Abstract
BACKGROUND
Falls can cause moderate to severe injuries such as hip fractures and head trauma in older adults. While declines in muscle strength and sensory function contribute to increased falls in older adults, skeletal muscle fatigue is often overlooked as an additional contributor to fall risk. The purpose of this investigation was to examine the effects of acute lower extremity muscle fatigue and age on reactive postural control in healthy adults.
METHODS
A sample of 16 individuals participated in this study (8 healthy older adults and 8 healthy young persons). Whole body kinematic and kinetic data were collected during anterior and posterior reproducible fall tests before (T0) and immediately after (T1) eccentric muscle fatiguing exercise, as well as after 15-minutes (T15) and 30-minutes (T30) of rest.
FINDINGS
Lower extremity joint kinematics of the stepping limb during the support (landing) phase of the anterior fall were significantly altered by the presence of acute muscle fatigue. Step velocity was significantly decreased during the anterior falls. Statistically significant main effects of age were found for step length in both fall directions. Effect sizes for all outcomes were small. No statistically significant interaction effects were found.
INTERPRETATION
Muscle fatigue has a measurable effect on lower extremity joint kinematics during simulated falls. These alterations appear to resolve within 15 minutes of recovery. The above deficits, coupled with a reduced step length, may help explain the increased fall risk in older adults.
Keywords: postural control, muscle fatigue, aging
1. INTRODUCTION
Falls are a major source of morbidity and disability in the aging population. Twenty to thirty percent of older adults who fall suffer moderate to severe injuries such as lacerations, hip fractures, and head traumas (Sterling et al., 2001). The economic burden of falls in older adults is very high and the direct medical cost of falls in 2012, adjusted for inflation, was $30 billion (CDC, 2012; Stevens et al., 2006). Despite the vast public health problem of falls in the older population, studies have generally neglected the impact that muscle fatigue may have on falls.
Acute muscle fatigue has been shown to alter postural control in healthy young and older individuals, which may increase the risk for falls (Bellew and Fenter, 2006; Nam et al., 2013; Paillard, 2012). More specifically, acute muscle fatigue is known to modify the peripheral proprioceptive system and the central processing of sensory inputs (Taylor et al., 2000) both of which are integral for reactive postural control.
Recovery from external perturbations requires reactive postural control, which is defined by modifying sensory and motor systems in response to changing tasks and externally induced postural demands (Shumway-Cook and Woollacott, 2012). The majority of falls in older adults occur in the context of tasks requiring reactive postural control (Niino et al., 2000). In addition, the chances of sustaining a fall are particularly high during slipping or tripping situations (Lord et al., 1993).
Several studies have experimentally examined the effect of acute muscle fatigue on reactive postural control in healthy older adults (Adlerton and Moritz, 2001; Davidson et al., 2009; Granacher et al., 2010; Mademli et al., 2008). Decrements such as increased center of mass (COM) displacements (Davidson et al., 2009), increased center of pressure (COP) displacements (Nam et al., 2013) and sway velocity (Parreira et al., 2013), increased antagonist muscle co-activation (Granacher et al., 2010), and decreased functional reflex activity (Granacher et al., 2010) have been demonstrated following acute muscle fatigue in older individuals in the context of externally induced perturbations. However, just one of these studies has examined the particular biomechanical aspects of postural control recovery at joint-specific anatomic locations (Mademli et al., 2008). This lack of biomechanical detail limits insight into the lower extremity segmental characteristics of postural instability. While Mademli et al. demonstrated alterations in the knee joint angle following muscle fatigue, they did not provide any indication of the duration of the fatigue effect. In general, investigations of muscle fatigue and postural control have neglected the recovery timeline of lower extremity proximal and distal joint kinematics.
The purpose of this investigation was to examine the effects of age and acute muscle fatigue on the biomechanic responses of reactive postural control. The secondary objective was to examine the timeline of postural control recovery following acute muscle fatigue. We hypothesized that regardless of age, acute muscle fatigue would result in declines in kinematic outcomes of reactive postural control but that the declines would be more substantial in older adults. We further hypothesized that these alterations would return to baseline within 20-minutes of post-exercise fatigue recovery (Yaggie and McGregor, 2002).
2. METHODS
2.1 Study Sample
Participants were recruited from the local community. Sample sizes were estimated using an effect size of 0.68 for peak center of pressure (COP) displacement following postural perturbations in previously published data (Davidson et al., 2009) and estimate tables (Portney and Watkins, 2009) based on an α level=0.05 and 90% power. Inclusion criteria for the healthy older group required that individuals be older than 50 years of age (Toebes et al., 2012) and between 18 and 35 years of age for the healthy young group. Participants were excluded if they had participated in vigorous exercise 24 hours prior to initiating the study. Subjects were also screened for musculoskeletal or neurologic impairments affecting postural stability via a health questionnaire and baseline functional mobility testing performed by a physical therapist trained in fall prevention. All participants were healthy, generally active individuals who were independent in activities of daily living. This study was reviewed by the University of Utah Institutional Review Board and participants provided informed consent prior to participation.
2.2 Postural Control Assessments
The reactive postural control task utilized a tether-release model, which forced the subjects to incorporate a protective step to regain stability. The tether-release protocol has been used previously to investigate balance recovery from anterior (Carty et al., 2011; Madigan, 2006; Madigan and Lloyd, 2005) and posterior falls (Hsiao and Robinovitch, 2001). The protocol for this study consisted of securing one end of a tether to a trunk harness at the level of the xiphisternal joint. The other end of the tether was connected to an inline force sensor and electromagnet that was fixed to the wall. Participants were asked to lean against the tether until 9–12% of their body mass registered through the inline force sensor. This value has been shown to exceed sway-based recovery abilities (Hsiao-Wecksler, 2008). Once the subjects were in position for the trial, they were given the following instruction: “When the tether is released try to recover your balance with a single step.” Release of the tether was randomized between 1 and 20 seconds from the time they were in position to limit anticipation of the release time. Five consecutive trials were performed in each of the anterior and posterior falling directions, with data analysis being performed on three successful trials for each direction. A trial was considered successful if the individual was able to recover from the tether-release independently in one step, without assistance from the overhead harness, and the joint markers were visible throughout the trial.
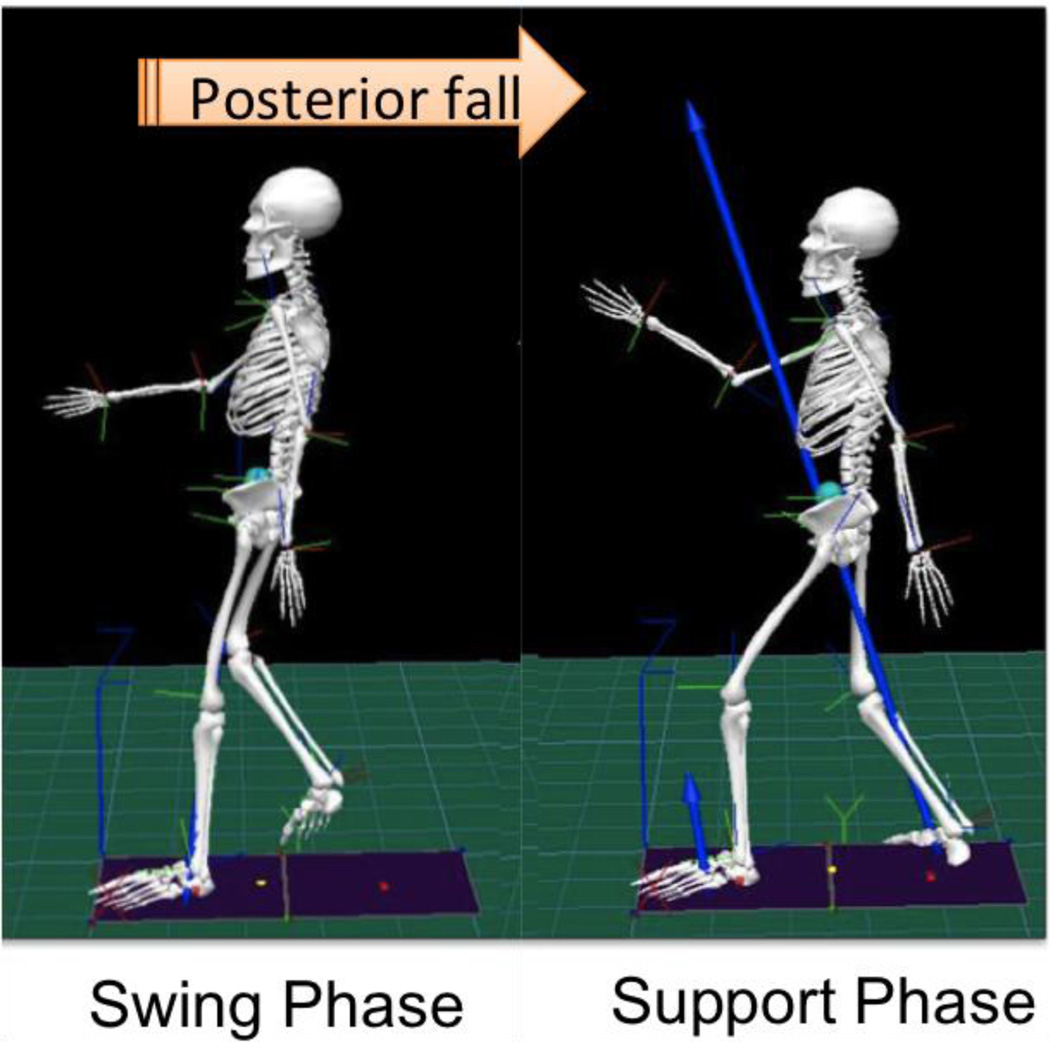
For analysis, the reactive postural control task was divided into the following phases: 1) The swing phase represented the time between when the stepping foot left the force platform to the point at which the same foot struck the second force platform upon landing (Figure 1). 2) The support phase represented the point from when the stepping foot struck the second force platform upon landing until the individual’s COM stopped moving in the direction of the fall.
Figure 1.
Diagram of a posterior tether-release task, demonstrating both swing and support phases.
2.3 Data Collection and Procedures
Following consent, subjects were instrumented with reflective markers based on a standardized gait analysis marker set defining 15 body segments (Plug-In-Gait marker set; Vicon Motion Systems; Oxford, UK). Data were acquired using a Vicon 10-camera motion analysis system (Vicon Motion Systems; Oxford, UK) and 2 AMTI OR6 series force platform systems (AMTI; Watertown, MA) at a rate of 250Hz. Data were recorded and synchronized using Vicon Nexus (Vicon Motion Systems; Oxford, UK) and post-processing occurred using Visual3D (C-Motion, Germantown, MD). Marker and force platform data were filtered using a fourth-order, low pass, zero-phaseshift Butterworth filter at 6 Hz and 20 Hz, respectively (Winter, 2009).
Reactive postural control assessments were performed before (T0) and immediately (<2mins) after muscle fatiguing exercise (T1), as well as after 15-minutes (T15) and 30-minutes (T30) of rest.
Prior to baseline testing participants were exposed to 1–3 trial sessions of the tether-release test in order to become familiar with the testing procedure, and to overcome the fear of falling. After performing the baseline (T0) assessments, markers on the posterior aspect of the trunk and pelvis were removed for seated fatiguing exercises (described below). To assure accurate re-application of the markers, the base of each marker was traced prior to removal. Immediately after exercise the markers were re-applied and the immediate post (T1) postural control assessments were performed. Following 15 minutes of rest the T15 assessments were performed. After a second 15 minute rest, the T30 postural control assessments were completed.
2.4 Postural Control Outcomes
Outcomes collected during the swing phase of the tether-release task included: step length normalized by height and step velocity. Outcome measures collected during the support phase included: hip, knee, and ankle joint angular displacements, peak COP displacement, and center of pressure-center of mass (COP-COM) difference. The COP-COM difference examined the difference between the COP position at its peak displacement in the medio-lateral direction and the COM at the concomitant time-point. A large COP-COM difference is indicative of robust postural control (Chang and Krebs, 1999; Martin et al., 2002). With changes in body position during the fall task the distance between COP and COM increases. A large COP-COM difference would indicate robust postural control, because the individual is able to allow straying of the COM outside of the functional base while recovering balance with a single step. Meanwhile, a small COP-COM difference represents a conservative approach to postural tasks, in that the performer does not feel stable enough to allow separation of the COP and COM.
2.5 Fatigue Protocol
The principal muscle groups fatigued in this study were those necessary for the control of postural responses during the reactive postural control task, namely the quadriceps and hip extensors. Fatigue was induced in the lower extremity using resistance exercise that was performed on a seated, isokinetic ergometer (EccentronTM, BTE Technologies, Inc., Hanover, MD). Participants resisted a motorized foot pedal that moved toward them at a self-selected pace between 20–40rpm causing eccentric muscle contractions about the knee and hip extensors. Fatigue was determined by real-time biofeedback of a 30% drop in individual participants’ maximal voluntary contraction (MVC), which has been shown to induce a deterioration in postural control following localized muscle fatigue (Paillard, 2012). This was accomplished in the present study by asking participants to "resist the pedals as hard as you can for 10 seconds" prior to beginning the fatiguing bout of exercise. Biofeedback was provided on a computer monitor with the average of 4 maximal effort pedal strokes being represented by a horizontal line. An additional line indicated a 30% decline in their baseline MVC. Subjects were required to perform the exercise until they dropped below the 30% level for 4 consecutive pedal strokes.
2.6 Statistical Analysis
Summary of the participant demographics and testing of the assumptions of the planned parametric analysis were performed using descriptive statistics and Shapiro-Wilk’s test, respectively. A one-way ANOVA was used to determine between-group differences on demographic variables and exercise components. Separate 2 (age group) × 4 (time period) ANOVAs with repeated measures on the time factor were used to determine between-group, within-group, and interaction effects for all postural control variables. If the assumption of sphericity was failed, a Greenhouse-Geiser correction was used. If significance was found, pairwise comparisons were performed on the time factor using the Bonferroni correction. Effect sizes were calculated using partial eta-squared. Effects were considered statistically significant when p<0.05. The above statistical analyses were performed using SPSS (version 21.0).
3. RESULTS
3.1 Participant Demographics
Sixteen participants were recruited from the local community including 8 healthy older adults (HO) and 8 healthy young adults (HY). Table 1 describes the demographic characteristics of each group. Statistical between-group differences were found for age (p<0.001) but the groups did not differ on sex, height, weight, or Body Mass Index. Descriptive characteristics of total work performed and total exercise time for each group can also be seen in Table 1. These data demonstrate that the HY group exercised longer and produced more muscular work than the HO cohort, but these differences were not statistically significant.
Table 1.
Characteristics of study participants.
| Characteristic | HO (n=8) | HY (n=8) | P value |
|---|---|---|---|
| Age (years) | 58.5 (4.6) [55.3–61.6] | 26.8 (3.7) [23.8–29.7] | 0.000 |
| Sex | 6M/2F | 3M/5F | 0.149 |
| Body height (m) | 1.8 (0.1) [1.75–1.91] | 1.7 (0.1) [1.66–1.81] | 0.094 |
| Body weight (kg) | 95.1 (20.7) [77.5–122.7] | 79.3 (25.4) [62.7–95.9] | 0.185 |
| BMI (kg/m2) | 28.3 (6.2) [23.9–32.6] | 25.7 (5.4) [21.6–29.8] | 0.376 |
| Total Work (joules) | 63,748 (80,513) [946–126,550] | 109,100 (85,733) [49,890–168,311] | 0.280 |
| Total Ex time (min) | 19.49 (17.7) [7.05–31.9] | 31.57 (15.3) [19.8–43.3] | 0.152 |
Values are presented as Mean xx (SD yy) [95% Confidence Intervals]
BMI - body mass index, Total Ex time – total exercise time
3.2 Effects of Age
Statistically significant main effects of age were found for step length in both the anterior and posterior fall directions (F=4.82, p=0.045, ηp2=.369/F=8.19, p=0.013, ηp2=.256) (Table 2/Table 3). Analysis of between-group means demonstrated that HY persons stepped further on average than HO individuals during the simulated falls.
Table 2.
Means and standard deviations for reactive postural control measures across all time-points during anterior tether-release.
| VARIABLE | GROUP | FATIGUE | P ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| Pre (T0) | Post1 (T1) | Post2 (T15) | Post3 (T30) | Group | Time | Interaction | ||
| PK_COP_DISP (cm) | HO | 12.1 (1.8) | 11.9 (2.2) | 11.7 (2.1) | 11.9 (2.1) | 0.788 | 0.774 | 0.764 |
| HY | 11.6 (2.2) | 11.6 (2.4) | 11.5 (1.9) | 11.9 (2.0) | ||||
| COP-COM_DIFF (cm) | HO | 17.3 (4.7) | 16.9 (2.2) | 15.6 (3.9) | 15.0 (4.0) | 0.949 | 0.010 | 0.691 |
| HY | 17.7 (4.2) | 16.6 (3.9) | 15.7 (5.1) | 15.2 (3.4) | ||||
| STEP LENGTH (cm) | HO | 27.8 (1.9) | 27.6 (3.1) | 28.0 (3.3) | 27.9 (3.6) | 0.045 | 0.835 | 0.861 |
| HY | 31.1 (2.9) | 31.1 (3.4) | 30.9 (2.6) | 31.0 (2.3) | ||||
| STEP VELOCITY (m/s) | HO | 1.15 (.10) | 1.14 (.06) | 1.06 (.12) | 1.06 (.09) | 0.408 | 0.008 | 0.256 |
| HY | 1.18 (.10) | 1.14 (.13) | 1.10 (.15) | 1.16 (.14) | ||||
| HIP_ANG_DISP (deg) | HO | 18.3 (5.3) | 23.8 (9.9) | 19.7 (4.0) | 23.8 (8.6) | 0.944 | 0.058 | 0.465 |
| HY | 20.3 (4.2) | 24.3 (8.0) | 21.1 (6.6) | 20.6 (6.2) | ||||
| KNEE_ANG_DISP (deg) | HO | 26.4 (8.1) | 36.2 (9.1) | 28.8 (5.6) | 30.2 (14.3) | 0.753 | 0.002 | 0.402 |
| HY | 31.4 (7.0) | 37.8 (8.1) | ▰29.0 (5.3) | 31.4 (5.7) | ||||
| ANKLE_ANG_DISP (deg) | HO | 17.5 (5.7) | 21.5 (5.2) | ▰16.3 (5.6) | 16.4 (8.5) | 0.842 | 0.003 | 0.299 |
| HY | 18.8 (5.2) | 23.7 (5.1) | ▰17.6 (4.6) | 17.5 (4.5) | ||||
Values are presented as Mean xx (SD yy) bold- indicates statistically significant main effect (p<0.05),
indicates return to baseline, PK_COP_DISP peak mediolateral center of pressure displacement toward the stance limb during the swing phase, COP-COM_DIFF center of pressure-center of mass difference during the support (landing) phase, HIP_ANG_DISP angular displacement of the stepping limb hip joint during the support (landing) phase, KNEE_ANG_DISP angular displacement of the stepping limb knee joint during the support (landing) phase, ANKLE_ANG_DISP angular displacement of the stepping limb ankle joint during the support (landing) phase
Table 3.
Means and standard deviations for reactive postural control measures across all time-points during posterior tether-release.
| VARIABLE | GROUP | FATIGUE | P ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| Pre (T0) | Post1 (T1) | Post2 (T15) | Post3 (T30) | Group | Time | Interaction | ||
| PK_COP_DISP (cm) | HO | 10.2 (2.3) | 10.0 (2.4) | 9.6 (1.9) | 9.4 (2.2) | 0.359 | 0.366 | 0.289 |
| HY | 10.6 (1.6) | 10.9 (2.4) | 11.2 (2.3) | 10.6 (2.6) | ||||
| COP-COM_DIFF (cm) | HO | 21.8 (4.0) | 20.0 (4.9) | 19.6 (5.0) | 19.2 (4.4) | 0.923 | 0.029 | 0.890 |
| HY | 21.2 (5.6) | 20.3 (4.1) | 18.8 (4.1) | 19.4 (5.3) | ||||
| STEP LENGTH (cm) | HO | 29.9 (2.3) | 30.4 (2.1) | 29.4 (1.8) | 29.2 (1.3) | 0.013 | 0.837 | 0.712 |
| HY | 33.2 (3.4) | 33.4 (3.6) | 33.4 (4.3) | 33.6 (3.8) | ||||
| STEP VELOCITY (m/s) | HO | 1.37 (.13) | 1.37 (.12) | 1.41 (.13) | 1.34 (.17) | 0.096 | 0.620 | 0.844 |
| HY | 1.48 (.15) | 1.47 (.11) | 1.49 (.14) | 1.47 (.12) | ||||
| HIP_ANG_DISP (deg) | HO | 24.4 (9.7) | 29.0 (13.2) | 26.7 (7.6) | 26.5 (11.0) | 0.356 | 0.463 | 0.554 |
| HY | 25.8 (6.8) | 25.7 (6.2) | 21.8 (5.1) | 23.7 (6.2) | ||||
| KNEE_ANG_DISP (deg) | HO | 38.7 (9.9) | 41.9 (9.3) | 43.9 (6.8) | 41.2 (8.2) | 0.683 | 0.162 | 0.302 |
| HY | 39.0 (7.9) | 44.9 (10.6) | 40.0 (9.1) | 41.2 (8.5) | ||||
| ANKLE_ANG_DISP (deg) | HO | 30.3 (7.4) | 31.0 (5.6) | 29.7 (7.1) | 30.4 (9.6) | 0.541 | 0.142 | 0.880 |
| HY | 29.1 (4.0) | 28.7 (3.0) | 29.1 (4.8) | 30.9 (5.2) | ||||
Values are presented as Mean xx (SD yy) bold- indicates statistically significant main effect (p<0.05), PK_COP_DISP peak mediolateral center of pressure displacement toward the stance limb during the swing phase, COP-COM_DIFF center of pressure-center of mass difference during the support (landing) phase, HIP_ANG_DISP angular displacement of the stepping limb hip joint during the support (landing) phase, KNEE_ANG_DISP angular displacement of the stepping limb knee joint during the support (landing) phase, ANKLE_ANG_DISP angular displacement of the stepping limb ankle joint during the support (landing) phase
3.4 Effects of Acute Muscle Fatigue
3.4.1 Anterior Reactive Response
Statistically significant main effects of time were found for several outcomes of the anterior tether-release, including COP-COM difference (F=4.26, p=0.010, ηp2=.233), step velocity (F=4.47, p=0.008, ηp2=.242), and both knee (F=5.91, p=0.002, ηp2=.297) and ankle (F=5.51, p=0.003, ηp2=.284) angular displacement of the stepping limb during the support phase. A trend was seen for hip angular displacement though it did not reach statistical significance (p=0.058) (Table 2). No statistically significant interaction effects were found.
3.4.2 Posterior Reactive Response
For the posterior tether-release, statistically significant main effects of time were found for COP-COM difference (F=3.31, p=0.029, ηp2=.192) (Table 3). No statistically significant interaction effects were found.
3.4.3 Recovery from Fatigue
Bonferroni adjusted pairwise comparisons (p<0.007) revealed that step velocity was decreased in T15 compared to baseline (p=0.006) and that knee and ankle angular displacements of the stepping limb in the support phase were increased in the immediate (T1) post-fatigue state compared to baseline (p=0.001/p=0.000). These T1 values returned to baseline after 15 minutes of rest (T15) in a statistically significant manner (p=0.001/p=0.000) (Figure 2).
Figure 2.
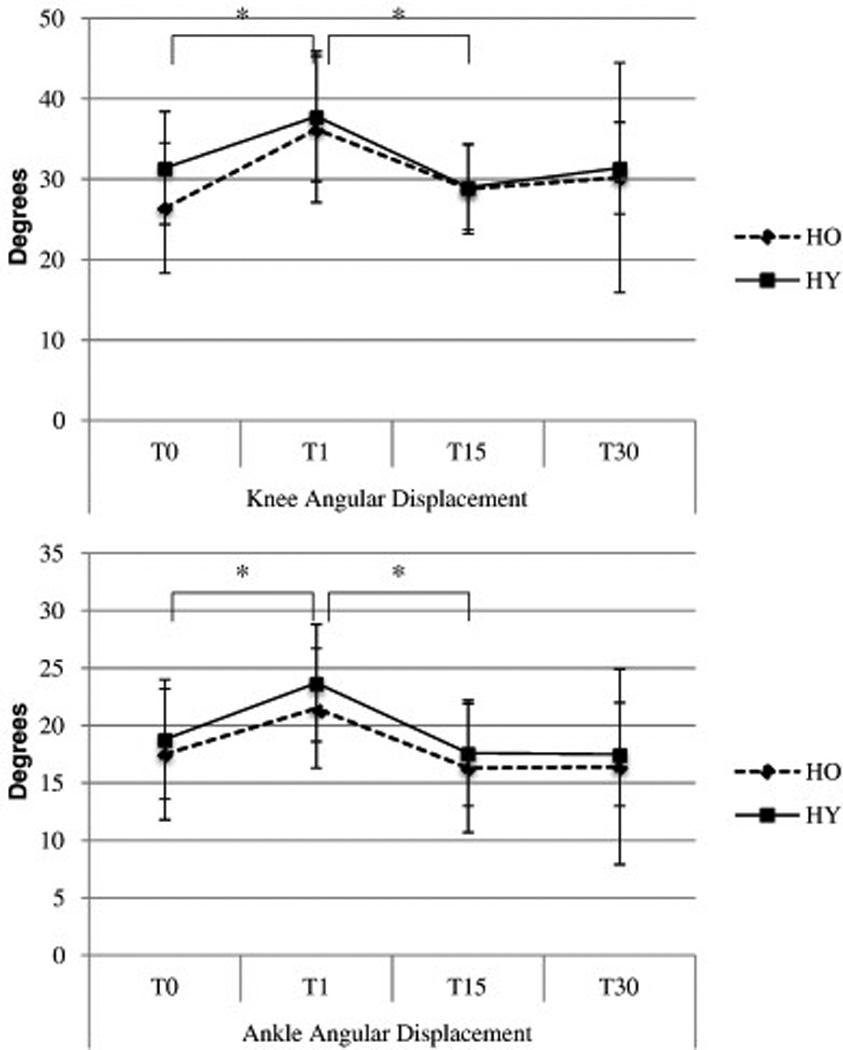
Angular displacements of the stepping limb knee and ankle joints during the support (landing) phase of the anterior tether-release in healthy old and young individuals.
* indicates statistically significant pairwise comparisons with Bonferroni correction (P<0.007), T0 baseline measurement, T1 immediate-post fatigue test, T15 15-minutes post-fatigue, T30 30-minutes post-fatigue
4. DISCUSSION
The purpose of this investigation was to characterize the effects of age and acute muscle fatigue on the biomechanics of reactive postural control in healthy adults. In general, the results indicate that acute muscle fatigue has deleterious effects on reactive postural control in healthy old and young individuals, and that some of these effects are alleviated within 15 minutes of rest.
4.1 Effects of Acute Muscle Fatigue
The effect of acute muscle fatigue on reactive postural control had a measurable effect on lower extremity joint kinematics. Specifically, increases in hip, knee and ankle angular displacement of the stepping limb during the support phase were found in the immediate post-fatigue test of the anterior tether-release. These findings are in agreement with work by Mademli et al. who reported increased knee flexion angles in the stepping limb during the support phase of an anterior release task in HY and HO individuals following acute muscle fatigue of the quadriceps femoris muscles (Mademli et al., 2008). Muscle fatigue induced by repetitive contractions causes a reduction in the force generating capability of the muscle (Gandevia, 2001). This may have resulted in the decreased capacity of the quadriceps and hip extensor muscles to maintain body weight through the stepping limb during the support phase of the tether-release.
Another neurophysiologic mechanism that could explain the increased joint angular displacement immediately after fatigue (T1) might be the reduction of functional reflex activity (FRA), which has been shown to contribute to the degree of joint laxity and joint stability (Granata et al., 2004). Additional research has demonstrated that ankle fatigue diminishes FRA in the tibialis anterior muscle of young and elderly men, resulting in reduced joint stability during the support phase of reactive gait perturbations (Granacher et al., 2010). The loss of joint stability in the presence of fatigue may be a significant contributor to the increased joint angular displacements seen in this study. Future research is needed to determine if such increased joint displacements are predictive of falls in HO persons seen at the end of the day (Doheny et al., 2012) or during slipping or tripping situations (Lord et al., 1993).
Regardless of age, the COP-COM difference during the support phase of the anterior and posterior tether-release tasks continued to get smaller at each time-point following the fatiguing intervention. A small COP-COM difference indicates that the individual attempts to minimize the separation of the COM from the COP during the fall, as if they’re uncertain of their ability to control COM motion. During an obstacle crossing task, a reduced COP-COM distance indicated a conservative reduction of the mechanical load on joints of the supporting limb (Hahn and Chou, 2004). Similarly, in the present study the significant decreases in COP-COM difference across time suggests that fatigue caused individuals to adopt a conservative strategy that limited the stability demands on the COP during recovery from simulated falls.
4.2 Effects of Age
Significant differences between the groups in this study occurred in the length of the protective step. HY adults employed a longer step length during recovery from the tether-release task than HO individuals. This phenomenon was also seen by Mademli et al., who found that HY individuals stepped 8.9cm further on average, than HO participants following acute muscle fatigue and recovery from a forward-leaning reproducible fall (Mademli et al., 2008). In an actual fall situation, the stepping strategy is used to bring the support base back into alignment under the COM (Maki and McIlroy, 1997). A reduced step length provides a much smaller degree of stabilization and is considered by some to be a maladaptive strategy for HO persons (Menz et al., 2003a, b, 2007).
4.3 Recovery from Fatigue
The negative effect of fatigue on joint kinematics during reactive postural control tasks appears to be brief. In this study both knee and ankle angular displacements of the stepping limb during the support phase returned to baseline after 15 minutes of rest (Figure 2). Independent of age, previous studies examining localized muscle fatigue have shown that postural control returns to baseline across all age groups between 75 seconds (Harkins et al., 2005) and 20 minutes (Yaggie and McGregor, 2002) with an average time of 8.2 minutes (Adlerton and Moritz, 2001; Harkins et al., 2005; Lin et al., 2009; Parreira et al., 2013; Yaggie and McGregor, 2002). The effect of fatigue on joint angular kinematics appears to concur with previous studies.
Fatigue had a protracted effect however, on step velocity and COP-COM difference in this study. The fact that some outcome measures failed to recover while others were ameliorated over time should not be surprising. Fatigue can result in changing postural strategies including different kinetic and kinematic approaches (Wilson et al., 2006). In addition, the factors that regulate balance are multifaceted (Shumway-Cook and Woollacott, 2012). Because the postural control system has a series of built in redundancies (Loeb et al., 1999) the system allows the individual to compensate for a decline in one factor by upregulating control in another (Tresch, 2007). In this manner, an individual can attempt to maintain a degree of postural stability despite the ominous demands on muscle due to fatigue (Wilson et al., 2006).
4.4 Limitations
Although this study found significant alterations in reactive postural response coordination as a result of acute muscle fatigue, these results should be interpreted with caution. The non-significant interaction effects in this study reflected smaller effect sizes than expected as a result of large between subject variability. Potential causes for this variability include the large number of male subjects in the HO cohort and the heterogeneous levels of fatigue within groups (see large standard deviations, confidence intervals in Table 1).
While significant main effects of age were found in the study it is likely that more robust effects could have been seen had the sample included a healthy older cohort that was more advanced in age. Most world countries have accepted the chronological age of 65 as a definition of “elderly” persons. Thus, the authors acknowledge that the small effect sizes and lack of interactions between age and fatigue could have been caused by the relatively young age of older participants.
4.5 Suggestions for Future Research
The results of this investigation and previous examinations of postural control in older individuals in fatigued states (Adlerton and Moritz, 2001; Davidson et al., 2009; Granacher et al., 2010) suggest that acute muscle fatigue has a negative effect on reactive postural control. When coupled with studies reporting the alteration of the effectiveness of sensory inputs and motor output of postural control (Taylor et al., 2000), these trials strongly suggest that fatigue has a measurable clinical effect on stability and potentially on fall risk and raises two important issues worthy of further research.
First, the aging population is becoming increasingly advised to seek clinical interventions for strength and mobility training (Gillespie et al., 2001; Stephens, 2010). Consequently, there is the potential for iatrogenic increases in fall risk following fatiguing exercise that, to our knowledge has not been explored especially in populations already at increased fall risk (e.g., frail elderly, persons with neurodegenerative diseases). Until more empirical data is developed, falls can be mitigated in clinical settings if fatiguing exercises are conducted at the beginning of an exercise session, with lighter exercise occupying the last 15 minutes. High risk clients should be monitored closely at all times regardless of exercise intensity.
Second, the degradation of postural control by acute muscle fatigue would appear to reveal a potential target for intervention. If exercise programs were explicitly designed to make lower extremity muscles more fatigue resistant, the participant might derive postural control benefits. To date, several chronic muscle endurance-training studies have been employed using a combination of postural control outcomes (Avelar et al., 2010; Ballard et al., 2004; Hess and Woollacott, 2005; Lopopolo et al., 2006; Melzer et al., 2003). However, these studies have employed balance correlates like static stance posture, gait speed and other clinical tests, which fail to incorporate measures of reactive postural control. Controlled trials are needed to examine the efficacy of training regimens on muscle fatigue induced instability in reactive postural control scenarios.
5. CONCLUSIONS
Acute muscle fatigue has a deleterious effect on the coordination of reactive postural responses, regardless of age. Older persons have reduced spatial measures of postural control recovery, which may influence fall risk. These results should heighten the awareness of clinicians regarding the potential negative effects of acute muscle fatigue and age for older populations during clinical exercise settings. Further research is needed to examine the effects of acute muscle fatigue on postural control in inherently balance-impaired populations.
HIGHLIGHTS.
Most falls in older adults occur during tasks requiring reactive postural control
Muscle fatigue alters spatial and kinematic measures of reactive postural control
Fatigue-induced alterations to postural control resolve within 15 minutes of rest
These effects may influence fall risk in older adults during/after exercise settings
Acknowledgments
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award Number KL2TR001103. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
All authors are free of conflicts of interest in this study.
Contributor Information
K. Bo Foreman, Email: bo.foreman@hsc.utah.edu.
Lee E. Dibble, Email: lee.dibble@hsc.utah.edu.
Reference
- Adlerton AK, Moritz U. How does calf-muscle fatigue and age affect vibration-perturbed one-legged stance? Adv Physiother. 2001;3:179–187. [Google Scholar]
- Avelar NC, Bastone AC, Alcantara MA, Gomes WF. Effectiveness of aquatic and non-aquatic lower limb muscle endurance training in the static and dynamic balance of elderly people. Rev Bras Fisioter. 2010;14:229–236. [PubMed] [Google Scholar]
- Ballard JE, McFarland C, Wallace LS, Holiday DB, Roberson G. The effect of 15 weeks of exercise on balance, leg strength, and reduction in falls in 40 women aged 65 to 89 years. J Am Med Womens Assoc. 2004;59:255–261. [PubMed] [Google Scholar]
- Bellew JW, Fenter PC. Control of balance differs after knee or ankle fatigue in older women. Arch Phys Med Rehabil. 2006;87:1486–1489. doi: 10.1016/j.apmr.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Carty CP, Mills P, Barrett R. Recovery from forward loss of balance in young and older adults using the stepping strategy. Gait Posture. 2011;33:261–267. doi: 10.1016/j.gaitpost.2010.11.017. [DOI] [PubMed] [Google Scholar]
- CDC. The cost of falls among older adults. [Accessed March 12, 2015];Centers for Disease Control and Prevention. 2012 http://www.cdc.gov/HomeandRecreationalSafety/Falls/fallcost.html.
- Chang H, Krebs DE. Dynamic balance control in elders: gait initiation assessment as a screening tool. Arch Phys Med Rehabil. 1999;80:490–494. doi: 10.1016/s0003-9993(99)90187-9. [DOI] [PubMed] [Google Scholar]
- Davidson BS, Madigan ML, Nussbaum MA, Wojcik LA. Effects of localized muscle fatigue on recovery from a postural perturbation without stepping. Gait Posture. 2009;29:552–557. doi: 10.1016/j.gaitpost.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Doheny EP, Greene BR, Foran T, Cunningham C, Fan CW, Kenny RA. Diurnal variations in the outcomes of instrumented gait and quiet standing balance assessments and their association with falls history. Physiol Meas. 2012;33:361–373. doi: 10.1088/0967-3334/33/3/361. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gillespie LD, Gillespie WJ, Robertson MC, Lamb SE, Cumming RG, Rowe BH. Interventions for preventing falls in elderly people. Cochrane Database Syst Rev. 2001:CD000340. doi: 10.1002/14651858.CD000340. [DOI] [PubMed] [Google Scholar]
- Granacher U, Gruber M, Forderer D, Strass D, Gollhofer A. Effects of ankle fatigue on functional reflex activity during gait perturbations in young and elderly men. Gait Posture. 2010;32:107–112. doi: 10.1016/j.gaitpost.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Granata KP, Wilson SE, Massimini AK, Gabriel R. Active stiffness of the ankle in response to inertial and elastic loads. J Electromyogr Kinesiol. 2004;14:599–609. doi: 10.1016/j.jelekin.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hahn ME, Chou LS. Age-related reduction in sagittal plane center of mass motion during obstacle crossing. J Biomech. 2004;37:837–844. doi: 10.1016/j.jbiomech.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Harkins KM, Mattacola CG, Uhl TL, Malone TR, McCrory JL. Effects of 2 ankle fatigue models on the duration of postural stability dysfunction. J Athl Train. 2005;40:191–194. [PMC free article] [PubMed] [Google Scholar]
- Hess JA, Woollacott M. Effect of high-intensity strength-training on functional measures of balance ability in balance-impaired older adults. J Manipulative Physiol Ther. 2005;28:582–590. doi: 10.1016/j.jmpt.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Hsiao ET, Robinovitch SN. Elderly subjects' ability to recover balance with a single backward step associates with body configuration at step contact. J Gerontol A Biol Sci Med Sci. 2001;56:M42–M47. doi: 10.1093/gerona/56.1.m42. [DOI] [PubMed] [Google Scholar]
- Hsiao-Wecksler ET. Biomechanical and age-related differences in balance recovery using the tether-release method. J Electromyogr Kinesiol. 2008;18:179–187. doi: 10.1016/j.jelekin.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Lin D, Nussbaum MA, Seol H, Singh NB, Madigan ML, Wojcik LA. Acute effects of localized muscle fatigue on postural control and patterns of recovery during upright stance: influence of fatigue location and age. Eur J Appl Physiol. 2009;106:425–434. doi: 10.1007/s00421-009-1026-5. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Brown IE, Cheng EJ. A hierarchical foundation for models of sensorimotor control. Exp Brain Res. 1999;126:1–18. doi: 10.1007/s002210050712. [DOI] [PubMed] [Google Scholar]
- Lopopolo RB, Greco M, Sullivan D, Craik RL, Mangione KK. Effect of therapeutic exercise on gait speed in community-dwelling elderly people: a metaanalysis. Phys Ther. 2006;86:520–540. [PubMed] [Google Scholar]
- Lord SR, Ward JA, Williams P, Anstey KJ. An epidemiological study of falls in older community-dwelling women: the Randwick falls and fractures study. Aust J Public Health. 1993;17:240–245. doi: 10.1111/j.1753-6405.1993.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Mademli L, Arampatzis A, Karamanidis K. Dynamic stability control in forward falls: postural corrections after muscle fatigue in young and older adults. Eur J Appl Physiol. 2008;103:295–306. doi: 10.1007/s00421-008-0704-z. [DOI] [PubMed] [Google Scholar]
- Madigan ML. Age-related differences in muscle power during single-step balance recovery. J Appl Biomech. 2006;22:186–193. doi: 10.1123/jab.22.3.186. [DOI] [PubMed] [Google Scholar]
- Madigan ML, Lloyd EM. Age-related differences in peak joint torques during the support phase of single-step recovery from a forward fall. J Gerontol A Biol Sci Med Sci. 2005;60:910–914. doi: 10.1093/gerona/60.7.910. [DOI] [PubMed] [Google Scholar]
- Maki BE, McIlroy WE. The role of limb movements in maintaining upright stance: the "change-in-support" strategy. Phys Ther. 1997;77:488–507. doi: 10.1093/ptj/77.5.488. [DOI] [PubMed] [Google Scholar]
- Martin M, Shinberg M, Kuchibhatla M, Ray L, Carollo JJ, Schenkman ML. Gait initiation in community-dwelling adults with Parkinson disease: comparison with older and younger adults without the disease. Phys Ther. 2002;82:566–577. [PubMed] [Google Scholar]
- Melzer I, Benjuya N, Kaplanski J. Effects of regular walking on postural stability in the elderly. Gerontology. 2003;49:240–245. doi: 10.1159/000070404. [DOI] [PubMed] [Google Scholar]
- Menz HB, Lord SR, Fitzpatrick RC. Acceleration patterns of the head and pelvis when walking are associated with risk of falling in community-dwelling older people. J Gerontol A Biol Sci Med Sci. 2003a;58:M446–M452. doi: 10.1093/gerona/58.5.m446. [DOI] [PubMed] [Google Scholar]
- Menz HB, Lord SR, Fitzpatrick RC. Acceleration patterns of the head and pelvis when walking on level and irregular surfaces. Gait Posture. 2003b;18:35–46. doi: 10.1016/s0966-6362(02)00159-5. [DOI] [PubMed] [Google Scholar]
- Menz HB, Lord SR, Fitzpatrick RC. A structural equation model relating impaired sensorimotor function, fear of falling and gait patterns in older people. Gait Posture. 2007;25:243–249. doi: 10.1016/j.gaitpost.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Nam HS, Park DS, Kim DH, Kang HJ, Lee DH, Lee SH, Her JG, Woo JH, Choi SY. The relationship between muscle fatigue and balance in the elderly. Ann Rehabil Med. 2013;37:389–395. doi: 10.5535/arm.2013.37.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niino N, Tsuzuku S, Ando F, Shimokata H. Frequencies and circumstances of falls in the National Institute for Longevity Sciences, Longitudinal Study of Aging (NILS-LSA) J Epidemiol. 2000;10:S90–S94. doi: 10.2188/jea.10.1sup_90. [DOI] [PubMed] [Google Scholar]
- Paillard T. Effects of general and local fatigue on postural control: a review. Neurosci Biobehav Rev. 2012;36:162–176. doi: 10.1016/j.neubiorev.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Parreira RB, Amorim CF, Gil AW, Teixeira DC, Bilodeau M, da Silva RA. Effect of trunk extensor fatigue on the postural balance of elderly and young adults during unipodal task. Eur J Appl Physiol. 2013;113:1989–1996. doi: 10.1007/s00421-013-2627-6. [DOI] [PubMed] [Google Scholar]
- Portney LG, Watkins MP. Foundations of clinical research: Applications to practice, 3rd. Upper Saddle River, NJ: Pearson/Prentice Hall; 2009. [Google Scholar]
- Shumway-Cook A, Woollacott MH. Motor control : translating research into clinical practice. 4th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012. [Google Scholar]
- Stephens J. A CDC Compendium of Effective Fall Interventions: What Works for Community-Dwelling Older Adults. 2nd ed. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- Sterling DA, O'Connor JA, Bonadies J. Geriatric falls: injury severity is high and disproportionate to mechanism. J Trauma. 2001;50:116–119. doi: 10.1097/00005373-200101000-00021. [DOI] [PubMed] [Google Scholar]
- Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and non-fatal falls among older adults. Inj Prev. 2006;12:290–295. doi: 10.1136/ip.2005.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Gandevia SC. Changes in muscle afferents, motoneurons and motor drive during muscle fatigue. Eur J Appl Physiol. 2000;83:106–115. doi: 10.1007/s004210000269. [DOI] [PubMed] [Google Scholar]
- Toebes MJ, Hoozemans MJ, Furrer R, Dekker J, van Dieen JH. Local dynamic stability and variability of gait are associated with fall history in elderly subjects. Gait Posture. 2012;36:527–531. doi: 10.1016/j.gaitpost.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Tresch MC. A balanced view of motor control. Nat Neurosci. 2007;10:1227–1228. doi: 10.1038/nn1007-1227. [DOI] [PubMed] [Google Scholar]
- Wilson EL, Madigan ML, Davidson BS, Nussbaum MA. Postural strategy changes with fatigue of the lumbar extensor muscles. Gait Posture. 2006;23:348–354. doi: 10.1016/j.gaitpost.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and motor control of human movement. 4th ed. Hoboken, N.J: Wiley; 2009. [Google Scholar]
- Yaggie JA, McGregor SJ. Effects of isokinetic ankle fatigue on the maintenance of balance and postural limits. Arch Phys Med Rehabil. 2002;83:224–228. doi: 10.1053/apmr.2002.28032. [DOI] [PubMed] [Google Scholar]