 20 min at 37°C to allow committed spores to complete the germination process prior to addition of low l-valine concentrations at time zero. (B) The percentages of spores that germinated in 120 min at various l-valine concentrations and with or without an initial 5-min pulse with 10 mM l-valine. All curves and data shown were from results determined with >300 individual spores. Spore germination percentages were determined and calculated as described in Materials and Methods and the legend to Fig. S1 in the supplemental material.
Effects of d-alanine incubation between germinant pulses on spore memory.
d-Alanine acts an inhibitor of germination via the GerA GR and blocks the interaction of GerA with l-valine or l-alanine (17). Indeed, d-alanine appears to bind to the GerA GR with higher affinity than l-alanine. Consequently, the exposure of spores to d-alanine between germinant pulses might eliminate spore memory. To test this possibility, after the 1st l-alanine pulse, spores were incubated with 0.5 mM d-alanine for 10 min, the d-alanine was removed, and the spores were incubated in K-HEPES buffer for 9 min and then given a 2nd l-alanine pulse. Spore memory was indeed accessed following the 2nd l-alanine pulse, although the spores germinated slowly (see Fig. S5 in the supplemental material). Thus, d-alanine does not eliminate spore memory, although its effects may be complicated due to its stronger affinity for the GerA GR than l-alanine as well as to its inhibition of GerA-dependent spore germination.
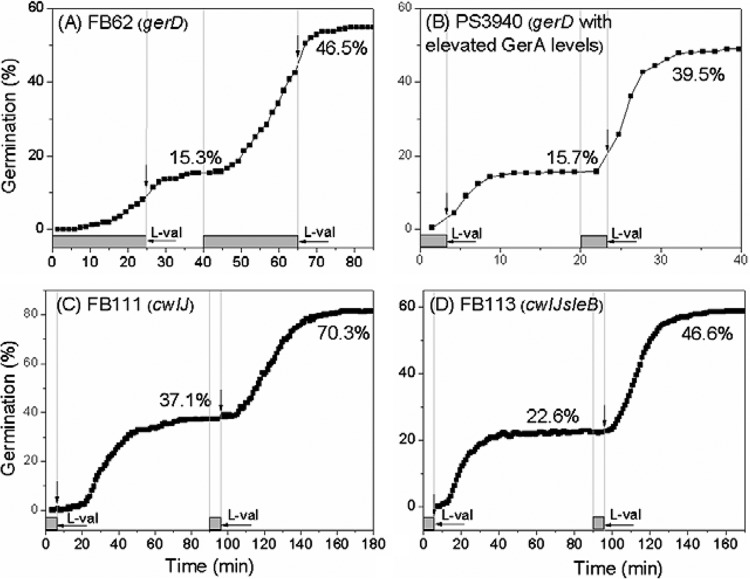
GerD and CLEs are not essential for spore memory of nutrient germinant pulses.
The auxiliary germination protein GerD plays an important role in the rapid GR-dependent response to nutrient germinants of spores, perhaps by facilitating GR assembly into a large protein complex, termed the germinosome, in the spore IM (7, 10). Consequently, GerD could be involved in spore memory of nutrient germinant exposure. To examine this possibility, spores of strain FB62 (with a gerD deletion) and strain PS3940 (also with a gerD deletion and overexpressing GerA ~8-fold) (18) were used. Although germination of FB62 spores was slow, as expected, using long germinant pulse times, these spores did show memory with l-valine–l-valine or AGFK-AGFK pulses (Fig. 4A; see also Table S3A and Fig. S6A in the supplemental material). PS3940 spores also showed memory with two pulses of l-valine or AGFK (Fig. 4B; see also Table S3A and Fig. S6B). Consequently, it is most likely that GerD and germinosome assembly are not essential for spore memory.
FIG 4 .
Effects of lack of GerD or CLEs on B. subtilis spore memory of germinant exposure. Spores from mutant strains were exposed to sequential pulses of 10 mM l-valine, germinant was removed after each pulse, and germination of >222 individual spores was monitored by DIC microscopy as described in Materials and Methods. Gray bars above horizontal axes indicate pulse durations, and vertical arrows above germination curves indicate the times when pulses were terminated. Percent germination after each pulse is given above the germination curves, and values for percent germination after the 2nd pulse were calculated as described in the legend to Fig. S1 in the supplemental material.
During the second stage of spore germination, the peptidoglycan cortex of spores is degraded by either of two CLEs, CwlJ and SleB; the spore core subsequently swells and takes up water (7, 10). Thus, we also checked if CLEs are where memory of a nutrient germinant pulse is stored. With two l-valine pulses, the spores of both cwlJ and cwlJ sleB mutants showed obvious memory (Fig. 4C and D), as did PS533 spores from which CwlJ had been removed by decoating (see Fig. S7 in the supplemental material). These results eliminated the possibility that CLEs alone are where memory is stored.
Spore memory with pulses of GR-independent and GR-dependent germinants.
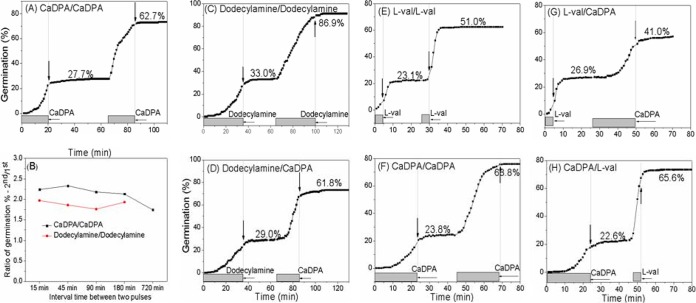
While memory was consistently evident in GR-dependent spore germination, suggesting that GRs may be involved in memory generation, storage, and access, loss of GerD, which greatly reduces rates of GR-dependent germination, did not abolish spore memory. So it is possible that memory is generated and stored downstream of GRs in the spore germination pathway. To gain further information relevant to the role of GRs in spore memory, the germination of multiple individual B. subtilis PS533 (wild-type) spores following two pulses of the GR-independent germinants CaDPA and dodecylamine levels were measured. When two sequential CaDPA pulses were used, there was ~2-fold more germination in the 2nd pulse than in the 1st one (Fig. 5A), and a similar result was obtained with two sequential dodecylamine pulses (Fig. 5C). Notably, (i) spore germination with these GR-independent germinants took place only during CaDPA or dodecylamine pulses and (ii) spore germination initiated faster with the 2nd pulse than with the 1st pulse (Fig. 5A and C; see also Table S1A in the supplemental material). When dodecylamine was used in the 1st pulse, a 2nd pulse with CaDPA also showed about 2-fold more germination than the 1st one and also about twice as much as a 1st CaDPA pulse (Fig. 5D; compare with Fig. 5A). However, a significant difference from GR-dependent germinations was that, with increases in intervals between two CaDPA pulses or two dodecylamine pulses, the amount of germination in the 2nd pulse decreased only minimally (Fig. 5B), suggesting that memory of a CaDPA or dodecylamine pulse decays extremely slowly, especially since the dodecylamine memory experiment was carried out at 50°C.
FIG 5 .
B. subtilis PS533 spore memory with two pulses of various combinations of GR-independent and GR-dependent germinants. (A to D) Spore memory with GR-independent and GR-independent germinant combinations. (A) PS533 (wild-type) spores were given a 20-min pulse of 60 mM CaDPA or a 35-min pulse of 0.8 mM dodecylamine followed by germinant removal, incubated in buffer at 37°C (for CaDPA) or 50°C (for dodecylamine) for a short period, and given a 2nd 20-min pulse of 60 mM CaDPA or a 35-min pulse of 0.8 mM dodecylamine, the germinant was removed, and the reaction mixture was incubated until germination stopped. (B) Spore memory decay with different times between two 60 mM CaDPA or two 0.8 mM dodecylamine pulses. Ratios of the percentage of germination due to the 2nd pulse to that due to the 1st pulse were determined and calculated as described in Materials and Methods and the legend to Fig. S1 in the supplemental material. (E to H) Spore memory with GR-independent and GR-dependent germinant pulses. Heat-activated PS533 spores were given a 4-min pulse of 10 mM l-valine or a 23-min pulse of 60 mM CaDPA followed by germinant removal, incubated for 22 min, and then given a 2nd 4-min pulse of 10 mM l-valine or a 23-min pulse of 60 mM CaDPA followed by germinant removal and incubated until 70 min (E and G) or 80 min (F and H). Curves shown are from data determined with ~300 individual spores. Gray bars indicate pulse durations, and vertical arrows indicate the times when the 2nd pulses were terminated.
Since memory was seen between two GR-independent germinant pulses, the ability of a GR-dependent germinant to induce memory for GR-independent germination and vice versa was also examined using heat-activated spores. Again, these spores showed ~2-fold more germination in the 2nd pulse than in the 1st one when sequential l-valine or CaDPA pulses were used (Fig. 5E and F). Surprisingly, when the 1st pulse was CaDPA, after a 2nd pulse with l-valine there was ~3-fold more germination than in the 1st pulse, although with a 1st l-valine pulse, after a 2nd CaDPA pulse there was only ~50% more germination than in the 1st l-valine pulse (Fig. 5G and H).
As with GR-dependent germination, the kinetic parameters of the germination of multiple individual PS533 spores given two sequential l-valine pulses and of consecutive l-valine and CaDPA pulses in either order were essentially the same for spores that germinated following the 1st and 2nd germinant pulses (see Table S1A in the supplemental material). However, when two sequential CaDPA pulses were used, the values of Tlag and Trelease in the 2nd pulse were much lower than those in the 1st pulse, although the values of ΔTrelease were unaffected (see Table S1A).
Effects of loss of the SpoVAEa and/or SpoVAF protein(s) on spore memory.
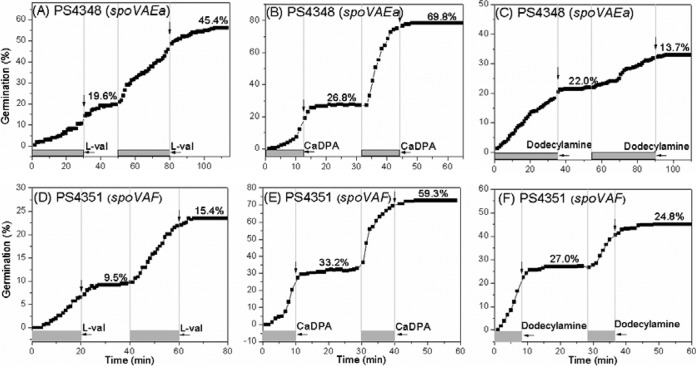
The differences in the stability of memory of GR-dependent germinant stimuli depending on the GR stimulated are consistent with GRs being the sites where memory is stored from a nutrient germinant pulse. It is also notable that a high-pressure treatment that activates GRs also generates memory and that this memory is also relatively unstable (19). However, memory was also generated by the GR-independent germinants CaDPA and dodecylamine, which likely act on the CLE CwlJ and on a specific protein in the SpoVA protein IM channel for CaDPA (6, 20), respectively, and this memory was much more stable than was memory of GR activation. These findings suggest there could be components involved in GR-dependent memory that are different from those involved in CaDPA or dodecylamine memory. To attempt to gain more-direct evidence for the latter suggestion, we used spores of two B. subtilis mutants that lack either both the SpoVAEa and the SpoVAF proteins or the SpoVAF protein alone (21, 22). These two proteins are well conserved in many but not all Bacillus species, and their loss has no effect on sporulation, in particular, on the rate of CaDPA uptake. Spores of a spoVAEa mutant also exhibit normal rates of CaDPA and dodecylamine germination, but rates of release of CaDPA in nutrient germination are ~4-fold lower than for wild-type spores. Strikingly, while loss of SpoVAEa plus SpoVAF had no effects on memory during l-valine or CaDPA germination, lack of this protein abolished memory in dodecylamine germination (Fig. 6A to C), and similar results were obtained with spores lacking only SpoVAF (Fig. 6D to F).
FIG 6 .
Effects of loss of the SpoVA proteins on B. subtilis spore memory. B. subtilis PS4348 (ΔspoVAEa strain) (A to C) or PS4351 (ΔspoVAF strain) (D to F) spores were given a 30-min (A) or 20-min (D) pulse of 10 mM l-valine, a 12-min (B) or 10-min (e) pulse of 60 mM CaDPA, or a 35-min (C) or 8-min (F) pulse of 0.8 mM dodecylamine followed by germinant removal, incubated in buffer at 37°C (l-valine, CaDPA) or 50°C (dodecylamine) for short periods, and given a 2nd same pulse, the germinant was removed and the spores were incubated until germination stopped, and spore germination levels were measured, all as described in Materials and Methods. Ratios of the percentage of germination due to the 2nd pulse to that due to the 1st pulse were determined and calculated as described in Materials and Methods and the legend to Fig. S1 in the supplemental material. Curves shown are from data determined with ~250 individual spores. Gray bars indicate pulse durations, and vertical arrows indicate the times when the 1st and 2nd pulses were terminated.
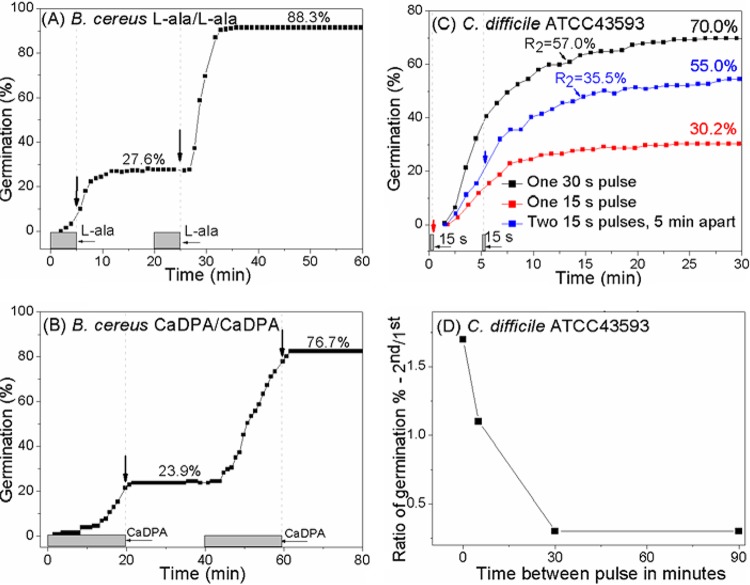
Bacillus cereus and Clostridium difficile spore memory.
To determine if spore memory is general in spore-forming bacteria, we also examined germination of B. cereus and C. difficile spores for memory. When B. cereus spores were given short l-alanine or CaDPA pulses, there was ~3-fold more germination in the 2nd l-alanine or CaDPA pulse than in the 1st pulse (Fig. 7A and B). When two l-alanine pulses were used, almost all spores germinated only after the pulses (see Fig. S1E in the supplemental material). However, when two CaDPA pulses were used, the vast majority of spores germinated only during CaDPA exposure, as seen with PS533 spores (Fig. 5A and 7B).
FIG 7 .
B. cereus and C. difficile spore memory. (A and B) B. cereus spore memory with GR-dependent or GR-independent germinants. B. cereus spores were given two pulses of either 10 mM l-alanine (A) or 60 mM CaDPA (B), and germination of ~300 individual spores was monitored by DIC microscopy. (C) C. difficile spores were exposed to one pulse of 15 s or 30 s of 0.25% taurocholate and 15.5 mM glycine at 37°C, followed by germinant removal, rinsing five times, and further incubation at 37°C in buffer until 30 min. The spores were also exposed to two sequential 15-s pulses separated by 5 min. The germination induced by the 2nd pulse is given in comparison to that induced by one 15-s pulse. (D) Spore memory decay as a function of time between 15-s pulses of 0.25% taurocholate plus 15.5 mM glycine. In panels A to C, the numerical labels indicate the fractions of spores germinating in response to the preceding pulse calculated as described in Materials and Methods and the legend to Fig. S1 in the supplemental material.
C. difficile is an anaerobic spore-forming, Gram-positive bacterium, and its spores can commit to germinate after exposure to taurocholate plus glycine (23). Short germinant exposures can trigger a high level of C. difficile spore germination. In this study, a 15-s germinant pulse followed by germinant removal, rinsing five times, and further incubation in buffer until 30 min gave 30% germination, and a 30-s pulse resulted in 70% germination. The 30-s germinant pulse can be regarded as two sequential 15-s pulses with no interval between, and when calculation of the number of the spores that germinated in the 2nd 15 s was corrected for the percentage of spores that had germinated with only one 15-s pulse, the proportion of germination in the 2nd 15 s of a 30-s germinant treatment was 57% (Fig. 7C). This suggests that memory is also generated by germinant pulses in C. difficile spores. However, when the interval between two 15-s pulses was increased, the ratios of the percentage of germination after the 2nd 15-s pulse to that after the 1st 15-s pulse fell rapidly from 1.7 to 0.3 (Fig. 7D), indicating that C. difficile spore memory decays faster than B. subtilis spore memory.
DISCUSSION
A general outline of a model to explain spore memory is that the 1st germinant pulse generates a change in some germination protein(s), with a change in the conformation seeming most appropriate, that activates this protein(s) for germination. In some spores, sufficient activated proteins are generated such that these spores reach the threshold commitment and proceed to germinate. However, in other spores, the 1st germinant pulse generates subthreshold levels of an activated germination protein(s). Consequently, these spores remain uncommitted, and the activated form of the germination protein(s) can decay to the unactivated state. However, if there is a 2nd germinant pulse before decay of the activated protein conformation, then generation of an additional activated protein(s) in the 2nd germinant pulse, together with residual activated proteins from the 1st pulse, generates more germination following the 2nd pulse than after the first one.
The proteins needed for normal germination include GRs, GerD, CLEs, and the SpoVA IM proteins that comprise a channel through which CaDPA is released early in germination (6, 7). Of these proteins, GerD was not essential for spore memory in nutrient germination. Perhaps the CLE CwlJ could store memory in CaDPA germination, since CwlJ is activated by CaDPA and CLE action precedes CaDPA release in CaDPA germination. However, it is also possible that partial cortex hydrolysis is how memory is stored in CaDPA germination, as complete cortex hydrolysis alone can cause the CaDPA channel to open, and memory generated in CaDPA germination was extremely stable, as partial cortex hydrolysis would be. However, CwlJ and SleB action were not essential for memory in nutrient germination, and CaDPA release precedes CLE action in both nutrient and dodecylamine germination. This leaves GRs and the SpoVA protein CaDPA channel as the most likely possibilities for memory generation and storage, at least for nutrient germination, and memory from GR activation could be stored in either activated GRs or the CaDPA channel. It is possible that some memory resides with the GRs, at least in GR-dependent germination, which is consistent with the different rates of memory decay that we observed for l-valine and l-asparagine pulses. However, since memory was also generated in CaDPA and dodecylamine germination, which does not require GRs, our model focuses on memory storage in CaDPA channel proteins (Fig. 8). Thus, in nutrient germination, the conformation state of one or more SpoVA channel proteins may be directly linked to the conformation change of adjacent GRs in the IM, and there is some evidence for direct interactions between GR and SpoVA proteins (22). Perhaps the maintenance of channel proteins in their subthreshold activation state induced by GRs depends on the maintenance of the activation state GRs, which may decay at different rates for different GRs. In addition to the points made above, the hypothesis that spore memory of exposure to a germinant stimulus is generated and stored in the SpoVA protein channels is consistent with additional results, including that (i) l-valine pulses can access memory generated by l-asparagine or AGFK pulses and vice versa; (ii) GR-independent CaDPA or dodecylamine pulses can access memory generated by GR-dependent pulses and vice versa, and the decay of memory generated by dodecylamine or CaDPA is extremely slow; (iii) the GR-independent germinant dodecylamine likely directly activates the CaDPA channel by binding to the SpoVAC protein (20); (iv) the absence of GerD which is essential for GR-dependent germination did not affect spore memory, and d-alanine incubation between l-alanine pulses also did not eliminate memory; (v) loss of the SpoVAEa and/or the SpoVAF protein had no effect on memory in GR-dependent or CaDPA germination but greatly decreased memory in dodecylamine germination; (vi) C. difficile spore germination also exhibited memory, and yet C. difficile spores have no IM GRs; and (vii) the CaDPA channel is known to exist in multiple states (Fig. 8), closed in dormant spores, opened wide at Tlag, and partially opened, allowing DPA leakage, at approximately the time of commitment (13). While the latter two states of the CaDPA channel do not decay back to the dormant state, it is easy to imagine that this channel can also exist in a fourth metastable activated state that can decay (Fig. 8). If the SpoVA channel is the primary site where spore memory is generated and stored, this predicts that there is a threshold of activated channels in the IM essential for commitment to germinate and thus that these channels act at least somewhat cooperatively (Fig. 8). Unfortunately, the precise structure and gating of this channel are not understood, but this information seems essential for a full understanding of both spore memory and spore germination itself.
FIG 8 .
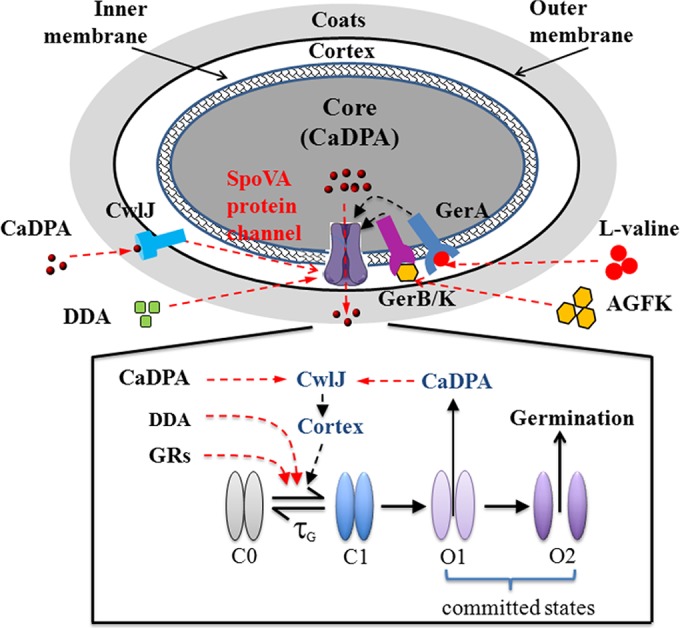
Channel-centered model of spore germination and memory. Multiple signals, including those from GRs, CLEs, and the direct binding of dodecylamine, stimulate the transition of the SpoVA CaDPA channel proteins in the IM from an inactive closed state (C0) to a metastable closed state (C1) that may either decay back to C0 or transition to the partially open state (O1), which marks commitment of the spore to germinate because this transition is not reversible. Cooperative interaction among the channel proteins may set a sharp threshold for the commitment step. Transition from the partially open O1 state to the fully open O2 state also occurs in a concerted fashion, possibly due to the activation of CwlJ by the release of CaDPA, which forms a positive-feedback loop, as cortex degradation facilitates activation of channel proteins. A subthreshold stimulation pulse leads to accumulation of SpoVA channel proteins in the C1 state without triggering commitment. Thus, transient stimulation can prime spores to germinate more easily upon further stimulation. The decay of this memory effect is controlled by τG in this simple model. Some fraction of the memory may reside in the GRs themselves in order to account for the different rates of memory decay observed with germinants that activate different GRs. Stimulation with CaDPA and dodecylamine (DDA) exhibits long-lived memory effects because they induce partial lysis of the cortex layer adjacent to the channel proteins in the IM, which is irreversible and decreases the threshold for germination upon subsequent restimulation.
Finally, the finding that memory of germinant stimuli in spores can significantly reduce the concentrations of nutrient germinants necessary to trigger spore germination indicates that spores with memory can sense and respond to very low nutrient germinant concentrations, similarly to the sensitization that is observed in bacterial chemotaxis (24).
MATERIALS AND METHODS
B. subtilis strains used and spore preparation.
The B. subtilis strains used in this work were (wild-type) PS832, a prototrophic 168 strain, and its isogenic derivatives, including (i) (wild-type) PS533, which contains plasmid pUB110, encoding resistance to kanamycin (25); (ii) PS3415, spores of which have 18-fold-elevated levels of a modified GerB, GerB* (11); (iii) FB62, a gerD deletion mutant (18); (iv) PS3940, a gerD deletion mutant with ~8-fold-elevated levels of GerA (18); (v) FB111, a cwlJ deletion mutant (26); (vi) FB113, a cwlJ sleB deletion mutant (26); (vii) PS4348, a spoVAEa deletion mutant (21) that lacks a protein essential for the normal rapid CaDPA efflux during spore germination but that has no role in DPA uptake in sporulation (spores of this strain probably lack the product of the downstream spoVAF gene); and (viii) PS4351, a spoVAF deletion mutant (21), lacking a SpoVA protein with no known function but expressing SpoVAEa at normal levels. Spores of B. cereus T originally obtained from H. O. Halvorson and C. difficile ATCC 43593 (23) were also assayed for spore memory.
Unless otherwise noted, spores of all B. subtilis strains and C. difficile were prepared on 2× Schaeffer's glucose (SG) medium agar plates without antibiotics (27) and on 70:30 sporulation media agar (28), respectively, and B. cereus spores were prepared as described previously (16). Where indicated, chemically decoated PS533 spores were used and prepared as described previously (29). All purified spores were stored at 4°C in water protected from light and were (98%) free of growing and sporulating cells, germinated spores, and cell debris as observed by phase-contrast microscopy.
Spore germination with germinant pulses.
Except for two consecutive CaDPA and/or dodecylamine pulses, B. subtilis spores were heat activated prior to nutrient germination by incubation in water at 70°C for 30 min and then by cooling on ice for at least 15 min. Analysis of spore memory of germinant exposures was performed essentially as described in previous work (14). Briefly, B. subtilis spores attached on the surface of a glass coverslip were germinated in 25 mM K-HEPES buffer (pH 7.4) with either a constant exposure to a germinant either for 60 to 90 min or exposure for various periods (15 s to 25 min) before the germinant was removed and spores were rinsed 5 times using vacuum pump suction (14). The rinsed spores were then incubated at 37°C in 25 mM K-HEPES buffer with a pH of 7.4 unless noted otherwise. After various incubation times, the spores were given a 2nd exposure to germinants for various times at 37°C in 25 mM K-HEPES buffer (pH 7.4) followed by germinant removal, rinsing by vacuum pump suction, and further incubation at 37°C in 25 mM K-HEPES buffer (pH 7.4). B. subtilis spores were also germinated at 37°C with 60 mM CaDPA–25 mM K-HEPES buffer (pH 7.4) and at 50°C with 0.8 mM dodecylamine–25 mM K-HEPES buffer (pH 7.4).
The method for measuring the germination of individual B. cereus and C. difficile spores was identical to that described above for B. subtilis spores. The germinants for B. cereus spores with or without a heat shock (exposure at 65°C at 30 min followed by cooling on ice for ~15 min) were, respectively, 10 mM l-alanine–25 mM K-HEPES buffer (pH 7.4) or 60 mM CaDPA–25 mM K-HEPES buffer (pH 7.4). C. difficile spore germination does not require heat activation, and the germinant mixture was 0.25% taurocholate–15.5 mM glycine–10 mM Tris-HCl (pH 7.4)–150 mM NaCl at 37°C.
Monitoring spore germination and data analysis.
Germination of multiple individual spores adhered on a microscope coverslip was monitored by DIC microscopy, and data were analyzed as described previously (30, 31). Most of the changes in spore germination seen by DIC microscopy are due to the release of spores’ large CaDPA depots, and the kinetic germination parameters for which averages were determined were Tlag, the time between germinant addition and fast CaDPA release; Trelease, the time between germinant addition and the end of CaDPA release; and ΔTrelease (i.e., Trelease − Tlag). For quantitation of spore memory with two germinant pulses, the percentages of spores that germinated in the 2nd pulse were corrected for the percentages of spores that had germinated before the 2nd pulse (see Fig. S1 in the supplemental material).
SUPPLEMENTAL MATERIAL
Schematic of spore memory experiments and memory in germination of PS533 spores and multiple individual PS832 and B. cereus spores. (A) Schematic diagram of spore germination memory experiment. Spores were spread on the surface of a coverslip glued to the sample container. The germinant solution was added with a pipette at T0 and T2 for a short period, removed with vacuum suction pump, rinsed five times, and incubated in buffer until T1 and T3, which are the times to complete all germination after the 1st and 2nd pulses, respectively. Phase-contrast images of a field of spores at various time points in l-valine germination are also shown. The number of spores that germinated in the 1st pulse between T0 and T1 was defined as Ngerm1, the number of spores that germinated in the 2nd pulse between T2 and T3 was defined as Ngerm2, and the total number of spores examined was defined as N0. The percentage of spores that germinated in the 1st pulse was (Ngerm1/N0) × 100% and that in the 2nd pulse was Ngerm2/(N0 − Ngerm1) × 100%. (B) B. subtilis PS533 (wild-type) spores were given two short nutrient germinant pulses, and germination was monitored as described in Materials and Methods. Gray bars above the horizontal axis in panels indicate pulse durations, and vertical arrows above germination curves indicate the times when pulses were terminated. (C to F) DIC image intensities of individual PS832 and B. cereus spores germinating with two germinant pulses. Spores were given various combinations of two germinant pulses, and the germination of ~20 individual spores was monitored by DIC microscopy as described in Materials and Methods. The DIC image intensity values of individual spores at 60 min (C to E) or 80 min (F) were subtracted from the image intensity values measured at various times, and the values were normalized to the image intensities of the spores at the beginning of the experiment. Individual germination parameters were determined as described in Materials and Methods, and times for Tlag, Trelease, and Tlys for one germinating spore are noted in panel C. Download
Effects of time and temperature during incubation between pulses on PS3415 (high GerB*) spore memory. (A) Spores were given two 1-min pulses of 0.8 mM l-asparagine at 37°C separated by 15, 20, or 45 min. Spores were also germinated with 0.8 mM l-asparagine for 2 min (■), followed by germinant removal and further incubation at 37°C. (B) Spores were exposed to a 1st 1-min pulse of 0.8 mM l-asparagine, the germinant was removed, and spores were incubated at 45, 37, or 15°C for 45 min and given a 2nd 1-min pulse of 0.8 mM l-asparagine, followed by germinant removal and further incubation at 37°C until 70 min. Spores were also germinated with 0.8 mM l-asparagine for 2 min (■), followed by germinant removal and further incubation. All curves shown are from data determined with ~300 individual spores. Gray bars indicate pulse durations, and vertical arrows indicate the times of termination of the 2nd pulses. Download
Effects of pH between germinant pulses on B. subtilis (wild-type) PS832 spore memory. Spores were given a 6-min pulse with either 3.5 mM l-valine (A) or 35 mM AGFK (B), followed by germinant removal, were incubated in 25 mM K-HEPES buffers at pH 7.4, 6.0, or 8.4 until 50 min, and were given a 2nd 6-min pulse of 3.5 mM l-valine (A) or 35 mM AGFK (B) in 25 mM K-HEPES buffer (pH 7.4), and germinants were removed and incubated at 37°C in 25 mM K-HEPES buffer (pH 7.4) until 80 min. All curves shown are from data determined with ~300 individual spores. Gray bars indicate pulse durations, and vertical arrows indicate the times of termination of the 2nd pulses. Download
Germination of spores with different l-valine concentrations with or without an initial pulse of a high l-valine concentration. (A) B. subtilis PS832 spores without an initial l-valine pulse were germinated with different concentrations of l-valine as described in Materials and Methods. (B) B. subtilis PS832 spores were given an initial 5-min pulse of 10 mM l-valine, suspended in HEPES buffer (pH 7.4) for 20 min after germinant removal and rinsing, and then germinated with different l-valine concentrations for 120 min. All curves shown are from results determined with >300 individual spores. Values for spore germination parameters were determined and calculated as described in Materials and Methods and the legend to Fig. S1, and values for percent germination after the 2nd germinant addition were corrected for the amount of spores that germinated following the first l-valine pulse, if given. Download
The effects of d-alanine on spore memory generated by l-alanine via the GerA GR. B. subtilis PS832 spores were exposed to a 1st 6-min pulse of 2.5 mM l-alanine, the germinant ws removed and rinsed three times and incubated in HEPES buffer (pH 7.4) with (□) or without (○) 0.5 mM d-alanine for 10 min, the d-alanine (if present) was removed, and the spores were again rinsed three times and incubated in HEPES buffer (pH 7.4) for another 9 min. The spores were then exposed to a 2nd 6-min pulse of 2.5 mM l-alanine, followed by germinant removal and further incubation until 50 min. Percent germination after each pulse is given above the germination curves, and values for percent germination after the 2nd pulse were calculated as described in the legend to Fig. S1. All curves are results from analysis of >300 individual spores. Download
Effects of lack of GerD on B. subtilis spore memory of germinant exposure. Spores from mutant strains were exposed to sequential pulses of 100 mM AGFK, the germinant was removed after each pulse, and germination of >222 individual spores was monitored by DIC microscopy as described in Materials and Methods. Gray bars above horizontal axes indicate pulse durations, and vertical arrows above germination curves indicate the times when pulses were terminated. Percent germination after each pulse is given above the germination curves, and values for percent germination after the 2nd pulse were calculated as described in the legend to Fig. S1. Download
Germination of decoated B. subtilis PS533 spores with two short l-valine pulses. Decoated PS533 (wild-type) spores were exposed to sequential pulses of 10 mM l-valine, the germinant was removed after each pulse, and germination of >288 individual spores was monitored by DIC microscopy, all as described in Materials and Methods. Gray bars above horizontal axes indicate pulse durations, and vertical arrows above germination curves indicate the times when pulses were terminated. Percent germination after each pulse is given above the germination curves, and values for percent germination after the 2nd pulse were calculated as described in the legend to Fig. S1. Download
(A) Mean values and standard deviations of germination parameters of B. subtilis PS832 and PS533 spores that germinated after various 1st and 2nd germinant pulses. (B) Mean values and standard deviations of germination parameters of B. subtilis (wild-type) PS832 spores incubated in at different pH values between germinant pulses. (C) Mean values and standard deviations of l-valine germination parameters of B. subtilis PS832 spores without or with brief preexposure to a high l-valine concentration.
Mean values and standard deviations of germination parameters of B. subtilis PS3415 (high GerB*) spores that germinated due to various 1st and 2nd germinant pulses and effects of incubation time and temperature between pulses.
(A) Mean values and standard deviations of germination parameters of B. subtilis FB62 (gerD), PS3940 (gerD, high GerA), FB111 (cwlJ), and FB113 (cwlJ and sleB) spores that germinated after two germinant pulses. (B) Mean values and standard deviations of germination parameters of B. cereus spores that germinated after two germinant pulses.
ACKNOWLEDGMENTS
This work was supported by a grant from the Army Research Office under contract no. W911NF-12-2-0024 and by a Department of Defense Multi-disciplinary University Research Initiative through the U.S. Army Research Laboratory and the U.S. Army Research Office under contract no. W911F-09-1-0286 (PS/YQL).
We are grateful to Aimee Shen for providing the C. difficile spores.
Footnotes
Citation Wang S, Faeder JR, Setlow P, Li Y. 2015. Memory of germinant stimuli in bacterial spores. mBio 6(6):e01859-15. doi:10.1128/mBio.01859-15.
REFERENCES
- 1.Burrill DR, Silver PA. 2010. Making cellular memories. Cell 140:13–18. doi: 10.1016/j.cell.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chouard P. 1960. Vernalization and its relations to dormancy. Annu Rev Plant Physiol 11:191–238. doi: 10.1146/annurev.pp.11.060160.001203. [DOI] [Google Scholar]
- 3.Heler R, Marraffini LA, Bikard D. 2014. Adapting to new threats: the generation of memory by CRISPR-Cas immune systems. Mol Microbiol 93:1–9. doi: 10.1111/mmi.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stock JB, Zhang S. 2013. The biochemistry of memory. Curr Biol 23:R741–R745. doi: 10.1016/j.cub.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paredes-Sabja D, Setlow P, Sarker MR. 2011. Germination of spores of Bacillales and Clostridiales species: mechanisms and proteins involved. Trends Microbiol 19:85–94. doi: 10.1016/j.tim.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol 101:514–525. doi: 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- 7.Setlow P. 2013. When the sleepers wake: the germination of spores of Bacillus species. J Appl Microbiol 115:1251–1268. doi: 10.1111/jam.12343. [DOI] [PubMed] [Google Scholar]
- 8.Logan NA. 2012. Bacillus and relatives in foodborne illness. J Appl Microbiol 112:417–429. doi: 10.1111/j.1365-2672.2011.05204.x. [DOI] [PubMed] [Google Scholar]
- 9.Mallozzi M, Viswanathan V, Vedantam G. 2010. Spore-forming Bacilli and Clostridia in human disease. Future Microbiol 5:1109–1123. doi: 10.2217/fmb.10.60. [DOI] [PubMed] [Google Scholar]
- 10.Setlow P. 2014. Germination of spores of Bacillus species: what we know and do not know. J Bacteriol 196:1297–1305. doi: 10.1128/JB.01455-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atluri S, Ragkousi K, Cortezzo DE, Setlow P. 2006. Cooperativity between different nutrient receptors in germination of spores of Bacillus subtilis and reduction of this cooperativity by alterations in the GerB receptor. J Bacteriol 188:28–36. doi: 10.1128/JB.188.1.28-36.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luu S, Setlow P. 2014. Analysis of the loss in heat and acid resistance during germination of spores of Bacillus species. J Bacteriol 196:1733–1740. doi: 10.1128/JB.01555-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Setlow P, Li Y. 2015. Slow leakage of Ca-dipicolinic acid from individual Bacillus spores during initiation of spore germination. J Bacteriol 197:1095–1103. doi: 10.1128/JB.02490-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang P, Liang J, Yi X, Setlow P, Li Y. 2014. Monitoring of commitment, blocking, and continuation of nutrient germination of individual Bacillus subtilis spores. J Bacteriol 196:2443–2454. doi: 10.1128/JB.01687-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart K-V, Yi X, Ghosh S, Setlow P. 2012. Germination protein levels and rates of germination of spores of Bacillus subtilis with overexpressed or deleted genes encoding germination proteins. J Bacteriol 194:3156–3164. doi: 10.1128/JB.00405-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi X, Setlow P. 2010. Studies of the commitment step in the germination of spores of Bacillus species. J Bacteriol 192:3424–3433. doi: 10.1128/JB.00326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasuda Y, Tochikubo K. 1984. Relation between d-glucose and d-alanine in the initiation of germination of Bacillus subtilis spore. Microbiol Immunol 28:197–207. doi: 10.1111/j.1348-0421.1984.tb00671.x. [DOI] [PubMed] [Google Scholar]
- 18.Pelczar PL, Igarashi T, Setlow B, Setlow P. 2007. Role of GerD in germination of Bacillus subtilis spores. J Bacteriol 189:1090–1098. doi: 10.1128/JB.01606-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong L, Doona CJ, Setlow P, Li Y-Q. 2014. Monitoring rates and heterogeneity of high-pressure germination of Bacillus spores by phase-contrast microscopy of individual spores. Appl Environ Microbiol 80:345–352. doi: 10.1128/AEM.03043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velásquez J, Schuurman-Wolters G, Birkner JP, Abee T, Poolman B. 2014. Bacillus subtilis spore protein SpoVAC functions as a mechanosensitive channel. Mol Microbiol 92:813–823. doi: 10.1111/mmi.12591. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Valdespino A, Li Y, Setlow B, Ghosh S, Pan D, Korza G, Feeherry FE, Doona CJ, Li Y-Q, Hao B, Setlow P. 2014. Properties and function of the SpoVAEa and SpoVAF proteins in Bacillus subtilis spores. J Bacteriol 196:2077–2088. doi: 10.1128/JB.01546-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vepachedu VR, Setlow P. 2007. Analysis of interactions between nutrient germinant receptors and SpoVA proteins of Bacillus subtilis spores. FEMS Microbiol Lett 271:42–47. [DOI] [PubMed] [Google Scholar]
- 23.Paredes-Sabja D, Shen A, Sorg JA. 2014. Clostridium difficile spore biology: sporulation, germination, and spore structural proteins. Trends Microbiol 22:406–416. doi: 10.1016/j.tim.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wadhams GH, Armitage JP. 2004. Making sense of it all: bacterial chemotaxis. Nat Rev Mol Cell Biol 5:1024–1037. doi: 10.1038/nrm1524. [DOI] [PubMed] [Google Scholar]
- 25.Setlow B, Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol 178:3486–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paidhungat M, Ragkousi K, Setlow P. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J Bacteriol 183:4886–4893. doi: 10.1128/JB.183.16.4886-4893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson WL, Setlow P. 1990. Sporulation, germination and outgrowth, p 391–450. In Harwood CR, Cutting AM (ed), Molecular biological methods for Bacillus. John Wiley, Chichester, United Kingdom. [Google Scholar]
- 28.Putnam EE, Nock AM, Lawley TD, Shen A. 2013. SpoIVA and SipL are Clostridium difficile spore morphogenetic proteins. J Bacteriol 195:1214–1225. doi: 10.1128/JB.02181-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagyan I, Noback M, Bron S, Paidhungat M, Setlow P. 1998. Characterization of yhcN, a new forespore-specific gene of Bacillus subtilis. Gene 212:179–188. doi: 10.1016/S0378-1119(98)00172-3. [DOI] [PubMed] [Google Scholar]
- 30.Zhang P, Kong L, Wang G, Setlow P, Li Y. 2010. Combination of Raman tweezers and quantitative differential interference contrast microscopy for measurement of dynamics and heterogeneity during the germination of individual bacterial spores. J Biomed Opt 15:056010–056019. doi: 10.1117/1.3494567. [DOI] [PubMed] [Google Scholar]
- 31.Zhang P, Garner W, Yi X, Li Y, Setlow P. 2010. Factors affecting variability in time between addition of nutrient germinants and rapid dipicolinic acid release during germination of spores of Bacillus species. J Bacteriol 192:3608–3619. doi: 10.1128/JB.00345-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic of spore memory experiments and memory in germination of PS533 spores and multiple individual PS832 and B. cereus spores. (A) Schematic diagram of spore germination memory experiment. Spores were spread on the surface of a coverslip glued to the sample container. The germinant solution was added with a pipette at T0 and T2 for a short period, removed with vacuum suction pump, rinsed five times, and incubated in buffer until T1 and T3, which are the times to complete all germination after the 1st and 2nd pulses, respectively. Phase-contrast images of a field of spores at various time points in l-valine germination are also shown. The number of spores that germinated in the 1st pulse between T0 and T1 was defined as Ngerm1, the number of spores that germinated in the 2nd pulse between T2 and T3 was defined as Ngerm2, and the total number of spores examined was defined as N0. The percentage of spores that germinated in the 1st pulse was (Ngerm1/N0) × 100% and that in the 2nd pulse was Ngerm2/(N0 − Ngerm1) × 100%. (B) B. subtilis PS533 (wild-type) spores were given two short nutrient germinant pulses, and germination was monitored as described in Materials and Methods. Gray bars above the horizontal axis in panels indicate pulse durations, and vertical arrows above germination curves indicate the times when pulses were terminated. (C to F) DIC image intensities of individual PS832 and B. cereus spores germinating with two germinant pulses. Spores were given various combinations of two germinant pulses, and the germination of ~20 individual spores was monitored by DIC microscopy as described in Materials and Methods. The DIC image intensity values of individual spores at 60 min (C to E) or 80 min (F) were subtracted from the image intensity values measured at various times, and the values were normalized to the image intensities of the spores at the beginning of the experiment. Individual germination parameters were determined as described in Materials and Methods, and times for Tlag, Trelease, and Tlys for one germinating spore are noted in panel C. Download
Effects of time and temperature during incubation between pulses on PS3415 (high GerB*) spore memory. (A) Spores were given two 1-min pulses of 0.8 mM l-asparagine at 37°C separated by 15, 20, or 45 min. Spores were also germinated with 0.8 mM l-asparagine for 2 min (■), followed by germinant removal and further incubation at 37°C. (B) Spores were exposed to a 1st 1-min pulse of 0.8 mM l-asparagine, the germinant was removed, and spores were incubated at 45, 37, or 15°C for 45 min and given a 2nd 1-min pulse of 0.8 mM l-asparagine, followed by germinant removal and further incubation at 37°C until 70 min. Spores were also germinated with 0.8 mM l-asparagine for 2 min (■), followed by germinant removal and further incubation. All curves shown are from data determined with ~300 individual spores. Gray bars indicate pulse durations, and vertical arrows indicate the times of termination of the 2nd pulses. Download
Effects of pH between germinant pulses on B. subtilis (wild-type) PS832 spore memory. Spores were given a 6-min pulse with either 3.5 mM l-valine (A) or 35 mM AGFK (B), followed by germinant removal, were incubated in 25 mM K-HEPES buffers at pH 7.4, 6.0, or 8.4 until 50 min, and were given a 2nd 6-min pulse of 3.5 mM l-valine (A) or 35 mM AGFK (B) in 25 mM K-HEPES buffer (pH 7.4), and germinants were removed and incubated at 37°C in 25 mM K-HEPES buffer (pH 7.4) until 80 min. All curves shown are from data determined with ~300 individual spores. Gray bars indicate pulse durations, and vertical arrows indicate the times of termination of the 2nd pulses. Download
Germination of spores with different l-valine concentrations with or without an initial pulse of a high l-valine concentration. (A) B. subtilis PS832 spores without an initial l-valine pulse were germinated with different concentrations of l-valine as described in Materials and Methods. (B) B. subtilis PS832 spores were given an initial 5-min pulse of 10 mM l-valine, suspended in HEPES buffer (pH 7.4) for 20 min after germinant removal and rinsing, and then germinated with different l-valine concentrations for 120 min. All curves shown are from results determined with >300 individual spores. Values for spore germination parameters were determined and calculated as described in Materials and Methods and the legend to Fig. S1, and values for percent germination after the 2nd germinant addition were corrected for the amount of spores that germinated following the first l-valine pulse, if given. Download
The effects of d-alanine on spore memory generated by l-alanine via the GerA GR. B. subtilis PS832 spores were exposed to a 1st 6-min pulse of 2.5 mM l-alanine, the germinant ws removed and rinsed three times and incubated in HEPES buffer (pH 7.4) with (□) or without (○) 0.5 mM d-alanine for 10 min, the d-alanine (if present) was removed, and the spores were again rinsed three times and incubated in HEPES buffer (pH 7.4) for another 9 min. The spores were then exposed to a 2nd 6-min pulse of 2.5 mM l-alanine, followed by germinant removal and further incubation until 50 min. Percent germination after each pulse is given above the germination curves, and values for percent germination after the 2nd pulse were calculated as described in the legend to Fig. S1. All curves are results from analysis of >300 individual spores. Download
Effects of lack of GerD on B. subtilis spore memory of germinant exposure. Spores from mutant strains were exposed to sequential pulses of 100 mM AGFK, the germinant was removed after each pulse, and germination of >222 individual spores was monitored by DIC microscopy as described in Materials and Methods. Gray bars above horizontal axes indicate pulse durations, and vertical arrows above germination curves indicate the times when pulses were terminated. Percent germination after each pulse is given above the germination curves, and values for percent germination after the 2nd pulse were calculated as described in the legend to Fig. S1. Download
Germination of decoated B. subtilis PS533 spores with two short l-valine pulses. Decoated PS533 (wild-type) spores were exposed to sequential pulses of 10 mM l-valine, the germinant was removed after each pulse, and germination of >288 individual spores was monitored by DIC microscopy, all as described in Materials and Methods. Gray bars above horizontal axes indicate pulse durations, and vertical arrows above germination curves indicate the times when pulses were terminated. Percent germination after each pulse is given above the germination curves, and values for percent germination after the 2nd pulse were calculated as described in the legend to Fig. S1. Download
(A) Mean values and standard deviations of germination parameters of B. subtilis PS832 and PS533 spores that germinated after various 1st and 2nd germinant pulses. (B) Mean values and standard deviations of germination parameters of B. subtilis (wild-type) PS832 spores incubated in at different pH values between germinant pulses. (C) Mean values and standard deviations of l-valine germination parameters of B. subtilis PS832 spores without or with brief preexposure to a high l-valine concentration.
Mean values and standard deviations of germination parameters of B. subtilis PS3415 (high GerB*) spores that germinated due to various 1st and 2nd germinant pulses and effects of incubation time and temperature between pulses.
(A) Mean values and standard deviations of germination parameters of B. subtilis FB62 (gerD), PS3940 (gerD, high GerA), FB111 (cwlJ), and FB113 (cwlJ and sleB) spores that germinated after two germinant pulses. (B) Mean values and standard deviations of germination parameters of B. cereus spores that germinated after two germinant pulses.