Abstract
Aims
In 2010, four subtypes (classical, proneural, mesenchymal, and neural) of glioblastoma multiforme (GBM) were defined by molecular genetic analyses. The objective of this study was to assess whether gliomas, independently of the type and grade, could be subdivided into protein-based subtypes.
Methods and results
A tissue microarray (TMA) approach was applied to incorporate tissue samples of low-grade and high-grade gliomas into five TMAs. High expression levels of epidermal growth factor receptor (EGFR), CD44, c-MER proto-oncogene tyrosine kinase (MERTK), platelet-derived growth factor receptor α, p53, oligodendrocyte transcription factor 2 (OLIG2) and isocitrate dehydrogenase 1 with the R132H mutation were assessed using immunohistochemistry (IHC). Glioma could be subdivided into four subtypes by IHC. The majority of the low-grade gliomas were of the proneural subtype, i.e. high p53 expression (63% of grade II). The classical subtype, with high EGFR and low p53 expression, was most common in GBMs (39%), followed by the proneural (29%) and mesenchymal (with high CD44 and MERTK expression) (29%) subtypes, a frequency that is in line with previously published data based on molecular genetics.
Conclusions
Assessment of the expression of the five proteins EGFR, CD44, MERTK, p53 and OLIG2 is sufficient for subtyping gliomas, and can be recommended for implementation in clinical practice for both low-grade and high-grade gliomas.
Keywords: glioma, immunohistochemistry, protein expression, subtyping
Introduction
Approximately 2% of all adult malignancies are brain tumours, and 80% of these are gliomas.1 They can occur at any age, regardless of gender or ethnicity. Currently, there is no cure for glioma, and, in spite of aggressive therapy, the morbidity is high. The most aggressive type of glioma, WHO grade IV, is also referred to as glioblastoma multiforme (GBM).2,3
According to the cell type, gliomas are divided into astrocytic, oligodendroglial, and ependymal.4 The neuropathological grading of gliomas is based upon the St Anne/Mayo system,5 including the assessment of features such as (i) cellular atypia (WHO grade II), (ii) mitotic figures (WHO grade III), (iii) endothelial proliferation, and/or (iv) necrosis (WHO grade IV), all visualized with haematoxylin and eosin (H&E) staining. While growing, these tumours (with the exception of ependymoma) tend to diffusely infiltrate the surrounding brain tissue, as recently demonstrated for gliomas of WHO grade II/III and secondary GBM, with isocitrate dehydrogenase 1 (IDH1) bearing the R132H mutation (IDH1R132H).6–8
Accumulation of genetic alterations is involved in the initiation and progression of gliomas, leading to great genetic heterogeneity of these tumours.9–12 In 2006, three molecular subtypes of GBM were defined on the basis of molecular profiles and the protein expression of tumour cells, i.e. proneural, proliferative, and mesenchymal.13 Later, a number of studies were published in which GBMs were divided into different subtypes on the basis of the activity of signal transduction pathways,9 gene expression analysis,14 or protein expression levels15,16 (Table 1).
Table 1.
High expression of proteins defining previously proposed subtypes of glioblastoma multiforme (GBM)
| Proposed subtypes |
|||||
|---|---|---|---|---|---|
| Proteins reported to be highly expressed | Philips et al.13 | Brennan et al.9 | Verhaak et al.14 | Motomura et al.16 | Le Mercier et al.15 |
| Epidermal growth factor receptor (EGFR) | Proliferative and Mesenchymal GBM | EGFR core | Classical GBM | Mixed | Classical like |
| CD44 | Mesenchymal GBM | Mesenchymal GBM | Astrocyticmesenchymal, Mixed | ||
| Proto-oncogene tyrosine-protein kinase MER | Mesenchymal GBM | ||||
| Isocitrate dehydrogenase 1 mutation | Proneural GBM | Oligodendrocyte precursor type (OPT) | |||
| OLIG2 | Proneural GBM | PDGF core | Proneural GBM | Differentiated oligodendro- cyte type and OPT | |
| P53 | Proneural GBM | OPT | Proneural like | ||
| Platelet derived growth factor (PDGF) receptor α | PDGF core | Proneural GBM | OPT | Proneural like | |
One of the proteins listed, which was of interest in all of the previous reports, is epidermal growth factor receptor (EGFR), which is known to be involved in cellular processes such as proliferation, survival, angiogenesis, invasion, and metastasis. Other proteins that have been reported to be characteristic of certain GBM subtypes are the cell surface proteins CD44, c-mer proto-oncogene tyrosine kinase (MERTK), and platelet-derived growth factor receptor α (PDGFRA).9,13–16 Furthermore, the tumour suppressor protein p53,14–16 oligodendrocyte transcription factor 2 (OLIG2)9,13,14,16 and IDH1 with the R132H mutation14,16 have also been reported to be of interest.
The current strategy for diagnosis of a brain tumour incorporates assessment of the histological type (astrocytic/oligodendrocytic/ependymal) and assessment of the grade (WHO grading system). Recent observations have indicated that the molecular signature of the tumour also appears to be of significance.13,14,17
The objective of the current study was to attempt to identify different glioma subtypes by assessing protein expression in surgical tissue samples of adult gliomas, using immunohistochemistry (IHC) and a tissue microarray (TMA) strategy. The patients in the cohort were all inhabitants of a defined central region of Sweden.
Materials and methods
Selection of Material
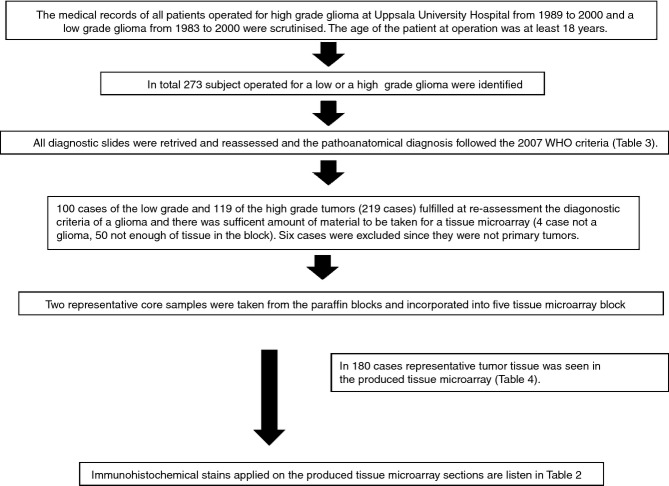
The general design of the study is delineated in Figure1. The medical records at the Department of Pathology and Cytology, Uppsala University Hospital, were searched for subjects with a diagnosis of low-grade or high-grade glioma of astrocytic or oligodendrocytic phenotype who underwent an operation between 1983 and 2000 (low grade glioma) or between 1989 and 2000 (high grade glioma). In total, 273 subjects were identified. All archived slides were retrieved and retrospectively reviewed, blind to the original diagnosis. The type and grading followed the WHO criteria from 2007.4 The material included small biopsies, and subtotal or presumably total resections. The patients were variably treated, i.e. no treatment, radiotherapy only, chemotherapy only, or a combination of radiotherapy and chemotherapy.
Figure 1.
Flowchart summarizing the logistics of the study.
Tissue Microarray Construction
In each case, two representative tumour regions were identified and marked on the original H&E-stained slides. TMAs were prepared as previously described.18 In total, five blocks with core samples were produced. The recipient blocks were cut into consecutive 4-μm-thick sections, and placed on SuperFrost Plus slides (Gerhard Menzel GmbH, Braunschweig, Germany); thereafter, the first section of each block was stained with H&E to assess the representativeness of the core, and this was followed by typing and grading of the tumour according to the WHO 2007 recommendations.
Immunohistochemistry
The antibodies, dilutions and pretreatments used are shown in Table 2. Subsequently, serial paraffin wax-embedded sections were rehydrated; endogenous peroxidase activity was quenched with 3% hydrogen peroxide for 10 min, and antigen retrieval was performed. Unspecific binding sites were blocked with Background Sniper (Biocare Medical, Concord, CA, USA) for 10 min. Thereafter, sections were incubated with primary antibodies overnight at 4°C. The PowerVision detection system (Immunologic, Duiven, the Netherlands) was used with the Romulin AEC or DAB chromogen kit (Biocare Medical).
Table 2.
Antibodies listed in alphabetical order, including dilution and pre-treatments applied
| Antibody | Source | Code | Clone | Epitope | Pretreatment | Dilution |
|---|---|---|---|---|---|---|
| CD44 cell-surface glycoprotein | Santa Cruz | sc-7297 | Polyclonal | Full-length | Citrate Buffer, pH 6.0, MW 3 × 5 min | 1:100 |
| Proto-oncogene tyrosine-protein kinase MER | Novus Biologicals | NB 110-57199 | Y323 | N-terminus | Citrate Buffer, pH 6.0, MW 3 × 5 min | 1:50 |
| Epidermal growth factor receptor | Invitrogen | 28-0005 | 31G7 | Extracellular domain | Proteinase K | 1:100 |
| Glial fibrillary acidic protein | DakoCytomation | Z0334 | Polyclonal | Full-length | 0.03% protease XXIV | 1:500 |
| Isocitrate dehydrogenase 1 | Dianova | DIA H09 | H09 | aa125-137 | Tris-EDTA, pH 9.0,MW 3 × 5 min | 1:500 |
| Ki67 | DakoCytomation | M7240 | MIB-1 | 345 and 395 Kd | Citrate Buffer, pH 6.0,MW 3 × 5 min | 1:100 |
| Microtubule-Associated protein 2 | Sigma-Aldrich | M4403 | HM-2 | Full-length | Citrate Buffer, pH 6.0,MW 3 × 5 min | 1:500 |
| Oligodendrocyte transcription factor 2 | Abnova | H00010215-M03 | 3C9 | Full-length | Citrate Buffer, pH 6.0,MW 3 × 5 min | 1:100 |
| Platelet derived growth factor receptor α | Santa Cruz | sc-338 | Polyclonal | C-terminus | Tris-EDTA, pH 9.0,MW 3 × 5 min | 1:100 |
| Tumour protein 53 | DakoCytomation | M7001 | DO-7 | Full-length | Citrate Buffer, pH 6.0,MW 3 × 5 min | 1:50 |
Light Microscopy
A case was included when at least 50% of the total area of at least one of the core samples remained on the slide, and if the core was representative regarding the original slide, i.e. tumour present.
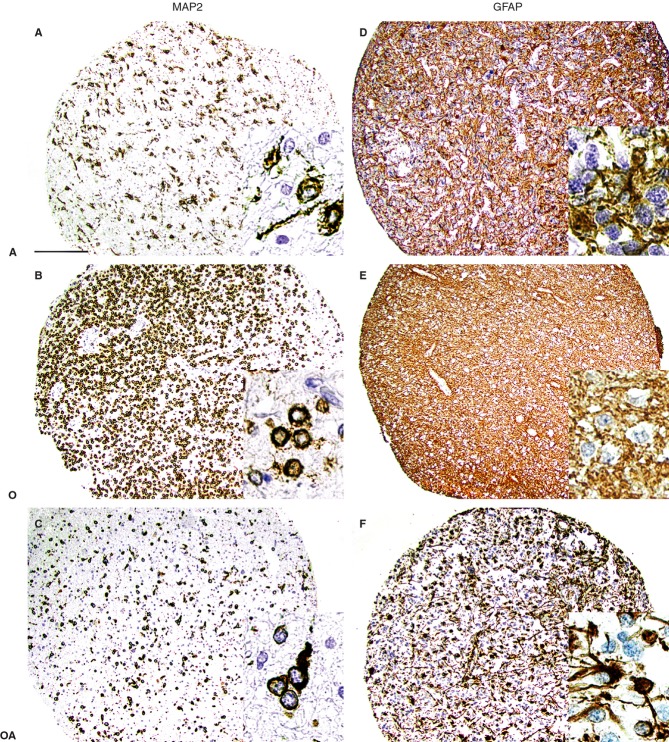
All IHC stains were assessed at magnifications of ×40 to ×400. The staining pattern of neoplastic cells with glial fibrillary acidic protein (GFAP) and microtubule-associated protein 2 (MAP2) was used to confirm the phenotype of the tumour cells, i.e. astrocytic, oligodendrocytic, or mixed (Figure2). Nuclear staining (Ki67 and p53) was scored on a scale from 0 to 3, as follows: 0, absence of labelled nuclei in the core; 1, up to 10 positive nuclei; 2, 10–30 positive nuclei; and 3, >30 positive nuclei. Scores of 2 and 3 were considered to indicate high expression of the protein. In sections stained for OLIG2, PDGFRA, CD44, MERTK, EGFR and IDH1R132H, the presence of nuclear, membrane or cytoplasmic staining was scored on a scale from 0 to 3, as follows: 0, absence of immunoreactivity (IR); 1, up to 10% of cells immunoreactive; 2, 10–50% of cells immunoreactive; and 3, >50% of cells immunoreactive. A score of 3 was considered to indicate high expression of these proteins. To confirm the reliability of the assessment of IHC stains, sections were reassessed several times by at least two evaluators blinded to the original results, and a consensus score was ascribed to each tumour sample.
Figure 2.
Staining results obtained with antibodies against microtubule-associated protein 2 (MAP2) and glial fibrillary acidic protein (GFAP). A, astrocytic differentiation; O, oligodendrocytic differentiation; OA, oligoastrocytic differentiation. Note the difference at both low and high (insert) magnification regarding the compartmentalization of the labelling (cell membrane versus cytoplasm) and the shape of the cell, depending on the glioma subtype (astrocytic versus oligodendrocytic) (A–C). Note the absence of GFAP labelling of the cell membrane in pure oligodendrocytic tumours (insert in E), in contrast to astrocytic or mixed tumours (D,F). Bar: 200 μm.
Photographs were taken with an Olympus BX46 microscope equipped with an Olympus DP72 digital camera, and images acquired using Cell Acquisition software (Olympus Optical, Tokyo, Japan). IBM SPSS Statistics 20 (IBM Corporation, Armonk, NY, USA) was used for statistical analysis. For significant differences, the non-parametric Kruskal–Wallis rank test and Spearman's correlation tests were used.
Results
Two hundred and nineteen cases were reassessed. The male/female ratio was 1.5, and the age at operation ranged from 19 to 80 years (mean ± standard error of the mean, 48 ± 1 years). The agreement rate for reassessment of original slides and following the WHO 2007 criteria regarding low-grade glioma was 77% (n = 128), and that regarding high-grade glioma was 98% (n = 91) (Table 3). Regarding grade, the highest agreement rate was noted for grade IV tumours (82%), and the poorest for grade III tumours (9%). A significant proportion of original grade III tumours were reassessed as being grade IV tumours, i.e. GBM. Regarding subtype, the highest agreement rate was for astrocytomas (76%), and the poorest was for oligoastrocytomas (7%).
Table 3.
Agreement rate between original diagnosis and the diagnosis given upon reassessment of original slides, following current WHO criteria
| Low-grade glioma |
High-grade glioma |
||||||
|---|---|---|---|---|---|---|---|
| AC | O | OAC | aAC | aO | GBM | Σ | |
| Original diagnosis | |||||||
| Astrocytoma (AC) | 36 | 5 | 8 | 2 | 1 | 128 | |
| Oligodendroglioma (O) | 8 | 19 | 3 | 5 | |||
| Oligoastrocytoma (OAC) | 17 | 7 | 3 | 5 | 2 | 7 | |
| Anaplastic (a) AC | 4 | 3 | 34 | 91 | |||
| aOAC | 4 | ||||||
| Glioblastoma (GBM) | 2 | 6 | 36 | ||||
| Glioma not defined | 2 | ||||||
| Σ | 100 | 119 | 219 | ||||
100% agreement given in bold.
Core samples from the 219 cases were included in the TMA. Most samples were surgical (67%), and the cores were taken from viable representative tumour areas. When the produced TMA sections were examined, in 39 cases there was insufficient material for assessment. Comparing results from reassessment of the TMA cores and the whole sections, agreement regarding the grade of the tumour was 100% (Table 4), whereas the highest agreement regarding subtype was for astrocytomas (87%).
Table 4.
Agreement rate between the diagnosis given upon reassessment of the whole original section and at the assessment of the tissue microarray (TMA) cores
| Diagnosis assessing TMA core |
||||||||
|---|---|---|---|---|---|---|---|---|
| Low-grade glioma |
High-grade glioma |
|||||||
| AC | O | OAC | aAC | aO | aOAC | GBM | Σ | |
| Diagnosis at reassessment | ||||||||
| Astrocytoma (AC) | 34 | 3 | 10 | 76 | ||||
| Oligodendroglioma (O) | 7 | 7 | 9 | |||||
| Oligoastrocytoma (OAC) | 2 | 2 | 2 | |||||
| Anaplastic(a) AC | 10 | 1 | 4 | 104 | ||||
| aO | 7 | 1 | 8 | |||||
| aOAC | 1 | |||||||
| Glioblastoma (GBM) | 72 | |||||||
| Σ | 76 | 104 | 180 | |||||
100% agreement given in bold.
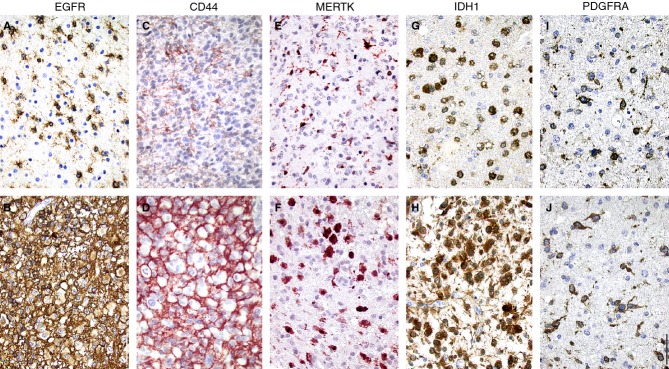
Representative photomicrographs of the staining results from application of the antibodies listed in Table 2 are presented in Figures2 and 3, and the results are summarized in Table 5. Only those cases that showed high protein expression are included in Table 5. The group of anaplastic oligodendrogliomas contained only two cases, and these were therefore excluded from the subsequent analyses. In 15 cases (8% of all tumours), two of which were GBMs (3% of GBMs), none of the proteins assessed was present to a high extent.
Figure 3.
Immunohistochemical detection of epidermal growth factor receptor (EGFR) and c-MER tyrosine kinase (MERTK) in anaplastic oligodendroglioma grade III, CD44 in glioblastoma multiforme grade IV, isocitrate dehydrogenase 1 with R132H mutation (IDH1) in oligodendroglioma grade II, and platelet-derived growth factor receptor α (PDGFRA) in astrocytoma grade II. A staining pattern with moderate immunoreactivity is shown in the upper panel (A,C,E,G,I), and a staining pattern with strong labelling is shown in the lower panel (B,D,F,H,J). Bar: 50 μm.
Table 5.
Percentages of cases with high protein expression while applying immunohistochemistry
| Diagnosis in TMA | n | EGFR | CD44 | MERTK | IDH1R132H | OLIG2 | P53 | PDGFRA |
|---|---|---|---|---|---|---|---|---|
| Astrocytoma (AC) II | 43 | 3010 | 245 | 84 | 562 | 505 | 314 | 345 |
| Oligodendroglioma (O) II | 12 | 384 | 03 | 134 | 783 | 254 | 504 | 145 |
| Oligoastrocytoma (OAC) II | 21 | 764 | 101 | 151 | 951 | 651 | 701 | 51 |
| Low-grade gliomas Σ | 76 | 4518 | 169 | 109 | 706 | 5110 | 459 | 2311 |
| AC III | 18 | 502 | 33 | 6 | 61 | 67 | 39 | 44 |
| O III | 2 | 1001 | 01 | 01 | 1001 | 01 | 01 | 01 |
| OAC III | 12 | 58 | 91 | 33 | 58 | 50 | 67 | 8 |
| Glioblastoma multiforme | 72 | 583 | 43 | 15 | 111 | 753 | 41 | 4 |
| High-grade gliomas Σ | 104 | 576 | 372 | 161 | 262 | 704 | 441 | 121 |
n, Number of cases; EGFR, epidermal growth factor receptor; MERTK, proto-oncogene tyrosine-protein kinase MER; IDH1R132H, isocitrate dehydrogenase 1 mutation R132H; PDGFRA, platelet derived growth factor receptor α.
Superscript indicates the number of cases not assessed.
The number of EGFR-positive cells varied significantly, from a few scattered strongly immunoreactive cells per core to labelling of almost all cells (Figure3A,B). Strong EGFR IR was noted in all glioma subtypes, independently of the WHO grade (Table 5). Forty-five per cent of low-grade gliomas, 57% of high-grade gliomas and 58% of GBMs showed high EGFR IR.
Sixteen per cent of low-grade gliomas, 37% of high-grade gliomas and 43% of GBMs showed high CD44 IR (Figure3C,D; Table 5), whereas strong CD44 IR was not seen in oligodendrogliomas, independently of grade.
Ten per cent of low-grade gliomas, 16% of high-grade gliomas and 15% of GBMs showed high MERTK IR (Figure3E,F; Table 5).
Both astrocytic and oligodendrocytic tumours were labelled using IDH1R132H antibody (Figure3G,H; Table 5). Sixty-seven per cent of grade II/III tumours and 11% of GBMs showed high IDH1R132H IR.
Fifty-one per cent of low-grade gliomas, 70% of high-grade gliomas and 75% of GBMs showed OLIG2-immunoreactive nuclei (Table 5).
Forty-five per cent of low-grade gliomas, 44% of high-grade gliomas and 41% of GBMs showed p53-immunoreactive nuclei (Table 5). The tumours of mixed phenotype (oligoastrocytic tumours) were most frequently p53-immunoreactive (69%), whereas only 33% of astrocytic tumours were p53-immunoreactive.
Platelet-derived growth factor receptor α antibody primarily labelled scattered cells in a tumour (Figure3I,J). Twenty-three per cent of low-grade gliomas, 12% of high-grade gliomas and 4% of GBMs showed PDGFRA-immunoreactive cells (Table 5). In 38% of astrocytic tumours, PDGFRA-immunoreactive cells were seen, as compared with 6% of oligoastrocytomas.
In order to assess the patterns of association, a correlation test was carried out. Only correlations that were high (r ≥ 0.4) and significant (P < 0.05) are shown in Table 6. The highest and most significant positive correlation was noted for EGFR/MERTK in grade II oligodendrogliomas, and the most significant negative correlation was noted for MERTK/IDH1R132H in grade III astrocytomas.
Table 6.
Correlation between protein expressions in different types of glioma
| Spearman's rank order of correlation |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrocytoma (A) grade II | A III | A Σ | Oligodendroglioma grade II | Oligoastrocytoma (OA) grade II | OA III | OA Σ | Glioblastoma multiforme | ||||||
| Proteins | r | P | r | P | r | P | r | P | R | P | r | r | r |
| EGFR/CD44 | NS | NS | NS | 0.699 | * | NS | NS | NS | NS | ||||
| EGFR/MERTK | NS | NS | NS | 0.883 | ** | NS | NS | NS | NS | ||||
| EGFR/IDH1R132H | NS | NS | NS | NS | NS | NS | NS | NS | |||||
| EGFR/OLIG2 | NS | NS | NS | 0.702 | * | NS | NS | NS | NS | ||||
| EGFR/P53 | NS | NS | NS | 0.654 | * | NS | NS | NS | NS | ||||
| EGFR/PDGFRA | NS | NS | NS | 0.787 | * | NS | NS | NS | NS | ||||
| CD44/MERTK | NS | NS | 0.412 | ** | 0.763 | * | 0.457 | * | NS | NS | NS | ||
| CD44/OLIG2 | NS | NS | NS | 0.730 | * | NS | NS | NS | NS | ||||
| CD44/P53 | 0.450 | ** | NS | NS | NS | NS | NS | NS | NS | ||||
| CD44/PDGFRA | 0.417 | ** | NS | NS | 0.651 | * | NS | NS | NS | NS | |||
| MERTK/IDH1 R132H | NS | −0.593 | ** | NS | NS | NS | NS | NS | NS | ||||
| MERTK/OLIG2 | NS | NS | NS | 0.801 | ** | NS | NS | NS | NS | ||||
| MERTK/PDGFRA | 0.425 | ** | NS | NS | 0.701 | * | NS | NS | NS | NS | |||
| IDH1/OLIG2 | NS | NS | NS | 0.594 | * | NS | NS | NS | NS | ||||
| OLIG2/PDGFRA | NS | NS | NS | 0.629 | * | NS | NS | NS | NS | ||||
| P53/OLIG2 | 0.446 | ** | NS | 0.400 | ** | 0.890 | ** | 0.501 | * | NS | NS | NS | |
| P53/PDGFRA | 0.502 | ** | 0.477 | * | 0.492 | ** | 0.684 | * | NS | NS | NS | NS | |
NS, Non significant; EGFR, epidermal growth factor receptor; MERTK, proto-oncogene tyrosine-protein kinase MER; IDH1 R132H, isocitrate dehydrogenase 1 mutation R132H; PDGFRA, platelet derived growth factor receptor α.
*P < 0.01.
**P < 0.001.
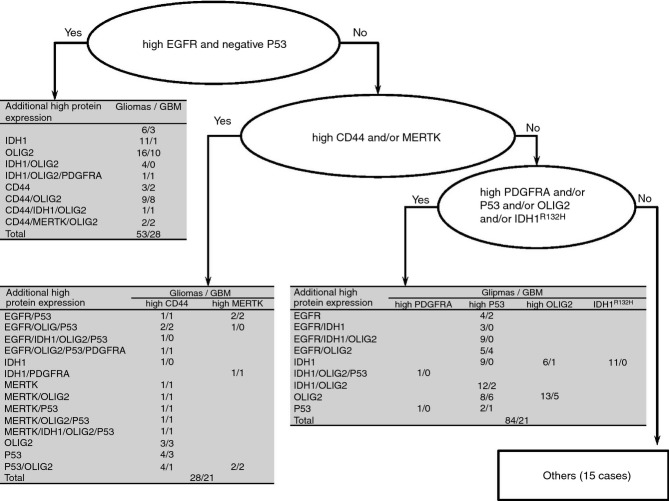
An algorithm was used for subtyping the glioma samples on the basis of their protein expression (Figure4; Table 7). The number of tumours with a pattern reminiscent of the classical and mesenchymal molecular subtypes increased with increasing WHO grade. In contrast, 63% of WHO grade II tumours showed a pattern reminiscent of the proneural molecular subtype, and 80% of the tumours that did not show overexpression of one of the analysed proteins were also of WHO grade II.
Figure 4.
Algorithm for grouping of the gliomas on the basis of their protein expression, reminiscent of the molecular subtypes defined by Verhaak et al.,14 (classical = high EGFR and negative p53; mesenchymal = high CD44 and/or MERTK; proneural = high PDGFRA and/or p53 and/or OLIG2 and/or IDH1) and a summary of the high protein expression observed in 180 gliomas.
Table 7.
Low and high-grade gliomas and the subtypes defined by protein expression
| WHO grade |
||||||||
|---|---|---|---|---|---|---|---|---|
| II | III | IV | Total | |||||
| Subtype14 | n | Age, years (range) | n | Age, years (range) | n | Age, years (range) | n | Age, years (range) |
| Classical | 14 | 50 ± 4 (31–71) | 11 | 54 ± 2 (34–62) | 28 | 61 ± 2 (35–77) | 53 | 56 ± 2 (31–77) |
| Proneural | 48 | 37 ± 1 (19–63) | 15 | 48 ± 4 (33–77) | 21 | 55 ± 4 (23–79) | 84 | 43 ± 2 (19–79) |
| Mesenchymal | 2 | 43 ± 0.5 (42–43) | 5 | 42 ± 4 (32–54) | 21 | 59 ± 3 (22–80) | 28 | 55 ± 3 (22–80) |
| Other | 12 | 44 ± 4 (25–70) | 1 | 48 | 2 | 45 ± 19 (26–64) | 15 | 45 ± 3 (25–70) |
Age – mean ± SE.
In order to detect significant differences in protein expression between groups, the non-parametric Kruskal–Wallis rank test was applied. Detailed results are given in Table 8. EGFR, p53 and CD44 IR frequently showed significant differences between subtypes and WHO grades. No significant differences were noted for PDGFRA, and no highly significant differences (<0.005) were noted for microtubule-associated protein 2 (MAP2).
Table 8.
Significant correlation of protein expression by means of Kruskall–Wallis rank test in different glioma subtypes and glioma WHO grades
| Significant correlations Kruskal–Wallis rank test |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteins assessed |
||||||||||||
| Subtypes14 | Correlated to | WHO grade | EGFR | IDH1R132H | OLIG2 | P53 | PDGFRA | CD44 | MERTK | GFAP | Ki67 | MAP2 |
| Classical high EGFR and negative P53 n = 53 | Proneural | II–IV | 0.000 | 0.000 | 0.006 | |||||||
| Mesenchymal | IV (GBM) | 0.000 | 0.000 | |||||||||
| Other | III–IV | 0.000 | 0.000 | |||||||||
| n = 98 | II | 0.000 | 0.001 | |||||||||
| Proneural high IDH1R132H and/or high OLIG2 and/or high P53 and/or high PDGFRA and/or n = 84 | Classical | II–IV | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.041 | 0.003 | |||
| Mesenchymal | IV (GBM) | 0.003 | 0.001 | 0.000 | 0.022 | 0.045 | ||||||
| Other | III–IV | 0.000 | 0.008 | 0.000 | 0.000 | 0.008 | ||||||
| n = 117 | II | 0.007 | 0.013 | 0.000 | 0.000 | 0.005 | ||||||
| Mesenchymal high CD44 and/or high MERTK n = 28 | Classical | II–IV | 0.000 | 0.000 | 0.000 | 0.008 | ||||||
| Proneural | IV (GBM) | 0.003 | 0.001 | 0.000 | 0.000 | 0.009 | ||||||
| Other | III–IV | 0.002 | 0.000 | 0.000 | 0.000 | 0.021 | ||||||
| n = 160 | II | 0.001 | 0.008 | |||||||||
| Other all low n = 15 | Classical | II–IV | 0.000 | 0.001 | 0.000 | 0.001 | 0.031 | 0.000 | 0.008 | |||
| Proneural | IV (GBM) | |||||||||||
| Mesenchymal | III–IV | 0.037 | ||||||||||
| n = 165 | II | 0.007 | 0.000 | 0.001 | 0.002 | 0.000 | ||||||
EGFR, Epidermal growth factor receptor; MERTK, proto-oncogene tyrosine-protein kinase MER; IDH1R132H, isocitrate dehydrogenase 1 mutation R132H; PDGFRA, platelet derived growth factor receptor α.
Italics signify ranges between P = 0.05–0.005.
Discussion
In 2006, it was proposed that malignant glial tumours such as GBM should be subtyped on the basis of their protein constituents. Thereafter, in 2010, subtyping of GBM into four defined molecular subgroups (classical, mesenchymal, proneural, and neural) was proposed (Table 9).14
Table 9.
Molecular subtypes of glioblastoma and their definitions as described by Verhaak et al.14
| Subtypes | High expression | Gene loss |
|---|---|---|
| Classical | EGFR | CDKN2A, PTEN, TP53 |
| Mesenchymal | CD44, CHI3L1/YKL40, MERTK, MET, NFκB pathway, TNF family | NF1, PTEN |
| Proneural | ASCL1, DCX, DLL3, IDH1R132H, NKX2-2,OLIG2, PDGFRA, SOX, TCF3, TCF4, TP53 mutation | |
| Neural | GABRA1, NEFL, SLC12A5, SYT1 | |
ASCL1, Achaete-scute homolog 1; CHI3L1/YKL40, chitinase 3-like protein 1; DCX, doublecortin; DLL3, delta-like 3; EGFR, epidermal growth factor receptor; GABRA1, gamma-aminobutyric acid receptor subunit alpha-1; IDH1R132H, isocitrate dehydrogenase 1 mutation R132H; MET, c-Met proto-oncogene; MERTK, proto-oncogene tyrosine-protein kinase MER; NEFL, neurofilament light polypeptide; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; NKX2-2, homeobox protein Nkx-2.2; OLIG2, oligodendrocyte transcription factor 2; PDGFRα, platelet-derived growth factor receptor alpha; SLC12A5, potassium-chlorid transporter member 5; SYT1, synaptotagmin 1; TCF, transcription factor; TNF, tumour necrosis factor; TP53, tumour protein 53.
Here, applying the IHC technique, we assessed the expression of some of the proteins that have been reported to be of significance for the subtyping of GBMs.9,13,16 According to our results, different protein-based ‘subtypes’ can indeed be identified by the application of IHC, independently of the grade or the histological phenotype of the tumour. Our findings indicate that assessment by means of a selective IHC panel should probably be included as part of routine practice in neuropathological diagnostics.
The molecular signature was not compared with the IHC profile in this study, owing to the lack of frozen tissue. Nevertheless, we believe that it was worth carrying out the study in order to assess the usability of archived paraffin-embedded material for the assessment of protein expression with IHC. Moreover, the frequency of cases with a certain protein subtype in our study was well in line with the frequency obtained in assessment of subtypes using molecular techniques.14
We did not include any data regarding the clinical outcome, owing to the heterogeneity of the included cases. The operations were performed within a timeframe of 17 years, and included material obtained using various surgical techniques (whole or partial resections of tumour or diagnostic biopsy); the consequent treatment comprised no treatment, radiotherapy only, different protocols of chemotherapy, or both radiotherapy and chemotherapy. It has previously been reported, regarding meningiomas, that the above issues are of significance when protein expression is assessed in relation to outcome.19 Thus, to assess reliable tumour characteristics (morphology, protein expression, and molecular genetics) in the future, prospective studies should be undertaken. The patients should be asked to provide consent for specific sampling of the tissue in addition to diagnostic material (fresh tissue for cell cultures, and fresh frozen tissue), collection of medical data including information regarding the surgical technique (biopsy, partial resection, and total resection), radiological findings at different stages, and treatment strategy. An autopsy should also be carried out, if possible, to assess the spread of a certain type of tumour. The constructed database should later be completed with information regarding survival. Previous reports have indicated that there is a certain level of discrepancy in diagnoses of tumours when the original and the reassessment results are compared.20–23 Both down-grading and up-grading of tumours have been reported. In line with previous reports, we also noted a certain level of discrepancy (85% agreement rate) when the archival material included in this study was reassessed. The primary reason for the discrepancy observed is probably the evolution of diagnostic criteria over time, as previously reported.24 Our findings, however, emphasize that, when studies are undertaken on archived material sampled from one or several centres, a reassessment of the diagnosis should be considered as an obligatory requirement to secure high quality and reliability of the work.
During the last few years, the number of studies applying TMAs in tumour research has increased dramatically. There are several benefits of using the TMA method, specifically: there is the possibility of including a large number of cases; each sample is handled methodologically in a similar way; the limited area to be assessed ensures a certain level of assessment uniformity; and, finally, assessment of thousands of cases is economically feasible. Thus, today, archival tumour tissues are often used for TMA construction. The use of the TMA technique in this study was successful; only 17% of the originally included cases were lost during processing, the tissue in the core samples was considered to be representative of the tumour in the whole section, and there was 100% agreement regarding the WHO grade (whole section versus TMA core).
Our aim was to assess whether gliomas could be subdivided into IHC types on the basis of their protein expression. Fifty-eight per cent of GBMs in this study were EGFR-immunoreactive, a finding that is within the previously reported range (40–73%).15,25–28 EGFR IHC results have been shown to reflect molecular genetic data; thus, EGFR IHC can certainly be applied.29–31 In our sample, 29% of all gliomas and 39% of all GBMs showed an IHC pattern (high EGFR expression; low p53 expression) reminiscent of the classical type. The frequency for GBM in our study is somewhat higher than previously reported (27% and 35%).15 This discrepancy in percentages might be attributable to selection bias. The expression of EGFR was easily demonstrated by means of IHC, and could therefore be routinely assessed. On the basis of our results, this approach should probably be applied in cases not only of high-grade glioma, but also of low-grade glioma. The significance of this subtyping in low-grade gliomas should be further investigated. It is noteworthy that the enrichment of proteins of significance for the proneural subtype of glioma has previously been described for WHO grade II gliomas.17
In this study, 16% of all gliomas and 29% of GBMs showed an IHC pattern reminiscent of the mesenchymal molecular subtype. The frequency for GBM in this study is the same as previously reported.14 It is notable that only a few low-grade gliomas showed this IHC profile, indicating that expression of these proteins might be of significance only in cases of GBM. Verhaak et al.14 defined the proneural subtype of GBM as a tumour with high expression of PDGFRA and/or OLIG2 and/or p53 and/or IDH1R132H. This subtype of GBM has previously been reported as representing approximately 20–30% of all GBMs.14 Interestingly, when IHC was applied, 29% of GBMs showed an IHC pattern reminiscent of the proneural molecular subtype.
In 44% of all gliomas and in 42% of GBMs, high expression of p53 was seen. In primary GBMs, the frequency of p53 positivity has been reported to range from 21 to 53%.15,32–35 This wide range is probably attributable to both sample selection and the methodology used. In line with p53, the reported frequency of OLIG2 expression in gliomas varies from low to 100%, independently of the detection techniques used. The major pitfall in the reported studies seems to be the low number of cases included (usually <20 GBMs).36–40 Here, we assessed OLIG2 expression in as many as 180 gliomas (72 GBMs), and high OLIG2 IR was noted in 51% of low-grade and 70% of high-grade tumours; our results for low-grade gliomas are in line with previous reports indicating an enrichment of proneural gene expression in WHO grade II gliomas with oligodendrogial differentiation.17
In this study, PDGFRA was expressed by 16% of all gliomas and by 12% of all high-grade gliomas (WHO grade III and GBM), a frequency in line with previously published data.28 Le Mercier et al.15 reported high PDGFRA expression in as many as 53% of all GBMs, as compared with 4% in our study. In these two studies, different antibody and assessment strategies were applied; thus, these results are not comparable. With regard to assessment strategies, Le Mercier et al. used computerized quantitation of labelled cells in a section, whereas we assessed the IR in TMA cores manually. Our findings are theoretically in line with previous reports indicating that PDGFRA amplification is not seen as overexpression of PDGFRA protein.41,42
The fourth protein assessed here, IDH1R132H, was present in 44% of all gliomas and in 11% of all GBMs. Thus, as expected, most of our GBMs were negative for IDH1R132H, indicating that they were primary GBMs.
On the basis of our results, PDGFRA IHC is not reliable, whereas assessment of both p53 IHC and OLIG2 IHC seems to be of interest. Assessment of IDH1R132H should probably always be included, in order to differentiate between a primary and a secondary GBM, to support the differentiation between WHO grade III and grade IV gliomas, and to visualize infiltrating neoplastic cells in the preserved brain tissue.7
No significant correlations were detected between patterns of protein expression and GBM. Most of the correlations noted were for the oligodendroglial WHO grade II tumours. There were only 12 cases; thus, the influence of selection bias cannot be excluded. It is noteworthy, however, that the expected positive correlations were seen between CD44/MERTK and the mesenchymal subtype, and between several proteins and the proneural subtype (Table 6). Thus, our findings imply that protein expression might be of interest in the low-grade gliomas, whereas it has no informative value in the high-grade tumours.
On the basis of our results, including analysis using the Kruskal–Wallis rank test, the proteins whose expression was most significant were EGFR, p53, CD44, and MERTK. EGFR was by far the most significant for identifying a protein subtype of glioma. The molecular subtypes have so far been implemented for GBM; however, here we noted that the assessment of protein expression was successfully applied for both high-grade and low-grade tumours. The significance of expression of these proteins regarding the fate of the tumour can only be assessed in prospective studies, i.e. comparison of protein expression in original and recurring tumours.
Our results suggest that expression of EGFR, p53, CD44, MERTK and OLIG2 can indeed define protein subtypes of glioma that are reminiscent of the previously defined molecular subtypes (classical, mesenchymal, and proneural). Assessment of expression of these five proteins by means of IHC is more economical and less time-consuming than the molecular approach. Furthermore, assessment of chromosome 1p19q codeletion, O(6)-methylguanine-DNA methyltransferase (MGMT) promoter methylation and serine/threonine-protein kinase B-Raf (BRAF) are certainly recommended for defining a glioma.43 Thus, current glioma diagnostics should probably include not only the WHO classification, but also the assessment of the above-mentioned proteins in order to stratify patients for specific treatment strategies.
Acknowledgments
We would like to thank the research engineer IngMarie Olsson and laboratory technologists Inga Hansson and Ulrika Larsson for their skilful technical assistance, and Meena Strömqvist for her critical reading of the manuscript. This study was supported by local ALF grants, and was approved by the local Uppsala ethical committee (Dnr 2002-330, 2005-542-31/1, and 2006/229).
Author contributions
I. Alafuzoff and S. N. Popova: study design, reassessment of all slides, and writing of the manuscript. G. Hesselager: neurosurgery. A. Smits, M. Bergqvis, and S. Ekman: selection of the cases from the medical records. I. Alafuzoff, S. N. Popova, L. Sooman, and A. Dimberg: collection of the slides from the archive and assessment of the TMAs. F. Ponten and P.-H. Edqvist: production of the tissue microarrays.
Conflicts of Interest
The authors report no conflicts of interest.
References
- 1.Dunbar E, Yachnis AT. Glioma diagnosis: immunohistochemistry and beyond. Adv. Anat. Pathol. 2010;17:187–201. doi: 10.1097/PAP.0b013e3181d98cd9. [DOI] [PubMed] [Google Scholar]
- 2.Niclou SP, Fack F, Rajcevic U. Glioma proteomics: status and perspectives. J. Proteomics. 2010;73:1823–1838. doi: 10.1016/j.jprot.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Steinbach JP, Blaicher HP, Herrlinger U, et al. Surviving glioblastoma for more than 5 years: the patient's perspective. Neurology. 2006;66:239–242. doi: 10.1212/01.wnl.0000194221.89948.a0. [DOI] [PubMed] [Google Scholar]
- 4.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daumas-Duport C, Scheithauer B, O'Fallon J, Kelly P. Grading of astrocytomas. A simple and reproducible method. Cancer. 1988;62:2152–2165. doi: 10.1002/1097-0142(19881115)62:10<2152::aid-cncr2820621015>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.Mellai M, Piazzi A, Caldera V, et al. Idh1 and idh2 mutations, immunohistochemistry and associations in a series of brain tumours. J. Neurooncol. 2011;105:345–357. doi: 10.1007/s11060-011-0596-3. [DOI] [PubMed] [Google Scholar]
- 7.Sahm F, Capper D, Jeibmann A, et al. Addressing diffuse glioma as a systemic brain disease with single-cell analysis. Arch. Neurol. 2012;69:523–526. doi: 10.1001/archneurol.2011.2910. [DOI] [PubMed] [Google Scholar]
- 8.Weller M, Wick W, von Deimling A. Isocitrate dehydrogenase mutations: a challenge to traditional views on the genesis and malignant progression of gliomas. Glia. 2011;59:1200–1204. doi: 10.1002/glia.21130. [DOI] [PubMed] [Google Scholar]
- 9.Brennan C, Momota H, Hambardzumyan D, et al. Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS ONE. 2009;4:e7752. doi: 10.1371/journal.pone.0007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikiforova MN, Hamilton RL. Molecular diagnostics of gliomas. Arch. Pathol. Lab. Med. 2011;135:558–568. doi: 10.5858/2010-0649-RAIR.1. [DOI] [PubMed] [Google Scholar]
- 11.Ohgaki H, Kleihues P. Genetic profile of astrocytic and oligodendroglial gliomas. Brain Tumor Pathol. 2011;28:177–183. doi: 10.1007/s10014-011-0029-1. [DOI] [PubMed] [Google Scholar]
- 12.Ozawa T, Brennan CW, Wang L, et al. PDGFRA gene rearrangements are frequent genetic events in PDGFRA-amplified glioblastomas. Genes Dev. 2010;24:2205–2218. doi: 10.1101/gad.1972310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Mercier M, Hastir D, Moles Lopez X, et al. A simplified approach for the molecular classification of glioblastomas. PLoS ONE. 2012;7:e45475. doi: 10.1371/journal.pone.0045475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motomura K, Natsume A, Watanabe R, et al. Immunohistochemical analysis-based proteomic subclassification of newly diagnosed glioblastomas. Cancer Sci. 2012;103:1871–1879. doi: 10.1111/j.1349-7006.2012.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper LA, Gutman DA, Long Q, et al. The proneural molecular signature is enriched in oligodendrogliomas and predicts improved survival among diffuse gliomas. PLoS ONE. 2010;5:e12548. doi: 10.1371/journal.pone.0012548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kampf C, Olsson I, Ryberg U, Sjostedt E, Ponten F. Production of tissue microarrays, immunohistochemistry staining and digitalization within the human protein atlas. J. Vis. Exp. 2012;63:3620. doi: 10.3791/3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karja V, Sandell PJ, Kauppinen T, Alafuzoff I. Does protein expression predict recurrence of benign World Health Organization grade I meningioma? Hum. Pathol. 2010;41:199–207. doi: 10.1016/j.humpath.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Combs SE, Schulz-Ertner D, Debus J, von Deimling A, Hartmann C. Improved correlation of the neuropathologic classification according to adapted World Health Organization classification and outcome after radiotherapy in patients with atypical and anaplastic meningiomas. Int. J. Radiat. Oncol. Biol. Phys. 2011;81:1415–1421. doi: 10.1016/j.ijrobp.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 21.Fromont G, Validire P, Prapotnich D, et al. Pathologic reassessment of prostate cancer surgical specimens before molecular retrospective studies. Clin. Cancer Res. 2011;17:836–840. doi: 10.1158/1078-0432.CCR-10-1046. [DOI] [PubMed] [Google Scholar]
- 22.Kraus JA, Wenghoefer M, Schmidt MC, et al. Long-term survival of glioblastoma multiforme: importance of histopathological reevaluation. J. Neurol. 2000;247:455–460. doi: 10.1007/s004150070175. [DOI] [PubMed] [Google Scholar]
- 23.Mucci NR, Akdas G, Manely S, Rubin MA. Neuroendocrine expression in metastatic prostate cancer: evaluation of high throughput tissue microarrays to detect heterogeneous protein expression. Hum. Pathol. 2000;31:406–414. doi: 10.1053/hp.2000.7295. [DOI] [PubMed] [Google Scholar]
- 24.Marucci G. The effect of WHO reclassification of necrotic anaplastic oligoastrocytomas on incidence and survival in glioblastoma. J. Neurooncol. 2011;104:621–622. doi: 10.1007/s11060-010-0523-z. [DOI] [PubMed] [Google Scholar]
- 25.Benito R, Gil-Benso R, Quilis V, et al. Primary glioblastomas with and without EGFR amplification: relationship to genetic alterations and clinicopathological features. Neuropathology. 2010;30:392–400. doi: 10.1111/j.1440-1789.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- 26.Hobbs J, Nikiforova MN, Fardo DW, et al. Paradoxical relationship between the degree of EGFR amplification and outcome in glioblastomas. Am. J. Surg. Pathol. 2012;36:1186–1193. doi: 10.1097/PAS.0b013e3182518e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rand V, Huang J, Stockwell T, et al. Sequence survey of receptor tyrosine kinases reveals mutations in glioblastomas. Proc. Natl Acad. Sci. USA. 2005;102:14344–14349. doi: 10.1073/pnas.0507200102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szerlip NJ, Pedraza A, Chakravarty D, et al. Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proc. Natl Acad. Sci. USA. 2012;109:3041–3046. doi: 10.1073/pnas.1114033109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dei Tos AP, Ellis I. Assessing epidermal growth factor receptor expression in tumours: what is the value of current test methods? Eur. J. Cancer. 2005;41:1383–1392. doi: 10.1016/j.ejca.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch FR, Varella-Garcia M, Bunn PA, Jr, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J. Clin. Oncol. 2003;21:3798–3807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 31.Horbinski C, Hobbs J, Cieply K, Dacic S, Hamilton RL. EGFR expression stratifies oligodendroglioma behavior. Am. J. Pathol. 2011;179:1638–1644. doi: 10.1016/j.ajpath.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brennan C. Genomic profiles of glioma. Curr. Neurol. Neurosci. Rep. 2011;11:291–297. doi: 10.1007/s11910-011-0198-7. [DOI] [PubMed] [Google Scholar]
- 33.Mendrysa SM, Ghassemifar S, Malek R. P53 in the CNS: perspectives on development, stem cells, and cancer. Genes Cancer. 2011;2:431–442. doi: 10.1177/1947601911409736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sulman EP, Guerrero M, Aldape K. Beyond grade: molecular pathology of malignant gliomas. Semin. Radiat. Oncol. 2009;19:142–149. doi: 10.1016/j.semradonc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Wang AL, Liu ZX, Li G, Zhang LW. Expression and significance of p53 protein and MDM-2 protein in human gliomas. Chin. Med. J. (Engl.) 2011;124:2530–2533. [PubMed] [Google Scholar]
- 36.Bouvier C, Bartoli C, Aguirre-Cruz L, et al. Shared oligodendrocyte lineage gene expression in gliomas and oligodendrocyte progenitor cells. J. Neurosurg. 2003;99:344–350. doi: 10.3171/jns.2003.99.2.0344. [DOI] [PubMed] [Google Scholar]
- 37.Ligon KL, Alberta JA, Kho AT, et al. The oligodendroglial lineage marker olig2 is universally expressed in diffuse gliomas. J. Neuropathol. Exp. Neurol. 2004;63:499–509. doi: 10.1093/jnen/63.5.499. [DOI] [PubMed] [Google Scholar]
- 38.Lu QR, Park JK, Noll E, et al. Oligodendrocyte lineage genes (olig) as molecular markers for human glial brain tumours. Proc. Natl Acad. Sci. USA. 2001;98:10851–10856. doi: 10.1073/pnas.181340798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marie Y, Sanson M, Mokhtari K, et al. Olig2 as a specific marker of oligodendroglial tumour cells. Lancet. 2001;358:298–300. doi: 10.1016/S0140-6736(01)05499-X. [DOI] [PubMed] [Google Scholar]
- 40.Ohnishi A, Sawa H, Tsuda M, et al. Expression of the oligodendroglial lineage-associated markers Olig1 and Olig2 in different types of human gliomas. J. Neuropathol. Exp. Neurol. 2003;62:1052–1059. doi: 10.1093/jnen/62.10.1052. [DOI] [PubMed] [Google Scholar]
- 41.Martinho O, Longatto-Filho A, Lambros MB, et al. Expression, mutation and copy number analysis of platelet-derived growth factor receptor A (PDGFRA) and its ligand PDGFA in gliomas. Br. J. Cancer. 2009;101:973–982. doi: 10.1038/sj.bjc.6605225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paulsson J, Lindh MB, Jarvius M, et al. Prognostic but not predictive role of platelet-derived growth factor receptors in patients with recurrent glioblastoma. Int. J. Cancer. 2011;128:1981–1988. doi: 10.1002/ijc.25528. [DOI] [PubMed] [Google Scholar]
- 43.Ma R, de Pennington N, Hofer M, Blesing C, Stacey R. Diagnostic and prognostic markers in gliomas – an update. Br. J. Neurosurg. 2013;27:311–315. doi: 10.3109/02688697.2012.752432. [DOI] [PubMed] [Google Scholar]