Abstract
The pathogenesis of pain in chronic pancreatitis is poorly understood, and its treatment can be a major clinical challenge. Surgical and other invasive methods have variable outcomes that can be unsatisfactory. Therefore, there is a great need for further discovery of the pathogenesis of pancreatitis pain and new therapeutic targets. Human and animal studies indicate a critical role for oxidative stress and activation of transient receptor potential (TRP) cation channel subfamily members TRPV1 and TRPA1 on pancreatic nociceptors in sensitization mechanisms that result in pain. However, the in vivo role of TRPV4 in chronic pancreatitis needs further evaluation. The present study characterized a rat alcohol/high fat diet (AHF) induced chronic pancreatitis model with hypersensitivity, fibrotic pathology, and fat vacuolization consistent with the clinical syndrome. The rats with AHF induced pancreatitis develop referred visceral pain-like behaviors, i.e. decreased hindpaw mechanical thresholds and shortened abdominal and hindpaw withdrawal latency to heat. In this study, oxidative stress was characterized as well as the role of TRPV4 in chronic visceral hypersensitivity. Lipid peroxidase and oxidative stress were indicated by increased plasma thiobarbituric acid reactive substances (TBARS) and diminished pancreatic manganese superoxide dismutase (MnSOD). The secondary sensitization associated with AHF induced pancreatitis was effectively alleviated by the TRPV4 antagonist, HC 067047. Similarity of the results to those with the peripherally restricted µ-opiate receptor agonist, loperamide, suggested TRPV4 channel activated peripheral sensitization. This study using a reliable model that provides pre-clinical correlates of human chronic pancreatitis provides further evidence that TRPV4 channel is a potential therapeutic target for treatment of pancreatitis pain.
Keywords: TBARS, rat, HC 067047, visceral pain, behavior, loperamide
Chronic pancreatitis features failure of its exocrine gland function and in some cases even its endocrine function (diabetes type 3c). Pain present in up to 90% of patients is another major clinical challenge and a primary cause of hospitalization. The pathogenesis of pain in this disorder is poorly understood and effectiveness of treatments long-term largely unsatisfactory. Therefore, there is great need for discovery of the pathogenesis of chronic pancreatitis pain and new therapeutic targets. Chronic pancreatitis can be due to long-term excessive alcohol/fat intake (Ammann et al., 1984). Human and animal studies indicate a critical role for neurogenic mechanisms since pancreatic nociceptors reportedly are particularly prone to sensitization (Bhutani and Pasricha, 2003; Li et al., 2013; Xu et al., 2006).
Activation of somatic and visceral nociceptors through transient receptor potential (TRP) channel family members has drawn attention due to their unique physiological functions and distributions in a wide range of tissues. Approximately 20 of the 30 mammalian transient receptor potential (TRP) channel subunits are expressed by specific neurons and non-neuronal cells within the digestive system (Holzer, 2011b). TRP channel activation plays an important role in mechanosensation and hyperalgesia, as well as chemesthesis, taste, regulation of gastrointestinal motility, absorptive and secretory processes, blood flow, and mucosal homeostasis. At the cellular level, TRP channels operate either as primary detectors of chemical and physical stimuli, as secondary transducers of ionotropic/metabotropic receptors, or as ion transport channels (Holzer, 2011a, b). Implication of some TRP channels in pathological processes has raised enormous interest in exploring them as therapeutic targets. This is particularly true for TRPV1, TRPA1, and TRPV4. While the roles of TRPV1 and TRPA1 as sensors in nerve terminals of C and Aδ fibers promoting somatic and/or visceral hypersensitivity have been extensively investigated (Bartho et al., 2004; Blackshaw et al., 2010; Kondo et al., 2009; Schwartz et al., 2013; Xu et al., 2007), the present study examines the role of TRPV4.
TRPV4 channels, as multimodal sensors, are reported to be involved in somatic and visceral nociception after activation by heat (27–34 °C threshold), chemicals, and mechanical insult and stretch, including hypotonicity (Alessandri-Haber et al., 2004; Blackshaw et al., 2010; Cenac et al., 2008). Intraductal administration of a TRPV4 channel agonist to the murine pancreas induces c-Fos expression in the spinal cord (Ceppa et al., 2010). In the same study deletion of the trpv4 gene inhibited transmission of input to the spinal cord and pain related behaviors associated with acute experimental pancreatitis induced by subcutaneous injection of caerulein.
In our previous in vitro study, TRPV4 channels were overexpressed in pancreatic stellate cells isolated from rats with AHF induced chronic pancreatitis. Overresponsiveness was reported to hypotonic stimuli, mimicking stretch as in edema during the course of cellular injury, and to biologically active compounds such as the lipid messenger, arachidonic acid. Activation resulted in intracellular calcium overload, initiating signaling cascades leading to sensitization (Ceppa et al., 2010; Zhang et al., 2013). The role of TRPV4 channels in chronic pancreatitis in vivo was under further study in the current study.
In the present study, the alcohol/high fat diet (AHF) induced chronic pancreatitis rat model was utilized to investigate oxidative stress and the ability of a TRPV4 antagonist to reduce behavioral hypersensitivity. We hypothesized that TRPV4 channels would be activated in the alcohol and fatty acid metabolite rich environment. In animals with AHF induced pancreatitis referred hypersensitivity was alleviated by both a TRPV4 antagonist and the peripherally restricted mu opioid receptor, loperamide.
2. Experimental Procedures
This study was performed in accordance with the Guidelines for the Care and Use of Laboratory Animals published by the National Institutes of Health. All experimental procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee.
2.1 Induction of Chronic Pancreatitis
A total 18 male Fisher 344 rats weighing between 240 – 250g (from Harlan Sprague-Dawley Inc, Indianapolis, IN) were used for this study. The number of animals required to achieve statistical significance is specified in each test description and represents reduction of animal use mandated by our IACUC. Animals were single caged and kept in a temperature constant (23° ± 2°C) room on a 12/12 hour reversed dark-light cycle. Rats were randomly divided into two groups and fed either an alcohol and high fat liquid diet (AHF) (n=12) or control rodent chow (Harlan Teklad 8626 with low soy content) (n=6). Chronic pancreatitis was induced with a high fat liquid diet (AHF) made from micro-stabilized rodent diet mix (LD 101A; Test-Diet, Richmond, IN). The commercial diet provides protein, fat, fiber, vitamins and minerals. Maltodextran (90 g), water (770 g), apple juice (100 g), and alcohol (w/v, 95% ethyl alcohol) is added to the mix. The dose of alcohol was progressively increased weekly from 4% alcohol for the first week, 5% for second week, and 6% for the third week, adjusting the mixture percentage with addition of less water. Corn oil (33 g) and 6% alcohol were added to the liquid diet in all subsequent weeks through the end of the experiment. A lard supplement (8 g/rat/day) was also given daily in a stainless steel condiment dish. The control group was fed standard rodent chow (Teklab 8626, Harlan, Indiana). All rats were given access to food and water ad libitum. Animals were observed closely, and no evidence of alcohol intoxication (no ataxia or lethargy) was noted. Food consumption was monitored daily and body weight monitored weekly. Rats fed with AHF diet consumed an average of ≈ 50 ml liquid diet per day and a lard supplement (≤ 8 g/rat/per day). The body weight gain of rats fed AHF diet progressed more slowly than rats fed standard chow in the later experimental weeks but did not exceed 20% weight difference between groups. Rats fed standard rodent chow gained 10 g body weight each week. The rats fed the AHF diet gained 2 – 5 g body weight each week.
2.2 Detecting Oxidative Stress
2.2.1 Blood plasma lipid peroxide analysis
Thiobarbituric acid reactive substances (TBARS) are formed as a byproduct of lipid peroxidation (i.e. as degradation products of fat) which can be detected by the TBARS assay using thiobarbituric acid as a reagent. The TBARS assay measures malondialdehyde (MDA) present in blood plasma samples as an index of lipid peroxidation and systemic oxidative stress. A 90 µl rat blood plasma sample was added to 1 ml of 20% Trichloroacetic acid (TCA), mixed gently and incubated on ice for 15 min. The mixture was centrifuged at 3000 rpm for 10 min at 4°C. The supernatant was discarded and the sediment mixed with 1.25 ml of 0.05M H2SO4 and 1.5 ml of 0.2% thiobarbituric acid (TBA). The glass test tubes were caped, vortexed and kept in a boiling water bath, 100°C, for 30 min. After cooling down, 2 ml of butanol was added to each tube and the color extracted in the butanol phase was read at 530 nm with a Spectrophotometer (SmartSpec Plus, BIO-RAD, Hercules, CA). The lipid peroxide content was calculated and expressed as nanomoles of TBA reactants/ml plasma (Buege and Aust, 1978; Deevska et al., 2012).
2.2.2 Immunohistochemical localization of MnSOD in pancreas tissue
Manganese superoxide dismutase (MnSOD, SOD2) protein utilized during oxidative stress was immunolocalized in defatted paraffin sections of paraformaldehyde immerse fixed pancreas with the ABC method (VECTASTAIN, ABC kit, VECTOR Laboratories, Inc. Burlingame, CA, USA). Tissue sections from all groups were simultaneously processed allowing quantitative analysis. After deparaffinization, antigen retrieval (10 mM sodium citrate buffer, pH 6.0) and blocking, the sections were incubated with the primary rabbit polyclonal antibody against human MnSOD (1:1000; Enzo Life Sciences, Inc. NY, USA) overnight at room temperature, followed by incubation with goat anti-rabbit biotinylated antibody for 1 hour. The sections were then incubated with avidin-biotin complex (ABC) reagent for an additional 1 hour. Finally the antibody-antigen interaction was visualized with a peroxidase-catalyzed reaction. Images from the pancreas tissues were acquired from consecutive non-overlapping fields using a Nikon E1000 microscope equipped with a Nikon DXM1200F digital camera and ACT-1 Program (Nikon Instruments, Inc., Melville, NY). Five images per animal (n=3) were taken at 20× magnification at full resolution with a single image dimension setting of 3600 × 2880 pixels. The immunostaining intensities of sections were analyzed with Image J (version 1.46r, NIH). Alternate sections were counterstained with Sirius red to visualize the collagen matrix deposition.
2.3 Assessments of Pain and Anxiety Related Behaviors
All behavioral tests were performed in the animals’ active dark period (i.e. 0900 h – 1500 h). Baseline testing began prior to AHF feeding, and weekly testing thereafter monitored progress of the pain and anxiety related behaviors. Mechanical threshold was measured on the foot (non-noxious stimulation) and heat withdrawal latency tested on the abdomen (noxious stimulation) once a week with the same start time. Mechanical threshold testing was done first followed by the heat withdrawal test.
Dark/light box preference test was carried out on a different day at baseline (before AHF feeding) and another day at the end of the study. The 44°C hot plate test was tested before and after drug treatment on another different day.
2.3.1 Secondary mechanical allodynia on the hindpaws
Secondary mechanical allodynia on the hindpaw was assessed by measuring the mechanical withdrawal threshold using von Frey filaments according to the “up/down” method described by Chaplan and colleagues (Chaplan et al., 1994). The animal was placed on a raised wire mesh table (76 × 38 cm), under a clear plastic ventilated rat restrainer (18 × 13 x15 cm) for a 30 min -acclimation period. The mechanical withdrawal threshold testing was done on the plantar surface of both hindpaws using a set of 8 von Frey monofilaments. The von Frey filaments were applied perpendicularly to the plantar surface with sufficient force to bend the monofilament slightly and held for about 5 seconds. A positive response was defined as an abrupt withdrawal (flick or moving away) of the foot during stimulation or immediately after the removal of stimulus. Whenever there was a negative or positive response, the next stronger or weaker filament was applied, respectively. The pattern of positive and negative responses was generated (at least 6 data points) and converted into a 50% mechanical threshold value (in gram) using a curve-fitting algorithm (Dixon, 1980). If the withdrawal threshold value was decreased it is an indication of secondary mechanical allodynia.
2.3.2 Secondary heat hyperalgesia on the abdomen
Secondary heat hyperalgesia was assessed by measuring the abdominal withdrawal latency (ABWL) in response to heat stimuli with a modified Hargreaves test (Vera-Portocarrero et al., 2003). The abdominal skin was shaved one day before the test each week. On the day of testing, the animals were placed separately in a clear plastic ventilated rat restrainer (18 × 13 x15 cm) on a glass-top table (approximately 2 mm thick glass) and allowed to acclimate to their new environment for at least 30 min before testing. A high-intensity light beam was applied to the shaved belly skin surface through the glass. The light beam projected through a 10 × 5 mm aperture from a movable metal box which contains a high-intensity projector lamp bulb (Quartzline Lamp;General Electric Co., Cleveland, OH) and attaches to an on/off switch and digital timer. An LCD window displayed the withdrawal latency (in seconds). To avoid any burn damage to the skin, the cutoff time for abdominal withdrawal latency was set to 40 s. A withdrawal event to radiant heat was defined as abdominal withdrawal (either abdominal musculature contraction or lifting of the abdomen through postural adjustment) accompanied by head turning toward the stimuli and licking of the affected abdominal area. The abdominal withdrawal latencies were tested in 3 trials with 5 min intervals between trials for averages. A cooling fan insured the temperature of the glass surface remained close to the initial starting temperature (22 – 24°C). The average of three readings was used as the abdominal withdrawal latency value (in seconds) in this study. A shortened abdominal withdrawal latency value was an indication of secondary thermal hyperalgesia.
2.3.3 Modified 44°C hotplate assay
Low-intensity heat stimulation was applied using a modified 44°C hotplate. Low-intensity heat stimulation was considered as noxious stimuli (Vierck et al., 2004; Yeomans and Proudfit, 1994). In this study, two hotplate analgesia meters were used with the thermal plates topped by a vented Plexiglas enclosure for each (26 cm × 26 cm × 28 cm) (Columbus Instruments, OH). One hot plate was set temperature at 38°C for pre-warming the feet to an initial uniform temperature and the other was set at 44°C as a moderate heat stimulus. After a 30 min acclimation to the room environment, each rat was placed on the 38°C warm-up plate for 10 min to equilibrate the foot temperature among all the rats to be tested before each was transferred onto the 44°C testing plate. The latency of the first hindpaw withdrawal event, the number and duration of hindpaw withdrawal events, and the rearing events were recorded during the 10-min 44°C plate assay. The event duration was defined as hindpaw withdrawal from the plate beginning when the hindlimb was lifted and was completed when the hindlimb made contact with the hot plate again. The events were plotted against time at 1 min intervals as an event/time curve. Rearing events were recorded, defined as erect posture with forelegs leaning against the wall of the enclosure. This event was considered a noxious-evoked escape response (Espejo and Mir, 1993; Espejo et al., 1994).
2.3.4 Light/dark box preference test
The light/dark box test is a widely used and well-validated assay for assessing anxiety levels in animals (Bilkei-Gorzo et al., 1998; Crawley and Goodwin, 1980; McCool et al., 2003). A light/dark box with two equally divided compartments was used. The two chambers were connected by a 10 × 10 cm doorway in the center of the partition to allow free access to the adjacent chamber. One chamber was darkened; the other one was illuminated by a fluorescent desk lamp outside the box. Rats were moved to the test room 30 min prior to the test. All tests are conducted between 9AM and 11AM. Animals were placed initially in the dark chamber facing away from the door leading to the adjacent light chamber; and behaviors were recorded for 10 min (600 s). The following behaviors were documented: latency (in seconds) to enter the light-side after initial placement in the dark-side; the number of chamber crossings (light/dark chamber transition), defined as at least partial passage between chambers with extension of at least one of the animal’s back leg from one chamber to the next; total time (in second) spent in the light chamber and the total rearing events in the 10 min experimental time course. Baseline testing was done prior group assignment.
2.4 Drugs
Rats received multiple drug doses (with Latin square of order 2 design) from low dose to high, with each drug dose given one week wash out time between tests. The drugs were effective for a maximum of 2–4 hour on the test day. No desensitizing effect was noticed.
HC 067047, a potent, selective antagonist of TRPV4 (2.5, 5 or 10 mg/kg; Tocris Bioscience, R&D Systems, Inc. Minneapolis, MN, USA), was dissolved with 2% dimethyl sulfoxide (DMSO) in normal saline. Loperamide hydrochloride is a piperidine derivative opioid ligand that selectively activates peripheral µ-opioid receptors without entering the CNS (1 mg/kg, Sigma-Aldrich, St. Louis, MO USA). Loperamide was dissolved with 10% (2-Hydroxyproply)-β-cyclodextrin in water. Naloxone methiodide (1 mg/kg; Sigma-Aldrich, St. Louis, MO USA), a peripherally acting opioid receptor antagonist which also does not cross the blood brain barrier was dissolved in normal saline. All drugs tested were administrated by an intraperitoneal injection in a volume of <10 ml/kg (Turner et al., 2011).
2.5 Statistics
GraphPad Prism version 6.1 for Windows (GraphPad Software, La Jolla California USA, www.graphpad.com) was used for all statistical analyses of the data. All data were expressed as means ± S.E. Comparisons among groups were performed with one-way ANOVA followed by Tukey’s multiple comparisons test. The comparison among groups over the time course was performed with two-way ANOVA and Bonferroni multiple comparisons test. Two-tailed t-tests were also used where appropriate. An alpha level p<0.05 was considered significant.
3. Results
3.1 Pancreatic Organ Shrinkage and Fat Replacement in Rats with AHF Pancreatitis
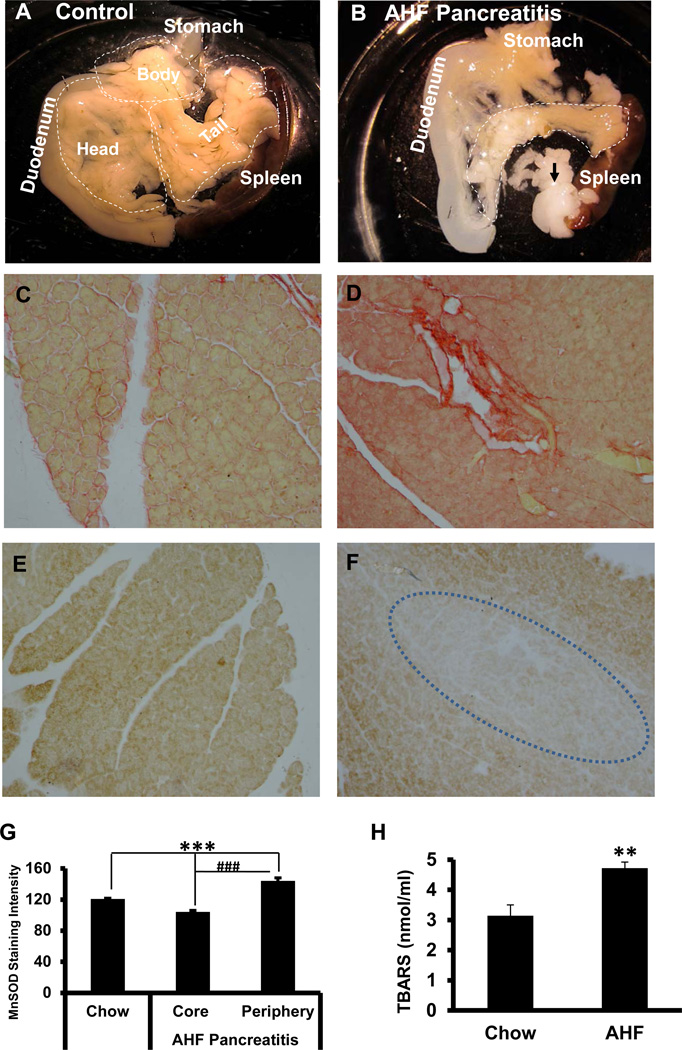
Pancreas tissues were taken from control chow fed rats and AHF pancreatitis rats after 12 weeks on the AHF diet. Freshly dissected pancreas of the AHF fed rats was significantly different in appearance compared to pancreas removed from rats fed regular chow (Fig. 1). As shown in Figure 1A, the pancreas of control rats was a large diffuse organ. The surrounding stomach, duodenum and spleen provided convenient division of the pancreas into three parts for histological processing: the biliary, duodenal (head) and gastrosplenic (tail) portions. The biliary portion was located around the biliopancreatic duct, mostly between the duct and the descending duodenum. The duodenal portion lay between the beginning of the mesojejenum and biliopancreatic duct. Both of these latter portions were in the mesoduodenum. The gastrosplenic portion, which was the largest portion, continued along the dorsal aspect of the stomach towards the visceral surface of the spleen and transverse colon (Kara, 2005).
Fig. 1. Pancreas Anatomy, Histology and Oxidative Indices.
A. Control pancreas. The normal pancreas is a large diffuse glandular organ surrounded by duodenum, stomach and spleen. Gross divisions include the head (duodenal), body (biliary), and tail (gastro-splenic).
B. AHF induced pancreatitis. The pancreas of rats with AHF induced pancreatitis is smaller and more translucent, indicative of tissue loss. A large portion of the gland is replaced by fat (black arrows).
C. Sirius red fibrosis staining. Sirius red staining of control pancreas tissue shows minimal fibrous collagen.
D. Numerous fibrotic regions are localized with Sirius red staining in pancreatic tissue from rats with AHF induced pancreatitis.
E. MnSOD immunostaining intensity in pancreas. MnSOD immunoreactivity is present in the pancreatic tissue of control rats fed standard rodent chow. F. Sparse MnSOD immunoreactivity is observed in the core of the pancreatic lobes in rats with AHF induced pancreatitis suggesting decreased mitochondrial anti-oxidative capacity. G. The bar graph shows quantitation of the pancreatic tissue staining intensity of the MnSOD immunoreactivity among groups (n=4/group; *** p<0.001, standard chow vs. injury core and periphery of pancreas in AHF fed rats; ### p< 0.001, pancreas core vs. periphery).
H. Plasma TBARS The metabolic byproduct thiobarbituric acid reactive substances (TBARS) in blood plasma, an indicator of lipid peroxidation, is significantly increased in rats with AHF induced pancreatitis (** p<0.01, chow vs. AHF fed, t-test).
Figure 1B provides an example of the pancreas taken from a rat after 12 weeks on the AHF diet. The size of the pancreas in rats with AHF diet induced pancreatitis was much smaller than those of the control rats. The sizes of all three regions of the pancreas were reduced in size. The mid portion of the gland was translucent indicative of tissue loss. Some of the lost tissue was replaced by fat.
3.2 Increased Blood Lipid Peroxide Level (TBARS) in Rats with AHF Pancreatitis
The thiobarbituric acid reactive substance (TBARS) was elevated markedly by the AHF diet (4.72 ± 0.2 nmol/ml) compared to that of the controls (3.14 ± 0.37 nmol/ml), indicating a statistically significant increase in lipid peroxidation and MDA content (n=6/ group, p<0.01, two-tailed t-test) (Fig. 1H). This result revealed increased lipid byproducts were circulating in the blood associated with the pancreatic tissue damage and was an indication of oxidative stress in the rats with AHF pancreatitis.
3.3 Uneven Distribution of MnSOD-like Immunoreactivity in Pancreas from Rats with AHF Pancreatitis
In the control chow fed rats a low level of MnSOD-like immunoreactivity was evenly distributed among the acinar and islet cells throughout the entire pancreas (Fig. 1E). Overall, in the tissues from the AHF pancreatitis group, the MnSOD-like-immunoreactivity was less intense or nearly negative in the core of the pancreatic lobes in the same regions with severe fibrosis or with tissue loss due to fat droplet de-fatting during paraffin processing. In contrast, more MnSOD-like immunoreactivity was found in the cytoplasm of acinar cells surrounding these injury core regions (Fig. 1F). Analysis of the immunoreactive intensities in these different regions in each group revealed that pancreas taken from rats with AHF induced pancreatitis had the highest staining intensity in the peripheral portions of the peri-injury area (143.96 ± 4.07). In contrast, the staining intensity in injury core areas decreased dramatically to an average intensity of 104.05 ± 2.08. This was a statistically significant decrease compared to pancreas peri-injury areas (n=5/group; p<0.001). The average staining intensity in pancreas of the control group was 120.68 ± 1.15, a statistically significant difference compared to both the injury core and the periinjury areas in the AHF pancreatitis group (p<0.001, n=5/group; one-way ANOVA followed by a Tukey’s multiple comparisons test Fig. 1G). This result from these batch processed pancreas tissues indicated better compensatory protection by MnSOD in the less damaged tissue areas of the AHF fed group compared to controls, while more enzyme utilization was ongoing or depleted in the more severely damaged areas under severe oxidative stress (injury core).
3.4 Rats with AHF Pancreatitis Progressively Develop Secondary Hypersensitization
3.4.1 Secondary mechanical allodynia of the hindpaws
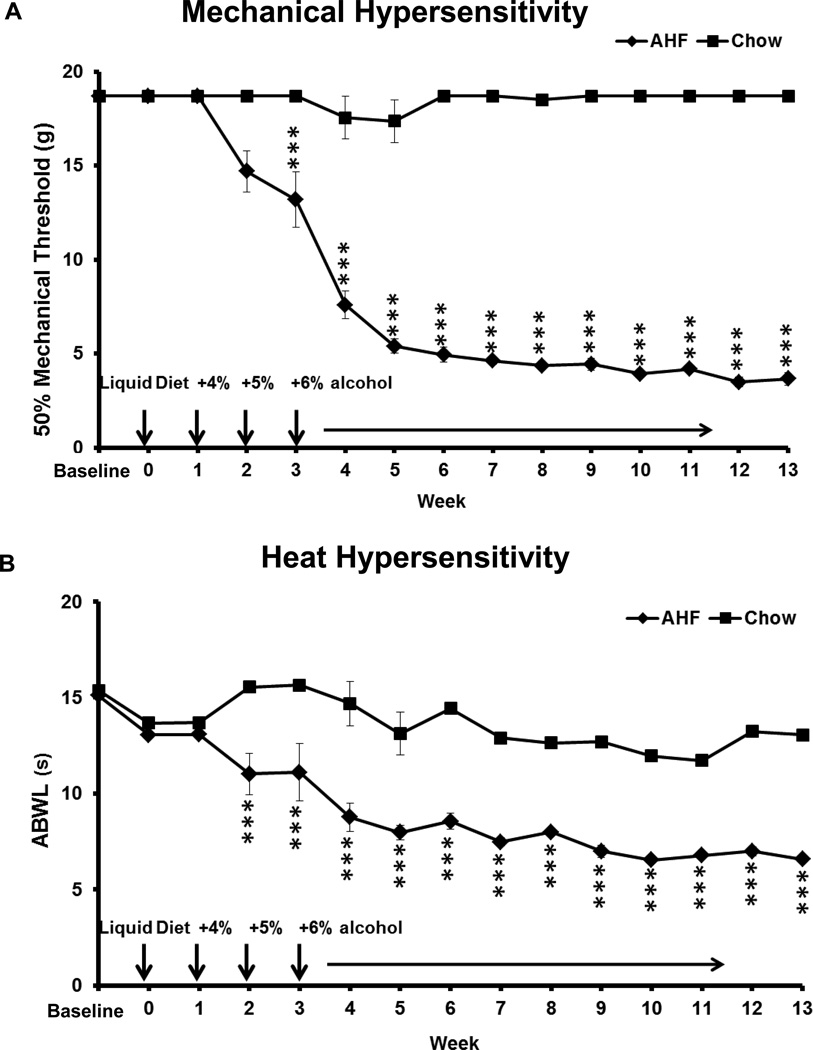
The baseline of the paw withdrawal threshold to mechanical stimulation (von Frey fiber test) was the same for both the standard chow fed rats (n=6) and the AHF fed (n=12) rat group, i. e. ≈18.72 g for both feet. Within weeks the AHF fed rats developed mechanical allodynia on their hindpaws (Fig. 2A). The mechanical threshold was progressively decreased beginning in week 3 and reached the maximum hypersensitivity in week 5. The hindpaw withdrawal threshold was decreased down to 3.66±0.35 g on both feet and remained at this level throughout the subsequent experimental weeks. This was a statistically significant difference compared to thresholds of the control chow group (17.38 ± 1.13 g) (p<0.001, two-way ANOVA, Bonferroni post-test). The decreased hindpaw mechanical threshold for the animals with AHF pancreatitis is a strong indication of secondary mechanical allodynia. Mechanical hypersensitivity is more consistently tested on the feet than on the abdominal skin of these rats which are often recumbent.
Fig. 2. Pain Related Behavioral Assessments.
A. Footpad mechanical hypersensitivity. The 50% paw withdrawal threshold (g) to mechanical stimulation (von Frey fiber test) was similar between rats fed standard chow and the AHF fed group at baseline. In the AHF group, the mechanical threshold was progressively decreased beginning in week 3 and reached maximum hypersensitivity in week 5. The paw mechanical allodynia was maintained through the entire experimental time course (**** p<0.0001, two-way ANOVA, Bonferroni post-test).
B. Abdominal heat hypersensitivity. The abdominal withdrawal latency (ABWL) was similar for standard chow control and AHF fed groups at baseline through week 1. Secondary heat hyperalgesia developed on the abdominal skin by the second week on the AHF diet. The abdominal withdrawal latency (ABWL) of the AHF induced pancreatitis group decreased from a baseline of 13.70 ± 1.1 s to 6.58 ± 0.32 s. This hypersensitivity level was maintained throughout the remainder of the experiment. This difference was statistically significant compared to the control chow fed group (****p<0.0001, two-way ANOVA, Bonferroni post-test).
3.4.2 Secondary heat hyperalgesia on the abdominal skin
There was no difference in the baseline abdominal withdrawal latencies (ABWL) of the control fed standard rodent chow (n=6) and AHF fed (n=12) groups (13.70 ± 1.1 vs. 13.39 ± 0.91 s, p>0.05; Fig. 2B). However, secondary heat hyperalgesia developed on the abdominal skin in the fifth week of feeding the AHF diet. The ABWL decreased from baseline (13.39 ± 0.91 to 6.58 ± 0.32 s) in rats with AHF pancreatitis, and the shortened ABWL remained at 6.58 ± 0.32 s for the rest of the experimental time course. This was a statistically significant decrease compared to the control chow group (13.41 ± 0.87 s; p<0.001, two-way ANOVA, Bonferroni post-test). Decreased response latency to heat stimuli on the abdominal skin of rats with pancreatitis is an indication of secondary heat hyperalgesia.
3.4.3 Increased nocifensive responses to 44°C hotplate stimulus
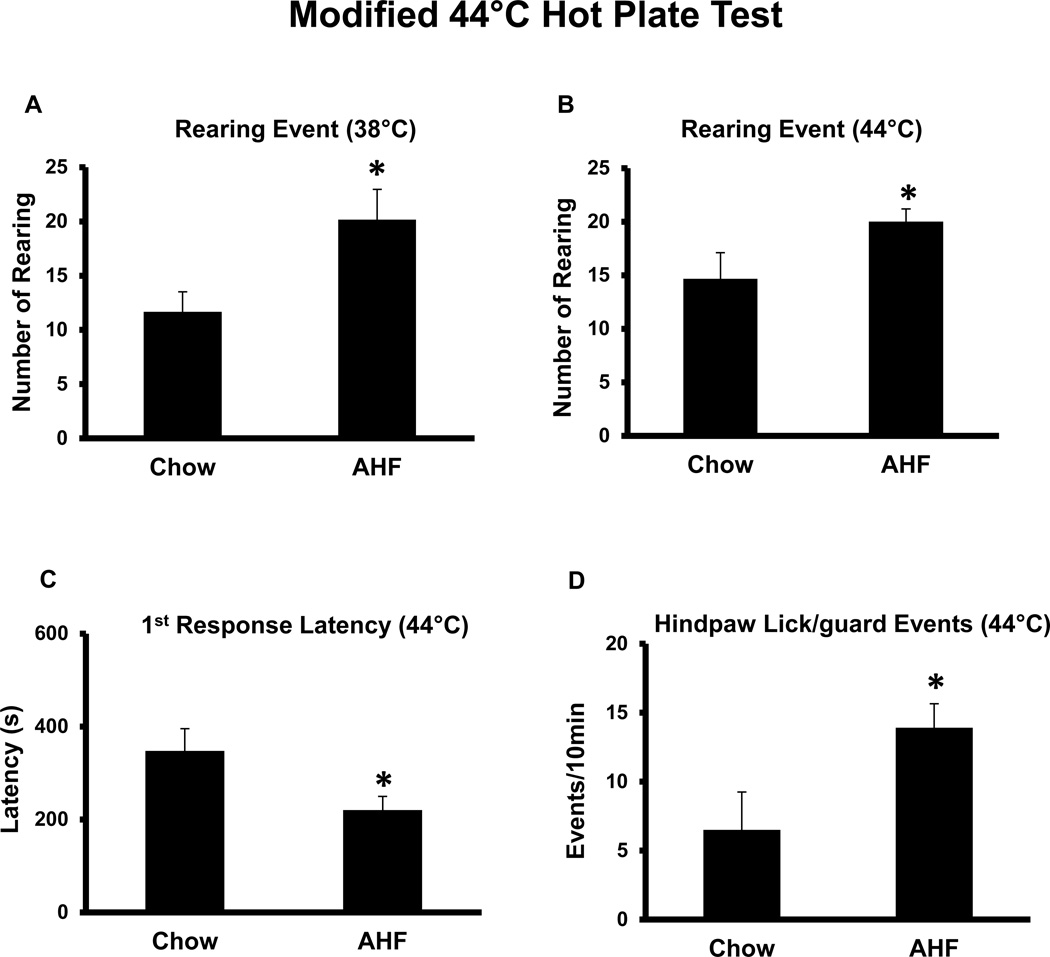
Nocifensive responses to noxious 44°C hotplate stimulus were assessed. After the feet were pre-warmed on the 38°C hotplate for 10 min, rats were then immediately transferred to the 44°C hotplate for testing. The nocifensive responses recorded were as follows (1 minute bins): the first response latency (hind-paw withdrawal latency), hindpaw licking/guarding events, and rearing events. The first hindpaw withdrawal latency of normal chow control rats was 347.7 7± 47.7 s. The accumulated hindpaw lick/guarding events recorded in the 10 min assay was 6.5 ± 2.8/10 min. In contrast, the first hindpaw withdrawal latency in AHF pancreatitis rats started as early as 220.41 ± 29.3 s (vs. normal chow control group, p<0.05, t-test). The accumulated hindpaw licking/guarding events were as high as 13.92 ± 1.73/10 min (vs. normal chow control group; p<0.05, t-test) (Fig. 3). Rearing events and leaning posture are considered escape responses and were tabulated during the 10 minutes 44°C hotplate stimulation. Rats with AHF pancreatitis displayed more rearing events (20.17 ± 1.98/10 min at 38°C; 20 ± 1.2/10 min at 44°C) than the control chow rats (12.33 ± 0.7/10 min at 38°C; 14.67 ± 2.4/10 min at 44°C; n=6/normal chow control; n=12/AHF pancreatitis)(p<0.05, Two-tailed t-test). This assay clearly revealed secondary heat hyperalgesia on the feet of rats with AHF pancreatitis.
Fig. 3. Nocifensive Responses to Noxious 44°C Hotplate Stimulus in Week 6.
A. Increased rearing events (10 min) in rats with AHF induced pancreatitis occurred on the 38°C and B. the 44°C hotplate. C. The first response hindpaw licking latency to the 44°C heat was significantly shorter in rats with AHF induced pancreatitis. D. The total number of hindpaw licking/guarding events per 10 min was dramatically increased in rats with AHF pancreatitis (* p<0.05, two-tailed t-test).
3.5 Rats with AHF Pancreatitis Did Not Show Anxiety-like Behavior in a Light/Dark Box Preference Test
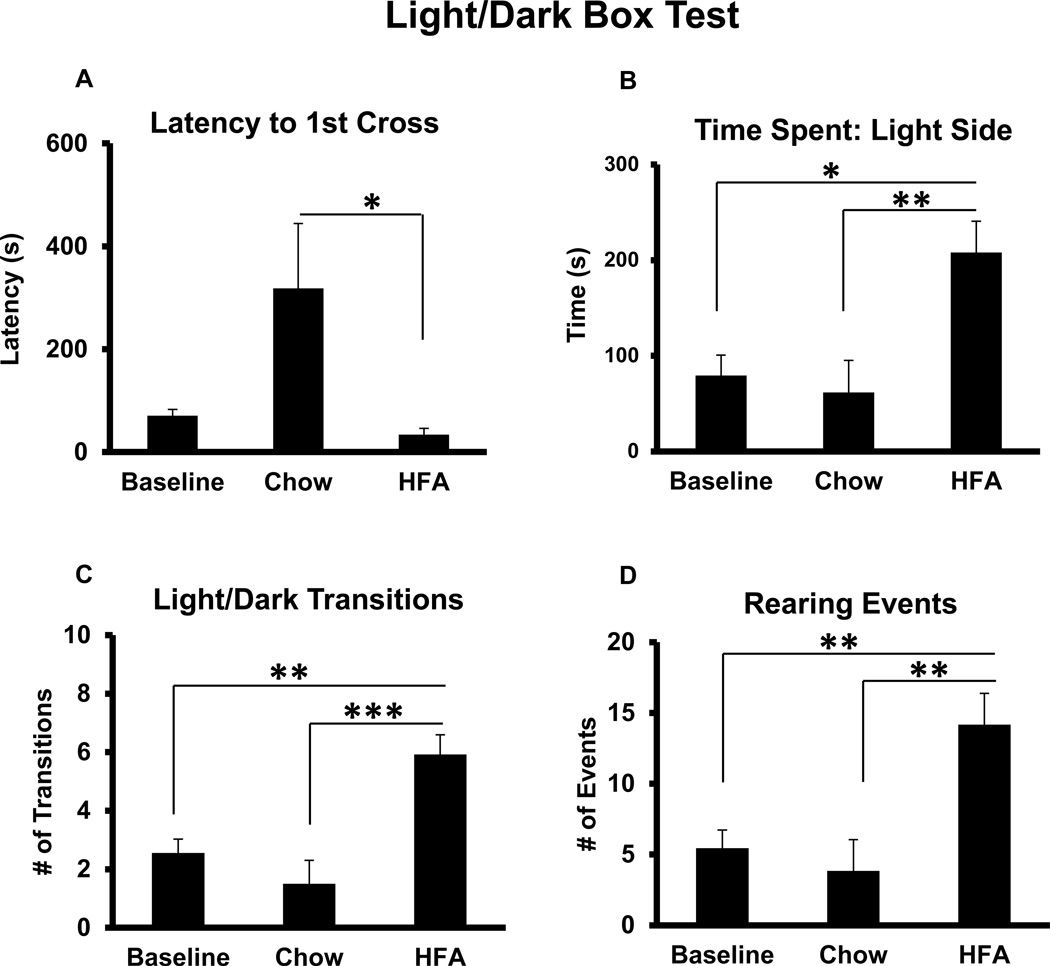
The light-dark box preference test was used to assess anxiety-like behaviors. The animals were tested at baseline before starting the AHF diet and every other week thereafter. The baseline light/dark preference parameters were the same in all groups (Fig. 4). During the last week of the experiments, rats were initially placed into the dark side of the box facing away from the light side of the box and their activities were monitored for 10 min. During the course of their exposure to the test apparatus, the normal chow control rats maintained behaviors similar to their baseline, i.e. they preferred to stay in the dark area 80–90% of the total testing time. The latency to first cross to the lighted area on the final testing day was as long as 318 ± 126.16 s (over 5 min) (Fig. 4A) The number of transitions between the light/dark compartments was as few as 1.5 ± 0.8/10 min, i.e. they sit in the dark side for most of the testing time course. However, rats with AHF pancreatitis, once placed into the dark side of the box, rush into the light side with the average latency to first cross of 33.8 ± 12.2 s (vs. normal chow control group; p<0.05, one-way ANOVA, Tukey’s multiple comparisons test)(Fig. 4A). Rats with AHF pancreatitis spent significantly more time in the light compartment than the normal chow control group or at baseline (208.11 ± 32.72 s vs. 61.7 ± 33.39 s p<0.01; or 79.39 ±21.32s p<0.05, oneway ANOVA, Tukey’s multiple comparisons test)(Fig. 4B). The increased time of light area occupancy for rats with AHF pancreatitis was accompanied by a greater number of transitions between the light and dark compartment. The number of transitions in rat with AHF pancreatitis (6±0.66/10 min) was significantly greater compared to the normal chow control group (1.5 ± 0.8/10 min) or at baseline (2.5 ±0.47/10 min) (vs. normal chow p<0.001; vs. baseline p<0.01; one-way ANOVA, Tukey’s multiple comparisons test)(Fig. 4C). Rats with AHF pancreatitis also performed more rearing events (exploratory activity) (14.17 ± 3.16/10 min) than did normal chow control rats (3.83 ± 2.23/10 min) or at baseline (5.4 ±1.3/10 min) (AHF vs. normal chow control, p<0.01; or vs. baseline, p<0.01; one-way ANOVA, Tukey’s multiple comparisons test)(Fig. 4D). These results demonstrate that rats with AHF pancreatitis had less anxiety due to long term alcohol consumption, consistent with our previous published study (Zhang et al., 2014).
Fig. 4. The Light/Dark Box Preference Test.
The baseline from each parameter shows the averaged baseline behaviors for all animals before group assignment. A. Rats with AHF induced pancreatitis had a shortened latency to 1st crossing to the light side of the box. B. Rats with AHF induced pancreatitis spent a considerably longer time in the light compartment than did rats fed with standard chow or at baseline. C. The number of transitions between the light and dark compartments was increased in rats with AHF pancreatitis compared to controls fed standard chow or at baseline. D. Rats with AHF pancreatitis had a great number of rearing events compared to the normal chow controls and at baseline.
3.6 Peripheral Mu Opioid Receptor Agonist Loperamide Attenuated Visceral Nociceptive Behaviors in Rats with Chronic AHF Pancreatitis
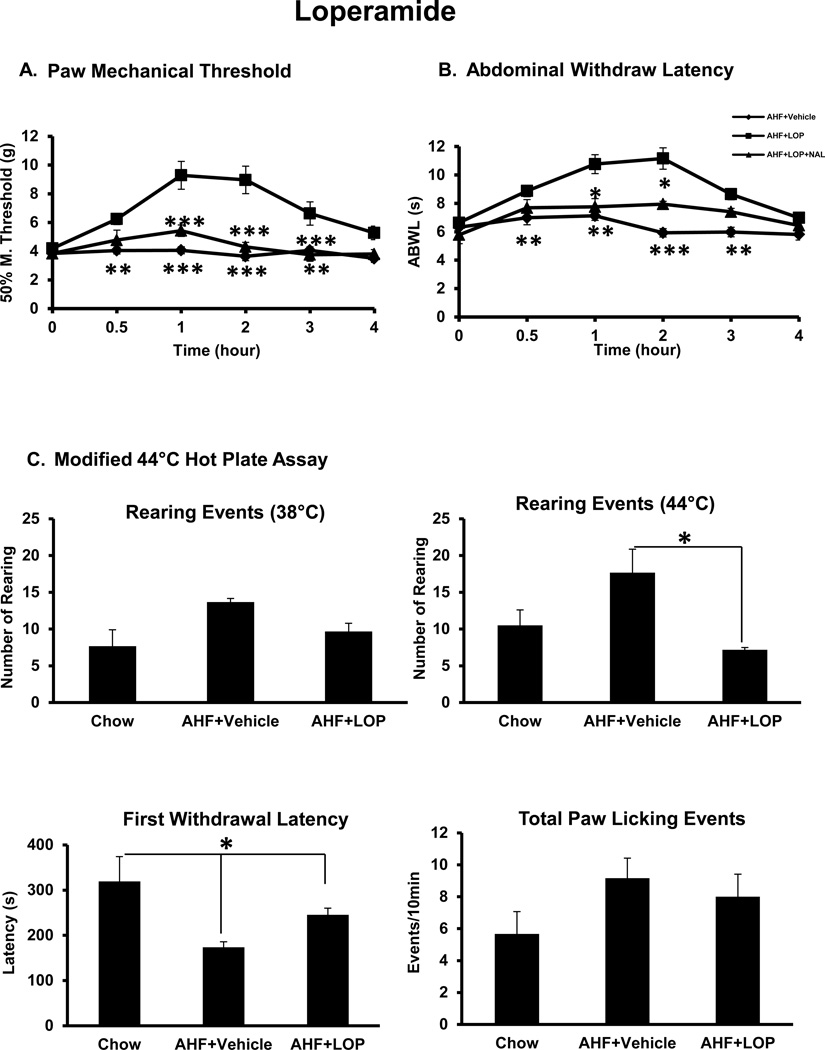
Loperamide, a peripherally restricted µ-opioid receptor agonist, was tested on all AHF pancreatitis rats (n=6/group, drug and vehicle treated groups). In this study, loperamide 1 mg/kg, i.p. effectively alleviated the hindpaw secondary mechanical allodynia. The mechanical threshold elevated from 4.2 ± 0.11 g before loperamide to 9.29 ± 0.96 g beginning at 0.5 hour, peaked at the 1 hour time point, and persisted for 3 hours. This was a statistically significant difference compared to the vehicle treated AHF pancreatitis group rats (n=6/group; p<0.001, two-way ANOVA with Bonferroni multiple comparisons test (Fig. 5A). The reduction by loperamide was completely reversed by naloxone methiodide. This was confirmation that rats with AHF pancreatitis display visceral nocifensive pain related responses that could be blocked by peripheral µ-opioid receptor activation.
Fig. 5. Effect of Peripherally Restricted Opiate, Loperamide.
A. Paw mechanical allodynia. Rats fed with AHF for 6 weeks, were given loperamide (1mg/kg, i.p). Loperamide effectively attenuated the paw mechanical allodynia in rats with AHF pancreatitis. The effect began at 0.5 hour, peaked at 1 hour, and persisted for 4 hours. This effect was reversed by naloxone methiodide (n=6/group; **p<0.01; *** p<0.001, two-way ANOVA, Bonferroni post-test). B. Abdominal heat hyperalgesia. Rats with AHF pancreatitis were given loperamide (1mg/kg, i.p). Treatment effectively attenuated abdominal heat hyperalgesia. The effect began at 0.5hour, peaked at 1 hour, and persisted for 4hours. This effect was reversed by naloxone methiodide (n=6/group; *p<0.05; ** p<0.01; *** p<0.001; two-way ANOVA, Bonferroni post-test). C. Noxious 44°C hotplate stimulus: Loperamide treatment reduced the number of rearing events of rats with AHF pancreatitis on the 44°C hotplate and prolonged the first response latency. There was a statistically significant difference comparing AHF pancreatitis rats with/without drug treatment (n=6/group; *p< 0.05 by two-way ANOVA).
The abdominal heat withdrawal latency in rats with AHF pancreatitis before loperamide drug treatment (1 mg/kg, i.p.) was 6.64 ± 0.2 s and increased to 11.16 ± 0.75 s. The effect began at the 0.5 h time point, reached a peak at the 1 hour time point, and persisted for 3 hours. There was a statistically significant difference between the treated and non-treated groups (n=6/group; p<0.001, two-way ANOVA with Bonferroni multiple comparisons test) (Fig. 5B). The effect of loperamide was completely reversed by naloxone methiodide, a nonselective antagonist at opioid receptors that does not cross the blood-brain barrier. The PWT was 5.43 ± 0.40 g and the abdominal withdrawal latency was 7.75 ± 0.58 s at the 1 hour post treatment time point (Fig. 5A, 5B). These results indicated that the secondary heat hypersensitivity induced by AHF pancreatitis was peripherally generated and could be reduced by mu opioid receptor activation.
During 44°C hot plate assay, nocifensive responses to noxious heat stimulus were also effectively controlled by loperamide treatment (1 mg/kg, i.p.) (Fig. 5C). The number of rearing events for rats with AHF pancreatitis treated with loperamide was 7.17/10 min versus 17.67/10 min without loperamide (n =6/group; p<0.05; one-way ANOVA, Tukey’s multiple comparisons test). Although the hindpaw licking/guarding events were not significantly different between groups, the first withdrawal latency was prolonged significantly, 245.17 s in treated rats with AHF pancreatitis vs. 173.67 s without treatment (n=6/group; p<0.05; one-way ANOVA followed by Tukey’s multiple comparisons test). This result revealed that the nocifensive responses to noxious heat stimuli were “pain” related and could be attenuated by activation of peripheral µ-opioid receptors. Less rearing on the 38°C degree plate for AHF rats in this study done at the end of the study compared to early in the study as shown in Fig. 3, may indicate increased susceptibility to the novel environment early in the study which was not an issue at the end of the study with this neutral temperature.
3.7 TRPV4 Antagonist, HC067047 Effectively Attenuated Pain Related Behaviors in Rats with AHF Chronic Pancreatitis in a Dose Dependent Manner
After confirmation of the presence of nocifensive behaviors in rats with AHF pancreatitis, the TRPV4 antagonist, HC067047, was tested.
3.7.1 Paw withdrawal threshold (PWT)
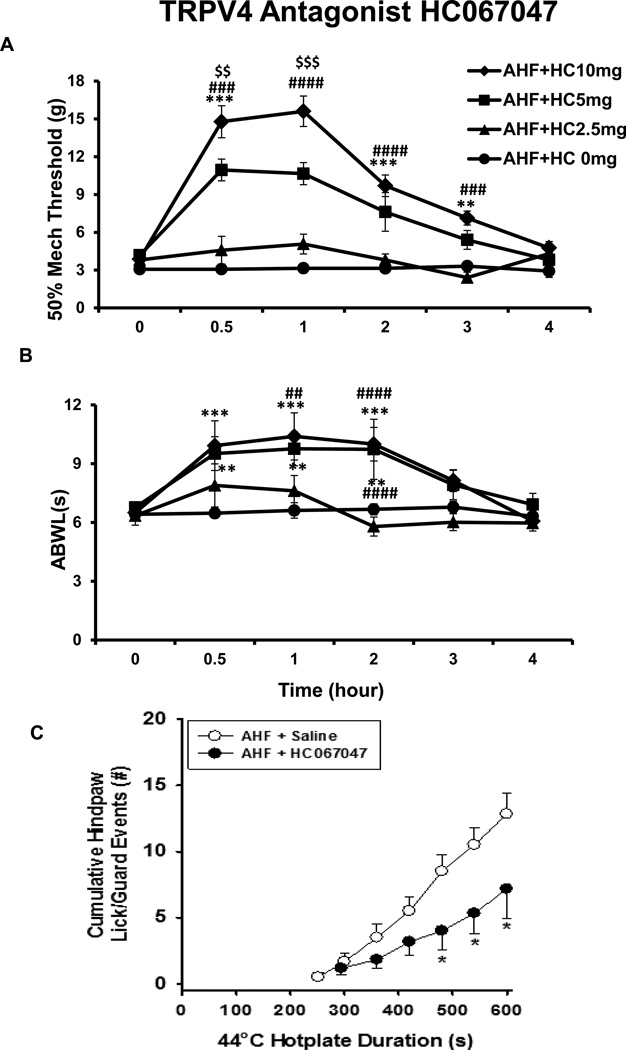
HC067047, a selective and potent TRPV4 channel antagonist, was given one time at the dose of 2.5, 5, or 10 mg/kg (i.p.) in the 7 – 9 week time frame after AHF diet feeding. The pain related behaviors (PWT and ABWL) were tested at 0, 0.5, 1, 2, 3 and 4 hour time points after the drug application. The paw mechanical threshold was elevated within 0.5 h in the treated rats with AHF pancreatitis. The PWT (in gram force) on both feet before drug treatment was 3.87 ± 0.29 g. The PWT increased up to 15.60 ± 1.2 g at the 1 hour time point, and the effect persisted for about 3 hours. The most effective doses were 5 and 10 mg/kg. There was a statistically significant difference at the 1 and 2 hour time points between the treated and non-treated groups (3.32 ± 0.3 g) (n=6/group, p<0.0001, respectively, two-way ANOVA, Bonferroni post-test) (Fig. 6A). This result demonstrated that HC 067047, a TRPV4 channel antagonist, effectively alleviates “pain” in a dose dependent manner.
Fig. 6. TRPV4 Antagonist HC067047.
A. Dose response curves of TRPV4 antagonist HC067047 on rat hindpaw hypersensitivity HC067047 effectively alleviated mechanical hypersensitivity of rats with AHF pancreatitis in a dose dependent manner beginning at 0.5 hour and persisting for 3 hours. The 5 and 10 mg/kg doses effectively elevated the paw withdrawal threshold. B. Dose response curve of HC067047 on rat abdominal heat hypersensitivity. HC067047 also effectively prolonged the abdominal withdrawal latency (ABWL) response of the rats with AHF pancreatitis to heat stimulus in a dose dependent manner beginning at 0.5 hour and persisting for 3 hours. Effective doses of HC067047 were 5 and 10 mg/kg. C. HC067047 reduced nocifensive responses of AHF pancreatitis rats to noxious 44°C hotplate. HC067047 (10 mg/kg) prolonged the latency to first response and reduced the number of total hindpaw licking/guarding events of rats with AHF pancreatitis on the 44°C hotplate. The event/time curve of the drug treatment group had a significant right-ward shift (*p<0.05, two-way ANOVA, Bonferroni post-test).
3.7.2 Abdominal withdrawal latency (ABWL)
The ABWL was tested, immediately followed the PWT testing, both before and after HC067047 administration, i.e. 0, 0.5, 1, 2, 3, 4 hour time points. The abdominal withdrawal latency was prolonged beginning at the 30 min time point, reached a peak at the 1 h time point, and persisted for 2 hours. The average ABWL increased to 10.40 ± 0.39 s in the drug treatment group. This was a statistically significant difference compared to the AHF pancreatitis group without drug treatment (6.62 ± 0.32 s) (n=6/group, p<0.001, two-way ANOVA, Bonferroni post-test) (Fig. 6B). The HC067047 was effective in a dose dependent manner, and the most effective doses were 5 and 10 mg/kg.
3.7.3 Nocifensive responses to noxious heat stimulus with 44°C hotplate
The HC067047 (at the dose of 10 mg/kg, i.p.) prolonged the first response latency (294.3 ± 67.05 s) and reduced the cumulative number of hind-paw lick/guard reflex events (7.16 ± 2.27/10 min) compared to non-treated rats with AHF pancreatitis (251.6 ± 31 s; 12.8 ± 1.55/10 min). The event/time curve of the treatment group showed a significant right-ward shift (n=6/group, p<0.05, two-way ANOVA, Bonferroni post-test) (Fig. 6C). These results revealed that blocking TRPV4 channels with HC067047, effectively attenuated the nocifensive responses to the noxious heat stimulus.
4. Discussion
In our previous studies, the AHF pancreatitis rat model featured a globally disrupted pancreatic pathology, including acinar and islet cell atrophy, progressive accumulation of lipid droplets in the tissue (vacuolization), and periductal, interlobular and intralobular fibrosis (McIlwrath and Westlund, 2015; Zhang et al., 2013). In week 10 on the AHF diet, rats begin to develop mild glucose intolerance. In the present study, we showed pancreatic organ shrinkage and fat replacement in pancreas of rats with AHF pancreatitis. These tissue morphological changes in rats with the AHF pancreatitis model are consistent with pathology in clinical samples from patients with alcoholic pancreatitis (Noronha et al., 1981a; Noronha et al., 1981b; Patel et al., 1980; Sarles et al., 1971). Increased blood lipid peroxide levels and manganese superoxide dismutase (MnSOD) reduction in the pancreas tissue indicated oxidative stress in this AHF pancreatitis model. Manganese superoxide dismutase is a nuclear encoded primary antioxidant enzyme localized exclusively in the mitochondrial matrix. The SOD is thought to be the first line of antioxidant defense and is highly efficient in protecting cells and tissue from oxidative stress by catalyzing the dismutation of superoxide radicals to form hydrogen peroxide and molecular oxygen (Leung and Chan, 2009; Li and Zhou, 2011; Sajewicz et al., 2006; Su et al., 2002).
Consistent with our previous study (Zhang et al., 2014), the rats with AHF pancreatitis showed less fear behavior than did the normal chow control rats in light/dark box preference testing, i.e. they spent more time in the light chamber and had increased numbers of transitions between the light/dark chambers and increased exploratory activity (rearing events). The long term ethanol consumption may have masked any pancreatitis induced anxiety (McCool et al., 2003). In a recent report, high-fat diet itself protected against stress-induced general anxiety- and depression-like behaviors (Finger et al., 2011). Fat, ethanol, and nicotine intake have been shown to increase the single- and double-labeling of the endogenous opioid peptide, enkephalin (ENK)- and c-Fos-immunoreactivity in precisely the same brain areas, the medial area of the paraventricular nucleus of the hypothalamus, central nucleus of the amygdala, and the core of the nucleus accumbens (Chang et al., 2014). These findings suggest that food rich in fat has effects in common with addictive drugs which are known to activate the same populations of ENK-expressing neurons in these nuclei of the hypothalamus and limbic system, thereby affecting behaviors such as social interaction, rewording seeking, and preference decision making.
Rats with AHF pancreatitis demonstrated typical progressive referred visceral pain-like behaviors indicated by decreased hindpaw mechanical threshold and shortened abdominal withdrawal latency in response to noxious heat stimulus. Rats with chronic AHF pancreatitis displayed pain associated anxiety-like activity in the 44°C hotplate assay with an increased number of rearing and leaning postures (attempting escape) as well as increased nocifensive responses to noxious heat stimuli. These behaviors are indicative of secondary nociceptive hypersensitization and were attenuated by the peripherally restricted MOR agonist, loperamide. These results provide evidence that peripheral nerves are primarily involved in the altered nociception in the AHF pancreatitis animal model. Sensitization is broadly classified into two types. Peripheral sensitization involves changes in the excitability of primary nociceptors. Central sensitization can occur in the spinal cord and/or higher levels in the brain. It is important to bear in mind that central sensitization inevitably accompanies ongoing increase in peripheral nerve input (Piomelli et al., 2014; Willis and Westlund, 1997; Willis, 2007).
These referred pain-like behaviors associated with chronic AHF pancreatitis were effectively alleviated by intraperitoneal injection of HC067047, a potent and selective TRPV4 antagonist. This is consistent with the previous study that TRPV4 channel is involved in acute cystitis visceral pain (Everaerts et al., 2010). The similarity of results for the TRPV4 channel antagonist to the results with peripherally acting loperamide suggests it is also acting peripherally. Signaling of pancreatic pain is transmitted via primary afferent nociceptors activated by proinflammatory mediators which induce neuroplastic alterations in peripheral nerve endings and damage tissue (Liddle and Nathan, 2004; Pasricha, 2012). Peripheral sensitization is part of the altered gating mechanism that regulates the access of nociceptive information to the spinal cord and the brain (Piomelli et al., 2014). Central sensitization also likely contributes to the pain associated with chronic pancreatitis.
The transmission and processing of pain signals relies critically on the activities of many ion channels expressed in afferent nerve fibers transmitting information about pain. Primary sensory afferents and their neighboring host-defense cells are a rich source of lipid-derived mediators that contribute to the sensation of pain caused by tissue damage and inflammation (Piomelli et al., 2014). In this study, we focused on TRPV4, the fourth member of the vanilloid subfamily of TRP channels. TRPV4 is a calcium-permeable cation channel that is detectable in both neuronal and non-neuronal cells. Recently, mounting of evidence has shown that TRPV4 channel gating possesses polymodal sensing properties, including response to mechanical, temperature, and lipid mediators. 1) Mechanical stress: The TRPV4 channel was initially cloned based on its sensitivity to hypotonic cell swelling, implicating the channel as a potential mechanosensor. It is now known that TRPV4 functions as an osmo- and mechano-sensitive channel or sensory complex (Liedtke and Friedman, 2003; Liedtke et al., 2003). The specific contribution to high threshold mechanosensory function makes TRPV4 currently a primary nociceptor-specific TRP channel in the digestive system (Blackshaw et al., 2010; Holzer, 2011a). 2) Temperature stress: The survival of animals, especially warm-blooded mammals, relies on the body’s abilities to sense and respond to changes in temperature. TRP channels may be the central components of this sensing ability. The TRPV channel members, TRPV1–4, are activated by warm to noxious heat temperatures and have been implicated in bodily temperature sensing. TRPV4 in vitro studies have shown responses to low temperatures in the 27 – 35°C range (Ferrandiz-Huertas et al., 2014). A study with TRPV4 knockout mice implicated TRPV4 as an essential heat sensor in vivo in generation of thermal hyperalgesia (35 – 50°C) (Todaka et al., 2004). 3) Lipid mediator stress: Fatty acid, arachidonic acid (AA), a lipid activator, and its downstream metabolites epoxy-eicosatrienoic acids (EETs) directly activate TRPV4 channels. Accumulating evidence indicates the AA cascade pathway and the epoxygenase metabolites play key roles in regulating TRPV4 gating activity endogenously (Nilius et al., 2004). In the present study utilizing the AHF chronic pancreatitis model, a rich alcohol/fatty acid metabolite environment provided long-term by the alcohol/high fat diet is activating associated inflammatory intracellular cascades. The animals display TRPV4 activation mediated mechanical allodynia and heat hyperalgesia which was effectively attenuated by the potent TRPV4 antagonist.
Increasing evidence has shown that TRPV4 is involved in inflammation and nociception. Recent reports suggest activation of TRPV4 channels in the gastrointestinal tract has proinflammatory effects (Holzer, 2011a). TRPV4 mRNA expression is up-regulated in patients with inflammatory bowel diseases (IBD) (Brierley et al., 2008) and in experimental IBD model in animals (Fichna et al., 2012).
5. Conclusions
This study presents strong evidence that blocking the TRPV4 channel effectively attenuates nociceptive responses in the chronic pancreatitis model and thus supplements literature supporting TRPV4 as a key player in amplification of nociception as well as inflammation. These studies along with previous studies with other visceral pain models identify TRPV4 as a potential therapeutic target. Pre-clinical study of this and other important targets with potential for drug development can be studied in this reliable, translational pancreatitis model which has provided additional information here about oxidative stress and pain-like behavioral correlates of human chronic pancreatitis. The similarity of results for the TRPV4 channel antagonist to those obtained with peripherally restricted loperamide suggests TRPV4 is also acting peripherally.
Highlights.
Pain like behaviors develop in an alcohol high fat fed rat pancreatitis model
Pain associated with AHF pancreatitis was alleviated by a TRPV4 channel antagonist
Pain associated with AHF pancreatitis was alleviated by the peripherally restricted µ-opiate receptor agonist, loperamide
Similarity of results for the TRPV4 channel antagonist to those with loperamide suggest TRPV4 is acting peripherally
TRPV4 channel is a potential therapeutic target for pancreatitis pain
Acknowledgments
This study was funded by NIH R01 5NS039041 (KNW), RO1 AG026711 (M.N-K.), and 2RO1 AG019223 (M.N-K.).
Abbreviations
- AHF
alcohol/ high fat diet
- TRPV4
Transient receptor potential cation channel subfamily V member 4
- TBARS
thiobarbituric acid reactive substances
- MnSOD
manganese superoxide dismutase
- MDA
malondialdehyde
- TCA
trichloroacetic acid
- TBA
thiobarbituric acid
- ABC
avidin-biotin complex
- ANOVA
analysis of variance
- ABWL
abdominal withdrawal latency
- DMSO
dimethyl sulfoxide
- CNS
central nervous system
- PWT
paw withdrawal threshold
- MOR
µ-opioid receptors
- ENK
enkephalin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
All authors declare no conflict of interest.
Author’s contribution:
All authors read and edited and have approved the final manuscript. LZ fed the animal, performed most of the behavioral tests, tissue immunostaining, data analysis, produced all the figs, and drafted and edited the manuscript; RHKIV performed some behavioral tests. GD performed the TBARS test and edited the manuscript; FM performed some behavioral tests and edited the manuscript; MNK provide materials for TBARS testing, read and edited the manuscript; KNW designed the high fat pancreatitis model, supervised the experiments, and edited the figures and manuscript.
References
- Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:4444–4452. doi: 10.1523/JNEUROSCI.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann RW, Akovbiantz A, Largiader F, Schueler G. Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology. 1984;86:820–828. [PubMed] [Google Scholar]
- Bartho L, Benko R, Patacchini R, Petho G, Holzer-Petsche U, Holzer P, Lazar Z, Undi S, Illenyi L, Antal A, Horvath OP. Effects of capsaicin on visceral smooth muscle: a valuable tool for sensory neurotransmitter identification. European journal of pharmacology. 2004;500:143–157. doi: 10.1016/j.ejphar.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Bhutani MS, Pasricha PJ. Neurolytic Approaches for the Treatment of Pain in Patients with Chronic Pancreatitis. Current treatment options in gastroenterology. 2003;6:375–379. doi: 10.1007/s11938-003-0040-7. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Gyertyan I, Levay G. mCPP-induced anxiety in the light-dark box in rats--a new method for screening anxiolytic activity. Psychopharmacology. 1998;136:291–298. doi: 10.1007/s002130050568. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Brierley SM, Hughes PA. TRP channels: new targets for visceral pain. Gut. 2010;59:126–135. doi: 10.1136/gut.2009.179523. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Page AJ, Hughes PA, Adam B, Liebregts T, Cooper NJ, Holtmann G, Liedtke W, Blackshaw LA. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology. 2008;134:2059–2069. doi: 10.1053/j.gastro.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods in enzymology. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Cenac N, Altier C, Chapman K, Liedtke W, Zamponi G, Vergnolle N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology. 2008;135:937–946. doi: 10.1053/j.gastro.2008.05.024. 946 e931–932. [DOI] [PubMed] [Google Scholar]
- Ceppa E, Cattaruzza F, Lyo V, Amadesi S, Pelayo JC, Poole DP, Vaksman N, Liedtke W, Cohen DM, Grady EF, Bunnett NW, Kirkwood KS. Transient receptor potential ion channels V4 and A1 contribute to pancreatitis pain in mice. American journal of physiology Gastrointestinal and liver physiology. 2010;299:G556–G571. doi: 10.1152/ajpgi.00433.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Barson JR, Liang SC, Leibowitz SF. Common effects of fat, ethanol, and nicotine on enkephalin in discrete areas of the brain. Neuroscience. 2014;277:665–678. doi: 10.1016/j.neuroscience.2014.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacology, biochemistry, and behavior. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Deevska GM, Sunkara M, Morris AJ, Nikolova-Karakashian MN. Characterization of secretory sphingomyelinase activity, lipoprotein sphingolipid content and LDL aggregation in ldlr−/− mice fed on a high-fat diet. Bioscience reports. 2012;32:479–490. doi: 10.1042/BSR20120036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annual review of pharmacology and toxicology. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Espejo EF, Mir D. Structure of the rat’s behaviour in the hot plate test. Behavioural brain research. 1993;56:171–176. doi: 10.1016/0166-4328(93)90035-o. [DOI] [PubMed] [Google Scholar]
- Espejo EF, Stinus L, Cador M, Mir D. Effects of morphine and naloxone on behaviour in the hot plate test: an ethopharmacological study in the rat. Psychopharmacology. 1994;113:500–510. doi: 10.1007/BF02245230. [DOI] [PubMed] [Google Scholar]
- Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, Hayward NJ, McNamara CR, Xue F, Moran MM, Strassmaier T, Uykal E, Owsianik G, Vennekens R, De Ridder D, Nilius B, Fanger CM, Voets T. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:19084–19089. doi: 10.1073/pnas.1005333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandiz-Huertas C, Mathivanan S, Wolf CJ, Devesa I, Ferrer-Montiel A. Trafficking of ThermoTRP Channels. Membranes. 2014;4:525–564. doi: 10.3390/membranes4030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichna J, Mokrowiecka A, Cygankiewicz AI, Zakrzewski PK, Malecka-Panas E, Janecka A, Krajewska WM, Storr MA. Transient receptor potential vanilloid 4 blockade protects against experimental colitis in mice: a new strategy for inflammatory bowel diseases treatment? Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2012;24:e557–e560. doi: 10.1111/j.1365-2982.2012.01999.x. [DOI] [PubMed] [Google Scholar]
- Finger BC, Dinan TG, Cryan JF. High-fat diet selectively protects against the effects of chronic social stress in the mouse. Neuroscience. 2011;192:351–360. doi: 10.1016/j.neuroscience.2011.06.072. [DOI] [PubMed] [Google Scholar]
- Holzer P. TRP channels in the digestive system. Current pharmaceutical biotechnology. 2011a;12:24–34. doi: 10.2174/138920111793937862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacology & therapeutics. 2011b;131:142–170. doi: 10.1016/j.pharmthera.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara ME. The anatomical study on the rat pancreas and its ducts with emphasis on the surgical approach. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft. 2005;187:105–112. doi: 10.1016/j.aanat.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Kondo T, Obata K, Miyoshi K, Sakurai J, Tanaka J, Miwa H, Noguchi K. Transient receptor potential A1 mediates gastric distention-induced visceral pain in rats. Gut. 2009;58:1342–1352. doi: 10.1136/gut.2008.175901. [DOI] [PubMed] [Google Scholar]
- Leung PS, Chan YC. Role of oxidative stress in pancreatic inflammation. Antioxidants & redox signaling. 2009;11:135–165. doi: 10.1089/ars.2008.2109. [DOI] [PubMed] [Google Scholar]
- Li C, Zhou HM. The role of manganese superoxide dismutase in inflammation defense. Enzyme research. 2011;2011:387176. doi: 10.4061/2011/387176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zhu Y, Shenoy M, Pai R, Liu L, Pasricha PJ. Anatomical and functional characterization of a duodeno-pancreatic neural reflex that can induce acute pancreatitis. American journal of physiology Gastrointestinal and liver physiology. 2013;304:G490–G500. doi: 10.1152/ajpgi.00012.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle RA, Nathan JD. Neurogenic inflammation and pancreatitis. Pancreatology. 2004;4:551–559. doi: 10.1159/000082180. discussion 559–560. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America 100 Suppl. 2003;2:14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Frye GD, Pulido MD, Botting SK. Effects of chronic ethanol consumption on rat GABA(A) and strychnine-sensitive glycine receptors expressed by lateral/basolateral amygdala neurons. Brain research. 2003;963:165–177. doi: 10.1016/s0006-8993(02)03966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwrath SL, Westlund KN. Pharmacological attenuation of chronic alcoholic pancreatitis induced hypersensitivity in rats. World journal of gastroenterology : WJG. 2015;21:836–853. doi: 10.3748/wjg.v21.i3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. American journal of physiology Cell physiology. 2004;286:C195–C205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- Noronha M, Bordalo O, Dreiling DA. Alcohol and the pancreas. II. Pancreatic morphology of advanced alcoholic pancreatitis. The American journal of gastroenterology. 1981a;76:120–124. [PubMed] [Google Scholar]
- Noronha M, Salgadinho A, Ferreira De Almeida MJ, Dreiling DA, Bordalo O. Alcohol and the pancreas. I. Clinical associations and histopathology of minimal pancreatic inflammation. The American journal of gastroenterology. 1981b;76:114–119. [PubMed] [Google Scholar]
- Pasricha PJ. Unraveling the mystery of pain in chronic pancreatitis. Nature reviews Gastroenterology & hepatology. 2012;9:140–151. doi: 10.1038/nrgastro.2011.274. [DOI] [PubMed] [Google Scholar]
- Patel S, Bellon EM, Haaga J, Park CH. Fat replacement of the exocrine pancreas. AJR American journal of roentgenology. 1980;135:843–845. doi: 10.2214/ajr.135.4.843. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Hohmann AG, Seybold V, Hammock BD. A lipid gate for the peripheral control of pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:15184–15191. doi: 10.1523/JNEUROSCI.3475-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajewicz W, Milnerowicz S, Nabzdyk S. Blood plasma antioxidant defense in patients with pancreatitis. Pancreas. 2006;32:139–144. doi: 10.1097/01.mpa.0000186247.81457.f7. [DOI] [PubMed] [Google Scholar]
- Sarles H, Lebreuil G, Tasso F, Figarella C, Clemente F, Devaux MA, Fagonde B, Payan H. A comparison of alcoholic pancreatitis in rat and man. Gut. 1971;12:377–388. doi: 10.1136/gut.12.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ES, La JH, Scheff NN, Davis BM, Albers KM, Gebhart GF. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:5603–5611. doi: 10.1523/JNEUROSCI.1806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SB, Motoo Y, Xie MJ, Mouri H, Asayama K, Sawabu N. Superoxide dismutase is induced during rat pancreatic acinar cell injury. Pancreas. 2002;24:146–152. doi: 10.1097/00006676-200203000-00005. [DOI] [PubMed] [Google Scholar]
- Todaka H, Taniguchi J, Satoh J, Mizuno A, Suzuki M. Warm temperature-sensitive transient receptor potential vanilloid 4 (TRPV4) plays an essential role in thermal hyperalgesia. The Journal of biological chemistry. 2004;279:35133–35138. doi: 10.1074/jbc.M406260200. [DOI] [PubMed] [Google Scholar]
- Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of substances to laboratory animals: routes of administration and factors to consider. Journal of the American Association for Laboratory Animal Science : JAALAS. 2011;50:600–613. [PMC free article] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Lu Y, Westlund KN. Nociception in persistent pancreatitis in rats: effects of morphine and neuropeptide alterations. Anesthesiology. 2003;98:474–484. doi: 10.1097/00000542-200302000-00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierck CJ, Jr, Kline Rt, Wiley RG. Comparison of operant escape and innate reflex responses to nociceptive skin temperatures produced by heat and cold stimulation of rats. Behavioral neuroscience. 2004;118:627–635. doi: 10.1037/0735-7044.118.3.627. [DOI] [PubMed] [Google Scholar]
- Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. Journal of clinical neurophysiology : official publication of the American Electroencephalograph Society. 1997;14:2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD., Jr The somatosensory system, with emphasis on structures important for pain. Brain research reviews. 2007;55:297–313. doi: 10.1016/j.brainresrev.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Xu GY, Winston JH, Shenoy M, Yin H, Pasricha PJ. Enhanced excitability and suppression of A-type K+ current of pancreas-specific afferent neurons in a rat model of chronic pancreatitis. American journal of physiology Gastrointestinal and liver physiology. 2006;291:G424–G431. doi: 10.1152/ajpgi.00560.2005. [DOI] [PubMed] [Google Scholar]
- Xu GY, Winston JH, Shenoy M, Yin H, Pendyala S, Pasricha PJ. Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology. 2007;133:1282–1292. doi: 10.1053/j.gastro.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Characterization of the foot withdrawal response to noxious radiant heat in the rat. Pain. 1994;59:85–94. doi: 10.1016/0304-3959(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kline RHt, McNearney TA, Johnson MP, Westlund KN. Cannabinoid receptor 2 agonist attenuates pain related behavior in rats with chronic alcohol/high fat diet induced pancreatitis. Molecular pain. 2014;10:66. doi: 10.1186/1744-8069-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LP, Ma F, Abshire SM, Westlund KN. Prolonged high fat/alcohol exposure increases TRPV4 and its functional responses in pancreatic stellate cells. American journal of physiology Regulatory, integrative and comparative physiology. 2013;304:R702–R711. doi: 10.1152/ajpregu.00296.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]