Abstract
Inhibition of α-glucosidase and α-amylase are key tactics for managing blood glucose levels. Currently, stronger, and more accessible inhibitors are needed to treat diabetes. Indeno[1,2-b] quinoxalines-carrying thiazole hybrids 1–17 were created and described using NMR. All analogues were tested for hypoglycaemic effect against STZ-induced diabetes in mice. Compounds 4, 6, 8, and 16 were the most potent among the synthesised analogues. These hybrids were examined for their effects on plasma insulin, urea, creatinine, GSH, MDA, ALT, AST, and total cholesterol. Moreover, these compounds were tested against α-glucosidase and α-amylase enzymes in vitro. The four hybrids 4, 6, 8, and 16 represented moderate to potent activity with IC50 values 0.982 ± 0.04, to 10.19 ± 0.21 for α-glucosidase inhibition and 17.58 ± 0.74 to 121.6 ± 5.14 μM for α-amylase inhibition when compared to the standard medication acarbose with IC50=0.316 ± 0.02 μM for α-glucosidase inhibition and 31.56 ± 1.33 μM for α-amylase inhibition. Docking studies as well as in silico ADMT were done.
Keywords: α-Amylase, α-Glucosidase, Fluorinated indeno-quinoxaline
Graphical Abstract
Introduction
Diabetes mellitus (DM) is becoming more and more prevalent all over the world. DM is a long-term metabolic disorder that happens when the pancreas does not make enough insulin, or the body doesn’t use it adequately1. Almost 463 million people are affected by it globally, and by 2030, that number is predicted to reach 578 million2. Diabetes mellitus is a collection of metabolic illnesses defined by hyperglycaemia resulting from abnormalities in insulin secretion, insulin action, or both. Diabetes’s chronic hyperglycaemia is linked to long-term harm, malfunction, and organ failure, particularly to the kidneys, eyes, heart, nerves, and blood vessels. Type II diabetes, or non-insulin-dependent diabetes mellitus (NIDDM), is characterised by impaired glucose homeostasis that leads to hyperglycaemia and is linked to neuropathy, macrovascular, and microvascular problems. NIDDM is a multifactorial, intricate disease3.
Long-term diabetes can result in numerous problems, including alterations to the blood vessels and cellular malfunction. Proteinuria, a steady loss in renal and hepatic functioning, and a higher risk of cardiovascular illnesses are all clinical symptoms of diabetes mellitus. Persistent hyperglycaemia boosts the formation of free radicals and weakens intracellular antioxidant defences, which causes oxidative stress and the activation of apoptotic pathways. This, in turn, controls the generation of several pro-inflammatory mediators that worsen damage to different organs and impair the course of diabetes mellitus4–7. Thus, creating new, safe, and biologically active anti-diabetic drugs is crucial.
Preventing problems from diabetes brought on by non-enzymatic glycation and the inhibition of blood glucose-regulating enzymes is the main objective of treatment for diabetes. The generation of insulin by pancreatic islets regulates this scenario. Furthermore, enzymes like α -glucosidase and α -amylase are employed to halt the decomposition of sugar8. Therefore, the inhibition of α -glucosidase is a major scientific focus for hyperglycaemic control in diabetic patients. Intestinal α -glucosidases break down dietary carbs into simple sugars so that they can be absorbed9. Inhibiting intestinal α-glucosidase is one approach of inhibiting carbohydrate breakdown and limiting carbohydrate absorption in diabetes to reduce hyperglycaemia. According to this strategy, acarbose, miglitol, and voglibose were produced as anti-diabetic medications a few years ago. However, since then, no significant development has been made in developing new α-glucosidase inhibitors as diabetic medications with enhanced effectiveness and fewer adverse effects10. To improve this area of anti-diabetic treatment, there is still a critical necessity to find novel α -glucosidase inhibitors to improve the activity and decrease the adverse side effects.
Moreover, the metallo-enzyme α-amylase is a member of the endo-amylase family and is responsible for catalysing the first step of starch hydrolysis into shorter oligosaccharides by cleaving α-D-(1–4) glycosidic linkages as it contains Ca2+ ions in its active site. Due to its ability to hydrolyse the 1,4-glycosidic linkage of starch and the actions that can be carried out because of hydrolysis, this enzyme attracted great interest. The use of a lot of carbohydrates is linked to certain major issues such as obesity, diabetes, and dental diseases11–14.
Additionally, a wide variety of biological properties, for example anti-inflammatory, antibacterial, anti-cancer, and antioxidant ones, are displayed by thiazole derivatives15. In addition, they have been revealed to have effective α-glucosidase inhibitory action. Some examples of this activity include the thiazole derivatives isatin-thiazole16, coumarin-thiazole17, thiazole-pyrrole18, and triazine-thiazole19,20. There is an insufficient amount of research available regarding the impact of isomerism on the inhibition of α-glucosidase, but those that do include research on kotalanol, iminosugars, hyacinthacins, bicyclitols, bicyclitol-deoxynojirimycin conjugates, perylene bisimide-deoxynojirimycin conjugates, perylene bisimide-deoxynojirimycin conjugates21.
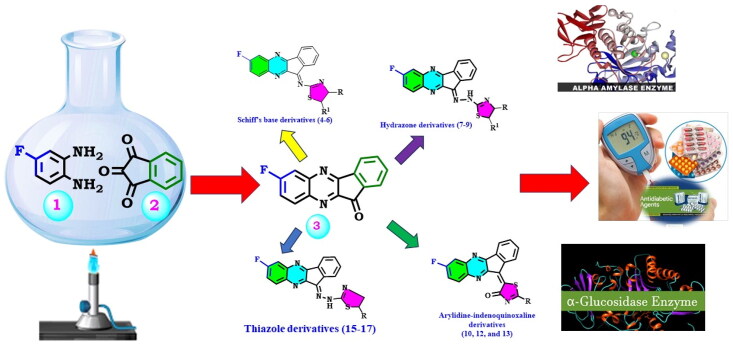
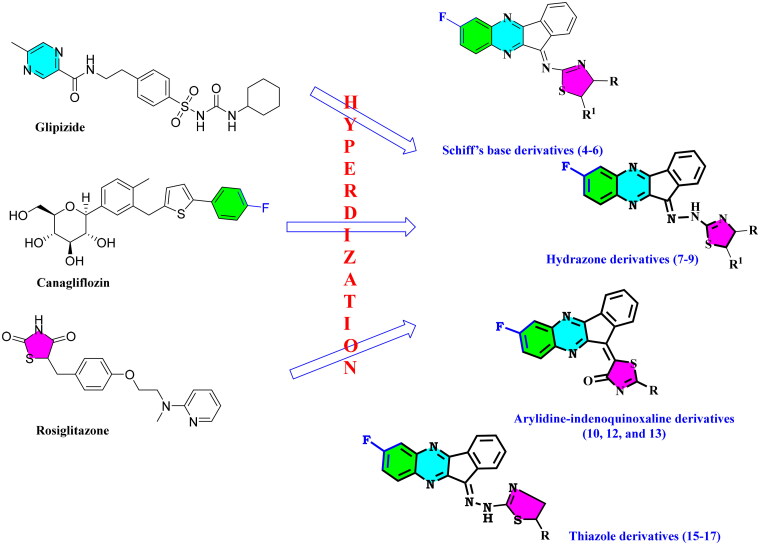
The current research is a part of our ongoing search for the discovery of novel hybrids to treat different diseases22–27. Herein we synthesised α-glucosidase and α-amylase inhibitors that might be used in the development of anti-diabetic medications (Figure 1).
Figure 1.
Synthetic approach for designing indenoquinoxaline hits 3–17.
Materials and methods
S1 in the supplementary materials28–49.
Result and discussion
Chemistry
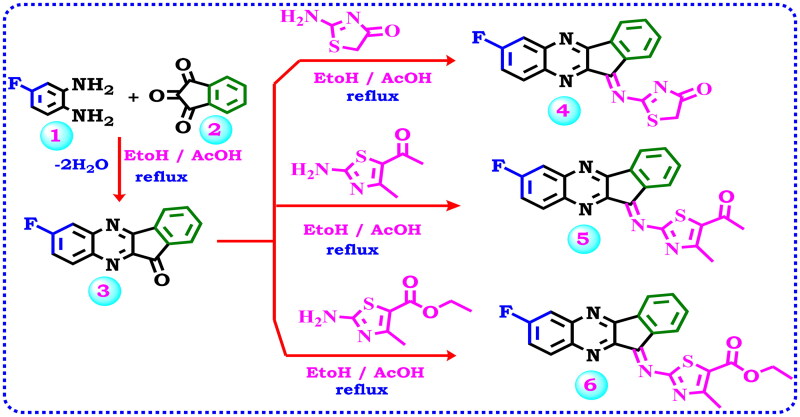
All the target substances were successfully synthesised according to Scheme 1–3, and their predicted structures were validated using analytical and spectroscopic data (IR,1H NMR,13C NMR). According to the literature protocol50, 7-fluoro-11H-indeno[1,2-b]quinoxalin-11-one (3) is prepared as a starting compound by cyclo-condensation of 4-fluoro-1,2-phenylenediamine (1) with ninhydrin 2 in hot methanol and catalytic quantities of acetic acid. Due to the importance of the biological activity of thiazole moiety, the author planned to design and construct a thiazole ring linked with the starting material 3 via keto function (C = O).
Scheme 1.
Illustrate the synthesis of indeno-quinoxaline derivative 3 and synthesis of new Schiff’s base derivatives 4–6.
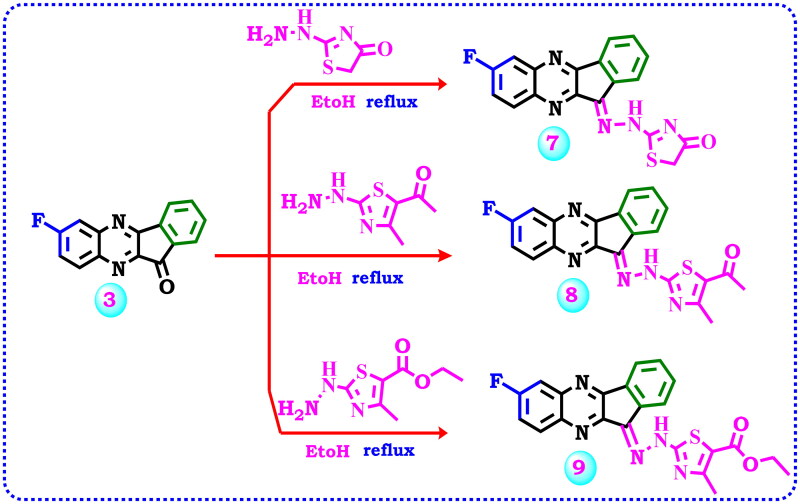
Scheme 2.
Strategy for the synthesis of hydrazone derivatives 7–9.
Scheme 3.
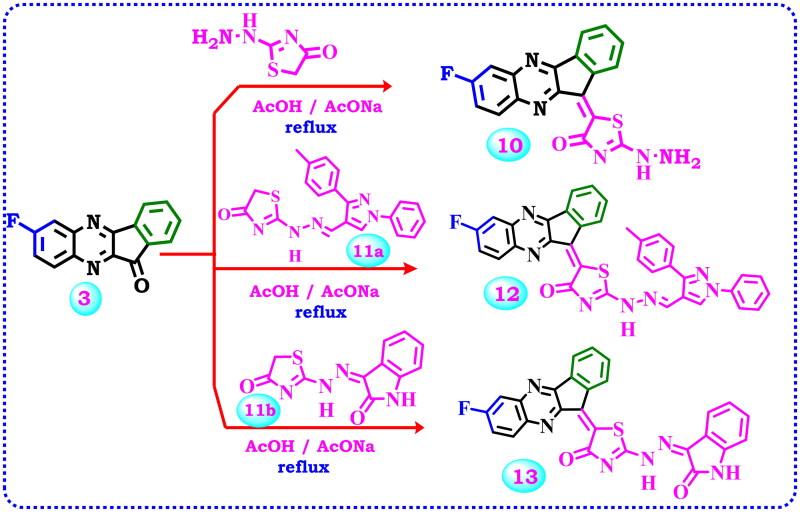
Proposed reactions for the synthesis of arylidine-indenoquinoxaline derivatives 10, 12, and 13.
As viewed in Scheme 1, the starting material fluoro-indenoquinoxaline (1) was subjected to react with 2-aminothiazol derivatives specified, 2-aminothiazol-4(5H)-one, 1–(2-amino-4-methylthiazol-5-yl)ethan-1-one or ethyl 2-amino-4-methylthiazole-5-carboxylate via a condensation reaction to afford the corresponding imine thiazole derivatives 4–6. The spectral data confirmed the expected structure of the products. The IR spectrum of compound 4 indicates the presence of one carbonyl group at 1736 cm−1 and the imino function C = N at 1604 cm−1. Additionally, its 1H NMR spectra designates two aliphatic protons of CH2 at δ 4.08 ppm beside the aromatic protons ranging from δ 7.69–8.24 ppm. The13C NMR spectrum revealed the aliphatic CH2 of the thiazolone ring at δ 37.46 ppm, the aromatic carbons ranged from δ 113.94–145.60 ppm, C = N at δ 163.57 ppm, carbonyl at δ 178.41, and signals at δ 179.02 ppm due to S-C = N function. Also, the IR spectrum of compound 5 revealed an absorption band at 1735 cm−1 corresponding to the carbonyl group due to acetyl thiazole, while compound 6 presented absorption bands for CH aromatic, CH aliphatic, C = O, and C = N groups at 3076, 2925, 1735, 1605 cm−1 respectively. The 1H NMR compound 5 demonstrated two singlet signals at δ 2.33, and 2.41 ppm relevant to the two methyl protons, as well as signals for the aromatic protons between δ 7.72 ppm to δ 8.25 ppm, while compound 6 displayed signals at δ 1.21, 2.38, and 4.13 ppm for two CH3 and CH2 of the ethyl group beside the signals of the aromatic protons. The13C NMR of compound 5 characterised the structure by the presence of two signals for aliphatic carbons, signals for aromatic carbons, and signals for (C-F), and (C = O) groups.
By the same manner, 7-fluoro-11H-indeno[1,2-b]quinoxalin-11-one (3) was allowed to react with some thiazolyl hydrazines such as 2-hydrazineylthiazol-4(5H)-one, 1–(2-hydrazineyl-4-methylthiazol-5-yl)ethan-1-one, or ethyl 2-hydrazineyl-4-methylthiazole-5-carboxylate, respectively, to produce the corresponding hydrazone derivatives 7–9, Scheme 2. The IR spectrum of compound 7 revealed bands at 3130, 3049, 2967, 1709, and 1612 cm−1 corresponding to NH, CH-aromatic, CH-aliphatic, carbonyl, and imino (C = N) groups, respectively. Moreover, its 1H NMR displayed a singlet signal at δ 4.06 ppm due to CH2 aliphatic protons of thiazolone and one singlet signal at δ 11.77 ppm for NH beside the aromatic protons. Also, 13C NMR confirmed the reaction product by the presence of a signal at δ 33.03 ppm related to CH2 of aliphatic carbon, and twelve signals for the aromatic carbons beside the signals specific to C = N, C-F, and carbonyl carbons at δ 160.05, 162.35, and 168.75 ppm, respectively. The IR spectrum of compound 8 displayed an absorption band at 3427 attributed to (NH), and a band at 1735 cm−1 due to (C = O). Moreover, its 1H NMR spectrum displayed two singlet signals at δ 1.94 and 2.29 ppm because of the presence of two methyl groups, a singlet signal at δ 7.96 ppm related to the NH group along with seven aromatic protons between δ 7.72 to 8.23 ppm, while 1H NMR spectrum of compound 9 revealed three signals as triplet, singlet, and quartette splitting signals at δ 1.27, 2.57, 4.23 ppm which represent the aliphatic protons of methyl and ethyl groups in addition to aromatic protons and the proton of the NH group which appeared at δ 7.90 ppm. Also, the 13C NMR spectrum of compound 9 displayed the existence of three carbons at δ 19.23, 21.02, and 61.94 ppm assignable for methyl and ethyl carbons in addition to the signals of the aromatic carbons and the signals representing (C = N), carbon attached to fluoride (C-F), (S-C = N), and carbonyl (C = O).
2-Hydrazineylthiazol-4(5H)-one has two nucleophilic active centres, hydrazine function and methylene CH2 function. The condensation of the keto group with hydrazine function in acidic medium afforded the corresponding hydrazone, while its condensation with methylene CH2 function in a basic medium, afforded the corresponding arylidine derivatives (C = C). Therefore, the starting material 3 was condensed with 2-hydrazineylthiazol-4(5H)-one in a basic medium using freshly prepared fused sodium acetate to produce the target 5–(7-fluoro-11H-indeno[1,2-b]quinoxalin-11-ylidene)-2-hydrazineylthiazol-4(5H)-one (10), Scheme 3. Elemental analysis and spectrum data proved the product. Along with a carbonyl band at 1711 cm−1, IR spectra showed NH2 and NH bands at 3415 and 3190 cm−1. Aside from two signals that emerged at δ 5.43 and 7.89 ppm due to NH2 and NH, 1H NMR also showed signals caused by aromatic protons. In addition to signals for the aromatic carbons, its 13C NMR provided signals at δ 172.16 for the carbonyl group, 161.58 ppm for carbon attached to fluoride atom, δ 158.91 for (S-C = N), and 157.13 ppm for (C = N) groups. Also as viewed in Scheme 3, the two reagents 11a and 11b were prepared according to previous work 51,52, and allowed to react with the starting material 3 in a basic medium using freshly prepared fused sodium acetate to produce the corresponding 12 and 13. The structure of 12 was confirmed by microanalyses and spectral data. The IR spectrum exhibited a band at 3422 cm−1 related to the NH group in addition to the carbonyl band at 1729 cm−1. The 1H NMR spectrum of the same compound showed the exchangeable signal of the NH group at δ 8.90 ppm and the signals for seventeen aromatic protons. Furthermore, its 13C NMR indicated three signals at δ 164.99, 172.31, and 174.44 ppm for S-C = N, C-F, and C = O groups, besides the aromatic carbons. Also, the 1H NMR spectrum of compound 13 elicited two singlet signals at δ 10.67 and 11.05 ppm attributed to 2NH protons, besides the signals associated with the aromatic Hs. Additionally, 13C NMR presented signals between δ 110.29 to 157.24 ppm, and four signals at δ 164.97, 168.86, 171.92, and 171.97 ppm related to (S-C = N), (C-F), and (2 C = O) groups correspondingly.
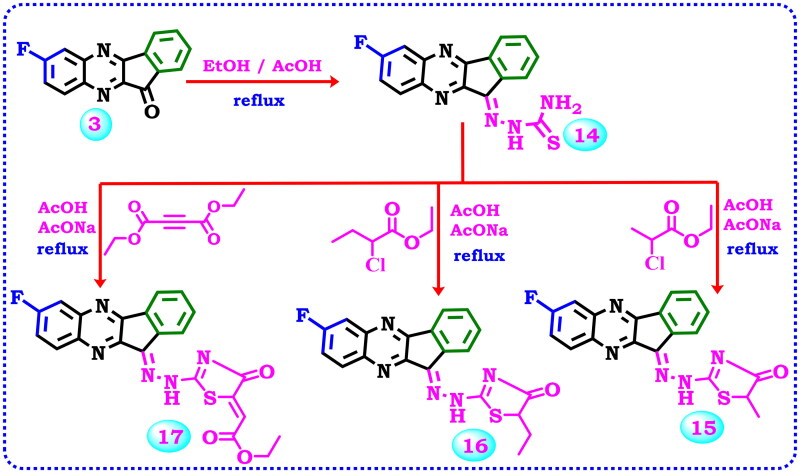
Thiosemicarbazone derivatives are very useful and widely used as a synthetic initial compound for thiazole synthesis. Consequently, compound 3 was allowed to react with thiosemicarbazide to produce the corresponding thiosemicarbazone derivative 14, Scheme 4. Its IR spectrum doesn’t have the C = O group absorption band present in compound 3 and at the same time revealed NH2 and NH group absorption bands at 3448, 3396, and 3275 cm−1 respectively. The 1H NMR spectrum exhibited two singlet signals at δ 8.15, and 12.48 ppm relevant to the NH2, and NH protons together with signals related to the aromatic protons. Furthermore, thiosemicarbazone derivative 14 was cyclo-alkylated to obtain many biologically active thiazole compounds. Thus, the interaction of compound 14 with an alkylating agent (ethyl 2-chloropropanoate, ethyl 2-chlorobutanoate) or diethyl but-2-ynedioate produced the corresponding different derivatives 15–17, Scheme 4. The IR spectrum of compound 15 exhibited bands at 3396 cm−1 for the NH2 group, and 1722 cm−1 due to the C = O group. The 1H NMR field confirmed its structure through the appearance of a singlet signal at δ 1.45 ppm corresponding to the hydrogens of the methyl, a quartette signal at δ 3.81 ppm representing CH of thiazolone, signals in the region δ 7.54–8.26 ppm showing the aromatic protons, in addition to singlet signal at δ 8.84 ppm due to NH group. While the 1H NMR spectrum of 16 displayed a triplet signal at δ 0.99 due to (CH3-CH2), a signal at δ 2.02 ppm corresponding to (CH3-CH2), triplet signal at δ 4.33 assignable to the CH in the thiazole, and a singlet signal at δ 12.64 for the NH group beside the signals of the aromatic protons. The 13C NMR spectrum showed the presence of two aliphatic carbons at δ 19.85, and 57.87 ppm, whereas the carbon attached to the flour and the carbonyl carbon were observed at δ 154.29, and 178.79 ppm respectively. Finally, The IR spectrum of thiazolone derivative 17 displayed absorption bands at 3263, 1727, and 1700 cm−1 related to NH, and two carbonyl groups respectively. Also, its 1H NMR spectrum revealed two signals at δ 1.27, and 4.24 ppm due to the ethyl group, besides a signal at δ 6.64 ppm for the CH-vinilic, and a signal at δ 7.98 ppm for the NH group, in addition to the aromatic protons. 13C NMR spectrum was characterised by signals at δ 14.57, 61.55, and 96.97 ppm representing the two aliphatic carbons of the ethyl group, and CH-vinylic, as well as signals at δ 158.28, 161.62, 165.01 ppm related to (C = N), (S-C = N), (C-F) carbons, and two signals at δ 166.24, 166.38 ppm assignable to (2 C = O) groups besides signals in the aromatic region owing to the aromatic carbons.
Scheme 4.
Synthesis of thiosemicarbazone derivative 14 and its cyclisation to afford the thiazole derivatives 15–17.
Biology
Blood glucose and plasma insulin level
Streptozotocin (STZ) causes a significant increase in blood sugar and a big drop in insulin levels in the plasma. When gliclazide and the newly synthesised hybrids were used, blood sugar levels declined significantly, and insulin levels rose compared to the STZ-only treatment group (p < 0.05) (Table 1).
Table 1.
Effect of two weeks of treatment with new hybrids 4–17 as well as Gliclazide on plasma glucose and insulin level in STZ-induced diabetes in mice.
| Parameters Groups | Glucose 3rd day (mg/dL) | Glucose 6th day | Glucose 9th day | Glucose 12th day | Glucose 15th day | Insulin (ng/mL) |
|---|---|---|---|---|---|---|
| NC | 98.67 ± 4.4b | 98.67 ± 4.4b | 98.67 ± 4.4b | 98.67 ± 4.4b | 98.67 ± 4.4b | 16.75 ± 0.5b,c |
| PC | 279.17 ± 10.2a | 357.50 ± 18.96a | 540 ± 26.6a,c | 519.17 ± 27.8a,c | 486.67 ± 11.4a,c | 3.14 ± 0.2a,c |
| Gliclazide | 358.33 ± 18.1a | 199.67 ± 5.8 | 165 ± 4.1b | 132.33 ± 3.7b | 118.33 ± 3.3b | 14.52 ± 0.4a,b |
| 4 | 311.33 ± 19.5a | 293.33 ± 17.8a | 304.17 ± 7.1a,b | 187 ± 3.4b | 162.83 ± 1.8 b | 11.44 ± 0.2a,b |
| 5 | 436.67 ± 51.6a | 566.67 ± 15.4a,b,c | 251.67 ± 13.5b | 300 ± 18.2a,b | 256.67 ± 19.9a,b | |
| 6 | 332.50 ± 18.4a | 363.83 ± 69a | 220 ± 13.9b | 177.50 ± 3.8b | 155.67 ± 3.6b | 12.28 ± 0.3a,b |
| 7 | 478.33 ± 44.7a,b | 543.33 ± 17.6a,c | 280.83 ± 27.5a,b | 230 ± 9.3b | 216.67 ± 10.6b | |
| 8 | 313.33 ± 4.9a | 238.33 ± 4.8 | 202.50 ± 3.6b | 214.83 ± 9.97b | 175 ± 11.5b | 10.5 ± 0.3a,b |
| 9 | 321.67 ± 35.5a | 300.83 ± 34.7a | 275.17 ± 24a,b | 355 ± 18.4a,c | 337.17 ± 11.4a,c | |
| 10 | 385.83 ± 61.2a | 381.67 ± 23.3a | 305.33 ± 16.4a,b | 408.33 ± 40.3a,c | 368.33 ± 33a,c | |
| 12 | 343.00 ± 52.8a | 288.33 ± 77.9 | 332.17 ± 79.1a,b | 251.67 ± 60.8b | 325 ± 15.6a,b,c | |
| 13 | 340.83 ± 39.2a | 240 ± 54.2 | 295 ± 80.3a,b | 226.67 ± 48.6b | 225 ± 46.7b | |
| 14 | 351.67 ± 16.6a | 258.33 ± 16.6 | 213 ± 10.3b | 387.50 ± 82.7a,c | 320.50 ± 79.9a,b,c | |
| 15 | 321.67 ± 24.7a | 356.67 ± 29.6a | 294.17 ± 31.3a,b | 318.33 ± 27.7a,b,c | 294.17 ± 31.3a,b,c | |
| 16 | 313.33 ± 23.8a | 254.50 ± 9.7 | 217.50 ± 6.3b | 202 ± 13.5b | 169 ± 1.5b | 9.34 ± 0.3a,b |
| 17 | 410 ± 57.6a | 350.83 ± 80a | 294.17 ± 60.9a,b | 323.33 ± 65.2a,b,c | 312.50 ± 63a,b,c |
Each value represents the mean of 6 mice ± SEM. Statistical analysis was carried out using one-way analysis of variance (ANOVA) followed by Tukey Kramer multiple comparisons test.
significantly different from normal control (NC) (p < 0.05)
significantly different from positive control (PC) (p < 0.05)
significantly different from standard (p < 0.05).
Kidney function parameters
STZ resulted in a nephropathy state manifested by a significant elevation of plasma urea and creatinine levels as compared to normal control mice. Treatment with gliclazide and 4, 6, 8, and 16 led to a significant lowering in plasma urea and creatinine levels as related to STZ only treated group p < 0.05 (Table 2).
Table 2.
Effect of two weeks of treatment with 4, 6, 8, and 16 on plasma levels of urea, creatinine, GSH, MDA, ALT, AST, and total cholesterol in STZ-induced diabetes in mice.
| Parameter Groups | Urea | Creat | GSH | MDA | ALT | AST | TC |
|---|---|---|---|---|---|---|---|
| NC | 55.1 ± 0.3 | 2.03 ± 0.1 | 4.56 ± 0.02 | 18.57 ± 0.2 | 44.4 ± 0.8 | 35.2 ± 0.3 | 65.3 ± 0.4 |
| PC | 91.6 ± 0.5a | 7.8 ± 0.1a | 0.998 ± 0.04a | 40.3 ± 0.8a | 96.7 ± 0.7a | 87.6 ± 0.7a | 150.3 ± 1a |
| Gliclazide | 57.5 ± 0.3a,b | 3.3 ± 0.1a,b | 3.15 ± 0.05a,b | 22 ± 0.2a,b | 53.7 ± 0.7a,b | 39.8 ± 0.3a,b | 74.4 ± 0.6a,b |
| 4 | 60.5 ± 0.4a,b | 5.1 ± 0.1a,b | 2.17 ± 0.03a,b | 23.9 ± 0.2a,b | 66 ± 0.4a,b | 46.4 ± 0.2a,b | 79.3 ± 0.4a,b |
| 6 | 59.5 ± 0.2a,b | 4.9 ± 0.1a,b | 2.5 ± 0.02a,b | 22.9 ± 0.3a,b | 59.6 ± 0.2a,b | 46.1 ± 0.3a,b | 77.5 ± 0.5a,b |
| 8 | 63.2 ± 0.3a,b | 5.9 ± 0.1a,b | 1.72 ± 0.03a,b | 27.7 ± 0.3a,b | 72.5 ± 0.9a,b | 54.2 ± 0.4a,b | 86.3 ± 0.7a,b |
| 16 | 62 ± 0.3a,b | 5.5 ± 0.1a,b | 1.99 ± 0.03a,b | 24.9 ± 0.2a,b | 68.2 ± 0.6a,b | 47.6 ± 0.2 a,b | 81.5 ± 0.3a,b |
Each value represents the mean of 6 mice ± SEM. Statistical analysis was carried out using one-way analysis of variance (ANOVA) followed by Tukey Kramer multiple comparisons test.
significantly different from normal control (NC) (p < 0.05)
significantly different from positive control (PC) (p < 0.05)
significantly different from standard (p < 0.05).
Oxidative stress markers
A biomarker for oxidative stress (MDA) was significantly higher in the diabetes group that wasn’t treated. Along with this, there was a big drop in plasma antioxidant (GSH). Treatment with gliclazide and hybrids 4, 6, 8, and 16 demonstrated a significant reduction of oxidative stress marker (MDA) and significant elevation in GSH as compared to the diabetic non-treated group, p < 0.05 (Table 2).
Liver function markers and cholesterol
The administration of STZ led to the deterioration of liver function biomarkers, as evidenced by a notable increase in AST and ALT levels. In addition to a significant elevation in total cholesterol, p < 0.05 (Table 2). Treatment of the diabetic group with gliclazide or hybrids 4, 6, 8, and 16 for 2 weeks resulted in a significant reduction of TC and liver function tests (AST and ALT), p < 0.05 (Table 2).
α-Glucosidase inhibition assay
The brush border of the small intestine contains the enzyme α-glucosidase, which controls the enzymatic hydrolysis of 1,4-linked polysaccharides with glucose as one of the main byproducts. Glucosidase is a target for the treatment of postprandial hyperglycaemia because of the significant role that glucose plays as one of the primary sources of energy in eukaryotes. The anti-diabetic medication acarbose belongs to a group of medications called α-glucosidase inhibitors (AGIs), which stop the gastrointestinal tract from absorbing glucose. In vitro α-glucosidase inhibitory activity of the target hybrids 4, 6, 8, and 16 was assessed in relation to the reference medication acarbose (IC50= 0.316 ± 0.02 μM). Table 3 provided a summary of the findings. Both substances were discovered to be strong inhibitors of the α-glucosidase enzyme, as shown below. However, the hydrazone derivative (1–(2-(2–(7-fluoro-11H-indeno[1,2-b]quinoxalin-11-ylidene)-hydrazineyl)-4-methyl-thiazol-5-yl)-ethan-1-one) (8) was found to be more active as α-glucosidase inhibitory activity with IC50 value of 0.982 ± 0.04 µM, followed by Schiff base derivative 6 with IC50 2.995 ± 0.09 μM. Additionally, the hydrazone derivative 16 has good activity as an inhibitor of α-glucosidase, which suggests that the most potent hybrids are those that contain indenoquinoxalene and thiazole connected with hydrazone, followed by Schiff base linker.
Table 3.
Effects of indenoquinoxaline-containing thiazole analogs 4, 6, 8, and 16 as well as acarbose on α-glucosidase activity.
| Ser. | Cpd. No. | α-Glucosidase IC50 (µm) | DF = 100 ± SD |
|---|---|---|---|
| 1 | 4 | 10.19 | 0.21 |
| 2 | 6 | 2.995 | 0.09 |
| 3 | 8 | 0.982 | 0.04 |
| 4 | 16 | 7.029 | 0.13 |
| Standard | Acarbose | 0.316 | 0.02 |
Results represent IC50 (Half maximal inhibitory concentration) of indenoquinoxaline-containing thiazole analogs ± SD as compared to acarbose on α-glucosidase activity.
α-Amylase inhibition assay
Changes to α-amylase activity have an impact on the utilisation of carbohydrates as a fuel source. This enzyme is necessary for the digestion of complex carbohydrates in humans. Inhibition of α-amylase is seen as a possible target for the treatment of diseases like diabetes, obesity, dental caries, and periodontal problems that are linked to carbohydrate intake. In vitro α-amylase inhibitory activity of compounds 4, 6, 8, and 16 was assessed in relation to the reference medication acarbose (IC50 = 31.56 ± 1.33 μM). Table 4 provided a summary of the findings. All the substances examined were discovered to be strong inhibitors of the α-amylase enzyme. Particularly, Schiff base derivative 6 and hydrazone derivative 8 show that these moieties are crucial for the anti-diabetic effects.
Table 4.
Effects of indenoquinoxaline-containing thiazole analogs 4, 6, 8, and 16 as well as acarbose on α-amylase activity.
| Ser. | Cpd. No. | α- Amylase IC50 (μM) | DF = 10 ±SD |
|---|---|---|---|
| 1 | 4 | 93.36 | 3.95 |
| 2 | 6 | 32.29 | 1.37 |
| 3 | 8 | 17.58 | 0.74 |
| 4 | 16 | 121.6 | 5.14 |
| Standard | Acarbose | 31.56 | 1.33 |
Results represent IC50 (Half maximal inhibitory concentration) of indenoquinoxaline-containing thiazole analogs ± SD as compared to acarbose on α-amylase activity.
In silico studies
In silico assessment of physicochemical and ADME properties
The compounds’ physicochemical parameters, lipophilicity, water-solubility, pharmacokinetics, drug-likeness, medicinal chemistry properties, and bioavailability score were calculated using SwissADME (http://www.swissadme.ch). Bioavailability is a quick assessment of substances to determine their suitability as an oral medication.
Using SwissADME, the physicochemical and ADME properties of the newly made thiazole-containing compounds 4, 5, 6, 7, 8, 9, 10, 12, 13, 14, 15, 16, and 17, as well as acarbose and gliclazide were studied (Table 5).
Table 5.
Physicochemical properties according to TPSA, and % ABS.
| Cpd. No. | TPSAa | % ABSb |
|---|---|---|
| 4 | 92.87 | 76.96 |
| 5 | 96.34 | 75.76 |
| 6 | 105.57 | 72.58 |
| 7 | 104.9 | 72.81 |
| 8 | 108.37 | 71.61 |
| 9 | 117.6 | 68.43 |
| 10 | 118.56 | 68.1 |
| 12 | 122.72 | 66.66 |
| 13 | 134 | 62.77 |
| 14 | 108.28 | 71.64 |
| 15 | 104.9 | 72.81 |
| 16 | 104.9 | 72.81 |
| 17 | 131.2 | 63.74 |
| Acarbose | 321.17 | −1.8 |
| Gliclazide | 86.89 | 79.02 |
topological polar surface area (Å2)
% absorption
Topological polar surface area (TPSA) (Table 5) values for all drugs are less than 140 A0, indicating high oral bioavailability. The estimated% ABS shows that all hits ranged between percentages of ABS were computed using the formula % ABS = 109 –(0.345 ×TPSA).
Rotatable bonds in all compounds are between 1 and 5, which suggests that the molecule is flexible concerning its bio target. Also, all compounds have hydrogen bond acceptor (HBA) sites less than 10. They also have less than 5 for hydrogen bond donor (HBD) sites. Additionally, Molar refractivity was from 40 to 130 for all compounds except compounds 12 and 13 (Table 6).
Table 6.
Physicochemical properties of the newly synthesised hits.
| Cpd. No. | Fraction Csp3a | No. of Rotatable bonds | HBAb | HBDc | iLOGPd | MRe |
|---|---|---|---|---|---|---|
| 4 | 0.06 | 1 | 6 | 0 | 2.59 | 98.74 |
| 5 | 0.1 | 2 | 6 | 0 | 3.39 | 107.51 |
| 6 | 0.14 | 4 | 7 | 0 | 3.83 | 113.4 |
| 7 | 0.06 | 2 | 6 | 1 | 2.32 | 101.54 |
| 8 | 0.1 | 3 | 6 | 1 | 2.98 | 111.29 |
| 9 | 0.14 | 5 | 7 | 1 | 3.43 | 117.18 |
| 10 | 0 | 1 | 6 | 2 | 2.34 | 100.88 |
| 12 | 0.03 | 5 | 7 | 1 | 4.37 | 178.67 |
| 13 | 0 | 2 | 7 | 2 | 4.37 | 141.48 |
| 14 | 0 | 2 | 4 | 2 | 2.58 | 89.74 |
| 15 | 0.11 | 2 | 6 | 1 | 2.82 | 106.35 |
| 16 | 0.15 | 3 | 6 | 1 | 2.74 | 111.15 |
| 17 | 0.09 | 5 | 8 | 1 | 2.74 | 121.58 |
| Acarbose | 0.92 | 9 | 19 | 14 | 0.63 | 136.69 |
| Gliclazide | 0.53 | 5 | 4 | 2 | 1.96 | 86.93 |
The ratio of sp3 hybridised carbons over the total carbon count of the molecule
number of hydrogen bond acceptors
number of hydrogen bond donors
lipophilicity; eMolar refractivity.
Table 7 for the drug similarity predictor demonstrates that all synthesised compounds meet Lipinski’s requirements for oral medications, except for compounds 12 and 13, which have just one infraction. While occasionally inadequate absorption can result from violating even one of these rules. A common method for assessing a compound’s drug-likeness is Lipinski’s rule of five. A drug-like compound should meet no more than one of the following requirements, according to Lipinski’s rule: ‘a molecular weight of less than 500 g/mol, a log p values of less than 5, no more than five hydrogen bond donors (HBD), and no more than 10 hydrogen bond acceptor (HBA) sites.’ Based on the screening method that follows Veber’s guidelines, all the hits pass the test for drug resemblance.
Table 7.
Bioavailability and drug-likeness predictions of substances 4–17 in addition to Acarbose and Gliclazide.
| Cpd. No. | Lipinski (violations) | Veber (violations) | Ghose (violations) | Egan (violations) | Muegge (violations) | Bioavailability Score | PAINS alerts | Synthetic Accessibility |
|---|---|---|---|---|---|---|---|---|
| 4 | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 | 3.44 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 | 3.54 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 | 3.7 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 | 3.43 |
| 8 | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 | 3.56 |
| 9 | 0 | 0 | 0 | 0 | 1 | 0.55 | 0 | 3.75 |
| 10 | 0 | 0 | 0 | 0 | 0 | 0.55 | 1 | 3.66 |
| 12 | 1 | 0 | 3 | 1 | 3 | 0.55 | 1 | 4.78 |
| 13 | 1 | 0 | 3 | 1 | 3 | 0.55 | 1 | 4.78 |
| 14 | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 | 3.03 |
| 15 | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 | 3.99 |
| 16 | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 | 4.09 |
| 17 | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 | 4.09 |
| Acarbose | 3 | 1 | 4 | 1 | 5 | 0.17 | 0 | 7.34 |
| Gliclazide | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 | 3.52 |
The Ghose, Egan, and Muegge guidelines say that all derivatives except compounds 12 and 13 follow the rule and could be used as medicines. Also, Muegge rules are broken in compound 9. All compounds that were looked at, had a score of 0.55 for bioavailability which is a high score.
(PAINS), which was made by SwissADME, showed no alerts for all hits except 10, 12, and 13. Those three only had one alert. Even though PAINS are important features to consider when making drugs to avoid false positives, overestimating them, and using them blindly could mean that promising hits are left out because of fake PAINS. All the analogs were given scores between 3.43 and 4.78 for how easy they are to produce, which means that they can be made in large quantities (Table 7).
Regarding the pharmacokinetic and medicinal chemistry aspects of the produced compounds (Table 8), it was determined that all derivatives have high gastrointestinal absorption, except compounds 9, 12, and 13. All of them can’t get through the blood-brain barrier. This means that these molecules that are targeted to the whole body will have few or no effects on the CNS. The investigation of the P-glycoprotein (P-gp) non-substrate candidature was another key issue throughout the preclinical analysis trial. Pgp functions as an efflux transporter, pumping medicines, other substances, and its substrate out of the cell (Figure 2).
Table 8.
Pharmacokinetic/ADME properties of the tested novel compounds.
| Cpd. No. | GI absorption | BBB permeant | Pgp substrate | CYP1A2 inhibitor | CYP2C19 inhibitor | CYP2C9 inhibitor | CYP2D6 inhibitor | CYP3A4 inhibitor |
|---|---|---|---|---|---|---|---|---|
| 4 | High | No | No | Yes | Yes | Yes | No | Yes |
| 5 | High | No | No | Yes | Yes | Yes | No | Yes |
| 6 | High | No | No | Yes | Yes | Yes | No | Yes |
| 7 | High | No | No | Yes | Yes | Yes | No | Yes |
| 8 | High | No | No | Yes | Yes | Yes | No | Yes |
| 9 | Low | No | No | No | Yes | Yes | No | Yes |
| 10 | High | No | No | Yes | No | No | No | Yes |
| 12 | Low | No | No | No | No | No | No | No |
| 13 | Low | No | No | No | No | No | No | No |
| 14 | High | No | No | Yes | No | Yes | No | Yes |
| 15 | High | No | No | Yes | Yes | Yes | No | Yes |
| 16 | High | No | No | Yes | Yes | Yes | No | Yes |
| 17 | High | No | No | Yes | Yes | Yes | No | Yes |
| Acarbose | Low | No | Yes | No | No | No | No | No |
| Gliclazide | High | No | Yes | No | No | No | No | No |
Figure 2.
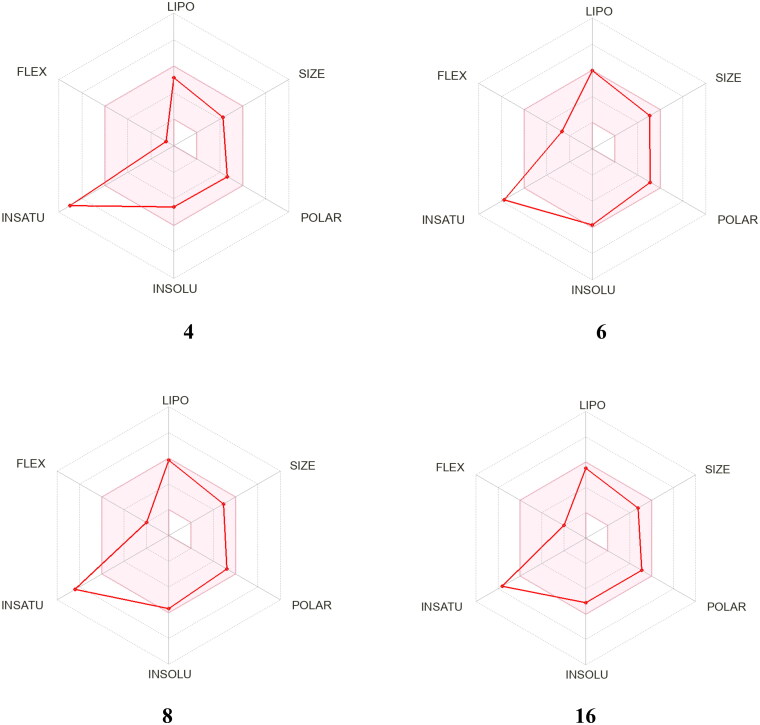
Diagrams showing how compounds 4, 6, 8, and 16 correlate to drugs. The coloured area is a good physical and chemical space for oral bioavailability. LIPO (lipophilicity); SIZE (molecular weight); POLAR (polarity); INSOLU (insolubility); INSATU (insaturation); FLEX (flexibility).
As a result, the hits were examined using the SwissADME website. We discovered that all the hits are not P-gp protein substrates, as shown in (Table 8), implying that these hits have a minimal probability of efflux out of the cell.
The metabolic profile obtained (Table 8) revealed that all the screened derivatives, except for compounds 9, 12, and 13, are inhibitors of the CYP1A2 enzyme, in addition to acarbose and gliclazide. Except for 10, 12, 13, 14, acarbose, and gliclazide, all are also CYP2C19 inhibitors. Compounds 10, 12, 13, acarbose, and gliclazide are not CYP2C9 inhibitors or substrates. All the hits are not CYP2D6 substrates. All drugs inhibit CYP3A4 except 12, 13, acarbose, and gliclazide.
Toxicity prediction
Safety is always crucial to consider while developing a new drug. Safety encompasses a variety of toxicities and unfavourable adverse effects that should be investigated during the drug development journey.
In this work, we investigate the toxicity of the most promising compounds 4, 6, 8, and 24 besides two antidiabetic drugs (acarbose and gliclazide) with the aid of the ProTox-II website (https://tox-new.charite.de/protox_II), and pkCSM website (http://biosig.unimelb.edu.au/pkcsm/prediction), and the results were shown in Tables 9, 10, and 11.
Table 9.
Toxicity of the most promising hits compared to acarbose and gliclazide.
| Cpd. No. | AMES toxicity | Max. tolerated dose (human) (log mg/kg/day) | hERG I inhibitor | hERG II inhibitor | Oral Rat Acute Toxicity (LD50) (mol/kg) | Oral Rat Chronic Toxicity (LOAEL) (log mg/kg_bw/day) |
|---|---|---|---|---|---|---|
| 4 | Yes | −0.409 | No | No | 2.682 | 0.862 |
| 6 | No | 0.187 | No | Yes | 2.672 | 0.733 |
| 8 | No | 0.093 | No | Yes | 2.816 | 0.74 |
| 16 | No | −0.204 | No | Yes | 2.505 | 0.745 |
| Acarbose | No | 0.616 | No | Yes | 3.176 | 7.01 |
| Gliclazide | No | −0.04 | No | No | 1.932 | 1.745 |
Table 10.
Continuation of toxicity of the most promising hits compared to acarbose and gliclazide.
| Cpd. No. | Hepatotoxicity | Skin Sensitisation | T.Pyriformis toxicity (log ug/L) | Minnow toxicity (log mM) |
|---|---|---|---|---|
| 4 | Yes | No | 0.413 | 0.321 |
| 6 | Yes | No | 0.287 | 0.729 |
| 8 | Yes | No | 0.294 | 0.605 |
| 16 | Yes | No | 0.385 | −0.905 |
| Acarbose | No | No | 0.285 | 15.114 |
| Gliclazide | Yes | No | 1.038 | 0.659 |
Table 11.
Toxicity prediction with ProTox-II website for the most active derivatives compared to acarbose and gliclazide.
| Cpd. No. | LD50 (mg/kg) | Toxicity Class | Carcinogenicity Prediction (Probability) | Immunotoxicity Prediction (Probability) | Mutagenicity Prediction (Probability) | Cytotoxicity Prediction (Probability) |
|---|---|---|---|---|---|---|
| 4 | 2000 | 4 | Inactive (0.54) | Inactive (0.94) | Inactive (0.58) | Inactive (0.72) |
| 6 | 300 | 3 | Inactive (0.51) | Inactive (0.97) | Inactive (0.68) | Inactive (0.68) |
| 8 | 807 | 4 | Inactive (0.53) | Inactive (0.96) | Active (0.58) | Inactive (0.75) |
| 16 | 2000 | 4 | Active (0.5) | Active (0.59) | Active (0.52) | Inactive (0.77) |
| Acarbose | 24000 | 6 | Inactive (0.84) | Active (0.99) | Inactive (0.76) | Inactive (0.70) |
| Gliclazide | 1750 | 4 | Inactive (0.79) | Inactive (0.99) | Inactive (0.79) | Inactive (0.66) |
Except for compound 4, none of the compounds in Table 9 including acarbose and gliclazide exhibited AMES toxicity in the pkCSM web tools. The maximum acceptable human dose for the components that are the most bioactive ranges from −0.409 to 0.616 log mg/kg/day. Additionally, it was discovered that none of the tested substances inhibit hERG I, which is a positive finding that makes the substances safer and lowers the risk factors for torsade de pointes (a deadly arrhythmia). Contrarily, the only compounds that do not block hERG II are compound 4 and gliclazide. Gliclazide’s acute oral rat toxicity values ranged from 1.932 mol/kg to 3.176 mol/kg, whereas acarbose’s acute oral rat toxicity (LOAEL) values ranged from 0.737 to 7.01 log mg/kg_ bw/day.
Hepatotoxicity is a rare reason for drug development to halt during the preclinical stage. In fact, liver toxicity is typically reversible in contrast to other organ toxicity, and sensitive serum enzyme assays can be used to monitor liver toxicity in humans. To confirm whether a substance is also hepatotoxic in humans, it is frequently tested on humans after it was discovered to be hepatotoxic in an animal species. In our investigation, gliclazide and all other evaluated substances are anticipated to be hepatotoxic. Hepatotoxicity was absent in just acarbose. However, none of the hits revealed any signs of cutaneous sensitivity. Tetrahymena pyriformis’s toxicity ranges from 0.285 to 1.038, making it a common indicator of toxicity endpoints.
As shown in (Table 11), the toxicity profile for the active chemicals 4, 6, and gliclazide demonstrated that they were not mutagenic, immune-toxic, carcinogenic, or cytotoxic, with LD50 values of 2000, 300, and 1750, respectively. Compound 8 was projected to be non-carcinogenic, non-immunogenic, and non-cytotoxic but active for mutagenicity, with an LD50 of 807 mg/kg. Furthermore, compound 16 was noncytotoxic, but it was mutagenic, immune-toxic, and carcinogenic in toxicity experiments, with an LD50 of 2000 mg/kg. Acarbose was also discovered to be immunogenic but not carcinogenic, mutagenic, or cytotoxic. Furthermore, the novel-generated quinoxaline derivatives 4, 8, and 16 were classified as class four toxicity, whereas derivative 6 was classified as class three toxicity.
Docking studies
To verify the accuracy of the docking procedure used in this docking investigation, self-docking of the co-crystalized ligand (acarbose) in the binding site was performed for α-amylase and α-glucosidase. The low RMSD between acarbose’s re-docked stance and native pose served as proof that the validation stage was successful. The RMSD for self-docking of α-amylase (PDB ID: 1B2Y) was 0.6608 Å. The RMSD value for self-docking of α-glucosidase (PDB ID: 5NN8) was 0.5693 Å. Additionally, as shown in Figure 3, the re-docked acarbose pose in both proteins demonstrated the capacity to overlap the native ligand posture and occupy important contacts made possible by the co-crystallized ligand with the active site hot spots in α-amylase and α-glucosidase (Table 12).
Figure 3.
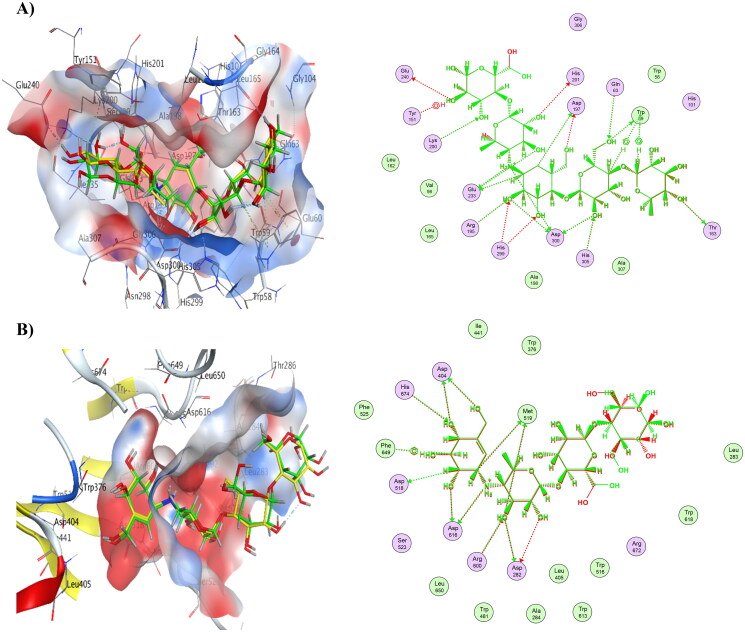
A) Overlay between co-crystallized ligand (yellow) and re-docked pose (green) of acarbose (RMSD= 0.66 Å) in α-amylase (PDB ID: 1B2Y), complex overlay and ligand interactions. B) Overlay between co-crystallized ligand (yellow) and re-docked pose (green) of acarbose (RMSD= 0.56 Å) in α-glucosidase (PDB ID: 5NN8), complex overlay and ligand interactions.
Table 12.
Binding energies S (Kcal mol−1), receptor interactions and distances in angstroms of acarbose, with α-amylase (PDB ID: 1B2Y) and α-glucosidase (PDB ID: 5NN8) (London dG as a scoring function).
| Ligand/ Protein | Receptor Amino Acid names and number | Type of interaction | Distance (Å) |
|---|---|---|---|
| α-Amylase | GLU 240 | H-donor | 2.78 |
| HIS 201 | H-donor | 2.67 | |
| GLU 233 | H-donor | 2.58 | |
| GLU 233 | H-donor | 2.63 | |
| ASP 300 | H-donor | 3.03 | |
| GLU 233 | H-donor | 3.26 | |
| ASP 300 | H-donor | 2.54 | |
| ASP 197 | H-donor | 3.37 | |
| ASP 197 | H-donor | 2.7 | |
| ASP 300 | H-donor | 3.18 | |
| TRP 59 | H-donor | 2.69 | |
| THR 163 | H-donor | 2.8 | |
| LYS 200 | H-acceptor | 2.77 | |
| ARG 195 | H-acceptor | 2.91 | |
| HIS 299 | H-acceptor | 3.06 | |
| HIS 299 | H-acceptor | 3.37 | |
| HIS 305 | H-acceptor | 2.76 | |
| GLN 63 | H-acceptor | 2.86 | |
| TYR 151 | H-pi | 4.59 | |
| TRP 59 | H-pi | 4.47 | |
| TRP 59 | H-pi | 3.92 | |
| α-Glucosidase | ASP 282 | H-donor | 2.77 |
| ASP 282 | H-donor | 2.65 | |
| MET 519 | H-donor | 3.18 | |
| ASP 616 | H-donor | 3.24 | |
| MET 519 | H-donor | 4.32 | |
| ASP 616 | H-donor | 2.55 | |
| ASP 404 | H-donor | 2.65 | |
| ASP 404 | H-donor | 2.54 | |
| ARG 600 | H-acceptor | 2.64 | |
| HIS 674 | H-acceptor | 2.87 | |
| PHE 649 | H-pi | 4.28 |
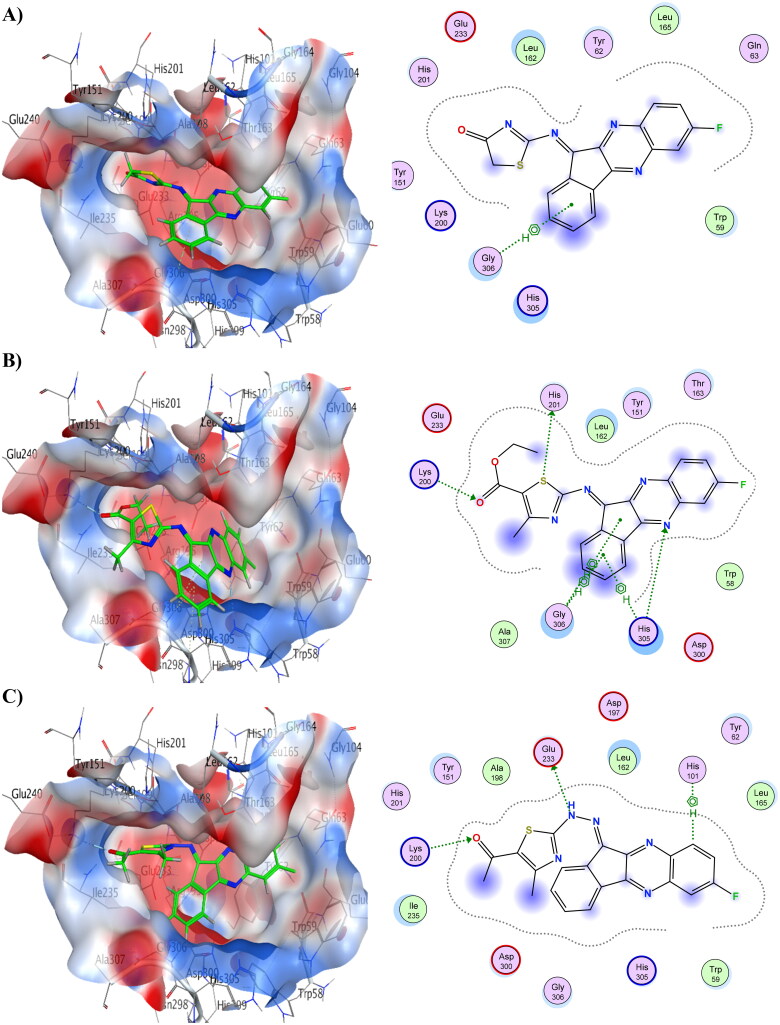
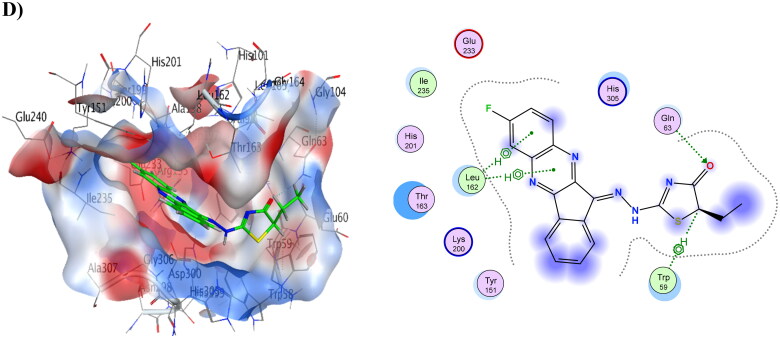
Compounds 6 and 8 showed the lowest energy in the case of α-amylase, with score values of −9.72 and −11.59 kCal/mol, respectively. This demonstrates that it agrees with the findings of the enzyme assay. Compound 8 was discovered to function as an H-bond acceptor from amino acid Lys200 as well as an H-bond donor to Glu233. The interaction between compound 8 and His101 was shown to be H-arene. Compound 6 displayed three H-bonds with the binding pocket, as well as three arene-H interactions with His305 and Gly306. Table 13 provides a summary of ligand interactions. Compound 16 revealed two H-arene contacts with Trp59, one H-bond with Gln63, and two arene-H interactions with Leu162. Compound 4 had just one arene-H interaction with Gly306, and it had the greatest energy score of −7.93 kCal/mol. Figure 4 shows ligand interactions and binding modalities.
Table 13.
Binding energies S (Kcal mol−1), receptor interactions, and distances in angstroms of compounds 4, 6, 8, and 16, with α-amylase (PDB ID: 1B2Y) and α-glucosidase (PDB ID: 5NN8) (London dG as a scoring function).
| Compound | Binding Energy (S) (kCal/mol) | Receptor Amino Acid | Type of interaction | Distance (Å) | Binding Energy (S) (kCal/mol) | Receptor Amino Acid | Type of interaction | Distance (Å) |
|---|---|---|---|---|---|---|---|---|
| 1B2Y | 5NN8 | |||||||
| 4 | −7.93 | GLY 306 | pi-H | 3.81 | −7.83 | HIS 674 | H-acceptor | 3.38 |
| 6 | −9.72 | HIS 201 | H-donor | 3.6 | −10.63 | ASP 616 | H-donor | 3.53 |
| HIS 305 | H-acceptor | 3 | ASP 645 | H-donor | 3.15 | |||
| LYS 200 | H-acceptor | 2.85 | TRP 481 | H-acceptor | 3.2 | |||
| HIS 305 | pi-H | 4.49 | HIS 674 | H-acceptor | 3.38 | |||
| GLY 306 | pi-H | 4.01 | ||||||
| GLY 306 | pi-H | 4.61 | ||||||
| 8 | −11.59 | GLU 233 | H-donor | 3.3 | −10.79 | ASP 616 | H-donor | 3.39 |
| LYS 200 | H-acceptor | 2.9 | ASP 616 | H-donor | 3.22 | |||
| HIS 101 | H-pi | 4.84 | TRP 481 | H-acceptor | 3.25 | |||
| HIS 674 | H-acceptor | 2.95 | ||||||
| LEU 283 | pi-H | 4.57 | ||||||
| 16 | −7.37 | GLN 63 | H-acceptor | 3.39 | −9.37 | ASP 616 | H-donor | 3.37 |
| TRP 59 | H-pi | 4.34 | ASP 616 | H-donor | 3.44 | |||
| TRP 59 | H-pi | 3.88 | TRP 481 | H-acceptor | 3.18 | |||
| LEU 162 | pi-H | 4.02 | LEU 83 | pi-H | 4.6 | |||
| LEU 162 | pi-H | 4.23 | ||||||
Figure 4.
3D binding modes & 2D ligand interactions with α-amylase (PDB ID: 1B2Y) of the most active compounds, (A) Compound 4, (B) Compound 6, (C) Compound 8, (D) Compound 16.
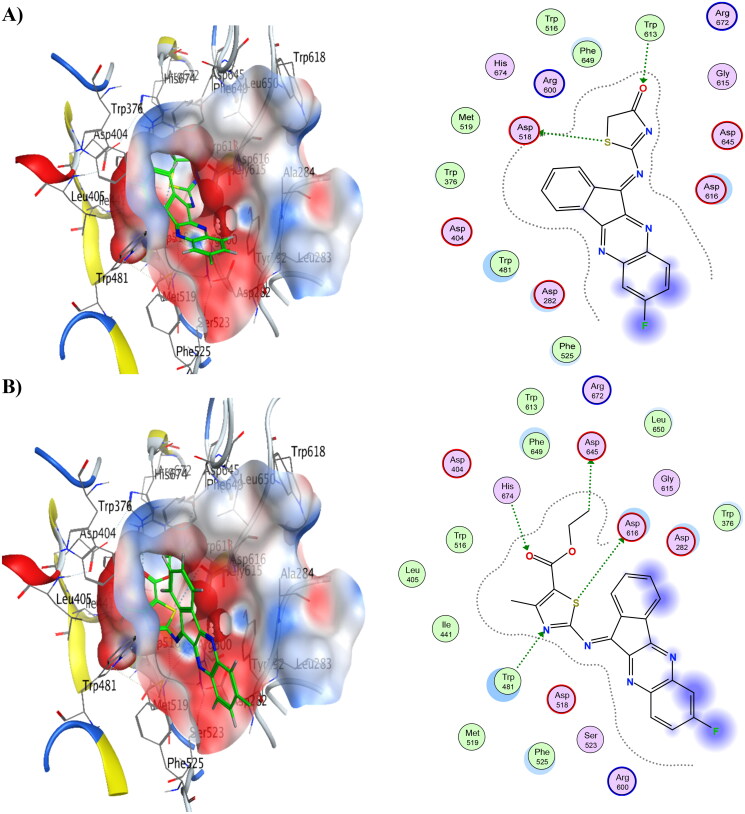
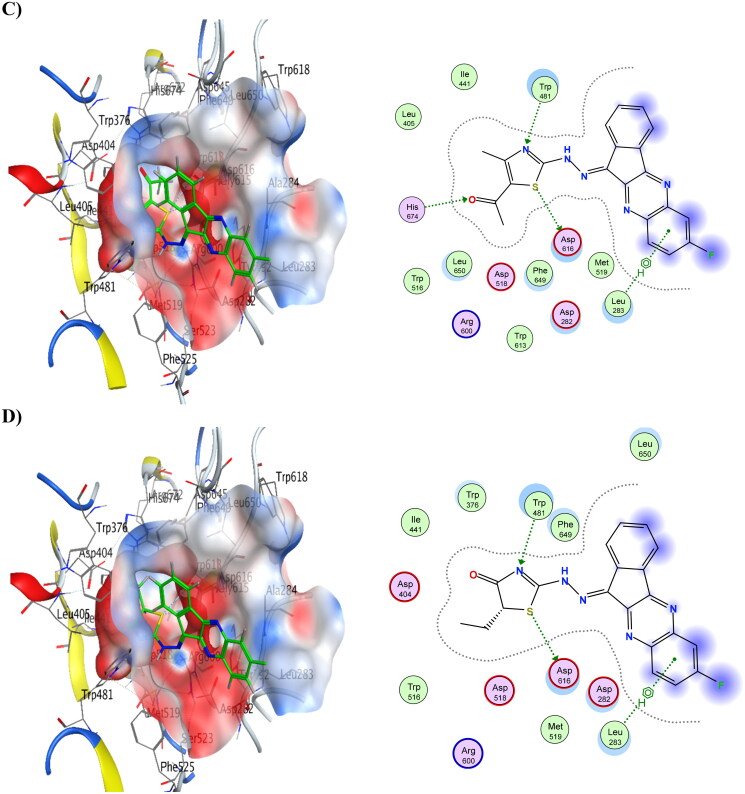
Moreover, compounds 6 and 8 showed the lowest energy in the case of α-glucosidase, with score values of −10.63 and −10.79 kCal/mol, respectively. This demonstrates that it agrees with the findings of the enzyme assay. Compound 6 was discovered to function as an H-bond acceptor from the amino acids Trp481 and His674 as well as an H-bond donor to the amino acids Asp616 and Asp645. Compound 8 revealed the same ligand interactions with the same amino acids as compound 6 (shown in Figure 5), in addition to a comparable manner of contact. However, compound 8 demonstrated an additional arene-H interaction with Leu283. Table 13 provides a summary of ligand interactions. Compound 16 had a score of −9.37 kCal/mol, which was slightly higher than average in terms of energy. It displayed three contacts with Asp616, Asp645, and Trp481 as well as an additional arene-H interaction with Leu83, which were shared by compounds 6 and 8 as well. With just one H-bond to His674, compound 4 has the highest energy score, measuring −7.83 kCal/mol.
Figure 5.
3D binding modes & 2D ligand interactions with α-glucosidase (PDB ID: 5NN8) of the most active compounds, (A) Compound 4, (B) Compound 6, (C) Compound 8, (D) Compound 16.
Conclusion
A series of novel indenoquinoxaline, Schiff’s base derivatives 4–6, hydrazone derivatives 7–9, arylidine-indenoquinoxaline derivatives 10, 12, and 13, and thiazole derivatives 15–17 was designed, synthesised, and subjected to biological evaluation as antidiabetic agents. The results indicated that there are four hybrids with good activity. Compounds 4, 6, 8, and 16 possess promising antidiabetic properties demonstrated by decreasing glucose level, increasing insulin level, also causes inhibition of α-amylase and α-glucosidase activities. In addition to their antioxidant, hypocholesteremic, renoprotective and hepatoprotective properties. When compared to acarbose, a reference medication (IC50=0.316 ± 0.02 and 31.56 ± 1.33 μM), the four compounds showed moderate to powerful activity with IC50 values of 0.982 ± 0.04, 10.19 ± 0.21, 17.58 ± 0.74, and 121.6 ± 5.14 μM, respectively. The Schiff base derivative 6 and the hydrazone derivative 8 demonstrate the importance of these moieties for the anti-diabetic actions as these derivatives show the best result when compared to the standard acarbose as α-amylase & α-glucosidase inhibitors. In addition, docking experiments were used to confirm the binding interactions, and all hybrid and standard medicines underwent in silico ADME and toxicity tests. Future work will be done for the most promising hybrids 6 and 8, to study another mode of actions as well as formulation in different dosage form to be tested.
Ethical approval
The least number of animals necessary to attain statistical significance was used. Mice were housed under a 12-hour light/dark cycle; a relative humidity of 50%; and a controlled temperature of 22 ± 1 °C; with free access to food and water. At the end of the experiment, mice were euthanatized by cervical dislocation. The protocol of the current research study is in line with the ARRIVE guidelines for the use and care of laboratory animals and approved by the research ethics committee for experimental and clinical studies at the Faculty of Pharmacy (Girls), Al-Azhar University (Permit number: ES 408, 1/6/2023).
Supplementary Material
Funding Statement
The project has been funded by the Taif University Researchers Supporting Project (TURSP) at Taif University, Taif, Saudi Arabia, under Grant (TURSP-2020/220). The authors extend their appreciation to Tife University, Saudi Arabia, for supporting this work through project number (TU-DSPP-2024-85).
Authors’ contributions
Conceptualisation, E.A.F., A.R., Y.A.A., A.M.M., and M.S.A.; Data curation, N.A.G., E.A.F., A.R., O.A.A.A., Y.A.A., A.M. M., and M.S.A.; Formal analysis, N.A.G., E.A.F., A.R., O.A.A.A., A.M. M., and M.S.A.; Investigation, N.A.G., E.A.F., A.R., O.A.A.A., Y.A.A. and M.S.A.; Methodology, N.A.G., E.A.F., A.R., A.M.M., and M.S.A.; Project administration, Y.A.A., and E.A.F.; Resources, E.A.F., O.A.A.A.; Software, E.A.F.; Supervision, E.A.F., Y.A.A.; Validation, E.A.F., and A.M.M.; Writing—original draft, E.A.F., N.A.G., A.R., A.M. M., and M.S.A.; Writing—review & editing, E.A.F., N.A.G., A.R. and M.S.A. All authors have read and agreed to the published version of the manuscript.
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data is contained within the article and supplementary material.
References
- 1.World Health Organization . Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a who consultation. Part 1, diagnosis and classification of diabetes mellitus. In. Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a who consultation. Part 1, Diagnosis and Classification of Diabetes Mellitus: World Health Organization. 1999;25:1–17. [Google Scholar]
- 2.Adaikalakoteswari A, Balasubramanyam M, Mohan V.. Telomere shortening occurs in Asian Indian type 2 diabetic patients. Diabetic Med. 2005;22(9):1151–1156. [DOI] [PubMed] [Google Scholar]
- 3.Miles PD, Levisetti M, Reichart D, Khoursheed M, Moossa A, Olefsky JM.. Kinetics of insulin action in vivo: identification of rate-limiting steps. Diabetes. 1995;44(8):947–953. [DOI] [PubMed] [Google Scholar]
- 4.Xu J, Zou M-H.. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120(13):1266–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahfoz AM, Hassanein NM, Shoka AA, El-Latif A, Hekma A.. Diabetic nephropathy: Review of novel experimental and clinical strategies. Bull Fac Pharm Cairo Univ. 2019;57(2):127–136. [Google Scholar]
- 6.El Maleky W, Mahfoz AM, Osman AO, Abd El-Latif HA.. Investigation of the impacts of zamzam water on streptozotocin-induced diabetic nephropathy in rats. in-vivo and in-vitro study. Biomed Pharmacother . 2021;138:111474. [DOI] [PubMed] [Google Scholar]
- 7.Mehmood R, Mughal EU, Elkaeed EB, Obaid RJ, Nazir Y, Al-Ghulikah HA, Naeem N, Al-Rooqi MM, Ahmed SA, Shah SWA, et al. Synthesis of novel 2, 3-dihydro-1, 5-benzothiazepines as α-glucosidase inhibitors: In vitro, in vivo, kinetic, sar, molecular docking, and qsar studies. ACS Omega. 2022;7(34):30215–30232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarald E, Joshi SB, Jain DC.. Diabetes and Herbal Medicines. IJPT. 2008;7(1):97–106. [Google Scholar]
- 9.Henrissat B, Bairoch A.. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J. 1993;293 (Pt 3) (Pt 3):781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dauw CA, Simeon L, Alruwaily AF, Sanguedolce F, Hollingsworth JM, Roberts WW, Faerber GJ, Wolf JS, Jr, Ghani KR.. Contemporary practice patterns of flexible ureteroscopy for treating renal stones: Results of a worldwide survey. J Endourol. 2015;29(11):1221–1230. [DOI] [PubMed] [Google Scholar]
- 11.Sales PMD, PMd S, Simeoni LA, PdOMD B, Silveira D. Α-amylase inhibitors: a review of raw material and isolated compounds from plant source. J Pharm Pharm Sci. 2012;15(1):141–183. . [DOI] [PubMed] [Google Scholar]
- 12.Nair SS, Kavrekar V, Mishra A. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. Eur J Exp Biol. 2013;3(1):128–132. [Google Scholar]
- 13.Sundarram A, Murthy TPK.. Α-amylase production and applications: a review. J Appl Environ Microbiol. 2014;2(4):166–175. [Google Scholar]
- 14.Taha M, Javid MT, Imran S, Selvaraj M, Chigurupati S, Ullah H, Rahim F, Khan F, Mohammad JI, Khan KM.. Synthesis and study of the α-amylase inhibitory potential of thiadiazole quinoline derivatives. Bioorg Chem. 2017;74(:179–186. [DOI] [PubMed] [Google Scholar]
- 15.Petrou A, Fesatidou M, Geronikaki A.. Thiazole ring—a biologically active scaffold. Molecules. 2021; 26(11):3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Z, Wang G, Wang J, Chen M, Peng Y, Li L, Deng B, Chen S, Li W.. Synthesis, biological evaluation, and molecular docking studies of novel isatin-thiazole derivatives as α-glucosidase inhibitors. Molecules. 2017;22(4):659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang G, He D, Li X, Li J, Peng Z.. Design, synthesis and biological evaluation of novel coumarin thiazole derivatives as α-glucosidase inhibitors. Bioorg Chem. 2016;65(:167–174. [DOI] [PubMed] [Google Scholar]
- 18.Ghani U, Albarrag A, Yurttaş L, Demirci F, Kaplancikli ZA.. Carbazoles and hydrazone‐bridged thiazole‐pyrrole derivatives as new inhibitors of α‐glucosidase. ChemistrySelect. 2018;3(27):7921–7925. [Google Scholar]
- 19.Wang G, Peng Z, Gong Z, Li Y.. Synthesis, biological evaluation, and docking studies of novel 5, 6-diaryl-1, 2, 4-triazine thiazole derivatives as a new class of α-glucosidase inhibitors. Bioorg Chem. 2018;78:195–200. [DOI] [PubMed] [Google Scholar]
- 20.Kumar H, Dhameja M, Kurella S, Uma A, Gupta P.. Synthesis, in-vitro α-glucosidase inhibition and molecular docking studies of 1, 3, 4-thiadiazole-5, 6-diphenyl-1, 2, 4-triazine hybrids: Potential leads in the search of new antidiabetic drugs. J Mol Struct . 2023;1273(:134339. [Google Scholar]
- 21.Mahapatra T, Nanda S.. Asymmetric synthesis of hydroxy-skipped bishomo-inositols as potential glycosidase inhibitors. Tetrahedron: Asymmetry. 2010;21(18):2199–2205. [Google Scholar]
- 22.AbdelFattah BA, Khalifa MMA, El-Sehrawi H, Fayed E, Bayoumi A, Said M.. Synthesis and anxiolytic activity of some novel 5-oxo-1, 4-oxazepine derivatives. LDDD. 2011;8(4):330–338. [Google Scholar]
- 23.Fayed EA, Ahmed HY.. Synthesis, characterization and pharmacological evaluation of some new 1, 4-diazepine derivatives as anticancer agents. Der Pharma Chem. 2016;8(13):77–90. [Google Scholar]
- 24.Fathy U, Abd El Salam HA, Fayed EA, Elgamal AM, Gouda A.. Facile synthesis and in vitro anticancer evaluation of a new series of tetrahydroquinoline. Heliyon. 2021;7(10):e08117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Othman EM, Fayed EA, Husseiny EM, Abulkhair HS.. Rationale design, synthesis, cytotoxicity evaluation, and in silico mechanistic studies of novel 1, 2, 3-triazoles with potential anticancer activity. New J Chem. 2022;46(25):12206–12216. [Google Scholar]
- 26.Ammar YA, Fayed EA, Bayoumi AH, Ezz RR, Alsaid MS, Soliman AM, Ghorab MM.. New chalcones bearing isatin scaffold: synthesis, molecular modeling and biological evaluation as anticancer agents. Res Chem Intermed. 2017; 43(12) :6765–6786. [Google Scholar]
- 27.Ramsis TM, Ebrahim MA, Fayed EA.. Synthetic coumarin derivatives with anticoagulation and antiplatelet aggregation inhibitory effects. Med Chem Res. 2023;32(11):2269–2278. [Google Scholar]
- 28.Trinder P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol. 1969; 22(2):158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orsonneau J-L, Massoubre C, Cabanes M, Lustenberger P.. Simple and sensitive determination of urea in serum and urine. Clin Chem. 1992;38(5):619–623. [PubMed] [Google Scholar]
- 30.Saibaba K, Srinivasa Rao P, Dakshina Murty K, Bhaskar MV.. Method evaluation of an enzymatic method for serum creatinine. Indian J Clin Biochem. 1997;12(2):139–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beutler E, Duron O, Kelly BM.. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61(:882–888. [PubMed] [Google Scholar]
- 32.Kei S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90(1):37–43. [DOI] [PubMed] [Google Scholar]
- 33.Piyachaturawat P, Poprasit J, Glinsukon T, Wanichanon C.. Gastric mucosal lesions in streptozotocin-diabetic rats. Cell Biol Int Rep. 1988;12(1):53–63. [DOI] [PubMed] [Google Scholar]
- 34.Walker HK, Hall WD, Hurst JW.. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Boston: Butterworths; 1990. [PubMed] [Google Scholar]
- 35.McCue PP, Shetty K.. Inhibitory effects of rosmarinic acid extracts on porcine pancreatic amylase in vitro. Asia Pac J Clin Nutr. 2004;13(1):101–106. [PubMed] [Google Scholar]
- 36.Daina A, Michielin O, Zoete V.. Swissadme: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7(1):42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kar S, Roy K, Leszczynski J.. In silico tools and software to predict admet of new drug candidates. In: In silico methods for predicting drug toxicity. New York (NY): Springer US; 2022. p. 85–115. [DOI] [PubMed] [Google Scholar]
- 38.Refai MY, Elazzazy AM, Desouky SE, Abu-Elghait M, Fayed EA, Alajel SM, Alajlan AA, Albureikan MO, Nakayama J.. Interception of epoxide ring to quorum sensing system in enterococcus faecalis and staphylococcus aureus. AMB Express. 2023;13(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fayed EA, Thabet A, Abd El-Gilil SM, Elsanhory HM, Ammar YA.. Fluorinated thiazole–thiosemicarbazones hybrids as potential ppar-γ agonist and α-amylase, α-glucosidase antagonists: design, synthesis, in silico admet and docking studies and hypoglycemic evaluation. J Mol Struct . 2024;1301:137374. [Google Scholar]
- 40.Fayed EA, Al-Arab EME, Saleh AS, Bayoumi AH, Ammar YA.. Design, synthesis, in silico studies, in vivo and in vitro assessment of pyridones and thiazolidinones as anti-inflammatory, antipyretic and ulcerogenic hits. J Mol Struct. 2022;1260:132839. [Google Scholar]
- 41.Fayed EA, Gohar NA, Farrag AM, Ammar YA.. Upregulation of bax and caspase‐3, as well as downregulation of bcl‐2 during treatment with indeno [1, 2‐b] quinoxalin derivatives, mediated apoptosis in human cancer cells. Arch Pharm. 2022;355(5):e2100454. [DOI] [PubMed] [Google Scholar]
- 42.Bax BD, Chan PF, Eggleston DS, Fosberry A, Gentry DR, Gorrec F, Giordano I, Hann MM, Hennessy A, Hibbs M, et al. Type iia topoisomerase inhibition by a new class of antibacterial agents. Nature. 2010;466(7309):935–940. [DOI] [PubMed] [Google Scholar]
- 43.Fayed EA, Nosseir ES, Atef A, El Kalyoubi SA. In vitro antimicrobial evaluation and in silico studies of coumarin derivatives tagged with pyrano-pyridine and pyrano-pyrimidine moieties as DNA gyrase inhibitors. Mol Divers. 2022;26(1):341–363. [DOI] [PubMed] [Google Scholar]
- 44.Fayed EA, Ebrahim MA, Fathy U, El Saeed HS, Khalaf WS.. Evaluation of quinoxaline derivatives as potential ergosterol biosynthesis inhibitors: design, synthesis, admet, molecular docking studies, and antifungal activities. J Mol Struct . 2022;1267:133578. [Google Scholar]
- 45.Othman EM, Fayed EA, Husseiny EM, Abulkhair HS.. The effect of novel synthetic semicarbazone-and thiosemicarbazone-linked 1, 2, 3-triazoles on the apoptotic markers, vegfr-2, and cell cycle of myeloid leukemia. Bioorg Chem. 2022;127:105968. [DOI] [PubMed] [Google Scholar]
- 46.Desouky SE, Abu-Elghait M, Fayed EA, Selim S, Yousuf B, Igarashi Y, Abdel-Wahab BA, Mohammed Alsuhaibani A, Sonomoto K, Nakayama J.. Secondary metabolites of actinomycetales as potent quorum sensing inhibitors targeting gram-positive pathogens: in vitro and in silico study. Metabolites. 2022;12(3):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fayed EA, Gohar NA, Bayoumi AH, Ammar YA.. Novel fluorinated pyrazole-based heterocycles scaffold: cytotoxicity, in silico studies and molecular modelling targeting double mutant egfr l858r/t790m as antiproliferative and apoptotic agents. Med Chem Res. 2023;32(2):369–388. [Google Scholar]
- 48.Fayed EA, Ebrahim MA, Fathy U, Elawady AM, Khalaf WS, Ramsis TM.. Pyrano-coumarin hybrids as potential antimicrobial agents against mrsa strains: design, synthesis, admet, molecular docking studies, as DNA gyrase inhibitors. J Mol Struct . 2024;1295:136663. [Google Scholar]
- 49.Ahangarpour A, Heidari H, Oroojan AA, Mirzavandi F, Esfehani KN, Mohammadi ZD.. Antidiabetic, hypolipidemic and hepatoprotective effects of arctium lappa root’s hydro-alcoholic extract on nicotinamide-streptozotocin induced type 2 model of diabetes in male mice. Avicenna J Phytomed. 2017;7(2):169. [PMC free article] [PubMed] [Google Scholar]
- 50.Ragab A, Abusaif MS, Gohar NA, Aboul-Magd DS, Fayed EA, Ammar YA.. Development of new spiro [1, 3] dithiine-4, 11′-indeno [1, 2-b] quinoxaline derivatives as s. Aureus sortase a inhibitors and radiosterilization with molecular modeling simulation. Bioorg Chem. 2023;131:106307. [DOI] [PubMed] [Google Scholar]
- 51.Raghuvanshi DS, Singh KN.. Microwave-assisted synthesis of some novel thiazolidinone and thiohydantoin derivatives of isatins. Phosphorus, Sulfur, Silicon Relat Elem. 2010;185(11):2243–2248. [Google Scholar]
- 52.K Ramshid P, Jagadeeshan S, Krishnan A, Mathew M, Asha Nair S, Radhakrishna Pillai M.. Synthesis and in vitro evaluation of some isatin-thiazolidinone hybrid analogues as anti-proliferative agents. MC. 2010;6(5):306–312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article and supplementary material.