Summary
The human-restricted pathogen Streptococcus pyogenes (Group A Streptococcus, GAS) is responsible for wide-ranging pathologies at numerous sites in the body, but has the proclivity to proliferate in individuals asymptomatically. The ability to survive in diverse tissues is undoubtedly benefited by sensory pathways that recognize environmental cues corresponding to stress and nutrient availability and thereby trigger adaptive responses. We investigated the impact that environmental signals contribute to cell-to-cell chemical communication (quorum sensing, QS) by monitoring activity of the Rgg2/Rgg3 and SHP-pheromone system in GAS. We identified metal limitation and the alternate carbon source mannose as two environmental indicators likely to be encountered by GAS in the host that significantly induced the Rgg-SHP system. Disruption of the metal regulator MtsR partially accounted for the response to metal depletion, whereas ptsABCD was primarily responsible for QS induction due to mannose, but each sensory system induced Rgg-SHP signaling apparently by different mechanisms. Significantly, we found that induction of QS, regardless of the GAS serotype tested, led to enhanced resistance to the antimicrobial agent lysozyme. These results indicate the benefits for GAS to integrate environmental signals with intercellular communication pathways in protection from host defenses.
Keywords: Pheromones, metal starvation, mannose, biofilms, innate immune defense
Introduction
The relationship between bacteria and their host may be peaceful, as in the case of commensals, or volatile and contentious, as in the case of pathogens. For some bacteria, it may at times be both, with the balance between ongoing interactions determining clearance, carriage, or progression to disease. Streptococcus pyogenes (Group A Streptococcus, GAS) is one of these organisms. Generally considered a strict human pathogen, GAS causes infections ranging from mild (impetigo, strep throat) to severe (septicemia, necrotizing fasciitis). However, up to ~15% of healthy individuals, depending on age, are asymptomatically colonized by this bacterium (Durmaz et al., 2003, Hoffmann, 1985, Martin et al., 2004, Shaikh et al., 2010). Though the oropharynx is thought to be the primary site of colonization, GAS may also be present in the gastrointestinal and genitourinary tracts, underscoring the importance of its ability to adapt to diverse and dynamic environments (Berkelman et al., 1982, Dei et al., 2010, McKee et al., 1966, Mogielnicki et al., 2000, Sobel et al., 2007, Verstraelen et al., 2011). The full complement of regulatory factors involved in its adaptation to different ecological niches, and the conditions that favor carriage over pathogenesis, and vice versa, are not completely understood. The recent identification of intercellular communication pathways utilizing short peptide pheromones and Rgg-family receptors in GAS provide a new avenue to explore the role of quorum sensing as a mechanism used by these bacteria to influence outcomes of microbe-host interactions.
Four genes encoding Rgg-like proteins are present in all GAS strains sequenced to date. Rgg1 (RopB) regulates the expression of the cysteine protease, SpeB, an important streptococcal virulence factor (Chaussee et al., 1999, Chaussee et al., 2002, Hollands et al., 2008, Lyon et al., 1998). Inhibition of Rgg1 activity by the vfr gene, possibly by a bioactive peptide, has been demonstrated; however, an Rgg1-cognate agonistic peptide has not been identified for this system (Ma et al., 2009, Shelburne et al., 2011). ComR (Rgg4), orthologous to the transcription factors governing natural competence in Mutans and Bovis group streptococci, interacts with a small peptide called SigX-inducing peptide (XIP) to upregulate transcription of competence genes (Mashburn-Warren et al., 2012, Mashburn-Warren et al., 2010). The final two Rggs, Rgg2 and Rgg3, together with neighboring short hydrophobic peptide (SHP) genes shp2 and shp3, regulate a common set of genes in a complementary fashion. Interestingly, SHP peptides bind to either of Rgg2 or Rgg3 and alter their regulatory activity. Under non-inducing conditions in which active SHPs are limited, Rgg3 acts as a repressor, binding highly conserved sequences present near the −35 site within shp2 and shp3 promoter regions. In contrast, Rgg2 is a transcriptional activator. Upon peptide binding, Rgg3 is thought to release the DNA, allowing occupation and transcriptional activation of the Rgg-SHP regulon, which includes the shp genes themselves, by peptide-bound Rgg2. We have shown in select strains of GAS that one consequence of expression of the Rgg-SHP regulon is increased biofilm formation, an event that may be relevant for colonization or persistence within the host (Aggarwal et al., 2014, Chang et al., 2011, Lasarre et al., 2013a, LaSarre et al., 2013b). However, a clear role for the Rgg2/3 pathway in terms of providing any benefit to the bacterium, particularly in consideration of the restrictive growth environment of the host, remains poorly explained.
The limitation of key nutrients available to potential pathogens is termed nutritional immunity (Weinberg, 1975) and is a common strategy of the host to diminish the habitability of microorganisms on susceptible tissues, complementing innate immune defenses. Key among inorganic nutrients that are commonly sequestered by the host are transition metals (e.g., iron, manganese, and zinc) required by microbes as cofactors of enzymes of vital metabolic pathways and of enzymes, like superoxide dismutase, that defend against reactive oxygen species (Kehl-Fie & Skaar, 2010, Hood & Skaar, 2012). At the same time, different niches within the body may vary in the kinds of nutrients available, including carbon sources and byproducts of other organisms’ metabolism (Freter, 1983, King, 2010, Ng et al., 2013). Thus, nutrient quantity and quality can be the prescient stimuli that trigger adaptive changes to promote survival of microbes in a changing environment. Despite advancements in our understanding of the operational mechanism of the Rgg-SHP signaling pathway, the precise conditions that lead to its utilization and expression in vivo remain unclear. In this work, we identify two environmental conditions likely to be encountered by GAS in the host that trigger Rgg-SHP system induction – metal limitation and alternate carbon source availability. Both conditions require Rgg2 and SHPs to modulate gene expression, and regulation by the latter stimulus is dependent upon the expression of the inducible mannose PTS system, ptsABCD. We extend our previous observation that expression of the Rgg-SHP regulon favors biofilm development and show that induced cells aggregate more readily and exhibit altered sensitivity to some antibiotics. Finally, we demonstrate that at least one consequence of Rgg-SHP regulon expression is resistance to lysozyme, an important antimicrobial host defense mechanism present in mucosal secretions and lysozomes of neutrophils and macrophages. This phenotype is conserved among multiple wild-type GAS serotypes and may promote GAS colonization of and persistence within the host.
Results
Metal deplete conditions induce Pshp expression
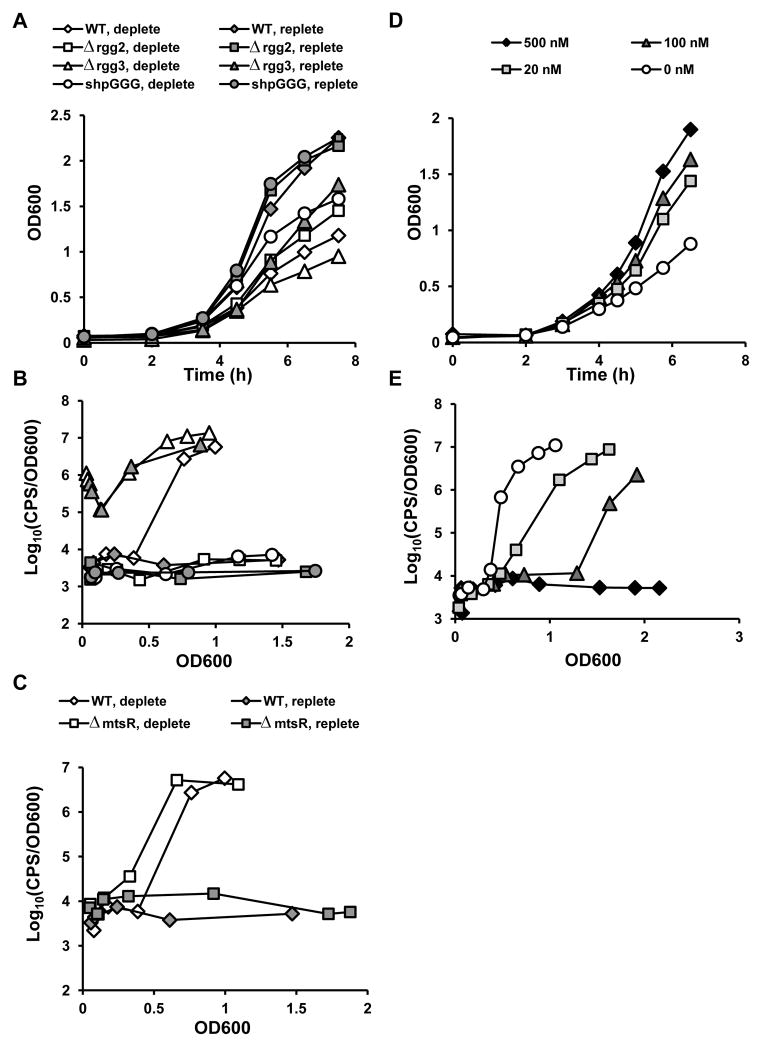
Limiting the availability of transition metals to pathogens is a host innate immune strategy that can also serve as an environmental signal leading to adaptive changes in bacterial gene expression. In GAS, multiple metal acquisition systems are present, including those for heme (Sia/Hts), ferric ferrichrome (Siu/Fts), and free cation (Mts) uptake (Janulczyk et al., 2003, Janulczyk et al., 1999, Bates et al., 2003, Montanez et al., 2005, Lei et al., 2003). Transcription of sia and mts has been shown to be under the regulation of the DtxR-family transcriptional repressor, MtsR, in response to iron and/or manganese levels (Hanks et al., 2006), and these two metals also affect binding of the repressor to the sia promoter (Bates et al., 2005). Microarray analyses comparing wild-type and ΔmtsR strains demonstrated that aroE.2, the gene directly downstream of shp3, is upregulated in the ΔmtsR mutant, and electromobility shift assays and DNaseI footprinting analysis confirmed that MtsR binds upstream of shp3, suggesting that this gene and the downstream operon could be regulated by low metal conditions (Toukoki et al., 2010). To test the possibility that metal limitation is an environmental signal regulating Rgg-SHP induction, a chemically-defined medium (CDM) described previously (Chang et al., 2011, van de Rijn & Kessler, 1980) was treated with Chelex-100 resin to remove divalent cations (CxCDM). In the original recipe for CDM, CaCl2, FeSO4, MgSO4, and MnSO4 are added as supplements. We found that bacteria could not grow in unsupplemented CxCDM or in CxCDM supplemented with calcium, iron, or manganese alone; furthermore, the addition of 1 mM MgSO4 was required to achieve ~50% of the final growth of fully-supplemented CDM (data not shown). Since iron and manganese are two metals to which MtsR has been reported to respond in GAS, we chose to focus on the effect of these cations on Rgg-SHP induction under conditions with fixed MgSO4 and CaCl2 content. Wild-type NZ131 and isogenic Δrgg2, Δrgg3, or shp2GGGshp3GGG (start codons mutated to abolish production of both peptides; abbreviated shpGGG hereafter; (Cook et al., 2013)) mutants containing an integrated Pshp3-luxAB reporter were grown to mid-log phase in CDM, washed with CxCDM containing dipyridyl to chelate residual metals, and diluted into CxCDM supplemented as indicated. Under these conditions, we observed high induction of the Pshp3-luxAB reporter in the wild-type parent when iron and manganese were omitted (Fig. 1A, 1B). Furthermore, in a ΔmtsR strain, reporter induction under metal deplete conditions occurred earlier than in the wild-type, consistent with the observation that this regulator binds DNA sequences upstream of shp3/aroE.2 (Toukoki et al., 2010) to repress transcription (Fig. 1C). Interestingly, deletion of MtsR was not sufficient to induce Pshp expression in metal replete conditions. In Δrgg3 and ΔmtsRΔrgg3 strains, the Pshp3-luxAB reporter exhibited immediate, high induction, similar to what occurs for the Δrgg3 mutant in metal-replete conditions (Fig. 1B, S1; (Chang et al., 2011)). Additionally, consistent with previous observations (Chang et al., 2011, Cook et al., 2013, LaSarre et al., 2013b), both Rgg2 and SHP pheromones are required for Pshp3 induction even when grown in metal-deplete conditions (Fig. 1B). Subsequent experiments testing supplementation with lower amounts of metals determined that wild-type cells are responsive to nanomolar concentrations of iron and manganese (Fig. 1D, 1E).
Figure 1. Rgg-SHP signaling under metal limitation.
(A) Growth and (B, C) luciferase activity of wild-type NZ131 and isogenic Pshp3-luxAB reporter strains grown in metal-deplete or replete conditions. Cells were grown to mid-log in CDM+1% glucose, washed three times with CxCDM containing 100 μM dipyridyl, then diluted into CxCDM supplemented with 50 μM CaCl2and 1000 μM MgSO4 (deplete) or 50 μM CaCl2, 1000 μM MgSO4, 20 μM FeSO4, and 30 μM MnSO4 (replete). (D) Growth and (E) luciferase activity of the wild-type NZ131 reporter strain grown in decreasing amounts of iron and manganese. CxCDM was supplemented with with 50 μM CaCl2, 1000 μM MgSO4, and equal amounts of FeSO4 and MnSO4 as indicated. Data are representative of experiments performed at least three times.
Under inducing conditions, the genes located directly downstream of shp2 and shp3 are highly expressed; however, their function remains unclear. Despite high conservation among GAS strains, the region downstream of shp2 contains a single annotated open reading frame encoding a protein of unknown function. Next to shp3 are located 10 genes of predicted biosynthetic function. This gene cluster is also conserved in all GAS, but among published genomes similar gene clusters appear only in S. porcinus, S. pseudoporcinus, and some B. thuringiensis strains. As this biosynthetic cluster is regulated by metal-deplete conditions, we tested whether Rgg-SHP-regulated genes are important for iron acquisition. To determine whether intracellular iron content increased after induction with synthetic peptide, strains were exposed to a range of concentrations of the iron-activated antibiotic, streptonigrin. No differences were observed between wild-type NZ131 treated with synthetic SHP3-C8 (C8) or a synthetic peptide with the same residues but in reversed order (rev), or Δrgg3 (Table S1). In contrast, the MIC of the ΔmtsR control strain was determined to be 4-fold lower than wild-type, consistent with previous observations that this mutant is hypersensitive to this compound (Bates et al., 2005). Although GAS is not thought to produce siderophores, we next used a modified Chrome Azurol S (CAS) assay to test for iron-chelating activity in Rgg-SHP inducing conditions (Perez-Miranda et al., 2007, Schwyn & Neilands, 1987). Strains were grown on CDM agar containing C8 peptide for 24 hours then overlaid with top agar containing the CAS substrate; Pseudomonas aeruginosa, a well-documented producer of siderophores, was included as a positive control. No color change was noted for GAS strains, indicating a lack of siderophore production (data not shown). Thus, while an environmental stimulus controlling the Rgg-SHP regulon appears to be metal availability, we have no evidence that these genes play a role in metal homeostasis at this time.
Growth on non-glucose carbon sources induces Pshp expression
In bacteria, the processes of nutrient import and metabolism are tightly linked with gene regulation events. It is well understood that depletion of glucose and the switch to a secondary carbon source lead to distinct changes in gene expression (Gorke & Stulke, 2008). Several transcriptomic studies have shown that GAS triggers the expression of genes involved in uptake of non-glucose carbon sources upon growth in host soft tissues, blood and saliva (Virtaneva et al., 2005, Graham et al., 2005, Shelburne et al., 2005, Graham et al., 2006), suggesting an important role for these molecules and their signaling effects in the GAS lifestyle.
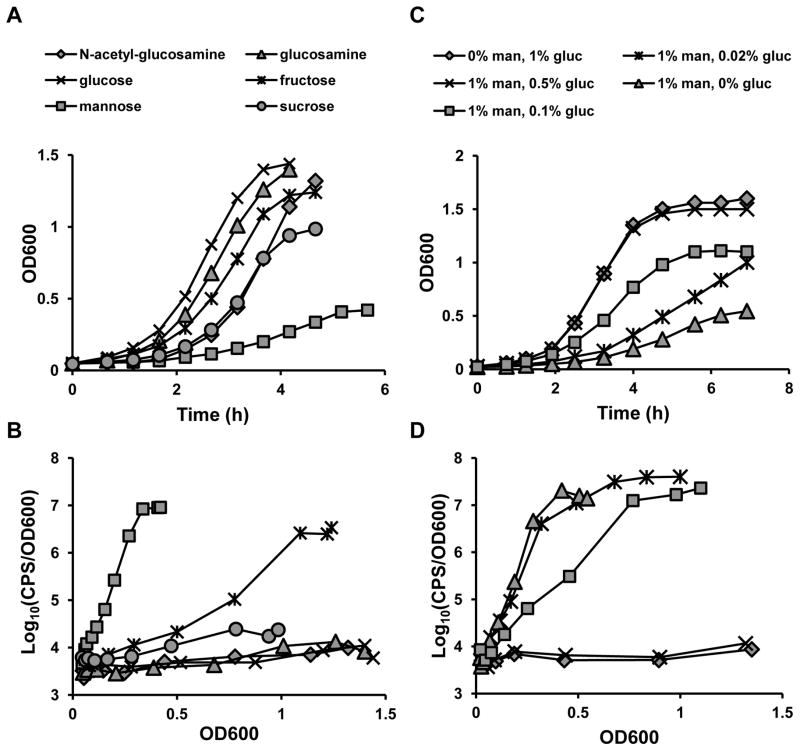
When cultured in CDM with glucose as the primary carbon source, shp gene expression is very low (Chang et al., 2011). To test whether Pshp expression could be induced by alternative carbon sources, reporter strains were suspended in a CDM from which glucose had been omitted, supplemented with a panel of different carbon sources (Biolog Phenotype Microarrays PM1 and PM2), and monitored for growth and luciferase activity over time in a microplate reader. As previously reported (Gera et al., 2014), GAS grew on only a subset of the carbon sources (60 of 190), and only a minority of these were able to support robust bacterial growth (Table S2). Of the non-glucose carbon sources that allowed growth in this screen, only mannose induced a robust increase in expression from Pshp, suggesting a specialized role for this sugar in the activation of Rgg-SHP signaling. As with other monosaccharides, mannose is imported into bacterial cells by the activity of the phosphoenolpyruvate (PEP):carbohydrate phosphotransferase system (PTS), a multiprotein complex comprised of two common proteins (Enzyme I (EI) and HPr) and a variety of enzyme II (EII) permease complexes, each one specializing in the import of a subset of mono- or disaccharides. PTS not only catalyze the transport and phosphorylation of sugars but can also phosphorylate and/or directly interact with other proteins to trigger changes in gene expression, and have been involved in regulating genes related with metabolism, chemotaxis and virulence in several bacterial pathogens (reviewed in (Deutscher et al., 2014)). In streptococcal species, PTS of the mannose family (PTS-Man) import several saccharides including glucose, fructose, mannose, galactose, glucosamine, and N-acetyl-glucosamine (NAG), but specificities vary depending on the precise system and species studied (Bidossi et al., 2012, Abranches et al., 2003, Moye et al., 2014, Gauthier et al., 1990). To validate the results of the phenotypic microarrays, we tested the effect of mannose and other PTS-Man-imported sugars on Pshp3 expression. Wild-type NZ131 containing the Pshp3-luxAB reporter was grown in CDM containing glucose, mannose, fructose, NAG, glucosamine or sucrose at a final concentration of 1%. Although slight increases in the lag phase were observed, particularly for bacteria grown in NAG or sucrose, the exponential growth rates of cultures supplemented with NAG, fructose, glucosamine, and sucrose were similar to that of cells grown in glucose. In contrast, the doubling time of cells grown in mannose was approximately twice that of cells grown in glucose (90 vs. 45 minutes, respectively; Fig. 2A). In agreement with the phenotypic microarray results, mannose elicited a robust increase in luciferase activity. Moderate reporter activity was also observed for fructose and to a lesser extent, sucrose (Fig. 2B). Consistent with our previous genetic studies and the data from metal-depletion experiments described above, full induction of the Pshp3-luxAB reporter also required rgg2 and shp genes (Fig. S2).
Figure 2. Rgg-SHP signaling in response to non-glucose carbon sources.
(A) Growth and (B) luciferase activity of a wild-type Pshp3 reporter strain grown in various sugars identified in a Biolog screen and thought to be transported by mannose-specific PTS systems. Cells were grown to mid-log in CDM containing 1% glucose, then diluted into fresh CDM containing the sugar indicated at a final concentration of 1%. (C) Growth and (D) luciferase activity of the reporter strain grown CDM supplemented with 1% mannose (man) and decreasing concentrations of glucose (gluc). Data shown are representative of experiments performed at least three times.
As a preferred carbon source, glucose represses the expression of systems involved in the use of secondary carbon sources through a process known as carbon catabolite repression (CCR). CcpA is the primary transcriptional repressor mediating CCR in GAS (Almengor et al., 2007, Shelburne et al., 2008). To test the effects of glucose over mannose on SHP signaling, increasing amounts of glucose were added to CDM containing 1% mannose, and growth and Pshp3-luxAB activity were monitored. We observed a dose-dependent increase in the growth rate of the reporter strain concurrent with dose-dependent repression of the Pshp3-lux reporter; hence shp induction by mannose is subject to CCR by glucose (Fig. 2C, D). Apart from glucose, sucrose, fructose, maltose, and lactose are the other main mono- and disaccharides of the human diet (Keim et al., 2005) and could be carbon sources available to GAS growing on the saliva-bathed surfaces of the oropharynx. Unlike glucose, addition of fructose, sucrose, and lactose at concentrations >10-fold molar excess of mannose did not inhibit the expression of the shp reporter (Fig. S3). Thus, mannose appears to be a specific signal that can be detected even in the presence of other dietary sugars.
Pshp expression during growth on mannose requires an inducible transport system, ptsABCD
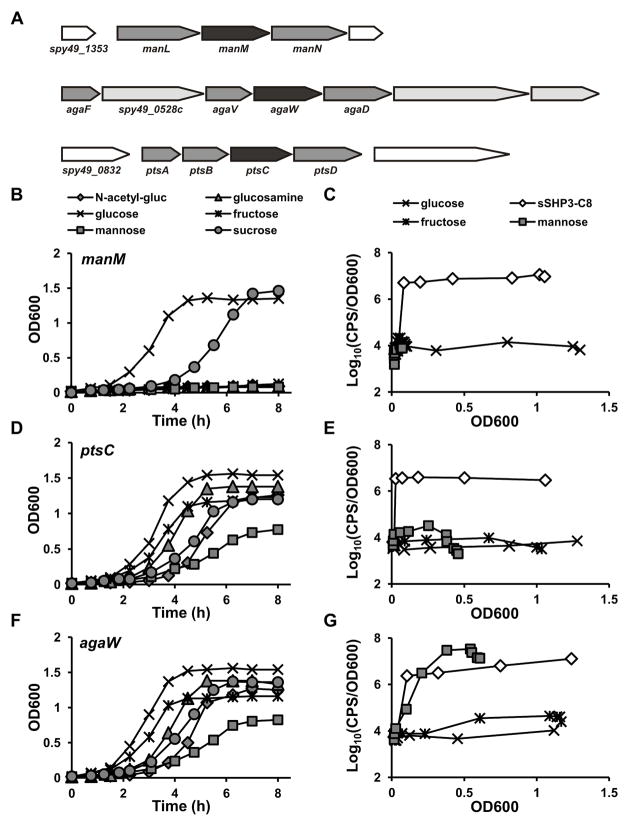
The GAS genome encodes three putative PTS-Man systems: manLMN, widely distributed in the Firmicutes and identified in other streptococci as the main importer of mannose (Abranches et al., 2003, Bidossi et al., 2012, Cochu et al., 2003, Lortie et al., 2000, Tong et al., 2011); ptsABCD, present only in some species of pyogenic streptococci; and a third system, agaFVWD, annotated as a N-acetyl-galactosamine importer, but shown to be required for growth on hyaluronate in Streptococcus pneumoniae (Bidossi et al., 2012) (Fig. 3A). To investigate whether a specific PTS system was required to import mannose and mediate Rgg-SHP signaling, mutants of the membrane-bound permease component (EIIC) of each PTS were constructed by insertional disruption. When tested on putative PTS-Man substrates, the manM strain was unable to grow on mannose, fructose, glucosamine, or N-acetyl-glucosamine, but was able to grow on non PTS-Man substrates glucose and sucrose (Fig. 3B). In contrast, growth of the ptsC and agaW mutants on all sugars was similar to that observed for wild-type (Fig. 2A, 3D, F). These results demonstrate that ManLMN is the primary PTS for import of mannose, fructose, glucosamine and NAG, and that neither PtsABCD nor AgaFVWD are sufficient to support robust growth on these substrates when ManLMN is absent. When we examined the effect of PTS disruption these strains’ ability to participate in Rgg-SHP signaling during growth on the specific PTS-Man substrates capable of inducing Pshp3, mannose and fructose, we observed that unlike wild-type and the agaW mutant, manM and ptsC exhibited minimal reporter induction when grown on mannose or fructose (Fig. 2B, 3C, E, G). However, interpretation of the manM mutant’s results was confounded by the severe growth defect of this strain on these sugars; it is possible this strain was not able to grow sufficiently to trigger signaling. In contrast, disruption of ptsC had minimal effect on growth yet induction of Pshp3 was dramatically reduced, suggesting this PTS transporter is essential to activate SHP signaling in response to these sugars (Fig. 3D, E). A transcriptional luciferase reporter for ptsA was constructed and showed that expression of this operon was also mannose-responsive and subject to CCR (Fig. S4A), and the signaling defect of the ptsC mutant could be fully complemented by expressing ptsABCD from a constitutive promoter on a multi-copy plasmid (Fig. S4B). Finally, when exposed to synthetic C8 peptide, a condition that bypasses the environmental cues needed to trigger Rgg-SHP signaling, all mutants responded similarly to wild-type (Fig. 3C, E, G). Taken together, these data suggest that all three mutants’ capacity to respond to pheromone is intact and that the importance of PtsABCD lies in sensing and/or transducing signals specifically related to carbon source availability.
Figure 3. Role of mannose PTS in Rgg-SHP signaling.
(A) Putative mannose PTS operons in S. pyogenes. Light gray genes indicate PTS components; IIC components targeted for insertional disruption are shown in dark gray. (B, D, F) Growth and (C, E, G) luciferase activity of manM (B, C), ptsC (D, E), and agaW (F, G) mutants grown in different carbon sources (at 1%). For luciferase experiments, 50 nM synthetic C8 peptide was added to CDM+1% glucose as a positive control for Pshp3 induction. Data are representative of experiments performed at least three times.
Effect of growth in metal-deplete conditions or non-glucose sources on expression of Rgg proteins
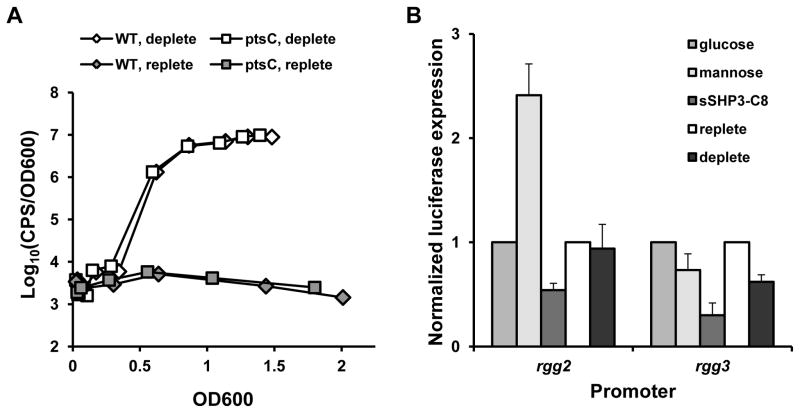
In previous studies, experiments examining induction of Rgg-SHP signaling have required the use of genetic mutants (e.g., Δrgg3) or the addition of synthetic pheromone. In assays performed with synthetic peptide, the kinetics of induction at Pshp2 versus Pshp3 have been indistinguishable by our current measurement techniques. Given that Rgg2 and Rgg3 bind both Pshp2 and Pshp3, expression of both shp genes is subject to autoinduction, and SHP2 and SHP3 are equivalent in their signaling potential in wild-type cells under conditions tested (Chang et al., 2011, Lasarre et al., 2013a, LaSarre et al., 2013b), we presume that expression at the two promoters in response to synthetic pheromone is virtually simultaneous. These observations were confirmed for reporter strains grown in metal-deplete or mannose-containing media (data not shown). However, the presence of an MtsR binding site upstream of shp3 but not shp2 suggested that initial activation of Rgg-SHP signaling in response to environmental cues may occur at one locus versus the other. To begin to test whether metal limitation and carbon source availability rely on separate mechanisms of induction, Pshp3 reporter activity in the ptsC mutant was observed during growth in metal-deplete conditions. In contrast to its inability to exhibit full induction in response to mannose, Pshp3-luxAB expression increased under metal limitation similar to wild-type (Fig. 4A), suggesting that the induction of Rgg-SHP signaling due to low metal conditions occurs independently of this PTS. We next examined the expression of rgg2 and rgg3 under the different conditions. While the addition of synthetic peptide to Prgg2-luxAB and Prgg3-luxAB reporter strains led to a slight decrease in the transcription of both genes, and expression of the reporters remained unchanged (Prgg2) or decreased slightly (Prgg3) when strains were grown in metal-deplete conditions, the pattern of expression of the two reporters diverged when cells were grown in mannose (Fig. 4B). Prgg3 expression decreased slightly, but there was a ~2.5-fold increase in the expression of Prgg2 relative to control conditions. We have previously shown that over-expression of rgg2 is sufficient to trigger induction of Rgg2/3 signaling (Chang et al., 2011), although whether this increase in expression translates into sufficient amounts of the transcription factor to induce signaling under these conditions has not been tested directly. Taken together, however, these data support a model in which the environmental signals of metal and carbon source availability are detected by separate mechanisms which converge at the step of pheromone production.
Figure 4. Metal limitation and carbon source availability elicit distinct responses upstream of pheromone production.
(A) Luciferase activity of wild-type and ptsC Pshp3-luxAB reporter strains grown in metal-deplete or replete conditions. CxCDM was supplemented with 50 μM CaCl2and 1000 μM MgSO4 (deplete) or 50 μM CaCl2, 1000 μM MgSO4, 20 μM FeSO4, and 30 μM MnSO4 (replete). Data are representative of experiments performed at least three times. (B) Luciferase expression from rgg2 and rgg3 promoters under different growth conditions. Bars 1–3: Luciferase activity in 1% mannose or 50 nM synthetic SHP3-C8 after three hours of growth was normalized to expression in 1% glucose. Bars 4–5: Luciferase activity of cells grown under metal deplete conditions was normalized to promoter activity in replete conditions. The mean and SD of at least three independent samples are shown.
Induction of Rgg-SHP signaling results in cell aggregation and altered susceptibility to antimicrobial agents
Previously, we demonstrated that the Δrgg3 mutant or wild-type cells stimulated with synthetic peptide formed robust biofilms in strain NZ131 (Chang et al., 2011). In the process of monitoring biofilm development, we noticed that induced cells also aggregate more quickly as assessed by a method measuring settling rates of cells (Fig. 5A). We reasoned that increased aggregation and biofilm formation could be linked to changes in cell-wall composition or cell-surface architecture due to variable display of surface structures, including proteins or glycans. To begin exploring differences in the cell wall of induced or uninduced cells, log-phase cells were exposed to a range of concentrations of chicken egg white lysozyme in 96-well plates and incubated overnight. When wild-type NZ131 was pretreated with C8 before exposure to the muramidase, we observed an eight to 16-fold increase in the minimum inhibitory concentration (data not shown). Subsequently, a killing assay was performed in which strains were exposed to a lethal concentration of lysozyme and plated for CFUs. Wild-type NZ131 was rapidly killed over the course of four hours; in contrast, the Δrgg3 mutant was resistant to lysozyme-mediated killing (Fig. 5B). In accordance with what we observed in the 96-well format assay, survival of wild-type cells induced with synthetic C8 peptide before exposure to lysozyme was similar to that of Δrgg3. To rule out the possibility that increased sensitivity to lysozyme was due to noticeable changes in growth rates seen during Rgg-SHP induction, wild-type cells were grown in subinhibitory concentrations of chloramphenicol or spectinomycin which resulted in a rate of growth comparable to that of C8-treated cultures; subsequent exposure to lysozyme demonstrated that antibiotic-treated cells were equally sensitive to lysozyme as untreated wild-type (Fig. S5). Finally, addition of C8 peptide to Δrgg2 had no effect on survival.
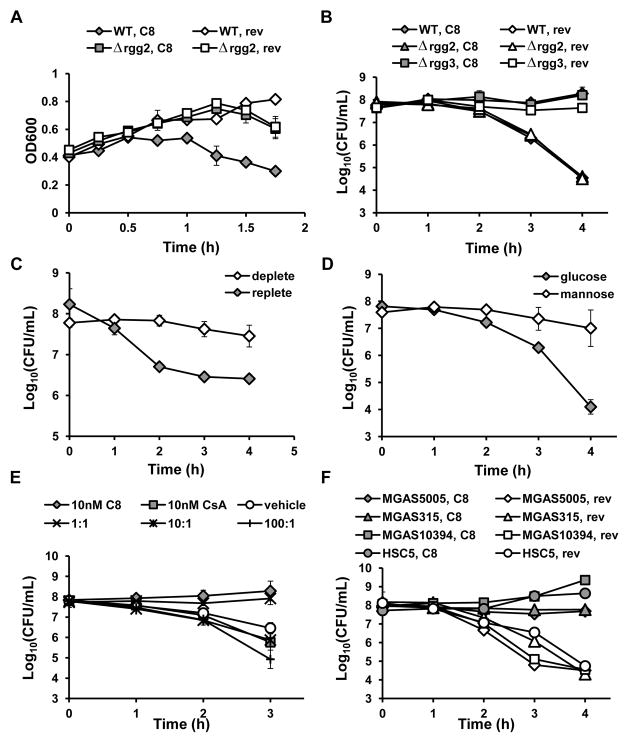
Figure 5. Lysozyme resistance is incurred through Rgg2/3-SHP induction.
(A) Effect of peptide treatment on bacterial aggregation. (B–E) Survival of GAS during challenge with 2 mg mL−1 lysozyme after (B) pre-treatment of wild-type NZ131 and isogenic rgg mutants with 100 nM synthetic C8 or reverse peptide; (C) pre-growth in metal-deplete (50 μM CaCl2, 1000 μM MgSO4) or replete (50 μM CaCl2, 1000 μM MgSO4, 20 μM FeSO4, 30 μM MnSO4) CxCDM; (D) pre-growth in CDM containing 1% glucose or 1% mannose; (E) pre-treatment with 10 nM synthetic C8, 10 nM cyclosporin A (CsA), or a combination of the two (1:1, 10 nM CsA + 10 nM C8; 10:1,100 nM CsA + 10 nM C8; 100:1, 1000 nM CsA + 10 nM C8). (F) Lysozyme challenge of different GAS serotypes after pre-treatment with 100 nM C8 or reverse peptide. Concentrations of lysozyme used were 10 mg mL−1, MGAS5005; 20 mg mL−1, MGAS10394, HSC5; or 50 mg mL−1, MGAS315. The mean and SD of duplicate (A) or triplicate (B–F) samples is shown, and data are representative of experiments performed at least twice.
A panel of antibacterial compounds was also tested for effects on cells expressing the Rgg-SHP regulon versus uninduced cells. No differences in MIC were observed for certain antibiotics that specifically target the cell wall or cell wall biosynthesis, including ampicillin, bacitracin, polymixin B, and vancomycin. However, a two-fold increase in the MIC of D-cycloserine, which disrupts the production of peptidoglycan monomers in the cytoplasm, was observed for cultures pre-treated with synthetic C8 but not reverse peptide or DMSO alone (Table S1). Because cycloserine acts within the cytoplasm, we also tested other antimicrobial compounds or chemicals that would have unfavorable effects in the cytosol. No differences in sensitivity were observed between induced and uninduced cultures exposed to chloramphenicol, rifampicin, or hydrogen peroxide. Surprisingly, SHP-treated cells also exhibited a modest increase in sensitivity to erythromycin, spectinomycin, and paraquat, and an eight to 16-fold increase in sensitivity to the aminoglycosides kanamycin and streptomycin (Table S1). The trends in decreased (cycloserine) and increased (erythromycin, kanamycin, spectinomycin, and streptomycin) sensitivity were also observed in the Δrgg3 mutant in which the system is constitutively activated and required intact Rgg-SHP signaling, as resistance of the Δrgg2 mutant was mostly unchanged by the addition of peptide.
Metal limitation and mannose independently induce lysozyme resistance
Initial experiments monitoring killing after lysozyme exposure were performed by artificially inducing Rgg-SHP signaling with synthetic peptide (Fig. 5B). We next tested whether growth of GAS in the environmental conditions observed to induce Pshp expression as described above was sufficient to confer resistance to lysozyme. For both metal depletion and mannose, reporter strains were grown under inducing conditions and monitored for Pshp3 induction before dilution into fresh media containing lysozyme. Samples were removed, serially diluted, and plated for CFUs at different time points. In both cases, Pshp3 induction in response to environmental cues correlated with increased survival after lysozyme exposure (Fig. 5C, D).
Inhibition of Rgg-SHP with a small molecule inhibitor abrogates development of lysozyme resistance
Recently, our lab identified cyclosporin A (CsA) as an inhibitor of Rgg-SHP signaling, specifically competing with SHP pheromones to bind the Rgg receptors (Aggarwal et al., 2015). To ask whether CsA could block the induction of lysozyme resistance in response to synthetic SHP, wild-type NZ131 was treated with the inhibitor, and lysozyme resistance was monitored by enumeration of CFU. When added at a 1:1 molar ratio with C8 peptide, CsA had minimal effect on the cells’ survival; as this assay was performed with wild-type cells that autoinduce shp2 and shp3 expression, it seems likely that CsA was outcompeted by endogenously-produced peptide. However, when CsA was added at 10- and 100-fold higher concentrations (100 nM and 1 μM, respectively) relative to C8, cells were killed in a manner resembling samples treated with CsA or vehicle alone (Fig. 5E). Although CsA has not been shown to affect bacterial growth at concentrations as high as 10 μM (Aggarwal et al., 2015), we did observe a slight increase in sensitivity to lysozyme relative to vehicle in cultures treated with CsA alone at high concentrations (Fig. S6).
Rgg-dependent regulation of lysozyme resistance is conserved in multiple GAS strains
The majority of our work to understand the Rgg-SHP system has used NZ131, an M49 serotype, as a model strain. To confirm that induction of Rgg-SHP contributes to resistance to lysozyme across multiple serotypes, we tested the following wild-type GAS strains: MGAS5005 (M1), MGAS315 (M3), MGAS10394 (M6), and HSC5 (M14). First, induction of Rgg-SHP signaling in response to synthetic peptide was confirmed in strains carrying a multi-copy Pshp3-luxAB reporter; all strains exhibited increased luciferase activity upon the addition of synthetic peptide, although they varied in maximum reporter induction in response to decreasing peptide, with NZ131 and HSC5 being the most sensitive to lower levels of pheromone (Fig. S7). When challenged with lysozyme, the strains also exhibited a range of innate resistance, with NZ131 being the most sensitive and MGAS315 the most resistant. However, treatment with synthetic C8, but not reverse peptide, led to enhanced survival in lysozyme of all of the strains (Fig. 5F). Thus, it appears that the Rgg-SHP-mediated lysozyme resistance is conserved across multiple GAS strains.
We next tested whether metal depletion or growth on mannose was sufficient to induce Rgg-SHP signaling and lysozyme resistance in the other GAS serotypes. Under metal-deplete conditions, Pshp3-luxAB induction was observed for MGAS5005, MGAS315, and HSC5, but not MGAS10394; however, all strains exhibited increased survival when challenged with lysozyme (Fig. S8). More strain variability was observed when cells were grown on mannose as the primary carbon source (Fig. S9). MGAS5005 and HSC5 reporter strains showed Pshp3 induction, but the former did not exhibit increased survival in lysozyme. MGAS315 exhibited very little reporter induction, yet cells were more lysozyme resistant. MGAS10394 failed to grow on mannose. Taken together, these data indicate that regulatory connections between upstream environmental signaling pathways and lysozyme resistance display variability in robustness among GAS serotypes, as is assessable using metal depletion and mannose culturing conditions optimized for NZ131, but the trend among these strains to enhance resistance in these conditions indicates that sensory pathways are generally conserved in multiple serotypes.
Discussion
Group A Streptococcus has proven adept in its interactions with its human host, yet our understanding of how this bacterium coordinates signals received from its environment to persist in the face of host immune defenses is incomplete. GAS is primarily regarded as an oropharyngeal colonizer, but it also causes skin disease, vulvovaginitis and perianal infections, and likely transits through the gastrointestinal tract due to swallowing of saliva and food. Thus, the environments it encounters may be more diverse than what is recognized clinically. Recognizing its location by detection of available nutrients would be an ideal cue to control appropriate colonization, immune-evasion, and competitive-advantage genes at appropriate times and places. We show here that one of the ways GAS responds to the specific signals of metal restriction and alternate carbon source availability is the upregulation of the Rgg-SHP quorum-sensing system, and that at least one of the consequences of this upregulation is an increased resistance to lysozyme.
Interestingly, the mechanisms by which metal and carbon source availability induce Rgg-SHP signaling appear to differ (Fig. 6). For the former, it seems likely that the metallorepressor, MtsR, senses decreasing iron or manganese concentrations and releases DNA, allowing increased transcription of shp3 and ultimately, the upregulation of Rgg-SHP signaling through autoinduction due to the positive feedback loop intrinsic to this and many other quorum-sensing systems. A straightforward explanation for how the non-glucose carbon sources mannose, and to a lesser extent fructose, may trigger system induction is less clear. A search for binding sites for CcpA, the primary regulator of CCR in GAS, in the promoter regions of shp peptides did not reveal any strong candidates. However, we observe a transient increase in the transcription of the activator protein, Rgg2, and our previous reports have indicated that over-expression of Rgg2 alone is sufficient to induce the system in ordinary laboratory growth conditions (i.e. 1% glucose and metal-replete conditions) (Fig. 4B) (Chang et al., 2011). Finally, our identification of an inducible mannose PTS, PtsABCD, as critical in the serotype M49 strain NZ131 for the induction of Rgg-SHP signaling, together with evidence that PTS and CCR regulators commonly influence gene expression beyond direct nutrient acquisition (Deutscher et al., 2014, Gorke & Stulke, 2008) raises the possibility that mannose-dependent induction has consequences on regulatory activities of this organism further and above its role in mannose uptake, perhaps through an intermediary transcriptional factor of the type seen to be regulated by PTS systems (Hondorp et al., 2013, Hammerstrom et al., 2015, Stulke et al., 1998). Though the manM mutant was unable to grow on mannose, preventing us from ruling out ManLMN as playing a role in mannose induction of Rgg2, additional support that the mannose response is due to PtsABCD comes from Shelburne et al (Shelburne et al., 2008) predicting the presence of a CRE site for CcpA binding and CCR control of the ptsABCD operon. The potential for mannose to be available in the body as a GAS carbon source and/or signal is supported by data in which GAS mannose catabolism genes are upregulated in transcriptomic studies of colonization in a macaque pharyngitis model as well as growth in blood, indicating the carbohydrate is an available nutrient source (Graham et al., 2005, Virtaneva et al., 2005). Additionally, a source of free mannose from degradation of mucins or other glycosylated host proteins by mannosidases and other glycosidases encoded by GAS or other organisms has been suggested and shown to be a viable strategy for carbon acquisition (Burnaugh et al., 2008, Ng et al., 2013, Shelburne et al., 2008, Siegel et al., 2014, Terra et al., 2010). Recently, a peptide-pheromone signaling pathway in S. pneumoniae was shown to be expressed when grown with galactose as a primary carbon source and not glucose (Hoover et al., 2015). Galactose, like mannose, is another prominent carbohydrate present in glycans on airway epithelia available to bacteria as carbon sources (Buckwalter & King, 2012). The apparent ability of galactose to induce quorum-sensing signaling provides a precedent for conditional activation of cell-cell signaling in streptococci.
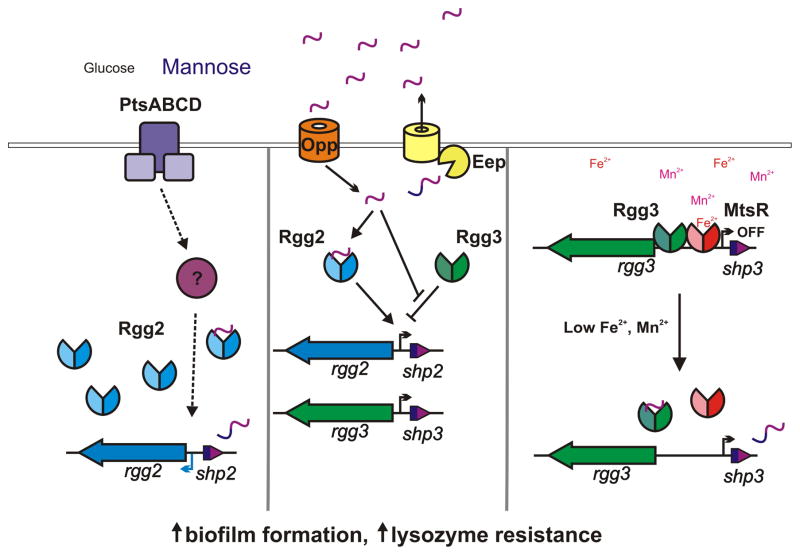
Figure 6. Proposed mechanisms for environmental activation of Rgg2/3 signaling.
Rgg2 (blue) and Rgg3 (green) are transcription factors that control expression of target promoters in response to peptide pheromones (center panel). Induction of this regulatory pathway generates biofilm production and lysozyme resistance. (Right panel) The metal-dependent transcriptional regulator MtsR (red) represses transcription of shp3 until iron or manganese concentrations are limiting, whereupon repression is relieved and SHP3 pheromones are generated to initiate the positive feedback loop of SHP production. (Left panel) Import of mannose through PtsABCD leads to enhanced expression of Rgg2 by an unknown mechanism, but we suggest includes an additional transcriptional regulator modified by transport of mannose.
The observations described in this text indicate that the mechanisms underlying the impetus of quorum sensing can initiate at one or the other side of the dual-sensor pathway (either Rgg2-SHP2 or Rgg3-SHP3), and provide a logical explanation for the complexity and apparent redundancy in having two nearly identical pheromones and two receptors encoded at two chromosomal loci. While only one ligand-receptor pair would seemingly suffice for purposes of controlling target-gene expression, perhaps two loci allows for a simple mechanism to integrate multiple environmental cues. While this scenario, on its face, does not evoke an idea of efficient use of genomic content, in GAS the Rgg2/3 circuitry is conserved absolutely among all available sequenced genomes (>200 genomes), attesting to an evolutionary selection to maintain this complex design.
Among the consequences of the induced quorum-sensing genes and their targets is an altered cellular surface resulting in an increased resistance to lysozyme. As we have reported previously, biofilm development is also observed, but by our assessment is reproducible for only a few strains that have been tested, namely NZ131 and GA19681. As indicated here, enhanced lysozyme resistance was observed following quorum-sensing induction for all five strains of varying serotype that we tested. The mechanism underlying lysozyme resistance, or pheromone-dependent biofilm development for that matter, remains a direction under investigation, though transcriptional regulation of GAS homologs of genes documented to modify peptidoglycan (pgdA, oatA) or teichoic acids (dltABCD), in other Gram-positive bacteria (Davis & Weiser, 2011, Kovacs et al., 2006, Kristian et al., 2005) have not thus far been promising leads in our studies (data not shown). Capsule, peptidoglycan and techoic acid structure, emm type or any other of the numerous surface features that vary between GAS strains would likely explain differences in basal sensitivities to lysozyme. Likewise, mechanisms that confer increased susceptibility to aminoglycosides and that modestly increased resistance to cycloserine remain unclear; however surface alterations leading to changes in surface charge or hydrophobicity may have impact on cellular permeability for these small molecules. Focus remains on genes within the two operons under direct control of Rgg2/3 and found immediately downstream of the shp2 and shp3 genes. Most intriguing is the operon located downstream of shp3 and containing aroE.2, whose expression is induced more than 100-fold when cells are treated with SHP (Chang et al., 2011). The function of these genes is unclear; however, the importance of this locus in survival in the host is suggested by the identification of the last gene of this operon, a putative efflux pump, in a signature-tagged mutagenesis screen for mutants attenuated in an invasive infection model of zebrafish (Kizy & Neely, 2009). This gene was also identified by Transposon-Site Hybridization (TraSH) following passage in blood (Le Breton et al., 2013). Additionally, hasB2 (spy49_0459) was found to contribute to hyaluronic acid capsule biosynthesis and compensated for hasB1 mutants (Cole et al., 2012). These enzymes serve as UDP-glucose dehydrogenases and up-regulation of hasB2 by Rgg-SHP signaling supports the prospect that glycosylation of a capsule or other substrate outside the cell is altered by pheromones. However, we have not found any obvious differences in capsule levels based on colony morphology phenotypes (unpublished). Prediction of any potential contribution provided by genetic material located downstream from shp2, which includes the small gene Spy49_0414c, whose expression is also greatly induced by pheromones, is less clear since no putative function is associated with this coding sequence. Thus, how genes known to be directly regulated by the Rgg2/3 system contribute to surfaces changes upon system induction remain unclear.
Regardless of the mechanism by which the Rgg2/3 pathway leads to lysozyme resistance, we naturally turn to the question of whether resistance contributes to infection or carriage, and if resistance can be blocked through disruption of the Rgg network. Quorum sensing pathways have garnered particular interest as therapeutic targets for their involvement in regulating specialized behaviors (including pathogenic factors), their inherent necessity to respond to extracellular chemical signals, and their potential susceptibility to agents that could disrupt signaling. Our lab is developing methodologies to identify small molecule modulators of Rgg proteins and to test the feasibility and effectiveness that these quorum-sensing pathways provide as new targets for anti-virulence therapeutics. Since human colonization by GAS is unlikely to be a preventable occurrence, perhaps microbial behavior can be manipulated through modulators of chemical communication.
Experimental Procedures
Bacterial strains and growth conditions
S. pyogenes was routinely grown in Todd-Hewitt (TH; BD) broth supplemented with 0.2% yeast extract (Y; Amresco) at 37°C without shaking (liquid) or in the presence of 5% CO2 (on plates). Starter cultures for all experiments were prepared as follows. Strains of interest were grown in THY broth overnight. In the morning, cultures were diluted 1:100 into a chemically defined medium (CDM; (Chang et al., 2011, van de Rijn & Kessler, 1980)) and allowed to grow to mid-logarithmic phase (OD600 = 0.4 to 0.7) at which time glycerol was added to a final concentration of 20%; aliquots were frozen and stored at −80° C. On experiment days, individual aliquots were thawed, diluted into fresh CDM to a starting OD600 of 0.01, and grown to densities as indicated in separate sections below. When appropriate, antibiotics were used at the following concentrations: chloramphenicol (cm, 3 μg mL−1), erythromycin (erm, 0.5 μg mL−1), spectinomycin (spec, 100 μg mL−1), kanamycin (kan, 200 μg mL−1). E. coli cloning strain BH10c (Howell-Adams & Seifert, 2000), was maintained in Luria broth (LB)c or on Luria agar with the following antibiotics used at the indicated concentrations: cm (10 μg mL−1), erm (500 μg mL−1), spec (100 μg mL−1).
Synthetic peptides
Synthetic peptides were purchased from Neo-Peptide (Cambridge, MA) and have been described previously (Chang et al., 2011, Lasarre et al., 2013a). All peptides were reconstituted in DMSO to a concentration of 2 mM and stored in aliquots at −80° C. Subsequent dilutions for working stocks (100 μM) were made in DMSO and stored at −20° C.
Low metal experiments
All low metal experiments were conducted in polypropylene tubes. Metal-depleted CDM was prepared as previously described (Chang et al., 2011) with the following changes: ultra pure water (ELGA Purelab Classic) was used, and CaCl2, FeSO4, Fe(NO3)3, MgSO4, and MnSO4 were omitted. This medium was then treated with 4.5% w/v Chelex 100 (Sigma; buffered with 0.5 M sodium acetate before use) with stirring at room temperature for four hours; the resulting Chelex-treated CDM (CxCDM) was filter sterilized. CaCl2 and MgSO4 were added to final concentrations of 50 μM and 1 mM, respectively, and FeSO4 and/or MnSO4 were supplemented as indicated; all metals were obtained from Sigma at ≥99% purity. Meanwhile, starter cultures as described above were diluted into CDM and allowed to grow to an OD600 of at least 0.3, at which time the cells were centrifuged at 2700×g for 10 minutes, and the pellets were washed three times with CxCDM containing 100 μM dipyridyl. Cells were then resuspended in CxCDM and diluted into metal-supplemented CxCDM to a final OD600 of 0.05. Cultures were incubated at 37°C without shaking. At each time point, a sample was removed to a semi-microcuvette, and the OD600 was measured on a SmartSpec 3000 (Biorad). 50 μL was also transferred to a 96-well white opaque plate (Greiner Bio-one), exposed to decyl aldehyde (Sigma) fumes for one minute, and counts per second (CPS) were quantified using a Veritas microplate luminometer (Turner Biosystems). Relative light units (RLU) were calculated by normalizing CPS to OD600.
Carbon source experiments
A glucose-free CDM was prepared by omitting glucose and bringing the volume up to 90% of the final value before filter sterilization. Carbon sources of interest were prepared as 10% (w/v) stocks and diluted into sugar-free CDM. Starter cultures as described above were diluted into CDM and allowed to grow to an OD600 of at least 0.3, pelleted, washed once to remove residual glucose, and diluted into fresh CDM containing the carbon source of interest at a starting OD600 of 0.05. At each time point, growth was monitored by measuring the OD600 using a Spectronic 20D+ (Thermo Scientific) and 50 μL of culture was removed to measure luciferase activity as described above. Relative light units (RLU) were calculated by normalizing CPS to OD600.
Cell aggregation assays
Starter cultures were diluted into THY media and allowed to grow until they reached early exponential phase. Cells were pelleted and resuspended to a final OD600 = 0.05 in fresh CDM containing 50 nM synthetic SHP3-C8 or 50 nM of the reverse peptide. Bacteria were grown at 37°C and briefly vortexed every 15 minutes until they reached an OD600 = 0.5. Cultures were then removed to room temperature, and duplicate 100 μL samples were taken from just below the meniscus and immediately measured for absorbance at 600 nm in a Synergy 2 microplate reader (Biotek).
MIC assays
96-well plates were prepared by performing two-fold serial dilutions of the chemicals of interest down each row in CDM, leaving the final row untreated. Starter cultures as described above were diluted into CDM and allowed to grow to an OD600 of ~0.1 at which time 100 nM of synthetic peptide, reverse peptide, or DMSO (vehicle) was added. After one hour of incubation at 37° C, the OD600 was measured and cultures were diluted to an OD600 of 0.05 in CDM containing peptide, reverse peptide, or DMSO as indicated. The diluted bacteria were added to the plates containing the serial dilutions of each chemical at a volume equal to that already present in each well, such that the resulting OD600 was ~0.025 and the final concentration of synthetic peptide present in each well was 50 nM. Plates were incubated at 37°C for 20 hours, after which the bacteria were resuspended by gentle pipetting, 75 μL were transferred to a fresh plate, and the OD600 was measured in a Synergy 2 microplate reader (Biotek). The MIC was defined as wells in which the final OD600 was 0.025 or less after blank subtraction.
Lysozyme killing assay
Starter cultures as described above were diluted into CDM and allowed to grow to an OD600 of ~0.1 at which time 100 nM of synthetic SHP3-C8 peptide (C8) or reverse peptide was added. After one hour of incubation at 37° C, the OD600 was measured and cultures were diluted to an OD600 of 0.1 in CDM containing peptide or reverse peptide. Freshly prepared chicken egg white lysozyme (Sigma) was added to diluted bacteria to a final concentration of 2 mg mL−1, and cultures were incubated at 37°C. At each time point, a sample was removed, serially diluted, plated on THY agar, and incubated at 37°C overnight to enumerate colony forming units (CFUs). For experiments testing the effect of cyclosporine A, a 1 mM stock of the drug (AMRI Global) was prepared in DMSO and stored at −20° C; CsA was added to cultures concurrently with C8 peptide. For experiments testing lysozyme resistance after growth in low metal conditions or mannose, cells were allowed to grow for one or three hours, respectively, after initial Pshp3-luxAB reporter induction, before being diluted to an OD600 of 0.1 in the same medium and challenged with lysozyme.
Supplementary Material
Acknowledgments
ZE491 and PA14 were kind gifts from from Z. Eichenbaum and M. Whiteley, respectively. We also thank the UIC Positive Thinkers and members of the Federle Lab for thoughtful discussions. Support for JC and MF was provided by NIH AI091779 and the Burroughs Wellcome Fund Investigators in the Pathogenesis of Infectious Diseases award. Support for JJ was provided by CONICYT Becas Chile.
Footnotes
The authors declare they have no conflicts of interest in publication of this work.
References
- Abranches J, Chen YY, Burne RA. Characterization of Streptococcus mutans strains deficient in EIIAB Man of the sugar phosphotransferase system. Appl Environ Microbiol. 2003;69:4760–4769. doi: 10.1128/AEM.69.8.4760-4769.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal C, Jimenez JC, Lee H, Chlipala GE, Ratia K, Federle MJ. Identification of Quorum-Sensing Inhibitors Disrupting Signaling between Rgg and Short Hydrophobic Peptides in Streptococci. MBio. 2015;6 doi: 10.1128/mBio.00393-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal C, Jimenez JC, Nanavati D, Federle MJ. Multiple length peptide-pheromone variants produced by Streptococcus pyogenes directly bind Rgg proteins to confer transcriptional regulation. J Biol Chem. 2014;289:22427–22436. doi: 10.1074/jbc.M114.583989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almengor AC, Kinkel TL, Day SJ, McIver KS. The catabolite control protein CcpA binds to Pmga and influences expression of the virulence regulator Mga in the Group A streptococcus. J Bacteriol. 2007;189:8405–8416. doi: 10.1128/JB.01038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates CS, Montanez GE, Woods CR, Vincent RM, Eichenbaum Z. Identification and characterization of a Streptococcus pyogenes operon involved in binding of hemoproteins and acquisition of iron. Infect Immun. 2003;71:1042–1055. doi: 10.1128/IAI.71.3.1042-1055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates CS, Toukoki C, Neely MN, Eichenbaum Z. Characterization of MtsR, a new metal regulator in group A streptococcus, involved in iron acquisition and virulence. Infect Immun. 2005;73:5743–5753. doi: 10.1128/IAI.73.9.5743-5753.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkelman RL, Martin D, Graham DR, Mowry J, Freisem R, Weber JA, Ho JL, Allen JR. Streptococcal wound infections caused by a vaginal carrier. JAMA. 1982;247:2680–2682. [PubMed] [Google Scholar]
- Bidossi A, Mulas L, Decorosi F, Colomba L, Ricci S, Pozzi G, Deutscher J, Viti C, Oggioni MR. A functional genomics approach to establish the complement of carbohydrate transporters in Streptococcus pneumoniae. PLoS One. 2012;7:e33320. doi: 10.1371/journal.pone.0033320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter CM, King SJ. Pneumococcal carbohydrate transport: food for thought. Trends Microbiol. 2012;20:517–522. doi: 10.1016/j.tim.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnaugh AM, Frantz LJ, King SJ. Growth of Streptococcus pneumoniae on human glycoconjugates is dependent upon the sequential activity of bacterial exoglycosidases. J Bacteriol. 2008;190:221–230. doi: 10.1128/JB.01251-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JC, LaSarre B, Jimenez JC, Aggarwal C, Federle MJ. Two group A streptococcal peptide pheromones act through opposing Rgg regulators to control biofilm development. PLoS Pathog. 2011;7:e1002190. doi: 10.1371/journal.ppat.1002190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussee MS, Ajdic D, Ferretti JJ. The rgg gene of Streptococcus pyogenes NZ131 positively influences extracellular SPE B production. Infect Immun. 1999;67:1715–1722. doi: 10.1128/iai.67.4.1715-1722.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussee MS, Sylva GL, Sturdevant DE, Smoot LM, Graham MR, Watson RO, Musser JM. Rgg influences the expression of multiple regulatory loci to coregulate virulence factor expression in Streptococcus pyogenes. Infect Immun. 2002;70:762–770. doi: 10.1128/iai.70.2.762-770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochu A, Vadeboncoeur C, Moineau S, Frenette M. Genetic and biochemical characterization of the phosphoenolpyruvate:glucose/mannose phosphotransferase system of Streptococcus thermophilus. Appl Environ Microbiol. 2003;69:5423–5432. doi: 10.1128/AEM.69.9.5423-5432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JN, Aziz RK, Kuipers K, Timmer AM, Nizet V, van Sorge NM. A conserved UDP-glucose dehydrogenase encoded outside the hasABC operon contributes to capsule biogenesis in group A Streptococcus. J Bacteriol. 2012;194:6154–6161. doi: 10.1128/JB.01317-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook LC, LaSarre B, Federle MJ. Interspecies communication among commensal and pathogenic streptococci. MBio. 2013;4 doi: 10.1128/mBio.00382-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KM, Weiser JN. Modifications to the peptidoglycan backbone help bacteria to establish infection. Infect Immun. 2011;79:562–570. doi: 10.1128/IAI.00651-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dei M, Di Maggio F, Di Paolo G, Bruni V. Vulvovaginitis in childhood. Best Pract Res Clin Obstet Gynaecol. 2010;24:129–137. doi: 10.1016/j.bpobgyn.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Deutscher J, Ake FM, Derkaoui M, Zebre AC, Cao TN, Bouraoui H, Kentache T, Mokhtari A, Milohanic E, Joyet P. The bacterial phosphoenolpyruvate:carbohydrate phosphotransferase system: regulation by protein phosphorylation and phosphorylation-dependent protein-protein interactions. Microbiol Mol Biol Rev. 2014;78:231–256. doi: 10.1128/MMBR.00001-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmaz R, Durmaz B, Bayraktar M, Ozerol IH, Kalcioglu MT, Aktas E, Cizmeci Z. Prevalence of group A streptococcal carriers in asymptomatic children and clonal relatedness among isolates in Malatya, Turkey. J Clin Microbiol. 2003;41:5285–5287. doi: 10.1128/JCM.41.11.5285-5287.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R. Mechanisms that control the microflora in the large intestine. In: Hentges DJ, editor. Human Intestinal Microflora in Health and Disease. New York: Academic Press; 1983. pp. 33–54. [Google Scholar]
- Gauthier L, Bourassa S, Brochu D, Vadeboncoeur C. Control of sugar utilization in oral streptococci. Properties of phenotypically distinct 2-deoxyglucose-resistant mutants of Streptococcus salivarius. Oral Microbiol Immunol. 1990;5:352–359. doi: 10.1111/j.1399-302x.1990.tb00440.x. [DOI] [PubMed] [Google Scholar]
- Gera K, Le T, Jamin R, Eichenbaum Z, McIver KS. The phosphoenolpyruvate phosphotransferase system in group A Streptococcus acts to reduce streptolysin S activity and lesion severity during soft tissue infection. Infect Immun. 2014;82:1192–1204. doi: 10.1128/IAI.01271-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorke B, Stulke J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol. 2008;6:613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- Graham MR, Virtaneva K, Porcella SF, Barry WT, Gowen BB, Johnson CR, Wright FA, Musser JM. Group A Streptococcus transcriptome dynamics during growth in human blood reveals bacterial adaptive and survival strategies. Am J Pathol. 2005;166:455–465. doi: 10.1016/S0002-9440(10)62268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham MR, Virtaneva K, Porcella SF, Gardner DJ, Long RD, Welty DM, Barry WT, Johnson CA, Parkins LD, Wright FA, Musser JM. Analysis of the transcriptome of group A Streptococcus in mouse soft tissue infection. Am J Pathol. 2006;169:927–942. doi: 10.2353/ajpath.2006.060112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerstrom TG, Horton LB, Swick MC, Joachimiak A, Osipiuk J, Koehler TM. Crystal structure of Bacillus anthracis virulence regulator AtxA and effects of phosphorylated histidines on multimerization and activity. Mol Microbiol. 2015;95:426–441. doi: 10.1111/mmi.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks TS, Liu M, McClure MJ, Fukumura M, Duffy A, Lei B. Differential regulation of iron- and manganese-specific MtsABC and heme-specific HtsABC transporters by the metalloregulator MtsR of group A Streptococcus. Infect Immun. 2006;74:5132–5139. doi: 10.1128/IAI.00176-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S. The throat carrier rate of group A and other beta hemolytic streptococci among patients in general practice. Acta Pathol Microbiol Immunol Scand B. 1985;93:347–351. doi: 10.1111/j.1699-0463.1985.tb02899.x. [DOI] [PubMed] [Google Scholar]
- Hollands A, Aziz RK, Kansal R, Kotb M, Nizet V, Walker MJ. A naturally occurring mutation in ropB suppresses SpeB expression and reduces M1T1 group A streptococcal systemic virulence. PLoS One. 2008;3:e4102. doi: 10.1371/journal.pone.0004102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondorp ER, Hou SC, Hause LL, Gera K, Lee CE, McIver KS. PTS phosphorylation of Mga modulates regulon expression and virulence in the group A streptococcus. Mol Microbiol. 2013;88:1176–1193. doi: 10.1111/mmi.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover SE, Perez AJ, Tsui HC, Sinha D, Smiley DL, DiMarchi RD, Winkler ME, Lazazzera BA. A new quorum-sensing system (TprA/PhrA) for Streptococcus pneumoniae D39 that regulates a lantibiotic biosynthesis gene cluster. Mol Microbiol. 2015 doi: 10.1111/mmi.13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell-Adams B, Seifert HS. Molecular models accounting for the gene conversion reactions mediating gonococcal pilin antigenic variation. Mol Microbiol. 2000;37:1146–1158. doi: 10.1046/j.1365-2958.2000.02067.x. [DOI] [PubMed] [Google Scholar]
- Janulczyk R, Pallon J, Bjorck L. Identification and characterization of a Streptococcus pyogenes ABC transporter with multiple specificity for metal cations. Mol Microbiol. 1999;34:596–606. doi: 10.1046/j.1365-2958.1999.01626.x. [DOI] [PubMed] [Google Scholar]
- Janulczyk R, Ricci S, Bjorck L. MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect Immun. 2003;71:2656–2664. doi: 10.1128/IAI.71.5.2656-2664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl-Fie TE, Skaar EP. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol. 2010;14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keim NL, Levin RJ, Havel PJ. Carbohydrates. In: Shils ME, Ross AC, Caballero B, Cousins RJ, editors. Modern nutrition in health and disease. Baltimore: Lippincott, Williams, and Wilkins; 2005. pp. 62–82. [Google Scholar]
- King SJ. Pneumococcal modification of host sugars: a major contributor to colonization of the human airway? Mol Oral Microbiol. 2010;25:15–24. doi: 10.1111/j.2041-1014.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- Kizy AE, Neely MN. First Streptococcus pyogenes signature-tagged mutagenesis screen identifies novel virulence determinants. Infect Immun. 2009;77:1854–1865. doi: 10.1128/IAI.01306-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, Halfmann A, Fedtke I, Heintz M, Peschel A, Vollmer W, Hakenbeck R, Bruckner R. A functional dlt operon, encoding proteins required for incorporation of d-alanine in teichoic acids in gram-positive bacteria, confers resistance to cationic antimicrobial peptides in Streptococcus pneumoniae. J Bacteriol. 2006;188:5797–5805. doi: 10.1128/JB.00336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristian SA, Datta V, Weidenmaier C, Kansal R, Fedtke I, Peschel A, Gallo RL, Nizet V. D-alanylation of teichoic acids promotes group a streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J Bacteriol. 2005;187:6719–6725. doi: 10.1128/JB.187.19.6719-6725.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasarre B, Aggarwal C, Federle MJ. Antagonistic Rgg regulators mediate quorum sensing via competitive DNA binding in Streptococcus pyogenes. MBio. 2013a;3 doi: 10.1128/mBio.00333-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaSarre B, Chang JC, Federle MJ. Redundant group a streptococcus signaling peptides exhibit unique activation potentials. J Bacteriol. 2013b;195:4310–4318. doi: 10.1128/JB.00684-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Breton Y, Mistry P, Valdes KM, Quigley J, Kumar N, Tettelin H, McIver KS. Genome-wide identification of genes required for fitness of group A Streptococcus in human blood. Infect Immun. 2013;81:862–875. doi: 10.1128/IAI.00837-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei B, Liu M, Voyich JM, Prater CI, Kala SV, DeLeo FR, Musser JM. Identification and characterization of HtsA, a second heme-binding protein made by Streptococcus pyogenes. Infect Immun. 2003;71:5962–5969. doi: 10.1128/IAI.71.10.5962-5969.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lortie LA, Pelletier M, Vadeboncoeur C, Frenette M. The gene encoding IIAB(Man)L in Streptococcus salivarius is part of a tetracistronic operon encoding a phosphoenolpyruvate: mannose/glucose phosphotransferase system. Microbiology. 2000;146(Pt 3):677–685. doi: 10.1099/00221287-146-3-677. [DOI] [PubMed] [Google Scholar]
- Lyon WR, Gibson CM, Caparon MG. A role for trigger factor and an rgg-like regulator in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. EMBO J. 1998;17:6263–6275. doi: 10.1093/emboj/17.21.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Bryant AE, Salmi DB, McIndoo E, Stevens DL. vfr, a novel locus affecting cysteine protease production in Streptococcus pyogenes. J Bacteriol. 2009;191:3189–3194. doi: 10.1128/JB.01771-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JM, Green M, Barbadora KA, Wald ER. Group A streptococci among school-aged children: clinical characteristics and the carrier state. Pediatrics. 2004;114:1212–1219. doi: 10.1542/peds.2004-0133. [DOI] [PubMed] [Google Scholar]
- Mashburn-Warren L, Morrison DA, Federle MJ. A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an Rgg regulator. Mol Microbiol. 2010;78:589–606. doi: 10.1111/j.1365-2958.2010.07361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashburn-Warren L, Morrison DA, Federle MJ. The cryptic competence pathway in Streptococcus pyogenes is controlled by a peptide pheromone. J Bacteriol. 2012;194:4589–4600. doi: 10.1128/JB.00830-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee WM, Di Caprio JM, Roberts CE, Jr, Sherris JC. Anal carriage as the probable source of a streptococcal epidemic. Lancet. 1966;2:1007–1009. doi: 10.1016/s0140-6736(66)92931-x. [DOI] [PubMed] [Google Scholar]
- Mogielnicki NP, Schwartzman JD, Elliott JA. Perineal group A streptococcal disease in a pediatric practice. Pediatrics. 2000;106:276–281. doi: 10.1542/peds.106.2.276. [DOI] [PubMed] [Google Scholar]
- Montanez GE, Neely MN, Eichenbaum Z. The streptococcal iron uptake (Siu) transporter is required for iron uptake and virulence in a zebrafish infection model. Microbiology. 2005;151:3749–3757. doi: 10.1099/mic.0.28075-0. [DOI] [PubMed] [Google Scholar]
- Moye ZD, Burne RA, Zeng L. Uptake and metabolism of N-acetylglucosamine and glucosamine by Streptococcus mutans. Appl Environ Microbiol. 2014;80:5053–5067. doi: 10.1128/AEM.00820-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, Naidu N, Choudhury B, Weimer BC, Monack DM, Sonnenburg JL. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature. 2013;502:96–99. doi: 10.1038/nature12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Miranda S, Cabirol N, George-Tellez R, Zamudio-Rivera LS, Fernandez FJ. O-CAS, a fast and universal method for siderophore detection. J Microbiol Methods. 2007;70:127–131. doi: 10.1016/j.mimet.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Shaikh N, Leonard E, Martin JM. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics. 2010;126:e557–564. doi: 10.1542/peds.2009-2648. [DOI] [PubMed] [Google Scholar]
- Shelburne SA, 3rd, Keith D, Horstmann N, Sumby P, Davenport MT, Graviss EA, Brennan RG, Musser JM. A direct link between carbohydrate utilization and virulence in the major human pathogen group A Streptococcus. Proc Natl Acad Sci U S A. 2008;105:1698–1703. doi: 10.1073/pnas.0711767105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne SA, 3rd, Olsen RJ, Makthal N, Brown NG, Sahasrabhojane P, Watkins EM, Palzkill T, Musser JM, Kumaraswami M. An amino-terminal signal peptide of Vfr protein negatively influences RopB-dependent SpeB expression and attenuates virulence in Streptococcus pyogenes. Mol Microbiol. 2011;82:1481–1495. doi: 10.1111/j.1365-2958.2011.07902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne SA, 3rd, Sumby P, Sitkiewicz I, Granville C, DeLeo FR, Musser JM. Central role of a bacterial two-component gene regulatory system of previously unknown function in pathogen persistence in human saliva. Proc Natl Acad Sci U S A. 2005;102:16037–16042. doi: 10.1073/pnas.0505839102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel SJ, Roche AM, Weiser JN. Influenza promotes pneumococcal growth during coinfection by providing host sialylated substrates as a nutrient source. Cell Host Microbe. 2014;16:55–67. doi: 10.1016/j.chom.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel JD, Funaro D, Kaplan EL. Recurrent group A streptococcal vulvovaginitis in adult women: family epidemiology. Clin Infect Dis. 2007;44:e43–45. doi: 10.1086/510678. [DOI] [PubMed] [Google Scholar]
- Stulke J, Arnaud M, Rapoport G, Martin-Verstraete I. PRD--a protein domain involved in PTS-dependent induction and carbon catabolite repression of catabolic operons in bacteria. Mol Microbiol. 1998;28:865–874. doi: 10.1046/j.1365-2958.1998.00839.x. [DOI] [PubMed] [Google Scholar]
- Terra VS, Homer KA, Rao SG, Andrew PW, Yesilkaya H. Characterization of novel beta-galactosidase activity that contributes to glycoprotein degradation and virulence in Streptococcus pneumoniae. Infect Immun. 2010;78:348–357. doi: 10.1128/IAI.00721-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Zeng L, Burne RA. The EIIABMan phosphotransferase system permease regulates carbohydrate catabolite repression in Streptococcus gordonii. Appl Environ Microbiol. 2011;77:1957–1965. doi: 10.1128/AEM.02385-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toukoki C, Gold KM, McIver KS, Eichenbaum Z. MtsR is a dual regulator that controls virulence genes and metabolic functions in addition to metal homeostasis in the group A streptococcus. Mol Microbiol. 2010;76:971–989. doi: 10.1111/j.1365-2958.2010.07157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I, Kessler RE. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980;27:444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraelen H, Verhelst R, Vaneechoutte M, Temmerman M. Group A streptococcal vaginitis: an unrecognized cause of vaginal symptoms in adult women. Arch Gynecol Obstet. 2011;284:95–98. doi: 10.1007/s00404-011-1861-6. [DOI] [PubMed] [Google Scholar]
- Virtaneva K, Porcella SF, Graham MR, Ireland RM, Johnson CA, Ricklefs SM, Babar I, Parkins LD, Romero RA, Corn GJ, Gardner DJ, Bailey JR, Parnell MJ, Musser JM. Longitudinal analysis of the group A Streptococcus transcriptome in experimental pharyngitis in cynomolgus macaques. Proc Natl Acad Sci U S A. 2005;102:9014–9019. doi: 10.1073/pnas.0503671102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg ED. Nutritional immunity. Host’s attempt to withold iron from microbial invaders. JAMA. 1975;231:39–41. doi: 10.1001/jama.231.1.39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.