Abstract
Background
The present study aimed to compare the expression of liver kinase B1 (LKB1) in prostate cancer (PCa) tissues and the paired adjacent tissues, then to evaluate the statistical relationship between LKB1 expression and prognosis of PCa patients.
Material/Methods
The relative expression of LKB1 at mRNA level was detected by quantitative real-time polymerase chain reaction (qRT-PCR). The expression of LKB1 at protein level was measured by immunohistochemistry (IHC) method. The relationship between LKB1 expression and clinicopathologic characteristics was estimated by chi-square test. Kaplan-Meier method was used to analyze the overall survival of PCa patients with different LKB1 expression. Cox regression analysis was performed to estimate the significance of LKB1 expression and clinicopathologic characteristics in the prognosis of PCa patients.
Results
The relative expression of LKB1 at mRNA level was significantly lower in PCa tissues than in the normal tissues (P<0.001). The LKB1 expression was proved to be affected by clinical stage (P=0.019) and PSA concentration (P=0.031) of PCa patients. Moreover, patients with negative LKB1 expression had shorter survival than those with positive expression. Cox regression analysis confirmed that LKB1 could be regarded as a prognostic biomarker for PCa patients (P=0.001, HR=3.981, 95% CI=1.698–9.336).
Conclusions
The expression of LKB1 was lower in PCa tissues and might be a predictor for the prognosis of PCa patients.
MeSH Keywords: Prognosis, Prostatic Neoplasms, Vascular Endothelial Growth Factor Receptor-2
Background
Prostate cancer (PCa) is one of the most common cancers among men over 50 years old and is the sixth leading cause of cancer-related deaths world-wide [1,2]. Although its incidence is higher in developed countries than in developing counties, it is still increasing in developing areas [3–5]. There are obvious regional and ethic differences in the pathogenesy of PCa. Currently, the diagnosis of PCa is mainly based on the combination of various procedures, and PCa is usually diagnosed as a localized disease [6,7]. Treatments for PCa predominantly include surgical castration, androgen-deprivation therapy (ADT), and radiation therapy (RT) [8,9]. However, these treatments have shortcomings and there is no effective strategy to treat metastasis and recurrence of Pca that cannot be treated by surgery or radiation therapy. Therefore, an innovative biomarker for therapies and prognosis of PCa patients is urgently needed.
Liver kinase B1 (LKB1), a serine/threonine kinase, is located at 19p 13.3 of human chromosomes [10]. It is known as a tumor suppressor and many studies have demonstrated that LKB1 plays an important role in regulating energy homeostasis, cell cycle progression, cell polarity, cell proliferation, senescence, DNA damage response, and differentiation [11–13]. It is also mutated in Peutz-Jeghers syndrome (PJS), which leads to an increasing risk of malignant tumors in multiple tissues [14]. In previous studies, LKB1 was reported to be a common mutated gene in various of cancers such as non-small cell lung carcinomas, cervical cancer, breast cancer, and pancreatic carcinoma [15–18]. It was also confirmed that LKB1 was related with prostate neoplasia and could suppress proliferation and invasion of PCa [19,20]. However, its role in the prognosis of PCa had never been determined.
This study aimed to detect the LKB1 expression in PCa tissues and the paired adjacent normal tissues both at mRNA level and protein level. Then we attempted to further explore whether LKB1 could serve as a prognostic factor for PCa patients, so as to understand the PCa progression, provide an efficient therapy method of Pca, and increase the survival rate of PCa patients.
Material and Methods
Patients and tissues specimens
Our study included 109 patients with PCa diagnosed at the Department of Urology Surgery of The Affiliated Hospital of Jining Medical College. None of them had ever received any chemical treatment or physical therapy before surgery. The present study was approved by the Ethics Committee of The Affiliated Hospital of Jining Medical College. All participants provided signed informed written consent in advance.
The tumor tissues and the paired adjacent tissues were collected from PCa patients. All the specimens were biopsy materials and frozen in liquid nitrogen immediately, then the samples were stored at −80°C for RNA extraction. A follow-up of 60 months was conducted. The overall survival time was defined as the time from day of surgery to the day of death. The follow-up information was obtained via a telephone or questionnaire and was updated every 2 months. Patients who died from other disease or accident were excluded from our study.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA from fresh PCa tissues and the paired adjacent tissues were extracted and purified using RNeasy Mini Kit (QIAGEN) according to the manufacturer’s directions. Reverse transcription was performed with a ReverTra Ace qPCR RT Kit (Toyobo Bio-Technology, Japan) according to the manufacturer’s instructions. qRT-PCR reaction was carried out in the Applied Biosystems 7900 Fast Real-Time PCR system (Applied Biosystems, Foster City, California, USA). The GAPDH was used as endogenous control. The relative expression of LKB1 at mRNA level normalized to GAPDH was evaluated by comparative cycle threshold (CT) method. All the experiments were conducted under optimal conditions and in triplicate.
Immunohistochemistry assay
Immunohistochemistry (IHC) was used to examine the expression of LKB1 at the protein level in all tissue samples. The tumor tissues and adjacent tissues were fixed in 10% formaldehyde and embedded in paraffin. Then the paraffin sections were cut into 4-μm sections, and were dewaxed and rehydrated with xylene and graded alcohol, respectively. The sections were washed with buffer solution for 5 min and then added into the primary antibody at 4°C overnight. The second antibody was added into the sections after being washed again. Finally, coloration was performed with DAB. The results are presented as the percentage of the staining cells (0 to 100%) in tissues. Staining under 20% of the tissue cells or no staining was included in the negative group (−), while the others belonged to the positive group (+).
Statistical analysis
All data processing was carried out using SPSS 18.0 software. The difference in LKB1 expression between PCa tissues and adjacent tissues was analyzed by t test. The chi-square test was used to analyze the relationship between LKB1 expression and clinicopathological characteristics. The association between LKB1 expression and overall survival, as well as the prognostic value of LKB1, were estimated by Kaplan-Meier and Cox regression analysis, respectively. P<0.05 was considered to be statistically significant.
Results
Low expression of LKB1 at mRNA level in PCa tissues
QRT-PCR was used to evaluate the expression of LKB1 in PCa tissues and the adjacent tissues. The expression level of LKB1 was normalized to GAPDH. The result demonstrated that the relative expression of LKB1 at mRNA level in PCa tissues was 0.59±0.25 (mean±SD), while that in the normal tissues was 1.32±0.59 (mean ±SD). A significant decrease in the expression of LKB1 at the mRNA level was found in PCa tissues (Figure 1, P<0.001).
Figure 1.
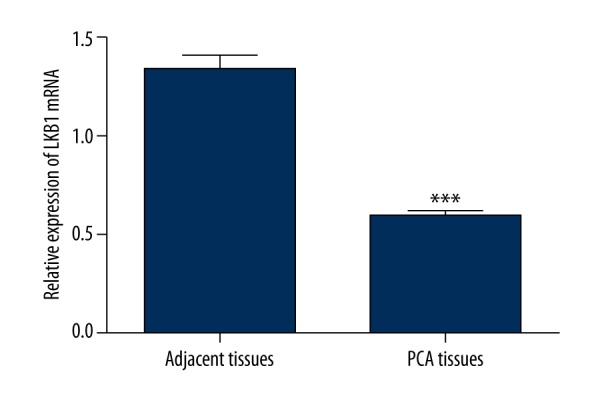
Relative expression of LKB1 at mRNA level was assessed by qRT-PCR in PCa tissues and adjacent normal tissues. The LKB1 expression was normalized to GAPDH. Expression level of LKB1 was significantly lower in PCa tissues compared to the adjacent normal tissues (P<0.001).
Decreased expression of LKB1 at protein level in PCa tissues
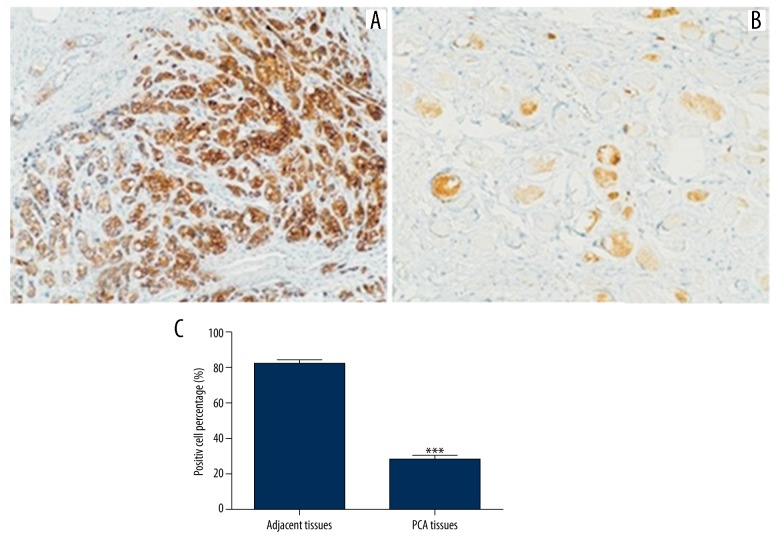
The LKB1 protein expression of all PCa tissues and a randomly selected 70 cases of adjacent tissues were assayed by IHC method. Figure 2 shows that staining degree was obviously diminished in PCa tissues compared with adjacent tissues. To obtain an exact result of protein expression level of the LKB1 gene in PCa patients, we analyzed the positive cell percentage in the 2 tissues. We found that positive cell percentage was 28.4% in PCa tissues and 81.4% in normal tissues. Both the staining degree and positive cell percentage indicated that the LKB1 protein expression in PCa tissues was significantly lower than that in the normal tissues (Figure 2, P<0.001).
Figure 2.
The expression of LKB1 protein in PCa tissues and adjacent tissues. The LKB1 protein expression level was lower in PCa tissues than in adjacent tissues (P<0.001). (A) Adjacent tissue; (B) PCa tissue; (C) Positive cell percentage.
Correlation between LKB1 expression and clinicopathological characteristics
The association between LKB1 expression and clinicopathological characteristics, including age, hematuria, urine retention, creatinine (μmol/L), clinical staging, and PSA (ng/ml), were evaluated to determine whether LKB1 participates in the development of PCa. The results showed that creatinine level (P=0.035), advanced clinical stage (P=0.019), and high concentration of PSA (P=0.031) were all related to low LKB1 expression (Table 1). However, no clinical relevance was observed between LKB1 and age, hematuria, or urine retention (Table 1, P>0.05).
Table 1.
The relationship between LKB1 expression and clinicopathological characteristics of PCa patients.
| Clinical features | Case (n) | LKB1 expression | χ2 | P | |
|---|---|---|---|---|---|
| Negative (n) | Positive (n) | ||||
| Age | 0.714 | 0.398 | |||
| ≤55 | 39 | 26 | 13 | ||
| >55 | 70 | 52 | 18 | ||
| Hematuria | 0.265 | 0.607 | |||
| Yes | 57 | 42 | 15 | ||
| No | 52 | 36 | 16 | ||
| Urine retention | 2.212 | 0.137 | |||
| Yes | 58 | 45 | 13 | ||
| No | 51 | 33 | 18 | ||
| Creatinine (μmol/L) | 4.469 | 0.035 | |||
| ≤110 | 46 | 28 | 18 | ||
| >110 | 63 | 50 | 13 | ||
| Clinical staging | 5.468 | 0.019 | |||
| T1+T2 | 51 | 31 | 20 | ||
| T3+T4 | 58 | 47 | 11 | ||
| PSA (ng/ml) | 4.645 | 0.031 | |||
| ≤6 | 56 | 35 | 21 | ||
| >6 | 53 | 43 | 10 | ||
Association between LKB1 expression and overall survival of PCa patients
During the follow-up, 47 of 78 (60.3%) patients with negative LKB1 expression died, whereas only 7 (22.6%) patients with positive LKB1 expression died. Kaplan-Meier analysis exhibited that patients with negative LKB1 expression had significantly lower overall survival than those with positive LKB1 expression (Figure 3, log rank test, P<0.001). Multivariate Cox regression analysis showed that clinical features had no significant relationship with the prognosis of PCa, but LKB1 expression (P=0.001, HR=3.981, 95% CI=1.698–9.336) was associated with the prognosis of PCa patients (Table 2). Therefore, we inferred that these clinical features could not act as markers for PCa prognosis, but LKB1 might be a novel indicator for the prognosis of PCa patients.
Figure 3.
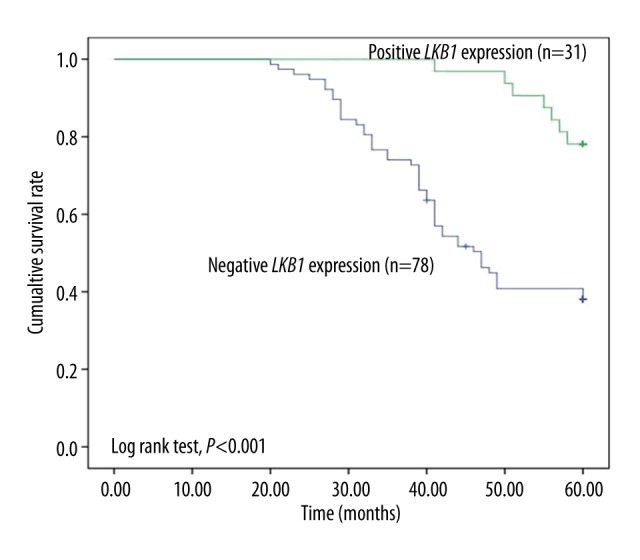
Kaplan-Meier analysis with the expression of LKB1 showed that patients with negative expression of LKB1 had a significantly shorter overall survival time than those with positive LKB1 expression (log-rank test, P<0.001).
Table 2.
Multivariate analysis for prognostic factors in prostate cancer via cox regression analysis.
| Variable | P value | HR | 95%CI |
|---|---|---|---|
| Hematuria | 0.188 | 0.659 | 0.354–1.227 |
| Urine retention | 0.155 | 0.655 | 0.365–1.173 |
| Clinical staging | 0.442 | 1.255 | 0.703–2.240 |
| LKB1 expression | 0.001 | 3.981 | 1.698–9.336 |
Discussion
PCa is a malignant tumor that presents in the prostatic tissues of the males and is the result of disordered growth of prostatic vesicle cells. Up to now, pathogens that induce PCa are still indefinite, which may be associated with the alteration of gene, such as the change of androgen receptor relative genes. It is usually a fatal disease for most patients diagnosed in advanced stages. Therefore, it is of great significance to explore effective diagnostic and prognostic markers for PCa.
Several molecular markers have been investigated in PCa tissues as predictive biomarkers [21–26]. For example, Rajal et al. reported that ERG was overexpression in PCa and Xu et al. also verified that ERG played a prognostic role in prostatic acinar adenocarcinoma [27,28]. Zheng et al. demonstrated that SFRP1 could be a prognostic biomarker in PCa patients [29]. In addition, LKB1 is a tumor suppressor gene with a molecular weight of 50 KD. It contains 10 exons and consists of kinase domain, N terminal regulatory domain, and C terminal regulatory domain. It has been studied in various diseases and was confirmed to play crucial roles in different cell processes. For instance, in the study of Inge et al. the inactivation of LKB1 could sensitize NSCLC to pharmacological aggravation of ER stress, and another report by Inge et al. found that LKB1 expression in NSCLC determined the sensitivity to 2-deoxyglucose [30,31]. Moreover, many studies suggested that the LKB1 gene may have a role in PCa, including the precursor lesions, as well as proliferation and invasion of PCa [19,20,32]. However, the association of the LKB1 gene with PCa development remains unclear. In this study, we detected the expression of LKB1 in PCa tissues and adjacent normal tissues both at mRNA level and protein level. Our study results demonstrate that LKB1 expression was reduced in PCa tissues and it might be a tumor suppressor in PCa. This result was consistent with the trend in lung cancer [33].
As LKB1 expression was linked with various cancers, thus it might be a potential prognostic marker. A previous study found that loss of LKB1 protein expression may be useful as a prognostic marker for breast carcinoma [34]. To clarify whether LKB1 had been involved in the development of PCa and its prognostic value in PCa patients, we analyzed the relationship between LKB1 expression and clinicopathologic characteristics as well as the overall survival of patients. It was shown that the LKB1 expression was related to clinical staging and PSA concentration tightly. In addition, Kaplan-Meier analysis shoed that the overall survival time of patients with low LKB1 expression was shorter than in those with high LKB1 expression. Cox regression analysis determined that there was a statistically significant relationship between LKB1 expression and the prognosis of PCa patients, indicating that LKB1 could be a prognostic biomarker for PCa patients.
As with other tumor suppressor genes, it is also difficult to identify the patients with or without low LKB1 expression, and potential mechanisms of LKB1 in various tumors are still unknown. Many studies have demonstrated that LKB1 functions in various cancers through the LKB1/AMPK signaling pathway [35–37]. Young-Ok Son et al. illustrated that cadmium induced autophagy through LKB1- AMPK signaling in skin epidermal cells [38]. Brown et al. showed that LKB1 expression was inhibited by estradiol-17β in MCF-7 cells [39]. Therefore, we presumed that the effect of LKB1 on PCa might be related to the LKB1/AMPK signaling pathway or estradiol-17β approach, but this hypothesis must be verified in further research.
Conclusions
In conclusion, LKB1 is a tumor suppressor in PCa via its decreased expression in PCa tissues. Statistical significance was found between LKB1 expression and the prognosis of PCa patients, suggesting that LKB1 may be a candidate prognostic marker for PCa patients.
Footnotes
Source of support: Departmnetal sources
References
- 1.Boyle P, Napalkov P. The epidemiology of benign prostatic hyperplasia and observations on concomitant hypertension. Scand J Urol Nephrol Suppl. 1995;168:7–12. [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Du LB, Li HZ, Wang XH, et al. Analysis of cancer incidence in Zhejiang cancer registry in China during 2000 to 2009. Asian Pac J Cancer Prev. 2014;15:5839–43. doi: 10.7314/apjcp.2014.15.14.5839. [DOI] [PubMed] [Google Scholar]
- 4.Jung KW, Won YJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 6.Ezquer A, Ortega Hrescak MC, Sanagua C, et al. Transrectal doppler ultrasound during prostate biopsy: clinical utility and limitations. Actas Urol Esp. 2015;39:13–19. doi: 10.1016/j.acuro.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Karantanos T, Evans CP, Tombal B, et al. Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur Urol. 2015;67:470–79. doi: 10.1016/j.eururo.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joniau S, Spahn M, Briganti A, et al. Pretreatment tables predicting pathologic stage of locally advanced prostate cancer. Eur Urol. 2015;67:319–25. doi: 10.1016/j.eururo.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Yu EY, Getzenberg RH, Coss CC, et al. Selective estrogen receptor alpha agonist GTx-758 decreases testosterone with reduced side effects of androgen deprivation therapy in men with advanced prostate cancer. Eur Urol. 2015;67:334–41. doi: 10.1016/j.eururo.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Zhao N, Wilkerson MD, Shah U, et al. Alterations of LKB1 and KRAS and risk of brain metastasis: comprehensive characterization by mutation analysis, copy number, and gene expression in non-small-cell lung carcinoma. Lung Cancer. 2014;86:255–61. doi: 10.1016/j.lungcan.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hezel AF, Bardeesy N. LKB1; linking cell structure and tumor suppression. Oncogene. 2008;27:6908–19. doi: 10.1038/onc.2008.342. [DOI] [PubMed] [Google Scholar]
- 12.Jansen M, Ten Klooster JP, Offerhaus GJ, Clevers H. LKB1 and AMPK family signaling: the intimate link between cell polarity and energy metabolism. Physiol Rev. 2009;89:777–98. doi: 10.1152/physrev.00026.2008. [DOI] [PubMed] [Google Scholar]
- 13.Vaahtomeri K, Makela TP. Molecular mechanisms of tumor suppression by LKB1. FEBS Lett. 2011;585:944–51. doi: 10.1016/j.febslet.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 14.Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–87. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 15.Ji H, Ramsey MR, Hayes DN, Fan C, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–10. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Chen H, Wang X, et al. Expression and transcriptional profiling of the LKB1 tumor suppressor in cervical cancer cells. Gynecol Oncol. 2014;134:372–78. doi: 10.1016/j.ygyno.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhuang Z, Wang K, Cheng X, et al. LKB1 inhibits breast cancer partially through repressing the Hedgehog signaling pathway. PLoS One. 2013;8:e67431. doi: 10.1371/journal.pone.0067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hezel AF, Gurumurthy S, Granot Z, et al. Pancreatic LKB1 deletion leads to acinar polarity defects and cystic neoplasms. Mol Cell Biol. 2008;28:2414–25. doi: 10.1128/MCB.01621-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu P, Cai F, Liu X, Guo L. LKB1 suppresses proliferation and invasion of prostate cancer through hedgehog signaling pathway. Int J Clin Exp Pathol. 2014;7:8480–88. [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson HB, McCarthy A, Collins CM, et al. Lkb1 deficiency causes prostate neoplasia in the mouse. Cancer Res. 2008;68:2223–32. doi: 10.1158/0008-5472.CAN-07-5169. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Wen J, Li R, et al. Gene expression profiling analysis of castration-resistant prostate cancer. Med Sci Monit. 2015;21:205–12. doi: 10.12659/MSM.891193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han X, Zhang JJ, Yao N, et al. Polymorphisms in NFKB1 and NFKBIA genes modulate the risk of developing prostate cancer among Han Chinese. Med Sci Monit. 2015;21:1707–15. doi: 10.12659/MSM.893471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Xie PG, Lin YL, et al. Aberrant methylation of PCDH10 predicts worse biochemical recurrence-free survival in patients with prostate cancer after radical prostatectomy. Med Sci Monit. 2014;20:1363–68. doi: 10.12659/MSM.891241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin YL, Xie PG, Wang L, Ma JG. Aberrant methylation of protocadherin 17 and its clinical significance in patients with prostate cancer after radical prostatectomy. Med Sci Monit. 2014;20:1376–82. doi: 10.12659/MSM.891247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niu WB, Gui SL, Lin YL, et al. Promoter methylation of Protocadherin8 is an independent prognostic factor for biochemical recurrence of early-stage prostate cancer. Med Sci Monit. 2014;20:2584–89. doi: 10.12659/MSM.893083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryniarski P, Paradysz A, Fryczkowski M. PSA mass as a marker of prostate cancer progression after radical prostatectomy. Med Sci Monit. 2011;17(2):CR104–9. doi: 10.12659/MSM.881395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah RB, Bentley J, Jeffery Z, DeMarzo AM. Heterogeneity of PTEN and ERG expression in prostate cancer on core needle biopsies: implications for cancer risk stratification and biomarker sampling. Hum Pathol. 2015;46:698–706. doi: 10.1016/j.humpath.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Xu B, Chevarie-Davis M, Chevalier S, et al. The prognostic role of ERG immunopositivity in prostatic acinar adenocarcinoma: a study including 454 cases and review of the literature. Hum Pathol. 2014;45:488–97. doi: 10.1016/j.humpath.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Zheng L, Sun D, Fan W, et al. Diagnostic value of SFRP1 as a favorable predictive and prognostic biomarker in patients with prostate cancer. PLoS One. 2015;10:e0118276. doi: 10.1371/journal.pone.0118276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inge LJ, Friel JM, Richer AL, et al. LKB1 inactivation sensitizes non-small cell lung cancer to pharmacological aggravation of ER stress. Cancer Lett. 2014;352:187–95. doi: 10.1016/j.canlet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Inge LJ, Coon KD, Smith MA, Bremner RM. Expression of LKB1 tumor suppressor in non-small cell lung cancer determines sensitivity to 2-deoxyglucose. J Thorac Cardiovasc Surg. 2009;137:580–86. doi: 10.1016/j.jtcvs.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 32.Chrisofos M, Papatsoris AG, Lazaris A, Deliveliotis C. Precursor lesions of prostate cancer. Crit Rev Clin Lab Sci. 2007;44(3):243–70. doi: 10.1080/10408360601177236. [DOI] [PubMed] [Google Scholar]
- 33.Roy BC, Kohno T, Iwakawa R, et al. Involvement of LKB1 in epithelial-mesenchymal transition (EMT) of human lung cancer cells. Lung Cancer. 2010;70:136–45. doi: 10.1016/j.lungcan.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Fenton H, Carlile B, Montgomery EA, et al. LKB1 protein expression in human breast cancer. Appl Immunohistochem Mol Morphol. 2006;14:146–53. doi: 10.1097/01.pai.0000176157.07908.20. [DOI] [PubMed] [Google Scholar]
- 35.Mirouse V, Billaud M. The LKB1/AMPK polarity pathway. FEBS Lett. 2011;585:981–85. doi: 10.1016/j.febslet.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 36.Fei W, Tian DR, Tso P, Han JS. Diet-induced obese rats exhibit impaired LKB1-AMPK signaling in hypothalamus and adipose tissue. Peptides. 2012;35:23–30. doi: 10.1016/j.peptides.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Luo L, Huang W, Tao R, et al. ATM and LKB1 dependent activation of AMPK sensitizes cancer cells to etoposide-induced apoptosis. Cancer Lett. 2013;328:114–19. doi: 10.1016/j.canlet.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Son YO, Wang X, Hitron JA, et al. Cadmium induces autophagy through ROS-dependent activation of the LKB1-AMPK signaling in skin epidermal cells. Toxicol Appl Pharmacol. 2011;255:287–96. doi: 10.1016/j.taap.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown KA, McInnes KJ, Takagi K, et al. LKB1 expression is inhibited by estradiol-17beta in MCF-7 cells. J Steroid Biochem Mol Biol. 2011;127:439–43. doi: 10.1016/j.jsbmb.2011.06.005. [DOI] [PubMed] [Google Scholar]