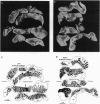
Abstract
Amblyopia can be induced by opacity of the ocular media (e.g., cataract), misalignment of the ocular axes (strabismus), or unequal refractive error in the eyes (anisometropia). Experiments in monkeys have shown that early monocular eyelid suture, a model of amblyopia caused by cataract, results in shrinkage of the eye's ocular dominance columns in striate cortex. This reduction of the geniculocortical projection from the deprived eye has been thought to explain in part the mechanism of amblyopia. We labeled the ocular dominance columns in monkeys with amblyopia by using cytochrome oxidase histochemistry. In animals rendered amblyopic by early unilateral eyelid suture, no pattern of cytochrome oxidase activity appeared in layer IVc. Outside layer IVc, alternating rows of light and dark patches were present; the pale patches fit in register with the shrunken ocular dominance columns of the deprived eye, which were labeled by autoradiography. Subsequent removal of one eye caused a striking cytochrome oxidase pattern to emerge in layer IVc that correlated precisely with the shrunken (deprived eye) and expanded (normal eye) ocular dominance columns. This correlation was shown by injecting one eye with [3H]proline. It has remained unsettled whether other forms of amblyopia are accompanied by shrinkage of ocular dominance columns. To address this issue, in an analogous clinical case, we examined the pattern of cytochrome oxidase activity in a human subject with a history of anisometropic amblyopia who suffered a lesion of one optic nerve shortly before death. The ocular dominance columns were normal in width, indicating that some forms of amblyopia occur without shrinkage of ocular dominance columns.
Full text
PDF

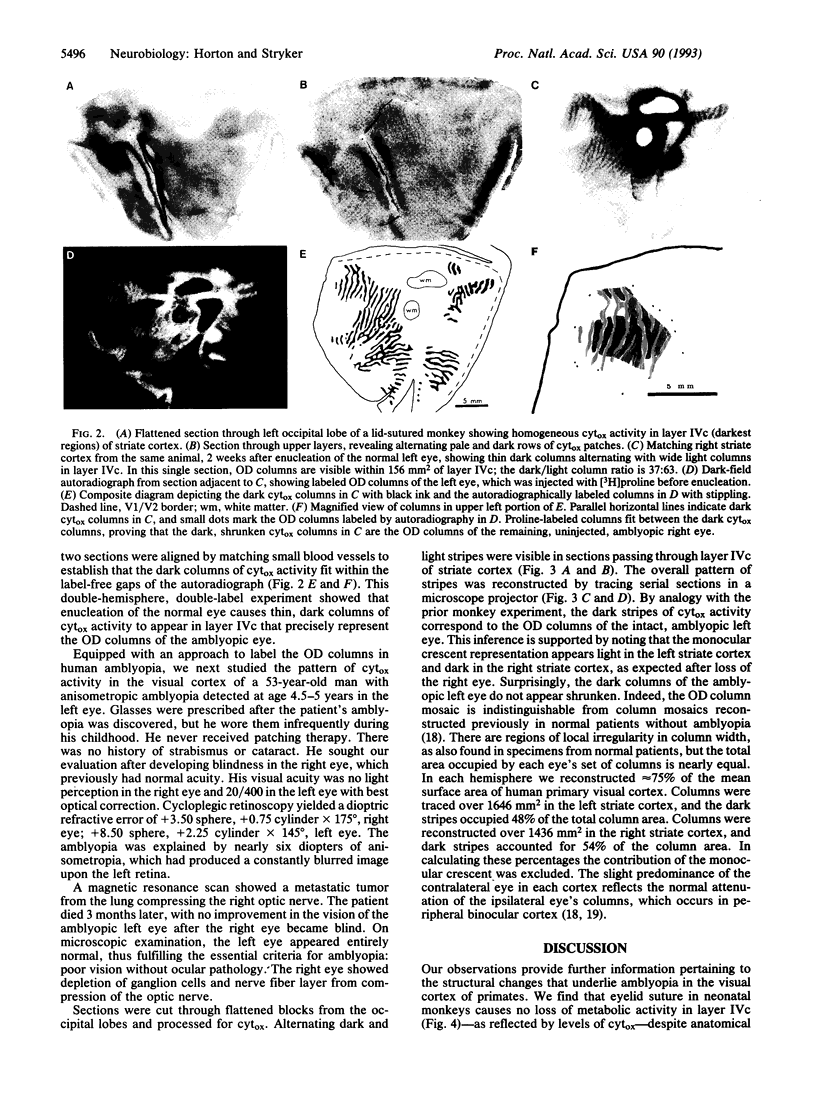
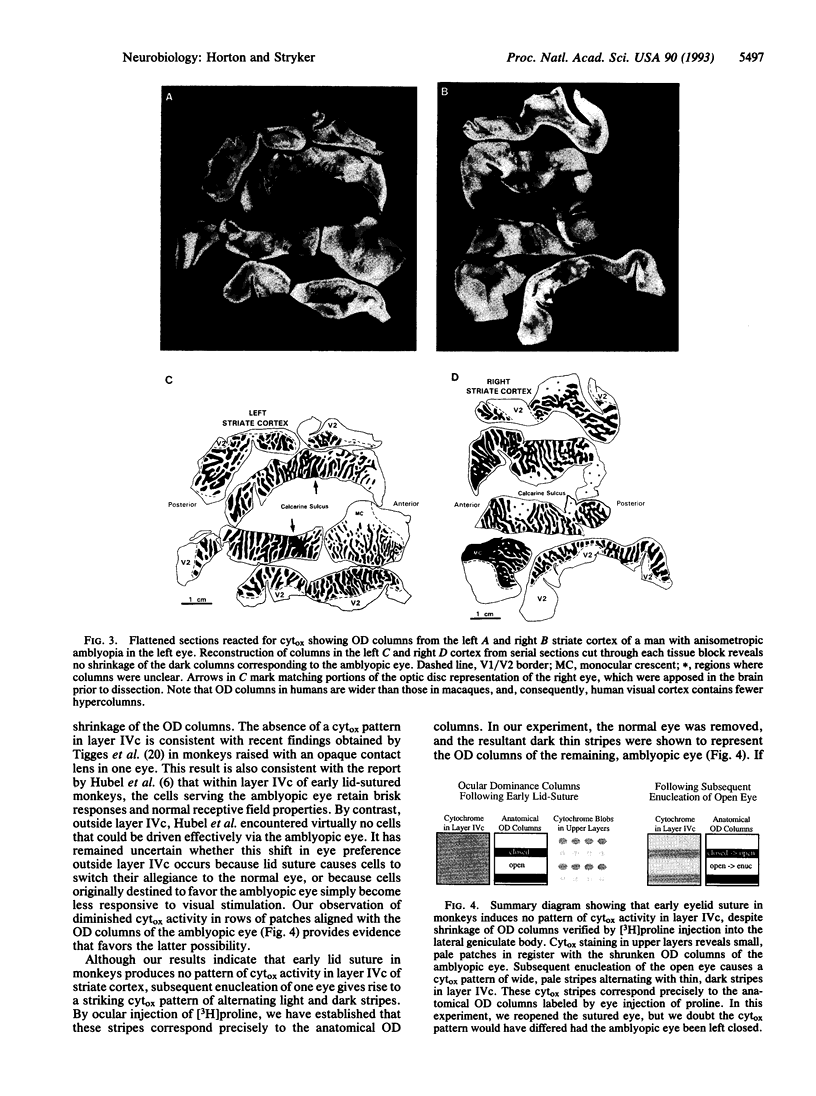

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakemore C., Garey L. J., Vital-Durand F. The physiological effects of monocular deprivation and their reversal in the monkey's visual cortex. J Physiol. 1978 Oct;283:223–262. doi: 10.1113/jphysiol.1978.sp012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynader M., Timney B. N., Mitchell D. E. Period of susceptibility of kitten visual cortex to the effects of monocular deprivation extends beyond six months of age. Brain Res. 1980 Jun 9;191(2):545–550. doi: 10.1016/0006-8993(80)91303-7. [DOI] [PubMed] [Google Scholar]
- Daw N. W., Fox K., Sato H., Czepita D. Critical period for monocular deprivation in the cat visual cortex. J Neurophysiol. 1992 Jan;67(1):197–202. doi: 10.1152/jn.1992.67.1.197. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. E., Hunt S. P., Wu J. Y. Immunocytochemical localization of glutamic acid decarboxylase in monkey striate cortex. Nature. 1981 Aug 13;292(5824):605–607. doi: 10.1038/292605a0. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. E., Movshon J. A., Eggers H. M., Gizzi M. S., Boothe R. G., Kiorpes L. Effects of early unilateral blur on the macaque's visual system. II. Anatomical observations. J Neurosci. 1987 May;7(5):1327–1339. doi: 10.1523/JNEUROSCI.07-05-01327.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J. C. Cytochrome oxidase patches: a new cytoarchitectonic feature of monkey visual cortex. Philos Trans R Soc Lond B Biol Sci. 1984 Jan 17;304(1119):199–253. doi: 10.1098/rstb.1984.0021. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Dagi L. R., McCrane E. P., de Monasterio F. M. Arrangement of ocular dominance columns in human visual cortex. Arch Ophthalmol. 1990 Jul;108(7):1025–1031. doi: 10.1001/archopht.1990.01070090127054. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Hedley-Whyte E. T. Mapping of cytochrome oxidase patches and ocular dominance columns in human visual cortex. Philos Trans R Soc Lond B Biol Sci. 1984 Jan 17;304(1119):255–272. doi: 10.1098/rstb.1984.0022. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Hubel D. H. Regular patchy distribution of cytochrome oxidase staining in primary visual cortex of macaque monkey. Nature. 1981 Aug 20;292(5825):762–764. doi: 10.1038/292762a0. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- LeVay S., Connolly M., Houde J., Van Essen D. C. The complete pattern of ocular dominance stripes in the striate cortex and visual field of the macaque monkey. J Neurosci. 1985 Feb;5(2):486–501. doi: 10.1523/JNEUROSCI.05-02-00486.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVay S., Stryker M. P., Shatz C. J. Ocular dominance columns and their development in layer IV of the cat's visual cortex: a quantitative study. J Comp Neurol. 1978 May 1;179(1):223–244. doi: 10.1002/cne.901790113. [DOI] [PubMed] [Google Scholar]
- LeVay S., Wiesel T. N., Hubel D. H. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980 May 1;191(1):1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Olson C. R., Freeman R. D. Profile of the sensitive period for monocular deprivation in kittens. Exp Brain Res. 1980;39(1):17–21. doi: 10.1007/BF00237065. [DOI] [PubMed] [Google Scholar]
- Shatz C. J., Stryker M. P. Ocular dominance in layer IV of the cat's visual cortex and the effects of monocular deprivation. J Physiol. 1978 Aug;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindale N. V., Vital-Durand F., Blakemore C. Recovery from monocular deprivation in the monkey. III. Reversal of anatomical effects in the visual cortex. Proc R Soc Lond B Biol Sci. 1981 Nov 24;213(1193):435–450. doi: 10.1098/rspb.1981.0074. [DOI] [PubMed] [Google Scholar]
- Tigges M., Boothe R. G., Tigges J., Wilson J. R. Competition between an aphakic and an occluded eye for territory in striate cortex of developing rhesus monkeys: cytochrome oxidase histochemistry in layer 4C. J Comp Neurol. 1992 Feb 8;316(2):173–186. doi: 10.1002/cne.903160204. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. SINGLE-CELL RESPONSES IN STRIATE CORTEX OF KITTENS DEPRIVED OF VISION IN ONE EYE. J Neurophysiol. 1963 Nov;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965 Nov;28(6):1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H., Lam D. M. Autoradiographic demonstration of ocular-dominance columns in the monkey striate cortex by means of transneuronal transport. Brain Res. 1974 Oct 18;79(2):273–279. doi: 10.1016/0006-8993(74)90416-8. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N. Postnatal development of the visual cortex and the influence of environment. Nature. 1982 Oct 14;299(5884):583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979 Jul 27;171(1):11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]