Abstract
PTP (protein-tyrosine phosphatase)-PEST is a ubiquitously expressed cellular regulator of integrin signalling. It has been shown to bind several molecules such as Shc, paxillin and Grb2, that are involved downstream of FAK (focal adhesion kinase) pathway. Through its specific association to p130cas and further dephosphorylation, PTP-PEST plays a critical role in cell-matrix interactions, which are essential during embryogenesis. We report here that ablation of the gene leads to early embryonic lethality, correlating well with the high expression of the protein during embryonic development. We observed an increased level of tyrosine phosphorylation of p130cas protein in E9.5 PTP-PEST−/− embryos, a first evidence of biochemical defect leading to abnormal growth and development. Analysis of null mutant embryos revealed that they reach gastrulation, initiate yolk sac formation, but fail to progress through normal subsequent developmental events. E9.5–10.5 PTP-PEST−/− embryos had morphological abnormalities such as defective embryo turning, improper somitogenesis and vasculogenesis, impaired liver development, accompanied by degeneration in both neuroepithelium and somatic epithelia. Moreover, in embryos surviving until E10.5, the caudal region was truncated, with severe mesenchyme deficiency and no successful liver formation. Defects in embryonic mesenchyme as well as subsequent failure of proper vascularization, liver development and somatogenesis, seemed likely to induce lethality at this stage of development, and these results confirm that PTP-PEST plays an essential function in early embryogenesis.
Keywords: PTP-PEST, Tyrosine phosphatase, Integrin, Mouse, Knockout, Embryonic stem cell, Vascularization, Neurogenesis, Mesenchyme, Liver
1. Introduction
Protein tyrosine phosphatases (PTPs) and kinases (PTKs) are important regulators of signalling during several fundamental biological events, such as cell migration and division. These processes are essential for proper embryonic development, especially when complex cell mechanisms must be finely regulated. Among tyrosine phosphatases, PTP-PEST is a member of a three-gene sub-family that also includes PEP (Matthews et al., 1992) and PTP-HSCF (hematopoietic stem cell factor) (Cheng et al., 1996). PTP-PEST and PEP share a typical phosphatase catalytic domain in the N-terminus, and a long C-terminal tail rich in proline residues, which allows several protein-protein interactions. Homology with PTP-HSCF is restricted to the phosphatase domain and the extreme carboxyl extremity, termed CTH domain (Spencer et al., 1997). PTP-PEST encodes a protein of 112 kDa localized mainly in cytoplasm and ubiquitously expressed in murine and human adult tissues. The protein is present in higher amounts in the hematopoietic system, particularly in thymus, spleen and liver, but is also found in brain and heart (Côté et al., 2002; Davidson et al., 1997). The murine gene is composed of 18 exons, which cover over 90 kb of the genome, on chromosome 5. Exons 2–11 encode the phosphatase domain consisting of approximately 300 amino acids (Charest et al., 1995). PTP-PEST interacts with a series of key adaptor signalling proteins, pointing to its possible function in cytoskeletal rearrangements. Among others, these include Grb2 (Charest et al., 1997), p130cas (Garton et al., 1997), Hef1 and Sin (Côté et al., 1998), paxillin (Shen et al., 1998; Côté et al., 1999), Csk kinase (Davidson et al., 1997), Shc (Habib et al., 1994; Charest et al., 1996) and PSTPIP (Côté et al., 2002). The most characterized substrates of PTP-PEST include p130cas and PSTPIP (Spencer et al., 1997; Yang and Reinherz, 2006), but other candidates have been identified such as c-Abl (Cong et al., 2000), FAK (Lyons et al., 2001), Shc and Pyk2 (Davidson and Veillette, 2001).
Integrin signalling mediates the clustering of cytoskeletal proteins into focal adhesion structures that permit movement of cells (Burridge and Chrzanowska-Wodnicka, 1996). Upon integrin engagement, tyrosine kinases such as FAK and Src are activated, leading to the tyrosine phosphorylation of adapter proteins like p130cas, Shc, Crk and paxillin. Being highly tyrosine phosphorylated, these molecules can initiate different signalling cascades. PTP-PEST has been proposed to dephosphorylate a panel of proteins in order to promote focal adhesion turnover, leading to rearrangement of actin cytoskeleton. In support of this model, our group has shown that fibroblasts isolated from PTP-PEST−/− embryos have an increased phosphorylation of p130cas, paxillin, FAK and PSTPIP proteins accompanied by cell spreading and impaired motility when plated on fibronectin (Angers-Loustau et al., 1999). Localized at the membrane during cell spreading, PTP-PEST modulates the activity of a small GTPase, Rac1 (Sastry et al., 2002), which is known to induce membrane ruffling, lamellipodia formation and focal adhesion complex organisation (Ridley and Hall, 1992). Indeed, PTP-PEST overexpression in fibroblasts abolishes Rac1 activity, while PTP-PEST−/− fibroblasts show an increased activity (Sastry et al., 2002). Whereas the role of PTP-PEST in the control of fibroblasts motility has been demonstrated, its importance in an entire organism is still to be determined.
Since cell migration is essential during embryonic development, ablation in the mouse of genes coding for proteins of extracellular matrix, actin cytoskeleton or integrin cascade often leads to early embryonic defects and lethality. PTP-PEST is ubiquitously expressed in adult tissues, most prominently in lymphoid organs, and so we examined here the effects of its ablation on mouse development and survival. We show that PTP-PEST is also expressed throughout embryonic development, and while heterozygous mice for the mutation develop normally, those lacking this phosphatase die around gestational day 10.5 due to multiple developmental defects. Null mutants present major deficiencies, which could be observed first at E9.5, including neuroepithelium degeneration, impaired vascular development accompanied by an important decrease in endothelial cell number, caudal development arrest with extensive pycnosis in newly-formed mesenchymal tissues, and absence of liver organogenesis at E10.5. These findings demonstrate that PTP-PEST is required for early steps of embryogenesis, particularly in the later phases of gastrulation, giving rise to a defective yolk sac, absence of caudal somites and neurogenesis, as well as failure of the liver to develop in the septum transversum.
2. Results
2.1. Normal expression of PTP-PEST during embryogenesis
We first examined the normal expression of the PTP-PEST gene during early embryogenesis, using sense and antisense PTP-PEST RNA probes in in situ hybridization experiments (Fig. 1). At E8.0 (Fig. 1A), PTP-PEST RNA was present in extra-embryonic structures, such as yolk sac, trophoblast, amnion, allantois and chorion, as well as throughout the embryo itself. At E9.0 (Fig. 1B), the embryonic expression was observed in mesenchyme and mesectoderm from head and pharyngeal arches. The neurectoderm was positively stained, both in mitotic zone and pseudostratified layer. At this stage, PTP-PEST RNA was also expressed in gut endoderm, in elements of hepatic primordium, in limb buds mesenchyme and in somites. Both neural crest-derived as well as primitive streak-derived mesenchyme expressed PTP-PEST RNA at these early phases. We also observed at this developmental phase, when the neuroepithelium presents a pseudostratified highly prolific layer, strong expression of PTP-PEST RNA in the mitotic zone of the ventricular layer. This could be seen both in the brain and spinal cord (Fig. 1B). At E10.0 (Fig. 1C), we observed high expression in branchial arches and limb buds as well as in the atrial chamber of the heart (Fig. 1D). The adjacent septum transversum was also positively stained. At E11.0 (Fig. 1E), we could more clearly see the expression of PTP-PEST in the hepatic primordium. At E12.0 (Fig. 1F), the midbrain stratified neuroepithelium hybridized with the probe in both mitotic zone and marginal zone at the periphery. The meninges, which derive from neural crest cells, also had strong expression of PTP-PEST. These results show that PTP-PEST is broadly expressed in embryos from E8.0 to E12.0.
Fig. 1.
Characterization of PTP-PEST expression during normal mouse embryonic development by in situ hybridization. Consecutive sections of E 8.0, 9.0, 10.0, 11.0 and 12.0 embryos (A–F) were sectioned and hybridized with a sense (left side panel) or antisense (right side panel) 33P labelled RNA probe specific for the catalytic domain of PTP-PEST. Broadly expressed PTP-PEST mRNA was observed at each developmental stage, both in embryonic and in extra-embryonic structures. A, amnion; Ac, atrial chamber; Al, allantois; Ba, brachial arcs; Bc, bulbus cordis; Ch, chorion; F, foregut; H, heart; Hp, hepatic primordium; Lb, limb bud; M, meninges; Maz, marginal zone; Mb, midbrain; Ms, mesectoderm; Mz, mitotic zone; N, neuroepithelium; Nt, neural tube; S, somites; Sc, spinal cord; St, septum transversum; T, trophoblast; YS, yolk sac.
2.2. Ablation of PTP-PEST function results in embryonic lethality
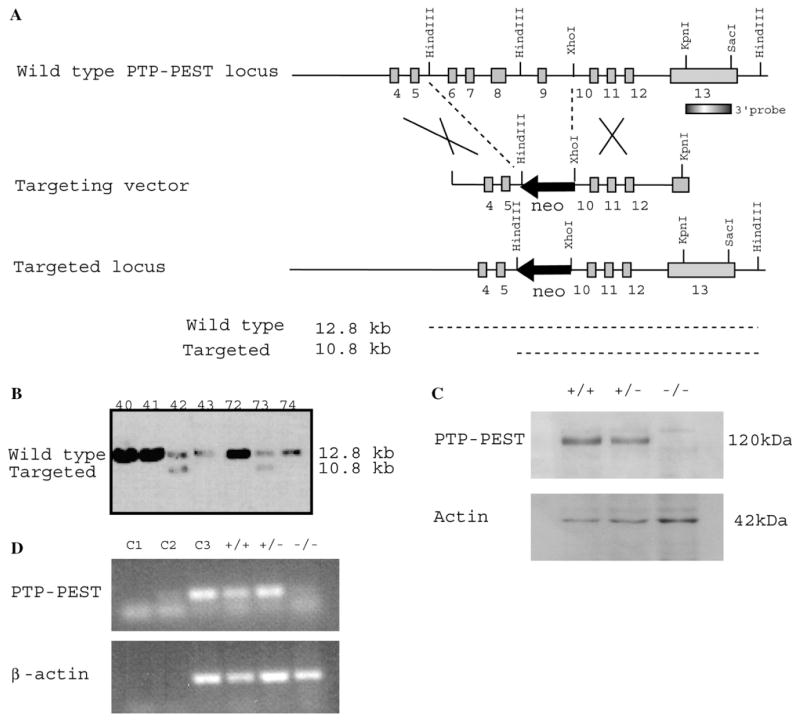
In order to elucidate the role of PTP-PEST in a mouse model, we first ablated the PTP-PEST gene function using targeted homologous recombination in embryonic stem (ES) cells. Following electroporation of the targeting construct (Fig. 2A), seven positive ES clones (PTP-PEST+/−) were obtained among 223 analyzed. By Southern blot analysis, they all showed a band of 10.8 kb (Fig. 2B) corresponding to the mutated allele, and a band of 12.8 kb from the normal allele, confirming deletion of exon 6–9 of the catalytic domain. Germ-line male chimeras were obtained from two independent cell lines (clones 42 and 73). Heterozygous animals do not show any phenotype, breed normally and have normal lifespan. Crossing PTP-PEST +/− animals revealed an absence of homozygous offspring in all litters (Table 1), and an expected 2:1 ratio of heterozygous: wild-type mice. Therefore, loss of PTP-PEST results in embryonic lethality.
Fig. 2.
Generation of PTP-PEST−/− mice. (A) Targeting strategy of PTP-PEST locus. A left arm fragment of genomic DNA encoding exons 4 and 5 of PTP-PEST was ligated to a neo cassette (in a reverse orientation in respect to the PTP-PEST sequence), which deletes exons 6–9 of the catalytic domain. A right arm encoding exons 10, 11 and 12 was ligated at the 5′ of the neo cassette. The linearized vector was used to electroporate 129 s/vJ ES cells. (B) Southern blot analysis of different targeted ES cell clones obtained following electroporation of the targeting vector and selection with G418. The numbers refer to the cell clones isolated. Two independent heterozygote cell lines (clones 42 et 73) were used to generate independent mice strains. (C) Western blot analysis of PTP-PEST protein expression in PTP-PEST+/+, PTP-PEST+/− and PTP-PEST−/− E8.5 embryos. The protein was identified with the rabbit polyclonal anti-PTP-PEST antibody clone 2530, which recognizes a 120 kDa band. (D) RT-PCR showing the absence of PTP-PEST exon 8 mRNA in PTP-PEST−/− E9.5 embryos. Equivalent use of cDNA was confirmed with the analysis of β-actin mRNA transcription. Three different controls were tested (C1, negative control with no cDNA sample, C2, negative control with cDNA from 5 μg tRNA as template in reverse transcription, C3, positive control with cDNA synthesized from 1 μg of PMEF RNA).
Table 1.
Distribution of genotypes from conceptus and pups following PTP-PEST +/− matings of 129 s/v:Balb/c background mice
| Stage | Total conceptuses | +/+ | +/− | −/− | Resorbed embryos |
|---|---|---|---|---|---|
| E 6.5 | 11 | 2 | 7 | 2 | 0 |
| E 7.5 | 19 | 7 | 10 | 2 | 0 |
| E 8.5 | 99 | 27 | 46 | 22 | 4 |
| E 9.5 | 263 | 63 | 131 | 52a | 16 |
| E 10.5 | 81 | 18 | 42 | 5a | 16 |
| E 11.5 | 30 | 7 | 19 | 4a | 0 |
| E 12.5 | 4 | 0 | 4 | 0 | 4 |
| 21-day-old mice | 78 (33%) | 159 (67%) | 0 |
Embryos with abnormal appearance.
To determine at which stage embryonic death occurs, we analyzed E6.5–E12.5 conceptuses produced by heterozygote matings (Table 1). PCR analysis of dissected embryos showed that expected ratios are obtained until E9.5. After this stage, ratio of PTP-PEST−/− embryos decreased, demonstrating that embryonic lethality occurs around E9.5 and E10.5. Absence of PTP-PEST protein was confirmed by Western blot (Fig. 2C) using a polyclonal PTP-PEST antibody (clone 2530) and a monoclonal actin antibody (clone AC40) as loading control; both PTP-PEST+/+ and PTP-PEST +/− E9.5 embryos had a specific band at 120 kDa in proportion to allele number, while PTP-PEST−/− did not express the protein, even when twice the amount of protein extract from mutant embryos was loaded. To verify that the gene itself was inactivated, we performed reverse-transcriptase (RT)-PCR on PTP-PEST+/+, PTP-PEST+/− and PTP-PEST−/− E9.5 embryos cDNA. The 143-bp PCR product corresponding to PTP-PEST mRNA (exon 8) was present in both PTP-PEST+/+ and PTP-PEST+/− but was absent in PTP-PEST−/− embryos (Fig. 2D). Equal amounts of cDNA were used in the PCR reaction, which was confirmed with an equivalent expression of β-actin mRNA (219-bp) in all samples.
2.3. Specific tyrosine-hyperphosphorylated proteins in PTP-PEST −/− embryos
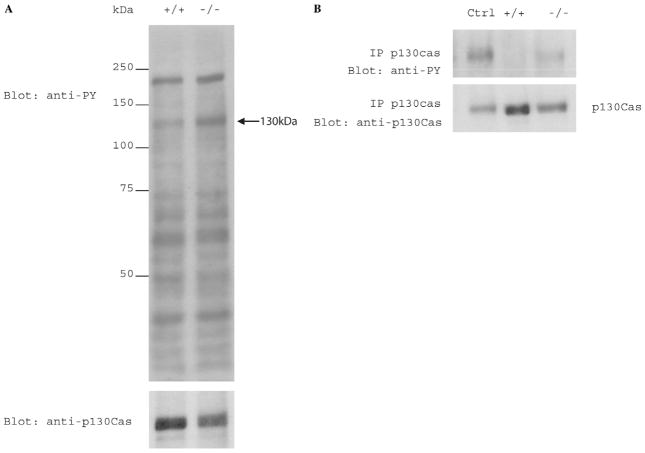
To investigate the physiological impact of PTP-PEST ablation in the embryo, we investigated the tyrosine phosphorylation status of proteins in total extracts from PTP-PEST −/− and wild-type embryos. Our results show that, in E9.5 PTP-PEST−/− embryos, at least one particular protein has increased in tyrosine phosphorylation (Fig. 3A). This protein runs at 130 kDa and is most probably p130cas. To verify the identity of this highly phosphorylated protein, we performed an immunoprecipitation experiment to specifically isolate p130cas from 30 μg of PTP-PEST+/+ and PTP-PEST−/− embryo lysates, using a monoclonal p130cas antibody covalently coupled to rProtein G-Agarose beads. The same amount of protein from primary mouse embryonic fibroblasts (PMEF) lysate was used as a positive control. Immunoprecipitated proteins were then subjected to SDS–PAGE, followed by immunoblotting with a monoclonal antiphosphotyrosine antibody (4G10) and subsequently with a monoclonal p130cas antibody. Our results confirm that p130cas protein is hyperphosphorylated in PTP-PEST−/− embryos (Fig. 3B) compared to wild-type. No p130cas phosphorylation was detected in normal embryos immunoprecipitates, while blotting with anti-p130cas shows that the protein has been well immunoprecipitated in both PTP-PEST+/+ and PTP-PEST−/− embryo lysates.
Fig. 3.
(A) Phosphotyrosine profile of PTP-PEST embryos. E9.5 PTP-PEST+/+ and PTP-PEST−/− embryos were lysed in cold buffer composed of 50 mM Tris pH7.5, 150 mM NaCl, 1% NP-40, 1× Protease Inhibitor, 1 mM Na2VO3 and 50 mM NaF. Equal amounts of total proteins were blotted with the phosphotyrosine specific antibody 4G10. Note the presence of highly tyrosine phosphorylated 130 kDa proteins. (B) Immunoprecipitation of p130cas proteins from embryonic lysates with monoclonal anti-p130cas antibody covalently coupled with rProtein G-Agarose beads. Tyrosine phosphorylation was assessed with the phosphotyrosine specific antibody 4G10. Membrane was blotted subsequently with anti-p130cas antibody.
2.4. Morphological defects of PTP-PEST−/− embryos
To verify that absence of PTP-PEST leads to embryonic defects, we investigated the integrity of embryos at E9.5–10.0, the time point where macroscopic abnormalities were the most striking (Fig. 4A). Observation of E9.5 PTP-PEST −/− embryos identified visible macroscopic defects, such as general growth retardation, caudal development arrest, failure to turn and a smaller cardiac region compared to wild-type embryos. Most PTP-PEST−/− embryos also show a severely kinked neural tube (Fig. 4B), with disorganized somites. To further investigate the possible onset of these abnormalities, histological sections of PTP-PEST −/− embryos from E7.5 to E10.5 were analyzed (Fig. 4C). At 7.5 days post fertilization, all embryos appeared morphologically intact (Fig. 4C-1 and 2). Gastrulation occurred normally, showing all three embryonic germ layers. An extensive maternal vasculature was established around the embryonic trophoblast giant cell layer. Similarly, the yolk sac formed normally, with the obvious presence of both extra-embryonic visceral endoderm and mesoderm. At E9.5 (Fig. 4C-3), we could observe degeneration of tissues with abundance of detached pycnotic cells compared to the wild-type counterpart (Fig. 4C-4). These detached cells could be seen clearly filling the neural canal. By E10.5 (Fig. 4C-5), PTP-PEST−/− embryos were deformed with a receding caudal region. We also observed that somites formed only in the cranial region. The caudal neural tube had degenerated, and somites were disorganized and had degenerated, compared to wild-type (Fig. 4C-6). Degeneration of cells could also be noted in the septum transversum, with no hepatic epithelial cell development. At this stage of development, initiation of a liver diverticulum in the septum transversum should be observed, as seen in the wild-type counterpart.
Fig. 4.
(A) Morphological defects of PTP-PEST null E9.5 embryos compared to wild-type. The mutant failed to turn and had severe developmental defects in the heart region, arrested in caudal development and general growth retardation. (B) E9.5 PTP-PEST−/− embryos had kinked neural tubes and disorganized somites. The wild-type control is at E8.5 stage, since the caudal development is delayed in null embryos. (C) Histological analysis of PTP-PEST null embryos. PTP-PEST−/− and PTP-PEST+/+ embryos were dissected from heterozygous mating. Conceptuses were embedded, sectioned at 6 μm and stained with haematoxylin/eosin. (1) E7.5 null conceptus showing a normal gastrulation with the three germ layers. (2) E7.5 wild-type conceptus. (3) Section through the caudal region of an E9.5 PTP-PEST−/− embryo. The neural canal (NC) was filled with detached pycnotic cells, showed by the arrows. Similar detached cells could be identified in the adjacent somites. (4) Section through the caudal region of an E9.5 PTP-PEST+/+ embryo. (5) Section through an E10.5 mutant embryo showing degenerating mesenchyme of septum transversum with no hepatic epithelial cell development. (6) Section through an E10.5 wild-type embryo showing normal liver development in septum transversum. Note also the well-organized somite formation, normal splanchnopleure (DM) of the hindgut (G) and normal highly cellular neural tube (NT). DA (dorsal aorta); HG (hindgut); N (notochord).
2.5. Reduced number of endothelial cells in PTP-PEST−/− yolk sac and aorta
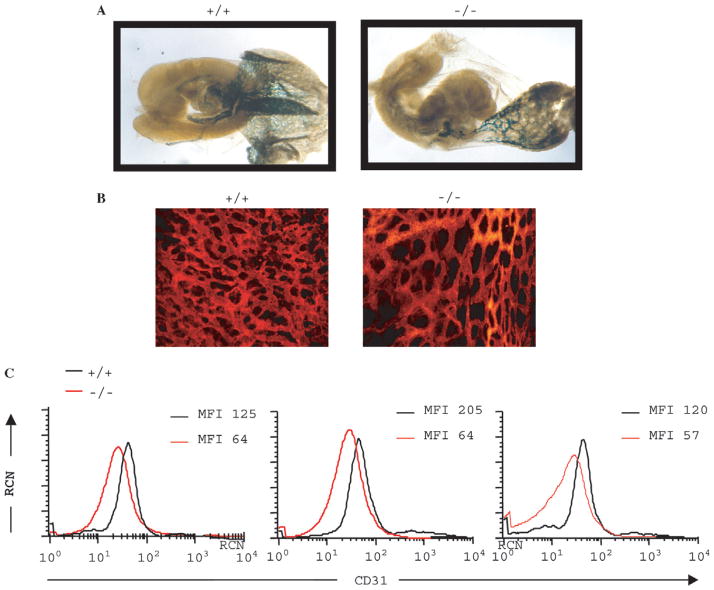
To investigate the possibility of a vascularization defect, we studied the pattern of expression of a specific endothelial cell promoter in a model combining PTP-PEST knockout and tek-lacZ transgene expression. The transgene, containing the lacZ reporter gene under the control of the endothelial specific tek promoter, was introduced by breeding tek-lacZ transgenic mice with PTP-PEST+/− mice. The embryos obtained from the mating were examined for endothelial expression. E9.5 PTP-PEST−/− transgenic embryos (Fig. 5A) were stained with X-Gal and had a decreased number of cells expressing lacZ in the yolk sac and aorta, compared with the wild-type embryos. This indicates that the homozygous embryos vascularization was impaired since fewer endothelial cells were present. In order to further characterize the capillary network of the yolk sac, endothelial cells were identified with R-phycoerytrin (R-PE)-conjugated anti-mouse CD31 (PECAM-1) monoclonal antibody (Fig. 5B). The organization of the endothelial cells in the yolk sac network was impaired in null embryos, showing less branching of vessels, which also were of larger calibre compared to vessels from wild-type embryos. The reduced number of endothelial cells in the yolk sac observed with CD31 staining was also confirmed by flow cytometry experiments (Fig. 5C). Following incubation of a yolk sac cell suspension with CD31 antibody and subsequent FACS analysis, we observed a decrease in endothelial cell number compared to other cell types. Three different comparisons between PTP-PEST+/+ and PTP-PEST−/− E10.0 yolk sacs had, respectively, a 49%, 69% and 53% decrease in endothelial cells mean fluorescence intensity (MFI). Other cell types expressing Ter119, CD11b or CD45 markers, such as erythrocytes and erythrocyte precursors, monocytes, macrophages and lymphocytes, were not altered (data not shown).
Fig. 5.
Characterization of the vascular system in PTP-PEST−/− embryos. (A) We introduced a LacZ reporter under the control of the vascular-specific Tek promoter (Tek-LacZ transgene) in PTP-PEST+/− mice. E9.5 PTP-PEST+/+ and PTP-PEST−/− embryos were stained with X-Gal to detect LacZ expression. Note the reduced LacZ staining in the null embryo. The yolk sac of PTP-PEST−/− embryos also contained fewer endothelial cells. (B) Whole-mount staining of E10.0 PTP-PEST+/+ and PTP-PEST−/− yolk sacs with R-PE-CD31 antibody. An abnormal organization of the network was observed. The magnification was 20×. (C) FACS plots showing decreased endothelial cell number in three different PTP-PEST−/− yolk sacs compared to PTP-PEST +/+, using R-PE-CD31 antibody as marker. RCN, Relative cell number; MFI, Mean fluorescence intensity.
2.6. Absence of hepatic albumin mRNA in PTP-PEST−/− embryos
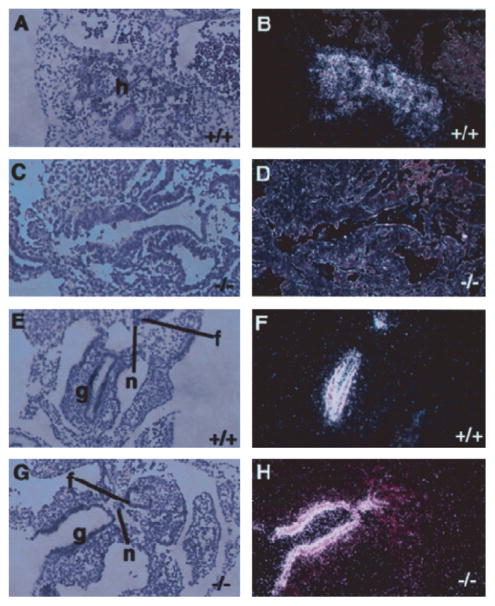
Since liver formation was also found to be defective by histological analysis, we wanted to determine if the foregut endoderm was capable of committing to a hepatic developmental program in the absence of PTP-PEST. We therefore performed in situ hybridization analyses to detect specific hepatic mRNAs. We first used a marker expressed at the onset of hepatocyte differentiation, albumin mRNA (Cascio and Zaret, 1991). The results revealed no expression of albumin mRNA from the hepatocytes of homozygous embryos at E9.5 (Fig. 6D), while the expression was detected in wild-type embryos (Fig. 6B). To verify the integrity of the other endoderm-derived cell types, we also analysed the expression of HNF3α, which is normally expressed in the developing liver, gut, notochord and floor plate of the neural tube (Ang et al., 1993; Monaghan et al., 1993; Sasaki and Hogan, 1993). HNF3α mRNA was clearly detected in the gut, the notochord and the floor plate of the neural tube (Fig. 6H). The same results were observed at E10.5 (data not shown), a stage at which the liver is one of the most prominent organs.
Fig. 6.
Expression of hepatic albumin and HNF3α mRNAs in PTP-PEST null embryos. In situ hybridization using antisense 33P-labelled RNA probes specific for albumin (A–D) or HNF3α (E–H) were performed on transverse sections through PTP-PEST+/+ (A, B, E, F) and PTP-PEST−/− E9.5 (C, D, G, H) embryos. A, C, E and G show bright field images of haematoxylin and eosin stained sections and the corresponding dark field images are shown in B, D, F and H. (A and B) Expression of albumin mRNA by hepatocytes of PTP-PEST+/+ embryos. (C and D) There is no expression of albumin mRNA in PTP-PEST−/− embryos. (E, F, G, H) Gut, floor plate of neural tube and notochord all stained positive for HNF3α marker, showing the tissue integrity. f; floor plate of neural tube; g, gut; h, developing hepatocytes; n, notochord. The magnification was 10×.
3. Discussion
We report that ablation of PTP-PEST function in the mouse results in embryonic lethality between E9.5 and E10.5, and reveal its important role for development. Even if earlier gastrulation requires proper cell proliferation and migration, lack of PTP-PEST does not seem to cause major defects before E9.5.
3.1. Ablation of PTP-PEST induces biochemical and morphological defects
In situ hybridization experiments (Fig. 1) show that PTP-PEST gene is expressed normally during early embryonic development. As soon as E8.0 (Fig. 1A), the RNA is ubiquitously expressed, and its expression is sustained during later stages (Fig. 1B–F). Presence of the RNA is also extended to extra-embryonic structures such as the yolk sac, trophoblast, amnion, allantois and chorion. Our results show that PTP-PEST is expressed throughout the embryo, which suggests that its ablation is likely to produce biochemical and morphological defects. Since lethality occurs around E9.5–10.5 (Table 1), PTP-PEST is not essential for early steps of development such as implantation, gastrulation, embryo and yolk sac formation. Other tyrosine phosphates as Shp2 (Saxton and Pawson, 1999) must be sufficient when initiation of these processes occurs. As shown in Fig. 3, the first evident impact of the lack of PTP-PEST in mouse embryo is the modulation in tyrosine phosphorylation status of p130cas. This substrate, and probably other substrates and binding partners of PTP-PEST, are hyperphosphorylated on tyrosine residues following PTP-PEST gene ablation. This supports our previous findings where fibroblasts isolated from null embryos had increased phosphorylation of p130cas, paxillin, FAK and PSTPIP proteins (Angers-Loustau et al., 1999), with increased cell spreading and impaired motility. Therefore, the normal in vivo expression of PTP-PEST observed in E9.5 mesenchyme, which is the precursor for embryonic fibroblasts, combined with its observed tyrosine phosphatase activity on p130cas in total embryos, suggests that the enzyme might also have an equivalent role in cell migration and adhesion in the embryo itself.
In parallel with abnormal phosphotyrosine activity in the embryo, morphological defects appear at E9.5, and are mainly characterized by degenerating cells near the somites and hindgut cavity, and in the neural tube. Therefore, there is a cellular defect in PTP-PEST−/− embryos, which does not allow cells to grow properly. Since PTP-PEST RNA is also expressed in mesenchymes as in endoderm, hepatic primordium, limb buds and somites, it is likely that degenerating cells would be present in all these tissues. This cell defect could be due to apoptosis of cells lacking proper contact with neighbouring cells or to improper mitosis, as observed in PTP-PEST−/− fibroblasts, which were shown to have hyperphosphorylation of PSTPIP associated with mitotic defects. Indeed, we found a large number of cells still attached at the cleavage furrow during the cytokinesis process (Angers-Loustau et al., 1999). Since PTP-PEST was shown to bind PSTPIP while it dephosphorylates WASP (Côté et al., 2002), a cytoskeletal protein localized at the cleavage furrow, absence of the protein could explain the observed hyperchromatic cells in null embryos. The cells might accumulate mitotic defects throughout development, explaining in part the extensive tissue degeneration observed and the lack of endothelial cells and liver precursor cells.
3.2. PTP-PEST is essential for proper yolk sac vascularization
The observed decrease in endothelial cell number in E9.5–10.0 null embryos is likely to induce defects in yolk sac vascularization and to impair heart function, leading to lethality at this stage. Since embryonic vascularization is essential for early steps of development, we wanted to verify if a vascular defect could also have contributed to the lethal phenotype. In fact, defects in vascular networks have been reported for other signalling gene disruptions involved in integrin cascade such as p130CAS, a PTP-PEST substrate (Honda et al., 1998). In mice lacking fibronectin, defects in mesoderm, neural tube and vascular development are observed at E8.0 (George et al., 1993). Vinculin knockout leads to heart and brain defects at the same time point (Xu et al., 1998). The targeted ablations of many integrin subunits also have similar effects, for example knockout of the α5 gene leads to lethality around E9.5–10.0, probably due to mesodermal (Yang et al., 1993) and vascular defects (Lin Goh et al., 1997). Therefore, our observations on PTP-PEST−/− embryos support a vascular deficiency, showing paucity of yolk sac endothelium and fewer, enlarged vessels.
Yolk sac formation starts at E6.5 during gastrulation, when mesodermal cells from the early posterior primitive streak migrate into the proximal region of the yolk sac (Lawson et al., 1991). To allow proper blood circulation, vessels within the yolk sac must proliferate and regress, undergo branching and migration, as well as being specified as veins or arteries, vessels or capillaries. They must also recruit supporting cells, like smooth muscle cells and pericytes, which are necessary to achieve a stable vascular network (Rossant and Howard, 2002). Such processes may be aberrant in PTP-PEST−/− embryos. Our flow cytometry results confirm that there is an important decrease in the number of endothelial cells in yolk sac of null embryos. Endothelial cells derived from primitive progenitors (hemangioblats) arise around E8.0 in the vascular plexus prior to blood island formation (Palis et al., 1995). Their proliferation and migration are essential to vascular development, in order to form capillaries as well as large vessels (Risau, 1997). Lack of this cell lineage could reflect a defect in progenitor cell differentiation, endothelial cell proliferation or survival. Therefore, our data support the hypothesis of a defect in vascular development, which could be explained by developmental arrest related to PTP-PEST absence.
3.3. PTP-PEST−/− embryos do not form a fetal liver
At E10.5, the hepatic endoderm normally delaminates from the foregut and the cells invade the surrounding septum transversum mesenchyme. Since the liver expresses high levels of PTP-PEST (Davidson et al., 1997), the lack of this phosphatase might have a major impact on the proper development and function of the liver. As a site of hematopoiesis in the embryo, absence of a fetal liver alone could explain the eventual embryonic lethality (Zaret, 2000). Moreover, an endothelial defect could also induce improper liver formation, since vascularization is also essential for its organogenesis (Matsumoto et al., 2001). Our data shows that there is no liver formation in PTP-PEST −/− embryos as well as no precursor cells expressing albumin. It is known that albumin is expressed in the endoderm in response to fibroblast growth factor signals from the adjacent cardiac mesoderm. Since contact of this tissue with the ventral foregut endoderm is necessary to induce hepatic specification, PTP-PEST could have a role in allowing cell interactions required for liver development. Because liver vasculature is also necessary prior to hematopoietic function, it is possible that a general defect in embryonic blood vessel formation would cause the observed lethality.
In summary, we show here that the gene-targeted ablation of PTP-PEST is lethal during embryonic development, probably due to primary defects in proliferation and migration during mesenchymal formation and histogenesis involving vascular or hematopoietic tissues. The lack of the protein results in developmental arrest more evident at E9.5. We observed impaired embryo turning, arrest in caudal development, neural tube and somites defects, growth retardation, vascular network abnormalities and the absence of a liver. Reduced numbers of endothelial cells and depletion of mesenchyme in the yolk sac, combined with the observed differences in network complexity, suggests that PTP-PEST could be involved in the process of angiogenesis of the embryo. Through its association with Grb2, PTP-PEST could be linked to the angiopoietin/Tie2 receptor signalling pathway (Jones and Dumont, 2000), which is essential for vascular remodelling (Rossant and Howard, 2002). Part of the integrin complex, PTP-PEST could also be essential for vessel formation, as are many endothelial integrins (Bouvard et al., 2001), particularly the α5 subunit (Yang et al., 1993). Similar embryonic lethality has been reported following targeted disruption of extracellular matrix genes, such as fibronectin, for which death occurs at E10.0 with multiple defects in morphogenesis (George et al., 1993; Georges-Labouesse et al., 1996). Vinculin disruption also causes embryonic lethality around E9.5, probably due to cardiac malformation (Xu et al., 1998). Defects in cell migration or adhesion to the extracellular matrix due to a lack of PTP-PEST could be the cause of embryonic lethality at this developmental stage, most probably due to an abnormal vascular system. Since the impact of the deletion of PTP-PEST results in an early lethality with major defects, a cre-lox study will help us define more precisely the different roles of this phosphatase in a variety of cells and tissues during development and in the adult.
4. Experimental procedures
4.1. Construction of the targeting vector
A PTP-PEST genomic clone was isolated from a lambda DASH II phage library of a 129 s/vJ mouse. The 17 kb targeting vector (Fig. 2A) was composed of exons 4 and 5 in its left arm, and of exons 10, 11 and 12 in the right arm. The PGK-neo cassette was inserted in between, in a reverse orientation, so that it eliminates exons 6 to 9, which code for the catalytic domain. Vector electroporation was done with 2 × 107 129 s/vJ cells (J1) and 50 μg of linearized vector, followed by selection of the integration event with G418 during 10 days. Targeted cells were identified by Southern blot analysis (Fig. 2B) following a HindIII digestion of ES cells DNA in 96 well culture dishes (Hogan et al., 1994). The probe, that was designed to recognize homologous recombination events, was a KpnI-SacI fragment of 700 bp outside the 3′ short arm of the targeting vector. Other internal probes such as the neo sequence were used to confirm the results, (data not shown). Heterozygous cells were then injected in E3.5 Balb/c blastocysts to give rise to chimeric animals. Germline transmission analysis was done by backcrossing these chimeras with Balb/c mice.
4.2. Genotyping of progeny and embryonic tissues
Progeny genotype was analyzed by Southern blot or PCR analysis, from mice tail tip biopsies done at weaning. The probe used is a 700 bp cDNA fragment of PTP-PEST cloned in pBluescript, digested with KpnI and SacI. Genotyping of embryos from E7.5 to E12.5 was done by PCR analysis from yolk sac or embryonic derived tissues. The mid-day of mouse plug is defined as E0.5. The tissues were first resuspended in 50 mM NaOH, followed by incubation at 95 °C during 15 min. Then, 0.08M Tris pH8.0 was added to the digested tissue, from which an aliquot was directly used in PCR reaction. PCR was performed using three primers: primer PEST1 5′-ATT CTG GAG CCA TGA GGA AGA-3′; primer PEST2 5′-CAC CAC CTC ACA GCA TAA ATG-3′; primer neo 5′-GAA TTC GCC AAT GAC AAG ACG-3′. The reaction was carried out first at 95 °C for 3 min of denaturation, followed by 35 cycles of 1 min at 95 °C, 1 min at 51 °C and 2 min at 72 °C, with a final single extension step of 5 min at 72 °C. Reactions were performed with 400 μM dNTP, 2mM magnesium chloride (MgCl2), 0.5 U DFS-Taq DNA polymerase (Bioron GmbH, Ludwigshafen, Germany), 0.5 μM of primers PEST1 and PEST2, and 0.25 μM of primer neo. The resulting band corresponding to wild-type allele was 500 bp, while the mutated allele one was 250 bp.
4.3. Histological analysis and in situ hybridization
Sections of normal embryos at different developmental stages (E8.0, E9.0, E10.0, E11.0 and E12.0) were obtained from Novagen (EMD Biosciences, Inc, Darmstadt, Germany). These sections were subjected to in situ hybridization (Wilkinson, 1992) using either a sense or antisense 33P-labeled RNA probe specific for the catalytic domain of PTP-PEST. Analyses of mutant embryos were done following breeding of PTP-PEST+/− mice, from E7.5 to E10.5 conceptuses. Deciduas were fixed in 10% buffered formalin phosphate (Fisher Scientific, Ottawa, Ontario, Canada), dehydrated and embedded, sectioned at 6 μm, and then stained with haematoxylin and eosin. Analysis of albumin and HNF3α (Hepatic nuclear factor 3α) mRNA expression in E9.5 PTP-PEST−/− and wild-type embryos was done by in situ hybridization, using antisense 33P-labeled probes specific for both proteins. Embryos were collected, fixed overnight with 4% paraformaldehyde in phosphate-buffered saline (PBS), dehydrated and embedded in paraffin. Sections were done at 5 μm, mounted and processed by in situ hybridization with 33P-labeled probes according to Wilkinson et al. (1987). Tissues were then rinsed at high stringency, counterstained with eosin and hematoxylin solution, mounted with Permount (Fisher, SP15-500 Toluene solution) and autoradiographed. The dark field image, obtained from a condenser, allowed the observation of the silver grains specific to albumin and HNF3α.
4.4. Protein analysis, RT-PCR and immunoprecipitation
PTP-PEST proteins from pooled E8.5 embryos were identified with the rabbit polyclonal anti-PTP-PEST antibody clone 2530. Loading consistency was ascertained with the monoclonal anti-actin antibody, clone AC-40 (Sigma-Aldrich, Saint-Louis, Missouri USA). Embryos were collected, directly lysed in SDS sample buffer (62.5 mM Tris–HCl pH6.8, 20% glycerol, 2% SDS, 5% β-mercaptoethanol, 0,025% bromophenol blue), incubated 5 min at 95 °C, centrifuged 2 min at 13,000 rpm and subjected to SDS–polyacrylamide gel electrophoresis (PAGE) on 10% acrylamide resolving gel and 5% stacking gel (Laemmli, 1970).
For RT-PCR, RNA was extracted from E9.5 embryos using TRIzol Reagent (GIBCO BRL, Missisauga, Ontario, Canada). Synthesis of complete cDNA was performed using 200 units of Superscript II (GIBCO BRL, Missisauga, Ontario, Canada) and Oligo (dT) 12–18 as template primer. Amplification of PTP-PEST was done with PTP-PEST EX8S 5′-GAATCCCGTCGGCTCTATCAGTTTC-3′ and EX8AS 5′-GGCACATCTTCATGTTCTTGG-3′ primers. The reaction was carried out first at 95 °C for 5 min of denaturation, followed by 35 cycles of 30 s at 95 °C, 45 s at 60 °C and 45 s at 72 °C, with a final single extension step of 5 min at 72 °C. Reactions were performed with 400 μMdNTP, 5 mMMgCl2, 0.5 U DFS-Taq DNA polymerase (Bioron GmbH, Ludwigshafen, Germany) and 0.5 μM of primers. The resulting band is a 143 bp fragment. Amplification of β-actin was done with 5′-β-actin 5′-AAAGACCTGTACGCCAACACAGTC-3′ and 3′-β-actin 5′-GTCATACTCCTGCTTGCTGATCCA-3′ primers. The reaction was carried out first at 95 °C for 5 min of denaturation, followed by 35 cycles of 1 min at 95 °C, 1 min at 59 °C and 1 min at 72 °C, with a final single extension step of 5 min at 72 °C. The reactions were performed with 400 μM dNTP, 4 mM MgCl2, 0.5 units of DFS-Taq DNA polymerase and 0.5 μM of primers. The resulting band was a 219 bp fragment.
The immunoprecipitation experiment was done using E9.5 embryos lysates, with primary mouse embryonic fibroblasts (PMEF) lysate as control. Embryos were collected in cold PBS and quickly frozen in liquid nitrogen. Two wild-type and four null embryos were pooled and lysed in 100 μl cold lysis buffer (50 mM Tris pH7.5, 150 mM NaCl, 1% NP-40, 1× Protease Inhibitor, 1 mM Na2VO3 and 50 mM NaF) by incubation with rotation for 10 min at 4 °C. A cleared lysate was obtained following centrifugation at 13,000 rpm for 10 min at 4 °C. Part of the cleared lysate was kept for BCA protein quantification (Pierce, Rockford Illinois, USA) and total cell lysate was analysed by SDS–PAGE. A lysate volume of 400 μl was subjected to immunoprecipitation with 30 μl of rProtein G-Agarose beads (Invitrogen, Carlsbad, California, USA) (pre-washed in lysis buffer) and 750 μg of covalently coupled monoclonal p130cas antibody (BD Transduction Laboratories, Mississauga, Ontario, Canada). After an incubation of 2 h at 4 °C, beads were sedimented and washed four times in cold lysis buffer. Immunoprecipitates were obtained by boiling samples for 2 min in 40 μl SDS-Sample Buffer. After a quick centrifugation in order to pellet beads, immunoprecipitates were analyzed by SDS–PAGE on 8% acrylamide resolving gel and 5% stacking gel (Laemmli, 1970). Equal amounts of total cell lysate proteins (2,5 μg) and immunoprecipitated proteins (10 μg) were subjected to Western blot. Tyrosine phosphorylation study was performed with antiphosphotyrosine monoclonal antibody clone 4G10 (Upstate, Lake Placid, New York, USA) and subsequently with a monoclonal p130cas antibody.
4.5. LacZ Staining of PTP-PEST−/− and wild-type embryos
Tek-LacZ transgene was introduced in PTP-PEST+/− mice by crossing them with a transgenic line expressing lacZ reporter gene under the transcriptional control of endothelial-specific tek promoter (Dumont et al., 1994) (The transgenic line was a kind gift from Dr Dan Dumont, Sunnybrook Hospital, Toronto, Ontario, Canada). Embryos resulting from the PTP-PEST+/− mating, also carrying the Tek-LacZ transgene, were fixed in glutaraldehyde and stained for LacZ activity by an overnight incubation with 5-bromo-4-chloro-3 indolyl β-D-galactopyranoside (X-Gal). Following post-fixation in 4% paraformaldehyde-PBS, embryos were photographed.
4.6. Yolk sac whole-mount CD31 immunostaining and flow cytometry analysis
To characterize the endothelial capillary network, E10.0 embryos yolk sacs were dissected at 4 °C in PBS and fixed in 4% paraformaldehyde-PBS. Non-specific proteins were blocked during 30 min at 4 °C with 3% milk, 0.025% Triton X-100 in PBS (PMT solution). Embryos were then incubated with R-phycoerythrin (PE)-conjugated rat anti-mouse CD31 (PECAM-1) monoclonal antibody (BD Biosciences, Mississauga, Ontario, Canada) in PMT solution during 2 h at room temperature, washed with PBS-Tween 0.1% at room temperature, mounted on slides and photographed with an inverted microscope.
Quantification of endothelial cells in the yolk sac was performed by flow cytometry analysis using E10.0 PTP-PEST+/+ PTP-PEST+/− and PTP-PEST−/− embryos. Yolk sacs were dissected in cold PBS and incubated in DMEM (with penicillin/streptomycin) (GIBCO) containing 0.1% collagenase and 20% fetal bovine serum (FBS) (Hyclone, Logan, Utah, USA) at 37 °C during 30 min. A single cell suspension was obtained by passing digested cells twice through a 23G needle and twice through a 30G needle. Cells were then counted, resuspended in FACS buffer (PBS, 2% FBS), and non-specific sites were blocked by an incubation of 5 min on ice with mouse Fc Block (CD16/CD32). Yolk sac cells were then labelled with two different combinations of fluorochrome-conjugated specific antibodies (BD Biosciences, Mississauga, Ontario, Canada). They were incubated for 30 min on ice with a combination of fluorescein isothiocyanate (FITC)-CD45 conjugated and R-PE-CD31 conjugated antibodies, and with a combination of FITC-CD45 conjugated, R-PE-Ter119 conjugated and allophycocyanin (APC)-CD11b conjugated antibodies. Data was obtained with a FACS Calibur machine (Becton Dickinson) and analyzed using CELLQUEST software.
Acknowledgments
The authors thank Dan Dumont for the transgenic TeklacZ mouse line, Jennifer M. Rossi for embryo dissections, and Ken Zaret for helpful comments. The authors also thank Vincent Giguere, Anna N. Moraitis and Jo-Ann Bader for help with in situ experiments using 33P-labelled probes, and Melanie Chagnon for help with immunoprecipitations. Jean-François Côté is a fellow of the National Cancer Institute of Canada, Annie Bourdeau is a fellow of the Lymphoma Research Foundation and Michel L. Tremblay is a scientist of the Canadian Institute for Health research and a recipient of the Jeanne and Jean-Louis Lévesque Chair in Cancer Research. This work was supported by an operating grant to MLT (Grant #MT12466) from the Canadian Institute of Health and Research.
References
- Ang SL, Wierda A, Wong D, Stevens KA, Cascio S, Rossant J, Zaret KS. The formation and maintenance of the definitive endoderm lineage in the mouse: involvement of HNF3/forkhead proteins. Development. 1993;119:1301–1315. doi: 10.1242/dev.119.4.1301. [DOI] [PubMed] [Google Scholar]
- Angers-Loustau A, Côté JF, Charest A, Dowbenko D, Spencer S, Lasky LA, Tremblay ML. Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. J Cell Biol. 1999;144:1019–1031. doi: 10.1083/jcb.144.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard D, Brakebusch C, Gustafsson E, Aszodi A, Bengtsson T, Berna A, Fassler R. Functional consequences of integrin gene mutations in mice. Circ Res. 2001;89:211–223. doi: 10.1161/hh1501.094874. [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Cascio S, Zaret KS. Hepatocyte differentiation initiates during endodermal-mesenchymal interactions prior to liver formation. Development. 1991;113:217–225. doi: 10.1242/dev.113.1.217. [DOI] [PubMed] [Google Scholar]
- Charest A, Wagner J, Muise ES, Heng HH, Tremblay ML. Structure of the murineMPTP-PEST gene: genomic organization and chromosomal mapping. Genomics. 1995;28:501–507. doi: 10.1006/geno.1995.1181. [DOI] [PubMed] [Google Scholar]
- Charest A, Wagner J, Jacobs S, McGlade CJ, Tremblay ML. Phosphotyrosine-independent binding of SHC to the NPLH sequence of murine protein-tyrosine phosphatase-PEST. Evidence for extended phosphotyrosine binding/phosphotyrosine interaction domain recognition specificity. J Biol Chem. 1996;271:8424–8429. doi: 10.1074/jbc.271.14.8424. [DOI] [PubMed] [Google Scholar]
- Charest A, Wagner J, Kwan M, Tremblay ML. Coupling of the murine protein tyrosine phosphatase-PEST to the epidermal growth factor (EGF) receptor through a Src homology 3 (SH3) domain-mediated association with Grb2. Oncogene. 1997;14:1643–1651. doi: 10.1038/sj.onc.1201008. [DOI] [PubMed] [Google Scholar]
- Cheng J, Daimaru L, Fennie C, Lasky LA. A novel protein tyrosine phosphatase expressed in lin(lo)CD34(hi)Sca(hi) hematopoietic progenitor cells. Blood. 1996;88:1156–1167. [PubMed] [Google Scholar]
- Cong F, Spencer S, Côté JF, Wu Y, Tremblay ML, Lasky LA, Goff SP. Cytoskeletal protein PSTPIP1 directs the PEST-type protein tyrosine phosphatase to the c-Abl kinase to mediate Abl dephosphorylation. Mol Cell. 2000;6:1413–1423. doi: 10.1016/s1097-2765(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Côté JF, Charest A, Wagner J, Tremblay ML. Combination of gene targeting and substrate trapping to identify substrates of protein tyrosine phosphatases using PTP-PEST as a model. Biochemistry. 1998;37:13128–13137. doi: 10.1021/bi981259l. [DOI] [PubMed] [Google Scholar]
- Côté JF, Turner CE, Tremblay ML. Intact LIM3 and LIM4 domains of paxillin are required for the association to a novel polyproline region (Pro2) of protein-tyrosine phosphatase-PEST. J Biol Chem. 1999;274:20550–20560. doi: 10.1074/jbc.274.29.20550. [DOI] [PubMed] [Google Scholar]
- Côté JF, Chung PL, Théberge JF, Hallé M, Spencer S, Lasky LA, Tremblay ML. PSTPIP is a substrate of PTP-PEST and serves as a scaffold guiding PTP-PEST toward a specific dephosphorylation of WASP. J Biol Chem. 2002;277:2973–2986. doi: 10.1074/jbc.M106428200. [DOI] [PubMed] [Google Scholar]
- Davidson D, Cloutier JF, Gregorieff A, Veillette A. Inhibitory tyrosine protein kinase p50csk is associated with protein-tyrosine phosphatase PTP-PEST in hemopoietic and non-hemopoietic cells. J Biol Chem. 1997;272:23455–23462. doi: 10.1074/jbc.272.37.23455. [DOI] [PubMed] [Google Scholar]
- Davidson D, Veillette A. PTP-PEST, a scaffold protein tyrosine phosphatase, negatively regulates lymphocyte activation by targeting a unique set of substrates. EMBO J. 2001;20:3414–3426. doi: 10.1093/emboj/20.13.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- Garton AJ, Burnham MR, Bouton AH, Tonks NK. Association of PTP-PEST with the SH3 domain of p130cas; a novel mechanism of protein tyrosine phosphatase substrate recognition. Oncogene. 1997;15:877–885. doi: 10.1038/sj.onc.1201279. [DOI] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse NE, Patel-King SR, Rayburn H, Hynes OR. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse EN, George EL, Rayburn H, Hynes RO. Mesodermal development in mouse embryos mutant for fibronectin. Dev Dyn. 1996;207:145–156. doi: 10.1002/(SICI)1097-0177(199610)207:2<145::AID-AJA3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Habib T, Herrera R, Decker SJ. Activators of protein kinase C stimulate association of Shc and the PEST tyrosine phosphatase. J Biol Chem. 1994;269:25243–25246. [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E, editors. A laboratory manual. 2. Cold Spring Harbor Laboratory Press; New York: 1994. Manipulating the mouse embryo. [Google Scholar]
- Honda H, Oda H, Nakamoto T, Honda Z, Sakai R, Suzuki T, Saito T, Nakamura K, Nakao K, Ishikawa T, Katsuki M, Yazaki Y, Hirai H. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat Gen. 1998;19:361–365. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- Jones N, Dumont DJ. Tek/Tie2 signaling: new and old partners. Cancer Metastasis Rev. 2000;19:13–17. doi: 10.1023/a:1026555121511. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Meneses JJ, Pedersen RA. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development. 1991;113:891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- Lin Goh K, Yang TJ, Hynes OR. Mesodermal defects and cranial neural crest apoptosis in α5 integrin-null embryos. Development. 1997;124:4309–4313. doi: 10.1242/dev.124.21.4309. [DOI] [PubMed] [Google Scholar]
- Lyons PD, Dunty JM, Schaefer EM, Schaller MD. Inhibition of the catalytic activity of cell adhesion kinaseβ by protein-tyrosine phosphatase-PEST-mediated dephosphorylation. J Biol Chem. 2001;276:24422–24431. doi: 10.1074/jbc.M011080200. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- Matthews RJ, Bowne DB, Flores E, Thomas ML. Characterization of hematopoietic intracellular protein tyrosine phosphatases: description of a phosphatase containing an SH2 domain and another enriched in proline-, glutamic acid-, serine-, and threonine-rich sequences. Mol Cell Biol. 1992;12:2396–2405. doi: 10.1128/mcb.12.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan AP, Kaestner KH, Grau E, Schutz G. Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development. 1993;119:567–578. doi: 10.1242/dev.119.3.567. [DOI] [PubMed] [Google Scholar]
- Palis J, McGrath KE, Kingsley PD. Initiation of hematopoiesis and vasculogenesis in murine yolk sac explants. Blood. 1995;86:156–163. [PubMed] [Google Scholar]
- Ridley A, Hall A. The small GTP-binding protein Rho regulates the assembly of focal adhesions and stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Rossant J, Howard L. Signaling pathways in vascular development. Annu Rev Cell Dev Biol. 2002;18:541–573. doi: 10.1146/annurev.cellbio.18.012502.105825. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Hogan BL. Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development. 1993;118:47–59. doi: 10.1242/dev.118.1.47. [DOI] [PubMed] [Google Scholar]
- Sastry SK, Lyons PD, Schaller MD, Burridge K. PTP-PEST controls motility through regulation of Rac1. J Cell Science. 2002;115:4305–4316. doi: 10.1242/jcs.00105. [DOI] [PubMed] [Google Scholar]
- Saxton TM, Pawson T. Morphogenetic movements at gastrulation require the SH2 tyrosine phosphatase Shp2. Proc Natl Acad Sci USA. 1999;96:3790–3795. doi: 10.1073/pnas.96.7.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Schneider G, Cloutier JF, Veillette A, Schaller MD. Direct association of protein-tyrosine phosphatase PTP-PEST with paxillin. J Biol Chem. 1998;273:6474–6481. doi: 10.1074/jbc.273.11.6474. [DOI] [PubMed] [Google Scholar]
- Spencer SD, Dowbenko D, Cheng J, Li WL, Brush J, Utzig S, Simanis V, Lasky LA. PSTPIP a tyrosine phosphorylated cleavage furrow-associated protein that is a substrate for a PEST tyrosine phosphatase. J Cell Biol. 1997;138:845–860. doi: 10.1083/jcb.138.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG. In situ hybridization: a practical approach. IRL; Oxford: 1992. [Google Scholar]
- Wilkinson DG, Bailes JA, Champion JE, McMahon AP. A molecular analysis of mouse development from 8 to 10 days post coitum detects changes only in embryonic globin expression. Development. 1987;99:493–500. doi: 10.1242/dev.99.4.493. [DOI] [PubMed] [Google Scholar]
- Xu W, Baribault H, Adamson ED. Vinculin knockout results in heart and brain defects during embryonic development. Development. 1998;125:327–337. doi: 10.1242/dev.125.2.327. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in α5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- Yang H, Reinherz EL. CD2BP1 modulates CD2-dependent T cell activation via linkage to protein tyrosine phosphatise (PTP)-PEST. J Immunol. 2006;176:5898–5907. doi: 10.4049/jimmunol.176.10.5898. [DOI] [PubMed] [Google Scholar]
- Zaret KS. Liver specification and early morphogenesis. Mech Dev. 2000;92:83–88. doi: 10.1016/s0925-4773(99)00326-3. [DOI] [PubMed] [Google Scholar]