Abstract
Epidemiological studies suggest a protective effect of cruciferous vegetables on breast cancer. Sulforaphane (SFN), an active food component derived from crucifers, has been shown to be effective in breast cancer chemoprevention. This study evaluated the chemopreventive effect of SFN on selective biomarkers from blood and breast tissues. In a 2-8-week double-blinded, randomized controlled trial, 54 women with abnormal mammograms and scheduled for breast biopsy were randomized to consume a placebo or a glucoraphanin (GFN) supplement providing SFN (n = 27). Plasma and urinary SFN metabolites, peripheral blood mononuclear cell (PBMC) histone deacetylase (HDAC) activity, and tissue biomarkers (H3K18ac, H3K9ac, HDAC3, HDAC6, Ki-67, p21) were measured before and after the intervention in benign, ductal carcinoma in situ (DCIS), or invasive ductal carcinoma (IDC) breast tissues. Within the supplement group, Ki-67 (p = 0.003) and HDAC3 (p = 0.044) levels significantly decreased in benign tissue. Pre-to-post-intervention changes in these biomarkers were not significantly different between treatment groups after multiple comparison adjustment. GFN supplementation was associated with a significant decrease in PBMC HDAC activity (p = 0.04). No significant associations were observed between SFN and examined tissue biomarkers when comparing treatment groups. This study provides evidence that GFN supplementation for a few weeks is safe but may not be sufficient for producing changes in breast tissue tumor biomarkers. Future studies employing larger sample sizes should evaluate alternative dosing and duration regimens to inform dietary SFN strategies in breast cancer chemoprevention.
Keywords: sulforaphane, cancer, chemoprevention, breast, DCIS
INTRODUCTION
Several lines of evidence indicate that increased consumption of cruciferous vegetables has a chemopreventive effect and may protect against several of the most common types of cancer, including breast cancer (1). Although the role of vegetable consumption in breast cancer risk remains controversial, several studies have demonstrated a decrease in breast cancer risk with increasing cruciferous vegetable intake (2, 3). Cruciferous vegetables and their constituent biologically active food components, including indoles and isothiocyanates (ITC), such as sulforaphane (SFN), appear to modulate breast cancer risk at multiple stages of carcinogenesis through a variety of biological mechanisms (4).
Ductal carcinoma in situ (DCIS) is a non-invasive form of breast cancer that accounts for about 20% of newly diagnosed cases of breast cancer (5, 6). DCIS lesions arise from terminal-duct-lobular units. Their presentation is considered a direct precursor, and thus a very high risk factor, for invasive cancer (6, 7). While there have been recent improvements in the treatment of breast cancer, epidemiological studies have shown that women are more likely to change their lifestyle behaviors and medication use following diagnosis of DCIS (8). Hence, there is a need for scientifically directed evaluation of the effect of alternative or supplemental therapies, such as dietary supplements that may effectively inhibit the progression of breast cancer in women. In this study, we evaluated the impact of SFN, obtained from a supplement containing its precursor glucosinolate, on molecular response biomarkers in blood and breast tissue (including tumor and non-tumor) from women that were scheduled for diagnostic biopsies following abnormal mammogram results. This is the first report of the effects of SFN on breast tissue physiology in women. Observations from this study will inform chemoprevention strategies in women with DCIS with or without a component of invasion, as well as women that present with benign tissue.
SFN exists in particularly high amounts in broccoli and broccoli sprouts (9) as the glucosinolate precursor, glucoraphanin (GFN). When the plant is consumed, GFN is converted to SFN by myrosinases released from plant tissue and present in the human gut (10). SFN has been shown to be an effective chemopreventive agent in both in vitro and in vivo models for breast cancer where SFN is able to selectively induce apoptosis and slow tumor growth (11-14). Mechanistic studies have identified several targets of SFN, including cell cycle proteins such as p21, which may be involved in its anti-cancer activities (15). SFN has also been shown to decrease levels of Ki-67, a marker of cell proliferation, in prostate tumor tissue and breast cancer cell xenografts (15, 16). Ki-67 is known as an important prognostic biomarker in women with breast cancer (17).
Recent work indicates that SFN targets epigenetic alterations and inhibits histone deacetylases (HDAC) (18, 19). HDACs, along with histone acetyltransferases (HAT), facilitate an important mechanism of gene regulation which involves the removal and addition, respectively, of acetyl groups from histone proteins. Inhibiting HDACs can lead to increased histone acetylation and re-expression of tumor suppressor genes (e.g., p21) that are often silenced in cancer cells (20, 21). Pharmacological HDAC inhibitors have demonstrated anti-cancer effects in breast cancer cells both in vitro and in vivo (22, 23). However, the adverse effects of these agents make them undesirable for long-term use in women with pre-invasive disease, such as DCIS (24). Intake of cruciferous vegetables and dietary SFN are considered safe and have not been associated with any serious adverse side effects (25). Therefore, we aimed to evaluate the efficacy of consuming a broccoli sprout extract in altering HDAC activity and improving biomarkers for prognosis in women with benign disease or DCIS with or without a component of invasion.
MATERIALS AND METHODS
Participants
This double-blind, randomized, placebo-controlled clinical trial was conducted in collaboration with clinicians and researchers at Oregon Health and Science University's (OHSU) Center for Women's Health Breast Center in Portland, OR. English-speaking women were recruited to participate in the study from OHSU, Kaiser Permanente Northwest and Epic Imaging Clinics. Inclusion criteria included: ≥ 21 years of age, diagnostic mammogram with results that required biopsy. Exclusion criteria included: invasive breast cancer without DCIS or atypical ductal hyperplasia (ADH), pregnancy (determined by clinically administered urine pregnancy test), patient reported breastfeeding, significant active medical illness, history of or active liver disease or baseline total bilirubin greater than institutional upper limit of normal, allergy to cruciferous vegetables, use of oral antibiotics (except doxycycline) within three months prior to randomization, oral steroid therapy at enrollment, current therapy with valproate acid or suberoyl + anilide + hydroxamic acid (SAHA), current and planned continuous use of SFN-containing supplements, herbal remedies or pharmaceutical HDAC inhibitors, additional surgical operations scheduled within 30 days of study start date, neoadjuvant radiation or chemotherapy for currently-diagnosed disease prior to or during study supplementation, or any condition possibly exacerbated by participating. Eligible women met with study coordinators at the OHSU Clinical and Translational Research Center (CTRC) to review the study's purpose and exclusion criteria. All participants provided informed consent. Study protocols were approved by OHSU and Kaiser Permanente Northwest committees for the protection of human subjects (ClinicalTrials.gov Identifier: NCT00843167).
Study Design
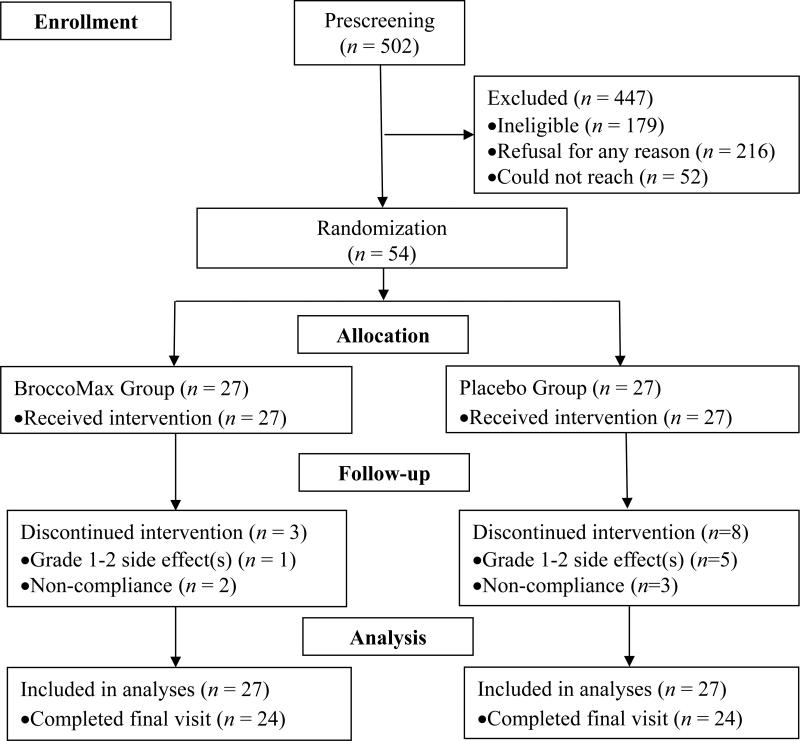
The study sample size flowchart is depicted in Figure 1 following CONSORT guidelines (26). Consented subjects (N = 54) were randomized to consume a minimum two-week supply of either ~250 mg of a broccoli seed extract containing GFN (BroccoMax™) (n = 27), or matching placebos containing ingredients of microcrystalline cellulose (n = 27). Subjects were instructed to take 2 pills 3×/day. BroccoMax™ supplements are reported to contain 30 mg GFN by manufacturers. SFN is rapidly cleared from the body, and multiple doses during the day may help maintain higher SFN plasma levels. Subjects always began supplementation following diagnostic biopsy and informed consent. For those diagnosed with DCIS or ADH with or without a component of invasive cancer, supplementation ended the day before operation. The maximum supplement intervention period was 8 weeks (56 days). Women with surgery scheduled earlier than 2 weeks post-biopsy were not eligible for enrollment, such that participation in the study would not delay surgery. Subjects assigned to the placebo received capsules identical to the BroccoMax™ capsules 3×/day. The total daily dose of GFN used in this study was 224 mg GFN (verified in-house), similar to the amount administered in our pilot study and other trials that achieved a significant increase in blood and urine ITC levels and reduced histone activity within one month with no reported adverse effects (25, 27, 28). All pills and containers were provided by Jarrow Formulas® (Los Angeles, CA) and dispensed by the OHSU Research Pharmacy. Administration of the intervention was extended up to an eight-week supply for women experiencing surgical operation delays not related to the dietary intervention or for whom operation was not indicated or chosen post-biopsy. After consenting to the research, subjects also completed family history and risk factor questionnaires, as well as two dietary history questionnaires: a modified National Cancer Institute (NCI) diet history questionnaire and the Arizona cruciferous vegetable food frequency questionnaire (CVFFQ) (29). Additional questionnaires were administered throughout the duration of each woman's participation on and 30 days after the intervention to monitor cruciferous vegetable intake, safety, and any changes in medications, supplement use or dietary intake. For any reported adverse event characterized as grade 3 or higher, according to the NCI Common Terminology Criteria for Adverse Events Version 3.0, the responsible clinician was notified, the event was determined related/not-related to the intervention, and the event was recorded. Adherence to study protocol (≥ 80%) was determined by Research Pharmacy count of returned pills.
Figure 1.
Trial enrollment flow chart
Sample Collection
Two, non-fasting, 30-ml whole blood specimens and a spot urine sample were collected from each participant at the baseline visit prior to starting the intervention and at the final visit. Blood was collected in BD lavender top tubes containing EDTA as the anticoagulant. Plasma was isolated by centrifugation, immediately acidified with 10% (v/v) pre-cooled trifluoroacetic acid (TFA), and stored at −80°C. Peripheral blood mononuclear cells (PBMC) were isolated using Histopaque (Sigma, St. Louis) separation, suspended in DMSO, and stored in liquid nitrogen until further analysis. Urine samples were acidified immediately with 10% (v/v) TFA and stored at −80°C. Diagnostic tissue specimens were formalin fixed, subjected to routine processing and paraffin embedding. All breast biopsy or surgical tissues were evaluated for the presence of DCIS and/or ADH or IDC immediately after these procedures by board certified pathologists.
Preparation of Mass Spectrometry Standards
Chemical standards for (R,S-) SFN and its metabolites (SFN-GSH, SFN-Cys, SFN-NAC) were purchased from LKT Laboratories, Inc. (St. Paul, MN) and Toronto Research Chemicals (Canada), respectively. Deuterated SFN-NAC (SFN-NAC-D3) and SFN-cysteinylglycine (SFN-CG) were prepared in-house as previously described (30). GFN and glucotropaeolin (GTP) were purchased from The Royal Veterinary School of Denmark and AppliChem (Darmstadt, Germany), respectively. All final standard dilutions were prepared in 0.1% (v/v) formic acid (FA) in H2O. Consistent and high (>80%) recoveries of ITC and glucosinolate standards from both biological matrices and 0.1% (v/v) FA in water were confirmed through a series of spike and recovery experiments using available internal standards.
Analysis of GFN Content within Broccoli Seed Extract Supplements
Each batch of BroccoMax™ supplements administered to subjects during the trial was analyzed in duplicate for GFN content. No significant batch-to-batch variation was detected. Our method was adapted from Tian, et al. (31). BroccoMax™ powder (~450-480 mg) was dissolved into 100% methanol and homogenized 5 min with an Omni homogenizer (Omni International, Kennesaw, GA). Mixtures were centrifuged (5 min, 25°C, 10,000 × g). Methanol extractions (3×/extract) were performed using supernatants. Preliminary experiments revealed > 95% GFN recovery within first 3 extractions (data not shown). All extracts were combined and filtered through Spin-X® centrifuge tube filters (VWR, Radnor, PA) by centrifugation (5 min, 25°C, 10,000 × g). Filtrates were diluted with 0.1% (v/v) FA in H2O to final concentrations of 250 μM GFN and stored at −20°C. GTP was used as an internal standard. Pill extracts (10 μl) were injected in duplicate. HPLC-MS/MS conditions were similar to those in our previous study (32), except that analysis was performed in negative ion mode using a 4-μm Synergi Hydro-RP, 80 Å, 150 × 2.0 mm reversed phase column (Phenomenex, Torrance, CA) with 0.2 μm guard column (Optimize Technologies, Inc., Oregon City, OR). The following precursor and product ions were used for detection: GFN (436 > 96/97), GTP (408 > 166). The final calculated GFN dose provided to participants was 224 mg GFN per day.
Isothiocyanate Analysis in Urine and Plasma
Following protein precipitation by centrifugation (3184 × g, 4 min, 2°C), plasma and urine supernatants were filtered twice through Spin-X® centrifuge tube filters (12,000 × g, 3.5 min, 2°C). Plasma filtrates were stored at −80°C. Urine filtrates were diluted 1:2 with 0.1% (v/v) FA in H2O prior to storage. Matched pre and post samples were analyzed for SFN, SFN-Cys (299 > 114), SFN-GSH (485 > 179), SFN-CG (356 > 114), and SFN-NAC (341.1 > 114) in duplicate following a 10-μl injection. Instrumentation and HPLC-MS/MS conditions were the same as used previously (32), except SFN metabolites were detected with an Applied Biosystems MDS Sciex 4000 Q TRAP HPLC-MS/MS system.
Urinary Creatinine Analysis
Creatinine concentrations were determined using the Jaffe reaction method as previous described (33). SFN and SFN metabolite concentrations in urine were normalized using creatinine concentrations to control for differences in urine volume.
PBMC HDAC Activity Analysis
Analyses were performed by the Cancer Chemoprevention Program's Core Laboratory at the Linus Pauling Institute. PBMC HDAC activity was evaluated using the positive control, sodium butyrate, as previously described (19). Substrates and standards for the assay were custom synthesized by AAPPTec, LLC (Louisville, KY). HDAC activity is expressed relative to PBMC protein content and negative control (DMSO).
Immunohistochemistry (IHC) Staining
IHC was performed on paraffin-embedded, breast biopsy samples pre-intervention and surgical samples post-intervention as described by Elsheikh, et al. (34). Briefly, slides of paraffin-embedded breast tissue specimens were deparaffinized in xylenes (3 × 3 min), rehydrated with graded alcohols, washed 10 min in Tris-buffered saline (pH 7.2-7.6), heated 10 min in a Russell-Hobbs programmable pressure cooker in 0.01 M citrate buffer (pH 6.0), treated 5 min with 3% aqueous H2O2 solution, blocked 1 h at 25°C in 3% goat serum, incubated 1 h at 25°C with primary antibodies for acetylated histone H3 lysine 9 (H3K9) (1:1000) and 18 (H3K18) (1:2000), p21 and Ki-67 (Abcam, Cambridge, MA), and HDAC6 and HDAC3 (Santa Cruz Biotechnology, Inc., Dallas, TX) followed by mouse Envision (Dako, Glostrup, Denmark), counterstained 1 min with Gill's hematoxylin, rinsed, dehydrated, and coverslipped using Permount. Benign breast tissues were scored by a collaborating pathologist separately from in situ disease or invasive carcinoma, where available. A modified Histo-score (H-score) was recorded, which involved semi-quantitative assessment of both staining intensity (graded as 1-3 with 1 representing weak staining, 2 moderate, and 3 strong) and percentage of positive cells.
Statistical Methods
All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Intent-to-treat analysis was performed. Baseline characteristics were expressed as means and standard errors (SE) for continuous variables, and counts (n) and percentages (%) for categorical variables, stratified by treatment group. The comparability of the two treatment groups for baseline characteristics were tested using independent two-sample t-tests for continuous variables and Chi-square tests for categorical variables. Any of the baseline characteristics found to be significantly different between groups, and also associated with the primary endpoints, were considered as possible adjustment variables in the final models.
The primary outcomes examined include isothiocyanate levels, HDAC activity, Ki-67, p21, and levels of acetylation of H3K9 and H3K18. Our primary interest was to determine whether changes from pre- to post-treatment were significantly different between placebo and supplement groups. The analysis was conducted separately for each primary endpoint as well as for each specimen type (e.g., blood, urine, normal tissue, cancer tissue). Shapiro-Wilks Normality tests were conducted for all continuous variables. Comparison between pre- and post-intervention levels of SFN metabolites and PBMC HDAC activity within each treatment group was conducted using either a paired t-test or Wilcoxon signed rank sum test. For urinary and blood SFN metabolites, Wilcoxon signed rank tests were conducted for the pre- to post-treatment changes between treatment groups. Tissue biomarkers were log2 transformed in order to obtain approximate normality.
To assess treatment group differences in the changes in primary outcome biomarkers, linear mixed effects models were conducted separately for each outcome to calculate adjusted least square means (LSMEANS) and 95% confidence intervals (95% CI), and to test the statistical significance of the difference between pre- and post- treatments within each group, as well as between treatment groups. The mixed effects model has the advantage of accommodating incomplete data as well as within-subject correlation due to repeated measurements (i.e., pre- and post-intervention). NSAIDs use was included as a covariate in all models due to the baseline difference between treatment groups. Length of intervention was also included in the mixed effect model. To adjust for multiple comparisons of the primary endpoints, we applied the method of False Discovery Rate (FDR) (35). The FDR p values were provided in addition to the standard p values for the overall treatment comparisons.
Adverse events and compliance between the treatment groups were analyzed using Chi-square tests or Fisher's exact test, as appropriate. Tests of statistical significance were conducted using two-sided tests, and a p-value ≤ 0.05 was considered statistically significant unless otherwise noted.
RESULTS
Patient Characteristics and Adverse Events
From December 23rd, 2008 to March 27th, 2013, a total of 54 participants aged 25-83 years (54 ± 12) were randomized into this trial at OHSU. The mean length of the intervention was 37 days (SD: 19 days). With regard to the number of women across the number of weeks, 34 (63%) of the women had intervention period between 2-8 weeks, 13 (24%) had intervention period < 2 weeks, and 7 (13%) had intervention period > 8 weeks. Supplementary Table 1 describes the baseline characteristics of the 54 patients stratified by treatment group. The supplement group reported significantly higher proportion of NSAIDs use than the placebo group (p = 0.002). There was no statistically significant difference in age, BMI, height, cruciferous vegetable intake, race, family history of breast cancer, smoking, alcohol, income, education, marital status or menopausal status. There was no difference between treatment groups for each specific type of adverse event and total number of adverse events (Supplementary Table 2). In addition, no statistically significant difference was observed in terms of compliance to the treatment plan between the two treatment groups (p = 0.88).
Urinary and Blood Biomarkers
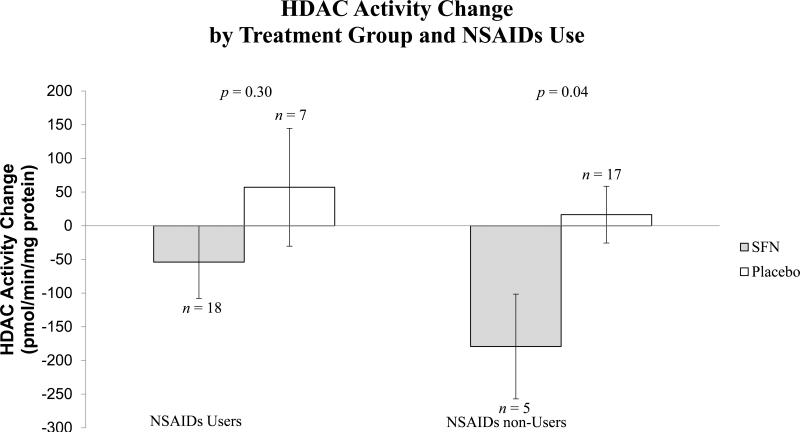
Supplementary Table 3 presents all continuous outcomes of isothiocyanates from urine and plasma and HDAC activity in PBMCs. Pre- to post-intervention changes in total urinary SFN isothiocyanates and in individual SFN metabolites (SFN-NAC, SFN-Cys, SFN-GSH, and SFN) were statistically higher in the SFN group compared to the placebo group. In plasma, pre- to post-intervention changes in total SFN isothiocyanates and individual SFN metabolites (SFN-NAC, SFN-GSH and SFN-CG) were statistically significant in the SFN group only. No SFN metabolites were detected in plasma from the placebo group. Changes in SFN-Cys levels in plasma were not significantly different between treatment groups. We also compared the means of pre- and post-intervention PBMC HDAC activity levels within each treatment group. For the SFN group, the average change in HDAC activity from pre- to post-intervention was a decrease of 80.39 pmol/min/mg protein (p = 0.11); for the placebo group, the average change from pre- to post-intervention was an increase of 27.52 pmol/min/mg protein (p = 0.40). Comparing the two groups, changes in HDAC activity were significantly different (p = 0.04). In a sub-analysis stratified by NSAIDs use, we observed a statistically significant difference in HDAC activity among non-NSAID users (p = 0.04), and no significant difference among NSAID users (Figure 2).
Figure 2. Comparison of PBMC HDAC activity change between intervention groups stratified by NSAIDs use.
Changes in HDAC activity from pre- to post-intervention between treatment groups were compared stratified by NSAIDs use using mixed effect model. Values shown indicate least-squares means of (lsmean ± SE) of pre-to-post change of HDAC activity.
IHC Biomarkers
Fifty (92%) women (24 in SFN group and 26 in placebo group) consented to analysis of breast biopsy tissue and were included in IHC analysis. Levels of H3K18ac, H3K9ac, HDAC3, HDAC6, Ki-67 and p21 were evaluated by IHC from pre-treatment biopsies followed by post-treatment biopsies lumpectomy or mastectomy specimens (when available). Interaction tests between NSAIDs use and treatment group did not show any statistical significance; therefore, NSAIDs use was adjusted in all models as a single variable, not as an interaction term. Supplementary Table 4 shows the log2-transformed LSMEANS of the tissue biomarkers by treatment groups and the p-values comparing pre-to-post changes of biomarkers between and within treatment groups after adjusting NSAIDs use and length of intervention. Through multiple comparison adjusted p-value using the Benjamini-Hochberg False Discovery rate, there was no statistical significance between treatment groups for pre-to-post changes of all the examined tissue biomarkers including H3K18ac, H3K9ac, HDAC3, HDAC6, Ki-67 and p21 levels in all the three tissue types. Before adjusting for false discovery rate, a significant difference in pre-to-post changes of Ki-67 was present between the two treatment groups among benign tissues, but not among DCIS or IDC tissues. Comparing pre- and post-treatment levels within each treatment group, there was a significant decrease in Ki-67 and HDAC3 in benign tissues in the SFN group and a significant decrease in H3K9ac in DCIS tissue in the placebo group.
DISCUSSION
In this analysis of 54 women who participated in this randomized, placebo-controlled trial, we found that SFN supplementation was associated with reduced PBMC HDAC activity. In addition, we observed significant pre-to-post changes in Ki-67 and HDAC3 within the SFN supplementation group. However, we did not observe significant differences between SFN and placebo groups for any of the tissue biomarkers examined including H3K9ac, H3K18ac, HDAC3, HDAC6, Ki-67 and p21.
Ki-67 is a marker of cell proliferation. We observed significant decreases in Ki-67 levels via IHC following SFN supplementation in benign tissue. The difference between treatment groups was not significant after adjusting for multiple comparisons; however, the change in the SFN group was significant and quite different than that of the placebo group, which had a non-significant increase in Ki-67 levels. There is evidence that Ki-67 gene expression is regulated in part through epigenetic mechanisms involving HDACs. For example, Stearns, et al. (36) reported significant decreases in Ki-67 gene expression, but not protein levels, in invasive breast tissue obtained from women treated with the pharmacological HDAC inhibitor, Vorinostat, compared to untreated subjects. Similar to this report, we also did not observe changes in Ki-67 protein levels following SFN supplementation in cancer tissue. It is possible that timing (pre-disease vs. disease) and disease stage may influence a cell's response to SFN.
There are several reports that SFN inhibits HDAC activity in cultured cells and animal models, but only a few human studies report decreases in HDAC activity with SFN consumption (18, 28, 37-39). One study in healthy individuals reported that PBMC HDAC activity was lower after consuming BroccoMax™ supplements compared to a placebo, though changes from pre-intervention levels were not statistically significant (39). Another human study reported larger decreases in PBMC HDAC activity in healthy adults following consumption of broccoli sprouts (28). That study used a small sample size (n = 3), so it is unclear whether or not the magnitudes of changes they observed are widely achievable in the population at similar SFN doses. Furthermore, Pledgie-Tracy, et al. (38) reported decreased HDAC activity in multiple breast cancer cell lines, including the DCIS-like cell line, T-47D. Specific metabolites of SFN are thought responsible for SFN-associated effects on HDACs. One study using several cell lines showed that SFN-NAC and SFN-Cys had the greatest HDAC inhibition effects compared to SFN and other SFN metabolites (19). In this study, we observed a significant difference in changes in PBMC HDAC activity between intervention groups, suggesting that the decreases observed may have been related to higher SFN exposure in the supplementation group. Because NSAIDs use was different between intervention groups, we compared changes in HDAC activity between groups among NSAIDs users and separately among NSAIDs non-users. Among NSAIDs non-users, mean fold change in HDAC activity was significantly different in supplement consumers compared to placebo consumers (Figure 2). NSAIDs inhibit synthesis of prostaglandins, which could suppress regulatory protein expression via recruitment of HDACs (40). Increased recruitment of HDACs to chromatin may prevent inhibition associated with SFN consumption, which is one potential explanation for our observations. Future studies on SFN with larger sample sizes are needed to confirm our findings.
Specific HDAC proteins have been reported to be inhibited by SFN. In colon cancer cell lines, HDAC3 and HDAC6 were among HDACs that showed the largest decrease in protein expression following SFN exposure (41). Clarke et al. (42) further demonstrated decreases in HDAC3 and HDAC6 in prostate cancer cells treated with SFN. We evaluated changes in HDAC3 and HDAC6 protein expression in breast biopsy tissue as targets of SFN. HDAC3 was significantly decreased in the supplement group, which may have contributed to the decreases in total HDAC activity we observed. HDAC6 was not decreased with SFN supplementation. Rajendran et al. (41) demonstrated that changes in HDAC protein expression following SFN treatment are time-dependent, where decreases in HDAC6 occurred after decreases in HDAC3.
Decreases in histone acetylation have been reported to occur with progression of normal breast epithelium to DCIS (43). Studies in prostate and breast cancer cells, and in an in vivo rat model of breast cancer, have shown that decreasing HDAC activity can result in increased histone and protein acetylation (44, 45). However, another study in breast cancer cells, reported that decreases in HDAC activity were not associated with increased histone H3 or H4 acetylation (38). In this study, we did not observe increased H3K18ac or H3K9ac, despite decreases in HDAC3 expression and total HDAC activity. We did observe a significant decrease in H3K9ac in DCIS tissue among placebo consumers, which could be related to cancer progression (43). Though it cannot be determined from the present data, GFN supplementation may have mitigated decreases in histone acetylation.
p21 is a major cell cycle regulator. Increased expression of p21 in breast cancer cells leads to cell cycle arrest (46). SFN increased p21 expression in breast cancer cell lines (15), and regulation of p21 expression has been shown to involve HDACs (47, 48). In this study, statistically significant changes in p21 protein expression were not observed in breast tissue with or without GFN supplementation. The intake threshold for increasing expression of p21 in human breast tissue is unclear and may not have been achieved by the doses consumed in this study. There could have also been other reasons why p21 may not have changed, including post-transcriptional and post-translational modifications as reviewed recently (49). Additionally, it is possible that regulation of p21 expression was altered in the tumors analyzed, altering responsiveness to changes in HDAC activity. Yet, no changes in p21 levels were observed in benign tissue either. Overall, our observations suggest that SFN does not alter cell proliferation during all stages of breast tumorigenesis.
Our study has several strengths. First, we analyzed biomarkers from both breast tissues and PBMCs among pre- and post-intervention samples. Second, we collected multiple types of breast tissue, including benign, DCIS and IDC, to examine potential effects of SFN in lesional and non-lesional breast tissue. Third, information on adverse events and changes to diet and medication use was collected at each visit among maximum 9 visits during the entire intervention period and ~30 days after participants’ surgical or post-intervention appointment. Study coordinators and subjects were both blinded to treatment assignment to minimize information bias. Study limitations include small sample size, limited tissue availability in some cases, and hospital-based study design. Importantly, most comparisons met the minimum sample size required to detect a biologically meaningful difference between intervention groups with ≥80% power. .
In conclusion, this study provides evidence for chemopreventive activity of SFN in human breast tissue. We demonstrated effects of SFN on known cancer biomarkers as well as epigenetic targets in vivo. Additional studies are needed to evaluate dose-responses and responses of other relevant molecular targets to consuming foods and supplements that provide SFN. While the supplements used in this study were well-tolerated, recent work indicated that other broccoli extract preparations may be more bioavailable and should be considered for use in future studies to enhance SFN absorption from these forms (33). Overall, this work provides important information for future larger population-based clinical trials investigating the impacts of consuming dietary sources of SFN.
Supplementary Material
Acknowledgments
We appreciate technical assistance from the Environmental Health Sciences Center at OSU and tissue staining assistance from Cara Poage and Jenny Miller with the OHSU Knight Cancer Institute Cancer Pathology Shared Resource. Dr. Megan Troxell remained engaged with the investigators and research team throughout the three-year long project about biomarker staining effectiveness, conducting literature reviews to uncover the most up-to-date methodologies, scoring multiple stacks of immunohistochemistry slides and taking the time to review the manuscript. OHSU Breast Center Nurses Christine Kemp and Brooke Hamby enthusiastically assisted research coordinators’ efforts (Alysia Cox, Amy Palma and Summer Carter) toward the recruitment of eligible study participants. We greatly appreciate all of the women who contributed their time and effort to join our study (Sulforaphane: A Dietary HDAC Inhibitor and Prevention of DCIS Progression) as well as those who expressed strong interest but who, for many reasons, were not able to participate. Jarrow Formulas® (Los Angeles, CA) generously donated the sulforaphane extract (BroccoMax™) we utilized to test our hypotheses. We also thank Drs. Brian Druker, Eric Orwoll, and Joseph. Beckman for their support of this project.
Grant support: This study was supported by the National Cancer Institute R21 CA132236-01A2 (J. Shannon and E. Ho), P01 CA090890 (E. Ho and J. Shannon), P30 CA069533, National Center for Advancing Translational Sciences of the National Institutes of Health UL1TR000128, and National Institute of Environmental Health Sciences P30 ES000210. Clinical Trial Registration: clinicaltrials.gov identifier: NCT00843167.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Clinical Trial Registry: NCT00843167
REFERENCES
- 1.Tortorella SM, Royce SG, Licciardi PV, Karagiannis TC. Dietary sulforaphane in cancer chemoprevention: The role of epigenetic regulation and HDAC inhibition. Antiox Redox Signal. 2015;22:1382–424. doi: 10.1089/ars.2014.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134:1134–8. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Kezhen L. Cruciferous vegetables intake is inversely associated with risk of breast cancer: A meta-analysis. Breast. 2013;22:309–13. doi: 10.1016/j.breast.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Fuentes F, Paredes-Gonzalez X, Kong AT. Dietary glucosinolates sulforaphane, phenethyl isothiocyanate, indole-3-carbinol/3,3′-diindolylmethane: Antioxidative stress/inflammation, Nrf2, epigenetics/epigenomics and in vivo cancer chemopreventive efficacy. Curr Pharmacol Rep. 2015;1:179–96. doi: 10.1007/s40495-015-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vatovec C, Erten MZ, Kolodinsky J, Brown P, Wood M, James T, et al. Ductal carcinoma in situ: A brief review of treatment variation and impacts on patients and society. Crit Rev Euk Gene Expr. 2014;24:281–6. doi: 10.1615/critreveukaryotgeneexpr.2014011495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: A systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102:170–8. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 7.Allegra CJ, Aberle DR, Ganschow P, Hahn SM, Lee CN, Millon-Underwood S, et al. National Institutes of Health State-of-the-Science Conference statement: Diagnosis and management of ductal carcinoma in situ September 22–24, 2009. J Natl Cancer Inst. 2010;102:161–9. doi: 10.1093/jnci/djp485. [DOI] [PubMed] [Google Scholar]
- 8.Sprague BL, Trentham-Dietz A, Nichols HB, Hampton JM, Newcomb PA. Change in lifestyle behaviors and medication use after a diagnosis of ductal carcinoma in situ. Breast Cancer Res Treat. 2010;124:487–95. doi: 10.1007/s10549-010-0869-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci. 1997;94:10367–72. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li F, Hullar MA, Beresford SA, Lampe JW. Variation of glucoraphanin metabolism in vivo and ex vivo by human gut bacteria. Br J Nutr. 2011;106:408–16. doi: 10.1017/S0007114511000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandi G, Schiavano GF, Zaffaroni N, De Marco C, Paiardini M, Cervasi B, et al. Mechanisms of action and antiproliferative properties of Brassica oleracea juice in human breast cancer cell lines. J Nutr. 2005;135:1503–9. doi: 10.1093/jn/135.6.1503. [DOI] [PubMed] [Google Scholar]
- 12.Gill CI, Haldar S, Porter S, Matthews S, Sullivan S, Coulter J, et al. The effect of cruciferous and leguminous sprouts on genotoxicity, in vitro and in vivo. Cancer Epidemiol Biomarkers Prev. 2004;13:1199–205. [PubMed] [Google Scholar]
- 13.Jackson SJ, Singletary KW. Sulforaphane: a naturally occurring mammary carcinoma mitotic inhibitor, which disrupts tubulin polymerization. Carcinogenesis. 2004;25:219–27. doi: 10.1093/carcin/bgg192. [DOI] [PubMed] [Google Scholar]
- 14.Johnston N. Sulforaphane halts breast cancer cell growth. Drug Discov Today. 2004;9:908. doi: 10.1016/S1359-6446(04)03267-2. [DOI] [PubMed] [Google Scholar]
- 15.Kanematsu S, Yoshizawa K, Uehara N, Miki H, Sasaki T, Kuro M, et al. Sulforaphane inhibits the growth of KPL-1 human breast cancer cells in vitro and suppresses the growth and metastasis of orthotopically transplanted KPL-1 cells in female athymic mice. Oncol Rep. 2011;26:603–8. doi: 10.3892/or.2011.1311. [DOI] [PubMed] [Google Scholar]
- 16.Shankar S, Ganapathy S, Srivastava RK. Sulforaphane enhances the therapeutic potential of TRAIL in prostate cancer orthotopic model through regulation of apoptosis, metastasis, and angiogenesis. Clin Cancer Res. 2008;14:6855–66. doi: 10.1158/1078-0432.CCR-08-0903. [DOI] [PubMed] [Google Scholar]
- 17.Inwald EC, Klinkhammer-Schalke M, Hofstädter F, Zeman F, Koller M, Gerstenhauer M, et al. Ki-67 is a prognostic parameter in breast cancer patients: Results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139:539–52. doi: 10.1007/s10549-013-2560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myzak MC, Dashwood WM, Orner GA, Ho E, Dashwood RH. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apcmin mice. FASEB J. 2006;20:506–8. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane. Cancer Res. 2004;64:5767–74. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- 20.Glaser KB, Staver MJ, Waring JF, Stender J, Ulrich RG, Davidsen SK. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: Defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol Cancer Ther. 2003;2:151–63. [PubMed] [Google Scholar]
- 21.Yang X, Ferguson AT, Nass SJ, Phillips DL, Butash KA, Wang SM, et al. Transcriptional activation of estrogen receptor α in human breast cancer cells by histone deacetylase inhibition. Cancer Res. 2000;60:6890–4. [PubMed] [Google Scholar]
- 22.Alao JP, Lam EW, Ali S, Buluwela L, Bordogna W, Lockey P, et al. Histone deacetylase inhibitor trichostatin A represses estrogen receptor alpha-dependent transcription and promotes proteasomal degradation of cyclin D1 in human breast carcinoma cell lines. Clin Cancer Res. 2004;10:8094–104. doi: 10.1158/1078-0432.CCR-04-1023. [DOI] [PubMed] [Google Scholar]
- 23.Bali P, Pranpat M, Swaby R, Fiskus W, Yamaguchi H, Balasis M, et al. Activity of suberoylanilide hydroxamic Acid against human breast cancer cells with amplification of her-2. Clin Cancer Res. 2005;11:6382–9. doi: 10.1158/1078-0432.CCR-05-0344. [DOI] [PubMed] [Google Scholar]
- 24.Lakshmaiah K, Jacob L, Aparna S, Lokanatha D, Saldanha S. Epigenetic therapy of cancer with histone deacetylase inhibitors. J Cancer Res Ther. 2014;10:469–78. doi: 10.4103/0973-1482.137937. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, et al. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: A clinical phase I study. Nutr Cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 26.Bolignano D, Mattace-Raso F, Torino C, D'Arrigo G, ElHafeez SA, Provenzano F, et al. The quality of reporting in clinical research: the CONSORT and STROBE initiatives. Aging Clin Exp Res. 2013;25:9–15. doi: 10.1007/s40520-013-0007-z. [DOI] [PubMed] [Google Scholar]
- 27.Kensler TW, Chen J, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 2005;14:2605–13. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 28.Myzak MC, Tong P, Dashwood WM, Dashwood RH, Ho E. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Exp Biol Med. 2007;232:227–34. [PMC free article] [PubMed] [Google Scholar]
- 29.Thomson CA, Newton TR, Graver EJ, Jackson KA, Reid PM, Hartz VL, et al. Cruciferous vegetable intake questionnaire improves cruciferous vegetable intake estimates. J Am Diet Assoc. 2007;107:631–43. doi: 10.1016/j.jada.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Clarke JD, Hsu A, Riedl K, Bella D, Schwartz SJ, Stevens JF, et al. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol Res. 2011;64:456–63. doi: 10.1016/j.phrs.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian Q, Rosselot RA, Schwartz SJ. Quantitative determination of intact glucosinolates in broccoli, broccoli sprouts, Brussels sprouts, and cauliflower by high-performance liquid chromatography–electrospray ionization–tandem mass spectrometry. Analyt Biochem. 2005;343:93–9. doi: 10.1016/j.ab.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 32.Clarke JD, Hsu A, Williams DE, Dashwood RH, Stevens JF, Yamamoto M, et al. Metabolism and tissue distribution of sulforaphane in Nrf2 knockout and wild-type mice. Pharm Res. 2011;28:3171–9. doi: 10.1007/s11095-011-0500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atwell LL, Hsu A, Wong CP, Stevens JF, Bella D, Yu T-W, et al. Absorption and chemopreventive targets of sulforaphane in humans following consumption of broccoli sprouts or a myrosinase-treated broccoli sprout extract. Mol Nutr Food Res. 2015;59:424–33. doi: 10.1002/mnfr.201400674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elsheikh SE, Green AR, Rakha EA, Powe DG, Ahmed RA, Collins HM, et al. Global histone modifications in breast cancer correlate with tumor phenotypes, prognostic factors, and patient outcome. Cancer Res. 2009;69:3802–9. doi: 10.1158/0008-5472.CAN-08-3907. [DOI] [PubMed] [Google Scholar]
- 35.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 36.Stearns V, Jacobs LK, Fackler MJ, Tsangaris TN, Rudek MA, Higgins M, et al. Biomarker modulation following short-term Vorinostat in women with newly diagnosed primary breast cancer. Clin Cancer Res. 2013;19:4008–16. doi: 10.1158/1078-0432.CCR-13-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis. 2006;27:9. doi: 10.1093/carcin/bgi265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pledgie-Tracy A, Sobolewski MD, Davidson NE. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther. 2007;6:1013–21. doi: 10.1158/1535-7163.MCT-06-0494. [DOI] [PubMed] [Google Scholar]
- 39.Clarke JD, Riedl K, Bella D, Schwartz SJ, Stevens JF, Ho E. Comparison of isothiocyanate metabolite levels and histone deacetylase activity in human subjects consuming broccoli sprouts or broccoli supplement. J Agric Food Chem. 2011;59:10955–63. doi: 10.1021/jf202887c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Q, Merkler KA, Zhang X, McLean MP. Prostaglandin F2α suppresses rat steroidogenic acute regulatory protein expression via induction of yin yang 1 protein and recruitment of histone deacetylase 1 protein. Endocrinology. 2007;148:5209–19. doi: 10.1210/en.2007-0326. [DOI] [PubMed] [Google Scholar]
- 41.Rajendran P, Delage B, Dashwood MW, Yu T-W, Wuth B, Williams DE, et al. Histone deacetylase turnover and recovery in sulforaphane-treated colon cancer cells: Competing actions of 14-3-3 and Pin1 in HDAC3/SMRT corepressor complex dissociation/reassembly. Mol Cancer. 2011:10. doi: 10.1186/1476-4598-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clarke JD, Hsu A, Yu Z, Dashwood RH, Ho E. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells. Mol Nutr Food Res. 2011;55:999–1009. doi: 10.1002/mnfr.201000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki J, Chen Y-Y, Scott GK, DeVries S, Chin K, Benz CC, et al. Protein acetylation and histone deacetylase expression associated with malignant breast cancer progression. Clin Cancer Res. 2009;15:3163–71. doi: 10.1158/1078-0432.CCR-08-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibbs A. Sulforaphane destabilizes the androgen receptor in prostate cancer cells by inactivating histone deacetylase 6. P Natl Acad Sci USA. 2009;106:16663–8. doi: 10.1073/pnas.0908908106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vigushin DM, Simak A, Pace PE, Mirsaidi N, Ito K, Adcock I, et al. Trichostatin A is a histone deacetylase inhibitor with potent antitumor activity against breast cancer in vivo. Clin Cancer Res. 2001;7:971–6. [PubMed] [Google Scholar]
- 46.Sambuccetti LC, Fischer DD, Zabludoff S, Kwon PO, Chamberlin H, Trogani N, et al. Histone Deacetylase Inhibition Selectively Alters the Activity and Expression of Cell Cycle Proteins Leading to Specific Chromatin Acetylation and Antiproliferative Effects. J Biol Chem. 1999;274:34940–7. doi: 10.1074/jbc.274.49.34940. [DOI] [PubMed] [Google Scholar]
- 47.Gui C. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci. 2004;101:1241–6. doi: 10.1073/pnas.0307708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang ZZC, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, et al. Combinatorial patters of histone acetylations and methylations in the human genome. Nature Genet. 2008;40:897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jung Y-S. Examination of the expanding pathways for the regulation of p21 expression and activity. Cell signal. 2010;22:1003–12. doi: 10.1016/j.cellsig.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.