Abstract
Background
Infection with Helicobacter pylori (H. pylori), a high-risk factor for gastric cancer, is frequently associated with chronic inflammation through activation of NFκB. TFF1 is a constitutively expressed protein in the stomach that has tumor suppressor functions and plays a critical role in maintaining mucosal integrity. In this study, we investigated the role of TFF1 in regulating the proinflammatory response to H. pylori infection.
Methods
For in vitro studies, we performed immunofluorescence, luciferase reporter assay, Western blot, and quantitative real-time PCR (qRT-PCR) to investigate activation of NFκB and its target genes in response to infection with H. pylori strains J166 and 7.13. In addition, we utilized the Tff1 knockout (KO) and Tff1 wild-type (WT) mice for infection with PMSS1 H. pylori strain.
Results
The reconstitution of TFF1 expression in gastric cancer cells significantly suppressed an H. pylori-mediated increase of NFκB-p65 nuclear staining, transcriptional activity and expression of proinflammatory cytokine genes (TNFα, IL1β, CXCL5, and IL4R) that were associated with reduction in expression and phosphorylation of NFκB-p65 and IKKα/β proteins. The in vivo studies using the Tff1-KO mouse model of gastric neoplasia confirmed the in vitro findings. Furthermore, they demonstrated an increase in chronic inflammation scores and frequency of invasive gastric adenocarcinoma in the Tff1-KO mice infected with H. pylori, as compared to uninfected Tff1-KO mice.
Conclusion
These findings underscore an important protective role of TFF1 in abrogating H. pylori-mediated inflammation, a crucial hallmark of gastric tumorigenesis. Therefore, loss of TFF1 expression could be an important step in the H. pylori-mediated gastric carcinogenesis.
Keywords: TFF1, NFκB, Helicobacter pylori, Inflammation, gastric cancer
Introduction
Gastric cancer is the third leading cause of cancer-associated death worldwide1. Several studies have reported frequent association of gastric adenocarcinoma with Helicobacter pylori (H. pylori) infection2, 3. H. pylori, a Gram-negative microaerophilic bacterium and a pathogen of the gastric mucosa, is harbored by approximately 50% of the world’s population. However, only approximately 1% of exposed individuals develop gastric cancer in response to chronic infection with H. pylori3. The mucosal inflammatory response is considered a hallmark of H. pylori infection in gastric tissues4.
NFκB is an important nuclear transcription factor that regulates the expression of several genes involved in cell proliferation, immune response, and inflammation5–7. In recent years, several studies have investigated the role of NFκB in inflammation and its link to cancer7. In fact, there are multiple growing lines of evidences that support the role of NFκB as a bridge between inflammation and cancer development5, 8. In conditions of chronic inflammation-related diseases such as ulcerative colitis, NFκB is super-activated with a high-risk of colon cancer5, 9. H. pylori bacteria, classified as a carcinogen, plays a major role in activating chronic inflammatory response that include activation of NFκB in the gastric mucosa10.
Trefoil factor 1 (TFF1) is expressed and secreted by epithelial cells that line the gastric mucosa11. Currently, it is widely accepted that TFF1 functions as a tumor suppressor in gastric carcinogenesis12, 13. Downregulation and loss of TFF1 expression occur in more than half of gastric adenocarcinomas; the most common molecular mechanisms include deletions, mutations, loss of heterozygosity or hypermethylation14–18. In addition, transcriptional regulation of TFF1 has been reported. The hypoxia inducible factor (HIF)-1 mediates the induction of TFF1 expression in gastric epithelial cells under hypoxic conditions19. On the other hand, the cofactor of BRCA1 (COBRA1) has been described as a transcriptional repressor of TFF1 in gastric cancers20. Our previous investigations demonstrated that TFF1 has many anti-tumorigenic functions in the prevention of gastric cancer. We showed that TFF1 plays an anti-inflammatory role through regulation of NFκB signaling in the multistep gastric tumorigenesis cascade21. In addition to its anti-inflammatory role, TFF1 suppresses cell proliferation and gastric tumorigenesis through regulation of β-catenin signaling22. Furthermore, we confirmed that TFF1 has a pro-apoptotic function by activating p53 through downregulation of miR-504, a negative regulator of p53 in gastric cancer12.
In the present study, we investigated whether TFF1 expression could modulate H. pylori-mediated inflammation in gastric cancer. Our study demonstrates that TFF1 plays a significant role in antagonizing H. pylori-induced activation of NFκB in vitro and in vivo. Silencing TFF1 expression in the Tff1 knockout (KO) mouse model fostered and accelerated the progression of gastric lesions to invasive adenocarcinoma.
Materials & Methods
Cell Culture and Reagents
Human gastric cancer AGS cells were obtained from American Tissue Culture Collection (ATCC, Manassas, VA) and were cultured in Ham’s F-12 supplemented with 10% FBS and 1% penicillin/streptomycin (Gibco, Carlsbad, CA) at 37°C in an atmosphere containing 5% CO2. These cells were evaluated weekly to ascertain conformity to their appropriate in vitro morphological characteristics. Specific antibodies against phospho-IKKα/β (Ser176/180), IKKα/β, phospho-NFκB-p65 (Ser536), NFκB-p65, and β-actin were purchased from Cell Signaling Technology (Beverly, MA). CagA specific antibody was purchased from Abcam (Cambridge, MA).
Reconstitution of TFF1 expression in gastric cancer cells
In order to reconstitute the expression of TFF1 in AGS cells to levels comparable to the expression in normal epithelial cells, we established AGS cell lines stably expressing pcDNA empty vector or human TFF1. The human TFF1 coding sequence was amplified using PCR and cloned in frame into pcDNA3.1 mammalian expression vector (Invitrogen, Carlsbad, CA) following standard protocols. AGS cells were transfected with pcDNA3.1-TFF1 or empty vector (control) using Fugene 6 (Roche Applied Science, Indianapolis, IN) following the manufacturer’s protocols. Stably transfected cells were selected using 0.5 mg/mL G418 (Invitrogen). After 3 weeks of selection, several cell colonies were isolated using cloning rings and then transferred to fresh plates. Single colony cultures were identified and analyzed by quantitative real-time RT-PCR (qRT-PCR). AGS-TFF1 clones that had expression levels of TFF1 comparable to normal gastric epithelial cells were used in the study21.
H. pylori bacterial strains and culture conditions
H. pylori CagA+ strains, “J166” a clinical isolate of human-derived H. pylori, and “7.13” a rodent adapted strain derived from B128 H. pylori23–25, were used in the in vitro studies. For the in vivo study, we used the wild-type rodent-adapted cag+ H. pylori strain PMSS1, a clinical isolate of a duodenal ulcer patient and the parental strain of the mouse-derivative Sydney strain 1 (SS1)26. All H. pylori cultures were performed on brucella agar (BBL/Becton Dickinson, Sparks, MD) supplemented with 5% heat-inactivated newborn calf serum (Invitrogen) and ABPNV (amphotericin B, 20 mg/liter; bacitracin, 200 mg/liter; polymyxin B, 3.3 mg/liter; nalidixic acid, 10.7 mg/liter; vancomycin, 100 mg/liter) antibiotics (Sigma-Aldrich, St. Louis, MO). H. pylori liquid cultures for mouse inoculation were grown in brucella broth with 5% NCS and antibiotic supplementation for approximately 24 h (optical density at 600 nm 0.35 to 0.45), pelleted by centrifugation, and suspended in brucella broth.
Immunofluorescence assay
AGS cell lines stably expressing TFF1 or empty vector were plated in 8-well chambers. After 48 h, cells were infected with either H. pylori strains J166 or 7.13. Cells were washed with PBS and fixed with fresh 4% paraformaldehyde solution for 15 min at room temperature. Cells were then washed twice with PBS, followed by incubation in 10% normal goat serum blocking solution (Zymed Laboratories) for 20 min at room temperature in a humidified chamber. Cells were then incubated in the specific primary antibody against NFκB-p65 (GenScript, Scotch Plains, NJ) diluted in PBS (1:400) for 2 h at room temperature in a humidified chamber. Cells were washed 3 times in PBS and incubated in fluorescein isothiocyanate (FITC)–tagged secondary antibody (1:1,000; Jackson Immunoresearch, West Grove, PA) for 45 min at room temperature in a humidified chamber. The cells were then washed in PBS, mounted with Vectashield/DAPI (Vector Laboratories, Burlingame, CA), and visualized using an Olympus BX51 fluorescence microscope (Olympus Co., Center Valley, PA). For quantification, at least 200 cells were counted from each experiment. Total cell number was measured with automatic particle counting in ImageJ software (http://www.uhnresearch.ca/facilities/wcif/imagej/) after setting an automatic threshold range. The image was transformed into a binary image and the total number of cells in each field was counted. The percentage of NFκB-p65 positive cells was calculated as the number of cells showing nuclear green staining divided by the total cell number showing DAPI nuclear blue staining × 100.
Luciferase reporter assay
To monitor the transcriptional activity of NFκB, we used the pNFκB-Luc reporter vector that contains multiple copies of the NFκB consensus sequence (Clontech Laboratories Inc., Mountain View, CA). AGS cells expressing TFF1 or pcDNA were seeded in 24 well plates overnight. Next day, cells were transiently transfected with 500 ng of NFκB-Luc and 250 ng of β-galactosidase as a control plasmid for transfection using Fugene 6 according to the manufacturer’s instructions (Roche Applied Science, Indianapolis, IN). After 48 h, cells were infected with J166 or 7.13 H. pylori strains (100:1) and luciferase and β-galactosidase activities were measured. The firefly luciferase activity was normalized to β-galactosidase activity and expressed as relative luciferase activity ± standard error of the mean (SEM).
Quantitative real-time RT-PCR
Total RNA was isolated using the RNeasy Mini kit (Qiagen, Germantown, MD), and single-stranded cDNA was subsequently synthesized using the Advantage RT-for-PCR Kit (Clontech). Genes specific for mouse and human primers were designed using the online software Primer 3 (http://frodo.wi.mit.edu/primer3/). The forward and reverse primers were designed to span two different exons for each gene (human: TNFα, IL1β, CXCL5, and IL4R; mouse: Tnfα, Il1β, Cxcl5, and Il4r) as previously described21. H. pylori detection was performed using primers specific for H. pylori ureB gene (urease) as described previously26, 27. All primers were purchased from Integrated DNA Technologies (Coralville, IA). qRT-PCR was performed using an iCycler (Bio-Rad, Hercules, CA), with the threshold cycle number determined by use of iCycler software version 3.0. Reactions were performed in triplicate and the threshold cycle numbers were averaged. The results of the genes expression were normalized to housekeeping genes, HPRT for human and actin for mouse, as described previously28. Expression ratios were calculated according to the formula 2(Rt−Et)/2(Rn−En) 21, where Rt is the threshold cycle number for the reference gene observed in the test samples, Et is the threshold cycle number for the experimental gene observed in the test samples, Rn is the threshold cycle number for the reference gene observed in the reference samples, and En is the threshold cycle for the experimental gene observed in the reference samples. Rn and En values were calculated as an average of all reference samples.
Western blotting
Cell lysates were prepared in RIPA buffer containing Halt Protease and Phosphatase Inhibitors Cocktail (Pierce Biotechnology, Inc., Rockford, IL) and were centrifuged at 4,390 g for 10 min at 4°C. Protein concentration was measured using a Bio-Rad Protein Assay (Bio-Rad). Equal amounts of proteins (10–15 µg) from each sample were subjected to SDS/PAGE and transferred onto nitrocellulose membranes. Target proteins were detected by using specific antibodies. The relative density of protein bands was normalized to β-Actin and presented as graphs produced from analysis of three independent blots by ImageJ software.
Animal infection and histologic evaluation
Tff1-KO mouse model of gastric tumorigenesis13, 21 and normal Tff1-WT, 6–8 weeks of age, were challenged with either sterile Brucella broth or H. pylori strain PMSS1 by oral gavage as previously described29. Mice were euthanized at 4, 24, 32, and 48 weeks post-challenge (8–10 mice per group). For evaluation of NFκB target genes, we used stomach tissues from mice that were challenged for a short time period of 4 weeks. We collected frozen and formalin fixed paraffin-embedded stomach tissue samples from all mice. Histopathological classification and grading of the inflammatory score in stomach from the mouse tissue bank (2006–2014) were performed by our pathologists on H&E stained sections21. All procedures were in accordance with Institutional Animal Care and Use Committee approved protocol at Vanderbilt University.
Statistical analysis
Using GraphPad Prism software, a One-way ANOVA Newman-Keuls Multiple Comparisons Test was performed to compare the differences between 3 groups or more, and a 2-tailed Student’s test was used to compare the statistical difference between 2 groups. To assess whether the difference of incidence of histological changes between uninfected and H. pylori-infected Tff1-KO mice is more than expected, we used the Chi-square (and Fisher's exact) test. The differences were considered statistically significant when p value was <0.05.
Results
TFF1 suppresses H. pylori-mediated NFκB activation in vitro
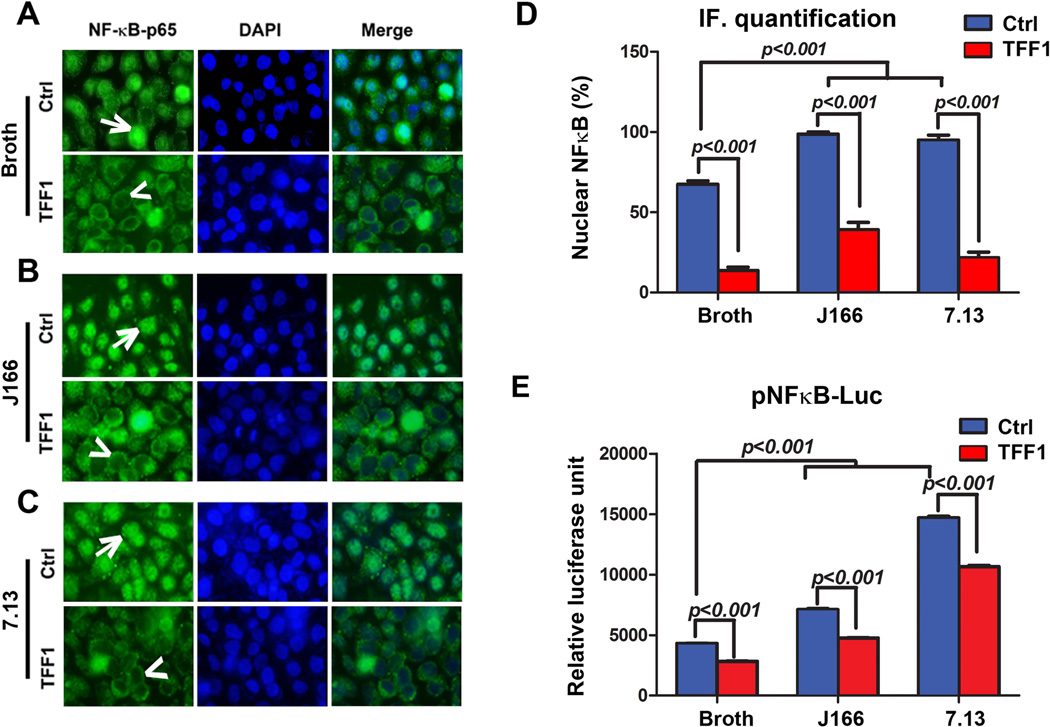
In order to investigate the role of TFF1 in regulating H. pylori-mediated activation of NFκB, immunofluorescence assay was performed. We used AGS cells stably expressing TFF1 or empty vector pcDNA infected with H. pylori J166 or 7.13 strains. Our results indicated a significant increase of the percentage of AGS-pcDNA cells showing NFκB-p65 nuclear staining following infection with H. pylori J166 or 7.13 strains (p<0.001). However, a significant decrease of NFκB-p65 nuclear staining was observed in AGS-TFF1 cells as compared to empty vector AGS-pcDNA after infection with H. pylori J166 or 7.13 strains (p<0.001, Figure 1A–D). These data indicate that TFF1 suppresses H. pylori-induced activation and nuclear translocation of NFκB-p65.
Figure 1. TFF1 reconstitution alters H. pylori-mediated nuclear translocation and transcriptional activation of NFκB.
A–C) In vitro immunofluorescence assay of NFκB-p65 in uninfected and H. pylori-infected AGS cells stably expressing pcDNA or TFF1 indicating nuclear localization of NFκB-p65 (green fluorescence, arrows) in AGS-pcDNA cells and absence of nuclear NFκB-p65 staining (arrowheads) in AGS-TFF1 cells. (A) Uninfected cells (Broth). (B) Cells infected with H. pylori J166 strain. (C) Cells infected with 7.13 strain. DAPI (blue) was used as a nuclear counterstain. Original magnification, ×40. D) Graph shows the quantification of nuclear NFκB-p65–positive staining in at least 200 counted cells, indicating an increase of NFκB-p65 nuclear staining in AGS-pcDNA cells, which decreases in AGS-TFF1 cells after infection. Results presented as percentage ± SEM. E) The luciferase reporter assay using a pNFκB-Luc reporter plasmid. H. pylori infection of AGS-pcDNA cells significantly increased the luciferase activity, which was reduced after reconstitution of TFF1. The bar graphs represent the mean ± SEM of 3 independent experiments.
To confirm the role of TFF1 in regulating H. pylori-mediated activation of NFκB, we examined the effect of TFF1 on the transcription activity of NFκB after H. pylori infection using the pNFκB-Luc reporter that contains multiple copies of the NFκB consensus sequence30. As expected, AGS-pcDNA control cells showed a significant increase of pNFκB-Luc activity after infection with H. pylori as compared to uninfected cells (p<0.001). However, this activation of pNFκB-Luc was significantly diminished in AGS-TFF1 cells as compared to AGS-pcDNA cells after infection with H. pylori J166 or 7.13 strains (p<0.001, Figure 1E).
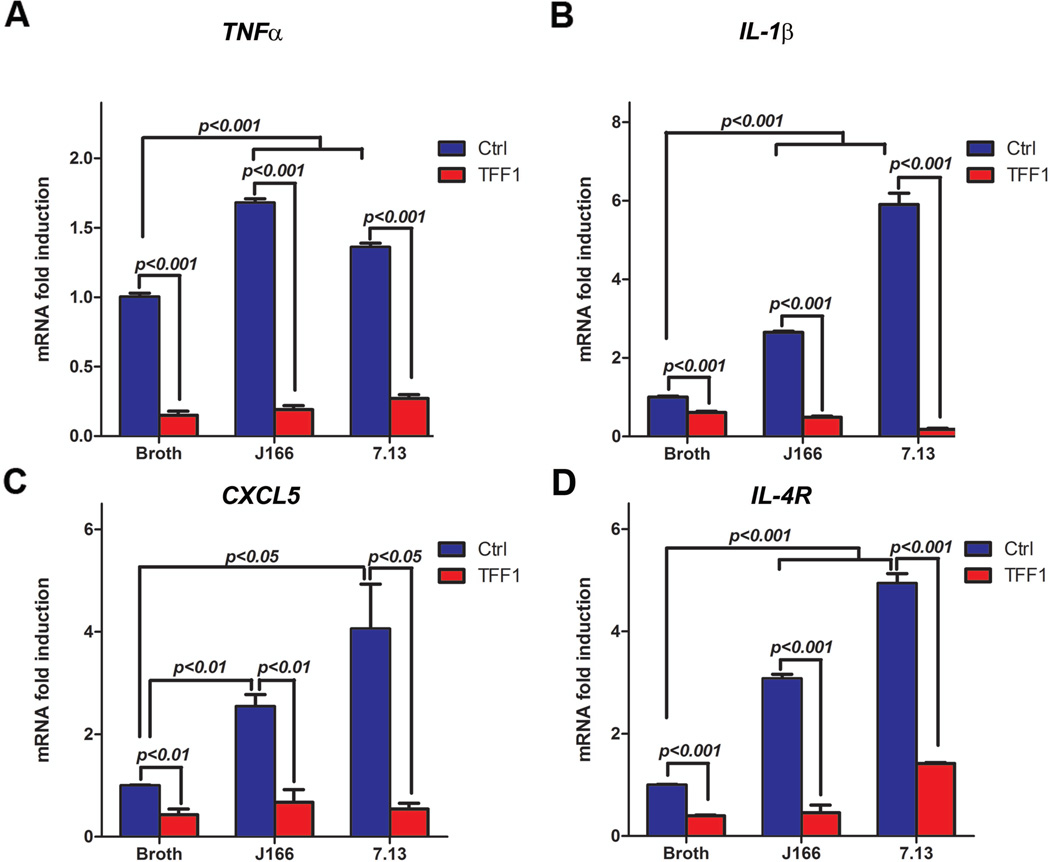
In epithelial cells, the activation of NFκB through H. pylori infection leads to increased expression and the secretion of proinflammatory cytokines, which in turn, results in inflammation31. To examine whether TFF1 can affect H. pylori-induced proinflammatory response, AGS cells stably expressing TFF1 or pcDNA empty vector were infected with H. pylori J166 or 7.13 strains for 3 h. Our qRT-PCR results showed a significant decrease in mRNA expression levels of four proinflammatory NFκB target genes (TNFα, IL1β, CXCL5, and IL4R) in AGS-TFF1 cells as compared to AGS-pcDNA cells following infection with either strains of H. pylori (Figure 2A–D). Collectively, these in vitro data suggest that TFF1 regulates H. pylori-mediated NFκB nuclear localization, transcription activation, and upregulation of proinflammatory target genes in gastric epithelial cells.
Figure 2. TFF1 suppresses H. pylori-induced upregulation mRNA expression of NFκB target genes.
A–D) qRT-PCR analysis showing a decrease in mRNA expression of proinflammatory NFκB target genes (TNFα, IL1β, CXCL5, and IL4R) in AGS-TFF1 cells relative to AGS-pcDNA cells, following infection with H. pylori. The bar graphs represent the mean ± SEM of 3 independent experiments.
TFF1 negatively regulates H. pylori-mediated activation of NFκB signaling
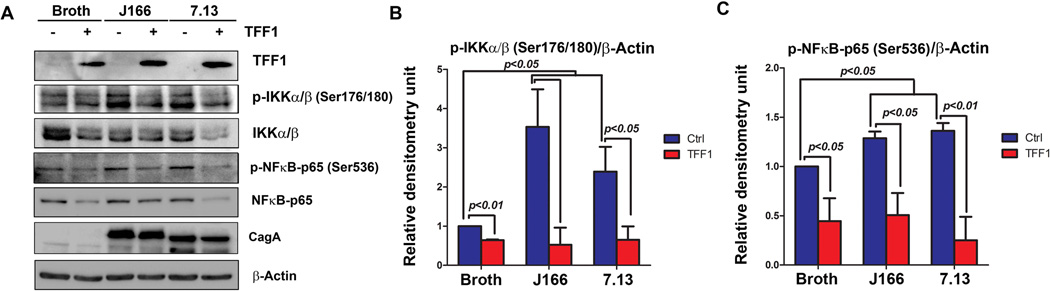
H. pylori is known to induce activation of NFκB canonical pathway in epithelial cells via its virulence factor CagA, which activates the IKK complex, leading to nuclear translocation of NFκB-p6532. We next examined the role of TFF1 in regulating the NFκB signaling pathway after H. pylori infection in gastric cancer cells. Western blot analysis demonstrated a decrease of p-IKKα/β (Ser176/180), p-NFκB-p65 (Ser536), IKKα/β and NFκB-p65 protein levels in AGS-TFF1 cells as compared to AGS-pcDNA control cells after infection with H. pylori J166 or 7.13 strains (Figure 3A–C). Altogether, these results demonstrate that TFF1 plays a negative role in regulating H. pylori-mediated activation of NFκB in gastric cancer cells.
Figure 3. TFF1 attenuates H. pylori-induced activation of NFκB signaling through dephosphorylation of IKKα/β and NFκB-p65 proteins.
A) Western blot analysis of the indicated proteins showed that the reconstitution of TFF1 decreases protein levels of p-IKKα/β and pNFκB-p65 after H. pylori infection. Protein loading was normalized for equal levels of β-actin and the infection with H. pylori was confirmed using CagA specific antibody. B–C) The relative density of p-IKK a/β (B) and p-NFκB-p65 (C) normalized to β-Actin are presented as graphs produced from analysis of three independent blots by ImageJ software. The results are expressed as mean ± SEM of at least 3 independent experiments.
H. pylori infection enhances gastric tumorigenesis in Tff1-KO mice
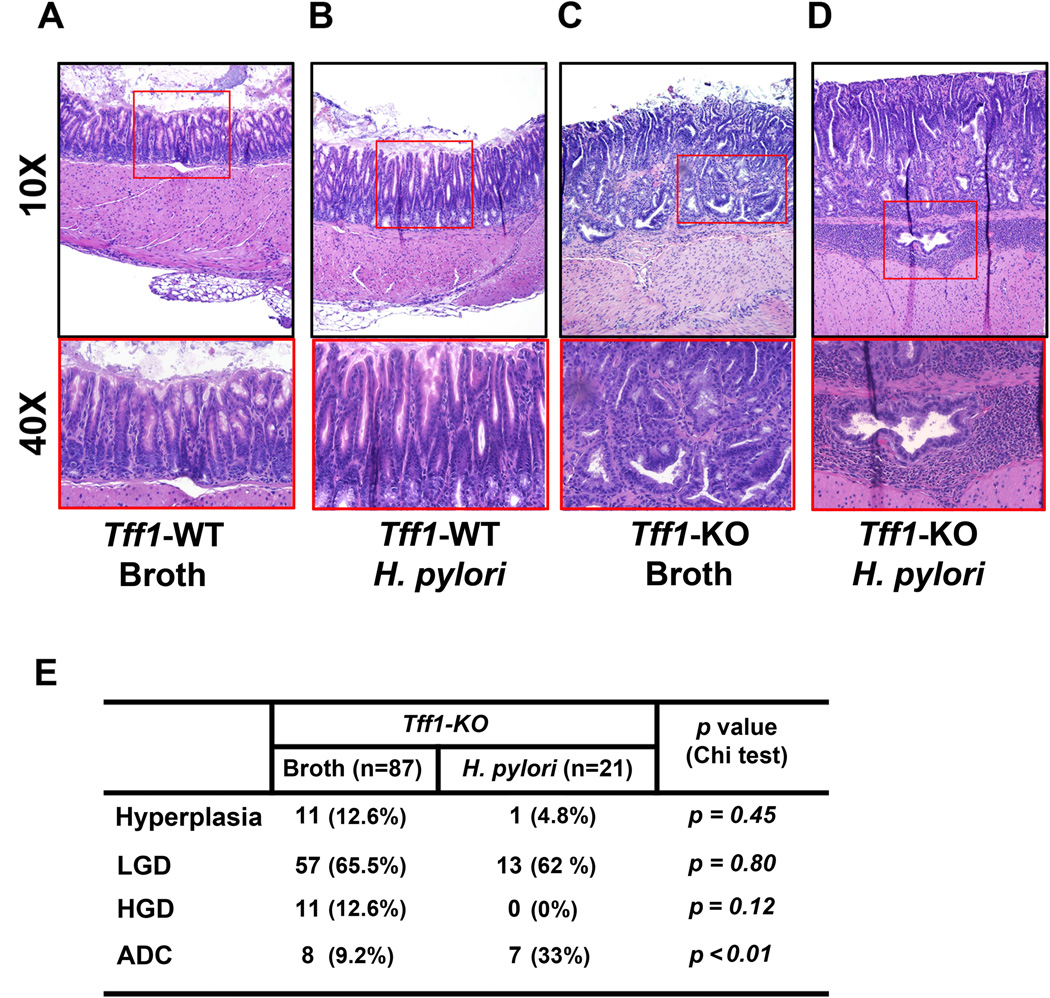
Our in vitro data showed that H. pylori-induced activation of NFκB, a hallmark of gastric tumorigenesis, was suppressed by TFF1. Therefore, we extended our study using the Tff1-KO mouse model of gastric tumorigenesis. We investigated whether H. pylori infection could affect the incidence of gastric cancer in this model. Histological analyses indicated that all uninfected Tff1-WT mice had normal gastric glands (Figure 4A). None of the H. pylori-infected Tff1-WT mice developed hyperplastic or dysplastic lesions (Figure 4B). On the other hand, all Tff1-KO mice developed gastric lesions, which were more advanced following H. pylori infection (Figure 4C&E). The results clearly indicated that H. pylori infection in Tff1-KO mice significantly enhanced the incidence of invasive gastric adenocarcinoma (33%) as compared to (9.2%) uninfected Tff1-KO mice (p<0.01, Figure 4E). These findings suggested that loss of Tff1 is a critical factor for H. pylori to promote cancer development.
Figure 4. H. pylori infection enhances gastric tumorigenesis in Tff1-KO mice.
A–D) H&E staining of representative histological features of antropyloric gastric mucosa from mice of matched age (30 weeks): Tff1-WT with normal gland (A), Tff1-WT infected with H. pylori PMSS1 strain showing gastritis (B), Tff1-KO mice showing high grade dysplasia (C), and Tff1-KO infected with H. pylori PMSS1 strain showing neoplastic antropyloric tissues expanded into the muscularis mucosa and formed invasive adenocarcinomas. (D) Original magnification, ×10 (top), ×40 (bottom). E) Table showing the percentage of histological distribution in gastric tissues from age-matched uninfected control (Ctrl) and H. pylori-infected Tff1-KO mice. The significance of correlation was determined by Chi square test.
H. pylori infection augments chronic inflammation and gene expression of proinflammatory cytokines in Tff1-KO mice
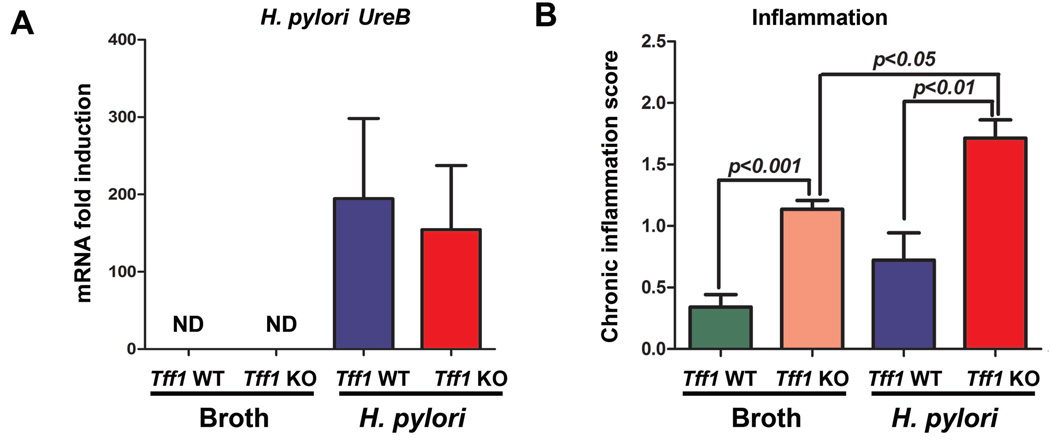
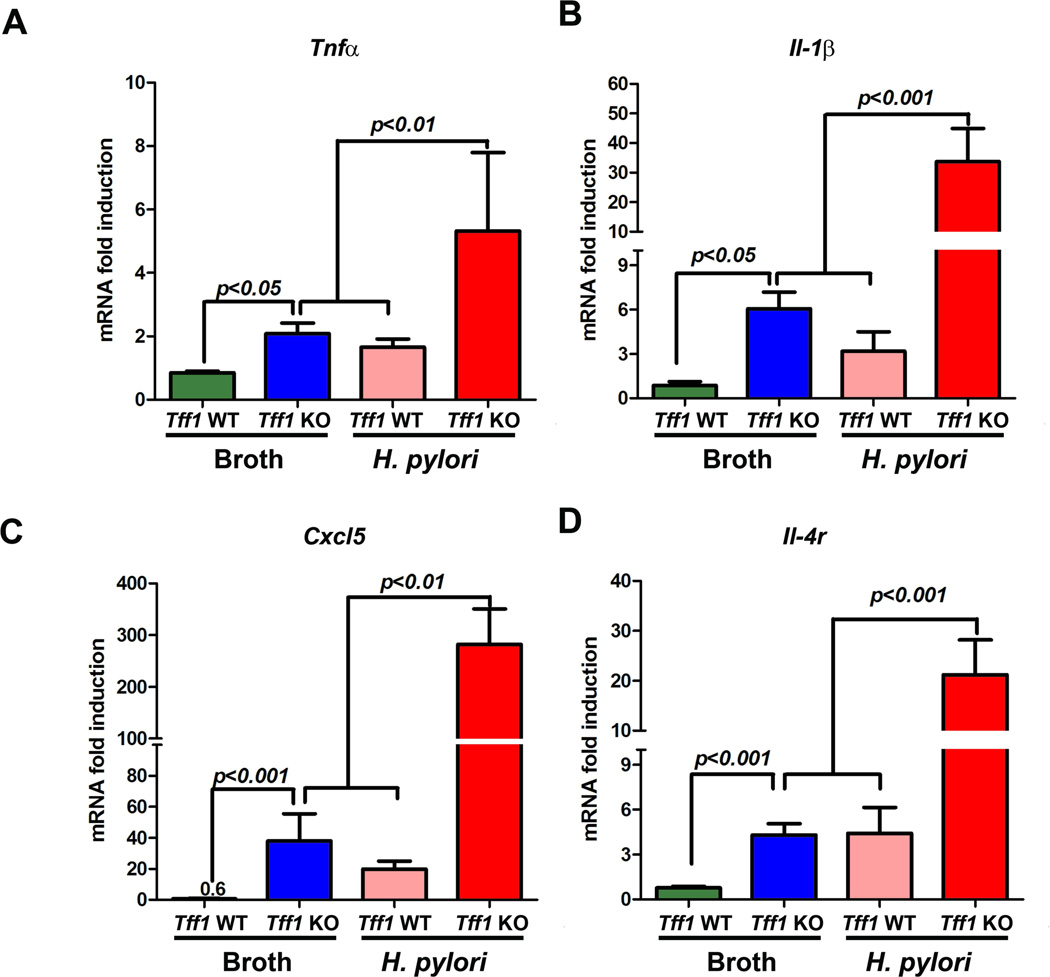
Inflammation and cancer development are well associated, and chronic inflammation represents the major pathologic basis for the majority of infection-induced malignancies33. Notably, H. pylori-induced chronic inflammation is mediated by an array of proinflammatory cytokines2, 34. Therefore, we investigated whether H. pylori-induced gastric tumorigenesis could be associated with an increase of chronic inflammation in Tff1-KO mice. We examined the chronic inflammatory scores in uninfected and H. pylori-infected Tff1-WT and Tff1-KO mice of matching age (10–12 weeks) (Figure 5A). We found that the inflammatory scores in Tff1-KO mice increase significantly (p<0.05) after infection with H. pylori as compared to uninfected Tff1-KO mice (Figure 5B). Importantly, the chronic inflammation scores were significantly (p<0.01) higher in the Tff1-KO mice than the Tff1-WT following H. pylori infection (Figure 5B). Next, we examined the mRNA gene expression of the proinflammatory cytokines (Tnfα, Il1β, Cxcl5, and Il4r), known mediators of chronic inflammation. The qRT-PCR data demonstrated a significant increase of mRNA expression of tested proinflammatory genes Tnfα (p<0.01, Figure 6A), Il1β (p<0.001, Figure 6B), Cxcl5 (p<0.01, Figure 6C), and Il4r (p<0.001, Figure 6D) in Tff1-KO mice infected with H. pylori as compared to uninfected Tff1-KO mice of matching age. In addition, the data showed that the mRNA expression of the aforementioned tested proinflammatory cytokines was significantly higher in Tff1-KO mice than Tff1-WT mice after H. pylori infection (Figure 5A–D). Taken together, these data indicated that H. pylori infection further enhances chronic inflammation and gene expression of proinflammatory cytokines in Tff1-KO mice.
Figure 5. H. pylori infection increases chronic inflammation in Tff1-KO mice.
A) qRT-PCR showing mRNA expression of H. pylori UreB (Ureas) as indication of H. pylori infection in Tff1-WT and Tff1-KO mice. The level of UreB gene expression in uninfected Tff1-WT and Tff1-KO mice was undetectable (ND). B) Chronic inflammation scores in uninfected and H. pylori-infected Tff1-WT and Tff1-KO mice of matched ages (10–12 weeks).
Figure 6. H. pylori infection increases mRNA expression of proinflammatory genes in Tff1-KO mice.
A–D) qRT-PCR analysis showing mRNA expression of proinflammatory NFκB target genes. The mRNA expression of Tnfα (A), Il1β (B), Cxcl5 (C), and Il4r (D) was significantly upregulated in H. pylori-infected Tff1-KO mice as compared to uninfected Tff1-KO mice (10–12 weeks of age). The bars denote the mean ± SEM, p<0.05 was considered statistically significant.
Discussion
The development of gastric cancer proceeds through a well-defined cascade of histological lesions that are associated with activation of oncogenic pathways, loss of tumor suppressor genes’ function, and chronic inflammation31, 35. This process is complex and is influenced by both host genetics and environmental factors36. H. pylori, a gastric pathogen that is classified as a carcinogen, plays an important role in developing gastric cancer through activation of NFκB, the core mediator of inflammation37, 38. While infection with H. pylori leads to the development of gastric inflammation, less than 1% of infected patients develop gastric adenocarcinoma36, 38, suggesting the presence of other important contributing factors that are required for promoting gastric tumorigenesis, such as loss of tumor suppressor genes.
TFF1, a tumor suppressor protein that protects gastric mucosa from injury, is frequently silenced in more than half of gastric adenocarcinomas through a combination of genetic and epigenetic mechanisms16, 39, 40. We have previously reported, using in vitro and in vivo models, that TFF1 loss promotes inflammation and gastric tumorigenesis21. Herein, we investigated whether TFF1 could affect the outcome of H. pylori infection and gastric carcinogenesis. We showed evidence, for the first time, that TFF1 can antagonize and suppress H. pylori-mediated activation of NFκB in vivo and in vitro and demonstrated that loss of TFF1 expression promotes the development of invasive gastric cancer following H. pylori infection.
The pathogenesis and virulence of H. pylori relies on the existence of cytotoxin-associated genes (cag), Pathogenicity Island (cag-PAI), and CagA is one of the bacterial oncoprotein encoded by cag-PAI41, 42. Once CagA is injected into gastric epithelial cells, it promotes NFκB activation37, 41. We have shown that H. pylori infection increases nuclear translocation and accumulation of NFκB-p65, indicating its activation, in AGS cells. However, the reconstitution of TFF1 expression abolished this activation and subsequently prevented the translocation of NFκB-p65 to the nucleus. These results confirmed our previously reported data about the role of TFF1 in suppressing TNFα-mediated activation of NFκB21. To validate the role of TFF1 in regulating H. pylori-mediated NFκB activation, we utilized the NFκB reporter as a measure of NFκB transcription activity, and confirmed that the reconstitution of TFF1 expression suppresses H. pylori-mediated activation of NFκB.
Several studies have reported that H. pylori-mediated NFκB activation involves the activity of IKKα and IKKβ kinases43. Of note, an earlier study has shown that TFF1 suppresses TNFα-induced NFκB activation through regulation of IKK pathway21. However, it remained unclear whether TFF1 expression is capable and sufficient to antagonize potent H. pylori-induced proinflammatory response. In this study, we showed that the reconstitution of TFF1 expression significantly counteracts H. pylori-induced activation of IKKα/β and NFκB-p65 proteins. These findings confirm the suppressive effect of TFF1 on H. pylori-mediated activation of NFκB signaling in gastric cancer cells.
Inflammation, in response to H. pylori infection, has been reported to play an important role in promoting gastric carcinogenesis (reviewed by41). NFκB responsive genes, including proinflammatory cytokines, are expressed at high levels in H. pylori-infected gastric mucosa41, 43. Similarly, we found that H. pylori infection induces mRNA expression of proinflammatory genes TNFα and IL1β. However, the reconstitution of TFF1 abolishes H. pylori-induced expression of these proinflammatory cytokines in gastric cancer cells. Several studies have demonstrated that the proinflammatory cytokines TNFα and IL1β, known to be induced by NFκB44, are associated with gastric disease in rodents after H. pylori infection45, 46.
Long exposure to proinflammatory cytokines and sustained activation of signaling pathways such as NFκB due to pathogen infection results in chronic inflammation that promotes malignancy and tumorigenesis5, 7, 8. Accordingly, our data demonstrated that H. pylori infection of Tff1-KO mice led to a significant increase of chronic inflammatory scores, expression of proinflammatory cytokines, and development of invasive gastric adenocarcinoma as compared to uninfected Tff1-KO mice. Notably, the high occurrence of gastric cancer in infected Tff1-KO mice could be attributed to the synergistic effect of loss of Tff1 expression and H. pylori infection. These findings confirm the in vitro cell model data and suggest that TFF1 might be a key molecule that suppresses H. pylori-induced chronic inflammation and carcinogenesis.
We previously reported that TFF1 suppresses NFκB activation through interfering with the formation of TNFR1 and TRAF2 protein complex and activation of IKKα/β proteins21. In addition, several studies have demonstrated that direct contact of H. pylori with gastric epithelial cells activates NFκB through regulation of a signaling pathway that involves IKKα/β and TRAF2 proteins10, 47. This suggests that modulation of NFκB activity by TFF1 and H. pylori involves regulation of TRAF2-IKKα/β pathway. Given the fact that TFF1 is a secreted protein11, it remains unclear whether TFF1 functions at the cell surface or intracellularly to regulate this pathway. Previous studies have claimed that the physical interaction between TFF1 protein and H. pylori promotes colonization of the bacterial organism in the stomach48, 49. This interaction in the mucous layer may also affect H. pylori virulence and/or the rate of attachment of H. pylori bacteria to the gastric epithelial cells. Additional detailed investigations are required to clarify the molecular mechanism by which TFF1 suppresses H. pylori-induced activation of NFκB.
In conclusion, our findings establish the interplay between TFF1 and H. pylori infection in gastric carcinogenesis. We showed that TFF1 plays an anti-inflammatory role by suppressing H. pylori-induced NFκB signaling pathway, thereby reducing gastric carcinogenesis. Accordingly, the presence of an intact and functional TFF1 protein could be a protective limiting factor in the H. pylori-mediated gastric carcinogenesis cascade.
Acknowledgements
This study was supported by grants from the Department of Veterans Affairs (WER), Vanderbilt SPORE in Gastrointestinal Cancer (P50 CA95103), Vanderbilt Ingram Cancer Center (P30 CA68485), and the Vanderbilt Digestive Disease Research Center (DK058404). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the Department of Veterans Affairs, National Institutes of Health, or Vanderbilt University.
Abbreviations used in this paper
- TFF1
trefoil factor 1
- H. pylori
Helicobacter pylori
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. Journal international du cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2(1):28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 3.El-Omar EM. The importance of interleukin 1beta in Helicobacter pylori associated disease. Gut. 2001;48(6):743–747. doi: 10.1136/gut.48.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polk DB, Peek RM., Jr Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10(6):403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A, Garlanda C, Allavena P. Molecular pathways and targets in cancer-related inflammation. Annals of medicine. 2010;42(3):161–170. doi: 10.3109/07853890903405753. [DOI] [PubMed] [Google Scholar]
- 6.Guo JL, Zheng SJ, Li YN, et al. Toxicarioside A inhibits SGC-7901 proliferation, migration and invasion via NF-kappaB/bFGF signaling. World journal of gastroenterology : WJG. 2012;18(14):1602–1609. doi: 10.3748/wjg.v18.i14.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 8.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Archiv : an international journal of pathology. 2005;446(5):475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 9.Fantini MC, Pallone F. Cytokines: from gut inflammation to colorectal cancer. Current drug targets. 2008;9(5):375–380. doi: 10.2174/138945008784221206. [DOI] [PubMed] [Google Scholar]
- 10.Maeda S, Yoshida H, Ogura K, et al. H. pylori activates NF-kappaB through a signaling pathway involving IkappaB kinases, NF-kappaB-inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterology. 2000;119(1):97–108. doi: 10.1053/gast.2000.8540. [DOI] [PubMed] [Google Scholar]
- 11.Thim L, May FE. Structure of mammalian trefoil factors and functional insights. Cellular and molecular life sciences : CMLS. 2005;62(24):2956–2973. doi: 10.1007/s00018-005-5484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soutto M, Chen Z, Saleh MA, et al. TFF1 activates p53 through down-regulation of miR-504 in gastric cancer. Oncotarget. 2014;5(14):5663–5673. doi: 10.18632/oncotarget.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefebvre O, Chenard MP, Masson R, et al. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science. 1996;274(5285):259–262. doi: 10.1126/science.274.5285.259. [DOI] [PubMed] [Google Scholar]
- 14.Beckler AD, Roche JK, Harper JC, et al. Decreased abundance of trefoil factor 1 transcript in the majority of gastric carcinomas. Cancer. 2003;98(10):2184–2191. doi: 10.1002/cncr.11789. [DOI] [PubMed] [Google Scholar]
- 15.Park WS, Oh RR, Park JY, et al. Somatic mutations of the trefoil factor family 1 gene in gastric cancer. Gastroenterology. 2000;119(3):691–698. doi: 10.1053/gast.2000.16483. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho R, Kayademir T, Soares P, et al. Loss of heterozygosity and promoter methylation, but not mutation, may underlie loss of TFF1 in gastric carcinoma. Lab Invest. 2002;82(10):1319–1326. doi: 10.1097/01.lab.0000029205.76632.a8. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka T, Nakamura J, Kitajima Y, et al. Loss of trefoil factor 1 is regulated by DNA methylation and is an independent predictive factor for poor survival in advanced gastric cancer. International journal of oncology. 2013;42(3):894–902. doi: 10.3892/ijo.2013.1759. [DOI] [PubMed] [Google Scholar]
- 18.Tomita H, Takaishi S, Menheniott TR, et al. Inhibition of gastric carcinogenesis by the hormone gastrin is mediated by suppression of TFF1 epigenetic silencing. Gastroenterology. 2011;140(3):879–891. doi: 10.1053/j.gastro.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez C, Santamatilde E, McCreath KJ, et al. Induction of trefoil factor (TFF)1, TFF2 and TFF3 by hypoxia is mediated by hypoxia inducible factor-1: implications for gastric mucosal healing. British Journal of Pharmacology. 2009;156(2):262–272. doi: 10.1111/j.1476-5381.2008.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McChesney PA, Aiyar SE, Lee OJ, et al. Cofactor of BRCA1: a novel transcription factor regulator in upper gastrointestinal adenocarcinomas. Cancer research. 2006;66(3):1346–1353. doi: 10.1158/0008-5472.CAN-05-3593. [DOI] [PubMed] [Google Scholar]
- 21.Soutto M, Belkhiri A, Piazuelo MB, et al. Loss of TFF1 is associated with activation of NF-kappaB-mediated inflammation and gastric neoplasia in mice and humans. The Journal of clinical investigation. 2011;121(5):1753–1767. doi: 10.1172/JCI43922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soutto M, Peng D, Katsha A, et al. Activation of beta-catenin signalling by TFF1 loss promotes cell proliferation and gastric tumorigenesis. Gut. 2015;64(7):1028–1039. doi: 10.1136/gutjnl-2014-307191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alm RA, Ling LS, Moir DT, et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397(6715):176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 24.Solnick JV, Hansen LM, Salama NR, Boonjakuakul JK, Syvanen M. Modification of Helicobacter pylori outer membrane protein expression during experimental infection of rhesus macaques. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(7):2106–2111. doi: 10.1073/pnas.0308573100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franco AT, Johnston E, Krishna U, et al. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer research. 2008;68(2):379–387. doi: 10.1158/0008-5472.CAN-07-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold IC, Lee JY, Amieva MR, et al. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology. 2011;140(1):199–209. doi: 10.1053/j.gastro.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roth DE, Velapatino B, Gilman RH, et al. A comparison of a string test-PCR assay and a stool antigen immunoassay (HpSA) for Helicobacter pylori screening in Peru. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2001;95(4):398–399. doi: 10.1016/s0035-9203(01)90194-4. [DOI] [PubMed] [Google Scholar]
- 28.El-Rifai W, Smith MF, Jr, Li G, et al. Gastric Cancers Overexpress DARPP-32 and a Novel Isoform, t-DARPP. Cancer Res. 2002;62(14):4061–4064. [PubMed] [Google Scholar]
- 29.Fox JG, Wang TC, Rogers AB, et al. Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology. 2003;124(7):1879–1890. doi: 10.1016/s0016-5085(03)00406-2. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh A, Saginc G, Leow SC, et al. Telomerase directly regulates NF-kappaB-dependent transcription. Nature cell biology. 2012;14(12):1270–1281. doi: 10.1038/ncb2621. [DOI] [PubMed] [Google Scholar]
- 31.Lamb A, Chen LF. Role of the Helicobacter pylori-induced inflammatory response in the development of gastric cancer. Journal of cellular biochemistry. 2013;114(3):491–497. doi: 10.1002/jcb.24389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun SC. Non-canonical NF-kappaB signaling pathway. Cell research. 2011;21(1):71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutation research. 2008;659(1–2):15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Ishijima N, Suzuki M, Ashida H, et al. BabA-mediated adherence is a potentiator of the Helicobacter pylori type IV secretion system activity. J Biol Chem. 2011;286(28):25256–25264. doi: 10.1074/jbc.M111.233601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sepulveda AR. Helicobacter, Inflammation, and Gastric Cancer. Current pathobiology reports. 2013;1(1):9–18. doi: 10.1007/s40139-013-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kodaman N, Pazos A, Schneider BG, et al. Human and Helicobacter pylori coevolution shapes the risk of gastric disease. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(4):1455–1460. doi: 10.1073/pnas.1318093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117(1):60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wroblewski LE, Peek RM, Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23(4):713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katoh M. Trefoil factors and human gastric cancer (review) Int J Mol Med. 2003;12(1):3–9. [PubMed] [Google Scholar]
- 40.McChesney PA, Aiyar SE, Lee OJ, et al. Cofactor of BRCA1: a novel transcription factor regulator in upper gastrointestinal adenocarcinomas. Cancer Res. 2006;66(3):1346–1353. doi: 10.1158/0008-5472.CAN-05-3593. [DOI] [PubMed] [Google Scholar]
- 41.Hatakeyama M. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell host & microbe. 2014;15(3):306–316. doi: 10.1016/j.chom.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nature reviews. Gastroenterology & hepatology. 2010;7(11):629–641. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirata Y, Maeda S, Ohmae T, et al. Helicobacter pylori induces IkappaB kinase alpha nuclear translocation and chemokine production in gastric epithelial cells. Infect Immun. 2006;74(3):1452–1461. doi: 10.1128/IAI.74.3.1452-1461.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor perspectives in biology. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim HJ, Hawke N, Baldwin AS. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006;13(5):738–747. doi: 10.1038/sj.cdd.4401877. [DOI] [PubMed] [Google Scholar]
- 46.Toyoda T, Yamamoto M, Takasu S, Ogawa K, Tatematsu M, Tsukamoto T. Molecular Mechanism of Gastric Carcinogenesis in Helicobacter pylori-Infected Rodent Models. Diseases. 2014:168–186. [Google Scholar]
- 47.Seo JH, Lim JW, Kim H. Differential Role of ERK and p38 on NF- kappa B Activation in Helicobacter pylori-Infected Gastric Epithelial Cells. Journal of cancer prevention. 2013;18(4):346–350. doi: 10.15430/JCP.2013.18.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reeves EP, Ali T, Leonard P, et al. Helicobacter pylori lipopolysaccharide interacts with TFF1 in a pH-dependent manner. Gastroenterology. 2008;135(6):2043–2054. 54 e1–54 e2. doi: 10.1053/j.gastro.2008.08.049. [DOI] [PubMed] [Google Scholar]
- 49.Clyne M, Dillon P, Daly S, et al. Helicobacter pylori interacts with the human single-domain trefoil protein TFF1. Proc Natl Acad Sci U S A. 2004;101(19):7409–7414. doi: 10.1073/pnas.0308489101. [DOI] [PMC free article] [PubMed] [Google Scholar]