Abstract
The delivery of HIV care in the initial rapid scale-up of HIV care and treatment was based on existing clinic-based models, which are common in highly resourced settings and largely undifferentiated for individual needs. A new framework for treatment based on variable intensities of care tailored to the specific needs of different groups of individuals across the cascade of care is proposed here. Service intensity is characterised by four delivery components: (i) types of services delivered, (ii) location of service delivery, (iii) provider of health services and (iv) frequency of health services. How these components are developed into a service delivery framework will vary across countries and populations, with the intention being to improve acceptability and care outcomes. The goal of getting more people on treatment before they become ill will necessitate innovative models of delivering both testing and care. As HIV programmes expand treatment eligibility, many people entering care will not be ‘patients’ but healthy, active and productive members of society 1. To take the framework to scale, it will be important to: (i) define which individuals can be served by an alternative delivery framework; (ii) strengthen health systems that support decentralisation, integration and task shifting; (iii) make the supply chain more robust; and (iv) invest in data systems for patient tracking and for programme monitoring and evaluation.
La délivrance des soins du VIH dans le déploiement initial rapide des soins et du traitement du VIH a été basée sur des modèles existants dans les cliniques, qui sont courants dans les régions bénéficiant d’importantes ressources et largement indifférenciées pour les besoins individuels. Un nouveau cadre est proposé ici pour le traitement basé selon les intensités variables de soins, adaptés aux besoins spécifiques des différents groupes de personnes à travers la cascade de soins. L’intensité des services est caractérisée par quatre éléments de délivrance: (1) les types de services délivrés, (2) l’emplacement de la délivrance des services, (3) Les prestataires des services de santé et (4) la fréquence des services de santé. La façon dont ces éléments sont développés dans un cadre de prestation de services peut varier selon les pays et les populations, l’intention étant d’améliorer les résultats d’acceptabilité et des soins. Le but d’obtenir plus de personnes sous traitement avant qu’ils ne tombent malades nécessitera des modèles innovateurs de prestation à la fois pour dépistage et pour les soins. Comme les programmes VIH étendent l’éligibilité au traitement, beaucoup de gens qui entrent dans les soins ne seront pas des “malades- mais des éléments sains de la société, actifs et productifs. Afin de tenir le cadre à l’échelle, il sera important de: (1) définir les individus qui peuvent être traités par un cadre alternatif de prestation, (2) renforcer les systèmes de santé qui soutiennent la décentralisation, l’intégration et le transfert des tâches; (3) rendre la chaîne d’approvisionnement plus robuste et (4) investir dans des systèmes de données pour le suivi des patients et pour le suivi et l’évaluation du programme.
Los servicios de atención del VIH durante el inicio de la primera etapa de rápida expansión del tratamiento y cuidados del VIH estaban basados en modelos clínicos existentes, comunes en lugares con abundancia de recursos y poco diferenciados en cuanto a necesidades individuales. Aquí se propone un nuevo marco para el tratamiento basado en intensidades variables de cuidados, hecho a medida según las necesidades específicas de los diferentes grupos de individuos a lo largo del tratamiento. La intensidad del servicio se caracteriza por cuatro componentes de entrega: (1) tipología de los servicios ofrecidos, (2) lugar de entrega de los servicios, (3) proveedor de los servicios sanitarios, y (4) frecuencia de los servicios sanitarios. El cómo estos componentes conforman un marco de entrega de servicios variará según el país y la población, con la intención de mejorar la aceptabilidad y los resultados de los cuidados. El objetivo de conseguir que más personas reciban tratamiento antes de que enfermen requerirá de modelos innovadores en la oferta tanto de pruebas para detección como de los cuidados. A medida que los programas para el VIH expandan los criterios de elegibilidad para el tratamiento, muchas de las personas que comiencen a recibir cuidados no serán “pacientes- sino miembros sanos, activos y productivos de la sociedad. Con el fin de expandir la escala de esta estructura, sería importante: (1) definir cuales individuos pueden ser atendidos dentro de un marco de entrega de servicios alternativo; (2) fortalecer los sistemas sanitarios que apoyan la descentralización, integración y delegación de funciones; (3) robustecer la cadena de proveedores; e (4) invertir en sistemas de datos para el seguimiento de pacientes y para la monitorización y evaluación de programas.
Keywords: AIDS; antiretroviral treatment, highly active; cascade; decentralisation; HIV; optimised care; patient-centred care; task shifting
Introduction
The widespread devastation caused by the HIV pandemic has led to unprecedented increases in overseas development aid for health, much of it earmarked for care and treatment-related services in low- and middle-income countries 2. The magnitude of HIV funding allowed for rapid strengthening of under-resourced health systems unaccustomed to providing chronic care and enabled the successful expansion of care and treatment services that have averted an estimated 5.5 million deaths since 1996 3,4. Further expansion of the emergency scale-up, as currently constituted, is constrained by the donor funding environment 5,6, and subsequent increases in donor resources are unlikely.
A sequel of this success story, however, is that health systems have become even more overburdened. The models of delivery for HIV care developed for the initial rapid scale-up of HIV services were based on traditional clinic-based service models, common in highly resourced settings, and largely not modified to reflect individual needs. Even as the number of people on ART has grown to almost 12 million in low- and middle-income countries, protocols for frequent clinic follow-up have been perpetuated with very few changes, regardless of how long an individual has been on antiretroviral treatment (ART) or their clinical status. After the early rapid growth in clinic sites, expansion has slowed and ever-growing numbers of people receive care in clinics often with insufficient numbers of doctors, clinical officers and nurses 7. As a result of traditional care models, HIV clinics are crowded and waiting times are long with many people waiting solely to pick up drug refills. Healthcare workers are overtaxed due to this high workload and, due to weak infrastructure, face challenges to provide care and follow-up according to the guidelines on which they have been trained.
These challenges have led to a mixed picture of effectiveness among the HIV care and treatment systems. On the one hand, individuals who have been linked to care and retained on ART achieve high rates of viral suppression 8–10. However, studies report substantial loss to follow-up across all steps of the care cascade 6,11. Overburdened health systems, lack of patient-focused services, resource limitations and mixed quality of care have led to efforts to modify the delivery of HIV care in a framework that addresses the causes of poor retention. Task shifting is one of the most common approaches 12. WHO has included task shifting in the 2013 Consolidated Guidelines as a way of providing care to a greater number of people at reduced cost or when there are insufficient healthcare workers in the public sector 13. Other programmes have focused on decentralisation, shifting care to primary health clinics and to the communities in which people live 14.
We describe a delivery framework which provides differential care and treatment services for specific, well-defined groups of people in an effort to improve service quality and access, adherence and retention, outcomes, efficiency, and cost of services. The framework has been variously termed optimised care, patient-centred/focused care, needs-based care or tiered care.
| Problem statements |
|---|
| 1. The scale-up of ART in low- and middle-income countries has led to overburdened health systems |
| •HIV clinics are overcrowded and waiting times are long |
| •Many countries lack sufficient clinical personnel to treat the increasing numbers of patients eligible for ART |
| •Health systems are geared to acute disease response rather than to providing chronic care |
| 2. The needs of people who are stable on and adherent to ART are different to those of people who are unwell or non-adherent |
| •Current models of care are not patient-centered |
| •People with widely divergent needs have only one access point to the clinic to receive care |
| •Stable people do not need regular contact with the healthcare facility |
| 3. Alternative care models implemented in resource-limited settings have not been taken to scale |
| •There are limited robust measures of impact and outcomes of alternative delivery frameworks |
A framework for delivering HIV care and treatment
Driven by a desire to provide care which people will use and to increase the efficiency and effectiveness of HIV care delivery, this framework aims to vary the intensity of both ART and pre-ART care based on individual need and to create more flexible, convenient and acceptable models of service delivery for patients, healthcare workers and health systems. In simple terms, the framework describes delivery of the right care at the right frequency to the right individuals by the right care providers in the right location at the right time. Although this concept is not new, it has not been extensively used by HIV care and treatment programmes in low- and middle-income countries to date.
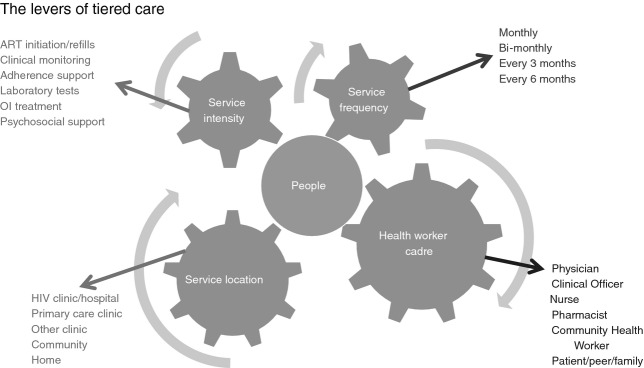
The framework involves providing differential intensity of care and treatment services across defined patient strata. Service intensity is characterised by four components, all centred on the needs of individuals: (i) types of services delivered; (ii) location of service delivery; (iii) provider of health services; and (iv) frequency of health services (Figure 1).
Figure 1.
Four levers to tailor or adapt care to people’s needs (service frequency, location, intensity and cadre).
Each of these components represents a flexible lever for adjusting or modifying a model of care to serve a specific patient stratum in a given geographic or health system setting. Health system variables, such as geography, level of health facility and available cadres of health workers, and individual variables (distance to the health facility, clinical condition, social and economic situation, education level, rural/urban context, and mobility pattern) determine how levers are applied in a given location. How the framework is implemented will vary across countries and populations to best serve the needs of individuals. Similarly, individual eligibility criteria will vary by heath setting, with the intention being to improve patient acceptability and care outcomes.
Different intensities of service can be delivered within a single location or between locations. Distribution of individuals into strata for optimised care is determined by the needs and preferences as defined by specific characteristics (Table 1). The distribution of individuals across care strata is dynamic due to the need for periodic up-referral or down-referral to more or less intensive care based on their current needs.
Table 1.
Key determinants of stratification into different levels of care
| Clinical determinants | Social/cultural determinants |
|---|---|
| Knowledge of HIV status | Individuals’ support network |
| HIV disease severity and current health status | Individuals’ preference for specific model of care |
| Duration of care or treatment | Distance from home to healthcare facility |
| Treatment tolerance and adherence | Sociocultural factors (family, work, or community barriers to care) |
Models of care can be organised into three categories based on the location at which people receive services. Centralised, facility-based models can provide differential care within a single health facility, such as reduced frequency of visits or substitution of a clinical assessment visit by a pharmacy-only medication refill visit. Decentralised models of care provide pre-ART and ART services either by down-referring stable people or initiating and managing people at more peripheral health facilities 15,16. Other models decentralise care even further by providing care directly in the community or in the home (Figure 2).
Figure 2.
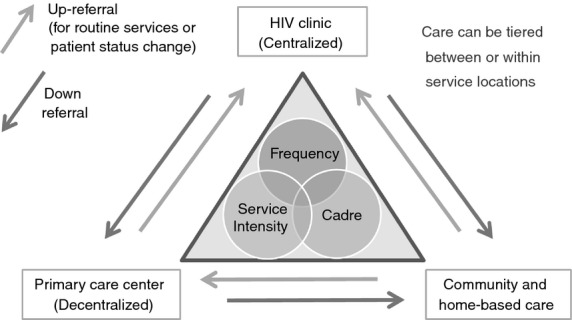
Categories of care models.
There are critical enabling services that are levers for successful HIV health delivery regardless of location, intensity, and frequency of care and who delivers that care. The need for psycho-social support, transportation, child care, nutrition, legal and other services may be as important as how long people wait in clinic.
Examples and evidence from the literature
Application of individual elements of this care framework, notably decentralisation and task shifting, has increased significantly during the past few years and has been widely endorsed by the WHO and other agencies. However, there are few models that represent differential HIV care intensity across patient strata in either the peer-reviewed literature or the grey/conference literature. While not a systematic review, the examples presented in Appendix 1 illustrate the key dynamics and outcomes of innovative models of care in the real world. The studies included in this analysis reported results from approximately 68 000 HIV-positive individuals in eight countries (Democratic Republic of the Congo, Kenya, Malawi, Mozambique, South Africa, Swaziland, Thailand and Uganda). See Appendix1 for a detailed listing of studies and results.
All of the models analysed differentiated individuals on the basis of clinical stability on treatment to determine eligibility for an alternative framework of care. Eligibility was generally restricted to adults with CD4 count above a certain threshold (ranging from ≥50 to ≥350), a certain length of time on ART (from ≥4 weeks to ≥18 months and adherent), undetectable viral load and/or other clinical considerations (no opportunistic infections, no adverse reactions, not pregnant). The studies generally reported on outcomes (including loss to follow-up, mortality and adherence), and some studies reported changes in resource use (health system and/or cost per person per year, number of clinic visits).
Examples of models and evidence of impact
One study examined the cost-effectiveness of the centralised, facility-based model in an urban HIV clinic 17. At the Infectious Diseases Institutes (IDI) in Kampala, Uganda, stable individuals are offered 3-monthly nurse visits, 6-monthly physician visits and monthly pharmacy-only ART refills. Individual outcomes were similar between those managed with monthly refill visits and standard monthly physician/nurse visits, but the cost per person per year fell from $610 per year to $496 for monthly refill-only visits, a decrease of nearly 20% 17.
A clinic-based model that used a six-monthly clinical appointments (SMA) programme was initiated at the Chiradzulu District Hospital in rural Malawi and supported by Médecins Sans Frontières (MSF) to reduce waiting times and clinic staff workload using visit spacing and pharmacy-only visits 18. This programme enrolled people stable on ART to receive 6-monthly clinical appointments with nurses and 3-monthly drug refill visits. Between January 2008 and mid-2013, 8528 adults were enrolled in SMA. Cohort retention at 36 months after SMA start was 94%; however, 2722 (33%) people had returned to standard clinical follow-up status. Reasons for SMA discontinuation and long-term treatment outcomes are being evaluated 18.
A number of studies evaluated the impact of a decentralised, facility-based model in which stable individuals were down-referred from the HIV clinic (where care was generally provided by a doctor or clinical officer) to a primary care health centre (where the care was generally provided by a nurse). Among the 39 000 individuals included in a meta-analysis of this approach, loss to follow-up per 100 patient years was 7.4 (95% CI 6.0–9.3) in the primary care centre group compared to 13.4 in the HIV clinic group and mortality per 100 patient years was 2.8 (95% CI 1.1–7.3) in the primary care centre group compared to 8.4 in the HIV clinic group 14.
At the Themba Lethu Clinic in Johannesburg, South Africa, stable individuals were down-referred to nurse-managed primary care clinics for treatment maintenance rather than being maintained at the HIV clinic 19–21. More than 2000 individuals were down-referred as of 2011, and a matched cohort analysis found that down-referred people were less likely to die (HR 0.2; 95% CI 0.04–0.8), or be lost to follow-up (HR 0.3; 95% CI 0.2–0.6) or experience viral rebound (RR 0.6; 95% CI 0.4–0.9) 19. The cost of care in primary clinics was 11% less than that in the HIV clinic 20. Similar care models have been introduced in rural areas of South Africa with similar outcomes 21.
A number of different approaches have decentralised care to the community or to the home. These models minimise the number of required clinic visits by utilising community health workers or peers to deliver care or treatment either at home or at a community meeting point. The community health workers ranged in education and training, and the qualifications and pay for community healthcare workers varied throughout the models. Some models used volunteers with few education requirements 22, while others recruited paid staff with college degrees 23. One model provided decision support tools to the community health workers 24. Two models used groups of people living with HIV (PLHIV) 25,26, while others used community health workers to deliver medication directly to the house 22,24,27 or distributed treatments in community meeting points 18. All models reported reduced loss to follow-up and reduced number of clinic visits among patients managed in the community or at home.
One decentralised model is of particular interest in urban, high-density areas due to the degree it has been scaled and evaluated. In the Western Cape of South Africa, MSF, driven by the need to provide better patient-centred care and to decongest over crowded HIV clinics, developed a model in which care, including ART drug refills, is provided either at the clinic or in community venues in a group setting 26. These groups, referred to as ART adherence clubs, are facilitated by a community healthcare worker. Forty-month retention in the clubs in Khayelitsha is 97% (club) vs. 83% (clinic) with a 67% reduction in virological rebound among those in clubs compared to clinics 26. While there is selection bias as those eligible for club care are, by definition, stable and adherent, adherence and retention have remained high despite a reduction in clinic visits. This model has been adopted by the Metro District Health Services1 from the initial MSF project in Khayelitsha to include 27 800 people (1/4 of total individuals in care by end June 2014) in the Cape Town metropolitan region 26. Roll-out of the same care model has commenced in some districts in Gauteng and Free State provinces, while Swaziland is likely to implement the model in 2015.
In Mozambique, MSF has collaborated with the Health Ministry to implement and scale Community ART Groups (CAGs) throughout the country 25. CAGs are groups of six individuals from which one rotating person in the group acts as the monthly ART collector for all members. Thus, each CAG member visits the clinic every 6 months. Eligible people must be stable on ART for >6 months and a CD4 count >200. Retention at 12, 24, 36 and 48 months, respectively, has been 97.7%, 96%, 93.4% and 91.8%, and mortality has been 2.1 per 100 person years 25. CAGs are being implemented at varied degrees of scale in Lesotho, Zimbabwe, Malawi and South Africa.
Limitations of the studies
The field of research on alternative delivery frameworks is nascent, and a number of important questions remain. The articles we found did not discuss the impact on people who remained in standard clinic care or the impact on care providers. Only two studies were randomised, and most were retrospective cohort studies. While models have been implemented in a number of countries, 6 of the 16 models and approximately 48 000 of the 68 000 people who were delivered care in this framework were in South Africa, often in urban settings. A model that is effective in urban South Africa, where resources and infrastructure are generally better, may not be reproducible with similar results in more resource-limited settings, such as Malawi, Mozambique, Zambia or even rural South Africa.
Implementation challenges
Challenges to implementing this framework include defining the most appropriate selection criteria for reduced intensity or non-clinic care, national and local regulatory and policy frameworks around reduced intensity of services, supply chain management and data systems for patient tracking and programme monitoring and evaluation.
Each country has their own regulatory frameworks that establish the scope of work for each cadre of healthcare worker. These regulations determine which cadre can initiate and/or manage antiretroviral therapy, dispense medications and perform laboratory tests. Further, regulations stipulate the frequency at which medications may be dispensed. These regulations significantly impact the ability to decentralise or temporally space care. For example, ARV dispensing for individual patients in Western Cape was maintained centrally at pharmacy level, while distribution of pre-packed and labelled ART was permitted at lower level facilities and through community-based adherence clubs. At present in many clinics in eastern, central and southern Africa, nurses cannot initiate ART, although WHO guidelines support it 28.
Supply chains and stock management must be sufficiently robust to ensure stable ART distribution for decentralised primary health centres and community-delivered ART along with longer durations of refills (ideally three monthly).
Robust data systems are necessary to track individuals across care sites as well as monitor overall programme effectiveness, in particular to ensure that retention in care can be tracked as patients move between care facilities or settings. Community-delivered ART requires simple and robust data collection. Unique identifiers, referral tools and data management systems are needed.
Conclusions
We believe this framework can guide policymakers into introducing and scaling up new approaches to delivery across the HIV cascade of care. The framework is driven by two needs: first, care that better meets the needs of people and assisting them to access care and remain in care for life; second, with donor funding for HIV expected to remain constant or decline in the coming years, this framework may provide a tool to provide this care more economically. The cost and cost-effectiveness of innovative models delivery of care needs further evaluation.
The framework, with its levers and patient-centredness, addresses the losses described by others across the cascade of testing, linkage and retention in care 6. Differentiated testing and linking strategies using new testing technologies such as oral self-test may hold promise in helping hard-to-reach populations know their HIV status 28–30. The framework is equally applicable to pre-ART care as it is to ART care.
Scale-up of innovative models of care should be monitored and evaluated through a robust implementation science framework targeting critical questions about most effective and efficient approaches to providing care in varied settings. As best practices are identified, normative bodies and lead implementers should continue to develop toolkits2 and guidelines to help countries and providers to implement these approaches.
Appendix 1
| Location, dates, summary (Source) | Scope/scale; rural/urban | Optimisation component | Stratification metric | ARV distribution frequency, location, and provider | Monitoring and clinical care | Clinical metric: intervention vs. SOC for similar population * denotes significant at P < 0.05 | Costs | System costs | Necessary supports |
|---|---|---|---|---|---|---|---|---|---|
| Centralised models | |||||||||
| Kampala, Uganda 29. June 2006–July 2007 Monthly pickup of medication at pharmacy, where routine screening is completed. SOC is monthly visit to clinic with physician | 578 in the intervention group. Urban | Health Service Provider | CD4 ≥ 200; ≥12 months of ART; self-reported adherence ≥95%; adherence to scheduled clinic visits for last 6 months; disclosed status to spouse; not pregnant; no substantial clinical event in last 6 months | Monthly in the pharmacy by a pharmacy-based nurse | Pharmacy-based nurse asked screening questions; Physician visit every 6 months | Favourable immune response after 1 year (CD4 ≥ 500): 18.9% vs. 19.6%; comparison group was a matched sample before PRP who were followed for at least 1 year after initiating ART | $496 per year vs. $610 per year Costs include: ART, other drugs, radiology, labs, health personnel, and overhead and capital | ||
| Decentralised models | |||||||||
| Free State, South Africa 21 January 2008–June 2010 In one cohort (top row) ART initiation and management was completed in nurse-led primary care clinic. In the other cohort (bottom row) ART management provided in nurse-led primary care clinic. SOC is initiation and management at physician-led HIV clinic | Initiation and management 5390 Rural and Urban | Health Service Provider, Location | CD4 between 51 and 200; no Stage IV infection; no previous ART ≥1 month; no drugs other than cotrimoxazole or vitamins, not bed-or wheelchair bound; Weight>40 kg; BMI<28 | Monthly in the primary care clinic by a nurse | Routine, not discussed in article; care provided in health centre by nurse | Mortality per 100 person years: 1.34 vs. 1.44 Programme retention: 63% vs. 58%* Random assignment by primary care clinic | Shorter commute to community clinic, not quantified in study | Significant training for nurses and nurse managers (4 sessions), plus 2.5 day train the trainer session | |
| Management 3029 Rural and Urban | Health Service Provider, Location | Undetectable VL; no severe side effects; no new opportunistic infections | Monthly in a primary care clinic by a nurse | Routine, not discussed in article; care provided in health centre by nurse | Suppressed VL: 71% vs. 70% Programme retention: 90% vs. 91% Random assignment by primary care clinic | Shorter commute to community clinic, not quantified in study | Significant training for nurses and nurse managers (4 sessions), plus 2.5 day train the trainer session | ||
| South Africa (3), Malawi (1), Swaziland (1), Thailand (1)14 Study data range from 2004 through 2009 Partial decentralisation – treatment initiation in a hospital with follow-up care provided by a health centre | 23 217 individuals decentralised; 15 980 in control; three studies focused only on adults, two on children, one on both Rural, peri-urban, and urban | Health Service Provider, Location | Varies, one study included only treatment naïve patients, three on stable patients with minimum time on ARV between 4 weeks and 11 months, and one with limited requirements | Studies did not vary frequency of care/ART distribution. Initiation was at the hospital by a doctor or clinical officer, while follow-up care provided at health centres by a nurse | Varies, but generally by nurse at health centre | Lost to care per 100 patient years: 7.4 vs. 13.4* Mortality per 100 patient years: 2.8 vs. 8.4* Note: these amounts are for 12-month follow-up of four of six studies. Account for nearly all participants. Two excluded studies are small and excluded b/c they do not provide 12-month time point | |||
| South Africa (1), Malawi (2), Ethiopia (2), Kenya, Mozambique, Rwanda, Tanzania, Lesotho 14 Study data range from 2004 through 2010 Full decentralisation – treatment initiation and management provided by health centre | 20 448 individuals fully decentralised; 48 096 control; four studies focused only on adults, one only on children, and one on both All studies include rural patients, two include urban patients as well | Task shifting, location | Varies, most studies do not note exclusion criteria, one study required individuals to be on treatment for <6 months, another required treatment naïve patients | Studies did not vary frequency of care/ART distribution. Initiation and follow-up were performed at a primary health centre. All studies used nurses, two also used physicians, three used medical officers, and two used medical assistants | Varies, but generally by nurse at health centre | Lost to care per 100 patient years: 8.1 vs. 27.0* Mortality per 100 patient years: 10.6 vs. 9.7 Note: these amounts are for 12-month follow-up of four of six studies. Account for nearly all participants. Two excluded studies are small and excluded b/c they do not provide 12-month time point | |||
| Chiradzulu District, Malawi 18 January 2008–June 2013 Intervention group could pick up medication at health centre every 3 months. Clinic visits every 6 months. Care at health centre provided by CHW. SOC is clinic visit every 1–2 months | 5 869 received intervention, which was 21% of active ART cohort; 2722 (33% of original enrollees) returned to standard clinical follow-up status. Rural | Health Service Provider, Frequency, Location | Stable adult patients - ≥15 on first-line ART for ≥12 months; CD4 ≥ 300; no OI or side effects; no pregnancy or breastfeeding | Clinic every 6 months vs. 1–2 months; 3-month ART refills at health centres by a community health worker | Monitored via standardised assessment tool at each visit; Clinic visits every 6 months | 36-month Retention: 94% vs. 83% Lost to follow-up (1, 2, 5 years):– 1.3%, 2.98% and 7.8%; Mortality (1, 2 and 5 years) –. 4%,. 9% and 2.8%. Comparison with those eligible for but not enrolled in intervention | Paid community health workers; supply chain that can accommodate 3-month prescriptions | ||
| Lubombo, Swaziland 30 January 2007 –November 2007 Intervention group received care in primary care health clinic by nurse. SOC is monthly visit to central HIV clinic and receiving care from clinical officer | 317 were included in the study of the 425 invited from the intervention clinic Rural | Health Service Provider, Location | ≥14; on ART for ≥4 weeks; CD4 ≥ 100; clinically suitable | Monthly at primary care clinic by a counsellor and nurse evolving to primary care nurse and staff | Blood test, clinical questionnaire; care provided at health centre by nurses | No missed appointments - 89.6% vs. 72%* Loss to follow-up: 2.8% vs. 1.3% Mortality: 0 vs. 2.5%* Comparison population were individuals who would have been eligible for the study, but receive care from a different clinical area | Average cost of round trip transportation was halved ($.74 vs. $1.5); 53% of intervention group said transportation cost was lowered. Other benefits reported include being nearer to home, shorter waits, better treatment by staff, better care | Initial training of primary care team | |
| South Africa 19,20 February 2008 through January 2009 (Study timeline, initiation intervention began in 2007) Care and medication distribution provided at nurse-led primary care clinic every 2 months. SOC is bi-monthly visits to HIV clinic with physician | 693 in study, approximately 2000 in total down-referred. Urban | Health Service Provider, Location | ART≥11 months; no opportunistic infections; CD4 > 200; stable weight as reflected by <5% weight loss between the last three visits; VL undetectable | Every 2 months at the primary care clinic by a primary care nurse | Weight loss; symptoms other visit to medical facility; blood test every 6 months; care provided at primary care health centre by nurse | Mortality per 100 patient years:. 3 vs. 1.6*; Lost to follow-up: 1.4% vs. 4.2%* Matched cohort using propensity scores based on gender, age, months on ART, ARV regimen, BMI, CD4 count | Costs reduced by 11% – $492 pppy vs. 551. Cost-effectiveness increased: $509 to $602 per person in care and responding to treatment Costs included: ARVs, other drugs, labs, outpatient visits, fixed costs | EHR system that enables communication between clinic and initiation site; 6-week ART-specific training for primary care health nurses | |
| Community and home-based models | |||||||||
| Khayelitsha, South Africa 18,26 11/2007 – 6/2013 Medications distributed via community health worker-led 30 person support groups bi-monthly. SOC is monthly visits with medical staff | 776 clubs have formed as of publication. 18 719 receiving care through the intervention, which is 19% of active ART cohort Urban | Health Service Provider, Location | Adult on 1st line for ≥18 months; two undetectable VL; CD4 > 200; Criteria for return to clinic care: Missed club visit (5 day grace) or clinically unstable including high VL | Every two months at meetings which take place either at clinic or community location, provided by community health workers | Bi-monthly weight, symptom based general assessments; attendance; nurse review twice per year (1 clinical, 1 blood test). Nurse attends meetings only during these sessions | Lost to care (including death, per 100 person years: 2.98 vs. 11.69* Virological rebound per 100 person years: 3.18 vs. 9.04* Comparison population had been on ARVs for a similar period of time | Shorter waiting times; higher acceptability of services; fewer missed appointments | $58 per year vs. $109 in SOC (unclear what is included, citation to a conference abstract) | Pharmacy staff to pre-package drugs for groups, well-trained lay-workers and support for lay-workers, registries |
| Kinshasa, Democratic Republic of the Congo 18 12/2010 – 5/2013 Medications distributed at community distribution points by peers every 3 months. SOC is visits to clinic (timing of SOC is not described.) | 2161 referred to community ART distribution sites, which is 43% of active ART cohort Urban | Health Service Provider, Frequency (?), Location | On 1st line ART for ≥6 months; CD4 ≥ 350; no OI or side effects | Every 3 months at community ART distribution points by peers | Basic health indicators monitored by peer distributor; annual clinical consultation and blood test (CD4) at clinic | Retention at 12 months, 24 months: 89.3%, 82.4%; reported retention of 75–85% reported elsewhere Lost to follow-up at 24 months: 7.6% | Reduction from 85 to 14 min to refill prescription; Transportation costs cut to 1/3 | HR costs lower, not quantified | Trained PLWH, supply chain that can support 3-month med delivery |
| Tete Province, Mozambique 18,25 2/2008 – 12/2012 PLWH form groups of six who share responsibility of picking up medications and distributing them to group monthly. SOC is monthly clinic visits by all | 8181 receiving medication through CAGs in study, which is 50% of active ART cohort within demonstration programme; Overall, 17 272 receiving care this way countrywide, including 276 children. Rural | Frequency, Location | On 1st line ART for ≥6 months; CD4 ≥ 200; no OI or side effects | Monthly, in the community for 5 of 6 members, while one member attends clinic to pick up meds for the group | Clinic visit every 6 months, which includes clinical consultation and blood test (CD4); group card record keeping | Retention at 12, 24, 36, 48 months: 97.7%, 96%, 93.4%, 91.8%; Mortality per 100 person years: 2.1 LTFU per 100 person years: 1.0 | Reduced costs and time burden on patients; 28% of members shared transportation costs | 49.6% reduction in clinic visits, 62% reduction of ART refill visits | Lay Health Service Providers to ensure links between community groups and health facilities |
| Kosirai, Western Kenya 24 March 2006 – March 2007 CHWs deliver medications, screen, and provide adherence support monthly at home. SOC is monthly clinic visits served by full medical staff | 100, 5% of active ART cohort in clinic that was studied. Rural | Health Service Provider, Location | ≥18 years old; clinically stable on ART for ≥3 months; no adherence issues; household members aware of patients’ HIV status; no WHO stage 3 or 4 condition; no pregnancy; no hospitalisations | Monthly, in the home by community health workers with secondary education, training and PDA with decision support tools | CCC assessed patient symptoms (using PDA) vital signs, adherence to ART, and opportunistic infection prophylaxis. Clinical consultation every 3 months with nurse, physician, and pharmacist. Blood test every 6 months | LTFU: 5.2% vs. 4.5% No significant difference of results as compared to SOC. Comparison population was based on random sample | 6.4 clinic visits vs. 12.6 | Half the clinic visits | CCCs with secondary education and mobile, computer-based decision support tools |
| Karabole, Uganda 22 March 2006-May 2009 Weekly, home-based monitoring and adherence counselling and monthly ARV delivery by unpaid volunteers with 6-monthly appointments at the clinic vs. monthly hospital visits in the standard of care | 185 enrolled in trial arm Rural | Health Service Provider, Frequency, Location | Eligible for treatment and willing to accept daily treatment support from a family member and weekly visits by a trained community volunteer | Monthly at home by trained community volunteers | Weekly monitoring by trained volunteers looking for adverse reactions, adherence (pill counts), and clinical problems. Six-monthly visits to clinic for blood work and clinical review. Health centre is staffed by two clinical officers, two nurses, and on midwife | Mortality: 17% vs. 12% VL suppression (ITT): 64.9% vs. 62.0% In multivariate analysis, the only factor significantly related to viral suppression was enrolment in home-based cohort. Odds ratio: 2.47 (1.02–6.04) | Clinic staff was trained on ART as part of the project; training for volunteers; boots, raincoats, bicycles for volunteers. Report forms for volunteers | ||
| Jinja, Uganda 23 February 2005 through January 2009 Home-based, monthly follow-up by trained field officers, with six-monthly clinic visits (after visits during months 2 and 6). SOC is 3-monthly visits with monthly ARV pickup | 859 enrolled in trial arm Rural and semi-urban | Health Service Provider, Location | Anyone eligible for treatment within 100 km from the clinic | Monthly at home by trained field officers | Monthly monitoring at home, plus clinic visits at months 2, 6, and every 6 months thereafter | Virological failure, LTFU, or withdrew: 24% vs. 27% Mortality (24 months): 14% vs. 14% | First Year: 29 vs. 60 Second Year: 18 vs. 54 This includes transportation, lunch, childcare costs, and lost work time | $793 vs. $838 This includes staff, transport, drugs, labs, sensitisation, training, utilities, supervision and overheads, and capital. Main cause of higher costs of facility-based model is increased contacts with staff. Home-based patients had 75% fewer clinic visits | 4 weeks of training for field officers over and above a college degree; motorcycles for field staff |
Footnotes
Metro District Health Services provides comprehensive primary health service, mainly to lower income groups in the Cape Town metropolitan region.
MSF has already developed a toolkit for the Khayelitsha ART adherence clubs and a toolkit for the CAGs.
References
- Eaton JW, Menzies NA, Stover J, et al. Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models. Lancet Glob Health. 2014;2(1):23–34. doi: 10.1016/S2214-109X(13)70172-4. doi: 10.1016/S2214-109X(13)70172-4. Epub 2013 Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J. Wexler A Kaiser Family Foundaiton. Financing the response to AIDS in low- and middle-income countries: International Assistance from Donor Governments in 2013. (Available from: https://kaiserfamilyfoundation.files.wordpress.com/2014/07/7347-10-financing-the-response-to-hiv-in-low-and-middle-income-countries.pdf )
- El-Sadr WM, Holmes CB, Mugyenyi P, et al. Scale-up of HIV treatment through PEPFAR: a historic public health achievement. J Acquir Immune Defic Syndr. 2012;60(Suppl 3):S96–S104. doi: 10.1097/QAI.0b013e31825eb27b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. 2013. Global report: UNAIDS report on the global AIDS epidemic 2013. (Available from: http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf )
- Katz IT, Bassett IV, Wright AA. PEPFAR in transition–implications for HIV care in South Africa. N Engl J Med. 2013;369:1385–1387. doi: 10.1056/NEJMp1310982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S. Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd MA, Cooper DA. Optimisation of HIV care and service delivery: doing more with less. Lancet. 2012;380:1860–1866. doi: 10.1016/S0140-6736(12)61154-4. [DOI] [PubMed] [Google Scholar]
- Barnabas RV, van Rooyen H, Tumwesigye E, et al. Initiation of antiretroviral therapy and viral suppression after home HIV testing and counselling in KwaZulu-Natal, South Africa, and Mbarara district, Uganda: a prospective, observational intervention study. Lancet HIV. 2014;1:e68–e76. doi: 10.1016/S2352-3018(14)70024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon JH, Elliott JH, Bertagnolio S, Kubiak R, Jordan MR. Viral suppression after 12 months of antiretroviral therapy in low- and middle-income countries: a systematic review. Bull World Health Organ. 2013;91:377–385E. doi: 10.2471/BLT.12.112946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elul B, Nuwagaba-Biribonwoha H, Basinga P, et al. High levels of adherence and viral suppression in a nationally representative sample of HIV-infected adults on antiretroviral therapy for 6, 12 and 18 months in Rwanda. PLoS Med. 2013;8 doi: 10.1371/journal.pone.0053586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15:1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredo T, Bateganya M, Pienaar ED, et al. 2012. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd,. (Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD007331.pub2/abstract )
- Holmes C, Pillay Y, Mwango A, et al. Health systems implications of the 2013 WHO consolidated antiretroviral guidelines and strategies for successful implementation. AIDS. 2014;28(Suppl 2):S231–S239. doi: 10.1097/QAD.0000000000000250. [DOI] [PubMed] [Google Scholar]
- Kredo T, Ford N, Adeniyi FB, et al. Cochrane Database of Systematic Reviews (John Wiley & Sons, Ltd, 2013). (Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD009987.pub2/abstract ) [DOI] [PMC free article] [PubMed]
- Fatti G, Grimwood A. Bock P. Better antiretroviral therapy outcomes at primary healthcare facilities: an evaluation of three tiers of ART services in four South African provinces. PLoS Med. 2010;5 doi: 10.1371/journal.pone.0012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decroo T, Panunzi I, das Dores C, et al. Lessons learned during down referral of antiretroviral treatment in Tete, Mozambique. J Int AIDS Soc. 2009;12:6. doi: 10.1186/1758-2652-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babigumira JB, Castelnuovo B, Lamorde M, et al. Potential impact of task-shifting on costs of antiretroviral therapy and physician supply in Uganda. BMC Health Serv Res. 2009;9:192. doi: 10.1186/1472-6963-9-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemelmans M, Baert S, Goemaere E. Community-supported models of care for people on HIV treatment in sub-Saharan Africa. Trop Med Int Health. 2014;19(8):968–997. doi: 10.1111/tmi.12332. . doi: 10.1111/tmi.12332. [DOI] [PubMed] [Google Scholar]
- Brennan A, Long L, Maskew M, et al. Outcomes of stable HIV-positive patients down-referred from doctor-managed ART clinics to nurse-managed primary health clinics for monitoring and treatment. AIDS. 2011;25:2027–2036. doi: 10.1097/QAD.0b013e32834b6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L, Brennan A, Fox MP, et al. Treatment outcomes and cost-effectiveness of shifting management of stable art patients to nurses in South Africa: an observational cohort. PLoS Med. 2011;8:e1001055. doi: 10.1371/journal.pmed.1001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairall L, Bachmann MO, Lombard C, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet. 2012;380:889–898. doi: 10.1016/S0140-6736(12)60730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipp W, Konde-Lule J, Saunders D, et al. Antiretroviral treatment for HIV in rural Uganda: two-year treatment outcomes of a prospective health centre/community-based and hospital-based cohort. PLoS Med. 2012;7 doi: 10.1371/journal.pone.0040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffar S, Amuron B, Foster S, et al. Rates of virological failure in patients treated in a home-based versus a facility-based HIV-care model in Jinja, southeast Uganda: a cluster-randomised equivalence trial. Lancet. 2009;374:2080–2089. doi: 10.1016/S0140-6736(09)61674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selke HM, Kimaiyo S, Sidle JE, et al. Task-shifting of antiretroviral delivery from health care workers to persons living with HIV/AIDS: clinical outcomes of a community-based program in Kenya. J Acquir Immune Defic Syndr. 2010;55:483–490. doi: 10.1097/QAI.0b013e3181eb5edb. [DOI] [PubMed] [Google Scholar]
- Decroo T, Koole O, Remartinez D, et al. Four-year retention and risk factors for attrition among members of community ART groups in Tete, Mozambique. Trop Med Int Health. 2014;19:514–521. doi: 10.1111/tmi.12278. [DOI] [PubMed] [Google Scholar]
- Luque-Fernandez MA, Van Cutsem G, Goemaere E, et al. Effectiveness of patient adherence groups as a model of care for stable patients on antiretroviral therapy in khayelitsha, Cape Town, South Africa. PLoS Med. 2013;8 doi: 10.1371/journal.pone.0056088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amuron B, Levin J, Birunghi J, et al. Mortality in an antiretroviral therapy programme in Jinja, south-east Uganda: a prospective cohort study. AIDS Res Ther. 2011;8:39. doi: 10.1186/1742-6405-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber A, McCarthy CF, Verani AR, et al. A survey of nurse-initiated and -managed antiretroviral therapy (NIMART) in practice, education, policy, and regulation in east, central, and Southern Africa. J Assoc Nurses AIDS Care. 2014;25:520–531. doi: 10.1016/j.jana.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Babigumira JB, Castelnuovo B, Lamorde M, et al. Cost effectiveness of a pharmacy-only refill program in a large urban HIV/AIDS clinic in Uganda. PLoS One. 2011;6:e18193. doi: 10.1371/journal.pone.0018193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys CP, Wright J, Walley J, et al. Nurse led, primary care based antiretroviral treatment versus hospital care: a controlled prospective study in Swaziland. BMC Health Serv Res. 2010;10:229. doi: 10.1186/1472-6963-10-229. [DOI] [PMC free article] [PubMed] [Google Scholar]