Abstract
Various reports have indicated that a number of viruses could infect neutrophils, but the multiplication of viruses in neutrophils was abortive. Based on our previous finding that avian influenza viral RNA and proteins were present in the nucleus of infected human neutrophils in vivo, we investigated the possibility of 2009 A (H1N1) influenza viral synthesis in infected neutrophils and possible release of infectious progeny from host cells. In this study we found that human neutrophils in vitro without detectable level of sialic acid expression could be infected by this virus strain. We also show that the infected neutrophils can not only synthesize 2009 A (H1N1) viral mRNA and proteins, but also produce infectious progeny. These findings suggest that infectious progeny of 2009 A (H1N1) influenza virus could be replicated in and released from human neutrophils with possible clinical implications.
Various in vitro and in vivo studies have shown that neutrophils can be infected with a number of viruses included influenza virus. For example, the migratory and phagocytic activities of murine neutrophils can be altered by an in vitro interaction with cytomegalovirus1, and activated neutrophils had vacuoles containing varicella-zoster virions and extended cytoplasmic projections toward virions2. A number of viruses attached and penetrated into neutrophils have been observed by Blackmon and Ginsberg3. The infection of influenza virus and its effect on neutrophil function such as suppressing endocytosis, accelerating apoptosis and inducing type I interferon signaling pathways have been extensively studied4,5,6,7,8,9. We also observed neutrophils infection by H5N1 virus in vivo10.
The successful replication of virus depends on host cells to provide functional organelles such as ribosome and endoplasmic reticulum to synthesize viral mRNA and proteins. Neutrophils, as mature polymorphonuclear leukocytes (PMNs), generally thought to have no significant biosynthetic capacity, synthesizing little if any RNA and protein11. It was reported that neutrophils have only few endoplasmic reticulum, mitochondria and ribosome12. Traditionally, it was believed that influenza viral infection of neutrophils is abortive, and infectious progeny are not produced. There was no detectable level of viral progeny in the supernatant of cytomegalovirus, varicella-zoster virus or even the A/WSN/33 (H1N1) strain of influenza virus infected neutrophils in vitro1,2,13.
However, Jack and Fearon reported that human peripheral blood neutrophils can selectively synthesize mRNA and proteins14. Several studies have also shown that the rate of RNA and protein synthesis increased upon neutrophil stimulation8,11,15,16,17. In our own study, we found that H5N1 viral proteins and nucleotide sequences were present in both the cytoplasm and the nucleus of infected neutrophils10. It has been shown that influenza virus synthesizes its messenger RNA in the nucleus of infected cells18,19. Based on the presence of human avian influenza virus RNA and proteins in the nuclei of infected human neutrophils, we speculate that human neutrophils may be infected by influenza virus, and serve as host cells for virus replication and progeny production.
To verify our hypothesis, we first examined the expression of sialic acid, the primary receptors for influenza virus, on neutrophils20. We further examined the infection, replication and progeny release of 2009 A (H1N1) virus in human neutrophils in vitro. Unexpectedly, we found that human neutrophils in vitro without detectable level of sialic acid expression could be infected by the virus. We also found that the infected neutrophils can not only synthesize 2009 A (H1N1) viral mRNA and proteins, but also produce infectious progeny. To our knowledge, this is the first observation of mature virions produced by neutrophils.
Results
The separated human neutrophils are of good quality
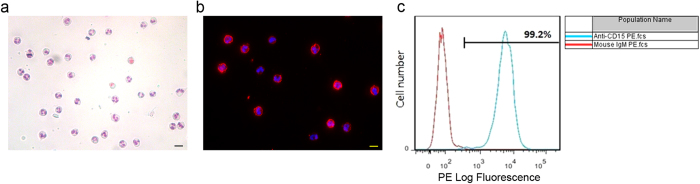
The quality of separated human neutrophils is essential for functional tests. The morphology of separated cells was highly consistent with specific polymorphonuclear characteristics of neutrophils which was identified with Giemsa staining (Fig. 1a). To further confirm the identity of neutrophils, immunofluorescence staining and flow cytometry were performed with mouse anti-CD15 (a marker of neutrophils) monoclonal antibody10. Figure 1b shows that the CD15+ cells had the neutrophil characteristic morphology of lobulated nuclei. Flow cytometry found that the purity of neutrophils reached 99.2% (Fig. 1c). Therefore, the quality of neutrophils separated is adequate for carrying out functional assays.
Figure 1. The separated human neutrophils are of good quality.
(a) The polymorphonuclear characteristic of human primary neutrophils was determined with Giemsa staining. (b) The expression of CD15 on separated cells was analyzed with immunofluorescence. The primary antibody was mouse anti-CD15, the secondry antibody was Alexa Fluor 555 conjugated goat anti-mouse (red), and the nuclei were stained with DAPI (blue). (c) Flow cytometry was performed to depict the purity of neutrophils with PE-conjugated anti-CD15 antibody (blue curve). PE-conjugated mouse IgM was used as the isotype control (red curve). Scale bar, 20 μm.
Influenza virus can enter neutrophils independent of sialic acid receptors
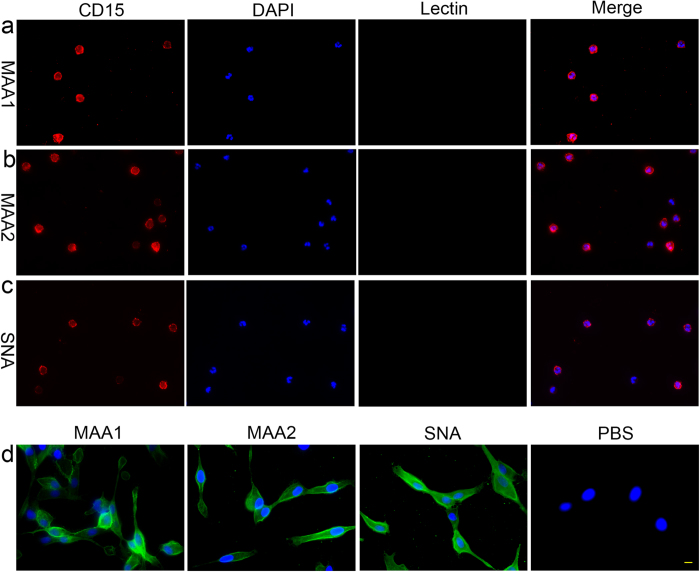
The expression of sialic acid on neutrophils and MDCK cells was examined with fluorescence microscopy using MAA I, MAA II and SNA stainings. As shown in Fig. 2a-c, no detectable level of sialic acid in α2–3 linkages or in α2–6 linkages was found on neutrophils with MAA I, MAA II or SNA staining. Meanwhile, antibody to CD15 was used to identify neutrophils. As a positive control, MDCK cells showed a strong expression of both avian influenza receptors (α2,3-linked sialic acids) and human influenza receptors (α2,6-linked sialic acids) to ensure the reliability of the technology (Fig. 2d).
Figure 2. No sialic acid residue, which is the primary receptor for influenza virus, was detectable on human neutrophils in vitro.
(a–c) The human primary neutrophils were incubated with biotinylated MAA I, MAA II or SNA, subsequently incubated with Dylight 488 labeled Streptavidin (green) and examined with immunofluorescence. No detectable level of sialic acid in α2–3 linkages (a&b) or sialic acid in α2–6 linkages (c) was found on neutrophils. The cells were stained with mouse anti-CD15 antibody (red) to identify the identity of neutrophils. (d) Lectin stain was also performed on MDCK cells as a positive control to ensure the reliability of the technology. Nuclei were stained with DAPI (blue). Scale bar, 20 μm.
To further clarify whether the entry of influenza virus into neutrophils is independent of sialic acid receptors, neutrophils were preincubated with neuraminidase (NA) prior to infection with influenza virus. Fig. S1 shows that the percentage of neutrophils pretreated with NA had nucleoprotein positive cells (infected cells) in number not less than that of the group of neutrophils without NA treatment.
2009 A (H1N1) viral mRNA and protein can be synthesized in human neutrophils in vitro
To examine the proliferation of 2009 A (H1N1) influenza virus in human neutrophils in vitro, real time quantitative PCR, Western blot and immunofluorescence staining were performed.
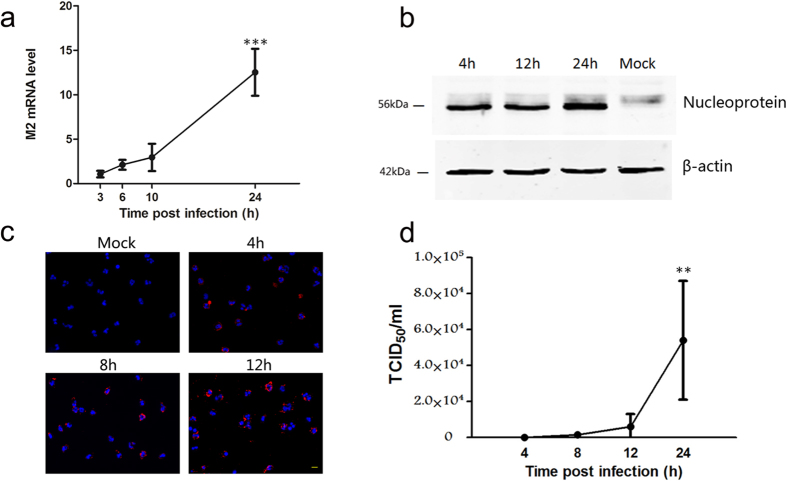
With real time quantitative PCR, we analyzed the expression of influenza A virus matrix 2 (M2) mRNA isolated from virus-exposed neutrophils. The M2 gene production was encoded by the spliced mRNA, which was only present within the infected cells9. As shown in Fig. 3a, along with prolonged post-infection duration, the expression levels of the M2 gene gradually increased and became very strong at 24 h after infection.
Figure 3. 2009 A (H1N1) Influenza Virus replicated in and released from Human neutrophils in vitro.
(a) Time course of 2009 A (H1N1) virus matrix 2 (M2) mRNA synthesis was analyzed with Real-time PCR. Data are shown as mean ± S.D. ***P < 0.001, indicate statistically highly significant differences of M2 mRNA expression at 24 h post infection comparing with its expression at 3 h, 6 h and 10 h post infection (P = 0.0001, 24 h vs 3 h post infection; P = 0.0002, 24 h vs 6 h post infection; P = 0.0008, 24 h vs 10 h post infection), n = 4. (b) The accumulation of virus Nucleoprotein at different time points post infection was examined with Western blot. (c) In situ analysis of 2009 A (H1N1) virus matrix 1 (M1) protein synthesis at different time points post infection with immunofluorescence. Scale bar, 20 μm. (d) The influenza virus titers of supernatants from infected neutrophils at different time points post infection were examined with the TCID50 assay. Data are shown as mean ± S.D. *P < 0.05, indicate statistically highly significant differences of virus titers at 24 h post infection comparing with virus titers at 4 h, 8 h and 12 h post infection (P = 0.0168, 24 h vs 4 h post infection; P = 0.0189, 24 h vs 8 h post infection; P = 0.029, 24 h vs 12 h post infection), n = 4.
With Western blot, we examined the expression of influenza A virus Nucleoprotein in virus-exposed neutrophils at 4 h, 12 h and 24 h post infection (Fig. 3b). Virus nucleoprotein displayed a constant level of protein accumulation with a little difference in protein accumulation levels observed at 4 h and 12 h post infection. After 24 h, protein expression levels increased significantly. The results were consistent with that of real time quantitative PCR.
With immunofluorescence, we examined the expression of influenza virus matrix 1 (M1) in cells at a series of time points post infection. As shown in Fig. 3c, along with prolonged post-infection duration, not only the number of infected cells constantly increased, but also the signal strength of positive cells apparently enhance.
Infectious progeny virus was released from viral infected human neutrophils in vitro
The culture supernatant of 2009 A (H1N1) virus-infected neutrophils was examined with the TCID50 assay to assess the production of infectious progeny virus and viral replication kinetics. Mature viral particles were found to be released from infected neutrophils and increased persistently as the duration prolonged (Fig. 3d). The infection and replication kinetics of H1N1 virus in neutrophils obtained from different donors were consistent.
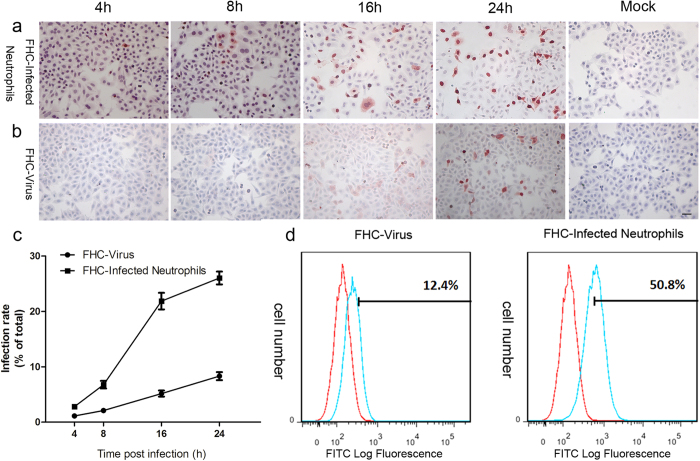
The infectivity of progeny virus released from neutrophils was also examined with cell co-culture of a different cell type (FHC). As depicted in Fig. 4a, following designated duration of cell co-culture with infected neutrophils, influenza virus Nucleoprotein expression was detected with immunocytochemistry in FHC cells. The expression level of virul Nucleoprotein in the FHC cells at 4 h post infection was very low. With the increase of viral replication cycles, infected neutrophils gradually released more mature viral particles. The numbers of infected FHC cells were significantly increased at 8, 16 and 24 h post infection. The amounts of virus nucleoprotein positive signals were remarkably enhanced at 24 h (Fig. 4a). Compared with the control group (Fig. 4b), in which FHC cells were directly incubated with parent virus propagated in embryonated eggs, we found that the rate of infected FHC cells co-cultured with virus-infected neutrophils was much higher (Fig. 4c). At 24 h post infection, flow cytometry demonstrated that the control group (FHC cells directly incubated with parent virus) had an average positive signal expression rate of 12.4%, and the experimental group (FHC cells co-cultured with virus-infected neutrophils) had an average rate of 50.8% (Fig. 4d), represent an increase of reflecting an infection rate increase of over fourfold.
Figure 4. The infectivity of progeny virus released from neutrophils was examined with cell co-culture.
(a,b) Immunocytochemistry shows time course of 2009 A (H1N1) virus Nucleoprotein expression in FHC cells co-cultured with infected neutrophils or incubated with parent 2009 A (H1N1) virus directly. Scale bar, 20 μm. (c) Statistical data of different cell infection rates between (a,b), n = 4. (d) Flow cytometry shows the comparison of different cell infection rates between FHC cells incubated with 2009 A (H1N1) virus directly and FHC cells co-cultured with infected neutrophils at 24 h post infection using FITC-conjugated mouse anti-Nucleoprotein antibody (blue curve), uninfected neutrophils were used as negative control (red curve).
The conditions of neutrophils before and after infection with 2009 A (H1N1) virus
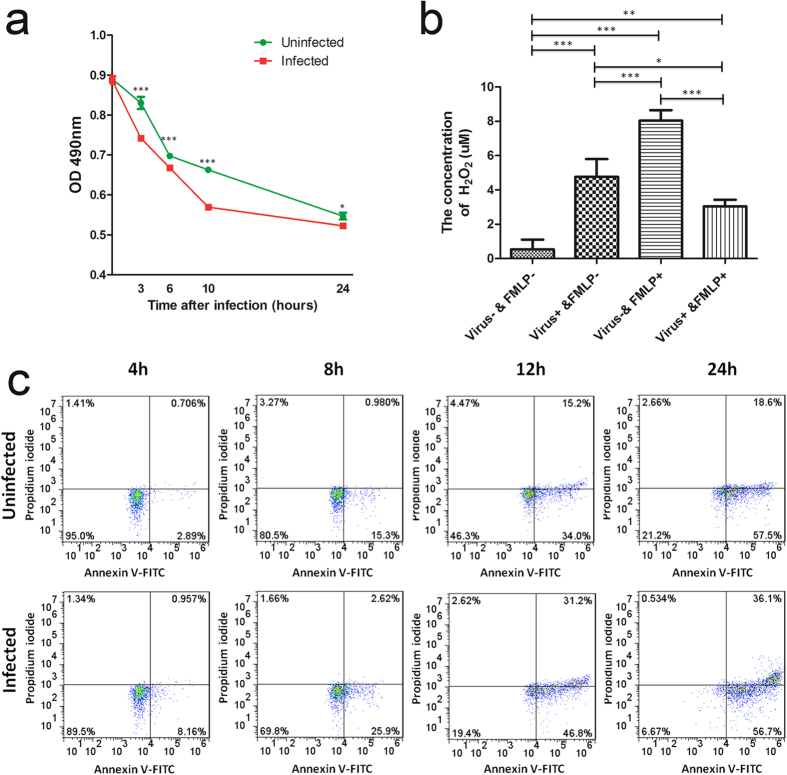
In order to better understand the interaction of 2009 A (H1N1) influenza virus with neutrophils, the conditions of neutrophils before and after infection were examined. As shown in Fig. 5a, influenza virus significantly reduced the viability of neutrophils at all time points post infection. Meanwhile, neutrophil apoptosis was accelerated following infection with influenza virus (Fig. 5c). The respiratory burst responses of neutrophils before and after infection were also examined through monitoring the production of H2O2. As shown in Fig. 5b, influenza virus itself induced a respiratory burst in which H2O2 were production (Virus+ & FMLP– vs Virus– & FMLP–), but it also depressed the ability of neutrophils to mount respiratory burst responses to FMLP (Virus + & FMLP + vs Virus– & FMLP+).
Figure 5. The conditions of neutrophils before and after infection with 2009 A (H1N1) virus.
(a) The viability of infected neutrophils was significantly lower than that of the uninfected neutrophils examined with MTS assay. Data are shown as mean ± S.D. *P < 0.05, ***P < 0.001, n = 3. Differences between infected and uninfected samples were analysed with Student’s t-test. (b) Hydrogen Peroxide production of neutrophils was higher following treatment with virus for 30 min than the untreated controls (Virus+ & FMLP– vs Virus– & FMLP–); but it also depressed H2O2 production of neutrophils to mount respiratory burst responses to FMLP (Virus+ & FMLP+ vs Virus- & FMLP+). Data are shown as mean ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001, n = 3. Differences among groups were analysed with One-way ANOVA. Comparisons between any two groups were performed with q-test (Newman-Keul’s test). (c) Influenza virus accelerated neutrophil apoptosis, which was proved with Annexin V-FITC/PI staining at different time points post infection.
Discussion
Our previous research found that H5N1 viral proteins and nucleotide sequences were present in both the cytoplasm and the nucleus of infected neutrophils of H5N1 virus infected patients10. This prompted us to speculate the possibility of influenza virus multiplication in human neutrophils. A number of studies on the ability of viral replication in neutrophils were reported about 30 years ago, but none found any evidence of successful viral replication1,2,13. To our knowledge, this is the first report of infectious viral progeny production and release by neutrophils.
Neutrophils have little biosynthetic activities as only a scanty endoplasmic reticulum and little ribosomes or mitochondria were present in these cells11,12. However, several studies observed that RNA and protein syntheses by neutrophils were increased after stimulation8,11,15,16,17 indicating that neutrophils still possess the abitity to supply sufficient biosynthetic components necessary for viral replication. The observed replication of 2009 H1N1 strain in neutrophils might indicate that this strain might be able to stimulate neutrophils to synthesize viral components and assemble infectious viral progeny. The reason for A/Nanchang/8002/2009 H1N1 but not A/WSN/33 (H1N1)13 successfully replicate in neutrophils remains to be investigated.
Cell surface sialic acid residues were known as primary receptors for influenza viruses20, but the distribution of sialic acid on neutrophils has not been carefully examined. We performed lectin staining with MAA and SNA on neutrophils and no detectible level of sialic acid, nor α-2,3-linked or α-2,6-linked was found, while positive controls ensured the reliability of our technical protocal. Two probabilities exist: Low level sialic acid residues might exist on neutrophil surface that might be below the detecting sensibility of lectin staining, or 2009 A (H1N1) virus strain can enter neutrophils without the classic salic acid receptors. As shown in Fig. S1, there was no difference in the percentage of infected cells between groups of neutrophils with and without NA pretreatment. This result indicates that even low level of sialic acid residues exist on neutrophil surface, sialic acid-dependent entry may not the main route for influenza virus to infect neutrophils. There is likely that other sialic acid receptors independent route(s) of entry might exit for influenza virus to infect neutrophils.
Cumulating evidence indicates that salic acid was not the sole gateway for influenza virus infection. It was reported that NWS-Mvi and parent influenza viruses could infect desialylated cells21. A/Memphis/1/71 (H3N2) influenza virus was found to bind to galactosyl ceramide (GalCer: Galβ1 → 1′Cer), as well as sulphatide, in virus overlay assays22. Macropinocytosis is yet another pathway for viruses to enter neutrophils without binding to specific receptors23. Phagocytosis is a major strategy for neutrophils to defend against invading pathogens, and this is also a possible pathway for viruses to enter neutrophils24. Inconsistence between the distributions of classic avian influenza virus receptor and infected cells in H5N1 virus infected patients was also reported25,26. The way and means of viral entry into neutrophils remain a subject of farther investigation.
One of the major cause of morbidity and mortality during influenza virus epidemics is bacterial superinfection27. Phagocyte dysfunction induced by influenza virus is one likely contributor to the development of bacterial superinfection, in addition to damage to respiratory epithelium28,29. Neutrophils and monocyte/macrophages infiltrate at the site of virus infection, and participate in the early inflammatory response. Both cell types exhibit depressed function in vivo during influenza virus infection30. Substantial evidences have been documented about the effect of influenza viruses on neutrophil function. Accelerated apoptosis5 and defects in chemotactic, oxidative and bacterial killing functions28 of neutrophils have been established in influenza virus infection. In addition, influenza virus itself can induce activation of neutrophils to generate a respiratory burst response31, but impair the ability of neutrophil respiratory burst respond to other stimuli32. In this study, we examined the conditions of neutrophils before and after infection by 2009 A (H1N1) strain virus. In accordance with other reports, 2009 A (H1N1) strain virus reduced cell viability, accelerated cell apoptosis, activated neutrophils itself and deactivated the ability of the cells to respond to FMLP (Fig. 5). Neutrophil dysfunction might be resulted from previous activation by influenza virus, inducing cell deactivation and viability reduction.
The pandemic 2009 A (H1N1) disease showed clinical symptoms similar to seasonal influenza, including fever, cough, sore throat, headache, myalgias, and arthralgias33. However, it also displayed symptoms that were not commonly seen in seasonal influenza including gastrointestinal symptoms such as diarrhea and vomiting, or neurological complications such as seizures, and encephalopathy34,35. Autopsies of deceased patients observed erythrophagocytosis and phagocytosis of inflammatory cells in various organs36 similar to those observed in patients infected with highly pathogenic avian influenza virus (HPAIV H5N1).
Our previous studies demonstrated multiple organ infections outside the lungs in H5N1 infected patients25. The pathologic findings of these autopsies included hemophagocytic activities in the spleen, liver, lymph node, and bone marrow37,38,39,40, white pulp atrophy with depletion of lymphocytes in the spleen25,38,39; apoptotic lymphocytes in the spleen and the intestine40; acute tubular necrosis25,39; activated Kupffer cells, cholestasis, fatty liver25,39,40, and edema of the brain25,39. What’s more, the H5N1 virus penetrated the placental barrier and infected the fetus25. Pandemic 2009 A (H1N1) virus was found to replicate in the intestine of the ferrets besides respiratory tract, a sign of multiple organ infection41. Previous reports demonstrated that infection by 2009 H1N1 virus was not confined to the upper respiratory tract but also involved the lungs and other organs, most importantly immune cells36,42. Many fatal patients had lymphopenia and elevated creatinine kinase (CK)43,44,45. Lymphocytic disruption caused an acute immunodeficiency that resulted in acute progressive respiratory syndrome including lower respiratory tract disease, respiratory failure, and ARDS with refractory hypoxaemia43,44. Based on our current findings that neutrophils can be infected by 2009 A (H1N1) and permit active viral replication, we hypothesize that infected immune cells including neutrophils may act as vehicles, transporting the virus to other organs and causing extra-pulmonary dissemination and multiple organ infection. The extent of immune system infection may be a determinant factor for the disease.
In conclusion, the present study shows that human neutrophils without detectable level of sialic acid expression on their surface can be infected by 2009 A (H1N1) influenza virus. Such virus can be replicated in and released by neutrophils and may play a role in the pathology and multiple organ infection with significant clinical implications.
Methods
Ethics Statement
All experiments involving human participants and chicken embryos were approved by the Ethical Committee of Shantou University Medical College. All participants have provided written informed consent. All methods were carried out in according to relevant national and international guidelines.
Separation of human neutrophils
Healthy adult Chinese donors’ heparinized whole blood (n = 10) was mixed with an equal volume of 3% dextran T-500 (Pharmacosmos, Holbaek, Denmark) in 0.9% NaCl and incubated 20 min at room temperature. Aspirated leukocyte-rich plasma and centrifuged 10 min at 1000 rpm, 5 °C. Discarded supernatant and resuspended cell pellet in a volume of 0.9% NaCl equal to the starting volume of blood. Layered 10 ml Ficoll-Hypaque solution (Solarbio, Beijing, China) beneath the cell suspension which ≤40 ml, and centrifuged 40 min at 1400 rpm, 20 °C with no brake. Aspirated the top layer as well as the Ficoll-Hypaque layer, leaving the neutrophil/RBC pellet, and removed residual RBC with red blood cell lysis buffer (TBDScience, Tianjin, China). After washing with Hanks buffered saline solution (Solarbio, Beijing, China), the cells were resuspended and cultured in IMDM (HyClone/Thermo Fisher Scientific Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, HyClone), 100 IU/ml of penicillin G and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA, USA) in a cell incubator (5% CO2 at 37 °C)46. The morphological characteristics and purity of neutrophils were examined with Giemsa staining, immunofluorescence staining and flow cytometry as described below.
Giemsa staining
To examine the morphological characteristics of purify cells, Giemsa staining (Solarbio, Beijing, China) was performed according to the manufacturer’s protocol. In brief, purified cells were fixed with methyl alcohol for 3 min, and then incubated with Giemsa stain for 20 min at room temperature. After washing with water and drying in the air, the slides were examined and photographed with a light microscopy (Leica, Wetzlar, Germany).
Lectin staining
The distribution of sialic acid in neutrophils and Madin-Darby canine-(MDCK) cells was examined with lectin staining. Human neutrophils and MDCK cells were fixed with 4% paraformaldehyde, and incubated with 5% BSA for 30 min at room temperature. The cells were incubated with biotinylated Maackia amurensis agglutinin (MAA I, MAA II, Vector laboratories, Burlingame, CA, USA), which binds to the avian influenza receptor Siaα2-3Galβ1-3GalNAc, or biotinylated Sambucus nigra agglutinin (SNA, Vector laboratories, Burlingame, CA, USA), which links to the human influenza receptor Siaα2-6Galβ1-3GalNAc47,48,49,50, and mouse anti-CD15 antibody (neutrophils marker) for neutrophils only at 4 °C overnight. After washing with PBS, they were incubated with Dylight 488 labeled Streptavidin (Vector laboratories, Burlingame, CA, USA) and Alexa Fluor 555 conjugated goat anti-mouse antibody (Life/Thermo Fisher Scientific Inc., Waltham, MA, USA) for neutrophils only for 1 h at room temperature. After a final wash, DAPI (Vector laboratories, Burlingame, CA, USA) was utilized to counterstain cell nuclei. A fluorescence microscope (Carl Zeiss, Oberkochen, Germany) was used for evaluation and photographing.
Neuraminidase treatment of neutrophils
After separation, 1 × 106 neutrophils were incubated with 0.128 U/ml of α2-3,6,8,9 Neuraminidase A (New England Biolabs, Inc., Ipswich, MA, USA) at 37 °C for 1 hour with constant mixing followed by washed three times with PBS and resuspended in influenza virus growth medium for infection.
Virus preparation and infection
Influenza virus A/Nanchang/8002/2009 H1N1strain was propagated in embryonated eggs, and the allantoic fluid was harvested. Virus titers were determined by tissue culture infection dose (TCID50) assay via infection of MDCK cells. Virus was stored at 70 °C until use51.
Infections were performed as previously described51. Briefly, we thawed virus stock in cool water which was then used at a infection (m.o.i.) of 1 plaque forming units (p.f.u.)/cell incubated with neutrophils or MDCK cells for 1 h at 33 °C in a CO2 incubator. After viral adsorption, unadsorbed viruses were washed away with warm HBSS (Solarbio, Beijing, China) and then cells were maintained in growth medium at 37 °C in a CO2 incubator before analysis.
Real time PCR and relative gene expression analysis
Total cellular RNA was extracted at the designed hours post infection with Trizol reagent (Takara, Dalian, China). cDNA was synthesized from mRNA with Random primers and MultiScribeTM reverse transcriptase (Applied Biosystems/Thermo Fisher Scientific Inc., Waltham, MA, USA). The mRNA levels of target gene were quantified by Real-time quantitative PCR analysis with an Applied Biosystems 7500 Real-Time PCR System. Amplification of β-actin was performed in parallel as an internal control. The primers used in this study were: Influenza A virus matrix 2 (M2) (accession number: CY089611.1): sense, 5′-GAGGTCGAAACGCCT-3′, antisense, 5′-CTGTTCCTTTCGATATTCTTCCC-3′9, β-actin (accession number: NM_001101.3): sense, 5′-AGCGAGCATCCCCCAAAGTT-3′, antisense, 5′- GGGCACGAAGGCTCATCATT-3′. The relative expression of M2 mRNA was determined with the method of 2−△△Ct52.
Immunofluorescence staining
Immunofluorescence staining was performed according to a protocol described previously with slightly modification53. At 4 h, 8 h, and 12 h post infection, neutrophils attached on glass slides coated with fibronectin (Sigma, St. Louis, MO, USA) in advance were fixed with 4% paraformaldehyde, permeabilized and blocked with 0.2%Triton X-100, 5%BSA. The slides were incubated overnight with mouse anti-CD15 monoclonal antibody (eBioscience, San Diego, CA, USA) or mouse anti-Influenza A virus matrix 1 (M1) antibody (Abcam, Cambridge, MA, USA) as the primary antibody. After washing in PBS, slides were incubated with Alexa Fluor 555 conjugated goat anti-mouse antibody (Life/Thermo Fisher Scientific Inc., Waltham, MA, USA). VECTASHIELD mounting medium DAPI (Vector laboratories, Burlingame, CA, USA) was applied to nuclear staining. The slides were examined and photographed with a fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
Western blot
Western blot was performed essentially the same as previously described54. Briefly, cells were lysed in RIPA buffer (Cell Signaling Technology, Boston, MA, USA) containing protease and phosphatase inhibitors (Roche, Penzberg, Germany). The concentrations of protein in the lysates were determined with Pierce BCA Protein Assay Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA) according to the manufacturer’s protocol. After electrophoresis on 10% SDS-PAGE Bio-Rad minigels, the aliquots proteins were transferred to nitrocellulose filter membrane (Whatman/GE Healthcare life sciences, Marlborough, MA, USA). The blots were blocked for 1 hour at room temperature in 5% skim milk in TBST, and incubated overnight with rabbit anti-Influenza A Virus Nucleoprotein antibody (GeneTex, Irvine, CA, USA) and mouse anti-β actin monoclonal antibody (ZSGB-BIO, Beijing, China) at a dilution of 1:10,00 as primary antibodies. IRDye 680-conjugated goat polyclonal anti-rabbit IgG (H+L) and IRDye 800-conjugated goat polyclonal anti-mouse IgG (H+L) (both from LiCor, Lincoln, NE, USA) were used as secondary antibodies at a dilution of 1:10,000. The blot was examined and photographed with a Odyssey imaging systems (LiCor, Lincoln, NE, USA).
Flow cytometry
PE-conjugated anti-CD15 monoclonal antibody (eBioscience, San Diego, CA, USA) and FITC-conjugated anti-Influenza A Virus Nucleoprotein monoclonal antibody (Abcam, Cambridge, MA, USA) were used for flow cytometry. For surface marker staining, neutrophils were blocked with 5% BSA to avoid nonspecific reaction and incubated with PE-conjugated anti-CD15 antibody. For the detection of intracellular virus Nucleoprotein, cells were fixed with 4% paraformaldehyde, permeabilized and blocked with 0.2%Triton X-100, 5%BSA in advance incubated with FITC-conjugated anti-Influenza A Virus Nucleoprotein antibody. After washing, cells were resuspended in HBSS (Solarbio, Beijing, China). Flow cytometry was performed using FACS Aria II instruments (BDBiosciences, San Jose, CA, USA) and Flow Jo software (Tree Star, Inc. USA) was used for data analysis.
Cell co-culture
The inner surface of 1.0 μm twelve-well millicell (Millipore, Billerica, MA, USA) inserts was coated with fibronectin (Sigma, St. Louis, MO, USA) to ensure neutrophil attachment. Human neutrophils were exposed to influenza virus in twelve-well millicell inserts at 37 °C for 1 h to allow viral infection. Then the culture supernatant was discarded and cells were washed with HBSS (Solarbio, Beijing, China) three times to make sure unbound viruses were removed. The inserts with virus-exposed neutrophils were put into twelve-well plates with cultured human colorectal mucosa cell line (FHC). Cells were collected at designated durations post infection and were analyzed with immunocytochemistry and flow cytometry. Immunocytochemistry was performed as described previously55 with rabbit anti-Influenza A Virus Nucleoprotein as primary antibody (Gene Tex, Irvine, CA, USA). Flow cytometry was performed as described above.
MTS assay
Separated neutrophils were plated in a 96-well plate and then infected with influenza virus. A time course experiment was performed to monitor cell viability with a Cell Titer 96 AQueous One Solution Cell Proliferation Assay Kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Uninfected neutrophils were examined in parallel as control.
Cell apoptosis analysis
At 4 h, 8 h, 12 h and 24 h post infection, neutrophils were collected and analyzed with a Annexin V-FITC/PI Apoptosis Detection Kit (Beyotime, Jiangshu, China) following the manufacturer’s instructions. Flow cytometry was performed using FACS Aria II instruments (BDBiosciences, San Jose, CA, USA) and Flow Jo software (Tree Star, Inc. USA) was used for data analysis. The same time points of uninfected neutrophils were examined as control.
Neutrophil activation measurement
H2O2 production was measured with a Hydrogen Peroxide Assay Kit (Beyotime, Jiangshu, China) following the manufacturer’s instructions. Activation was measured by incubating neutrophils with influenza virus for 30 min or 10–7 M N-formyl-L-methionyl-L-leucyl-L-phenylalanine (FMLP, Sigma, St. Louis, MO, USA) for 5 min. Deactivation was assessed by first incubating neutrophils with influenza virus for 30 min followed by activated with 10–7 M FMLP for 5 min. Neutrophils without any treatment was used as negative control.
Statistical Analysis
Statistical analyses were performed using Prism (GraphPad Software, La Jolla, CA, USA). All experiments were repeated in neutrophils derived from at least three different individuals. The data were expressed as the mean ± S.D. and compared with Student’s t-test or One-way ANOVA. Comparisons of each group were performed with q-test (Newman-Keul’s test). The significant level was set at P < 0.05.
Additional Information
How to cite this article: Zhang, Z. et al. Infectious Progeny of 2009 A (H1N1) Influenza Virus Replicated in and Released from Human Neutrophils. Sci. Rep. 5, 17809; doi: 10.1038/srep17809 (2015).
Supplementary Material
Acknowledgments
We thank Yun Wang, Jing Li, Jin Huang, Chun Ruan, and Zuoqing Su for assistance with the experiments. This work was supported by grants from the Major Program of National Natural Science Foundation of China (81030033).
Footnotes
Author Contributions J.G. designed the study, supervised experiments and revised the manuscript. Z.Z. and T.H. performed experiments and manuscript writing. F.Y., X.L., C.Z., X.C. and D.J.K. contributed to experimental design and data analysis.
References
- Bale J. F. Jr., O’Neil M. E. & Greiner T. The interaction of murine cytomegalovirus with murine neutrophils: effect on migratory and phagocytic activities. Journal of leukocyte biology 38, 723–734 (1985). [DOI] [PubMed] [Google Scholar]
- Szanton E. & Sarov I. Interaction between polymorphonuclear leukocytes and varicella-zoster virus-infected cells. Intervirology 24, 119–124 (1985). [DOI] [PubMed] [Google Scholar]
- Blackmon J. R. & Ginsberg H. S. Reactions of influenza viruses with guinea pig polymorphonuclear leucocytes. I. Virus-cell interactions. Virology 2, 618–636 (1956). [DOI] [PubMed] [Google Scholar]
- Abramson J. S. et al. Suppression of endocytosis in neutrophils by influenza A virus in vitro. The Journal of infectious diseases 154, 456–463 (1986). [DOI] [PubMed] [Google Scholar]
- Colamussi M. L., White M. R., Crouch E. & Hartshorn K. L. Influenza A virus accelerates neutrophil apoptosis and markedly potentiates apoptotic effects of bacteria. Blood 93, 2395–2403 (1999). [PubMed] [Google Scholar]
- Daigneault D. E. et al. Influenza A virus binding to human neutrophils and cross-linking requirements for activation. Blood 80, 3227–3234 (1992). [PubMed] [Google Scholar]
- Ivan F. X. et al. Neutrophils infected with highly virulent influenza H3N2 virus exhibit augmented early cell death and rapid induction of type I interferon signaling pathways. Genomics 101, 101–112, 10.1016/j.ygeno.2012.11.008 (2013). [DOI] [PubMed] [Google Scholar]
- Wang J. P. et al. Toll-like receptor-mediated activation of neutrophils by influenza A virus. Blood 112, 2028–2034, 10.1182/blood-2008-01-132860 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufford M. M. et al. Influenza-infected neutrophils within the infected lungs act as antigen presenting cells for anti-viral CD8(+) T cells. PloS one 7, e46581, 10.1371/journal.pone.0046581 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. et al. Neutrophils may be a vehicle for viral replication and dissemination in human H5N1 avian influenza. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 47, 1575–1578, 10.1086/593196 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Vassalli J. D. & Reich E. RNA and protein synthesis in human peripheral blood polymorphonuclear leukocytes. The Journal of experimental medicine 149, 284–289 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Ullyot J. L. & Farquhar M. G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. The Journal of experimental medicine 134, 907–934 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy L. F., Lyles D. S. & Abramson J. S. Synthesis of viral proteins in polymorphonuclear leukocytes infected with influenza A virus. Journal of clinical microbiology 26, 1267–1270 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack R. M. & Fearon D. T. Selective synthesis of mRNA and proteins by human peripheral blood neutrophils. Journal of immunology 140, 4286–4293 (1988). [PubMed] [Google Scholar]
- Cline M. J. Ribonucleic acid biosynthesis in human leukocytes. Effects of phagocytosis on RNA metabolism. Blood 28, 188–200 (1966). [PubMed] [Google Scholar]
- Cline M. J. Ribonucleic acid biosynthesis in human leukocytes: the fate of rapidly labeled RNA in normal and abnormal leukocytes. Blood 28, 650–664 (1966). [PubMed] [Google Scholar]
- Tiku K., Tiku M. L. & Skosey J. L. Interleukin 1 production by human polymorphonuclear neutrophils. Journal of immunology 136, 3677–3685 (1986). [PubMed] [Google Scholar]
- Herz C., Stavnezer E., Krug R. & Gurney T. Jr. Influenza virus, an RNA virus, synthesizes its messenger RNA in the nucleus of infected cells. Cell 26, 391–400 (1981). [DOI] [PubMed] [Google Scholar]
- Samji T. Influenza A: understanding the viral life cycle. The Yale journal of biology and medicine 82, 153–159 (2009). [PMC free article] [PubMed] [Google Scholar]
- Weis W. et al. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature 333, 426–431, 10.1038/333426a0 (1988). [DOI] [PubMed] [Google Scholar]
- Stray S. J., Cummings R. D. & Air G. M. Influenza virus infection of desialylated cells. Glycobiology 10, 649–658 (2000). [DOI] [PubMed] [Google Scholar]
- Suzuki T. et al. Sulphatide binds to human and animal influenza A viruses, and inhibits the viral infection. The Biochemical journal 318 (Pt 2), 389–393 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J. & Helenius A. Gulping rather than sipping: macropinocytosis as a way of virus entry. Current opinion in microbiology 15, 490–499, 10.1016/j.mib.2012.05.016 (2012). [DOI] [PubMed] [Google Scholar]
- Papayannopoulos V. & Zychlinsky A. NETs: a new strategy for using old weapons. Trends in immunology 30, 513–521, 10.1016/j.it.2009.07.011 (2009). [DOI] [PubMed] [Google Scholar]
- Gu J. et al. H5N1 infection of the respiratory tract and beyond: a molecular pathology study. Lancet 370, 1137–1145, 10.1016/S0140-6736(07)61515-3 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J. M. et al. Tropism of avian influenza A (H5N1) in the upper and lower respiratory tract. Nature medicine 13, 147–149, 10.1038/nm1529 (2007). [DOI] [PubMed] [Google Scholar]
- Kilbourne E. D. in Influenza 255-289 (Springer, 1987). [Google Scholar]
- Hartshorn K. L. et al. Effects of influenza A virus on human neutrophil calcium metabolism. Journal of immunology 141, 1295–1301 (1988). [PubMed] [Google Scholar]
- Jakab G. J. & Green G. M. Defect in intracellular killing of Staphylococcus aureus within alveolar macrophages in Sendai virus-infected murine lungs. The Journal of clinical investigation 57, 1533–1539, 10.1172/JCI108423 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I. & Snyderman R. Inflammation: Basic Principles and Clinical Correlates. (Lippincott Williams & Wilkins, 1999). [Google Scholar]
- Hartshorn K. L., Collamer M., White M. R., Schwartz J. H. & Tauber A. I. Characterization of influenza A virus activation of the human neutrophil. Blood 75, 218–226 (1990). [PubMed] [Google Scholar]
- Hartshorn K. L. et al. Neutrophil deactivation by influenza A virus. Role of hemagglutinin binding to specific sialic acid-bearing cellular proteins. Journal of immunology 154, 3952–3960 (1995). [PubMed] [Google Scholar]
- Stadlmann J., Pabst M. & Altmann F. Analytical and Functional Aspects of Antibody Sialylation. Journal of clinical immunology 30 Suppl 1, S15–19, 10.1007/s10875-010-9409-2 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novel Swine-Origin Influenza, A. V. I. T. et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. The New England journal of medicine 360, 2605–2615, 10.1056/NEJMoa0903810 (2009). [DOI] [PubMed] [Google Scholar]
- Centers for Disease C. & Prevention. Neurologic complications associated with novel influenza A (H1N1) virus infection in children - Dallas, Texas, May 2009. MMWR. Morbidity and mortality weekly report 58, 773–778 (2009). [PubMed] [Google Scholar]
- Soto-Abraham M. V. et al. Pathological changes associated with the 2009 H1N1 virus. The New England journal of medicine 361, 2001–2003, 10.1056/NEJMc0907171 (2009). [DOI] [PubMed] [Google Scholar]
- Peiris J. S. et al. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 363, 617–619, 10.1016/S0140-6736(04)15595-5 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungchusak K. et al. Probable person-to-person transmission of avian influenza A (H5N1). The New England journal of medicine 352, 333–340, 10.1056/NEJMoa044021 (2005). [DOI] [PubMed] [Google Scholar]
- To K. F. et al. Pathology of fatal human infection associated with avian influenza A H5N1 virus. Journal of medical virology 63, 242–246 (2001). [DOI] [PubMed] [Google Scholar]
- Uiprasertkul M. et al. Apoptosis and pathogenesis of avian influenza A (H5N1) virus in humans. Emerging infectious diseases 13, 708–712, 10.3201/eid1305.060572 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines T. R. et al. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science 325, 484–487, 10.1126/science.1177238 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund P. et al. Pandemic H1N1 2009 influenza A virus induces weak cytokine responses in human macrophages and dendritic cells and is highly sensitive to the antiviral actions of interferons. Journal of virology 84, 1414–1422, 10.1128/JVI.01619-09 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, W. H. Clinical management of human infection with pandemic (H1N1) 2009: revised guidance. World Health Organization Web site. http://www. who. int/csr/resources/publications/swineflu/clinical_management_ h1n1. pdf. Accessed November (2009).
- Patel M., Dennis A., Flutter C. & Khan Z. Pandemic (H1N1) 2009 influenza. British journal of anaesthesia 104, 128–142, 10.1093/bja/aep375 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Cherit G. et al. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA: the journal of the American Medical Association 302, 1880–1887, 10.1001/jama.2009.1536 (2009). [DOI] [PubMed] [Google Scholar]
- Coligan J. E. V. (loose leaf) (John Wiley and Sons, New York). [Google Scholar]
- Suzuki Y. Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses. Biological & pharmaceutical bulletin 28, 399–408 (2005). [DOI] [PubMed] [Google Scholar]
- Stevens J. et al. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science 312, 404–410, 10.1126/science.1124513 (2006). [DOI] [PubMed] [Google Scholar]
- Rogers G. N. et al. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature 304, 76–78 (1983). [DOI] [PubMed] [Google Scholar]
- Skehel J. J. & Wiley D. C. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annual review of biochemistry 69, 531–569, 10.1146/annurev.biochem.69.1.531 (2000). [DOI] [PubMed] [Google Scholar]
- Coico R. V. (loose-leaf) (Wiley, Hoboken, N.J., 2006). [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408, 10.1006/meth.2001.1262 (2001). [DOI] [PubMed] [Google Scholar]
- Jiang C. et al. Immunoglobulin G expression in lung cancer and its effects on metastasis. PloS one 9, e97359, 10.1371/journal.pone.0097359 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Song Y. et al. Human surfactant protein A2 gene mutations impair dimmer/trimer assembly leading to deficiency in protein sialylation and secretion. PloS one 7, e46559, 10.1371/journal.pone.0046559 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J. et al. Fab fragment glycosylated IgG may play a central role in placental immune evasion. Human reproduction 30, 380–391, 10.1093/humrep/deu323 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.