Highlight
GhCFE1A decorated the ER network and was linked to actin cables. Overexpressing GhCFE1A repressed the initiation of fibre cells, and also resulted in short fibres in transgenic cotton lines.
Key words: Actin cytoskeleton, cotton fibre, endoplasmic reticulum, fibre elongation, fibre initiation, GhCFE1A.
Abstract
Fibre cell initiation and elongation is critical for cotton fibre development. However, little is known about the regulation of initiation and elongation during fibre cell development. Here, the regulatory role of a novel protein GhCFE1A was uncovered. GhCFE1A is preferentially expressed at initiation and rapid elongation stages during fibre development; in addition, much higher expression of GhCFE1A was detected at the fibre initiation stage in fibreless cotton mutants than in the fibre-bearing TM-1 wild-type. Importantly, overexpression of GhCFE1A in cotton not only delayed fibre cell elongation but also significantly reduced the density of lint and fuzz fibre initials and stem trichomes. Yeast two-hybrid assay showed that GhCFE1A interacted with several actin proteins, and the interaction was further confirmed by co-sedimentation assay. Interestingly, a subcellular localization assay showed that GhCFE1A resided on the cortical endoplasmic reticulum (ER) network and co-localized with actin cables. Moreover, the density of F-actin filaments was shown to be reduced in GhCFE1A-overexpressing fibres at the rapid elongation stage compared with the wild-type control. Taken together, the results demonstrate that GhCFE1A probably functions as a dynamic linker between the actin cytoskeleton and the ER network, and plays an important role in fibre cell initiation and elongation during cotton fibre development.
Introduction
Cotton is the primary renewable source of natural textile fibre. Cotton fibres are single-celled trichomes derived from the ovule epidermis which are morphologically divided into two types: lint and fuzz. About 25% of the epidermal cells of the cotton seed coat differentiate and develop into unicellular long and mature fibres (lint) that can be spun into yarn (Basra and Malik, 1984; Kim and Triplett, 2001). In Gossypium hirsutum, lint fibre cells extend from the seed coat either before or on the day of anthesis, with a final length of ~30mm, whereas fuzz fibres initiate a few days later and fail to elongate appreciably (<5mm; Kim and Triplett, 2001; Lee et al., 2006, 2007).
Cotton fibre development undergoes four distinct but overlapping stages: fibre initiation, elongation, secondary cell wall biosynthesis, and drying and maturation (Basra and Malik, 1984; Ruan and Chourey, 1998). The number of fibre cells per ovule is established in the initiation phase, and the length and strength of fibre are determined mainly during the stages of elongation and secondary cell wall synthesis. During cotton fibre elongation, the thin actin arrays and thick and long actin cables and bundles parallel to the growing axis of the fibre cells form a complicated network, which orchestrates the trafficking of secretory vesicles and other large organelles and plays a critical role in the process of fibre elongation (Li et al., 2005; J. Wang et al., 2010). A previous study showed that 15 GhACT cDNAs encoding putative actin proteins were found to be differentially expressed in various tissues. Specifically, GhACT1 is predominantly expressed in fibre cells, and its suppression by RNA interference (RNAi) disrupted the actin cytoskeleton network, causing reduced fibre elongation (Li et al., 2005). In addition, the dynamic actin cytoskeleton is regulated by a number of actin-binding proteins. Cotton LIM domain-containing proteins were reported to play important roles in modulating the actin cytoskeleton (Han et al., 2013; Li et al., 2013, 2014). GhPLIM1 and GhWLIM5 can directly bind actin and bundles F-actin in vitro (Li et al., 2013, 2014); indeed, WLIM1a plays dual roles during cotton fibre development, acting as a actin bundler to promote cell elongation at the fibre cell elongation stage and as a transcriptional factor to trigger lignin biosynthesis at the secondary cell wall biosynthesis stage (Han et al., 2013). AnxGb6 was reported to interact with actin GbACT1 (Huang et al., 2013). In addition, overexpression of an actin-binding protein-encoding gene, GhPFN2, caused early terminatation of cell elongation, producing obvious short fibres (J. Wang et al., 2010), and down-regulation of the actin depolymerizing factor-encoding gene GhADF1 resulted in a greater abundance of actin filaments in the cortical region of the transgenic fibre cells, producing fibres with increased length and strength (Wang et al., 2009).
It is well known that the actin cytoskeleton plays a crucial role in endoplasmic reticulum (ER) movement and distribution in plant cells (Voeltz et al., 2002; Borgese et al., 2006; Sparkes et al., 2009). The cortical ER, which is a highly dynamic structure in plants composed of a polygonal tubular network, was first described by electron microscopists in the 1960s (Porter and Machado, 1960; Sparkes et al., 2009, 2011). In Arabidopsis thaliana, mutations of the membrane-bound GTPase ROOT HAIR DEFECTIVE3 (RHD3), which was reported to mediate ER fusion maintaining ER morphology, caused defects in cell expansion and elongation, resulting in a short root and short, wavy root hairs (Wang et al., 1997, 2002; Stefano et al., 2012; Zhang et al., 2013); another study reported that maMYB, a member of the plant-specific R2R3-MYB family, localized to the ER, and silencing of maMyb led to a reduction of root hair length (Slabaugh et al., 2011). However, thus far, there is little known about the role of the ER network in cotton fibre cell development.
In this study, a gene with unknown function was isolated and designated as GhCFE1A, which is preferentially expressed at the initiation and elongation stage during cotton fibre development. Interestingly, it was found that GhCFE1A decorates the ER network and also binds to actin cables. Furthermore, overexpression of GhCFE1A resulted in reduced density of lint and fuzz fibre initials and impaired fibre elongation.
Materials and methods
Plant materials
Fibre-bearing wild-type (7235, TM-1) G. hirsutum and three fuzzless–lintless cotton mutants (MD17, SL1-7-1, and Xu142 fl), G. barbadense (Hai7124), G. herbaceum, and G. raimondii were field-cultivated in Nanjing, China, using normal cotton farming practices. Developing ovules and fibres were excised from flower buds or bolls and ovules on selected days before or after anthesis relative to the day of anthesis (0 DPA). Roots, stems, and leaves were collected from 2-week-old seedlings cultured in a growth chamber. All frozen materials were stored at –70 °C.
Gene cloning and sequence analysis
The full-length GhCFE1A cDNA sequence was isolated from a 7235 cDNA library, which was constructed as described previously (H.H. Wang et al., 2010). To obtain the genomic sequences of GhCFE1 homeologues in TM-1, Hai7124, and two diploid progenitor cotton species, G. herbaceum L. (A genome), and G. raimondii Ulbrich (D genome), primers (GhCFE1-full-F/R) were designed to amplify the open reading frame (ORF) using the genomic DNA as a template. All the fragments were sequenced after they were cloned into T-vectors. The conserved protein domains were searched in the National Center for Biotechnology Information (NCBI) database. The GhCFE1A signal peptide was predicted using the web-based program SignalP 3.0 (http://www.cbs.dtu.dk/services/SignalP). For sequence alignment analysis, the genomic and cDNA sequences and peptides of GhCFE1 homologues were aligned with ClustalX (Thompson et al., 1997) and, for phylogenetic analysis, a phylogenetic tree was constructed with MEGA5 software (Tamura et al., 2011).
Southern blot hybridization and molecular mapping
Genomic DNA isolation and Southern blotting were carried out according to the methods described by H.H. Wang et al. (2010). The probe fragment for detecting the copy number was amplified by GhCFE1 primers (GhCFE1-RT-F/R) containing one XbaI restriction site, and genomic DNA of TM-1 was digested with HindIII, EcoRI, and XbaI restriction enzymes. Transgenic cotton plants were detected using the NPTII fragment as a probe, and genomic DNA was digested with EcoRI.
GhCFE1D was mapped using the BC1 [(TM-1×Hai7124)×TM-1] interspecific mapping population. The pair of primers consisting of amplified regions with Dt subgenome polymorphisms between TM-1 and Hai7124 was used to survey 138 individuals of the BC1 mapping population; however, there were no At subgenome polymorphisms between the two mapping parents. The polymorphic loci were integrated in the backbone map (Guo et al., 2008) by JoinMap version 3.0 (Van Ooijen and Voorrips, 2001).
RT–PCR and qRT-PCR
The expression levels of GhCFE1 homeologues were analysed by reverse transcription–PCR (RT–PCR) or real-time quantitative RT–PCR (qRT-PCR). The cotton elongation factor gene GhEF1α was used as a standard control in the RT–PCR. In the qRT-PCR, expression was normalized against the expression of His3. Total RNA was extracted using the cetyl trimethylammonium bromide (CTAB)–acidic phenol extraction method (Jiang and Zhang, 2003). A total of 2 μg of RNA per reaction from different tissues was reverse-transcribed from an oligo(dT)18 primer using M-MLV reverse transcriptase (Promega, Madison, WI, USA; Cat# M1701) according to the manufacturer’s recommendations. The cDNA samples were diluted 10-fold and 2 μl of the dilution was used as the template. qRT-PCR was performed with 40 cycles using a LightCycler FastStart DNA Master SYBR Green I kit (Roche, Basel, Switzerland) in an ABI7500 sequence detection system according to the manufacturer’s protocol (Applied Biosystems, http://www.appliedbiosystems.com). For all reactions, three technical replicates were performed in each of the three biological experiments. The gene-specific primers used in the study are listed in Supplementary Table S1 available at JXB online. The relative expression level of the GhCFE1 homeologues was calculated by the equation Y=2^-ΔCt (where ΔCt is the difference between the Ct values of the His3 products and the GhCFE1 homeologue product; i.e. ΔCt=CtGhCFE1–CtHis3.). The relative expression levels of transcription regulators were determined by the ΔΔCt method (Livak and Schmittgen, 2001).
Plasmid construction and plant transformation
Sense and antisense GhCFE1A plant expression vectors were constructed using two pairs of primers with BamHI (SacI) and SacI (BamHI) (enzymes denoted in parentheses were used to construct the antisense vectors and the enzymes outside the parentheses were used for constructing the sense vectors) restriction sites. The amplified sense GhCFE1A product was inserted into the pBI121 and pBI121-E6 [a pBI121 derivative where the Cauliflower mosaic virus (CaMV) 35S promoter was replaced by a GhE6 promoter] vectors at the BamHI/SacI sites to replace the GUS (β-glucuronidase) gene, while the amplified antisense GhCFE1A product was only inserted into the pBI121-E6 vector. The constructed vectors and pBI121 were transformed into G. hirsutum accession W0 by Agrobacterium tumefaciens strain LBA4404 (Li et al., 2009). The transformed plants containing the empty vector pBI121 were used as the null control.
Heterologous expression of GhCFE1A in Escherichia coli and co-sedimentation assay
After removing the predicted signal peptide (amino acids 1–26), the remaining GhCFE1A sequence was inserted into the BamHI/XhoI sites of the pET-30a (+) vector (Qiagen, Valencia, CA, USA), resulting in translational fusion of the protein with six histidine residues at the N-terminus. The E. coli strains carrying pET-30a (+)-GhCFE1A were cultured until the optical density at 600nm (OD600) reached 0.4. Then isopropyl-β-d-thiogalactopyranoside (IPTG, 0.6mM) was added to induce the expression of the fusion protein. The proteins obtained in the induced and uninduced E. coli cultures were analysed using SDS–PAGE. His-tagged GhCFE1A proteins were purified using nickel–nitrilotriacetic acid resin following the procedures described by the manufacturer (Qiagen). As the purified proteins consisted of two bands with different size proteins in SDS–PAGE, GhCFE1A was digested with BamHI and XhoI and cloned into the pGEX-6P-1 vector (GE Healthcare). Glutathione S-transferase (GST)–CFE1A recombinant proteins were expressed in E. coli following similar procedures to those described above and purified using glutathione–Sepharose following the procedures described by the manufacturer (GE Healthcare). A co-sedimentation assay was conducted according to Han et al. (2013). The proteins in the supernatants and pellets were separated by SDS–PAGE.
Protein extraction, quantification, and immunoblot analysis
Total proteins were extracted from the ovules, fibres, and leaves using plant protein extraction buffer [40mM HEPES pH 7.5, 10mM KCl, 3mM MgCl2, 0.4M sucrose, 1mM EDTA, 1mM dithiothreitol (DTT), 0.2% Triton X-100, 1mM phenylmethylsulphonyl fluoride (PMSF)] and quantified by the Bradford method (Bradford, 1976). Equal amounts of protein for transgenic and wild-type plants were subjected to SDS–PAGE followed by western blot analysis. A polyclonal antiserum was raised in rabbits against a synthetic peptide corresponding to residues 233–246 (HLKKSDTWENHGRD) of GhCFE1A (GenScript). Corresponding secondary IRDye 800CW-labelled goat anti-rabbit IgG (H+L) antibodies were used for detection.
Scanning electron microcopy and fibre quality measurement
To compare the lint and fuzz fibre initiation difference between transgenic and wild-type plants, ovules and seeds were collected at 0 and 4 DPA, respectively, from similar positions on the cotton plants and fixed in 3% (v/v) glutaraldehyde (pH 7.2). After serial dehydration for 30min each in 30, 50, 70, 80, 90, and 100% ethanol solutions, the samples were transferred into isoamyl acetate and dried at the critical point. The dried cotton ovules at 0 DPA and seeds at 4 DPA with the lint fibre removed were sputter-coated with gold on the surface, and then viewed and photographed with a scanning electron microscope (SEM; Hitachi S-3000N). After cotton ginning and cottonseed delinting, three independent fibre samples and cotton seeds (with or without fuzz) from each T3 generation of the transgenic lines and wild-type cotton were weighed and counted to determine the lint index (fibre weight of 100 seeds) and the fuzz index (fuzz weight of 100 seeds). The fibres were then sent to the Center of Cotton Fibre Quality Inspection and Testing, Ministry of Agriculture (Anyang, Henan Province, China) to determine the quality of the cotton fibre. Seeds at 8, 10, 13, 15, 18, 20, 23, and 25 DPA were chosen to measure the fibre length for the dynamic curve analysis of fibre development, as reported previously (Gipson and Ray, 1969). Data were processed in Microsoft Excel using Student’s t-test.
Yeast two-hybrid screening
The coding sequences of GhCFE1A with the signal peptide removed and partial sequence-containing domain conserved were amplified using gene-specific primers (Supplementary Table S1 at JXB online), and fused with the GAL4-DNA-binding domain in the pGBKT7 vector (Clontech) for constructing the bait vectors. A prey library of cotton ovules and fibres (0 DPA ovules; 5, 10, 15, and 20 DPA fibres) was constructed by fusing cDNAs with the GAL4-activation domain in the pGADT7-Rec vector (Clonetech). The yeast two-hybrid assay was performed according to the manufacturer’s instructions. The plasmids of positive clones were extracted, and the cDNA sequences integrated in these plasmids were sequenced. The full length of the interacting genes was fused with the GAL4-activation domain in the pGADT7 vector (Clontech), and co-transformed into Saccharomyces cerevisiae AH109 with the bait vector to test their interaction.
Co-localization assay and live cell imaging analysis
The ORF of GhCFE1A was amplified and cloned into pGWB5 and pGWB6 to obtain the CFE1A–green fluorescent protein (GFP) and GFP–CFE1A constructs, respectively, by Topo cloning and subsequent recombination reaction as described by Liu et al. (2014). The binary vectors were transiently co-expressed in leaves of Nicotiana benthamiana with ABD2-mCherry or red fluorescent protein (RFP)–HDEL via agroinfiltration (Waadt and Kudla, 2008). The images were taken by a spinning-disc confocal microscope (UltraView VoX, Perkin Elmer) as described by Liu et al. (2014).
Fluorescent staining, and microscopic and quantitative analyses of the actin cytoskeleton
Fibre-bearing seeds were carefully dissected from fresh bolls obtained at 5 and 8 DPA. F-actin staining by Alexa Fluor® 488 phalloidin (Molecular Probes, Invitrogen) was performed according to the manufacturer’s instructions. The seeds were washed twice with phosphate-buffered saline (PBS; pH 7.0) for 10min each. After fixing with 4% (w/v) paraformaldehyde in PBS for 10min, the fibres were carefully cut from the ovules and washed twice or more with PBS, and then incubated for 20–30min in PBS containing 1U of Alexa Fluor® 488 phalloidin, 0.1% (v/v) Triton X-100, 40mM HEPES (pH 7.0), 1mM MgCl2, 3mM DTT, and 0.3mM PMSF. After briefly rinsing in PBS, the fibres were mounted onto glass slides and examined under a Leica DMl fluorescence microscope. At image acquisition, all settings, including excitation wavelength and emission filters (488nm/band-pass 505–530nm for Alexa-Fluor 488), were fixed. To evaluate actin alignment, occupancy analysis, a statistical parameter, was employed to quantify actin density, according to a previously described method (Higaki et al., 2010).
Accession numbers
Sequence data from this article can be found in the GenBank data libraries under accession numbers: AF072404 (GhCFE1D, originally named as GhCFE1, isolated by Yamamoto and Baird, 1998), DQ073045 (GhCFE1A), XP_002521431.1 (RCOM_1063000), XP_0025 31045.1 (RCOM_0517470), XP_002273372.2 (LOC100244739), CBI23474.3 (VIT_00006712001), XP_002284551.1 (LOC10026 7532), XP_002316652.1 (POPTRDRAFT_833990), XP_0023304 25.1 (POPTRDRAFT_811252), XP_002316932.1 (POPTRDRA FT_771875), NP_176321.2 (At1g61260); NP_200241.1 (At5g54300), KF018237 (CFE1A A1), KF018238 (CFE1D D5), KF018239 (CFE1A TM-1), KF018240 (CFE1D TM-1), KF018241 (CFE1A Hai7124), KF018242 (CFE1D Hai7124), KF018243 (GhACTa), KF018244 (GhACTb).
Results
CFE1 genes in diploid and allotetraploid cotton
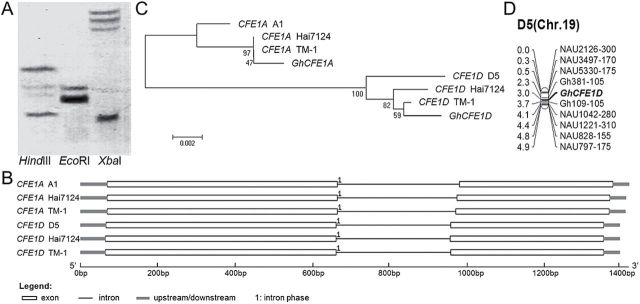
A cotton fibre cDNA library was constructed in a previous study, using ovules (1 and 3 DPA) and fibres (5–25 DPA) from G. hirsutum accession 7235, a germplasm line with elite fibre quality (H.H. Wang et al., 2010). Through random sequencing of clones from the library, a 1274 nucleotides long cDNA clone was obtained. This cDNA encodes a predicted polypeptide of 331 amino acids which shares very high homology (96% identity) with GhCFE1 (Yamamoto and Baird, 1998). Bioinformatics analysis predicted that either of the two encoded proteins contains a signal peptide encoded by its N-terminal fragment (first 26 amino acid residues). Additionally, Southern blotting of genomic DNA from G. hirsutum accession TM-1 (AADD) showed that the gene cloned in this study is present as two copies in the tetraploid species (Fig. 1A), implying that the novel gene and GhCFE1 are two homoeologous loci in allotetraploid cotton. To investigate this, CFE1 genomic loci were cloned and sequenced from TM-1 and G. barbadense cv. Hai7124 (AADD), and close relatives of the progenitor species, G. herbaceum L. (A genome) and G. raimondii Ulbrich (D genome). Gene structure and sequence alignment analysis confirmed that these CFE1 genes, with a single copy from the diploid species and two homeologues from each allotetraploid species, contained only one intron spliced at the same site (the 200th amino acid, Fig. 1B) and are classified into two groups, A genome- and D genome-like, which were designated CFE1A and CFE1D, respectively. Phylogenetic analysis of CFE1A and CFE1D homologues suggested that the gene originally cloned from the cDNA library in this study was the transcript from the A subgenome and designated GhCFE1A, and GhCFE1 (GhCFE1D) isolated by Yamamoto and Baird (1998) was from the D subgenome in tetraploid cotton (Fig. 1C). Based on the single nucleotide polymorphisms (SNPs) of CFE1D sequences between the two mapping parents, TM-1 and Hai7124 (Guo et al., 2007), GhCFE1D was mapped on chromosome D5 (chromosome 19; Fig. 1D), implying that GhCFE1A is present on the homeologous chromosome A5 (chromosome 5).
Fig. 1.
Genomic organization of CFE1 genes in diploid and allotetraploid cotton. (A) Southern blot analysis of the gene isolated in this study from a normalized cDNA library in allotetraploid cotton. Genomic DNA was digested with HindIII, EcoRI, and XbaI, and hybridized with a 458bp fragment of the novel gene which contains one XbaI restriction site. (B) Gene structure analysis of CFE1 genomic sequences from G. herbaceum (A1), G. raimondii (D5), G. barbadense (AADD, Hai7124), and G. hirsutum (AADD, TM-1). The exons and introns are represented by different characters. (C) Phylogenetic analysis based on nucleic acid sequences of CFE1 genes from different cotton species. ‘GhCFE1A’, cDNA sequence isolated in this study; ‘GhCFE1D’, cDNA sequence isolated by Yamamoto and Baird (1998; originally named GhCFE1); ‘CFE1A A1’, genomic CFE1 sequence in G. herbaceum (A1); ‘CFE1D D5’, genomic CFE1 sequence in G. raimondii (D5); ‘CFE1A TM-1’ and ‘CFE1D TM-1’, A and D subgenome genomic CFE1 sequence in G. hirsutum TM-1(AADD), respectively; ‘CFE1A Hai7124’ and ‘CFE1D Hai7124’, A and D subgenome genomic CFE1 sequence in G. barbadense Hai7124 (AADD), respectively. (D) Chromosomal localization of GhCFE1D, the homeologous gene of GhCFE1A.
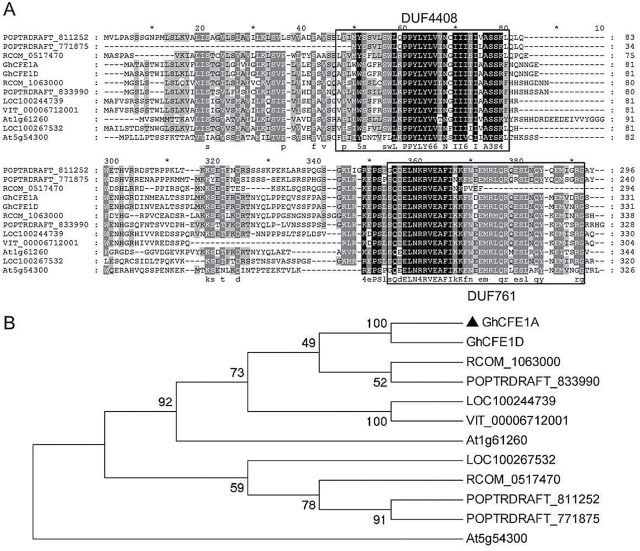
Motif analysis indicated that GhCFE1 homeologues in cotton contains two conserved domains of unknown function (DUFs), DUF761 (residues 293–330; Fig. 2A) and DUF4408 (residues 42–75) which is found at the N-terminus of members of the DUF761 family. The GhCFE1-homologous peptides from Ricinus communis, Vitis vinifera, and other species were identified by a database search. The proteins show high homology, especially at the N- and C-terminal domains, suggesting that these regions are important for CFE function. The protein from Ricinus species exhibited the highest similarity to GhCFE1, with 59% amino acid sequence identity (Fig. 2B).
Fig. 2.
Two conserved domains with unknown function (DUFs) were found among the CFE proteins from different plant sources. (A) Multiple sequence alignment analysis of GhCFE1A/D and other plant CFE proteins uncharacterized and not functionally analysed from Ricinus communis (RCOM_1063000 and RCOM_0517470), Vitis vinifera (LOC100244739, LOC100267532, and VIT_00006712001), Populus trichocarpa (POPTRDRAFT_833990, POPTRDRAFT_811252, and POPTRDRAFT_771875), and Arabidopsis thaliana (At1g61260 and At5g54300). Accession numbers are listed in the Materials and methods. (B) Phylogenetic analysis of CFE proteins. The Neighbor–Joining tree was generated from the amino acid sequences mentioned above, and the numbers next to each node give bootstrap values from 1000 replicates.
GhCFE1 is correlated with cotton fibre initiation and elongation
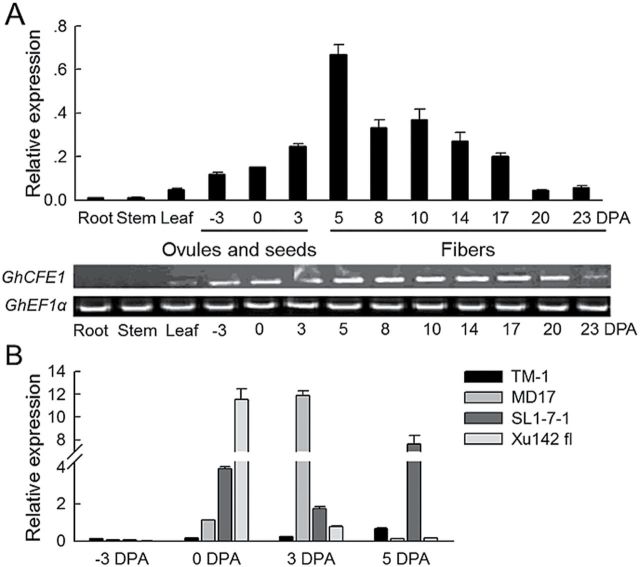
Both RT–PCR and qRT-PCR data for the GhCFE1 expression pattern were consistent with each other. GhCFE1 transcripts were detected in the immature ovules of TM-1 prior to the formation of fibre initials and reached the highest level in the fibre cells at 5 DPA (Fig. 3A). At the rapid elongation stage, the expression was moderate, but the transcript abundance sharply decreased from 17 to 23 DPA in fibre cells. The roots and stems showed almost no expression, and the leaves expressed GhCFE1 at very low levels. Moreover, GhCFE1 transcripts were dozens of times higher in 0, 3, and 5 DPA ovules of the fl mutants (MD17, SL1-7-1, and Xu142 fl) than in the corresponding fibre-bearing TM-1 ovules at the same developmental stage (Fig. 3B). These results indicate that the expression of GhCFE1 is associated with fibre initiation and elongation; moreover, the much higher transcripts of GhCFE1 detected in fuzzless–lintless mutants of cotton suggest that its excessive expression may not be conducive to the formation of lint and fuzz fibres.
Fig. 3.
Expression patterns of GhCFE1. (A) The expression levels of GhCFE1 in root, stem, leaf, ovules, and seeds at –3, 0, and 3 DPA, and fibres at 5–23 DPA. Error bars represent the standard deviation of triplicate experiments, and His3 was used as an internal control in quantitative RT-PCR (qRT-PCR). EF1α was used as an internal control in RT–PCR. (B) The transcripts of GhCFE1 were highly enriched in the fuzzless–lintless mutants (MD17, SL1-7-1, and Xu142 fl) in the early stage of cotton fibre development. Error bars represent the standard deviation of triplicate experiments, and His3 was used as an internal control.
Increased GhCFE1A expression represses cotton seed fibre and stem trichome development
Two overexpression constructs, driven by the 35S constitutive promoter and E6 fibre-specific promoter, were generated (Supplementary Fig. S1 at JXB online) and introduced into cotton by A. tumefaciens-mediated transformation. The empty vector pBI121 was used as the control. A total of nine independent transformed lines (T0), including four 35S-sense-GhCFE1A transgenic lines (named 35SSC plants) and five E6-sense-GhCFE1A transgenic lines (named E6SC plants) were selected. Positive transformants were confirmed by PCR detection of NPTII, 35S-GhCFE1A, and E6-GhCFE1A, respectively (Supplementary Fig. S2A), and Southern blot analysis (Supplementary Fig. S2B). The GhCFE1 transcript levels were significantly higher in the ovules (0 DPA) and fibres (5–15 DPA) of all the T3 homozygous GhCFE1A overexpression plants, especially in the 35SSC transgenic lines, as compared with their corresponding wild-type plants (Fig. 4A; Supplementary Fig. S3). This finding is consistent with the protein levels of GhCFE1 (Fig. 4B), and the size of the GhCFE1 band was determined by heterologous expression of GhCFE1A in E. coli (Supplementary Fig. S4). Moreover, GhCFE1 was sharply up-regulated in the root, stem, and leaf of the four 35SSC lines (Fig. 4A, B).
Fig. 4.
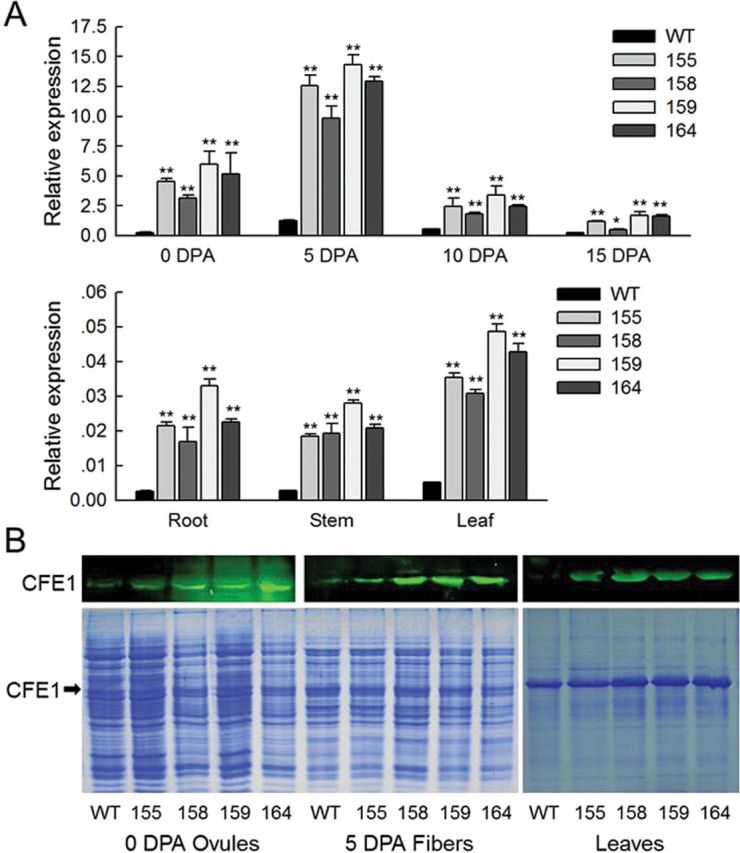
The transcript and protein level analysis of GhCFE1 in wild type (WT) and GhCFE1A-overexpressing cotton. (A) qRT-PCR analysis of expression levels of GhCFE1 in 0 DPA ovules, 5–15 DPA fibres, roots, stems, and leaves of WT and 35S-sense-GhCFE1A (35SSC) transgenic (lines 155, 158, 159, and 164) plants. Error bars represent the standard deviation of triplicate experiments, and His3 was used as an internal control (*P<0.05, **P<0.01, by Student’s t-test). (B) Western blotting with antibodies against GhCFE1 reveals increased abundance of GhCFE1 in 0 DPA ovules, 5 DPA fibres, and leaves of 35SSC transgenic lines (155, 158, 159, and 164). The size of the GhCFE1 band was determined by heterologous expression of GhCFE1A in E. coli (Supplementary Fig. S4 at JXB online), and is indicated by the black arrows. (This figure is available in colour at JXB online.)
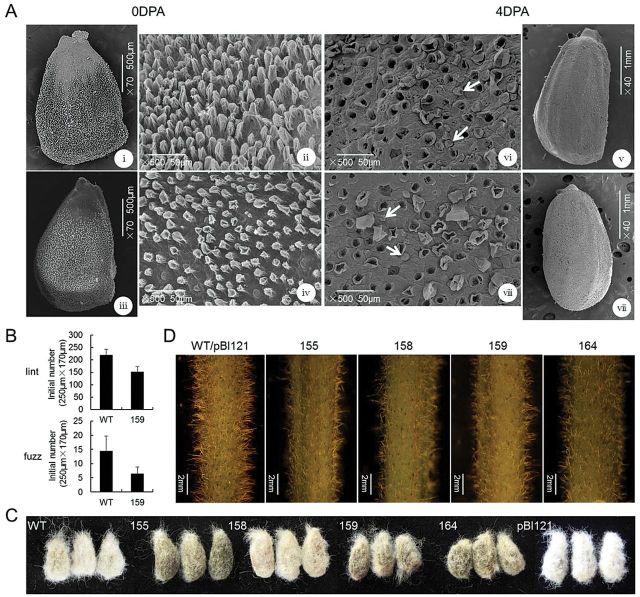
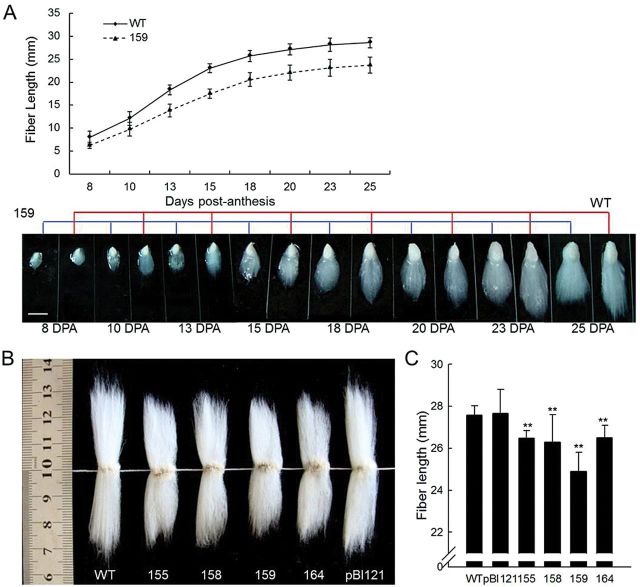
To identify further the effect of overexpression of GhCFE1A, the 35SSC transgenic plants of the T3 generation were analysed for lint and fuzz development. Under electron microscopy, the initial patterns of the lint and fuzz fibre cells on the surface of the ovules at 0 DPA and the seeds at 4 DPA with the lint fibre removed to make the fuzz fibre visible, respectively, were compared between the wild-type and 35SSC line 159 (Fig. 5A). The density of both lint and fuzz fibres was significantly decreased in line 159 (153.11±19.37 lint fibre and 6.42±2.32 fuzz fibre initiations in an area of 250 μm×170 μm) as compared with that in the wild-type plants (219.33±23.32 lint fibre and 14.45±5.25 fuzz fibre initiations in an area of the same size; Fig. 5B). Similar results were found following statistical analysis of data in other lines (Supplementary Table S2 at JXB online). Upon detaching the mature lint fibres from seeds, the T3 transgenic seeds were found to contain fewer fuzz fibres than wild-type and control plants (Fig. 5C). Meanwhile, the overexpression of GhCFE1A also altered the initial density of stem trichomes, which was obviously lower in all the 35SSC plants as compared with the wild-type plants (Fig. 5D).
Fig. 5.
Characterization and phenotype analysis of cotton seed and stem trichome initials. (A) Scanning electron microscope (SEM) images of (i–iv) 0 DPA ovules and (v–viii) 4 DPA seeds, with the lint fibre removed to make the fuzz fibre visible, illustrate the morphological differences of lint and fuzz fibre initials between the wild type (WT; i and ii; v and vi) and 35SSC line 159 (iii and iv; vii and viii). The SEM images were taken at a similar position in the middle of the ovules and seeds. White arrows indicate fuzz fibre cells (vi and viii). (B) Lint and fuzz fibre initials were counted from SEM images of ovules and seeds obtained at 0 and 4 DPA, respectively. The values were averaged over 20 images of 10 selected ovules over an area of 250 μm×170 μm for each line and the wild-type cotton. (C) Photographs of seeds from 35SSC lines, the wild type, and control (pBI121) plants with the mature lint fibres detached. (D) The stem trichomes in 35SSC transgenic cotton lines (155, 158, 159, and 164) have lower density than those in the wild-type cotton.
Additionally, comparison of the fibre length between the GhCFE1A-overexpressing lines and wild-type plants revealed that lint fibre elongation was retarded in line 159 at 0 DPA (Fig. 5A) and the subsequent development stages (8–25 DPA, Fig. 6A), ultimately leading to much shorter mature fibres in 35SSC transformants than in the control plants (Fig. 6B). The survey of fibre quality in the transgenic lines and control plants showed that the transgenic line 159 had significantly shorter fibre length (24.90±0.90mm) than the wild-type (27.58±0.43mm) and control plants (27.67±1.13mm), and the fibre length of the other 35SSC transgenic lines also obviously decreased (26.47±0.37, 26.28±1.31, and 26.49±0.59mm for lines 155, 158, and 164, respectively, Fig. 6C). However, no significant alterations in strength and micronaire were observed in the transgenic plants. The fibre initial index and the fibre length of E6SC transgenic plants can be viewed in Supplementary Tables S2 and S3 at JXB online, and the level of reduction of fibre initiation and elongation is lower than in 35SSC plants. All the observations for GhCFE1A-overexpressing plants were associated with an increase of GhCFE1A transcript and protein (Figs 4–6).
Fig. 6.
Retardation of the fibre elongation process in the GhCFE1A-overexpressing cotton lines. (A) Dynamic curve of fibre development and phenotypes of fibre-bearing seeds in the wild type (WT) and transgenic line 159. Fibre length was quantified and the values were averaged over 10 fibre-bearing ovules of each of the three selected individual plants at every stage for the transgenic line (159) and the wild-type cotton. The stages are indicated at the bottom of the graphs. Scale bar=10mm. (B) The mature lint fibres from 35SSC transgenic cotton lines (155, 158, 159, and 164) were shorter than those from the null control (pBI121) or wild-type fibres, particularly line 159. (C) Average fibre length of the null control, WT, and 35SSC transgenic (lines 155, 158, 159, and 164) fibres. Error bars indicate the standard deviation of triplicate experiments (**P<0.01, by Student’s t-test). (This figure is available in colour at JXB online.)
Suppression of GhCFE1A exhibits no obvious phenotype alteration
A knockdown construct driven by the E6 promoter (pBI121E6-antiGhCFE1A; Supplementary Fig. S1 at JXB online) was introduced into cotton, and six T0 E6-antisense-GhCFE1A transgenic lines (termed E6ASC plants) were obtained. Segregating T1 plants from independent transformants were repeatedly selfed to produce T3 seeds of transgenic homozygotes. The down-regulation of GhCFE1 expression was confirmed by qRT-PCR (Supplementary Fig. S5A). The data showed that, especially at 10 DPA, the expression level in all the transgenic fibres was significantly reduced, which was consistent with the expression pattern of the E6 promoter (John, 1995). However, phenotypic examination showed no significant differences in the number of fuzz and lint fibre initiations at 0 and 4 DPA (Supplementary Table S2), in the density of the fuzz fibres attached to the mature seeds (Supplementary Fig. S5B), and in fibre length (Supplementary Fig. S5C) between the E6ASC and wild-type plants.
GhCFE1A decorates cortical ER, interacts with actin, and functions in fibre cell elongation
To explore how GhCFE1A protein may block the development of cotton fibre cells, GhCFE1A without the N-terminal signal peptide was fused with the GAL4-DNA-binding domain to screen a yeast two-hybrid library transformed with a cDNA library of TM-1 ovules (0 DPA) and fibres (5–20 DPA). Six candidate interaction proteins were obtained by screening 3.8×107 colonies (Supplementary Table S4 at JXB online). Of these, one clone containing a 234 amino acid long actin protein was identified. Li et al. (2005) previously isolated 15 GhACT cDNA clones from the cotton cDNA library and determined using qRT-PCR analysis that GhACT1, GhACT2, GhACT4, GhACT5, and GhACT11 are predominantly expressed in fibre cells. However, sequence analysis revealed that GhACT4 and GhACT11 encoded the same putative amino acid sequence. To elucidate the interaction between the GhCFE1A and actin proteins predominantly expressed in fibre cells, the complete ORF sequences of four GhACT genes were cloned from TM-1 fibres, including GhACT2, GhACT4, and other two GhACT genes (GhACTa and GhACTb, whose encoded proteins share 98% and 99% amino acid sequence identities with GhACT1 and GhACT5, respectively) and fused them with the GAL4-activation domain. Meanwhile, new baits containing truncated GhCFE1A fragments with only one of the two DUFs, designated as ΔN (residues 76–331) and ΔC (residues 27–292, Fig. 7A) were constructed. Co-transformation of GAL4-DNA-BD–GhCFE1A and GAL4-AD–GhACT in yeast cells showed that GhCFE1A interacted with these four actin proteins in vivo (Fig. 7B); however, no interaction was detected between the truncated GhCFE1A fragments and ACTs (Fig. 7B). To investigate whether GhCFE1A and actin would be able to co-localize, constructs encoding GhCFE1A with a C- and N-terminal GFP tag, respectively, driven by the 35S promoter were constructed and transiently co-transformed into N. benthamiana leaf epidermal cells with ABD2-mCherry. Confocal microscopy showed that CFE1A–GFP and GFP–CFE1A not only formed filamentous structures in pavement cells which co-localized with actin bundles visualized by transiently expressing ABD2-mCherry, but also formed a polygonal tubular network morphologically similar to the ER (Fig. 7C; Supplementary Fig. S6A at JXB online). To confirm this localization, CFE1A–GFP and GFP–CFE1A were transiently co-expressed in N. benthamiana leaf epidermal cells with an ER luminal marker RFP–HDEL. The green fluorescent signal of CFE1A–GFP and GFP–CFE1A overlapped perfectly with the red fluorescent signal of RFP–HDEL, as shown in Fig. 7D and Supplementary Fig. S6B. The co-localization pattern was further confirmed by time-lapse imaging (Supplementary Movie S1 at JXB online).
Fig. 7.
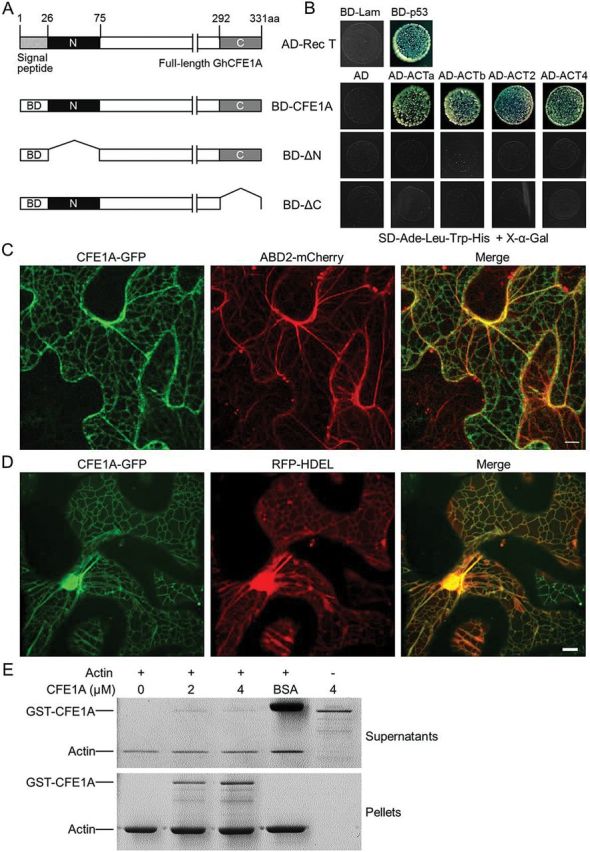
GhCFE1A physically interacts with actin. (A) Structure of the GhCFE1A protein and schematic diagrams of truncated fragments fused with the GAL4-DNA-binding domain (BD) used as bait in yeast two-hybrid analysis. ΔN is the GhCFE1A protein with the N-terminal fragment deleted and ΔC is the protein without the C-terminal fragment. (B) Yeast two-hybrid assay showing that GhCFE1A interacts with GhACTa, GhACTb, GhACT2, and GhACT4. The GAL4-activation domain- (AD) fused interaction partners were retransformed into the yeast strains carrying different baits. Yeast transformants were assayed for growth on X-α-Gal synthetic complete medium without Ade, Leu, Trp, and His (SD-Ade-Leu-Trp-His). BD-p53, a fusion between the GAL4-DNA-BD and murine p53. AD-Rec T, a fusion between the GAL4-AD and large T-antigen, which interacted with p53 as the positive control. BD-Lam, a fusion of the GAL4-DNA-BD with human lamin C with AD-Rec T was used as the negative control. (C) CFE1A–GFP co-expressed with an actin filaments marker, ABD2-mCherry in tobacco (Nicotiana benthamiana) leaf epidermal cells. Scale bar=10 μm. (D) CFE1A–GFP co-expressed with an ER luminal marker, RFP–HDEL in tobacco leaf epidermal cells. Scale bar=10 μm. (E) Co-sedimentation assay of the actin binding activity of GhCFE1A. The presence of GST–CFE1A in the pellet indicates its co-sedimentation with F-actin.
The ability of GhCFE1A to interact with actin was also examined in vitro. A co-sedimentation assay showed that GST-tagged GhCFE1A co-sedimented with actin filaments, whereas bovine serum albumin (BSA) remained in supernatants and GST–CFE1A could not sediment in the absence of actin (Fig. 7E). These results demonstrate that GhCFE1A locates on the ER network and interacts with actin both in vivo and in vitro; furthermore, the two conserved DUFs in GhCFE1A are required for the interaction.
To characterize the F-actin structure in GhCFE1A overexpression lines, the actin alignment in 35SSC transgenic fibres at the elongation stages (5 and 8 DPA) was determined by staining by phalloidin with green fluorescent signal and compared with that of the wild-type fibres. As shown in Fig. 8A, the actin filaments in the wild type existed longitudinally or obliquely and formed a complicated net-like structure at 5 DPA. With subsequent elongation of fibre cells, the actin cytoskeleton was comprised of relatively thick arrays or cables and displayed an increasingly complicated network. In comparison, actin filaments in the GhCFE1A-overexpressing fibres were obviously reduced in terms of the number of filaments, and no net-like structure were observed at 5 and 8 DPA. Changes in actin alignment were further quantified. Compared with that of wild-type fibres, the occupancy values were dramatically reduced in transgenic fibres (Fig. 8B). Thus, a decrease in actin alignment could potentially explain the short fibre phenotype in GhCFE1A overexpression cotton lines.
Fig. 8.
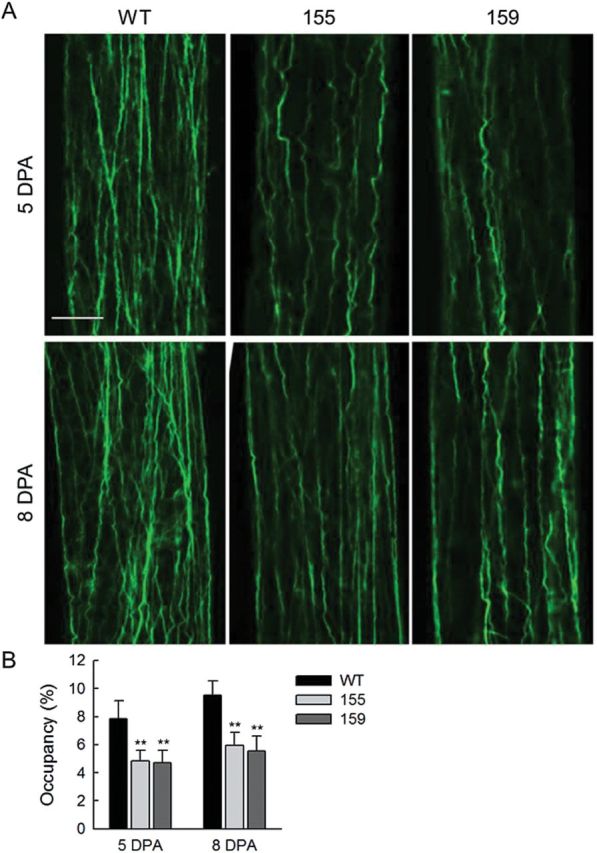
F-actin organization in the wild-type and GhCFE1A-overexpressing fibre cells. (A) F-actin structure in five and eight DPA wild-type (WT) and GhCFE1A-overexpressing (lines 155 and 159) fibres. Scale bar=10 μm. (B) Values of occupancy showing the index of actin alignment in wild-type and GhCFE1A-overexpressing fibres. Error bars represent the standard deviation of >30 individual cells (**P<0.01, by Student’s t-test).
Discussion
Since the DUF761 domain proteins, GhCFE1, GhCFE2, and GhCFE3, were first identified and described as cotton fibre expressed proteins (Yamamoto and Baird, 1998), no further study has been conducted to investigate the in vivo function of these proteins. In the present study, it was revealed that GhCFE1A, the A genome homeologue of GhCFE1 in Upland cotton, decorated the ER network and was linked to actin cables. Overexpressing GhCFE1A repressed the initiation of fibre cells, and also resulted in short fibres in the transgenic cotton lines.
GhCFE1A is associated with trichome initiation
Each cotton fibre is a single cell derived from the epidermal layer of seed coat. Seed hair development in cotton shares many similarities with leaf trichome development in Arabidopsis (Lee et al., 2007; Guan et al., 2011). However, it still remains unclear how the protodermal cells turn into fibres in cotton. In this study, it is shown that overexpressing GhCFE1A in cotton caused an obvious decrease in the number of lint and fuzz fibres on the surface of ovules and seeds or lower lint and fuzz indices; in addition, it resulted in a reduced number of trichomes on stems (Figs 4, 5; Supplementary Table S2 at JXB online). Interestingly, these findings are correlated with the up-regulated expression of GhCFE1A in the fuzzless–lintless mutants (MD17, SL1-7-1, and Xu142 fl) compared with linted–fuzzed TM-1 (Fig. 3B). Therefore, the data suggested that GhCFE1A probably negatively regulates fibre cell initiation.
Previous studies have uncovered several regulators, such as GaMYB2, GhMYB25, GhMYB109, GhTTG3, and GaHOX1, which are known to regulate cotton fibre initiation and Arabidopsis trichome development (Wang et al., 2004; Humphries et al., 2005; Guan et al., 2008; Pu et al., 2008; Machado et al., 2009). GhCFE1A may function downstream of these transcription factors; however, further studies are required to explore the molecular mechanism of GhCFE1A in fibre initiation.
GhCFE1A is localized to the ER and negatively regulates cotton fibre elongation through decreasing actin alignment
CFE proteins are a superfamily with unknown function, and even the homologues of this family in Arabidopsis are uncharacterized. GhCFE1A was shown to interact with GhACT by yeast two-hybrid assay, which was further confirmed by in vitro co-sedimentation assay. However, the truncated GhCFE1A fragments with only the N- or C-terminal domain conserved had no interaction with GhACTs, implying the functional role of GhCFE1A with complete domains. Moreover, it was uncovered that GhCFE1A decorated the ER network, and was also associated with actin bundles. To the best of the authors’ knowledge, this represents the first report of a protein linking the ER and actin cytoskeleton and regulating cotton fibre development.
In plant cells, the ER network is a pleomorphic, dynamic structure that pervades the entire cytoplasm (Sparkes et al., 2011), and involves protein and phospholipid synthesis, their translocation, and their integration into the membrane (Voeltz et al., 2002; Sparkes et al., 2011). It is known that dynamic streaming of the ER network is dependent on the actin cytoskeleton in plant cells (Voeltz et al., 2002; Borgese et al., 2006; Sparkes et al., 2009). The actin cytoskeleton has been demonstrated to participate in intracellular transport for cell elongation (Hussey et al., 2006; Staiger and Blanchoin, 2006). During cotton fibre cell elongation, the F-actin cytoskeleton is predominantly organized as thin arrays parallel to the growing axis of the fibre cells; during the elongation of fibre cells, it shows a complicated network consisting of thick and long cables and bundles (Li et al., 2005; J. Wang et al., 2010). Cotton fibres are highly specialized single cells which undergo enormous growth and finally increase their length 1000–3000 times the diameter of the cell (Meinert and Delmer, 1977), which would need highly active protein and phospholipid synthesis and intracellular transport. So, the ER network and actin cytoskeleton must be orchestrated to meet the requirements of synthesis and transport of the large amounts of cell wall and membrane materials.
GhCFE1A may represent an example of a protein that mediates the interplay between the ER network and the actin cytoskeleton. The GhCFE1A transcripts accumulated predominantly in the fibres, especially at the early stages of development and elongation, and its overexpression led to decreased cell length associated with fewer actin filaments and no formation of a net-like structure at early elongation stages. It is probable that overexpression of GhCFE1A may affect its bridging function, subsequently disorganize the actin cytoskeleton, and finally lead to impairment of cotton fibre cell elongation. Similarly, mutation of a Lumina ER protein-encoding gene, RHD3, also resulted in short root hairs (Schiefelbein and Somerville, 1990; Wang et al., 1997; Stefano et al., 2012). On the other hand, the actin motor protein, myosin XI-K, was reported to play a major role in ER network remodelling, with XI-1 and XI-2 having minor roles (Ueda et al., 2010). Moreover, root hair length in class XI myosin-deficient plants was reduced ~10-fold (Peremyslov et al., 2010). However, it still needs to be determined whether GhCFE1A interacts with the RHD3 or class XI myosins during cotton fibre elongation.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Construction of sense and antisense expression vectors of GhCFE1A.
Figure S2. Molecular confirmation of the independent transgenic lines.
Figure S3. GhCFE1 expression in ovules and developing fibres of E6SC transgenic cotton.
Figure S4. SDS–PAGE analysis of GhCFE1A recombination protein in Escherichia coli.
Figure S5. Inhibition of GhCFE1 in cotton exhibits no obvious phenotype changes.
Figure S6. GhCFE1A is located on the cortical endoplasmic reticulum (ER) and co-localizes with actin bundles.
Movie S1. Time-lapse confocal scanning microscopy of tobacco (Nicotiana benthamiana) leaf epidermal cells co-expressing CFE1A–GFP and RFP–HDEL.
Table S1. Oligonucleotides used in this study.
Table S2. Statistics of lint and fuzz physical parameters in 35SSC, E6SC, and E6ASC transgenic lines.
Table S3. Comparison of fibre quality parameters between transgenic lines and null control or wild-type plants.
Table S4. Clones identified by yeast two-hybrid library screening.
Acknowledgements
The authors would like to thank Dr Daolong Dou from the College of Plant Protection in Nanjing Agricultural University for helpful comments and key experimental guidance, and Dr Shanjin Huang from the Institute of Botany, Chinese Academy of Sciences, for providing purified actin proteins. The authors have no conflict of interest to declare. This work was financially supported in part by the National Natural Science Foundation of China (31471539, 31371676), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- Basra AS, Malik CP. 1984. Development of the cotton fiber. International Review of Cytology 89, 65–113. [Google Scholar]
- Borgese N, Francolini M, Snapp E. 2006. Endoplasmic reticulum architecture: structures in flux. Current Opinion in Cell Biology 18, 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Gipson JR, Ray LL. 1969. Fiber elongation rates in five varieties of cotton (Gossypium hirsutum L.) as influenced by night temperatures. Crop Science 9, 339–341. [Google Scholar]
- Guan XY, Lee JJ, Pang MX, Shi XL, Stelly DM, Chen ZJ. 2011. Activation of Arabidopsis seed hair development by cotton fiber-related genes. PLoS One 6, e21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan XY, Li QJ, Shan CM, Wang S, Mao YB, Wang LJ, Chen XY. 2008. The HD-Zip IV gene GaHOX1 from cotton is a functional homologue of the Arabidopsis GLABRA2 . Physiologia Plantarum 134, 174–182. [DOI] [PubMed] [Google Scholar]
- Guo WZ, Cai CP, Wang CB, et al. 2007. A microsatellite-based, gene-rich linkage map reveals genome structure, function and evolution in Gossypium . Genetics 176, 527–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo WZ, Cai CP, Wang CB, Zhao L, Wang L, Zhang TZ. 2008. A preliminary analysis of genome structure and composition in Gossypium hirsutum . BMC Genomics 9, 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han LB, Li YB, Wang HY, et al. 2013. The dual functions of WLIM1a in cell elongation and secondary wall formation in developing cotton fibers. The Plant Cell 25, 4421–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki T, Kutsuna N, Sano T, Kondo N, Hasezawa S. 2010. Quantification and cluster analysis of actin cytoskeletal structures in plant cells: role of actin bundling in stomatal movement during diurnal cycles in Arabidopsis guard cells. The Plant Journal 61, 156–165. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wang J, Zhang L, Zuo K. 2013. A cotton annexin protein AnxGb6 regulates fiber elongation through its interaction with actin 1. PLoS One 8, e66160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries JA, Walker AR, Timmis JN, Orford SJ. 2005. Two WD-repeat genes from cotton are functional homologues of the Arabidopsis thaliana TRANSPARENT TESTA GLABRA1 (TTG1) gene. Plant Molecular Biology 57, 67–81. [DOI] [PubMed] [Google Scholar]
- Hussey PJ, Ketelaar T, Deeks MJ. 2006. Control of the actin cytoskeleton in plant cell growth. Annual Review of Plant Biology 57, 109–125. [DOI] [PubMed] [Google Scholar]
- Jiang JX, Zhang TZ. 2003. Extraction of total RNA in cotton tissues with CTAB–acidic phenolic method. Cotton Science 15, 166–167. [Google Scholar]
- John ME. 1995. Characterization of a cotton (Gossypium hirsutum L.) fiber-mRNA (Fb-E6). Plant Physiology 107, 1478–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Triplett BA. 2001. Cotton fiber growth in planta and in vitro. Models for plant cell elongation and cell wall biogenesis. Plant Physiology 127, 1361–1366. [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Hassan OS, Gao W, Wei NE, Kohel RJ, Chen XY, Payton P, Sze SH, Stelly DM, Chen ZJ. 2006. Developmental and gene expression analyses of a cotton naked seed mutant. Planta 223, 418–432. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Woodward AW, Chen ZJ. 2007. Gene expression changes and early events in cotton fibre development. Annals of Botany 100, 1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FF, Wu SJ, Chen TZ, Zhang J, Wang HH, Guo WZ, Zhang TZ. 2009. Agrobacterium-mediated co-transformation of multiple genes in upland cotton. Plant Cell, Tissue and Organ Culture 97, 225–235. [Google Scholar]
- Li L, Li Y, Wang NN, Li Y, Lu R, Li XB. 2014. Cotton LIM domain-containing protein GhPLIM1 is specifically expressed in anthers and participates in modulating F-actin. Plant Biology 17, 528–534. [DOI] [PubMed] [Google Scholar]
- Li XB, Fan XP, Wang XL, Cai L, Yang WC. 2005. The cotton ACTIN1 gene is functionally expressed in fibers and participates in fiber elongation. The Plant Cell 17, 859–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Jiang J, Li L, Wang XL, Wang NN, Li DD, Li XB. 2013. A cotton LIM domain-containing protein (GhWLIM5) is involved in bundling actin filaments. Plant Physiology and Biochemistry 66, 34–40. [DOI] [PubMed] [Google Scholar]
- Liu T, Tian J, Wang GD, Yu YJ, Wang CF, Ma YP, Zhang XX, Xia GX, Liu B, Kong ZS. 2014. Augmin triggers microtubule-dependent microtubule nucleation in interphase plant cells. Current Biology 24, 2708–2713. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2 –ΔΔCt method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Machado A, Wu Y, Yang Y, Llewellyn DJ, Dennis ES. 2009. The MYB transcription factor GhMYB25 regulates early fibre and trichome development. The Plant Journal 59, 52–62. [DOI] [PubMed] [Google Scholar]
- Meinert MC, Delmer DP. 1977. Changes in biochemical composition of the cell wall of the cotton fiber during development. Plant Physiology 59, 1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peremyslov VV, Prokhnevsky AI, Dolja VV. 2010. Class XI myosins are required for development, cell expansion, and F-actin organization in Arabidopsis . The Plant Cell 22, 1883–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter KR, Machado RD. 1960. Studies on the endoplasmic reticulum. IV. Its form and distribution during mitosis in cells of onion root tip. Journal of Biophysical, and Biochemical Cytology 7, 167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu L, Li Q, Fan X, Yang W, Xue Y. 2008. The R2R3 MYB transcription factor GhMYB109 is required for cotton fiber development. Genetics 180, 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YL, Chourey PS. 1998. A fiberless seed mutation in cotton is associated with lack of fiber cell initiation in ovule epidermis and alterations in sucrose synthase expression and carbon partitioning in developing seeds. Plant Physiology 118, 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein JW, Somerville C. 1990. Genetic control of root hair development in Arabidopsis thaliana. The Plant Cell 2, 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabaugh E, Held M, Brandizzi F. 2011. Control of root hair development in Arabidopsis thaliana by an endoplasmic reticulum anchored member of the R2R3-MYB transcription factor family. The Plant Journal 67, 395–405. [DOI] [PubMed] [Google Scholar]
- Sparkes I, Hawes C, Frigerio L. 2011. FrontiERs: movers and shapers of the higher plant cortical endoplasmic reticulum. Current Opinion in Plant Biology 14, 658–665. [DOI] [PubMed] [Google Scholar]
- Sparkes IA, Frigerio L, Tolley N, Hawes C. 2009. The plant endoplasmic reticulum: a cell-wide web. Biochemical Journal 423, 145–155. [DOI] [PubMed] [Google Scholar]
- Staiger CJ, Blanchoin L. 2006. Actin dynamics: old friends with new stories. Current Opinion in Plant Biology 9, 554–562. [DOI] [PubMed] [Google Scholar]
- Stefano G, Renna L, Moss T, McNew JA, Brandizzi F. 2012. In Arabidopsis, the spatial and dynamic organization of the endoplasmic reticulum and Golgi apparatus is influenced by the integrity of the C-terminal domain of RHD3, a non-essential GTPase. The Plant Journal 69, 957–966. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28, 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The ClustalX Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 24, 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Yokota E, Kutsuna N, Shimada T, Tamura K, Shimmen T, Hasezawa S, Dolja VV, Hara-Nishimura I. 2010. Myosin-dependent endoplasmic reticulum motility and F-actin organization in plant cells. Proceedings of the National Academy of Sciences, USA 107, 6894–6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ooijen JW, Voorrips RE. 2001. JoinMap® Version 3.0, software for the calculation of genetic linkage maps . Wageningen, The Netherlands: Plant Research International. [Google Scholar]
- Voeltz GK, Rolls MM, Rapoport TA. 2002. Structural organization of the endoplasmic reticulum. EMBO Reports 3, 944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Kudla J. 2008. In planta visualization of protein interactions using bimolecular fluorescence complementation (BiFC). Cold Spring Harbor Protocols 2008, t4995. [DOI] [PubMed] [Google Scholar]
- Wang H, Lee MM, Schiefelbein JW. 2002. Regulation of the cell expansion gene RHD3 during Arabidopsis development. Plant Physiology 129, 638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lockwood SK, Hoeltzel MF, Schiefelbein JW. 1997. The ROOT HAIR DEFECTIVE3 gene encodes an evolutionarily conserved protein with GTP-binding motifs and is required for regulated cell enlargement in Arabidopsis. Genes and Development 11, 799–811. [DOI] [PubMed] [Google Scholar]
- Wang HH, Guo Y, Lv FN, Zhu HY, Wu SJ, Jiang YJ, Li FF, Zhou BL, Guo WZ, Zhang TZ. 2010. The essential role of GhPEL gene, encoding a pectate lyase, in cell wall loosening by depolymerization of the de-esterified pectin during fiber elongation in cotton. Plant Molecular Biology 72, 397–406. [DOI] [PubMed] [Google Scholar]
- Wang HY, Wang J, Gao P, Jiao GL, Zhao PM, Li Y, Wang GL, Xia GX. 2009. Down-regulation of GhADF1 gene expression affects cotton fibre properties. Plant Biotechnology Journal 7, 13–23. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang HY, Zhao PM, Han LB, Jiao GL, Zheng YY, Huang SJ, Xia GX. 2010. Overexpression of a profilin (GhPFN2) promotes the progression of developmental phases in cotton fibers. Plant and Cell Physiology 51, 1276–1290. [DOI] [PubMed] [Google Scholar]
- Wang S, Wang JW, Yu N, Li CH, Luo B, Gou JY, Wang LJ, Chen XY. 2004. Control of plant trichome development by a cotton fiber MYB gene. The Plant Cell 16, 2323–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto E, Baird WV. 1998. Three cotton fiber-expressed cDNAs (Accession Nos.AF072404, AF072405, and AF072406) (PGR 98–144). Plant Physiology 117, 1525–1528.9750105 [Google Scholar]
- Zhang M, Wu F, Shi J, Zhu Y, Zhu Z, Gong Q, Hu J. 2013. ROOT HAIR DEFECTIVE3 family of dynamin-like GTPases mediates homotypic endoplasmic reticulum fusion and is essential for Arabidopsis development. Plant Physiology 163, 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.