Abstract
Although a vaccine against hepatitis B virus (HBV) has been available since 1982, it is estimated that 600,000 people die every year due to HBV. An affordable oral vaccine could help alleviate the disease burden and to this end the hepatitis B surface antigen (HBsAg) was expressed in maize. Orally delivered maize material induced the strongest immune response in mice when lipid was extracted by CO2 supercritical fluid extraction (SFE), compared to full fat and hexane-extracted material. The present study provides a biochemical and biophysical basis for these immunological differences by comparing the active ingredient in the differently treated maize material. Purified maize-derived HBsAg underwent biophysical characterization by gel filtration, transmission electron microscopy (TEM), dynamic light scattering (DLS), UV-CD, and fluorescence. Gel filtration showed that HBsAg forms higher-order oligomers and TEM demonstrated virus-like particle (VLP) formation. The VLPs obtained from SFE were more regular in shape and size compared to hexane or full fat material. In addition, SFE-derived HBsAg showed the greatest extent of α-helical structure by far UV-CD spectrum. Fluorescence experiments also revealed differences in protein conformation. This work establishes SFE-treated maize material as a viable oral vaccine candidate and advances the development of the first oral subunit vaccine.
Keywords: Hepatitis B surface antigen, Zea mays, mucosal vaccine, supercritical fluid extraction, TEM, virus-like particle
1. Introduction
Over 20 years ago an effective parenteral vaccine was developed and commercialized against hepatitis B virus (HBV) yet the vaccine is still cost-prohibitive, inaccessible, or ineffective for many segments of the world's population [1, 2]. The commercial vaccine is a subunit vaccine comprised of the hepatitis B surface antigen (HBsAg) produced in yeast, and injected as a three-dose series. The adjuvanted, purified protein displays thermoinstability, losing immunogenicity when frozen and thawed [3, 4], or when stored for a week at 45°C [5]. A low-cost thermostable oral vaccine may help overcome accessibility and efficacy hurdles presently faced by the commercial vaccine.
Many attempts have been made to produce an HBsAg vaccine in an edible formulation, most notably in edible plants [2]. Expression in potato demonstrated the best proof-of-concept for an oral vaccine, but antigen concentrations were too low to achieve seroconversion rates equivalent to the injected vaccine in human volunteers [6]. The potato delivery system was also non-optimal in that 100g of raw potato needed to be ingested for a single dose, and attempts to improve digestibility by boiling the potato resulted in a 25-fold decrease in immunogenicity [7]. Expression of HBsAg using recombinant maize grain has overcome many of these setbacks, demonstrating much higher levels of expression in a more palatable and digestible formulation [8]. In addition, the maize system has resulted in strong immunologic responses at both systemic and mucosal sites [8, 9] and can likely achieve this on a commercial scale for less than $0.01 per dose for the raw material [2] in a thermostable formulation [10].
Formation of virus-like particles (VLPs) is one means towards inducing an effective immunogenic response. It is well-established that the small form of HBsAg is immunogenic in an oligomerization-dependent manner when produced in mammalian systems [11, 12] and this correlation holds for HBsAg produced in plants [13]. Structural characterization of hepatitis B VLPs formed by HBsAg oligomerization has been undertaken by various researchers and shows that the particles display an octahedral symmetry [14] and that significant structural changes occur upon heat treatment [15]. It has also been shown that processing steps following production, purification, and reassembly of HBsAg into VLPs can affect the structural properties of the vaccine produced in yeast [16, 17]. In the oral maize delivery system, no purification is required, but removal of lipids is a preferred step to produce a highly thermostable vaccine [10]. However, depending on the delipidation method, immunogenicity of the maize material can either increase or decrease, relative to the full fat material [8]. For example, extraction of lipids using hexane as a solvent leads to lower immunogenicity in mice relative to full fat material, while extraction using supercritical CO2 (SFE) results in greater immunogenicity, as determined by fecal IgA, serum IgA, serum IgG and serum mIU/mL. SFE is a method for extracting lipids using a solvent, typically CO2, in a supercritical state [18]. It is considered to be safer from an environmental, as well as health, perspective as it is less toxic than other organic solvents and there are no concerns over residual solvent remaining in the sample [19]. The present study examines the biochemical characteristics of a next-generation maize-produced HBsAg oral vaccine. The biochemical basis for differential immunogenicity seen between hexane, SFE, and full fat treatments was defined, and definitive evidence for proper VLP formation in maize grain was obtained. This paper supplies the necessary data to advance the maize-based vaccine towards being the first subunit oral vaccine to be commercialized.
2. Materials and Methods
2.1 Maize material
All maize material was derived from hybrid grain containing the HBG20 construct, as previously described [8, 10]. Briefly, the HBG20 construct was designed to drive expression of HBsAg with the 3-kb globulin1 promoter sequences. The HBsAg protein was fused at the N-terminus to a codon optimized type B barley alpha amylase signal sequence. Commercially obtained maize material (hybrid line 9806) was used as a negative control.
2.2. Seed processing
Germ used for analysis was fractionated by hand and de-lipidated, as previously described [8]. Briefly, HBG hybrid seeds were soaked in water for 5 days at 4 °C followed by extraction of germ by hand, and overnight drying at 37 °C to a final moisture content of 6–15%. The dried germ was ground into fine powder with cornmeal consistency, and defatted by either hexane extraction or supercritical fluid extraction (SFE). Hexane extractions were conducted as described in earlier work [8, 9]. SFE treatment consisted of CO2 extraction at 350 bar, 40–53 °C vessel temperature, using a 5L vessel in an SFT-250 (Supercritical Fluid Technologies, Newark, DE).
2.2 Extraction of HBsAg
1 g of ground maize material was washed twice with 10 mL of 1×PBS containing 0.05% Tween (PBST) buffer at 4°C for 15 minutes, centrifuged at 4100g for 10 minutes, and the supernatant discarded. Subsequently, HBsAg extraction was done with 10 mL of 1×PBS containing 1% Triton X-100 at 4 °C for 15 minutes, centrifuged at 4100g for 10 minutes, and the supernatant containing the protein extract collected and filtered through a 0.45μm syringe filter.
2.3 Purification of HBsAg
The protein was precipitated from the extract by addition of ammonium sulfate (AS) (Sigma #A4418-1) such that the final concentration of AS in the extract was 30% w/v. The precipitate was centrifuged at 4100g for 10 minutes and the pellet obtained was re-dissolved into 2.0 mL of 20 mM phosphate buffer pH 7.0. The AS-purified sample was centrifuged at 12,300g for 10 minutes and was then loaded onto a HiTrap DEAE FF- 5mL column (GE#17-5154-01) pre-equilibrated with 20 mM phosphate buffer pH 7.0. The bound protein was eluted by using a gradient increase of NaCl concentration from 0 to 1 M. The purity of protein was determined by SDS-PAGE.
2.4 Gel filtration chromatography
Gel filtration was performed on an ÄKTA FPLC system (Amersham) using the Superdex 200 10/300 GL (GE#17-5175-01) column equilibrated with 1×PBS. AS-purified protein (500 μl, ~0.5mg of protein) was loaded on the column and protein was eluted with 1×PBS at a flow rate of 0.5 mL/min.
2.5 Western blot
Protein samples containing 50 mM DTT in the sample buffer were first resolved on a Bis-Tris SDS-PAGE gel and subsequently transferred to a nitrocellulose membrane. The HBsAg was detected by using a rabbit anti-HBsAg primary antibody (Genway #18-511-245179, 1:1000 dilutions) and a polyclonal alkaline phosphate (AP) conjugated anti-rabbit secondary antibody (Sigma #A3687, 1:20,000 dilution). Colorimetric detection of the secondary antibody was performed using an AP conjugate substrate kit (Bio Rad #170-6432).
2.7 Far UV-CD analysis
Yeast-derived HBsAg (Meridian # R86872) and HBsAg purified from maize germ were dialyzed overnight against 20 mM phosphate buffer pH 7.4 and centrifuged at high speed to remove any particulate matter. All protein samples were diluted in buffer and the absorption spectrum measured in the 250-350 nm range. The far UV-CD spectrum of each protein, with an A280 value of ~1.0, after subtracting the negligible absorbance at 330-340 nm, was recorded on a Jasco J-710 spectropolarimeter using a 0.1cm path-length cuvette and excitation wavelengths ranging from 190 nm–260 nm.
2.8 Fluorescence analysis
Yeast-derived HBsAg (Meridian # R86872) and HBsAg purified from maize germ were dialyzed overnight in 20 mM phosphate buffer pH 7.4, centrifuged at high speed to remove any particulate matter and diluted to an A280 of ~0.1 after accounting for the negligible absorbance at 330-340 nm attributable to potential light scattering effects. Fluorescence measurements were made in a 1 cm cuvette with a Cary Eclipse spectrofluorimeter (Varian) at room temperature. Scans were executed at an excitation wavelength of 290 nm and a band width of 5 nm with a scan speed of 120 nm/min.
2.9 N-terminal sequencing
Purified HBsAg was used for N-terminal sequencing using the Edman degradation method [20]. The N-terminal sequencing was performed on a 494 Procise protein/peptide sequencer with an on-line 140C PTH Amino Acid Analyzer (Perkin Elmer Applied Biosystem).
2.10 Mass-spectrometry analysis
The purified HBsAg protein was subjected to trypsin as well as chymotrypsin digestion. Trypsin-digested samples were analyzed by a MALDI-TOF MS/MS mass spectrometer using a QSTAR XL quadrupole TOF mass spectrometer (AB/MDS Sciex, Toronto, Canada) equipped with a MALDI ion source. Chymotrypsin-digested sample were analyzed on an Orbitrap Elite LC-MS system. The resulting MS data were analyzed using the MASCOT (MatrixScience, London, UK, version 2.2.06) and a custom protein database containing the HBsAg protein sequence.
2.11 Immunolocalization of HBsAg by TEM
Full fat embryo samples were hand-dissected and fixed with 0.1% glutaraldehyde (w/v) and 2% paraformaldehyde (w/v) in 0.1 M cacodylate buffer (pH 7.2) for 2 hours at 4°C. Samples were rinsed in 0.1M cacodylate buffer and dehydrated in a graded ethanol series, infiltrated and embedded using LR White resin (Electron Microscopy Sciences, Ft. Washington, PA). Resin blocks were polymerized for 48 hours at 4°C under UV light. Thin (60-90 nm) sections were made through embryo blocks by placing 4–6 thick sections together on ProbeOn-Plus slides and stored in a slide box and then placing thin sections on 5 carbon film/nickel (Ni) grids. For immunogold labelling, sections were blocked in PBS containing 1% fish gelatin and 3% BSA for 30 minutes, and were labeled using a rabbit anti–HBsAg primary antibody (Genway#18-511-245179, 1:500 dilutions), and goat anti-rabbit IgG EM-grade 6nm (Electron Microscopy Science #25104, 1:20 dilutions) as secondary antibody. Images of sections on grids were made using a 200kV JEOL 2100 scanning and transmission electron microscope (Japan Electron Optics Laboratories, LLC, USA, Peabody, MA).
2.12 Electron microscopic analysis of VLPs
HBsAg particles purified via AS precipitation followed by gel filtration were used for TEM analysis. HBsAg particles were negatively stained by using 0.5% uranyl acetate and TEM images were acquired on a 200kV JEOL 2100 scanning and transmission electron microscope.
2.13 HBsAg ELISA
HBsAg was detected by ELISA, as previously described [4]. Briefly, a sandwich ELISA is conducted with a monoclonal capture antibody (Meridian Life Sciences, Memphis, TN, cat # C01246M) and a polyclonal HRP-conjugated detection antibody (Meridian Life Sciences, cat # B65811P).
2.14 Dynamic light scattering (DLS)
Gel filtration purified HBsAg was dialyzed in 1X PBS buffer and diluted to 0.01mg/ml for DLS measurements. Hydrodynamic particle size measurements of VLPs were done in 4 μL cyclic olefin copolymer disposable microcuvettes (Wyatt technology Santa Barbara, CA) at 25 °C using DynaPro NanoStar dynamic light scattering instrument (Wyatt technology, Santa Barbara, CA). The data were analyzed using DYNAMICS software (Wyatt technology, Santa Barbara, CA).
3. Results
3.1 Localization and expression of HBsAg
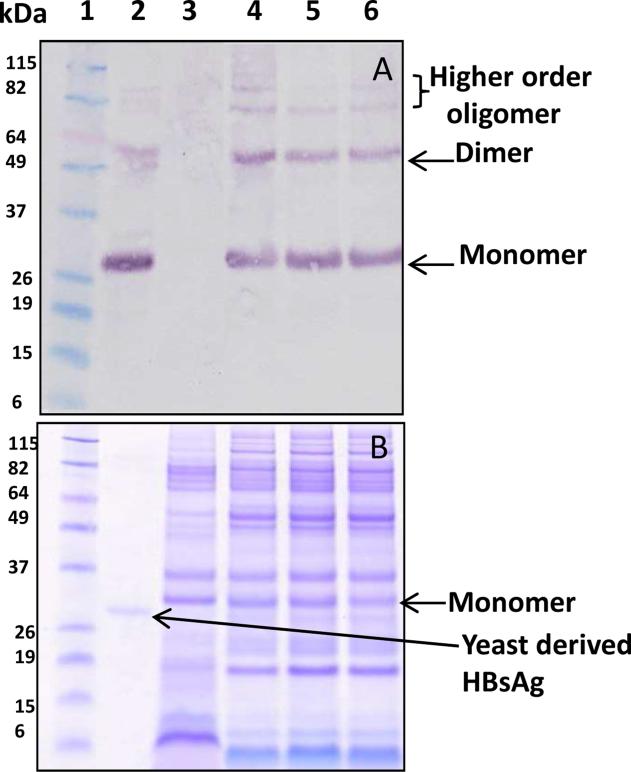
One of the challenges to developing effective plant-derived oral vaccines is the very low accumulation levels of antigen in the edible plant tissue. Membrane-bound proteins are often difficult to express in heterologous systems [21], and this is especially true for HBsAg, which has historically ranged from 6 ng/g to 60 μg/g in plant tissue [22, 23]. Recently, hybrid maize lines were produced that were able to accumulate HBsAg at very high concentrations by fusing HBsAg to the barley alpha amylase signal sequence (BAASS) and by preferentially expressing the antigen in the germ fraction of the seed [8]. Expression of HBsAg in the germ fraction was detected at a level of 1023 μg/g by ELISA, the highest reported expression of HBsAg in a plant system to date. Expression in the germ was confirmed by TEM immunolocalization (Supplementary Fig.1) in whole hand-dissected germ tissue and in a western blot of crude germ extracts. Protein extracts were prepared from maize germ that was differentially processed by hexane extraction, SFE treatment, or without any treatment (i.e. full-fat germ). The western blot showed the presence of monomer, dimer and higher order oligomers of HBsAg (Fig 1). The maize-derived monomeric form of HBsAg migrated around 27 kDa rather than 24 kDa suggesting that the 24 amino acid signal sequence (BAASS) was retained as a fusion to HBsAg. This was verified by N-terminal sequencing of the purified protein, which showed the presence of the first 7 amino acids (MANKHLS) of the BAASS, confirming the presence of the signal sequence in the expressed protein. The mass spectrometry data further confirmed the expected sequence of the BAASS:HBsAg fusion protein based on purified samples (Table 1).
Fig. 1. Oligomerization propensity of HBsAg in protein extract obtained from variously processed maize germ.
(A) The anti-HBsAg western blot under reducing conditions showing the presence of monomer, dimer and higher order oligomers. Equal volumes (20 μl) of full fat, hexane and SFE treated samples were loaded. Protein standard marker (lane 1); yeast-derived HBsAg as positive control (lane 2); non-transgenic maize seed extract as negative control (lane 3); maize germ extract from full fat (lane 4), hexane-treated (lane 5) and SFE-treated (lane 6) samples. (B) The corresponding SDS-PAGE gel used to transfer protein to the western blot.
Table 1. Peptides identified by mass spectrometry analysis of HBsAg.
Purified HBsAg protein was digested by sequencing grade trypsin and chymotrypsin and was analyzed as described in the Materials and Methods.
| Peptide sequence identified by chymotrypsin digestion | Probability | Mascot Ion score |
|---|---|---|
| (K)HLSLSLF(L) (BAASS sequence) | 87% | 26.92 |
| (W)TSLNFLGGAPTcPGQNL(Q) | 60% | 10.28 |
| (W)TSLNFLGGAPTcPGQNLQSPT(S) | 75% | 15.19 |
| (F)SLLTRIL(T) | 90% | 36.57 |
| (L)TRILTIPQSLDSW(W) | 94% | 37.06 |
| (C)TKPSDGNcAcIPIPSSW(A) | 98% | 26.02 |
| (L)LPGTSTTSTGPcK(T) | 100% | 107.21 |
| (C)cTKPSDGNcAcIPIPSSW(A) | 100% | 38.35 |
| Peptide sequence identified by trypsin digestion | ||
| (R)FLWEWASVR(F) | 98% | 36 |
3.2 Purification of HBsAg
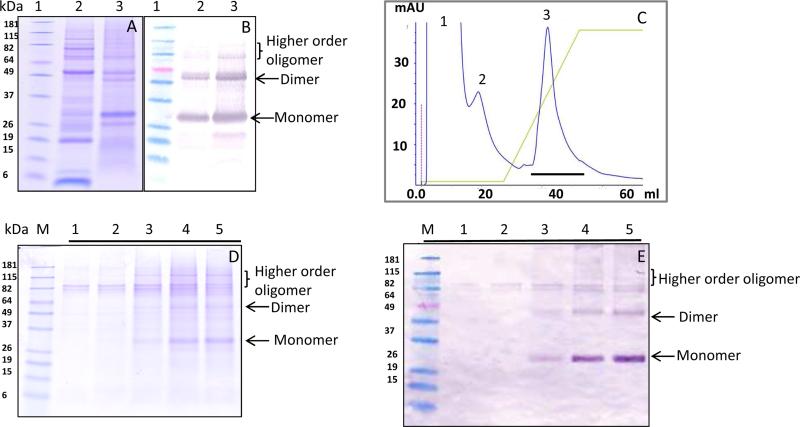
Purification of HBsAg was achieved via a two-step process. Ammonium sulphate (AS) in the range of 20-60% w/v was used for precipitation of HBsAg. Initial precipitation of HBsAg occurred at approximately 20% AS and complete precipitation was achieved at 40% AS (data not shown). At 30% AS most of the HBsAg was precipitated while significant purification was also achieved at this concentration (Fig 2A). The precipitation of HBsAg was also confirmed by western blot using an anti-HBsAg specific antibody (Fig. 2B). Hence, for all further purification 30% AS was used as an optimum concentration for precipitation of HBsAg. In most cases, purification of recombinantly expressed HBsAg has been achieved via hydrophobic interaction chromatography or ion exchange chromatography [24, 25]. The attempt to purify HBsAg using hydrophobic interaction chromatography was not very successful (data not shown). Hence, further purification of HBsAg was performed by anion exchange chromatography. The bound protein (Fig. 2C, fraction 3) was eluted by a NaCl salt gradient and yielded purified HBsAg as shown by SDS-PAGE (Fig 2D, lanes 3-5). The presence of HBsAg in the eluted fraction was confirmed by western blot (Fig 2E). The western blot showed the presence of monomer, dimer and other higher order oligomers in the purified fraction (Fig 2E, lanes 3-5).
Fig. 2. Purification of SFE treated HBsAg.
(A) Ammonium sulphate (AS) purified protein resolved on SDS-PAGE under reducing conditions. Protein standard (lane 1); SFE-treated seed extract (lane 2); AS-precipitated protein (lane 3). (B) Anti-HBsAg western blot showing the presence of HBsAg after AS precipitation. (C) Anion exchange chromatogram showing the elutions of unbound protein (peaks 1 and 2) and bound protein (peak 3). The salt gradient is indicated by the green line. (D) SDS-PAGE gel of the anion exchange-purified protein. Lanes 1 to 5 represent various fractions of peak 3. (E) The anti-HBsAg western blot of samples used in panel D demonstrating the presence of HBsAg in the purified fraction.
3.3 Comparison of full-fat, SFE and hexane treated samples
In order to determine the effect of lipid removal on the higher order structure of HBsAg in maize material, purified samples from full fat, hexane-treated, and SFE-treated material were compared using various techniques.
3.3.1 Gel filtration analysis
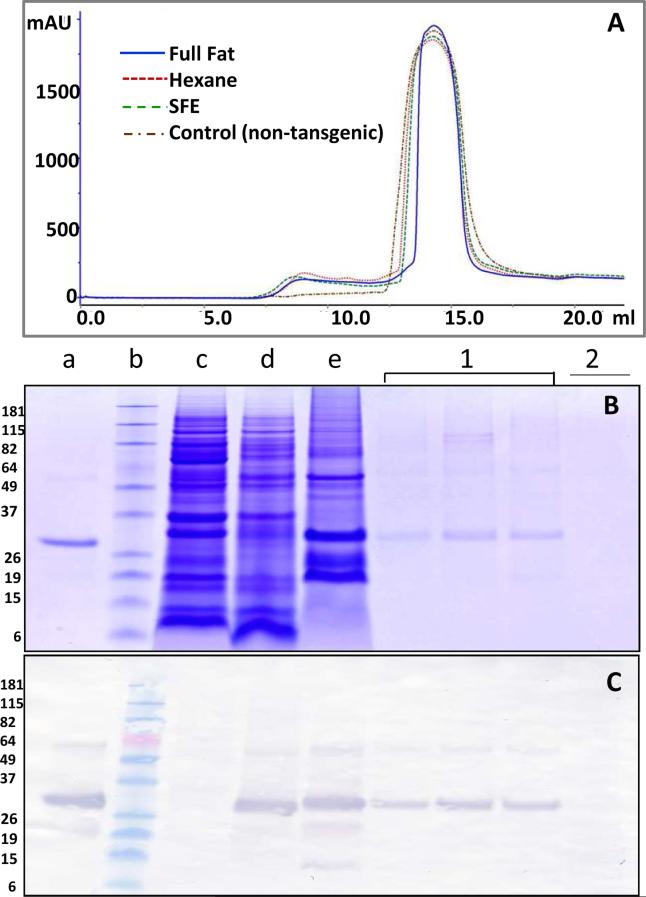
Gel filtration was employed to elucidate the size of AS-purified HBsAg obtained from hexane-extracted, SFE-treated and full-fat maize germ. The chromatograms were similar for all three samples (Fig. 3A). SDS-PAGE and western blot with HBsAg-specific antibody (Figs. 3 B and 3C, lanes marked 1 correspond to peak 1 fractions from gel filtration) showed that the HBsAg from the SFE-treated sample eluted in the void volume (size exclusion limit of the column is ~ 1.3 × 106 Da), indicating its occurrence as higher order oligomers (Fig 3). The non-transgenic maize control sample, treated similarly as the transgenic maize sample, was also run on gel-filtration column. The control sample only showed peak 2 while peak 1 was absent (Figure 3A) However, little or no protein was detected on Coomassie stained SDS-PAGE gel for the peak 2 fraction (Fig 3B). Mass spectrometric analysis of this fraction also did not indicate the presence of proteins and strongly suggested that nonproteinaceous compounds with strong absorption at 280 nm were the predominant components. Interestingly, SDS-PAGE and western blots of the gel filtration fraction of hexane-treated and full fat samples also showed the presence of nearly all the HBsAg in the void volume (data not shown).
Fig. 3. Gel filtration of HBsAg.
(A) Gel filtration chromatogram for SFE-treated material (green line), hexane-treated (red line) , full fat material (blue line) and non-transgenic maize control (brown line). (B) SDS-PAGE of protein eluted from the gel filtration. Yeast-derived HBsAg was used as a positive control (lane a); protein standard marker (lane b); non-transgenic maize seed extract as negative control (lane c); transgenic maize seed extract (lane d); AS-purified HBsAg (lane e); fractions of protein eluted from gel filtration peak 1 (lane 1); protein eluted from gel filtration peak 2 (lane 2). (C) The corresponding anti-HBsAg western blot demonstrating the presence of HBsAg. The SDS PAGE and western blots presents data collected for SFE treated material and are representative of data collected for hexane treated and full fat material.
3.3.2 Size and structure of VLPs
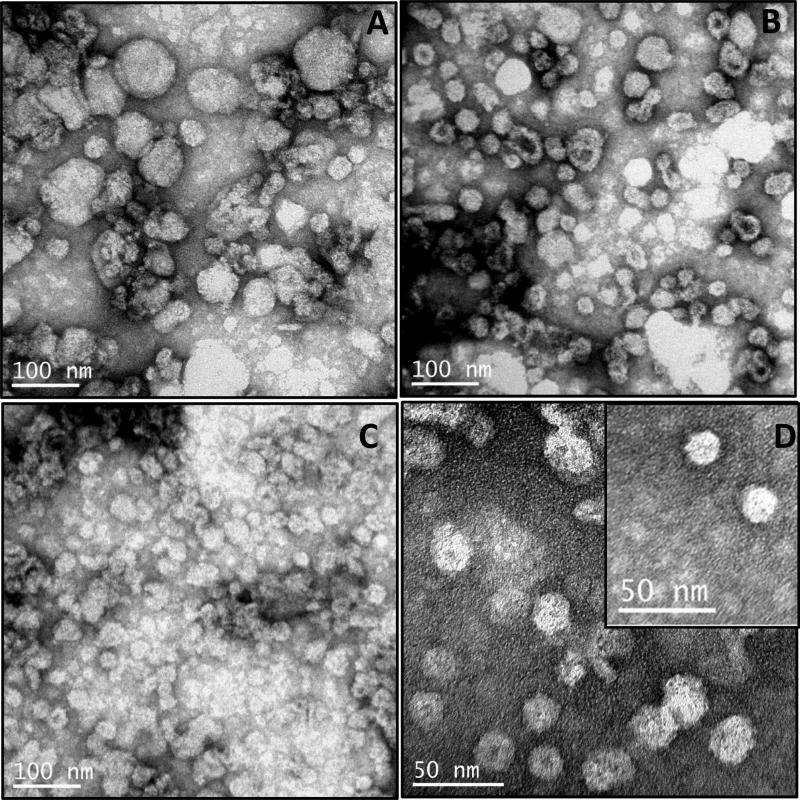
The effect of various treatments of maize germ on the structure and size of VLPs was studied by TEM. A sample of HBsAg derived from the void volume fraction of gel filtration was used for this analysis. Formation of VLPs was clearly evident in all three treatments i.e. full fat, hexane-treated and SFE-treated germ (Fig. 4).
Fig.4. Electron microscopy of virus-like particles (VLPs).
TEM images of VLPs formed by HBsAg present in the void volume fractions of gel filtration of proteins obtained from (A) full fat germ; (B) hexane-treated germ; (C) SFE-treated germ; (D) VLPs of SFE-treated germ shown at higher magnification. (E) Elute obtained from peak 2 of the gel filtration chromatography (negative control). (F) Protein extract from non-transgenic maize material (negative control). (G) VLPs of yeast derived HBsAg (Meridian)(positive control). VLPs/proteins were negatively stained with 0.5% uranyl acetate and visualized by TEM.
Proteins eluted in the second peak fractions of gel filtration and a non-transgenic maize protein extract served as negative controls. The VLPs obtained from all three treatments varied widely in size. The VLP sizes obtained from full fat maize germ (Fig. 4A) were the least uniform, ranging between 20–100nm, VLP sizes of hexane-treated maize germ (Fig. 4B) ranged from 20–30nm, and VLP sizes in SFE-treated germ were the most uniform, all approximately 20nm in size (Fig. 4C,D). In addition, the shape of the VLPs from SFE-treated material was more uniform and symmetrical, while the VLPs from full fat were large globular structures and from hexane-treated material were less uniform. No VLPs could be seen in either the proteins obtained from the second peak fraction of gel filtration or in the non-transgenic maize seed extract (Fig 4 E and 4F, respectively). The yeast derived HBsAg also showed presence of VLPs, as reported earlier [16] (Fig 4G).
Dynamic Light Scattering (DLS) is a well-established technique to measure hydrodynamic size and polydispersity of proteins[26]. DLS experiments indicate that the hydrodynamic diameter of VLPs obtained from differently processed maize germ range from 32 to 38 nm (Fig 5). It should also be noted that the size of VLPs from SFE-treated material (32nm) is very close to that of yeast derived HBsAg (30.5 nm). DLS analysis also confirmed that the VLPs obtained from FF sample were polydisperse compared to SFE and hexane-treated samples (Fig 5). Additionally, the DLS data indicate presence of aggregates in maize-derived HBsAg that is almost absent in a commercial preparation of HBsAg from yeast. This may be attributed in part to an ultracentrifugation step in the purification scheme of the yeast HBsAg that results in removal of aggregated material. Also, the use of ammonium sulfate (AS) during purification, and the presence of salt in the purified samples may result in formation of aggregated particles. Recently, it has been shown that the presence of salt can lead to aggregation of HBsAg particles into oligomeric particles [17].
Fig 5. Dynamic light scattering (DLS) of VLPs.
DLS for the size distribution of VLPs obtained from (A) full fat; (B) hexane-treated; (C) SFE-treated maize germ samples and (D) the yeast-derived HBsAg. The DLS analysis was done using 0.01mg/ml of HBsAg in PBS buffer in 4 μL cyclic olefin copolymer disposable microcuvettes at 25 °C using DynaPro NanoStar dynamic light scattering instrument. The polydispersity index for each sample was < 0.05 suggesting that size distribution of VLPs measured by DLS was narrow.
3.3.3 Secondary Structure of HBsAg
The far UV-CD spectra of HBsAg obtained from maize germ were compared with the yeast-derived HBsAg. The spectra of the maize-derived HBsAg obtained from the variously treated samples suggested a predominantly α-helical structure as indicated by minima at 208 nm and 222nm. However, the form and amplitude of the spectra differed, indicating that the helical content was different in all the cases (Fig 6).
Fig. 6. Far UV-CD spectra of HBsAg.
The far UV-CD spectra of anion exchange-purified HBsAg from full fat (FF), hexane-treated (Hexane), or SFE-treated (SFE) maize germ samples were compared with the yeast-derived (Yeast) HBsAg. The far UV-CD spectra were acquired in 20 mM phosphate buffer pH 7.4 with an A280 value of ~1.0 using Jasco J-710 spectropolarimeter.
While the spectrum from maize HBsAg obtained after SFE treatment was similar to that of the yeast-derived HBsAg the spectrum obtained from the hexane-treated germ or the full-fat sample had reduced amplitudes at 208 and 222nm.
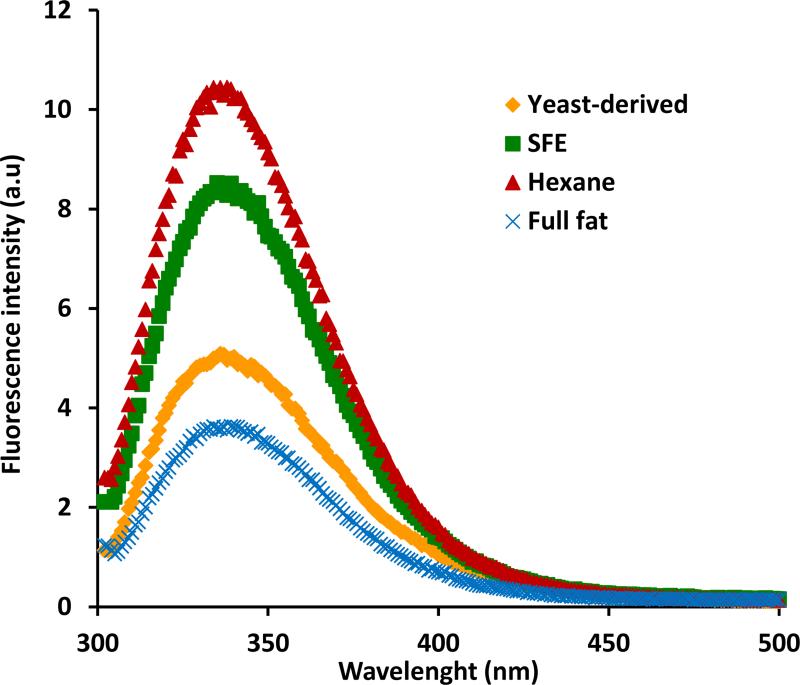
3.3.4 Fluorescence spectroscopy
Fluorescence spectroscopy is a sensitive technique for comparing conformational states of proteins or conformational changes within a given protein and is particularly influenced by the microenvironment of tyrosine (Tyr) and tryptophan (Trp) residues [27]. When excited at 290 nm, the emission spectra of HBsAg purified from variously treated maize germ all show a maximum emission around 334 nm that is similar to the value reported by others [15, 31, 33]. However, the quantum yield of fluorescence was significantly higher for the hexane and SFE treated samples compared to yeast derived and full fat samples and is suggestive of conformational differences among the proteins (Fig 7). Although one of the primary causes of quenched fluorescence can be attributed to inner filter effects, we rule this out as a possibility since all the protein samples have been diluted to the same absorbance at 280 nm (~0.1) and are devoid of particulate matter (see Materials & Methods). Indeed, the markedly reduced fluorescence intensity of the full fat sample is more likely associated with bound lipids and masking of tryptophan residues (see discussion below).
Fig 7. Fluorescence spectra of HBsAg.
The fluorescence spectra of purified HBsAg from full fat, hexane-treated, or SFE-treated maize germ samples were compared with the yeast-derived HBsAg. Fluorescence measurements were made in 20 mM phosphate buffer pH 7.4 with an A280 value of ~0.1 in a 1 cm cuvette with a Cary Eclipse spectrofluorimeter with an excitation wavelength of 290nm.
4. Discussion
Oral subunit vaccines are a biomedical platform that promises to overcome limitations inherent in parenteral administration platforms. While biochemical characterization has been described for an early HBsAg oral vaccine candidate in potato [28-30], no such characterization has been completed for maize-produced HBsAg. The higher concentrations of HBsAg achieved by the maize-based system, relative to other plant-based oral vaccine systems, provides a more robust immunogenic response [8, 10]. While part of the success is due to the higher HBsAg levels obtained in maize, there are also other factors that can influence the immune response based on the preparation of the maize material. Here we have presented a biochemical, biophysical and structural characterization of the active ingredient present in the maize-derived oral vaccine candidate.
HBsAg particles contain around 30% lipids by mass [16, 31, 32] and it has been shown that association of lipids with HBsAg is very important, as these molecules not only affect the self-assembly of HBsAg into proper VLPs but also its immunogenicity [16, 33-34]. The cellular environment in which HBsAg is expressed, and processing conditions used on the raw plant material, can potentially affect the immunogenicity of the plant material since both these factors could influence the association of lipid molecules to HBsAg and the formation of VLPs.
Recently we have shown that both the removal of oil and method of extraction of oil can impact the immunogenicity of the maize-derived HBsAg [8]. The maize materials from which lipids have been removed by SFE treatment were found to be more antigenic compared to hexane-treated or full-fat material. In order to study the effect of lipid removal on HBsAg, protein extracted from variously treated maize germ were analyzed by HBsAg-specific western blot under reducing conditions (in presence of 50 mM DTT). No significant difference in oligomerization was noticed under these experimental conditions. (Fig.2). The presence of dimer and higher order oligomers suggest the presence of highly stable disulphide bonds in the maize-derived HBsAg, although the existence of other types of covalent bonds cannot be ruled out [34]. Disulphide bonding has been shown to play a key role in determining the antigenicity of HBsAg [35], and it is therefore not surprising that oral vaccine material derived from all three treatments can induce immunological responses [8] albeit to varying degrees. The presence of dimer and higher order oligomer were also observed in purified protein fractions (Fig.3).
HBsAg is eluted mostly in the gel filtration void volume fraction which suggests the presence of oligomers (Fig 4). Importantly, this fraction shows the formation of VLPs in all three differentially processed maize materials. The formation of VLPs was confirmed by TEM analysis (Fig 4). Strikingly, the type of VLPs obtained by differently processed maize germ varied widely, providing a possible explanation for immunogenic differences demonstrated between the samples [8]. The VLPs obtained from full fat maize germ were very heterogeneous in size, globular in shape, and ranged from 20-100nm in diameter, much larger than the 22 nm VLPs formed upon native HBsAg assembly (Fig. 4A). One explanation for the irregularities in size and shape could be the presence of large amounts of lipid in the full fat germ. In addition, when most of the lipids are removed by SFE treatment, uniform ~20 nm particles were detectable (Fig 4C, D). The hexane-extracted sample contained VLPs in the size range of 20 nm but their shape was irregular which is believed to be due to excessive removal of lipids during oil extraction that are important for maintaining the structural integrity of the VLPs (Fig 4B). As expected, no VLPs could be seen in the control samples (Fig 4E, F). Differences in shape and uniformity may be due to the preferential removal of different types of lipids during oil extraction, or in the case of the full fat germ, lipids may be hindering proper structural folding of the antigen.
The secondary structures of HBsAg obtained from the differently processed maize germ were also found to be quite different, as measured by far UV-CD spectroscopy. While the conformations of the SFE-treated material and the yeast-derived HBsAg were similar (Fig. 6), the full fat and hexane-treated samples displayed spectra indicative of a change in conformation consistent with a reduced α-helical content. This loss of HBsAg structure in the maize material correlates with a decrease in immunogenicity and may be indicative of further abnormalities within the VLPs. Similar secondary changes have been reported when lipids were removed by using octylglucoside from HBsAg [24].
The higher fluorescence emission intensity observed in hexane and SFE treated samples suggest that the removal of lipids may expose some of the buried tryptophan residues within the hydrophobic part of the protein (Fig 7). Desombere et al. [36] have demonstrated that partial delipidation can improve the anti-HBs response by increasing T-cell antigenicity of the HBsAg. Therefore the enhanced antigenicity observed with the SFE treated sample may also be attributed to partial delipidation of HBsAg particles that is evident from the enhanced fluorescence intensity. Together, the CD and fluorescence data indicate that the partial delipidation due to the treatment of maize germ by supercritical fluid does not result in major changes to the gross secondary structure but the tertiary structure may be perturbed. The diminished fluorescence intensity in the full fat sample may be attributed to lipids that could potentially shield tryptophan residues.
To conclude, this is the first report demonstrating that maize-derived HBsAg is capable of forming VLPs, and that the type of HBsAg higher order structure depends on the method used to prepare material for oral delivery. These results could determine the course of oral vaccine development in the future.
Supplementary Material
Highlights.
A maize-derived oral HBsAg vaccine is capable of forming virus like particles (VLPs)
The specific method of oil extraction affects secondary structure and VLP formation
Oil removal by SFE treatment resulted in proper formation of VLPs
Acknowledgement
We thank Tracey Stewart for providing help with TEM analysis. This project was supported by NIH grant 2R44AI068239-03A1.
Abbreviations
- VLP
Virus-like particle
- SFE
Supercritical fluid extraction
- HBV
hepatitis b virus
- HBsAg
hepatitis B surface antigen
- BAASS
barley alpha amylase signal sequence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int. J. Epidemiol. 2005;34:1329–1339. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 2.Hayden CA. An oral vaccine for hepatitis B: challenges, setbacks, and breakthroughs. In: Howard JA, Hood EE, editors. Commercial plant-produced recombinant protein products: case studies. Springer; accepted. [Google Scholar]
- 3.Kumru OS, Joshi SB, Smith DE, Middaugh CR, Prusik T, Volkin DB. Vaccine instability in the cold chain: mechanisms, analysis and formulation strategies. Biologicals. 2014;42:237–259. doi: 10.1016/j.biologicals.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Clapp T, Munks MW, Trivedi R, Kompella UB, Braun LJ. Freeze-thaw stress of Alhydrogel ® alone is sufficient to reduce the immunogenicity of a recombinant hepatitis B vaccine containing native antigen. Vaccine. 2014;32:3765–3771. doi: 10.1016/j.vaccine.2014.05.037. [DOI] [PubMed] [Google Scholar]
- 5.Van Damme P, Cramm M, Safary A, Vandepapelière P P, Meheus A. Heat stability of a recombinant DNA hepatitis B vaccine. Vaccine. 1992;10:366–367. doi: 10.1016/0264-410x(92)90064-q. [DOI] [PubMed] [Google Scholar]
- 6.Thanavala Y, Mahoney M, Pal S, Scott A, Richter L, Natarajan N, Goodwin P, Arntzen CJ, Mason HS. Immunogenicity in humans of an edible vaccine for hepatitis B. Proc. Natl. Acad. Sci. USA. 2005;102:3378–3382. doi: 10.1073/pnas.0409899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong Q, Richter L, Yang YF, Arntzen CJ, Mason HS, Thanavala Y. Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc. Natl. Acad. Sci. USA. 2001;98:11539–11544. doi: 10.1073/pnas.191617598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayden CA, Smith EM, Turner DD, Todd KK, Wong JC, Walker JH, Tizard IR, Jimenez-Flores R, Howard JA. Supercritical fluid extraction provides an enhancement to the immune response for orally-delivered hepatitis B surface antigen. Vaccine. 2014;32:1240–1246. doi: 10.1016/j.vaccine.2014.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayden CA, Streatfield SJ, Lamphear BJ, Fake GM, Keener TK, Walker JH, Clements JD, Turner DD, Tizard IR, Howard JA. Bioencapsulation of the hepatitis B surface antigen and its use as an effective oral immunogen. Vaccine. 2012;30:2937–2942. doi: 10.1016/j.vaccine.2012.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayden CA, Egelkrout EM, Moscoso AM, Enrique C, Keener TK, Jimenez-Flores R, Wong JC, Howard JA. Production of highly concentrated, heat-stable hepatitis B surface antigen in maize. Plant Biotechnol. J. 2012;10:979–984. doi: 10.1111/j.1467-7652.2012.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vyas GN, Rao DR, Ibrahim AB. Australia antigen (hepatitis B antigen). A conformational antigen dependent on disulfide bonds. Science. 1972;178:1300–1301. doi: 10.1126/science.178.4067.1300. [DOI] [PubMed] [Google Scholar]
- 12.Mangold CM, Unckell F, Werr M, Streeck RE. Secretion and antigenicity of hepatitis B virus small envelope proteins lacking cysteines in the major antigenic region. Virology. 1995;211:535–543. doi: 10.1006/viro.1995.1435. [DOI] [PubMed] [Google Scholar]
- 13.Sojikul P, Buehner N, Mason HS. A plant signal peptide–hepatitis B surface antigenfusion protein with enhanced stability andimmunogenicity expressed in plant cells. Proc. Natl. Acad. Sci. USA. 2003;100:2209–2214. doi: 10.1073/pnas.0438037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert RJC, Beales L, Blond D, Simon MN, Lin BY, Chisari FV, Stuart DI, Rowlands DJ. Hepatitis B small surface antigen particles are octahedral. Proc. Natl. Acad. Sci. USA. 2005;102:14783–14788. doi: 10.1073/pnas.0505062102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greiner VJ, Manin C, Larquet E, Ikhelef N, Gréco F, Naville S, Milhiet PE, Ronzon F, Klymchenko A, Mély Y. Characterization of the structural modifications accompanying the loss of HBsAg particle immunogenicity. Vaccine. 2014;32:1049–1054. doi: 10.1016/j.vaccine.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Q, Li S, Yu H, Xia N, Modis Y. Virus-like particle-based human vaccines: quality assessment based on structural and functional properties. Trends Biotechnol. 2013;31:654–663. doi: 10.1016/j.tibtech.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Zhang Y, Quan C, Luo J, Yang Y, Yu M, Kong Y, Ma G, Su Z. Aggregation and antigenicity of virus like particle in salt solution-A case study with hepatitis B surface antigen. vaccine. 2015 doi: 10.1016/j.vaccine.2015.03.078. doi: 10.1016/j. [DOI] [PubMed] [Google Scholar]
- 18.Brunner G. Supercritical fluids: technology and application to food processing. J. Food Eng. 2005;67:21–33. [Google Scholar]
- 19.Beckman EJ. Supercritical and near-critical CO2 in green chemical synthesis and processing. J. of Supercrit. Fluid. 2004;28:121–191. [Google Scholar]
- 20.Edman P. Method for Determination of the Amino Acid Sequence in Peptides. Acta Chem. Scand. 1950;4:283–293. [Google Scholar]
- 21.Grisshammer R. Understanding recombinant expression of membrane proteins. Curr. Opin. Biotech. 2006;17:337–340. doi: 10.1016/j.copbio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Kapusta J, Modelska A, Figlerowicz M, Pniewski T, Letellier M, Lisowa O, Yusibov V, Koprowski H, Plucienniczak A, Legocki AB. A plant-derived edible vaccine against hepatitis B virus. FASEB J. 1999;13:1796–1799. doi: 10.1096/fasebj.13.13.1796. [DOI] [PubMed] [Google Scholar]
- 23.Pniewski T, Kapusta J, Bociąg P, Wojciechowicz J, Kostrzak A, Gdula M, Fedorowicz-Strońska O, Wójcik P, Otta H, Samardakiewicz S, Wolko B, Płucienniczak A. Low-dose oral immunization with lyophilized tissue of herbicide-resistant lettuce expressing hepatitis B surface antigen for prototype plant-derived vaccine tablet formulation. J. Appl. Genetics. 2011;52:125–136. doi: 10.1007/s13353-010-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patil A, Khanna N. Novel membrane extraction procedure for the purification of hepatitis B surface antigen from Pichia pastoris. J. Chromatogr. B. 2012;898:7–14. doi: 10.1016/j.jchromb.2012.03.041. [DOI] [PubMed] [Google Scholar]
- 25.Gurramkonda C, Zahid M, Nemani KS, Adnan A, Gudi SK, Khanna N, Ebensen T, Lünsdorf H, Guzmán CA, Rinas U. Purification of hepatitis B surface antigen virus-like particles from recombinant Pichia pastoris and in vivo analysis of their immunogenic properties. J. Chromatogr. B. 2013;940:104–11. doi: 10.1016/j.jchromb.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 26.Lorber B, Fischer F, Bailly M, Roy H, Kern D. Protein analysis by dynamic light scattering: methods and techniques for students. Biochem. Mol. Biol. Educ. 2012;40:372–382. doi: 10.1002/bmb.20644. [DOI] [PubMed] [Google Scholar]
- 27.Lakowicz JR. Principles of Fluorescence Spectroscopy. second ed. Plenum Publishing Corporation; 1999. [Google Scholar]
- 28.Mason HS, Lam DM, Arntzen CJ. Expression of hepatitis B surface antigen in transgenic plants. Proc. Natl. Acad. Sci. USA. 1992;89:11745–11749. doi: 10.1073/pnas.89.24.11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith ML, Richter L, Arntzen CJ, Shuler ML, Mason HS. Structural characterization of plant-derived hepatitis B surface antigen employed in oral immunization studies. Vaccine. 2003;21:4011–4021. doi: 10.1016/s0264-410x(03)00268-8. [DOI] [PubMed] [Google Scholar]
- 30.Huang Z, LePore K, Elkin G, Thanavala Y, Mason HS. High-yield rapid production of hepatitis B surface antigen in plant leaf by a viral expression system. Plant Biotechnol. J. 2008;6:202–209. [Google Scholar]
- 31.Gavilanes F, Gomez-Gutierrez J, Aracil M, Gonzalez-Ros JM, Ferragur JA, Guerrero E, Peterson DL. Hepatitis B surface antigen: Role of lipids in maintaining the structural and antigenic properties of protein components. Biochem. J. 1990;265:857–864. doi: 10.1042/bj2650857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diminsky D, Schirmbeckt R, Reimannt J, Barenholz Y. Comparison between hepatitis B surface antigen (HBsAg) particles derived from mammalian cells (CHO) and yeast cells (Hansenula polymorpha): composition, structure and immunogenicity. Vaccine. 1997;15:637–647. doi: 10.1016/s0264-410x(96)00239-3. [DOI] [PubMed] [Google Scholar]
- 33.Greiner VJ, Egelé C, Oncul S, Ronzon F, Manin C, Klymchenko A, Mély Y. Characterization of the lipid and protein organization in HBsAg viral particles by steady-state and time-resolved fluorescence spectroscopy. Biochimie. 2010;92:994–1002. doi: 10.1016/j.biochi.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 34.Kang HJ, Baker EN. Intramolecular isopeptide bonds:protein crosslinks built for stress? Trends Biochem. Sci. 2011;36:229–237. doi: 10.1016/j.tibs.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Mangold CM, Streeck RE. Mutational analysis of the cysteine residues in the hepatitis B virus small envelope protein. J. Virol. 1993;67:4588–4597. doi: 10.1128/jvi.67.8.4588-4597.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desombere I, Willems A, Gijbels Y, Leroux-Roels G. Partial delipidation improves the T-cell antigenicity of hepatitis B virus surface antigen. J. Virol. 2006;80:3506–3514. doi: 10.1128/JVI.80.7.3506-3514.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.