Abstract
Allopolyploids, formed by hybridization and chromosome doubling, face the immediate challenge of having duplicated nuclear genomes that interact with the haploid and maternally inherited cytoplasmic (plastid and mitochondrial) genomes. Most of our knowledge of the genomic consequences of allopolyploidy has focused on the fate of the duplicated nuclear genes without regard to their potential interactions with cytoplasmic genomes. As a step toward understanding the fates of nuclear-encoded subunits that are plastid-targeted, here we examine the retention and expression of the gene encoding the small subunit of Ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco; rbcS) in multiple populations of allotetraploid Tragopogon miscellus (Asteraceae). These polyploids formed recently (~80 years ago) and repeatedly from T. dubius and T. pratensis in the northwestern United States. Examination of 79 T. miscellus individuals from 10 natural populations, as well as 25 synthetic allotetraploids, including reciprocally formed plants, revealed a low percentage of naturally occurring individuals that show a bias in either gene (homeolog) loss (12%) or expression (16%), usually toward maintaining the maternal nuclear copy of rbcS. For individuals showing loss, seven retained the maternally derived rbcS homeolog only, while three had the paternally derived copy. All of the synthetic polyploid individuals examined (S0 and S1 generations) retained and expressed both parental homeologs. These results demonstrate that cytonuclear coordination does not happen immediately upon polyploid formation in Tragopogon miscellus.
Introduction
Allopolyploidy is a major mode of plant speciation and results from the union of two or more diverse, but generally closely related, genomes by hybridization and genome duplication [1, 2]. Genomic data indicate that all angiosperms may be regarded as polyploid, if paleopolyploid events are taken into account [3–5]. Allopolyploid genomes experience both immediate and long-term evolutionary changes, which may involve a variety of genetic and epigenetic interactions leading to genome alteration, regulatory incompatibilities, chromosomal abnormalities, and reproductive challenges [6–14]. Polyploidy has been considered a driver of modifications in gene function, potentially resulting in four fates for the duplicated genes (homeologs): (I) both copies are preserved and retain their original functions, (II) one copy maintains the original function whereas the other copy is silenced, or (III) the two copies diverge such that each copy contributes only a part of the original gene function (subfunctionalization) or (IV) one copy attains a novel function (neofunctionalization) [15–21].
In newly formed allopolyploids, coordination between the haploid maternally inherited cytoplasmic (plastid and mitochondrial) and the duplicated biparentally inherited nuclear genomes is required to facilitate genomic stability [8]. Indeed, ‘cytonuclear interactions’ are considered responsible for post-zygotic hybrid incompatibilities and speciation [22–24] and have also caused striking differences in floral traits in reciprocal diploid hybrids [25, 26]. Cytonuclear coordination may also be a contributor to the directional genomic changes and preferential expression of some genes in reciprocally formed polyploids [27–29].
Recent studies of Rubisco (Ribulose-1,5-bisphosphate carboxylase/oxygenase), which comprises a nuclear-encoded subunit (rbcS) and a chloroplast-encoded subunit (rbcL), in allopolyploids have revealed a dynamic nature to the evolution of the nuclear component [30, 31]. In several allopolyploid systems, the duplicated nuclear gene copies (homeologs) of rbcS undergo gene conversion in favor of maintaining the maternally derived copy even when the parental chloroplast sequences of rbcL are not divergent [30]. Additionally, a common feature of this system is that allopolyploids show preferential expression of the maternal rbcS homeolog when both copies are maintained in the genome [30, 31]. How early following polyploid formation this cytonuclear coordination might be established is not known as the polyploids studied to date are several hundred thousand to several million years old [30, 31].
An excellent model system for studying the early stages of allopolyploid cytonuclear coordination is offered by Tragopogon (Asteraceae). Following the introduction of three diploid species from Europe (Tragopogon dubius, T. pratensis, and T. porrifolius) to the Palouse region of eastern Washington State/western Idaho, USA, in the early 1900s, two allopolyploid species were formed. Tragopogon mirus (T. dubius × T. porrifolius) and T. miscellus (T. dubius × T. pratensis) both formed repeatedly in the past 80 years in western North America with T. miscellus also forming reciprocally, yielding short-liguled (T. dubius ♂ × T. pratensis ♀) and long-liguled (T. dubius ♀ × T. pratensis ♂) forms [32–34]. Recurrent formation of both allopolyploids and restricted gene flow among origins [35–38] offer an opportunity to determine if independently formed polyploids develop similar cytonuclear coordination. Previous studies have identified a myriad of genomic and transcriptomic modifications in the Tragopogon allopolyploids in the short time since their formation, including differential expression of homeologous loci, homeolog loss and silencing, differential proteomes [1, 39–46], and extensive chromosomal variation, such as aneuploidy and intergenomic translocations [13, 47]. Moreover, the formation of synthetic polyploids of Tragopogon has allowed the analysis of genomic modifications at early stages of polyploid formation [48].
Here, we use the Rubisco system (rbcS and rbcL) to examine cytonuclear coordination in naturally occurring and synthetic Tragopogon miscellus allopolyploids, representing independent and reciprocal formations. We characterize rbcS in the Tragopogon diploid parental species to answer the following questions: (1) How divergent are rbcS and rbcL in the T. miscellus progenitors? (2) Is there differential retention of rbcS homeologs in T. miscellus? (3) When both parental copies of rbcS are retained, do the naturally occurring and synthetic polyploids of T. miscellus show equal or biased expression of the rbcS homeologs?
Materials and Methods
Plant material
The populations sampled for Tragopogon dubius Scop. T. pratensis L. and T. miscellus Ownbey are listed in S1 Table, as are the synthetic lineages of T. miscellus examined. For T. miscellus, we included individuals from nine short-liguled populations (T. pratensis maternal parent) and one long-liguled population (T. dubius maternal parent), the latter representing the only extant natural population of this form. To assess potential variability in the diploid progenitors, genomic DNA and cDNA were included for multiple individuals of T. dubius (12) and T. pratensis (8) from different populations (S1 Table). For T. miscellus, four synthetic lineages (25 individuals) and ten populations (79 individuals total) were sampled. Plant material for most of the polyploids and diploids was the same as that used in Tate et al. [45, 46] and Buggs et al. [40]. For the synthetics, mature seeds were grown under standard glasshouse conditions at Massey University (Palmerston North, New Zealand); these lines were generated by Tate et al. [48]. For expression analyses, only a subset of individuals (31) was studied due to limited availability of fresh material for RNA extraction (S1 Table).
DNA and RNA extraction
Both DNA and RNA were extracted from leaf tissue 28 days after seed germination. For DNA, a modified CTAB extraction protocol was used [49]. For RNA extraction, leaf tissue was flash-frozen in liquid nitrogen and ground in a 1.5-ml tube using a sterile pestle. Total RNA was extracted using the RNeasy Plant Mini kit (Qiagen, UK). First-strand cDNA was synthesized from 200 ng of total RNA using SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen, CA, USA).
Primer design, PCR and sequencing of rbcL and rbcS-1
Full length rbcL was amplified from the diploid progenitors and T. miscellus using primers rbcL1 and rbcL2 ([50, 51] primer names as in [52]). PCR reactions were conducted in a 25-μl total volume containing 10X Thermopol buffer (New England Biolabs, USA), 10 mM dNTPs, 5 μM each primer, 0.5 Unit NEB Taq polymerase and ~50 ng of either genomic DNA or cDNA template. The following PCR profile was used: 95°C for 5 min, 48°C for 45 sec, 72°C for 1 min followed by 35 cycles at 95°C for 1 min, 48°C for 45 sec (2 sec added in each successive cycle) and 72°C for 1 min, with a final extension at 72°C for 10 min [52].
Initial amplification of rbcS was accomplished by designing PCR primers from an alignment of T. dubius ESTs (Tdu01-5MS1_K18.e, Tdu01-3MS1_B10.e, Tdu01-2Ms1_K16.e) to Lactuca sativa rbcS (AF162210) using Primer3 [53]. Primers (rbcS-2F and rbcS-2R) for one copy, hereafter rbcS-1, were used for the initial amplification of both genomic and cDNA of the diploids (Table 1). A second rbcS-like sequence was identified in the T. dubius EST database, but this copy is apparently a pseudo-gene as it is truncated (missing 5’ UTR through exon 1) with several premature stop codons and indels as compared to the full-length rbcS-1 and rbcS sequences from other Asteraceae (S1 Fig). For this second copy, rbcS-2, 5’ genome walking (using methods described later for rbcS-1) revealed the presence of a long ~700-bp intron-like sequence (data not shown) that has not been found in any angiosperm group to date [54]. Likewise, this rbcS-2 copy is also truncated in T. pratensis. Amplification of rbcS-1 was conducted using the following PCR profile: 95°C for 5 min, 95°C for 1 min, 53°C for 1 min, 72°C for 1 min for 5 cycles, followed by 44 cycles of 95°C for 1 min, 48°C for 1 min, 72°C for 1 min and a final extension at 72°C for 7 min. PCR products of rbcS-1 from genomic DNA of T. dubius and T. pratensis were cloned using the TOPO TA Cloning Kit (Invitrogen, CA, USA).
Table 1. rbcS-1 primers designed in this study.
| Primer Name | Experiment(s) | Primer/oligo Sequence (5’to 3’) |
|---|---|---|
| GS1 | 5’ Genome walking | ATCATACCTTCATGCACTGCACTCTTCCAC |
| GS2 | 5’ Genome walking | AGGAAAAGTCATTGGCCTTCTTGGTGACTG |
| AP1 | 5’ Genome walking | GTAATTCGCATCACTATAGCTC |
| AP2 | 5’ Genome walking | ACTATAGCTCACCGCTGGT |
| NA44 | 5’ Genome walking | GTAATTCGCATCACTATAGCTCACCGCTGGTCGACGGCCCGGGCTGGT |
| NA45 | 5’ Genome walking | PO4-ACCAGCCC-NH2 |
| Inv. Fwd 1 | 3’ RACE | TGGACCTCAATCGGGTTTAT |
| Inv. Fwd 2 | 3’ RACE | CAAGAAGGAGTACCCCAACG |
| 3'RACE adapter | 3’ RACE | GACTCGAGTCGACATCG |
| 3'RACE oligodT adapter | 3’ RACE | GACTCGAGTCGACATCGATTTTTTTTTTTTTTTTTV |
| rbcS-2F | Sequencing and CAPS | AATGGCTTCCATCTCCTCCT |
| rbcS-3F | Sequencing and CAPS | TTTCCCAGTCACCAAGAAGG |
| rbcS-2R | Sequencing and CAPS | AGGCAACTTCCACATTGTCC |
| rbcS-8R | Sequencing and CAPS | CGATTGAGGTCCATCCAAAG |
| rbcS-F1 | Sequencing | CAAAACATACCCATAACGTATCAGCC |
| rbcS-R3 | Sequencing | AGCAGAAACATAAATTTTTATTATTATCATC |
| HS-Pra-Snp3 | Homeolog-specific RT-PCR (forward) | AAGGCCAATGACTTTTCCTCCCGC |
| HS-Dub-Snp3A | Homeolog-specific RT-PCR (forward) | AAGGCCAATGACTTTTCCTCCCAT |
| HS-R3 | Homeolog-specific RT-PCR (reverse) | CGAACATAGGCAACTTCCACATTGTCC |
Ten positive clones per sample were sequenced. Prior to sequencing, PCR products were treated with exonuclease I (5 Units) and shrimp alkaline phosphatase (0.5 Unit). Cycle sequencing was performed using Big Dye v.3.1 (Applied Biosystems, Inc.), and purified products were sequenced on an ABI DNA Analyzer 3770 at the Massey Genome Service (Palmerston North, New Zealand) using both T3 and T7 plasmid primers. Sequencing results were analyzed in Sequencher v.5.1 (Gene Codes Corporation, Michigan, USA). Based on the alignment of these cloned sequences with available T. dubius ESTs, a new reverse primer (rbcS-8R) was designed further downstream to amplify a longer portion of rbcS-1 from synthetic and naturally occurring T. miscellus polyploids; these longer fragments of rbcS-1 were then sequenced directly using the aforementioned sequencing protocol with both forward (rbcS-2F) and reverse (rbcS-8R) primers (Table 1).
Genomic and cDNA CAPS analysis
Sequences of rbcS-1 for the diploid parents were aligned to determine sequence variation that could differentiate parental homeologs in T. miscellus. The programs dCAPS Finder 2.0 [55] and NEB Cutter v.1.0 [56] were used to identify diagnostic restriction sites between parental rbcS-1 sequences. Genomic and cDNA cleaved amplified polymorphic sequence (CAPS) analyses were performed for rbcS-1 using the forward primer rbcS-3F and reverse primer rbcS-8R (Table 1). The amplified region included exon 1 (from aligned position 433 bp), intron 1 and exon 2 (to position 1054 bp) (Fig 1). The resulting PCR products from T. dubius and T. pratensis were 462 bp for cDNA and 622 bp (T. dubius) and 628 bp (T. pratensis) from genomic DNA. PCR products were digested with MseI, which cuts the cDNA of T. dubius at one position (resulting in fragment sizes 375 bp and 87 bp) and does not cut T. pratensis. For genomic DNA, T. dubius is cut at three positions (resulting in fragment sizes 272 bp, 167 bp, 154 bp and 29 bp), while T. pratensis is cut at two positions (resulting in fragment sizes 327 bp, 272 bp and 29 bp). For PCRs of both genomic and cDNA, a digestion reaction was set up in a total volume of 10 μl containing 1 μl of the PCR product, 1X buffer 4 (New England Biolabs, USA), 100 μg/ml Bovine Serum Albumin and 20 Units of MseI enzyme (New England Biolabs, USA). Reactions were incubated at 37°C for 3 hours as specified by the manufacturer. The digested products were run on a 2% agarose gel, stained with ethidium bromide and analyzed using a Gel Doc 2000 system (Bio-Rad, UK). After establishing the protocols for the diploid parents, rbcS-1 was PCR-amplified from the naturally occurring and synthetic polyploids of T. miscellus and digested following the same protocols. We also included an artificial hybrid DNA or cDNA template, which contained an equal mixture of the two parental DNAs or cDNAs for genomic and cDNA CAPS, respectively. As a control to verify equal expression of parental homeologs for cDNA CAPS, actin was amplified (actinF: 5’-GGAGCAGAGAGATTCCGTTG-3’, actinR: 5’-CTCTCTGGAGGAGCAACCAC-3’) and digested with BspHI (S2 Fig) following the CAPS protocol above. The PCR conditions used to amplify actin were the same as those for rbcS-1.
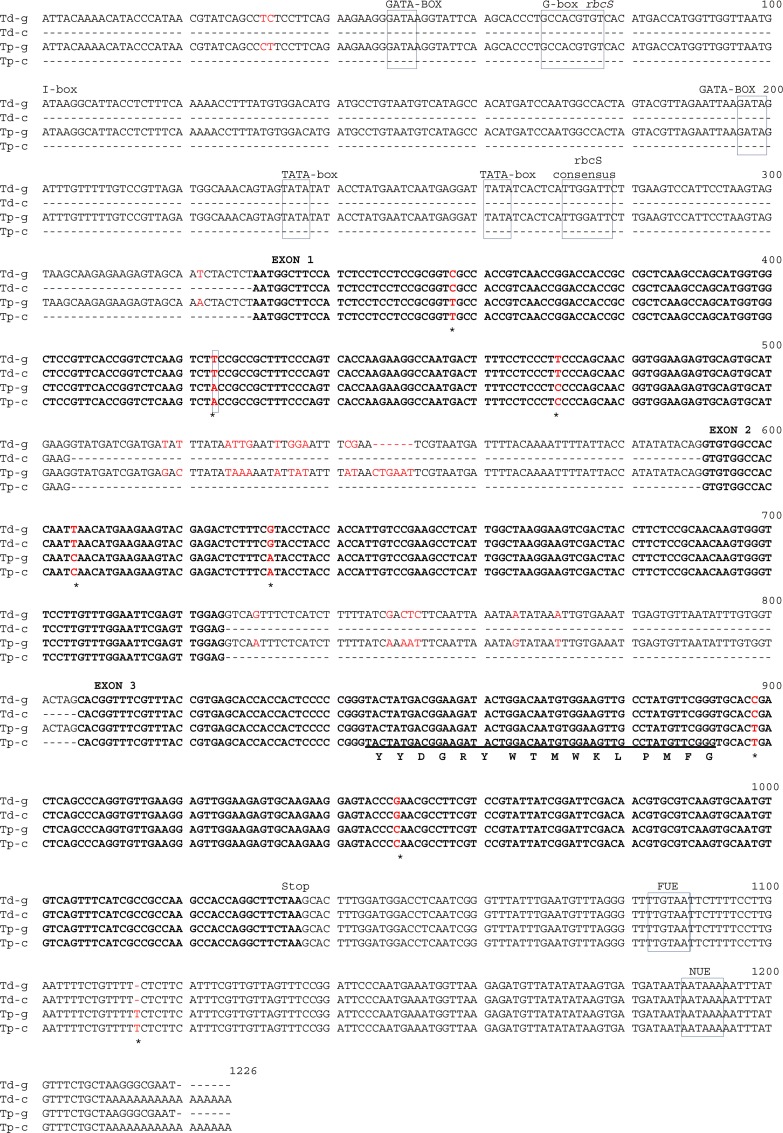
Fig 1. rbcS-1 gene structure and locations of SNPs between Tragopogon dubius and T. pratensis.
Upstream 5’ elements, the stop codon, and downstream 3’ elements are labelled above the nucleotide sequences. The conserved hexadecapeptide sequence [54] is highlighted and the amino acid residues indicated below the sequences. SNPs between the parental sequences are highlighted in red text and an asterisk (exons), with the one non-synonymous change surrounded by a box. Td-g = Tragopogon dubius-genomic; Td-c = Tragopogon dubius-cDNA; Tp-g = Tragopogon pratensis-genomic; Tp-c = Tragopogon pratensis-cDNA. FUE = far upstream element, NUE = near upstream element.
5ʹ Genome walking and 3’ RACE of rbcS-1
To obtain full-length rbcS-1 sequence, we employed a 5’genome walking technique to amplify upstream unknown gene sequence (using a homemade kit following the GenomeWalker manual, Clontech Laboratories) [57]. Two outward-facing gene-specific primers were designed near the 5’ end of the T. dubius rbcS-1 sequence to act as reverse primers (GS1 and GS2, Table 1). Long and short oligos to form an adapter and adapter-specific primers (to act as forward primer) were designed as described by the GenomeWalker user manual (NA44 and NA45, Table 1). Tragopogon dubius genomic DNA was digested with three different blunt-cutting enzymes: EcoRV, ScaI and DraI (New England Biolabs) independently using 2.5 μg of genomic DNA, 80 Units of restriction enzyme and 10X buffer (New England Biolabs) in a total volume of 100 μl. Reactions were incubated at 37°C for 16–18 hours. These digestion reactions were cleaned by ethanol precipitation in the presence of 20 μg glycogen and 3M sodium acetate. Adapters were ligated to the cleaned, digested genomic DNA in a total volume of 8 μl containing 25 μM adapter, 10X ligation buffer, 3 Units of T4 DNA ligase (New England Biolabs) and 0.5 μg of purified DNA. Primary PCR was conducted in 50-μl total volume using 10 mM dNTPs, 10X PCR buffer (Takara Biotechnology, Japan), 10 μM adapter primer AP1 (Forward) and gene-specific primer GS1 (Reverse) (Table 1) and 1 Unit of Takara Ex Taq polymerase (Takara Biotechnology, Japan). Cycling conditions for the primary PCR were as follows: first 7 cycles at 94°C for 25 sec, 72°C for 3 min, then the remaining 32 cycles at 94°C for 25 sec, 67°C for 3 min, then final extension at 67°C for 7 min. Primary PCR products from the first round were diluted 1:50 in ddH2O. In the secondary PCR, 10 μM nested or internal adapter primer AP2 (forward) and gene-specific primers GS2 (reverse) were used (Table 1), and 2 μl of diluted primary PCR product were used as template. The secondary PCR profile was as follows: 94°C for 25 sec, 72°C for 3 min for 5 cycles and 94°C for 25 sec, 67°C for 3 min for the next 20 cycles, then final extension at 67°C for 7 min. Secondary PCR products were separated on a 1% agarose gel, and products from each library were cloned and sequenced using the protocols described above. The resulting sequences were aligned to the previously obtained partial rbcS-1 sequence of T. dubius.
To obtain the 3’ end of the rbcS-1 gene, 3’ RACE was used. Two gene-specific nested inverse primers were designed near the 3’ end of the known rbcS-1 gene sequence (Inv. Fwd 1 and Inv. Fwd 2, Table 1). First-strand cDNA from T. dubius was made using an oligo(dt) incorporating a 3’ RACE-specific primer sequence at the 5’ end (3’ RACE oligodT adapter, Table 1). After synthesizing T. dubius cDNA, primary PCR for 3’ RACE was conducted in a 25-μl total volume containing 5 μM gene-specific inverse primer (Inv. Fwd 2) as a forward primer and 5 μM 3’ RACE adapter primer as a reverse primer, 10X PCR buffer, 10 mM dNTPs and 1 Unit Takara Ex Taq polymerase. The PCR profile was as follows: 95°C for 1 min, 53°C for 1 min, 72°C for 1 min for 5 cycles, followed by 44 cycles at 95°C for 1 min, 48°C for 1 min, 72°C for 1 min, with a final extension at 72°C for 7 min. This primary PCR product was diluted 100X and used as template for nested PCR. The nested PCR mix contained all of the above reagents, except 5 μM nested primers (Inv. Fwd 1 and 3’ RACE adapter primer, Table 1) was used. Cycling conditions were the same as the 3’ RACE primary PCR. Products from the nested PCR were cloned, sequenced and aligned with the previous rbcS-1 gene sequence from T. dubius. Once the complete rbcS-1 gene sequence for T. dubius was obtained, new primers were designed (rbcS-F1 and rbcS-R3, Table 1) for the amplification and sequencing of the complete rbcS-1 gene from genomic DNA and cDNA of T. pratensis.
Prediction of rbcS-1 gene structure
Gene structure of rbcS-1 was predicted using Augustus (Version 2.6) [58] and GENSCAN [59]. These programs were used to confirm the transcription start site (TSS), exons, introns and other regulatory sequences as determined by cDNA sequencing of the complete rbcS-1 gene. Plant Promoter Analysis Navigator (PlantPAN) [60] was used to identify promoter sequences of rbcS-1, putative transcription factor binding sites in the promoter region and conserved motifs in the promoter (S2 Table).
Homeolog-specific RT-PCR
Homeolog-specific RT-PCR was conducted to amplify each of the diploid parental homeologs of rbcS-1 from cDNA of the Tragopogon miscellus polyploids. Homeolog-specific (HS) primers were based on SNPs identified between parental rbcS-1 homeologs [61]. Homeolog specificity was assured by adding a mismatch one nucleotide away from the 3’ end of each of the two forward HS primers (T. dubius: HS-dub-Snp3A, T. pratensis: HS-Pra-Snp3, Table 1). A common reverse primer was designed downstream of the polymorphic site (HS-R3, Table 1), corresponding to a highly conserved region in exon 3 (Fig 1). For the present experiment, forward primers were designed at the third SNP in exon 1 (corresponding to position 471 bp, Fig 1). PCR conditions were as follows: 95°C for 1 min, 60°C for 45 sec, 72°C for 1 min for 35 cycles with a final extension at 72°C for 10 min. PCR was conducted in a 25-μl total volume containing 10X PCR buffer, 10 mM dNTPs, 5 μM each primer, 0.5 Unit Taq polymerase (New England Biolabs, USA) and 15 ng/μl template (cDNA). The amount of template cDNA included in the PCR was quantified using a Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific, USA) and normalized in the PCR reaction. Resulting PCR products were run on a 1.5% agarose gel and scored for presence/absence of parental homeologs or inspected to determine relative intensity of resulting bands. Different numbers of PCR cycles (30, 35, 40, and 45) were tested to determine the potential effect of amplification cycles. As no differences were observed among the different cycle numbers, the same PCR profile was used for all cDNA amplifications. Artificial hybrid cDNA, which was a 50:50 mix of T. dubius and T. pratensis cDNA, was again included as a control for equal amplification of the parental homeologs.
Results
rbcS gene family
Two rbcS gene copies, rbcS-1 and rbcS-2, were identified from the Tragopogon dubius EST database. These two rbcS genes are fairly divergent from each other, with several SNPs, insertions and deletions in the genic regions and even more variability at the 3’ UTRs (S1 Fig). Of these two rbcS genes, rbcS-1 was determined to be a functional copy, while the second rbcS gene, rbcS-2, was considered a pseudogene and a truncated copy as it had premature stop codons compared to the rbcS-1 amino acid alignment. Several attempts at 5’genome walking experiments yielded non-rbcS genomic sequences (e.g., plastid atpB sequence) at its flanking ends where conserved sequence would have been expected. We found a similar scenario with T. pratensis. Hence, we focused on rbcS-1 to examine potential cytonuclear coordination in T. miscellus. Sequences of rbcS-1 sequences of T. dubius and T. pratensis were deposited in GenBank (accession numbers: KT879189, KT879190, respectively).
The total length of the rbcS-1 sequence with coding and non-coding regions, including upstream promoter elements and downstream terminator signals, was 1212 bp in Tragopogon dubius and 1219 bp in T. pratensis. Fig 1 shows the full-length genomic sequences of these two diploid species with the conserved 5’ upstream elements, canonical hexadecapeptide sequence [54] and SNPs between them highlighted. No intraspecific variation in genomic sequences of rbcS-1 was detected among individuals of either Tragopogon dubius or T. pratensis.
Divergence of rbcS-1 and rbcL between the diploids and their pattern of inheritance and retention in T. miscellus
Comparative sequence analysis of rbcS-1 in T. dubius and T. pratensis revealed seven SNPs in the exons and a 1-bp indel in the 3’ UTR between polyadenylation signals at 1114 bp (Fig 1). Six of these SNPs were synonymous substitutions, with the second SNP at 424 bp a non-synonymous change resulting in a threonine in T. pratensis and a serine in T. dubius. Non-coding regions (upstream promoter regions and introns) were also found to contain multiple SNPs and indels (Fig 1) between the diploids. Analysis of the predicted protein structure of the rbcS-1 sequence using the protein homology/analogy recognition engine Phyre2 V 2.0 [62] revealed that the non-synonymous SNP resides in an alpha-helix and does not cause any difference in predicted protein structure between rbcS-1 parental homeologs. Genomic rbcL (1415 bp) sequences from both diploid parents were compared, and only one SNP was discovered at 703 bp, resulting in a synonymous substitution (Genbank accessions: KT897489, KT897491).
To determine the pattern of retention of these subunits, genomic copies of both rbcS-1 and rbcL were analyzed from 25 synthetic polyploid individuals (representing five independently generated lineages) and 79 naturally occurring polyploids from 10 populations of T. miscellus. In the case of rbcL, all synthetic and naturally occurring polyploids had the maternally derived sequence (i.e., T. pratensis for the short-liguled form and T. dubius for the long-liguled form; T. miscellus Genbank accessions: KT897488, KT897490). For rbcS-1, all synthetic polyploids and 69 of the naturally occurring polyploids had both T. dubius and T. pratensis rbcS-1 homeologs, as determined by additivity of the genomic CAPS analysis (Fig 2A). Inspection of the chromatograms resulting from directly sequenced rbcS-1 products from these same T. miscellus individuals also revealed additivity of peaks at SNPs between the parents. Ten polyploid individuals from six natural populations [Spangle (2), Garfield (1), Albion (3), Moscow (2), Pullman (1) and Troy (1)] had only one homeolog present in the genomic DNA. Of nine short-liguled individuals, six had the maternally derived rbcS-1 homeolog (T. pratensis), and three had the paternally derived copy (T. dubius, Fig 2A, Table 2, S1 Table). One long-liguled individual from Pullman retained the T. dubius (maternal) genomic homeolog only (Fig 2A, Table 2).
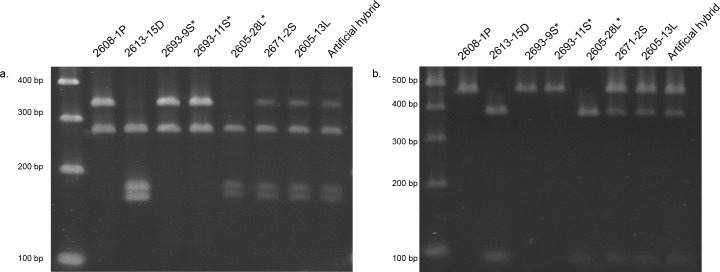
Fig 2. Genomic (a) and cDNA (b) cleaved amplified polymorphic sequence (CAPS) results for representative samples of naturally occurring Tragopogon miscellus polyploids and the diploid parents, T. dubius (D) and T. pratensis (P).
T. pratensis is the maternal parent of the short-liguled (S) individuals, and T. dubius is the maternal parent of the long-liguled (L) individuals. An asterisk (*) indicates homeolog loss in T. miscellus. The artificial hybrid contained equal mixture of T. dubius and T. pratensis genomic DNA (a) or cDNA (b). Population codes are detailed in S1 Table.
Table 2. Naturally occurring individuals of Tragopogon miscellus that showed bias in the retention and/or expression of parental rbcS-1 homeologs.
| Population | Maternal parent | Lineage | Retention of rbcS homeologs | Expression of rbcS homeologs |
|---|---|---|---|---|
| Spangle | T. pratensis | 2693–7 | Both | T. pratensis > T. dubius |
| Spangle | T. pratensis | 2693–9 | T. pratensis only | T. pratensis only |
| Spangle | T. pratensis | 2693–11 | T. pratensis only | T. pratensis only |
| Oakesdale | T. pratensis | 2671–2 | Both | T. pratensis > T. dubius |
| Oakesdale | T. pratensis | 2671–11 | Both | T. pratensis > T. dubius |
| Garfield | T. pratensis | 2688–8 | T. pratensis only | T. pratensis only |
| Garfield | T. pratensis | 2688–12 | Both | T. pratensis > T. dubius |
| Moscow | T. pratensis | 2604–17 | T. pratensis only | T. pratensis only |
| Moscow | T. pratensis | 2604–22 | T. pratensis only | T. pratensis only |
| Moscow | T. pratensis | 2604–43 | Both | T. pratensis > T. dubius |
| Albion | T. pratensis | 2625–3 | T. dubius only | - |
| Albion | T. pratensis | 2625–6 | T. dubius only | - |
| Albion | T. pratensis | 2625–8 | T. dubius only | - |
| Troy | T. pratensis | 2682–5 | T. pratensis only | - |
| Pullman | T. dubius | 2605–9 | Both | T. dubius > T. pratensis |
| Pullman | T. dubius | 2605–28 | T. dubius only | T. dubius only |
| Pullman | T. dubius | 2605–46 | Both | T. dubius > T. pratensis |
A dash (-) indicates that material was not available to study a particular individual for both retention (genomic DNA) and expression (cDNA).
Expression of rbcS-1 homeologs in T. miscellus polyploids
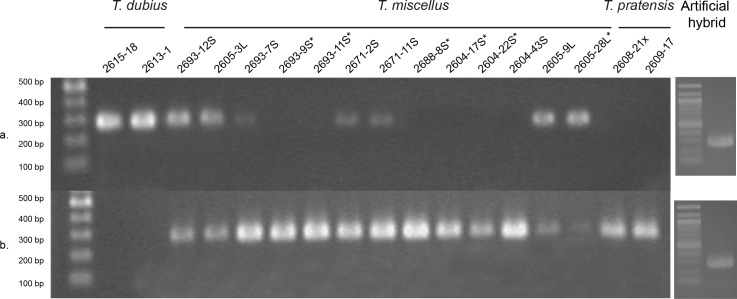
Relative expression of parental rbcS-1 homeologs was determined by cDNA CAPS (Fig 2B) and HS-RT-PCR (Fig 3) analyses. Because the cDNA CAPS results did not appear to show any appreciable differences in homeolog expression, HS-RT-PCR was employed as a potentially more sensitive method to detect differential homeolog expression. For the six synthetic polyploid individuals examined, all showed equal expression of the parental homeologs (S1 Table). Of the 31 naturally occurring polyploid individuals examined, five showed deviation from additive expression (Table 2, Figs 2B and 3), as determined by comparison of the relative intensity or presence/absence of parental homeologs in the polyploids, using the positive controls as a baseline for those comparisons. In all cases where both genomic copies were detected, the maternally derived rbcS-1 homeolog was expressed at a greater level (Fig 3). The five individuals showing differential expression were from different populations and represented one long-liguled individual from Pullman and four short-liguled individuals [Moscow (1), Oakesdale (2), and Spangle (1)]. All T. miscellus individuals that showed biased expression of the rbcS-1 homeologs were found to have equal expression of T. dubius and T. pratensis actin homeologs (S2 Fig).
Fig 3. Homeolog-specific HS-RT-PCR of rbcS-1 for representative individuals of Tragopogon miscellus and diploid progenitors T. dubius and T. pratensis.
RT-PCR results using T. dubius-specific primers (a) and T. pratensis-specific primers (b). T. pratensis is the maternal parent of the short-liguled (S) individuals, and T. dubius is the maternal parent of the long-liguled (L) individuals. An asterisk (*) indicates homeolog loss in T. miscellus. The artificial hybrid contained equal mixture of T. dubius and T. pratensis cDNA. Population codes are detailed in S1 Table.
Discussion
Characterization of rbcS-1 in Tragopogon diploid species
In angiosperms, the rbcS small subunit is fairly divergent among species and is often encoded by a multigene nuclear family [63–67], compared to the plastid rbcL, which is highly conserved and present in single copy [66]. Indeed, only one SNP distinguished the Tragopogon progenitor rbcL copies, and this resulted in a synonymous substitution. As is the case in most other eudicots [30, 63], the rbcS-1 gene in Tragopogon consists of three exons separated by two short introns. The second copy found in T. dubius and T. pratensis (rbcS-2) may represent a pseudogene on its way to being lost from the genome. In most other angiosperms, the rbcS gene family ranges in size from four (Arabidopsis) to more than 22 (wheat) copies [64, 67, 68]. Generally, only one or two members of the rbcS gene family are strongly expressed in the angiosperms surveyed to date, and these genes contribute more than half of the total rbcS transcripts [69, 70]. Indeed, concerted evolution of the rbcS gene family is probably a common phenomenon even in diploid taxa [30]. In other members of Asteraceae, Lactuca sativa (tribe Cichorieae) has six rbcS genes [71], and Flaveria species (Heliantheae) contain from 5–16 genes [72], while Helianthus (Heliantheae) [73] and Chrysanthemum (Anthemideae) [69] each has only one rbcS gene. Thus, rbcS may be diverse in copy number even within the same plant family or tribe (Tragopogon is a member of the Cichorieae), perhaps due to general processes of gene loss, genome downsizing, concerted evolution or a mere lack of expansion of the gene family [74, 75].
Interspecific rbcS-1 sequence variation was low between the parental diploids Tragopogon dubius and T. pratensis (2.5% sequence divergence, 0.5% amino acid divergence), compared to other genera (Gossypium [31], Arabidopsis [64], Triticum [68]). Only one non-synonymous substitution was detected between T. dubius and T. pratensis rbcS-1 homeologs; this SNP resided in the α-helix of the predicted protein and did not result in a change in protein structure or folding. It is not obvious if this change has an influence on the seemingly maternal bias toward homeolog retention and expression in the T. miscellus polyploids.
Genomic loss and expression of rbcS-1 homeologs biased towards the maternal parent in T. miscellus polyploids
From an evolutionary perspective, the dynamic nature of polyploid genomes is well known [8, 76, 77]. Homeolog loss is one genetic modification commonly observed following genome duplication in a diverse array of polyploid species (Brassica [11], Triticum [14], Tragopogon [44], Gossypium [78], Arabidopsis [79]). The results presented here are generally consistent with previous findings of preferential retention and expression of maternal homeologs in Tragopogon [40, 44, 45, 80]. In polyploids, homeolog losses may be associated with dosage compensation to efficiently maintain gene regulatory mechanisms [81]. In the case of cytonuclear coordination involving multi-subunit complexes, like Rubisco, loss of the paternal homeolog and retention of the maternal copy may facilitate the regulatory coordination between the maternal and paternal genomes. However, in Tragopogon, this coordination is not immediate upon polyploid formation as the synthetics and most of the naturally occurring polyploids still retain and express both parental homeologs. Compared to other polyploid systems that are much older [30, 31], we also did not find any evidence for unique mutations (autapomorphies) in the natural or synthetic Tragopogon miscellus rbcS sequences. Examination of other cytonuclear complexes would lend insight to the potential to maintain genomic balance between nuclear homeologs and their cytoplasmic counterparts.
Changes in duplicate gene expression are another consequence of allopolyploidization [1, 8, 82, 83], which may involve biased expression of the parental homeologs in the polyploids. This bias may be balanced, with an equal number of genes showing bias towards each parent, or unbalanced, with more genes displaying bias towards one parent [28, 84–87]. Previous studies on Tragopogon identified alterations in expression of homeologous loci (i.e., T. dubius loci silenced more often than T. pratensis) [1, 44, 88]. In this study, expression of parental rbcS-1 homeologs was biased toward the maternal parent, although again very few individuals showed this pattern and it was not immediately upon allopolyploid formation.
The successful establishment of F1 hybrids and allopolyploids requires coordination between the maternally inherited cytoplasmic (plastid and mitochondrial) and the biparentally inherited nuclear genomes to facilitate genomic stability [22, 89]. Cytoplasmic factors, including a variety of nucleo-cytoplasmic co-evolutionary pathways, have been considered responsible for post-zygotic hybrid incompatibilities and therefore a driver of plant speciation [23]. Here we show that this coordination may be a slower process and does not occur immediately upon formation in Tragopogon, however, given that the naturally occurring Tragopogon miscellus populations are less than 80 years old (~40 generations as they are biennials), sorting out potential cytonuclear incompatibilities may only take a few generations to begin. Examination of the synthetic lineages over successive generations would lend valuable insight as to when these changes start to occur.
The biased retention and expression of maternal rbcS-1 homeologs in individuals from different populations indicates repeatability of this evolutionary trajectory because each population of T. miscellus represents an independent formation [33, 36, 38, 90]. However, within a population, the observed homeolog losses may result from the same historical event; thus, our estimates of absolute losses may be lower (six rather than ten). Although the majority of the individuals showed maternal bias, three individuals from the Albion population were an exception. These short-liguled individuals retained the paternal (T. dubius) rbcS-1 genomic homeolog, instead of the T. pratensis copy. Unfortunately, fresh material was not available to study rbcS-1 expression in individuals from this population, so we do not know if the paternal bias is restricted to genome loss or extends to homeolog expression as well for individuals that retained both homeologs. In a previous study of homeolog loss in T. miscellus [45], this population showed a greater number of homeolog losses (individual 2625–3 in particular) than all other populations. Although in general there seems to be a recurrent pattern toward maternal bias, in some populations different rbcS/rbcL parental combinations might be beneficial to facilitate cytonuclear interactions.
Given that the predicted protein structure of both parental rbcS-1 homeologs is the same and the rbcL progenitor copies only differ by one synonymous SNP, exactly what has driven differential expression of the maternal copy of rbcS-1 in the naturally occurring polyploids is not yet understood. There are several possible explanations for the expression biases observed. First, the polymorphisms observed between rbcS-1 homeologs in the promoter region (e.g., one SNP was found eight nucleotides away from the transcription start site) might be responsible for differential regulation of rbcS-1 homeologs, and later, their interaction with the rbcL-encoded subunit. Dean et al. [63] found that specific rbcS copies in Petunia (Solanaceae) contained ‘enhancer-like’ elements in the promoter region that resulted in quantitative differences in expression levels, even when there was a high degree of similarity in coding sequence among other copies. This region, termed box II, was also identified in other solanaceous genera (tomato, Solanum, and tobacco, Nicotiana), and rbcS copies with this motif were expressed at a greater level than were other copies. Tragopogon also contains this enhancer-like motif, but no SNPs between the parents were identified in this region. Perhaps the other polymorphisms in the promoter region contribute to the expression differences observed here.
A second explanation for the expression bias is that the one non-synonymous change in exon I (threonine in T. pratensis and serine in T. dubius) may result in differential selection on the rbcS-1 copies under some conditions. Further research involving protein-protein interactions between rbcS/rbcL subunits in T. miscellus would be helpful to clarify the complexities of these cytonuclear interactions.
Supporting Information
The conserved hexadecapeptide motif (YYDGRYWTMWKLPMFG) is indicated in red text.
(PDF)
A.H. stands for artificial hybrid which was a 1:1 mixture of T. dubius and T. pratensis cDNA.
(PDF)
Data are summarized from genomic DNA and cDNA CAPS and homeolog-specific RT-PCR. Note: Letters “D” and “P” correspond to the diploid parents T. dubius and T. pratensis, respectively. A ‘D’ or a ‘P’ indicates that only one parental homeolog was detected in genomic DNA or expressed. P>D indicates that the T. pratensis homeolog showed higher relative expression than the T. dubius rbcS-1 homeolog in the T. miscellus individual and vice versa for D>P.
(PDF)
(PDF)
Acknowledgments
We thank Muhammad Faisal for assistance in predicting promoter and terminator elements, Afsana Islam for her guidance in genome walking experiments, and Prashant Joshi for technical assistance. This work was supported in part by a Massey University Research Fund grant to JT. The Higher Education Commission (HEC) of Pakistan is gratefully acknowledged for a Ph.D. scholarship to TS.
Data Availability
Sequence data have been deposited to GenBank under accession numbers: KT897488-KT897491 and KT879189-KT879190.
Funding Statement
A PhD scholarship to TS from the Higher Education Commission of Pakistan funded TS. Massey University Research Fund to JT funded the research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Buggs RJA, Chamala S, Wu W, Gao L, May GD, Schnable PS, et al. Characterization of duplicate gene evolution in the recent natural allopolyploid Tragopogon miscellus by next-generation sequencing and Sequenom iPLEX MassARRAY genotyping. Mol Ecol 2010;19: 132–146. 10.1111/j.1365-294X.2009.04469.x [DOI] [PubMed] [Google Scholar]
- 2. Te Beest M, Le Roux JJ, Richardson DM, Brysting AK, Suda J, Kubesova M, et al. The more the better? The role of polyploidy in facilitating plant invasions. Ann bot 2012;109: 19–45. 10.1093/aob/mcr277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soltis DE, Albert VA, Leebens-Mack J, Bell CD, Paterson AH, Zheng CF, et al. Polyploidy and angiosperm diversification. Am J Bot 2009;96: 336–348. 10.3732/ajb.0800079 [DOI] [PubMed] [Google Scholar]
- 4. Yang XH, Ye CY, Cheng ZM, Tschaplinski TJ, Wullschleger SD, Yin WL, et al. Genomic aspects of research involving polyploid plants. Plant Cell Tiss Organ 2011;104: 387–397. 10.1007/s11240-010-9826-1 [DOI] [Google Scholar]
- 5. Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, et al. Ancestral polyploidy in seed plants and angiosperms. Nature 2011;473: 97–100. Epub 100. 10.1038/nature09916 [DOI] [PubMed] [Google Scholar]
- 6. Yoo MJ, Szadkowski E, Wendel JF. Homoeolog expression bias and expression level dominance in allopolyploid cotton. Heredity 2013;110: 171–180. 10.1038/hdy.2012.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gaeta RT, Pires JC, Iniguez-Luy F, Leon E, Osborn TC. Genomic changes in resynthesized Brassica napus and their effect on gene expression and phenotype. Plant Cell 2007;19: 3403–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nature Reviews Genetics 2007;8: 93–103. [DOI] [PubMed] [Google Scholar]
- 9. Comai L, Tyagi AP, Winter K, Holmes-Davis R, Reynolds SH, Stevens Y, et al. Phenotypic instability and rapid gene silencing in newly formed Arabidopsis allotetraploids. Plant Cell 2000;12: 1551–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma XF, Gustafson JP. Allopolyploidization-accommodated genomic sequence changes in Triticale. Ann Bot 2008;101: 825–832. 10.1093/aob/mcm331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiong Z, Gaeta RT, Pires JC. Homoeologous shuffling and chromosome compensation maintain genome balance in resynthesized allopolyploid Brassica napus . PNAS 2011;108: 7908–7913. 10.1073/pnas.1014138108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hegarty MJ, Barker GL, Wilson ID, Abbott RJ, Edwards KJ, Hiscock SJ. Transcriptome shock after interspecific hybridization in Senecio is ameliorated by genome duplication. Curr Biol 2006;16: 1652–1659. 10.1016/j.cub.2006.06.071 [DOI] [PubMed] [Google Scholar]
- 13. Chester M, Gallagher JP, Symonds VV, da Silva AVC, Mavrodiev EV, Leitch AR, et al. Extensive chromosomal variation in a recently formed natural allopolyploid species, Tragopogon miscellus (Asteraceae). PNAS 2012;109: 1176–1181. 10.1073/pnas.1112041109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feldman M, Levy A, Chalhoub B, Kashkush K. Genomic plasticity in polyploid wheat In: Soltis PS, Soltis DE, editors. Polyploidy and genome evolution. Berlin Heidelberg: Springer; 2012. p. 109–135. [Google Scholar]
- 15. Prince VE, Pickett FB. Splitting pairs: the diverging fates of duplicated genes. Nat Rev Genet 2002;3: 827–837. [DOI] [PubMed] [Google Scholar]
- 16. Doyle JJ, Flagel LE, Paterson AH, Rapp RA, Soltis DE, Soltis PS, et al. Evolutionary genetics of genome merger and doubling in plants. Annu Rev Genet 2008;42: 443–461. 10.1146/annurev.genet.42.110807.091524 [DOI] [PubMed] [Google Scholar]
- 17. Conant GC, Wolfe KH. Turning a hobby into a job: How duplicated genes find new functions. Nat Rev Genet 2008;9: 938–950. 10.1038/nrg2482 [DOI] [PubMed] [Google Scholar]
- 18. Edger PP, Pires JC. Gene and genome duplications: the impact of dosage-sensitivity on the fate of nuclear genes. Chromosome Res 2009;17: 699–717. 10.1007/s10577-009-9055-9 [DOI] [PubMed] [Google Scholar]
- 19. Lynch M, Conery JS. The evolutionary fate and consequences of duplicate genes. Science 2000;290: 1151–1155. [DOI] [PubMed] [Google Scholar]
- 20. Blanc G, Wolfe KH. Functional divergence of duplicated genes formed by polyploidy during Arabidopsis evolution. Plant Cell 2004;16: 1679–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roulin A, Auer PL, Libault M, Schlueter J, Farmer A, May G, et al. The fate of duplicated genes in a polyploid plant genome. Plant J 2013;73: 143–153. 10.1111/tpj.12026 [DOI] [PubMed] [Google Scholar]
- 22. Fishman L, Willis JH. A cytonuclear incompatibility causes anther sterility in Mimulus hybrids. Evolution 2006;60: 1372–1381. 10.1554/05-708.1 [DOI] [PubMed] [Google Scholar]
- 23. Levin DA. The cytoplasmic factor in plant speciation. Syst Bot 2003;28: 5–11. 10.2307/3093933 [DOI] [Google Scholar]
- 24. Sloan DB. Using plants to elucidate the mechanisms of cytonuclear co-evolution. New Phytol 2015;205: 1040–1046. [DOI] [PubMed] [Google Scholar]
- 25. Oehlkers F. Cytoplasmic inheritance in the genus Streptocarpus lindley . Adv Genet 1964;12: 329–370. 10.1016/s0065-2660(08)60418-6 [DOI] [Google Scholar]
- 26. Grant V. The genetic structure of races and species in Gilia . Adv Genet 1956;8: 55–87. 10.1016/s0065-2660(08)60499-x [DOI] [Google Scholar]
- 27. Song KM, Lu P, Tang KL, Osborn TC. Rapid genome change in synthetic polyploids of Brassica and its implications for polyploid evolution. PNAS 1995;92: 7719–7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adams KL, Cronn R, Percifield R, Wendel JF. Genes duplicated by polyploidy show unequal contributions to the transcriptome and organ-specific reciprocal silencing. PNAS 2003;100: 4649–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wolf JB. Cytonuclear interactions can favor the evolution of genomic imprinting. Evolution 2009;63: 1364–1371. [DOI] [PubMed] [Google Scholar]
- 30. Gong L, Olson M, Wendel JF. Cytonuclear evolution of Rubisco in four allopolyploid lineages. Mol Biol Evol 2014;31: 2624–2636. 10.1093/molbev/msu207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gong L, Salmon A, Yoo M-J, Grupp KK, Wang Z, Paterson AH, et al. The cytonuclear dimension of allopolyploid evolution: An example from cotton using rubisco. Mol Biol Evol 2012;29: 3023–3036. 10.1093/molbev/mss110 [DOI] [PubMed] [Google Scholar]
- 32. Ownbey M. Natural hybridization and amphiploidy in the genus Tragopogon . Am J Bot 1950;37: 487–499. [Google Scholar]
- 33. Soltis DE, Soltis PS. Polyploidy: recurrent formation and genome evolution. Trends in Ecol Evol 1999;14: 348–352. [DOI] [PubMed] [Google Scholar]
- 34. Ownbey M, McCollum GD. Cytoplasmic inheritance and reciprocal amphiploidy in Tragopogon . Am J Bot 1953;40: 788–796. [Google Scholar]
- 35. Soltis DE, Soltis PS. The dynamic nature of polyploid genomes. PNAS 1995;92: 8089–8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Soltis DE, Soltis PS, Pires JC, Kovarik A, Tate JA, Mavrodiev E. Recent and recurrent polyploidy in Tragopogon (Asteraceae): cytogenetic, genomic and genetic comparisons. Biol J Linn Soc 2004;82: 485–501. [Google Scholar]
- 37. Soltis PS, Soltis DE. Multiple origins of the allotetraploid Tragopogon mirus (Compositae)—rDNA evidence. Syst Bot 1991;16: 407–413. 10.2307/2419333 [DOI] [Google Scholar]
- 38. Symonds VV, Soltis PS, Soltis DE. Dynamics of polyploid formation in Tragopogon (Asteraceae): recurrent formation, gene flow, and population structure. Evolution 2010;64: 1984–2003. 10.1111/j.1558-5646.2010.00978-x [DOI] [PubMed] [Google Scholar]
- 39. Buggs RJA, Chamala S, Wu W, Tate JA, Schnable PS, Soltis DE, et al. Rapid, repeated, and clustered loss of duplicate genes in allopolyploid plant populations of independent origin. Curr Biol 2012;22: 248–252. 10.1016/j.cub.2011.12.027 [DOI] [PubMed] [Google Scholar]
- 40. Buggs RJA, Doust AN, Tate JA, Koh J, Soltis K, Feltus FA, et al. Gene loss and silencing in Tragopogon miscellus (Asteraceae): comparison of natural and synthetic allotetraploids. Heredity 2009;103: 73–81. 10.1038/hdy.2009.24 [DOI] [PubMed] [Google Scholar]
- 41. Buggs RJA, Renny-Byfield S, Chester M, Jordon-Thaden IE, Viccini LF, Chamala S, et al. Next-generation sequencing and genome evolution in allopolyploids. Am J Bot 2012;99: 372–382. 10.3732/ajb.1100395 [DOI] [PubMed] [Google Scholar]
- 42. Buggs RJA, Zhang LJ, Miles N, Tate JA, Gao L, Wei W, et al. Transcriptomic shock generates evolutionary novelty in a newly formed, natural allopolyploid plant. Curr Biol 2011;21: 551–556. 10.1016/j.cub.2011.02.016 [DOI] [PubMed] [Google Scholar]
- 43. Koh J, Chen S, Zhu N, Yu F, Soltis PS, Soltis DE. Comparative proteomics of the recently and recurrently formed natural allopolyploid Tragopogon mirus (Asteraceae) and its parents. New Phyto 2012;196: 292–305. 10.1111/j.1469-8137.2012.04251.x [DOI] [PubMed] [Google Scholar]
- 44. Koh J, Soltis PS, Soltis DE. Homeolog loss and expression changes in natural populations of the recently and repeatedly formed allotetraploid Tragopogon mirus (Asteraceae). BMC Genomics 2010;11: 97 10.1186/1471-2164-11-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tate JA, Joshi P, Soltis KA, Soltis PS, Soltis DE. On the road to diploidization? Homoeolog loss in independently formed populations of the allopolyploid Tragopogon miscellus (Asteraceae). BMC Plant Biol 2009;9: 80 10.1186/1471-2229-9-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tate JA, Ni Z, Scheen A-C, Koh J, Gilbert CA, Lefkowitz D, et al. Evolution and expression of homeologous loci in Tragopogon miscellus (Asteraceae), a recent and reciprocally formed allopolyploid. Genetics 2006;173: 1599–1611. 10.1534/genetics.106.057646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lim KY, Soltis DE, Soltis PS, Tate J, Matyasek R, Srubarova H, et al. Rapid chromosome evolution in recently formed polyploids in Tragopogon (Asteraceae). PLoS ONE 2008;3: e3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tate JA, Symonds VV, Doust AN, Buggs RJA, Mavrodiev E, Majure LC, et al. Synthetic polyploids of Tragopogon miscellus and T. mirus (Asteraceae): 60 Years after Ownbey's discovery. Am J Bot 2009;96: 979–988. 10.3732/ajb.0800299 [DOI] [PubMed] [Google Scholar]
- 49. Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 1987;19: 11–15. [Google Scholar]
- 50. Hillis DM, Moritz C, Mable BK, editors. Molecular systematics Sunderland, MA, USA: Sinauer Associates, Inc; 1996. [Google Scholar]
- 51. Olmstead RG, Michaels HJ, Scott KM, Palmer JD. Monophyly of the Asteridae and identification of their major lineages inferred from DNA sequences of rbcL . Ann MO Bot Gard 1992;79: 249–265. 10.2307/2399768 [DOI] [Google Scholar]
- 52. Panero JL, Crozier BS. Primers for PCR amplification of Asteraceae chloroplast DNA. Lundellia 2003;6: 1–9. [Google Scholar]
- 53. Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology Humana Press, Totowa, NJ, USA: 2002. p. 365–386. [DOI] [PubMed] [Google Scholar]
- 54. Dean C, Elzen PVd, Tamaki S, Black M, Dunsmuir P, Bedbrook J. Molecular characterization of rbcS multigene family in Petunia . Mol gen Genet 1987;206: 465–74. [Google Scholar]
- 55. Neff MM, Turk E, Kalishman M. Web-based primer design for single nucleotide polymorphism analysis. Trends Genet 2002;18: 613–615. 10.1016/s0168-9525(02)02820-2 [DOI] [PubMed] [Google Scholar]
- 56. Vincze T, Posfai J, Roberts RJ. NEBcutter: a program to cleave DNA with restriction enzymes. Nucleic Acids Res 2003;31: 3688–3691. 10.1093/nar/gkg526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Siebert PD, Chenchik A, Kellogg DE, Lukyanov KA, Lukyanov SA. An improved PCR method for walking in uncloned genomic DNA. Nucleic Acids Res 1995;23: 1087–1088. 10.1093/nar/23.6.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stanke M, Diekhans M, Baertsch R, Haussler D. Using native and syntenically mapped cDNA alignments to improve de novo gene finding. Bioinformatics 2008;24: 637–644. 10.1093/bioinformatics/btn013 [DOI] [PubMed] [Google Scholar]
- 59. Burge C, Karlin S. Prediction of complete gene structures in human genomic DNA. J Mol Bio 1997;268: 78–94. [DOI] [PubMed] [Google Scholar]
- 60. Chang WC, Lee TY, Huang HD, Huang HY, Pan RL. PlantPAN: Plant promoter analysis navigator, for identifying combinatorial cis-regulatory elements with distance constraint in plant gene groups. BMC Genomics 2008;9: 561. doi: 56110.1186/1471-2164-9-561 10.1186/1471-2164-9-561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li BH, Kadura I, Fu DJ, Watson DE. Genotyping with TaqMAMA. Genomics 2004;83: 311–320. 10.1016/j.ygeno.2003.08.005 [DOI] [PubMed] [Google Scholar]
- 62. Kelley LA, Sternberg MJE. Protein structure prediction on the web: a case study using the Phyre server. Nature Protocols 2009;4: 363–371. 10.1038/nprot.2009.2 [DOI] [PubMed] [Google Scholar]
- 63. Dean C, Pichersky E, Dunsmuir P. Structure, evolution and regulation of rbcS genes in higher plants. Ann Rev Plant Phys 1989;40: 415–439. 10.1146/annurev.pp.40.060189.002215 [DOI] [Google Scholar]
- 64. Krebbers E, Seurinck J, Herdies L, Cashmore A, Timko M. Four genes in two diverged subfamilies encode the ribulose-1,5-bisphosphate carboxylase small subunit polypeptides of Arabidopsis thaliana . Plant Mol Bio 1988;11: 745–759. 10.1007/bf00019515 [DOI] [PubMed] [Google Scholar]
- 65. Miziorko HM, Lorimer GH. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem 1983;52: 507–535. 10.1146/annurev.bi.52.070183.002451 [DOI] [PubMed] [Google Scholar]
- 66. Spreitzer RJ, Salvucci ME. Rubisco: Structure, regulatory interactions, and possibilities for a better enzyme. Ann Rev Plant Biol 2002;53: 449–475. 10.1146/annurev.arplant.53.100301.135233 [DOI] [PubMed] [Google Scholar]
- 67. Spreitzer RJ. Role of the small subunit in ribulose-1,5-bisphosphate carboxylase/oxygenase. Arch Biochem Biophys 2003;414: 141–149. 10.1016/s0003-9861(03)00171-1 [DOI] [PubMed] [Google Scholar]
- 68. Sasanuma T. Characterization of the rbcS multigene family in wheat: subfamily classification, determination of chromosomal location and evolutionary analysis. Mol Genet Genom 2001;265: 161–171. 10.1007/s004380000404 [DOI] [PubMed] [Google Scholar]
- 69. Outchkourov NS, Peters J, Jong J, Rademakers W, Jongsma MA. The promoter-terminator of chrysanthemum rbcS1 directs very high expression levels in plants. Planta 2003;216: 1003–1012. 10.1007/s00425-002-0953-8 [DOI] [PubMed] [Google Scholar]
- 70. Izumi M, Tsunoda H, Suzuki Y, Makino A, Ishida H. RBCS1A and RBCS3B, two major members within the Arabidopsis RbcS multigene family, function to yield sufficient rubisco content for leaf photosynthetic capacity. J Exp Bot 2012;63: 2159–2170. 10.1093/jxb/err434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Goumenaki E, Taybi T, Borland A, Barnes J. Mechanisms underlying the impacts of ozone on photosynthetic performance. Environ Exp Bot 2010;69: 259–266. 10.1016/j.envexpbot.2010.04.011 [DOI] [Google Scholar]
- 72. Kapralov MV, Kubien DS, Andersson I, Filatov DA. Changes in rubisco kinetics during the evolution of C4 photosynthesis in Flaveria (Asteraceae) are associated with positive selection on genes encoding the enzyme. Mol Bio Evol 2011;28: 1491–1503. 10.1093/molbev/msq335 [DOI] [PubMed] [Google Scholar]
- 73. Waksman G, Lebrun M, Freyssinet G. Nucleotide-sequence of a gene encoding sunflower ribulose-1,5-bisphosphate carboxylase oxygenase small subunit (rbcS). Nucleic Acids Res 1987;15: 7181–7181. 10.1093/nar/15.17.7181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang X, Shi X, Hao B, Ge S, Luo J. Duplication and DNA segmental loss in the rice genome: implications for diploidization. New Phytol 2005;165: 937–946. 10.1111/j.1469-8137.2004.01293.x [DOI] [PubMed] [Google Scholar]
- 75. Wolfe KH. Yesterday's polyploids and the mystery of diploidization. Nat Rev Genet 2001;2: 333–341. [DOI] [PubMed] [Google Scholar]
- 76. Wendel JF. Genome evolution in polyploids. Plant Mol Biol 2000;42: 225–249. [PubMed] [Google Scholar]
- 77. Soltis PS, Soltis DE. The role of genetic and genomic attributes in the success of polyploids. PNAS 2000;97: 7051–7057. 10.1073/pnas.97.13.7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wendel JF, Flagel LE, Adams KL. Jeans, genes, and genomes: cotton as a model for studying polyploidy In: Soltis PS, Soltis DE, editors. Polyploidy and Genome Evolution. New York: Springer; 2012. [Google Scholar]
- 79. Matsushita SC, Tyagi AP, Thornton GM, Pires JC, Madlung A. Allopolyploidization lays the foundation for evolution of distinct populations: evidence from analysis of synthetic Arabidopsis allohexaploids. Genetics 2012;191: 535 10.1534/genetics.112.139295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Malinska H, Tate JA, Matyasek R, Leitch AR, Soltis DE, Soltis PS, et al. Similar patterns of rDNA evolution in synthetic and recently formed natural populations of Tragopogon (Asteraceae) allotetraploids. BMC Evol Bio 2010;10: 291. doi: 291 10.1186/1471-2148-10-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Birchler JA, Veitia RA. The gene balance hypothesis: implications for gene regulation, quantitative traits and evolution. New Phyto 2010;186: 54–62. 10.1111/j.1469-8137.2009.03087.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Flagel LE, Wendel JF. Evolutionary rate variation, genomic dominance and duplicate gene expression evolution during allotetraploid cotton speciation. New Phyto 2010;186: 184–193. 10.1111/j.1469-8137.2009.03107.x [DOI] [PubMed] [Google Scholar]
- 83. Bottley A, Xia GM, Koebner RMD. Homoeologous gene silencing in hexaploid wheat. Plant J 2006;47: 897–906. 10.1111/j.1365-313X.2006.02841.x [DOI] [PubMed] [Google Scholar]
- 84. Wang J, Tian L, Lee H-S, Wei NE, Jiang H, Watson B, et al. Genomewide nonadditive gene regulation in Arabidopsis allotetraploids. Genetics 2006;172: 507–517. 10.1534/genetics.105.047894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chen ZJ, Pikaard CS. Transcriptional analysis of nucleolar dominance in polyploid plants: Biased expression/silencing of progenitor rRNA genes is developmentally regulated in Brassica . PNAS 1997;94: 3442–3447. 10.1073/pnas.94.7.3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Grover CE, Gallagher JP, Szadkowski EP, Yoo MJ, Flagel LE, Wendel JF. Homoeolog expression bias and expression level dominance in allopolyploids. New Phytol 2012;196: 966–971. 10.1111/j.1469-8137.2012.04365.x [DOI] [PubMed] [Google Scholar]
- 87. Chaudhary B, Flagel L, Stupar RM, Udall JA, Verma N, Springer NM, et al. Reciprocal silencing, transcriptional bias and functional divergence of homeologs in polyploid cotton (Gossypium). Genetics 2009;182: 503–517. 10.1534/genetics.109.102608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sehrish T, Symonds VV, Soltis DE, Soltis PS, Tate JA. Gene silencing via DNA methylation in naturally occurring Tragopogon miscellus (Asteraceae) allopolyploids. BMC Genomics 2014;15: 701 10.1186/1471-2164-15-701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Barr CM, Fishman L. The nuclear component of a cytonuclear hybrid incompatibility in Mimulus maps to a cluster of pentatricopeptide repeat genes. Genetics 2011;184: 455–465. 10.1534/genetics.109.108175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Soltis PS, Plunkett GM, Novak SJ, Soltis DE. Genetic-variation in Tragopogon species—additional origins of the allotetraploids T. mirus and T. miscellus (Compositae). Am J Bot 1995;82: 1329–1341. 10.2307/2446255 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The conserved hexadecapeptide motif (YYDGRYWTMWKLPMFG) is indicated in red text.
(PDF)
A.H. stands for artificial hybrid which was a 1:1 mixture of T. dubius and T. pratensis cDNA.
(PDF)
Data are summarized from genomic DNA and cDNA CAPS and homeolog-specific RT-PCR. Note: Letters “D” and “P” correspond to the diploid parents T. dubius and T. pratensis, respectively. A ‘D’ or a ‘P’ indicates that only one parental homeolog was detected in genomic DNA or expressed. P>D indicates that the T. pratensis homeolog showed higher relative expression than the T. dubius rbcS-1 homeolog in the T. miscellus individual and vice versa for D>P.
(PDF)
(PDF)
Data Availability Statement
Sequence data have been deposited to GenBank under accession numbers: KT897488-KT897491 and KT879189-KT879190.