SUMMARY
TP53 is the most frequently mutated gene in human cancer, and small molecule reactivation of mutant p53 function represents an important anti-cancer strategy. A cell-based high-throughput small molecule screen identified chetomin (CTM) as a mutant p53 R175H reactivator. CTM enabled p53 to transactivate target genes, restored MDM2 negative regulation, and selectively inhibited the growth of cancer cells harboring mutant p53 R175H in vitro and in vivo. We found that CTM binds to Hsp40 and increases the binding capacity of Hsp40 to the p53 R175H mutant protein causing a potential conformational change to a wt-like p53. Thus, CTM acts as a specific reactivator of the p53 R175H mutant form through Hsp40. These results provide new insights into the mechanism of reactivation of this specific p53 mutant.
Graphical Abstract
INTRODUCTION
The tumor suppressor p53 is mutated in at least half of human cancers (Joerger and Fersht, 2008; Levine and Oren, 2009). Loss of p53 function plays a pivotal role in the initiation as well as the progression of cancers. Recent large-scale genomics analysis found that some cancer types exhibit very high frequencies of p53 mutation, including ovarian cancer (95%), lung squamous cell carcinoma (84%), head and neck cancer (67%), and esophageal adenocarcinoma (65%) (Lawrence et al., 2014). p53 functions as a transcription factor and is activated in response to various cellular stresses, such as DNA damage, oncogene activation and hypoxia (Joerger and Fersht, 2008; Levine and Oren, 2009). Once activated, p53 induces its downstream target genes and promotes cell cycle arrest, apoptosis, senescence and DNA repair (Allen et al., 2014; Joerger and Fersht, 2008; Levine and Oren, 2009). Thus, p53 is a tumor suppressor gene and is frequently called the guardian of the genome (Lane, 1992). The majority of clinically useful traditional anticancer drugs includes DNA damaging agents, and the activities rely on functional wild-type p53 for anticancer effects. In addition, mutant p53 has a gain of function phenotype that promotes more aggressive cancer forms. Thus, cancer cells harboring p53 mutants have been reported to exhibit chemoresistance to many conventional anticancer agents (Muller and Vousden, 2014; Willis et al., 2004). This dramatic dependence on functional p53 argues that restoring p53 function is an important approach for cancer therapy, and several earlier studies have reported targeting mutant p53 using small molecules, peptides and adenovirus (Chen et al., 2010; Hong et al., 2014; Mandinova and Lee, 2011).
In the present study, we have identified a natural compound, chetomin (CTM) from a fungus extract as a novel p53 R175H mutant reactivator in a cell-based high throughput screen. We found that CTM restores p53 function and can transactivate and induce p53 target genes in vitro and in vivo through the p53-Heat shock protein 40 (Hsp40) axis.
RESULTS
Identification of CTM as a mutant p53 R175H reactivator
To identify small molecules that reactivate mutant p53, a luciferase reporter-based, high-throughput chemical screen against the R175H structural mutant was performed. For the screen, we established a stable cell line with mutant p53 R175H and a luciferase reporter carrying the p53 DNA binding site of the PUMA promoter in p53-null H1299 lung cell carcinoma cells (H1299-mtp53 R175H/PUMA-luc). We first verified the responsiveness of this luciferase reporter cell line by infection with Ad-p53 expressing wild-type p53, which significantly increased luciferase activity, whereas treatment with Ad-GFP showed no effect (Figure 1A). We then performed high-throughput chemical screening, as outlined in Figure 1B, with a chemical library containing 20,000 compounds and 36,256 natural extracts (from the NCI's Natural Products Repository) to identify compounds that increase luciferase activity of the PUMA promoter. Five top hits (#1 - 5) that consistently showed more than 2.5 fold increased luciferase activity compared to DMSO control were chosen (Figure 1C). All five candidates were from the fungal extract library. We performed validation experiments and found that fungal extracts #4 and #5 showed the strongest effect. Extracts #4 and #5 were effective in inducing PUMA promoter activity in a dose-dependent manner (Figure S1A and B, left top panels) and showed significantly higher cytotoxic effects towards mutant p53 R175H cells such as KLE, FAMPAC, SK-BR-3, AU565 and TOV-112D than towards p53 null cells including SK-OV-3, HCT116−/− and H1299 (Figure S1A and B, left middle panels). Although p53 gene in HCT116−/− cell line is not fully deleted, it has been reported as a p53-deficient cell line (Bunz et al., 1998; Murray-Zmijewski et al., 2006). In addition, when treated to mouse embryonic fibroblasts (MEFs) expressing one of p53 R172H, R172P (the mouse equivalent to R175 in human), wt-p53, or to p53 null MEFs, extracts #4 and #5 were both able to induce significant cell death only in the mutants p53 R172P and R172H MEFs, unlike the known mutant-p53 reactivator MIRA-1 (Bykov et al., 2005) (Figure S1A and B, left bottom panels). In response to extract #4, mRNA and protein expression levels of p53 target genes such as p21 and PUMA were induced in cancer cell lines that harbor mutant p53 R175H, while there was little effect in cancer cells with wt-p53 or in p53 null cells. Extract #5 also showed similar results in protein expression level of p53 target genes (Figure S1A and B: right panels). Based on these results, extracts #4 and #5 were chosen for further investigation.
Figure 1. Identification of CTM as a mutant p53 R175H reactivator.
(A) H1299-mutant p53 R175H cells with luciferase reporter carrying the p53 DNA binding site of PUMA promoter was generated and tested for luciferase activity with adenovirus (Ad)-GFP or -p53. (B) Screening strategy used in this study. (C) High-throughput chemical screening was performed in duplicate, and relative luciferase activity was calculated. (D) Fractionation of natural extracts #4 and #5 by HPLC methods and luciferase activity assays of resulting fractions of natural extracts. Each natural extract was subjected to HPLC fractionation [#4, fractions 1-7; #5, fractions 1-9]. Each fraction from extracts #4 and #5 was analyzed by luciferase assay using H1299-mutant p53 R175H cells with luciferase reporter carrying the p53 DNA binding site of PUMA promoter. Cells were treated with each fraction at indicated concentrations for 15 hours. Luciferase activity was then measured. Data shown are mean ± S.D. in triplicate and measured at the same time. (E) Chemical structure of chetomin with absolute stereochemistry predicted through optical rotation calculations. (F) Global minimum conformation of chetomin calculated using DFT at the SCRF(chloroform)-wB97XD/6-311++G(d,p) level of theory. See also Table S1. (G) CTM was analyzed by luciferase assay using H1299-mutant p53 R175H cells with luciferase reporter carrying the p53 DNA binding site of PUMA promoter. Cells were treated with CTM at indicated concentrations for 15 hours, after which luciferase activity was measured. Data shown are mean ± S.D. in triplicate and measured at the same time. Adenoviruses Ad-GFP and Ad-p53 were used as negative and positive controls for luciferase assay, respectively. See also Figure S1.
In order to identify the active molecule(s) from the natural extracts, we fractionated the extract #4 (fractions 1 to 7) and #5 (fractions 1 to 9) by HPLC (Figure 1D) and tested PUMA promoter reporter activity in H1299-mtp53R175H/PUMA-luc cells. Treatment with fraction 4 of extract #4 or fraction 6 of extract #5, respectively, demonstrated the highest luciferase activity (Figure 1D). We then analyzed these two fractions by nuclear magnetic resonance (NMR) spectroscopy, and as a result, an identical small molecule – chetomin (CTM) – was identified from both fractions. CTM is produced by several species in the fungal genus Chaetomium (Waksman and Bugie, 1944). The structure of CTM and 3D image of the CTM global minimum conformation are shown in Figure 1E and 1F, respectively (Table S1). While the relative stereochemistry of CTM is known, its absolute configuration has not been determined. Thus, optical rotation calculations were performed in a similar manner to a previous report (Cherblanc et al., 2011). Through using density functional theory (DFT) with the SCRF(chloroform)-wB97XD/6-311++G(d,p) level of theory (Chai and Head-Gordon, 2008) and assuming the absolute stereochemistry of CTM depicted in Figure 1E, a Boltzmann-weighted optical rotation [α]D +299° was obtained (Tables S1-4), which is of the same sign and similar magnitude to the experimental optical rotation for CTM ([α]D25 +278° CHCl 3) (Fujimoto et al., 2004). Therefore, the CTM absolute stereochemistry shown in Figure 1E is predicted to be correct. In order to confirm that CTM is the molecule responsible for the activities of extracts #4 and #5, we tested the effects of purified commercial CTM on the PUMA promoter activity in H1299-mtp53R175H/PUMA-luc cells. As a result, CTM indeed increased PUMA promoter activity in a dose-dependent manner (Figure 1G). Meanwhile, it did not show any effect on NF-κB luciferase activity (Figure S1C). These results suggest that CTM is a strong candidate small molecule capable of restoring p53 activity from mutant p53 R175H.
Anticancer effects and induction of p53 target genes by CTM in cancer cell lines expressing R175H p53 mutant
To investigate the anti-cancer activity of CTM, we treated human tumor cell lines of different p53 status including mutant p53 R175H (structural mutation), p53 R273H (contact mutation), wild type p53 (wt p53) and p53 null, as well as normal cells. CTM exhibited a higher cytotoxicity to the mutant p53 R175H cell lines than towards mutant p53 R273H, wt p53, or p53 null cell lines (Figure 2A). In mutant p53 R175H cells, such as CAL-33, HuCCT1, FAMPAC, KLE and TOV-112D, mRNA expression of p53 target genes such as p21, PUMA and/or MDM2 was significantly induced upon CTM treatment. In fact, the level of induction was comparable to that observed in wt p53 containing positive control HCT116 cells treated with etoposide (ETO) (Figure 2B). Meanwhile, the mRNA expression level of these genes showed little response to CTM in HCT116 (wt), H1299 (null) and PANC-1 (R273H) cells (Figure 2B). CTM also significantly increased protein expression levels of p21 and PUMA in a dose-dependent manner in mutant p53 R175H cells, such as CAL-33, HuCCT1, FAMPAC and KLE (Figure 2C), whereas slight or no induction was observed in OVCAR-3 (R248Q), A431 (R273H), HCT116 (wt), MCF7 (wt), H1299 (null) and HCT116−/− (null) cells (Figures 2D). These results suggest that CTM exerts anticancer effect with higher specificity towards cancer cells harboring mutant p53 R175H.
Figure 2. CTM preferentially suppresses cancer cells with p53 R175H and induces p53 target genes.
(A) CTM shows high anticancer activity in mutant p53 R175H cells. Normal and cancer cells including p53 wild type, p53 null, mutant p53 R175H and R273H were treated with CTM for 24 hours at indicated concentrations. Cells were stained with Sulforhodamine B and measured for cell viability. Error bars represent the range of duplicates. (B) p53 target genes are highly induced in mutant p53 R175H cells. Cancer cells (R175H: CAL-33, HuCCT1, FAMPAC, KLE and TOV-112D; wild type: HCT116; null: H1299; R273H: PANC-1) with various status of p53 were treated with CTM (150 nM) for indicated times. Total RNA was extracted and subjected to quantitative real-time PCR with specific primers for p21, PUMA and MDM2. HCT116 cells were treated with etoposide (50 μM) for indicated time points as a positive control. Data shown are mean ± S.D. in triplicates and measured at the same time. (C and D) CTM-mediated p53 target protein induction in mutant p53 R175H cells. Cancer cells (R175H: CAL-33, HuCCT1, FAMPAC and KLE; R248Q: OVCAR-3; R273H: A431; wild type: HCT116; null: H1299) with various status of p53 were treated with CTM for 18 hours, and cell lysates were analyzed by western blotting with indicated antibodies. Ad-p53 was used as a positive control.
CTM specifically targets mutant p53 R175H and restores p53 wild type-like properties
To confirm the specificity of CTM to mutant p53 R175H, we knocked-down mutant p53 R175H by siRNA and observed at the protein level that the induction of p53 target genes p21, PUMA, and Noxa in response to CTM was impaired in R175H cells, including TOV-112D, KLE and CAL-33 (Figure 3A, top panel). A similar result was also observed when p53 was knocked-down by shRNA in FAMPAC (R175H) cells (Figure S2A). In contrast, introduction of mutant p53 R175H to p53 null cells (H1299 and HCT116 p53−/−) resulted in induction of p21 and PUMA at the protein level upon CTM treatment, while induction of p21 or PUMA was not observed when mutant p53 R273H was introduced into HCT116 p53−/− cells (Figure 3A, bottom panel). Taken together, these results demonstrate that CTM induces p53 target genes in a mutant p53 R175H-dependent manner.
Figure 3. Mutant p53 reactivation effect of CTM is mediated through p53 R175H.
(A) Knockdown of mutant p53 R175H impairs induction of p53 target genes by CTM. Cells were transfected with siRNA (si control or sip53) and treated with CTM for 18 hours. Overexpression of mutant p53 R175H increased protein expression level of p53 target genes. H1299 (p53-null) cells were transfected with pcDNA3-empty or mutant p53 R175H plasmid. Stable cells were treated with CTM for 18 hours. HCT116 p53−/− cells overexpressing mutant p53 R175H or R273H by tetracycline-inducible system were treated with CTM (150 nM) for 18 hours. Mutant p53 was overexpressed by doxycycline for 48 hours prior to CTM treatment. (B) ChIP analysis shows that CTM treatment restores the transactivation function of p53 in mutant p53 R175H cells. Cells were treated with DMSO, etoposide (50 μM), or CTM (200 nM) and cross-linked. Sheared chromatin was immunoprecipitated with p53 antibody. Eluted DNA was examined by quantitative real-time PCR using primers that specifically target p53 binding site in the promoter. Data shown are mean ± S.D. in triplicate. See also Figure S2.
We next investigated whether CTM restores DNA-binding activity of mutant p53 R175H protein by chromatin immunoprecipitation (ChIP) assay. HCT116 cells containing wt p53 showed significant p53 occupancy at p21, PUMA and MDM2 promoters in response to the DNA damaging reagent ETO, while CTM showed little effect (Figure 3B). However, H1299 cells transfected with mutant p53 R175H and CAL-33 (R175H) cells showed increased p53 promoter occupancy at p21, PUMA, and MDM2 promoters upon CTM treatment. In order to test if this was due to a CTM-specific effect, ETO was treated and the occupancy of mutant p53 was examined through ChIP assay in H1299 cells transfected with mutant p53 R175H. As a result, ETO treatment showed little effect on mutant p53 occupancy, corroborating the importance of chetomin as a mutant p53-specific compound (Figure S2B). Thus, it appears that CTM can restore DNA-binding activity of mutant p53 R175H protein.
Notably, we observed that upon CTM treatment, p53 protein level significantly decreased in mutant p53 R175H cells, while remaining relatively stable in OVCAR-3 (R248Q) and A431 (R273H) cells (Figure 2C and D). Based on this finding, we came to suspect that the restoration of wt p53 function by CTM might have resulted in increased negative regulation by MDM2, leading to decreased level of the mutant p53 R175H protein. In order to test this idea, we assessed the impact of MDM2 negative regulation on CTM-treated mutant p53 R175H using Nutlin-3, a well-known MDM2 antagonist. Nutlin-3 treatment alone did not affect p53 protein level, whereas CTM treatment alone resulted in a decrease in p53 level in mutant p53 R175H cells (CAL-33, KLE, HuCCT1 and FAMPAC) but not in A431 (R273H) and OVCAR-3 (R248Q) cells. However, when p53 R175H cells (CAL-33, KLE, HuCCT1 and FAMPAC) were treated with both Nutlin-3 and CTM, the decrease in p53 was inhibited (Figure 4A, Figure S3A). We also observed that MDM2 protein level was induced upon CTM treatment, and the binding between mutant p53 and MDM2 protein was significantly increased upon CTM treatment but inhibited upon addition of Nutlin-3 in CAL-33 cells (Figure 4B). Furthermore, to examine if co-treatment of Nutlin-3 and CTM, which appears to lead to stabilization of functionally restored p53 R175H, might result in synergistic effects on p53 target gene induction, we checked mRNA levels of p53 target genes through qRT-PCR assay in HuCCT1 cells and CAL-33 cells treated with either CTM or Nutlin-3 alone, or with both CTM and Nutlin-3. As a result, while Nutlin-3 alone did not cause increase, co-treatment of Nutlin-3 and CTM resulted in increased levels of mRNA, demonstrating additive or synergistic effects in a gene-dependent manner (Figure S3B). These results indicate that CTM restores mutant p53 R175H function to wt-like p53, thus activating the induction of MDM2, which then binds to and negatively regulates p53 R175H.
Figure 4. CTM restores p53 wild type-like properties in mutant p53 R175H.
(A) p53 level is decreased upon CTM treatment due to MDM2 negative regulation in mutant p53 R175H cells, but not in mutant p53 R275H and R248Q cells. Cells were treated with Nutlin-3 and/or CTM at indicated concentrations and time. (B) CTM treatment increased MDM2 protein level and binding capacity to p53 protein in R175H cells. Cells were treated with etoposide, CTM and/or Nutlin-3 as described, and co-immunoprecipitation was performed with cell lysate using anti-p53 or -MDM2 antibody. Inputs and co-IP were analyzed with indicated antibodies. See also Figure S3.
Antitumor effect of CTM in xenograft tumor model
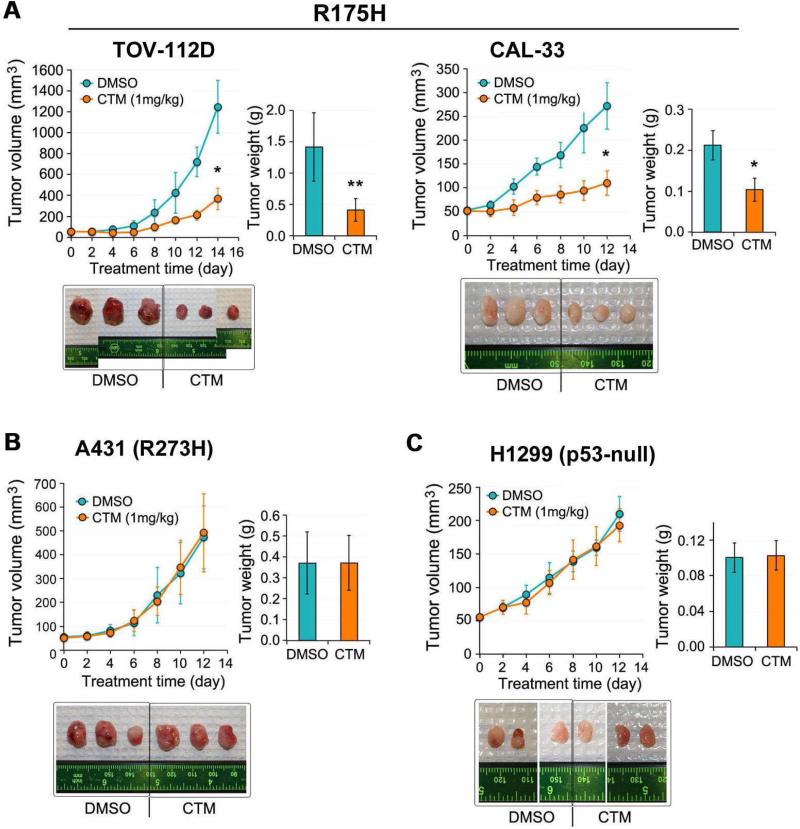
To investigate whether CTM can reactivate the mutant p53 R175H in vivo, mouse xenografts of various tumor cell lines carrying mutant p53 R175H, p53 R273H or p53 null were generated. In mutant p53 R175H-carrying TOV-112D and CAL-33 tumors, CTM treatment resulted in significant reduction of tumor volume (up to 71% and 59% at endpoint, respectively) and weight (71% and 51% at endpoint, respectively) (Figure 5A). However, CTM did not inhibit in vivo tumor growth of A431 (R273H) (Figure 5B) and H1299 (p53 null) (Figure 5C) tumors. These findings further support the idea that the antitumor effect of CTM is specific to the p53 R175H mutation.
Figure 5. CTM suppresses tumor growth in vivo in a p53 R175H mutant dependent manner.
Various types of p53 cells were used for xenograft model-(A) TOV-112D (p53-R175H) and CAL-33 (p53 -R175H), (B) A431 (p53-R273H), (C) H1299 (p53-null). Tumors were allowed to grow to 50 mm3 before intraperitoneal injection of DMSO or CTM at 1 mg/kg/day for indicated days. Tumor volume and weight were measured. Examined mice numbers are as follows: TOV-112D (DMSO control: n=7, and CTM-treated: n=6), CAL-33 (DMSO control: n=9, and CTM-treated: n=9), A431 (DMSO control: n=6, and CTM-treated: n=6), H1299 (DMSO control: n=8, and CTM-treated: n=8). Data shown are mean ± S.D. Student's t-test, *P<0.001, **P<0.005.
Hsp40 is a CTM target and mediator of mutant p53 R175H reactivation
To explore the mechanism by which CTM affects mutant p53 R175H, we investigated whether CTM could directly bind to mutant p53 R175H protein. However, in a gel shift assay to assess DNA binding activity of mutant p53 R175H upon CTM treatment, no increase in direct DNA binding ability was observed (Figure S4A and B). This suggests that CTM does not bind to or directly affect the mutant p53 R175H protein. Thus, we investigated potential CTM binding partners with a co-immunoprecipitation-coupled mass spectrometry analysis to identify the direct targets of CTM (Figure S4C). Mass spectrometry data demonstrated that most known p53-binding partners were decreased in unique peptide number upon CTM treatment, a finding in accordance with the previously observed decrease in p53 protein level (Table S5) (Avantaggiati et al., 1997; Bates et al., 2005; Gaiddon et al., 2001; Lee et al., 2002; Yuan et al., 2010). However, upon CTM treatment, some p53 binding partners such as YBX1, WDR33 and Hsp40 homolog (DNAJC8) showed increased binding to p53 (King et al., 2001; Okamoto et al., 2000; Stelzl et al., 2005).
As previously reported, heat shock proteins, which have been identified as p53 binding partners, function as a chaperone or co-chaperone proteins to regulate protein conformation and stability (King et al., 2001; Rosser and Cyr, 2007; Sugito et al., 1995). Therefore, we focused on the role of Hsp40 in reactivation of mutant p53 R175H upon CTM treatment. Consistent with the mass spectrometry data, only Hsp40, but not Hsp90, showed increased binding affinity to mutant p53 upon CTM treatment (Figure 6A). The induction of Hsp40 protein was observed only upon CTM treatment and not in response to treatments with known DNA damaging agents (ETO and camptothecin, CPT) or the known mutant p53 reactivators {MIRA-1 (Bykov et al., 2005) and PRIMA-1 (Bykov et al., 2002)} (Figure S4D). Notably, knockdown of Hsp40 by siRNA impaired the protein level induction of p53 target genes upon CTM treatment (Figure 6B). We also observed that CTM enhanced Hsp40 binding to mutant p53 R175H protein in a dose-dependent manner in an in vitro binding assay using recombinant proteins (Figure 6C, top panels), which was not seen with PRIMA-1 or MIRA-1 (Figure S4E). Interestingly, this in vitro binding assay also showed that upon CTM addition, p53-R175H/Hsp40 complex was detected by wild type-specific antibody PAb1620 (Figure 6C, lower panels). Intrigued by these observations, we next determined the effects of CTM on the DNA binding activity of p53 R175H in the presence of Hsp40. When using purified recombinant Hsp40 and mutant p53-DBD-R175H for gel shift assay, CTM showed little effect. However, when the nuclear extract of TOV-112D (R175H) cell was used to repeat this experiment, CTM treatment increased the DNA binding activity of p53-DBD-R175H (Figure S5). As another approach, using Biacore assay, we confirmed that CTM binds to Hsp40 with a KD value of 3.7 μM based on surface plasmon resonance data analysis (Figure 6D and Figure S6A). Next, the binding affinity of CTM towards Hsp40 and p53 R175H was examined. Varying concentrations of CTM were injected in addition to Hsp40 over immobilized p53R175H. Although the addition of CTM resulted in a slight increase of binding affinity between Hsp40 and p53 R175H, the level of increase was not sufficient to determine the KD towards this complex (Figure S6B). This observation is consistent with the aforementioned gel shift assay results, where little effect of CTM was observed when testing with purified Hsp40 and p53 R175H proteins. The positive gel shift assay result obtained when using nuclear extract of TOV-112D (R175H) cell (Figure S5) implies the involvement of a yet unidentified factor(s) present in vivo that contributes to the higher potency of CTM in vivo. Binding of CTM to mutant p53 R175H and Hsp70 were also tested, but no significant interaction was detected. In addition, no interaction was detected between Hsp40 and other small molecules such as ETO or NSC319726, a previously reported reactivator of mutant p53 R175H (Yu et al., 2012) (Figure 6D). These results corroborate the specificity of CTM towards Hsp40 and p53 R175H in the process of reactivating p53 R175H.
Figure 6. Binding of CTM to the Hsp40 protein is required for the CTM-mediated reactivation of mutant p53 R175H.
(A) Hsp40 expression is increased and its binding capacity to mutant p53 is enhanced upon CTM treatment in CAL-33 (R175H) cells. Cells were treated with CTM (200 nM) for 8 hours and co-immunoprecipitation was performed with cell lysate using anti-p53 antibody. Inputs and co-IP were analyzed with indicated antibodies. (B) Hsp40 depletion impairs protein induction of p53 target genes upon chetomin treatment. CAL-33 cells were transfected with siRNA (si control and si-p53) and treated with CTM for 18 hours. Cell lysates were analyzed by western blotting. (C) CTM treatment increases the binding capacity of Hsp40 protein to mutant p53 R175H in vitro. Top panel: Recombinant proteins of mutant p53 R175H (250 nM) and His-Hsp40 (1 μM) were incubated with or without CTM at increasing concentrations (1, 2 and 4 μM), and pull-down assays were performed with anti-p53 antibody DO1, which recognizes both wild type and mutant p53. Bottom panel: Recombinant proteins of His-mutant p53 R175H (55 nM) and His-Hsp40 (1 μM) were incubated with or without CTM (4 μM), and pull-down assays were performed with either anti-p53 antibody DO1 or anti-p53 antibody PAb1620 (wild type-specific). (D) CTM binds to Hsp40 in a concentration-dependent manner. Physical interaction between CTM and Hsp40, mt-p53 R175H, or Hsp70 was tested through Biacore assay. A 2-fold dilution series of CTM, ranging from 0 μM to 40 μM, was tested for binding. In addition, physical interaction between Hsp40 and either NSC319726 (40 μM) or etoposide (40 μM) was tested through Biacore assay. Each series of experiment was tested in duplicates. See also Figure S4-S6 and Table S5.
DISCUSSION
Several small molecules, including PRIMA-1, MIRA-1 and CP-31398, have been reported as mutant p53 reactivators (Bykov et al., 2002; Bykov et al., 2005; Foster et al., 1999). However, only PRIMA-1MET (PRIMA-1 analog), also known as APR-246, is currently in clinical trials (phase Ib, NCT02098343) and has a report on a completed phase I study (Hoe et al., 2014). Thus, discovering small molecules that restore function to mutant p53 remains an important research goal for generating drug leads and developing new therapeutics. Mutant p53 has several hot spot mutations that are categorized into two classes: contact mutations and structural mutations (Bullock and Fersht, 2001; Joerger and Fersht, 2008). While either type results in the loss of p53 transactivation function, contact mutations, such as R248 and R273, directly inhibit the ability of p53 to bind DNA, while structural mutations, such as R175, alter the conformation of p53 protein to abrogate DNA binding.
Recently, two compounds, NSC319726 (Yu et al., 2012) and stictic acid (Wassman et al., 2013), were reported as mutant p53 reactivators. The mechanism of mutant p53 R175H reactivation by NSC319726 was reported to be through its zinc ion chelating and redox changing function. Stictic acid (Wassman et al., 2013) was identified through an ensemble-based virtual screening approach, and its mechanism of reactivation is through docking of the small molecule in the open L1/S3 p53 binding pocket around Cys124, Cys135 and Cys141. These two reports indicate multiple mechanisms for reactivating mutant p53. We also focused on the structural mutant p53 R175H with an intact DNA binding site over contact mutants, as the likelihood of finding a small molecule effecting a p53 conformational change back to wild type seemed higher than correcting a contact mutant with lost DNA-binding ability. In addition, among the various hot spot mutations, R175H is the most frequent (Leroy et al., 2013).
In this study, we examined 20,000 chemical compounds and 36,256 natural product extracts, and identified CTM through cell-based screening using a luciferase reporter assay. CTM indeed induced p53 target genes such as p21, PUMA and/or MDM2 and showed anti-cancer effects in an R175H-specific manner in vitro and in vivo. Moreover, its ability to induce MDM2 resulted in increased p53-MDM2 binding and p53 degradation, which was inhibited by Nutlin-3. CTM also increased p53 occupancy on p53 target promoter binding sites in mutant p53 R175H cells. The conformational change of the mutant p53 R175H to wild type was shown by its increased detection by wild type p53-specific antibody, PAb1620. Collectively, these results strongly suggest that CTM can reactivate mutant p53 and restore it to wt-like function, including restoration of MDM2 negative regulation.
We observed that R175H mutant-harboring cell lines are in general more sensitive to CTM than cell lines with R273H mutant or wild-type p53 (Figure 2A). In addition, among different R175H mutant cells, there was a different degree of sensitivity to CTM, suggesting that there may be more factors other than R175H mutation contributing to the sensitivity towards CTM, although further details remain to be elucidated. For instance, it was previously reported that CTM exerts anti-tumor activity through targeting the interactions between HIF-1alpha and p300 (Kessler et al., 2010; Kung et al., 2004; Staab et al., 2007) in conditions where HIF-1alpha is stabilized. Although our experiments were not carried out in such conditions, and thus argues against this possibility, we still cannot exclude the possibility of other factors contributing to the anti-tumor effects of CTM observed in our settings. CTM is a member of the epidithiodiketopiperazine family of natural products. Chaetocin, another member of this family, has been previously reported to show similar activities as CTM in targeting HIF-1alpha and p300 interaction (Cook et al., 2009). When we tested the effects of chaetocin, we observed similar effects as CTM on p53 R175H destabilization and p21 gene induction in p53 R175H harboring cells (Figure S6C). This result demonstrates the value of CTM as a lead compound.
The mechanism by which CTM reactivates mutant p53 R175H does not appear to involve direct binding between CTM and p53 R175H protein. However, mass spectrometry results suggested Hsp40 as a promising target of CTM, which possibility is further supported by previous reports showing that Hsp40 can bind to wild type and mutant p53 proteins and act as a chaperone to stabilize unfolded p53 proteins (King et al., 2001; Rosser and Cyr, 2007; Sugito et al., 1995). We demonstrated that CTM increases the binding of Hsp40 to mutant p53 R175H upon treatment and that reactivation of mutant p53 R175H is suppressed by knockdown of Hsp40. We also confirmed through Biacore assay that CTM directly binds Hsp40 protein and that the CTM-Hsp40-p53 R175H in vivo complex can be recognized by the wild type p53-specific antibody PAb1620. All of these findings indicate that CTM-Hsp40 functions to revert mutant p53 R175H to wild type-like conformation. Previous studies have described other small molecules that reactivated mutant p53 through heat shock proteins. PRIMA-1 treatment resulted in translocation of Hsp90α to the nucleus and enhanced binding between Hsp90 α and mutant p53 (Rehman et al., 2005). Similarly, its analog PRIMA-1MET is reported to induce Hsp70 and co-localization with mutant p53 in the nucleoli (Rokaeus et al., 2007). Thus, these studies support a role for heat shock proteins in the refolding and reactivation of mutant p53 protein.
In conclusion, this study shows that CTM can restore mutant p53 R175H to wild type-like p53 function through direct interaction and activation of Hsp40, demonstrating the critical role of Hsp40 in reactivating mutant p53 R175H in cancer cells, and providing novel insights into mutant p53 R175H reactivation.
EXPERIMENTAL PROCEDURES
Cell lines and culture conditions
Cells were cultured in media containing 10% fetal bovine serum (FBS) (Gibco), 100 U/ml penicillin and 10 μg/ml streptomycin at 37°C. DMEM (Cellgro) was used for FAMPAC (human pancreatic cancer cells), HCT116 p53+/+ (human colorectal cancer cells), HCT116 p53−/− (human colorectal cancer cells), SK-OV-3 (human ovarian cancer cells), H1299 (human lung cancer cells), PC3 (human prostate cancer cells), PANC-1 (human pancreatic cancer cells), A431 (human epidermoid carcinoma cells), A549 (human lung cancer cells), MCF7 (human breast cancer cells), and TOV-112D (human ovarian cancer cells). DMEM/F12 (Cellgro) media was used for CAL-33 (human tongue cancer cells), KLE (human endometrial cancer cells), and SK-BR-3 (human breast cancer cells). RPMI 1640 (Cellgro) was used for AU-565 (human breast cancer cells), HuCCT1 (human bile duct cancer cells), and RXF393 (human renal cell carcinoma). MEM (Cellgro) was used for CCD-8Lu (lung fibroblast), WI-38 (lung fibroblast), and CCD-18Co (colon fibroblast). Various p53 status of mouse embryonic fibroblasts (MEF; wt p53, R172P, R172H and p53 null) were kindly provided by Z. Yuan (Harvard School of Public Health). The HCT116 p53−/− with tetracycline-inducible mutant p53-R175H and -R273H were gifts from X. Chen (University of California, Davis). p53 status of the cell lines were previously reported in reference websites (http://p53.free.fr/, http://www-p53.iarc.fr, and http://cancer.sanger.ac.uk/cancergenome/projects/cell_lines/) or confirmed as previously documented (Sjogren et al., 1996).
Plasmids and adenovirus constructs
The pcDNA3-Flag-HA mutant p53 R175H plasmid was kindly provided by X. Chen (University of California, Davis). p53-expressing adenovirus (Ad-p53) and GFP-expressing adenovirus (Ad-GFP) were generated as previously reported (He et al., 1998). For the PUMA-Luc reporter plasmid, p53 binding site of the PUMA promoter in a plasmid obtained from J. Manfredi (originally from Lin Zhang) was subcloned into pGL4.20-Luc vector (Yu et al., 2001).
Screening
H1299 cells were transfected with pcDNA3-Flag-HA mutant p53 R175H and luciferase reporter plasmid pGL4.20-PUMA-luc carrying the p53 responsive element of human PUMA promoter to establish a stable cell line, H1299-mtp53R175H/PUMA-luc cells. High throughput chemical screening was performed as previously described with modifications (Raj et al., 2011). Cells were plated in 25 μl of medium containing 9,000 cells per well into 384-well plate using an automated plate filler. Twenty-four hours after plating, 20,000 small molecules (Compound library from Chembridge) or 36,256 natural extracts (from the NCI's Natural Products Repository) were pin transferred from stock plates to the 384-well assay plates containing cells. The final concentration of small molecule and natural extract were 10 μM and 1 μg/ml, respectively. The assay plates were incubated with compounds or natural extracts for 15 hours, and luciferase activities were measured using 25 μl of luciferase assay reagent (Steady-Glo Luciferase Assay System, Promega). Luminescence was measured with an automated plate reader after shaking the assay plate at room temperature for 3 minutes to allow full signal generation from the lysed cells. All small molecules and natural extracts were tested in duplicates.
Chemicals
The following chemicals were purchased and dissolved in DMSO: CTM (Sigma-Aldrich), etoposide (Sigma-Aldrich), camptothecin (Sigma-Aldrich), PRIMA-1 (Sigma-Aldrich), MIRA-1 (Santa Cruz Biotechnology), Nutlin-3 (EMD Millipore), NSC 319726 (Selleck Chemicals).
Cell viability assay
Cell viability was determined by Sulforhodamine B Based In Vitro Toxicology Assay Kit (Sigma-Aldrich). Cells were plated in 6-well plates, and after reaching 60-70% confluency, the cells were treated with chemicals at indicated concentrations and hours in the figures and figure legends. Staining and quantitative analysis were performed according to the manufacturer's method. All experiments were performed in duplicates.
Total RNA extraction and quantitative RT-PCR analysis
Total RNA was extracted using Qiagen RNA extraction kit, converted to cDNA using iScript ™cDNA Synthesis Kit (Bio-Rad) and analyzed by q-PCR using gene-specific primers. Primer sequences used were as follows: p21 (FW: GGCGGCAGACCAGCATGACAGATT, RV: GCAGGGGGCGGCCAGGGTAT), PUMA (FW: GACCTCAACGCACAGTACGAG, RV: AGGAGTCCCATGATGAGATTGT), MDM2 (FW: GAATCATCGGACTCAGGTACATC, RV: TCTGTCTCACTAATTGCTCTCCT), 36B4 (FW: CAGATTGGCTACCCAACTGTT, RV: GGGAAGGTGTAATCCGTCTCC). Q-PCR was performed using an iCycler iQ™5 real time detection system (Bio-Rad Laboratories) with LightCycler 480 SYBR Green I Master (Roche) according to the manufacturer's instruction. The quantitative value was normalized by 36B4 expression. All experiments were performed in triplicates.
Immunoblotting
Cells were lysed with 1% Triton X-100 lysis buffer (20 mM Tris-HCl at pH 7.4, 5 mM EDTA, 10 mM Na4P2O7, 100 mM NaF, 2 mM Na3VO4, 1% Triton X-100) supplemented with protease inhibitor cocktail (Roche). Equal amount of total cellular proteins per sample was subjected to SDS-PAGE and transferred to a nitrocellulose membrane (Bio-Rad Laboratories). Antibodies for immunoblotting included anti-p53 (DO-1, Santa Cruz Biotechnology), p21 (Cell Signaling), PUMA (Cell Signaling), MDM2 (Calbiochem), Noxa (Calbiochem), Hsp40 (Santa Cruz Biotechnology), Hsp90 (Enzo Life Sciences), and β-actin (Sigma). Bands were detected using Western Lightning Plus ECL (PerkinElmer) or SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific). All experiments were performed independently at a minimum of three times.
siRNA and shRNA experiments
Vectors expressing shRNAs [pLKO.1-shLuc and pLKO.1-shp53 (TRCN0000003753, Sigma-Aldrich)] and siRNAs [siControl (12935-499, Invitrogen), sip53 (VHS40367, Invitrogen), siHsp40 (DNAJB1, Santa Cruz Biotechnology)] were used. All shRNA and siRNA constructs were introduced into cells by transfection with Lipofectamine 2000 or 3000 (Invitrogen) and Lipofectamine RNAiMax (Invitrogen), respectively, according to the manufacturer's protocol.
Chromatin immunoprecipitation (ChIP)
Cells were seeded in 10-cm dishes and began treatment of compound at 70% confluency for 6 hours. Cells were harvested, and ChIP was carried out according to the manufacturer's instructions using Chromatin Immunoprecipitation Assay Kit (EMD Millipore). Immunoprecipitation was performed at 4°C overnight with anti-p53 antibody (DO-1, Santa Cruz Biotechnology). Q-PCR amplifications were carried out using the following specific primers: p21 (FW: CTCACATCCTCCTTCTTCAG, RV: CACACACAGAATCTGACTCCC), PUMA (FW: GCGAGACTGTGGCCTTGTGT, RV: CGTTCCAGGGTCCACAAAGT), MDM2 (FW: GGTTGACTCAGCTTTTCCTCTTG, RV: GGAAAATGCATGGTTTAAATAGCC). The amount of co-precipitating DNA was normalized to inputs. All experiments were performed in triplicates.
Co-immunoprecipitation and mass spectrometry
Immunoprecipitation was performed as previously described (Namba et al., 2013) with modifications. Briefly, cells were washed with PBS and incubated with PBS containing 1 mM dithiobis[succinimidyl propionate] (DSP; Thermo Scientific) for 30 min at room temperature. The reaction was then quenched by 10 mM of Tris (pH 7.5) for 15 min at room temperature. Cells were lysed with 0.5% Triton X-100 lysis buffer (20 mM Tris-HCl at pH 7.4, 5 mM EDTA, 10 mM Na4P2O7, 100 mM NaF, 2 mM Na3VO4, 0.5% Triton X-100) with protease inhibitor cocktail (Roche). Immunoprecipitation was performed using anti-p53 antibody (DO-1, Santa Cruz Biotechnology) with Protein A/G PLUS-Agarose (Santa Cruz Biotechnology) or agarose-conjugated anti-p53 antibody (DO-1 AC, Santa Cruz Biotechnology). Immunoprecipitated samples were subjected to mass spectrometry at the Taplin Biological Mass Spectrometry Facility of Harvard Medical School.
Animal experiments
All animal experiments were reviewed and approved by the Massachusetts General Hospital Subcommittee on Research Animal Care. For xenograft tumor models, cancer cell lines TOV-112D (5×106 cells/mouse), CAL-33 (5×106 cells/mouse), H1299 (1×107 cells/mouse) and A431 (3×106 cells/mouse) were injected subcutaneously into the flanks of nude mice (NCr nude, 5-6 week old). Tumor dimensions were measured, and volume was calculated by length (L) and width (W) using the formula (volume = π/6 × L × W2). Tumors were allowed to grow to 50 mm3 prior to intraperitoneal injection of CTM at 1 mg/kg for indicated days shown in the figures and figure legends. Examined mice numbers are as follows: TOV-112D (DMSO control: n=7, and CTM-treated: n=6), CAL-33 (DMSO control: n=9, and CTM-treated: n=9), A431 (DMSO control: n=6, and CTM-treated: n=6), and H1299 (DMSO control: n=8, and CTM-treated: n=8).
Recombinant proteins
Full length wild type p53 and p53 R175H were subcloned into pGEX-6P-1 vector. Full length Hsp40 (DNAJB1) was subcloned into pET-28a vector. These bacterial expression constructs were used to transform Escherichia coli BL21 (New England BioLabs). Cells were induced with 0.05 mM isopropylthiogalactoside at 25 °C for 24 hours. Recombinant proteins of interes t were bound to GST beads (Glutathione Sepharose 4B, GE Healthcare Life Science) or His beads (TALON Metal Affinity Resin, Clontech). GST-R175H was incubated with PreScission Protease (GE Healthcare Life Science) to purify full length p53 proteins.
Biacore assay and analysis
Hsp40, mt p53 R175H or Hsp70 was immobilized on CM5 sensor chip by amine coupling, and compound binding was assayed through a BIAcore 3000 SPR system (GE Healthcare). CM5 sensor chips were coated with each of the purified proteins to a final resonance value of 1,000-2,000 response unit (RU). Various concentrations of compound in binding buffer (100 μg/ml BSA, 0.01 % Triton X-100, 2% DMSO in PBS, pH 7.4) were injected for 90 sec or 180 sec at a flow rate of 40 μl/min, and each series of experiments was tested in duplicates. Sensorgram analyses were carried out through BIAevaluation software (GE Healthcare). All of the experiments were performed in duplicates.
In vitro binding and pull-down assay
In vitro binding assay was performed as previously described (King et al., 2001; Takada et al., 2012) with some modifications. Briefly, purified recombinant p53 R175H (250 nM) or His-p53 R175H (55 nM, Thermo Scientific) and His-Hsp40 (1 μM) in 90 μl of assay buffer [phosphate buffered saline pH 7.4, bovine serum albumin (100 μg/ml), 0.01% Triton X-100] with protease inhibitor cocktail (Roche) were incubated with the indicated concentrations of CTM in 10 μl of DMSO at 4°C for overnight. Subsequently, 900 μl of binding buffer and p53 antibody (DO-1, Santa Cruz Biotechnology) with Protein A/G PLUS-Agarose (Santa Cruz Biotechnology) were added, followed by immunoprecipitation. All experiments were performed independently at a minimum of three times.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 6 Software using Student's t-test.
Highlights.
A Cell-Based Small Molecule Screen Identifies Ctm As A Mutant P53 R175H Reactivator
CTM inhibits growth of cancer cells harboring mutant p53 R175H in vitro and in vivo
CTM enables to transactivate p53 targets and restores MDM2 negative regulation
CTM binds Hsp40, enhances its binding to p53 R175H, and restores wt-like function
Significance.
TP53 gene is mutated in more than 50% of human cancers, including ovarian cancer, head and neck cancer, lung cancer, and so on. Among the most frequently found mutations is R175H, which alters the conformation of p53 and disrupts its negative regulation, as well as target gene induction, thus promoting tumorigenesis. Therefore, pharmacologically restoring mutant p53 R175H to wild-type activity is anticipated to be an effective strategy for targeting cancer. Here we report chetomin (CTM) as a novel reactivator of mutant p53 R175H identified by high-throughput cell-based screening of chemical compounds and natural product libraries. CTM restores DNA binding activity of mutant p53 R175H, and also restores MDM2-mediated negative regulation of mutant p53 R175H. Furthermore, our findings suggest that the mechanism of action of chetomin requires interaction with heat shock protein Hsp40. Therefore, we not only report a lead compound for mutant p53 reactivator drug discovery, but also present a novel approach that involves heat shock protein in restoring mutant p53 function for anti-cancer therapeutics.
Acknowledgments
We thank Z. Yuan for MEF cells, X. Chen and J. Manfredi for mutant p53 plasmids and tet-inducible cell lines, J. Manfredi for p53 binding site of PUMA promoter plasmid, M. Taipale and S. Lindquist for materials and help on some heat-shock protein activity experiments, H. Li and S.J. Lee for help on Biacore studies, and W. Gu for in vitro gel-shift experiments. M.H. and T.N. were supported by postdoctoral fellowships for research abroad from Japan Society for the Promotion of Science. M.H. was also supported by a research fellowship for research abroad from Uehara Memorial Foundation. T.R.R was supported by an F32 postdoctoral fellowship from the NIH (GM108415). The research was supported by NIH grants CA142805, CA149477, CA80058 and GM086258. Quantum-chemical calculations were run on the Odyssey cluster supported by the FAS Division of Science, Research Computing group at Harvard University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
M.H., S.Y.H. and S.C. performed experiments, analyzed the experimental data and wrote the manuscript. S.C. and T.R.R. fractionated the fungal extracts, identified CTM. S.B., K.W.Y., J.H.L., K.C., A.U.G., J.Z. and T.N. performed some of the critical experiments. V.K., D.J.N. and A.M. helped high throughput screening and analyzed the data. M.E.M. helped with research design. A.M., J.C., and S.W.L designed experiments, analyzed the data and co-wrote the paper, which was reviewed by all authors.
REFERENCES
- Allen MA, Andrysik Z, Dengler VL, Mellert HS, Guarnieri A, Freeman JA, Sullivan KD, Galbraith MD, Luo X, Kraus WL, et al. Global analysis of p53-regulated transcription identifies its direct targets and unexpected regulatory mechanisms. Elife. 2014;3:e02200. doi: 10.7554/eLife.02200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avantaggiati ML, Ogryzko V, Gardner K, Giordano A, Levine AS, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- Bates GJ, Nicol SM, Wilson BJ, Jacobs AM, Bourdon JC, Wardrop J, Gregory DJ, Lane DP, Perkins ND, Fuller-Pace FV. The DEAD box protein p68: a novel transcriptional coactivator of the p53 tumour suppressor. Embo J. 2005;24:543–553. doi: 10.1038/sj.emboj.7600550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock AN, Fersht AR. Rescuing the function of mutant p53. Nat Rev Cancer. 2001;1:68–76. doi: 10.1038/35094077. [DOI] [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- Bykov VJ, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, Bergman J, Wiman KG, Selivanova G. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med. 2002;8:282–288. doi: 10.1038/nm0302-282. [DOI] [PubMed] [Google Scholar]
- Bykov VJ, Issaeva N, Zache N, Shilov A, Hultcrantz M, Bergman J, Selivanova G, Wiman KG. Reactivation of mutant p53 and induction of apoptosis in human tumor cells by maleimide analogs. J Biol Chem. 2005;280:30384–30391. doi: 10.1074/jbc.M501664200. [DOI] [PubMed] [Google Scholar]
- Chai J-D, Head-Gordon M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008;10:6615–6620. doi: 10.1039/b810189b. [DOI] [PubMed] [Google Scholar]
- Chen F, Wang W, El-Deiry WS. Current strategies to target p53 in cancer. Biochem Pharmacol. 2010;80:724–730. doi: 10.1016/j.bcp.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Cherblanc F, Lo YP, De Gussem E, Alcazar-Fuoli L, Bignell E, He Y, Chapman-Rothe N, Bultinck P, Herrebout WA, Brown R, Rzepa HS, Fuchter MJ. On the determination of the stereochemistry of semisynthetic natural product analogues using chiroptical spectroscopy: desulfurization of epidithiodioxopiperazine fungal metabolites. Chem. Eur. J. 2011;17:11868–11875. doi: 10.1002/chem.201101129. [DOI] [PubMed] [Google Scholar]
- Cook KM, Hilton ST, Mecinovic J, Motherwell WB, Figg WD, Schofield CJ. Epidithiodiketopiperazines block the interaction between hypoxia-inducible factor-1alpha (HIF-1alpha) and p300 by a zinc ejection mechanism. J Biol Chem. 2009;284:26831–26838. doi: 10.1074/jbc.M109.009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BA, Coffey HA, Morin MJ, Rastinejad F. Pharmacological rescue of mutant p53 conformation and function. Science. 1999;286:2507–2510. doi: 10.1126/science.286.5449.2507. [DOI] [PubMed] [Google Scholar]
- Fujimoto H, Megumi S, Okuyama E, Ishibashi M. Immunomodulatory constituents from an ascomycete, Chaetomium seminudum. J Nat Prod. 2004;67:98–102. doi: 10.1021/np0302201. [DOI] [PubMed] [Google Scholar]
- Gaiddon C, Lokshin M, Ahn J, Zhang T, Prives C. A subset of tumor-derived mutant forms of p53 down-regulate p63 and p73 through a direct interaction with the p53 core domain. Mol Cell Biol. 2001;21:1874–1887. doi: 10.1128/MCB.21.5.1874-1887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe KK, Verma CS, Lane DP. Drugging the p53 pathway: understanding the route to clinical efficacy. Nat Rev Drug Discov. 2014;13:217–236. doi: 10.1038/nrd4236. [DOI] [PubMed] [Google Scholar]
- Hong B, Prabhu VV, Zhang S, van den Heuvel AP, Dicker DT, Kopelovich L, El-Deiry WS. Prodigiosin rescues deficient p53 signaling and antitumor effects via upregulating p73 and disrupting its interaction with mutant p53. Cancer Res. 2014;74:1153–1165. doi: 10.1158/0008-5472.CAN-13-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger AC, Fersht AR. Structural biology of the tumor suppressor p53. Annu Rev Biochem. 2008;77:557–582. doi: 10.1146/annurev.biochem.77.060806.091238. [DOI] [PubMed] [Google Scholar]
- Kessler J, Hahnel A, Wichmann H, Rot S, Kappler M, Bache M, Vordermark D. HIF-1alpha inhibition by siRNA or chetomin in human malignant glioma cells: effects on hypoxic radioresistance and monitoring via CA9 expression. BMC Cancer. 2010;10:605. doi: 10.1186/1471-2407-10-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King FW, Wawrzynow A, Hohfeld J, Zylicz M. Co-chaperones Bag-1, Hop and Hsp40 regulate Hsc70 and Hsp90 interactions with wild-type or mutant p53. Embo J. 2001;20:6297–6305. doi: 10.1093/emboj/20.22.6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung AL, Zabludoff SD, France DS, Freedman SJ, Tanner EA, Vieira A, Cornell-Kennon S, Lee J, Wang B, Wang J, et al. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell. 2004;6:33–43. doi: 10.1016/j.ccr.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, Meyerson M, Gabriel SB, Lander ES, Getz G. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Kim JW, Seo T, Hwang SG, Choi EJ, Choe J. SWI/SNF complex interacts with tumor suppressor p53 and is necessary for the activation of p53-mediated transcription. J Biol Chem. 2002;277:22330–22337. doi: 10.1074/jbc.M111987200. [DOI] [PubMed] [Google Scholar]
- Leroy B, Fournier JL, Ishioka C, Monti P, Inga A, Fronza G, Soussi T. The TP53 website: an integrative resource centre for the TP53 mutation database and TP53 mutant analysis. Nucleic Acids Res. 2013;41:D962–969. doi: 10.1093/nar/gks1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9:749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandinova A, Lee SW. The p53 pathway as a target in cancer therapeutics: obstacles and promise. Sci Transl Med. 2011;3:64rv61. doi: 10.1126/scitranslmed.3001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller PA, Vousden KH. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell. 2014;25:304–317. doi: 10.1016/j.ccr.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray-Zmijewski F, Lane DP, Bourdon JC. p53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ. 2006;13:962–972. doi: 10.1038/sj.cdd.4401914. [DOI] [PubMed] [Google Scholar]
- Namba T, Tian F, Chu K, Hwang SY, Yoon KW, Byun S, Hiraki M, Mandinova A, Lee SW. CDIP1-BAP31 complex transduces apoptotic signals from endoplasmic reticulum to mitochondria under endoplasmic reticulum stress. Cell reports. 2013;5:331–339. doi: 10.1016/j.celrep.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Izumi H, Imamura T, Takano H, Ise T, Uchiumi T, Kuwano M, Kohno K. Direct interaction of p53 with the Y-box binding protein, YB-1: a mechanism for regulation of human gene expression. Oncogene. 2000;19:6194–6202. doi: 10.1038/sj.onc.1204029. [DOI] [PubMed] [Google Scholar]
- Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rehman A, Chahal MS, Tang X, Bruce JE, Pommier Y, Daoud SS. Proteomic identification of heat shock protein 90 as a candidate target for p53 mutation reactivation by PRIMA-1 in breast cancer cells. Breast Cancer Res. 2005;7:R765–774. doi: 10.1186/bcr1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokaeus N, Klein G, Wiman KG, Szekely L, Mattsson K. PRIMA-1(MET) induces nucleolar accumulation of mutant p53 and PML nuclear body-associated proteins. Oncogene. 2007;26:982–992. doi: 10.1038/sj.onc.1209858. [DOI] [PubMed] [Google Scholar]
- Rosser MFN, Cyr DM. Do Hsp40s Act as Chaperones or Co- Chaperones? Springer; New York: 2007. Chapter 4. [Google Scholar]
- Sjogren S, Inganas M, Norberg T, Lindgren A, Nordgren H, Holmberg L, Bergh J. The p53 gene in breast cancer: prognostic value of complementary DNA sequencing versus immunohistochemistry. Journal of the National Cancer Institute. 1996;88:173–182. doi: 10.1093/jnci/88.3-4.173. [DOI] [PubMed] [Google Scholar]
- Staab A, Loeffler J, Said HM, Diehlmann D, Katzer A, Beyer M, Fleischer M, Schwab F, Baier K, Einsele H, et al. Effects of HIF-1 inhibition by chetomin on hypoxia-related transcription and radiosensitivity in HT 1080 human fibrosarcoma cells. BMC Cancer. 2007;7:213. doi: 10.1186/1471-2407-7-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, Stroedicke M, Zenkner M, Schoenherr A, Koeppen S, et al. A human protein-protein interaction network: a resource for annotating the proteome. Cell. 2005;122:957–968. doi: 10.1016/j.cell.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Sugito K, Yamane M, Hattori H, Hayashi Y, Tohnai I, Ueda M, Tsuchida N, Ohtsuka K. Interaction between hsp70 and hsp40, eukaryotic homologues of DnaK and DnaJ, in human cells expressing mutant-type p53. FEBS Lett. 1995;358:161–164. doi: 10.1016/0014-5793(94)01417-y. [DOI] [PubMed] [Google Scholar]
- Takada K, Zhu D, Bird GH, Sukhdeo K, Zhao JJ, Mani M, Lemieux M, Carrasco DE, Ryan J, Horst D, et al. Targeted disruption of the BCL9/beta-catenin complex inhibits oncogenic Wnt signaling. Science translational medicine. 2012;4:148ra117. doi: 10.1126/scitranslmed.3003808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman SA, Bugie E. Chaetomin, a New Antibiotic Substance Produced by Chaetomium cochliodes: I. Formation and Properties. J Bacteriol. 1944;48:527–530. doi: 10.1128/jb.48.5.527-530.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassman CD, Baronio R, Demir O, Wallentine BD, Chen CK, Hall LV, Salehi F, Lin DW, Chung BP, Hatfield GW, et al. Computational identification of a transiently open L1/S3 pocket for reactivation of mutant p53. Nat Commun. 2013;4:1407. doi: 10.1038/ncomms2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis A, Jung EJ, Wakefield T, Chen X. Mutant p53 exerts a dominant negative effect by preventing wild-type p53 from binding to the promoter of its target genes. Oncogene. 2004;23:2330–2338. doi: 10.1038/sj.onc.1207396. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- Yu X, Vazquez A, Levine AJ, Carpizo DR. Allele-specific p53 mutant reactivation. Cancer Cell. 2012;21:614–625. doi: 10.1016/j.ccr.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Villagra A, Peng L, Coppola D, Glozak M, Sotomayor EM, Chen J, Lane WS, Seto E. The ATDC (TRIM29) protein binds p53 and antagonizes p53-mediated functions. Mol Cell Biol. 2010;30:3004–3015. doi: 10.1128/MCB.01023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]