Abstract
The subset of metastatic colorectal adenocarcinomas that harbor BRAF V600E mutations are aggressive tumors with significantly shortened survival and limited treatment options. Here we present a colorectal cancer patient whose disease progressed through standard chemotherapy and who developed liver metastasis. Comprehensive genomic profiling (FoundationOne®) identified a BRAF V600E mutation in the liver lesion, as well as other genomic alterations consistent with colorectal cancers. Combination therapy of dabrafenib and trametinib with standard cytotoxic chemotherapy resulted in a durable major ongoing response for the patient. This report illustrates the utility of comprehensive genomic profiling with personalized targeted therapy for aggressive metastatic colorectal adenocarcinomas.
Keywords: oxaliplatin, colorectal adenocarcinoma, combination targeted therapy, BRAF mutations
Case presentation
A 33-year-old female with a history of celiac sprue presented to the clinic for routine follow-up, and a suspicious mass was seen on colonoscopy. Original colonoscopy showed a 3–4 cm lesion around the hepatic flexure that appeared malignant. Biopsies confirmed a moderately differentiated adenocarcinoma. Further staging with CT scanning of chest, abdomen, and pelvis showed unremarkable liver and lungs, small lymph nodes adjacent to the area of thickening in the proximal transverse colon, and an enlarged 2 cm lymph node anterior to the superior mesenteric vein with no other pelvic masses or adenopathy. The CEA level preoperatively was 1.0 mcg/L. The patient proceeded to laparoscopic right hemicolectomy, and pathologic examination identified an invasive moderately differentiated adenocarcinoma with mucinous features with tumor invading through the muscularis, through pericolic adipose tissue to the serosal surface. Margins were negative, there was positive lymphovascular invasion, appendix was benign, and five of 31 lymph nodes were positive for metastatic adenocarcinoma, pathologic stage T4aN2a. Postoperative scans showed no evidence of metastatic disease, and the patient underwent adjuvant chemotherapy with 12 cycles of FOLFOX. Oxaliplatin was suspended after ten cycles due to peripheral neuropathy. Follow-up CT scans 7 months postresection revealed a new heterogeneous mass in the anterior liver measuring 3.3 cm with an additional small attenuation focus in the posterior right lobe of the liver. PET/CT showed the liver lesion to be FDG avid with an SUV of 12.6 and liver biopsy confirmed metastatic adenocarcinoma consistent with the known colonic primary tumor. The patient underwent resection of the liver lesion with negative margins. Restaging scans 6 months later showed new enlarged lymph nodes as well as new metastatic liver lesions. At this time, KRAS mutation status was confirmed. The patient began systemic therapy with FOLFIRI and cetuximab, which was transitioned to FOLFIRI with bevacizumab due to the development of a significant rash while the patient was on cetuximab. On follow-up, the patient developed severe chest and abdominal pain and was admitted to the hospital for further workup of symptoms. While hospitalized, the patient underwent restaging scans, which demonstrated new lymph node lesions in the anterior mediastinum as well as multiple new bilobar liver lesions consistent with progressive disease (Figures 1 and 2). Liver biopsy again confirmed metastatic colorectal cancer (CRC), and the specimen was submitted for comprehensive genomic profiling (CGP). The patient continued on FOLFIRI without bevacizumab, pending CGP results.
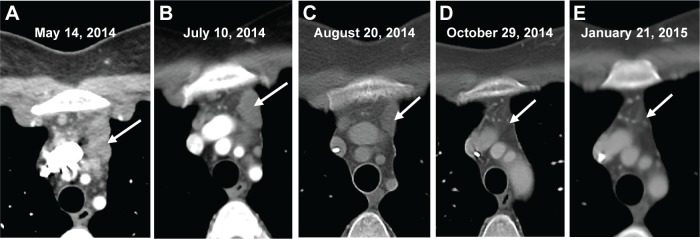
Figure 1.
Contrast-enhanced CT of the chest demonstrating the response to treatment of unresectable metastatic colorectal carcinoma.
Notes: (A) Mediastinal conglomerate mass of enlarged lymph nodes (arrow). (B) Conglomerate masses (arrow) upon initiation of dabrafenib/trametinib/oxaliplatin therapy. (C) Interval decrease in size of mediastinal nodes (arrow) after 1 month of therapy. (D) Interval resolution of mediastinal lymph nodes (arrow) after 3 months of therapy. (E) Absent mediastinal lymphadenopathy (arrow) at 6 months after therapy.
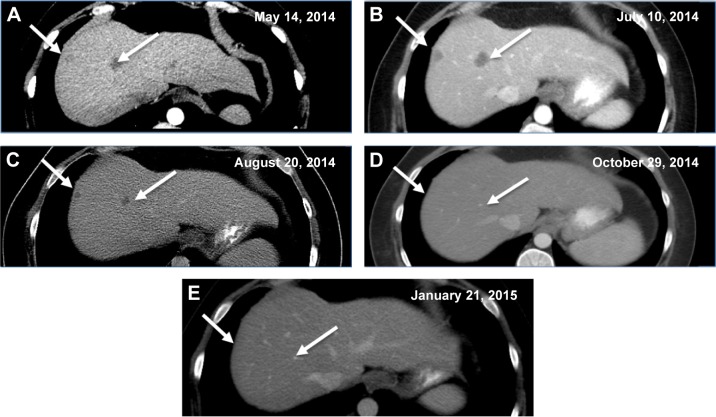
Figure 2.
Contrast-enhanced CT of the abdomen demonstrating resolution of hepatic lesions in an unresectable metastatic colorectal carcinoma.
Notes: (A) Hepatic lesions (arrows) prior to initiation of dabrafenib/trametinib/oxaliplatin therapy. (B) Hepatic lesions (arrows) upon initiation of therapy. (C) Interval decrease in size of liver lesions (arrows) after 1 month of therapy. (D) Interval resolution of liver lesions (arrows) after 3 months of therapy. (E) Absent liver lesions (arrows) at 6 months after therapy.
Methods
Percutaneous liver biopsy was performed and submitted for histopathology as well as CGP using the FoundationOne® assay (Foundation Medicine, Inc., Cambridge, MA, USA). In brief, hybridization capture of 3,230 exons from 182 cancer-related genes and 37 introns of 14 genes commonly rearranged in cancer was applied to ≥50 ng of DNA extracted from formalin-fixed, paraffin-embedded tumor and sequenced to a median coverage depth of 344x.1 Informed patient consent was obtained and approval from Western IRB (WIRB) #20140659; CCD001 protocol - Identifying Molecular Drivers of Cancer was obtained.
Results
CGP demonstrated that the recurrent liver metastasis harbored seven genomic alterations: BRAF V600E as well as MYC amplification, CDKN2A (p16INK4a) A57V, TP53 R175H, SMAD4 R361C, and two APC frameshift insertions/deletions (S1545fs*1, T1556fs*3). No evidence was seen of a hypermutated genome suggestive of mismatch repair deficiency. Based on the presence of BRAF V600E and TP53 R175H mutations, the patient was started on dabrafenib (Tafinlar; GlaxoSmithKline plc, London, UK) 75 mg twice daily, trametinib (Mekinist; GlaxoSmithKline plc) 1 mg daily, and oxaliplatin 85 mg/m2 IV every 2 weeks.
The patient experienced fever and chills and developed a rash shortly after starting therapy but quickly improved after dose reduction of dabrafenib to 75 mg once daily. CT scan 1 month after treatment initiation demonstrated a significant radiographic response with decreased mediastinal lymphadenopathy and no new hepatic or mediastinal lesions (Figures 1A and B and 2A and B vs Figures 1C and 2C), and imaging at 3 months showed a partial response with continued disease regression of both mediastinal lymphadenopathy and focal liver lesions (Figures 1D and 2D). Imaging at 8 months showed near-complete resolution of disease with continued regression of mediastinal adenopathy and complete regression of liver lesions (Figures 1E and 2E). Since the last clinical examination, the patient has continued on therapy in an uninterrupted fashion, with the following side effects: intermittent low-grade fevers, abdominal pain, and an ongoing but readily manageable rash.
Discussion
Advanced CRC is a heterogeneous disease composed of multiple clinicopathologic entities, for which descriptive and diagnostic criteria are evolving.2 Regardless, metastatic CRC carries a dire prognosis with a poor 5-year survival rate, although, recently, resection of hepatic oligometastases has increased survival relative to chemotherapy treatment alone.3 The presence of BRAF V600E in a subset of CRC initially raised hopes for benefit from targeted monotherapy, but early clinical trials of vemurafenib monotherapy, a BRAF V600E-specific inhibitor, administered to CRC patients with the BRAF V600E mutation have been unsuccessful.4 Recent approaches to treat these patients using various combinations of targeted therapy have shown promising clinical results, albeit limited.5,6
BRAF V600E is a well-established oncogenic driver, most notably in melanoma, where it is present in 50% of cases and predicts response to vemurafenib.7 In CRCs, the BRAF gene is mutated in roughly 6%–15% of cases, depending on the composition of the study population, and cosegregates to some degree with clinicopathologic features including proximal anatomic origin, female sex, and higher grade.8 The presence of an activating BRAF mutation in metastatic CRC confers a worse prognosis in patients with otherwise resectable disease as well as worse overall and progression-free survival when treated with standard chemotherapy.9,10
Monotherapy with BRAF V600E-specific inhibitors such as vemurafenib, which are efficacious in melanoma patients harboring BRAF V600E mutations, rarely induces an antitumor response in patients with advanced CRC. The differential de novo resistance of CRC may originate from the observation that, unlike melanoma, BRAF V600E-mutated CRC supports a feedback loop through upregulation of the EGFR pathway, which confers resistance to single-agent BRAF inhibitors because of high levels of hepatic growth factor (HGF) expression from the normal colon, driving activation of cMET.11,12 In support of this, combined inhibition of BRAF and EGFR has achieved meaningful responses in a small subset of advanced CRC cases harboring the BRAF V600E mutation.6 The present case has an excellent response relative to these studies, but it is unclear whether this is due to the therapies used, the underlying genomic profile, or a match between the therapy and genomic profile.
Inhibition of BRAF in combination with MAPK signaling also has a biologic rationale as a strategy to overcome the monotherapy resistance.13 The present patient was treated with dabrafenib, a BRAF V600E-specific inhibitor, and trametinib, a MEK pathway inhibitor, agents approved for simultaneous use in BRAF V600E-mutated melanoma. This same combination of targeted therapies induced 12% partial response and 2% complete response in a recent trial of BRAF V600E-mutation colorectal carcinoma, with 51% of patients achieving stable disease, but the duration of response is still pending.14 This trial used just targeted therapy; in contrast, the present case was targeted therapy and a chemotherapy backbone, and this regimen continues to be effective despite dose reduction due to toxicity. The other alterations found in the present patient’s genomic profile could possibly affect the efficacy of this regimen, but CGP of many BRAF V600E CRC cases treated with the therapy used here will be needed to understand if the genomic profile modifies the efficacy of the treatment regimen.
In our study, the patient failed multiple systemic chemotherapy regimens for adjuvant and metastatic disease with a rapidly progressive unrelenting disease course, indicative of metastatic CRC harboring a BRAF mutation. The median survival of patients who fail initial therapy for metastatic disease is 12.5 months, and is only 4.3 months when a BRAF mutation is present.14,15 The ongoing response to combination targeted therapy with oxaliplatin represents the longest period of disease control for this patient, with relatively minimal toxicities, despite being implemented as salvage therapy. This case exemplifies the benefit of CGP for patients with metastatic CRC and the implication of combination targeted therapies for BRAF V600E-mutant CRC. Further investigation will be needed to extend the findings presented here.
Footnotes
Disclosure
CM, RY, DL, JC, DM, PJS, JSR, JAE, VAM, and SMA are employed by and have equity interest in Foundation Medicine, Inc. TJG has received research support from Bristol-Myers Squibb and Bayer Oncology. The authors report no other conflicts of interest in this work.
References
- 1.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31(11):1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kemeny NE. Treatment of metastatic colon cancer: “the times they are A-changing”. J Clin Oncol. 2013;31(16):1913–1916. doi: 10.1200/JCO.2013.49.4500. [DOI] [PubMed] [Google Scholar]
- 4.Connolly K, Brungs D, Szeto E, et al. Anticancer activity of combination targeted therapy using cetuximab plus vemurafenib for refractory BRAF V600E-mutatnt metastatic colorectal carcinoma. Curr Oncol. 2014;21:151–154. doi: 10.3747/co.21.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600E-mutant colorectal cancer. J Clin Oncol. 2015 Sep 21; doi: 10.1200/JCO.2015.63.2471. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaeger R, Cercek A, O’Reilly EM, et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutation metastatic colorectal cancer patients. J Clin Oncol. 2015;21(6):1313–1320. doi: 10.1158/1078-0432.CCR-14-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 8.Gonsalves WI, Mahoney MR, Sargent DJ, et al. Alliance for Clinical Trials in Oncology. Patient and tumor characteristics and BRAF and KRAS mutations in colon cancer, NCCTG/Alliance N0147. J Natl Cancer Inst. 2014;106(7) doi: 10.1093/jnci/dju106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karagkounis G, Torbenson MS, Daniel HD, et al. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer. 2013;119(23):4137–4144. doi: 10.1002/cncr.28347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teng HW, Huang YC, Lin JK, et al. BRAF mutation is a prognostic biomarker for colorectal liver metastasectomy. J Surg Oncol. 2012;106(2):123–129. doi: 10.1002/jso.23063. [DOI] [PubMed] [Google Scholar]
- 11.Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2(3):227–235. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straussman R, Morikawa T, Shee K, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahronian LG, Sennott EM, Van Allen EM, et al. Clinical acquired resistance to RAF inhibitor combinations in BRAF-mutant colorectal cancer through MAPK pathway alterations. Cancer Discov. 2015;5(4):358–367. doi: 10.1158/2159-8290.CD-14-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corcoran RB, Atreya CE, Falchook GS, et al. Phase 1–2 trial of the BRAF inhibitor dabrafenib (D) plus MEK inhibitor trametinib (T) in BRAF V600 mutant colorectal cancer (CRC): updated efficacy and biomarker analysis. J Clin Oncol. 2014;32:5s. suppl; abstr 3517. [Google Scholar]
- 15.Souglakos J, Philips J, Wang R, et al. Prognostic and predictive value of common mutations for treatment response and survival in patients with metastatic colorectal cancer. Br J Cancer. 2009;101(3):465–472. doi: 10.1038/sj.bjc.6605164. [DOI] [PMC free article] [PubMed] [Google Scholar]