Abstract
The literature has been highly informative for when to use actigraphy and its validity in pediatric research. However, minimal literature exists on how to perform actigraphy, especially in special populations. We determined whether providing actigraphy training to parents and coordinators increased the nights of actigraphy data that could be scored. We compared two studies in children with autism spectrum disorders, one which provided a basic level of training in a single site trial and the other which provided more detailed training to parents and coordinators in a multisite trial. There was an increase in scorable nights in the multisite trial containing a one-hour structured parent training session. Our results support the use of educational tools in clinical trials that use actigraphy.
Keywords: autism spectrum disorders, sleep, insomnia, clinical trials
Actigraphy has been used in both research and clinical settings to evaluate sleep quality in the home environment (Berger et al., 2008; Kotagal & Pianosi, 2006; Natale, Plazzi, & Martoni, 2009; Sánchez-Ortuño, Edinger, Means, & Almirall, 2010). It is a less costly measure than polysomnography and can provide weeks of data in the patient’s naturalistic setting as opposed to data from a single night’s stay in an unfamiliar setting (e.g., sleep laboratory) (Beebe, et al., 2008; Blackwell, Ancoli-Israel, Redline, & Stone, 2011; Goldman, et al., 2009; Goldman, Bichell, Surdyka, & Malow, 2012; Peterson, et al., 2012). While the literature has been highly informative in terms of indications for when to use actigraphy, (Insana, Gozal, & Montgomery-Downs, 2010; Littner, et al., 2003; Martin, & Hakim, 2011; Morgenthaler, et al., 2007) including a comprehensive review on the validity of actigraphy in pediatric research (Meltzer, Montgomery-Downs, Insana, & Walsh, 2012), the literature is sparse on how to work with parents and participants to collect actigraphy data.
The goal of this manuscript is to describe a practical approach to performing actigraphy, focusing on training of the caregiver and coordinators. Elements related to participant tolerability (e.g., length of wear and placement of device) are also described. This approach was developed by our sleep research program for use in our single (Malow, et al., 2012; Reed, et al., 2009), and multicenter studies of children with autism spectrum disorders (Adkins, et al., 2012; Malow, et al., 2013). However, aspects of our approach are generalizable to the broader pediatric population. To determine success with our approach, we compared a relevant metric — scorable nights — in one study conducted before and a separate study conducted after implementation of caregiver and coordinator training. Our hypothesis was that children participating in the study conducted after implementation of our approach would have a higher proportion of scorable nights than children of caregivers who did not receive actigraphy training.
Methods
Studies and Participants
We reviewed study processes and scorable nights used in two intervention studies conducted by our sleep research laboratory on children with autism spectrum disorder (ASD), meeting DSM-IV criteria (APA, 2000) on clinical interview, and confirmed by the Autism Diagnostic Observation Schedule (Lord, et al., 2000). Our population included 75% with autistic disorder, 8% with Pervasive Developmental Disorder-Not Otherwise Specified, and 17% with Asperger’s Syndrome. Children had sleep onset delay, defined as taking at least 30 minutes to fall asleep on 3 or more nights a week, confirmed by actigraphy. Both studies analyzed the effect of sleep education provided to the parent on the child’s sleep onset latency, with the first study conducted prior to the development of our comprehensive approach to actigraphy research. Both studies were approved by the Institutional Review Boards at the respective site.
Study 1, a pilot trial conducted at a single site (Vanderbilt University) between March 2006 and May 2008, had 20 participants with ASD, ages 4–10 years (Reed, et al., 2009). Parents received three 2-hour sleep education weekly sessions (in groups of 3–5 parents) to review daytime habits that affect sleep, develop a visual bedtime routine, and teach parents how to effectively interact with their children to manage bedtime resistance and night wakings. Study 2, a randomized clinical trial with 80 participants with ASD ages 2–10 years, was conducted between January 2010 and May 2012 (Malow, et al., 2013). This multicenter study included Vanderbilt University, the University of Colorado, and the University of Toronto. Children were randomly assigned to individual sleep education sessions (one 1-hour sessions) or small group sessions (two 2-hour sessions one week apart) with a similar curriculum to Study 1. Two phone calls were made to follow-up on progress, one and two weeks after education was completed. Participants wore actigraphy devices from the same manufacturer (Philips Respironics, Bend, Oregon) and data were analyzed using the same software (Philips Respironics, Actiware Version 5). The AW-64 actigraph was used in Study 1 and the Actiwatch Spectrum was used in Study 2. The Spectrum is an updated version of the AW-64 actigraph that provides a digital readout and a plastic band, whereas the AW-64 is a black box with no digital readout and a grosgrain wrist band. Although there are a variety of actigraphy products available commercially, we chose to use these actigraphs based on our prior use of these devices in our sleep research program. In both studies, parents completed a daily sleep diary that included comments about bedtime, wake time, and reasons a child did not wear the device. A comments column was provided to note any unusual circumstances that may have interfered with sleep (e.g, vacation). Participants wore the actigraph in their home setting to capture the typical sleep patterns of both weekday and weekend schedules. This was an important consideration in both studies because we wanted to capture the variability of sleep patterns over a sufficient period as recommended by the American Academy of Sleep Medicine (Morgenthaler, et al., 2007). Participants were asked to wear the device for 24 hours, although those unable to tolerate the actigraph for long periods were allowed to limit wearing of the actigraph from 1 hour prior to bedtime until 30 minutes after wake time the following morning.
Actigraphy Training Tools
Tools used in Study 2 (and our ongoing studies) are included in the Appendices. Rationale for using these tools is summarized in Table 1. Appendix Figure 1 provides an outline of the training that the study coordinator conducted with the parent in Study 2 (and our ongoing studies). This training includes logistical aspects (such as identifying times to reach the parent regarding collection of actigraphy data) as well as training, through the use of scenarios, on how to accurately complete the sleep and actigraphy diary forms (Appendix Figure 2 shows an accurately completed example). The importance of the child wearing the watch every night is emphasized. Another critical point reviewed with the parents is when to mark bedtime, making the distinction between activities that are part of the bedtime routine (such as gentle massage or reading to the child) vs. activities that the parent performs after bedtime, while the child is attempting to go to sleep. For example, a child exhibiting bedtime resistance may require multiple visits from a parent to soothe the child. Because these occur after the bedtime routine is completed, it is important to teach the parent to press the event marker at bedtime, rather than during or after these visits to soothe the child, which could underestimate sleep latency.
Table 1.
Actigraphy Training Tools to Increase Scorable Nights
| Tools | Rationale |
|---|---|
| 1. Clearly defined bedtime | Accuracy in scoring sleep onset latency |
| 2. Model event marking | Accuracy in scoring lights out and wake time |
| 3. Show actigraph with event markers | Illustrates importance of event marking and wearing the watch in the proper time period. |
| 4. Quiz | Determines parent comprehension and need for additional education. |
| 5. Practice recording on sample sleep diary | Provides parent with hands-on training and allows coordinator to assess parent knowledge. |
| 6. Practice watch worn at home Complete sleep diary | Tests tolerability to wrist wear and identifies problems with data collection before study data collection begins. |
| 7. Feedback to parent after practice watch | Provides additional education and ensures that watch is functioning properly. |
Appendix Material Figure 3 is a quiz to determine comprehension. Administering a quiz helps identify areas requiring additional education. A score lower than 80% prompts an immediate review of the topics missed on the quiz. Appendix Figure 4 includes examples that emphasize to the parent the need to press the event marker (to mark bedtime), to place the device on their children for at least an hour prior to bedtime, and to leave the device on after waking (to ensure sufficient baseline of activity counts).
Actigraphs and identification of rest intervals
Although two different actigraph models (AW-64 for the first study and Actiwatch Spectrum for the second study) were employed for our two studies, comparative analysis conducted by Philips Respironics concluded no differences existed for any of the sleep statistics between models (Koninklijke Philips Electronics N.V., 2009). Software sampling rates and settings were consistent for both studies. The Spectrum unit digital readout displays time and visual feedback when the event marker button is pushed. The AW-64 has a metal indentation that marks events. On both watches the epoch length, or sampling rate, was set to 60 seconds, based on our previous studies (Adkins, et al., 2012; Malow, et al., 2012). A sleep interval of 10 epochs (10 min) for onset of sleep and an awake threshold setting of 40 (medium) were utilized. The medium threshold is the default setting for the software that has been validated by other research (Chae, et al., 2009; Kushida, et al., 2001; Peterson, et al., 2012). Validated software, Actiware 5 (Philips Respironics, Inc.) algorithms were used to estimate sleep parameters. The data were downloaded to a desktop computer using a docking station. Manual scoring of actigraphy data was used exclusively. Event markers superseded diary entries unless there was reason to suspect inaccuracies. Parents were trained to push the event marker prior to recording the time on a sleep diary, reflected in our scoring rationale. Discrepent times may be due to a delay in recording times with recollection error. Failing to record a bedtime or event marker resulted in an unscored night.
In study 2, and rescored study 1 data, we modified our scoring protocol to allow for nights to be scored if either the event marker or the sleep diary was missing as long as (a) both the event marker and the sleep diary information were present on other nights and (b) showed consistency in indicating bedtime. For example, if discrepant times recorded on the sleep diary suggested that the diary was reconstructed at a later time (e.g. times consistently ending in “0” or “30”), nights with missing event markers remained unscored rather than utilizing a potentially inaccurate recorded time. If the diary indicated corroboration with the event marker times, the recorded times were presumed more likely to be accurate and were used to score a night with a missing event marker. Parents were instructed to make a note in the comments column if the event marker was forgotten and pushed at a later time. In this instance, the time recorded on the diary was used for scoring.
In agreement with Boyne et al. (2013), we chose not to use the autoscore function, The autoscore scoring method uses activity count changes to determine bedtime, which may be misleading. Many participants exhibited long sleep onset latencies, or trouble settling. The definition of bedtime given during our actigraphy training identifies bedtime as the moment the parent determines interactions are over and falling asleep will be attempted, even if unsuccessfully. In lieu of autoscoring, we used sleep diary comments regarding parent/child interactions to facilitate determination of reliability of event markers or recorded sleep diary times.
Training Parents
The study protocols differed in specific ways related to actigraphy training of participants and method of data collection and scoring. In Study 1, the coordinator showed the actigraphs and sleep diaries to the parent during the consent visit. The parents then took the materials home to begin baseline actigraphy collection. After the intervention (behavioral sleep education) was provided, the child wore a post intervention watch. Prior to the child wearing the post-interventional watch, the coordinator called the parents to remind them how to use the watch and to complete the sleep diary, both of which were mailed to the participant’s home after the phone call. Parents were encouraged to call with any questions or concerns that arose during the study. In Study 2, parents were provided with a one-hour structured actigraphy training session at the clinic. Comprehension was checked after instruction with a quiz. In addition, parents participated in a practice session in which they recorded bedtimes on the sleep diary. In study 1, the actigraph was worn for seven nights before and after the intervention consisting of an educational session with the parent to review sleep habits; in study 2, it was worn for 21 nights before (including a “practice” week after which feedback was given to the parent) and 14 nights after completing the sleep intervention. The minimum number of nights (seven) that the actigraph was worn was based on the pediatric literature (reviewed in Meltzer, & Westin, 2011; and Meltzer, et al., 2012). We chose to collect more nights in study 2 because (a) we incorporated a practice watch and (b) we included sites less familiar with actigraphy procedures. However, for consistency in our analysis, we compared the first seven nights of actigraphy collected in each study.
In Study 1, children who were not able to tolerate the actigraphy watch placement on the wrist wore the actigraph on the ankle (2.4%), given literature supporting use of this alternative placement in ASD (Sitnick, Goodlin-Jones & Anders, 2008). In Study 2, participants who were not able to tolerate the wrist placement wore the actigraph inside a pocket sewn onto the shoulder of a tee shirt (28.0%), using methodology which was validated in previous research (Adkins, et al., 2012; Souders, et al., 2009). Parents were encouraged to acclimate their child to wearing the watch by introducing plastic bracelets and toy watches. Placing a toy watch on a favorite stuffed animal at the same time the child wore their study watch helped some children accept this study procedure more readily. After approximately a month, the time allowed for sleep education to occur and suggestions implemented in the home, a third watch was worn. This post intervention watch measured improvement in sleep onset due to strategies learned. In Study 1, we required both a sleep diary and event marker for scoring (although as noted below, in our analysis of study 1 data we used consistent scoring rules with Study 2).
Training Coordinators
For Study 2, we also conducted hands-on training for study coordinators at our investigator meeting with role playing (trainer and parent) and video recording of the mock training session. This video was made available to coordinators after the conference. We developed a manual of operations and internet accessible (e.g, YouTube) training videos over a secure link (featuring study personnel) showing research staff how to collect actigraphy data, download it locally, and electronically transfer it to the centralized scoring site. The manual of procedures (MOP) included computer screen photos to illustrate steps for configuring and downloading actigraphy and the subsequent electronic transfer of the actigraphy data. The MOP specified a uniform method of naming electronic databases to accurate identify the subject identification code and time period of data collection. Typical procedural errors were reviewed to minimize their occurrence. (e.g., selecting the configure button in error instead of retrieve during downloading, which would erase previously recorded data). Coordinators were alerted to configure specific epoch lengths and data collection durations, to conduct time zone and battery status checks, and to complete required fields of subject identification in the new participant dialog box.
Data Analysis
For both studies, the proportion of scorable nights was calculated in order to determine whether Study 2 has a higher proportion of scorable nights. The numerator was the number of nights scored. The denominator was the number of nights of actigraphy to be collected as specified by the protocol. Mann-Whitney U test was used for comparison.
To address inter-rater reliability issues and differing scoring approaches, the data from Study 1 were rescored using scoring criteria developed for Study 2 by a single scorer. To ensure the same number of nights in the denominator of the scorable night ratio for each study, groups of seven night periods for all watches used in Study 2 were compared to the seven nights of data collected in Study 1. The reasons a night could not be scored were also tabulated and reported as proportions of the total attempted nights of actigraphy collection, and compared between studies.
For Study 2, we also analyzed overall scorable nights by site using one-way analysis of variance (ANOVA). The data were analyzed using SPSS (IBM, version 21) software. Level of significance was set at p < 0.05.
Results
In Study 1, there were 20 participants with a mean (standard deviation) age of 5.8 years (2.6) and 16 (80%) males. In Study 2, there were 80 participants with a mean age 5.4 years (2.7) and 64 (80%) males.
To account for the different number of nights of data collection in the baseline period prior to sleep education intervention and eliminate concern for a “practice effect,” the first seven nights of data from Study 2 (the “practice watch”) were compared to the seven nights of data collected in Study 1. In Study 2, only two children wore the practice watch only; both of these children were withdrawn from the study by their parents’ choice due to family illness.
For each study week, scorable nights were consistently higher in Study 2 than Study 1 (Table 2). One-way analysis of variance test showed no difference in scorable nights between the three research sites in Study 2 [F= 0.93; p = 0.4].
Table 2.
Scorable nights in each study using seven night increments
| Time Period | Study week |
Study 1 Mean (SD) a scorable nights |
Study 2 Mean (SD) a scorable nights |
Mann-Whitney U test p-value |
|---|---|---|---|---|
| Baseline | ||||
| 1 | 0.73 (0.35) | 0.91 (0.20) | 0.04b | |
| 2 | 0.90 (0.19) | 0.04b | ||
| 3 | 0.76 (0.31) | 0.69b | ||
|
| ||||
| Post-intervention | ||||
| 1 | 0.58 (0.39) | 0.90 (0.17) | 0.002c | |
| 2 | 0.75 (0.30) | 0.08c | ||
Mean (SD=standard deviation)
Comparing mean scorable nights in baseline period (by week) between study 1 and study 2
Comparing mean scorable nights in post-intervention period (by week) between study 1 and study 2
Table 3 shows the reasons why nights were not scored. For each reason, the average number of nights that were not scored were compared between the two studies. Reasons showing either a trend or significant difference between study 1 and 2 included the watch not being worn, missing or discrepant data, and incomplete sleep diaries. Examples included (1) placement of the watch on the child either too close to sleep onset to obtain a baseline of activity counts, or after sleep onset; (2) the child’s bedtime (“lights out”) was not provided on the sleep diary or event marker, or (3) sleep diary times that were discrepant from the event marker times. Watch malfunctions were infrequent (5% in study 1 and 3% in study 2) and did not differ between studies.
Table 3.
Mean nights not scored in each study tabulated by reason not scored
| Reason not scored | Study 1 Mean (SD) nights not scored | Study 2 Mean (SD) nights not scored | Mann-Whitney U p-value |
|---|---|---|---|
| Watch not worn | .60 (.10) | .30 (.93) | 0.07 |
| Missing or discrepant data | .35 (.67) | .19 (.70) | 0.06 |
| Watch malfunction | .35 (1.65) | .16 (.96) | .78 |
| Incomplete sleep diaries | .65 (2.06) | .04 (.34) | .04 |
SD = standard deviation
The software algorithm compares activity levels prior to and subsequent to the current epoch to score the interval as either wake or sleep. Therefore the actigraph should optimally be placed on the child at least 30–60 minutes prior to falling asleep. As mentioned in the methods, parents were asked to place the device on their child for 24 hours, although only 29% were successful. The remainder wore the actigraph prior to bedtime, and the device was removed in the morning after awakening. The proportion of nights scored (standard deviation) did not differ between children that wore the actigraphy for 24 hours [0.81 (0.28),] and children who could only wear the device an hour prior to lights out [0.81 (0.23)] (p = 0.85). The proportion of scorable nights differed between ankle placement [0.59 (0.42)] and wrist placement [0.81 (0.28)] (p=0.03), but not between the shoulder placement [0.76 (0.28)] and wrist placement [0.81 (0.28)](p=0.12).
Discussion
In this manuscript, we describe a practical approach to performing actigraphy in children with autism spectrum disorders. Given the challenges of performing actigraphy in this population, our work should have applicability to broader pediatric populations. Our study is unique because, to our knowledge, no practical guide exists for investigators to use in working with participants and families to collect accurate actigraphy data. Our study provides this information and extends it to multi-site clinical trials in which site study coordinators download and transfer data to a centralized scoring site.
The increase in scorable nights in Study 2 likely reflected the structured educational hands-on training given to parents regarding watch placement, completion of sleep diaries, and the use of event markers, as well as the importance of the child wearing the watch every night. Showing the parent the actigraphy and sleep diary with brief verbal instruction during a consenting visit, as employed in Study 1, was not as effective in optimizing scorable nights. The increase in data was not due to the practice effect because the first seven nights of data collection were compared in both studies. In fact, wearing a watch for too long may result in “watch fatigue,” as supported by fewer scorable nights in the third week (vs. weeks one or two) of the pre-intervention period in Study 2 and in the second week (vs. week one) of the post-intervention period in Study 2. It is possible that watch fatigue may be improved with a booster actigraphy session performed by study coordinators. We are now limiting pre- and post- actigraphy collection to 1–2 weeks. The one exception to this rule is studies that involve dose escalation, in which we have been successful in collecting data over many weeks (Malow, et al., 2012). Offering the shoulder placement of the actigraph may retain a larger number of children with tactile sensitivities without compromising data integrity. Reducing time of wear (placing actigraph on the child 1 hour before bedtime rather than 24-hour use), may also retain a larger number of children – however, it is critical to ensure that the parent places the actigraph on the child prior to bedtime to obtain a sufficient waking baseline of activity.
As mentioned in the methods section, in Study 2, we modified our protocol to allow for nights to be scored if either the event marker or the sleep diary was missing as long as (a) both the event marker and the sleep diary information were present on other nights and (b) showed consistency in indicating bedtime. This modification alone did not influence scorable nights between studies, as when we rescored Study 1 data using the Study 2 rules, we still found a higher proportion of scorable nights in Study 2 (as compared to Study 1). However, we do believe taking both event marker and sleep diary information into account is a useful modification that other actigraphy researchers may wish to practice in their work to optimize data interpretation.
Fortifying an actigraphy program with tested training methods and tools may increase integrity of data, especially when conducting multi-site clinical trials. It is notable that despite having multiple sites, the scorable nights of actigraphy data were higher in Study 2. One limitation to our study was the lack of fidelity checks on the actigraphy training session with the parent conducted by different study coordinators. However, the three research sites participating in Study 2 did not differ in the average ratio of scorable nights of actigraphy despite varying degrees of familiarity with actigraph use amongst study personnel. Another limitation of our study was that we used different models of the Philips Respironics actigraphy device, Spectrum and AW-64, in our studies. While both were validated by the manufacturer to show consistent collection in data between the two models, manufacturer validation was primarily conducted on stationary mechanical devices, which may not represent sleep patterns in pediatric populations. Furthermore, the Spectrum watch used in Study 2 is larger in size and might be expected to cause decrease in tolerability. However, it may have been perceived as more “interesting” (i.e., digital readout, visual feedback, ergonomic features), than the AW-64 model used in Study 1.
In summary, we present a practical guide for investigative teams conducting actigraphy research in pediatric special populations that has applicability for the general pediatric population. In future studies, we plan to include the adolescent population, where assistance from parents may be minimal or absent during the actigraphy collection process. We also plan to expand our use of webinar training for both research staff and parents/participants.
Supplementary Material
Figure 1. Study Flow Charts.
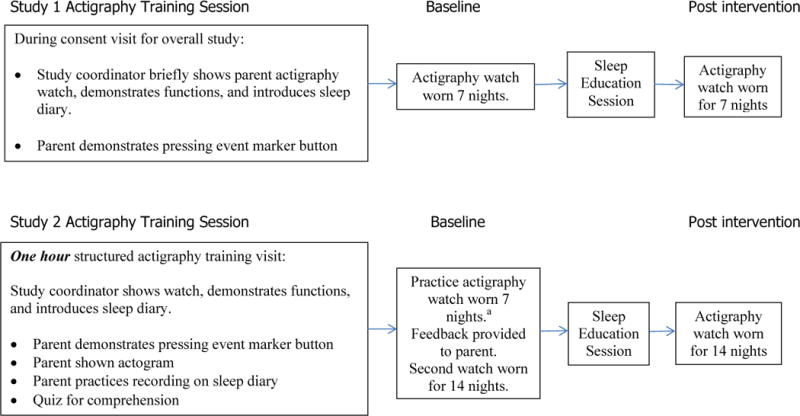
a To account for the different number of nights of data collection in the baseline period prior to sleep education intervention, the first seven nights of data from Study 2 (the “practice watch”) were compared to the seven nights of data collected in Study 1
Acknowledgments
This research was conducted as part of the Autism Speaks Autism Treatment Network. Further support came from a cooperative agreement (UA3 MC 11054) from the U.S. Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health Research Program, to the Massachusetts General Hospital. The views expressed in this publication do not necessarily reflect the views of Autism Speaks, Inc. Additional support was provided by the Organization for Autism Research and CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Financial Disclosure: The authors report no conflicts of interest or financial relationships relevant to this article.
References
- Adkins KW, Goldman SE, Fawkes DB, Surdyka K, Wang L, et al. A pilot study of shoulder placement for actigraphy in children. Behavioral Sleep Medicine. 2012;10(2):138–147. doi: 10.1080/15402002.2011.596598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins KW, Molloy C, Weiss S, Reynolds A, Goldman SE, Burnette C, et al. Effects of a Standardized Pamphlet on Insomnia in Children with Autism Spectrum Disorders. Pediatrics. 2012;130(Supplement 2):S139–44. doi: 10.1542/peds.2012-0900K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- Beebe D, Fallone G, Godiwala N, Flanigan M, Marin D, Schaffner L, et al. Feasibility and behavioral effects of an at-home multi-night sleep restriction protocol for adolescents. Journal of Child Psychology and Psychiatry. 2008;49(9):915–923. doi: 10.1111/j.1469-7610.2008.01885.x. [DOI] [PubMed] [Google Scholar]
- Berger A, Wielgus K, Young-McCaughan S, Fischer P, Farr L, Lee K. Methodological Challenges When Using Actigraphy in Research. Journal of Pain Symptom Management. 2008;36(2):191–199. doi: 10.1016/j.jpainsymman.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T, Ancoli-Israel S, Redline S, Stone K. Factors that may influence the classification of sleep-wake by wrist actigraphy: The MrOS sleep study. Journal of Clinical Sleep Medicine. 2011;4:357–367. doi: 10.5664/JCSM.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyne K, Sherry DD, Gallagher PR, Olsen M, Brooks LJ. Accuracy of computer algortithms and the human eye in scoring actigraphy. Sleep Breath. 2013;17:411–417. doi: 10.1007/s11325-012-0709-z. [DOI] [PubMed] [Google Scholar]
- Chae KY, Kripke DF, Poceta JS, Shadan F, Jamil SM, Cronin JW, et al. Evaluation of immobility time for sleep latency in actigraphy. Sleep Medicine. 2009;10:621–625. doi: 10.1016/j.sleep.2008.07.009. [DOI] [PubMed] [Google Scholar]
- De la Vega R, Miro J. The assessment of sleep in pediatric chronic pain sufferers. Sleep Medicine Review. 2013;17(3):185–92. doi: 10.1016/j.smrv.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Goldman SE, Bichell T, Surdyka K, Malow BA. Sleep in children and adolescents with angelman syndrome: Association with parent sleep and stress. Journal of Intellectual Disability Research. 2012;56(6):600–608. doi: 10.1111/j.1365-2788.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SE, Surdyka K, Cuevas R, Adkins KW, Wang L, Malow BA. Defining the sleep phenotype in children with autism. Developmental Neuropsychology. 2009;34:560–573. doi: 10.1080/87565640903133509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde M, O’Driscoll D, Binette S, Galang C, Tan S, Verginis N, et al. Validation of Actigraphy for determining sleep and wake in children with sleep disordered breathing. Journal of Sleep Research. 2007;16:213–216. doi: 10.1111/j.1365-2869.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- Insana S, Gozal D, Montgomery-Downs H. Invalidity of one actigraphy brand for identifying sleep and wake among infants. Sleep Medicine. 2010;11(2):191. doi: 10.1016/j.sleep.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson N, Kirchner L, Rosen C, Storfer-Isser A, Cartar L, Ancoli-Israel S, et al. Sleep estimation using wrist actigraphy in adolescents with and without sleep disordered breathing: A comparison of three data modes. Sleep. 2007;30(7):899–905. doi: 10.1093/sleep/30.7.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koninklijke Philips Electronics N.V. Equivalence of Activity Recordings and Derived Sleep Statistics: Actiwatch-64, Actiwatch 2 and Actiwatch Spectrum 2009 [Google Scholar]
- Kotagal S, Pianosi P. Sleep disorders in children and adolescents: Clinical Review. BMJ. 2006:332–828. doi: 10.1136/bmj.332.7545.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disorder patients. Sleep Medicine. 2001;2:389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Lam J, Mahone E, Mason T, Scharf S. Defining the Roles of Actigraphy and Parent Logs for Assessing Sleep Variables in Preschool Children. Behavioral Sleep Medicine. 2011;9(3):184–193. doi: 10.1080/15402002.2011.583906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littner M, Kushida CA, Anderson WM, Bailey D, Berry RB, Davila DG, et al. Practice Parameters for the Role of Actigraphy in the Study of Sleep and Circadian Rhythms: An Update for 2002. Sleep. 2003;26(3):337–341. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook E, Jr, Leventhal B, DiLavore P, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Malow BA, Adkins KW, McGrew SG, Wang L, Goldman SE, Fawkes DB, et al. Melatonin for sleep in children with autism: A controlled trail examining dose, tolerability, and outcomes. Journal of Autism and Developmental Disorders. 2012;42(8):1729–1737. doi: 10.1007/s10803-011-1418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malow BA, Adkins KW, Reynolds A, Weiss S, Loh A, Fawkes DB, et al. Parent-based sleep education improves sleep onset delay in children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2013 doi: 10.1007/s10803-013-1866-z. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Hakim AD. Wrist Actigraphy. Chest. 2011;139(6):1514–1527. doi: 10.1378/chest.10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer LJ, Montgomery-Downs HE, Insana SP, Walsh CM. Use of actigraphy for assessment in pediatric sleep research. Sleep Medicine Reviews. 2012 Oct;16:463–475. doi: 10.1016/j.smrv.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer LJ, Westin AML. A comparison of actigraphy scoring rules used in pediatric research. Sleep Medicine. 2011;12(8):793–796. doi: 10.1016/j.sleep.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30(4):519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- Natale V, Plazzi G, Martoni M. Actigraphy in the Assessment of Insomnia: A Quantitative Approach. Sleep. 2009;32(6):767–771. doi: 10.1093/sleep/32.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J, Spirito A, McGuinn M. The children’s sleep habits questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–1051. [PubMed] [Google Scholar]
- Pacquet J, Kawinska A, Carrier J. Wake detection capacity of actigraphy during sleep. Sleep. 2007;30(10):1362–9. doi: 10.1093/sleep/30.10.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B, Reed M. Value of actigraphy in clinical trials: How technology, service, and science can help improve the value of actigraphy as a cost-effective measurement for drug development. Koninklijke Philips Electronics NV 2012 [Google Scholar]
- Peterson B, Chiao P, Pickering E, Freeman J, Zammit G, Ding Y, et al. Comparison of actigraphy and polysomnography to assess effects of zolpidem in a clinical research unit. Sleep Medicine. 2012;13:419–424. doi: 10.1016/j.sleep.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Reed H, McGrew SG, Artibee K, Surdyka K, Goldman SE, Frank K, et al. Parent-based sleep education workshops in autism. Journal of Child Neurology. 2009;24(8):936–45. doi: 10.1177/0883073808331348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Ortuño M, Edinger J, Means M, Almirall D. Home Is Where Sleep Is: An Ecological Approach to Test the Validity of Actigraphy for the Assessment of Insomnia. Journal of Clinical Sleep Medicine. 2010;6(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- Sitnick S, Goodlin-Jones B, Anders T. The Use of Actigraphy to Study Sleep Disorders in Preschoolers: Some Concerns about Detection of Nighttime Awakenings. Sleep. 2008;31(3):395–401. doi: 10.1093/sleep/31.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souders M, Mason T, Valladares O, Bucan M, Levy S, Mandell D, et al. Sleep behaviors and sleep quality in children with autism spectrum disorders. Sleep. 2009;32(12):1566–78. doi: 10.1093/sleep/32.12.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


