Summary
Alzheimer’s disease (AD) is the most common cause of dementia, and there is currently no cure. The “β-amyloid cascade hypothesis” of AD is the basis of current understanding of AD pathogenesis and drug discovery. However, no AD models have fully validated this hypothesis. We recently developed a human stem cell culture model of AD by cultivating genetically modified human neural stem cells in a three-dimensional (3D) cell culture system. These cells were able to recapitulate key events of AD pathology including β-amyloid plaques and neurofibrillary tangles. In this review, we will discuss the progress and current limitations of AD mouse models and human stem cell models as well as explore the breakthroughs of 3D cell culture systems. We will also share our perspective on the potential of dish models of neurodegenerative diseases for studying pathogenic cascades and therapeutic drug discovery.
What is Alzheimer’s Disease?
Alzheimer’s disease (AD), which currently affects 5.2 million people in the United States, is the most common cause of dementia, and has become the 6th leading cause of death [1]. However, no cure is currently available. AD patients typically show gradual cognitive impairments including loss of memory, language skills and abilities to focus and reason [2]. β-amyloid plaques and neurofibrillary tangles (NFT) observed in brains of AD patients are key pathological markers of AD. The β-amyloid plaques are composed of aggregated β-amyloid peptides (Aβs), which is derived by proteolytic cleavage from the β-amyloid precursor protein (APP) [2, 3]. The NFT is composed of hyperphosphorylated tau proteins (p-tau), a microtubule binding protein. In pathological conditions such as AD, tau proteins are hyperphosphorylated, released from axonal microtubules and form insoluble fibers (paired helical filaments, PHF) and aggregated in cell bodies and apical dendrites to form the NFT. Interestingly, NTFs are observed in other neurodegenerative conditions such as progressive supranuclear palsy, corticobasal degeneration and frontotemporal dementia (FTD), while β-amyloid plaques are unique to AD [3].
What major obstacles are Alzheimer’s researchers grappling with?
The “β-amyloid cascade hypothesis” of AD has provided the groundwork for current understanding of AD pathogenesis [4–6]. With strong experimental support from many studies, this hypothesis is the framework for most current clinical trials for potential AD therapies [7]. The β-amyloid hypothesis proposes that excessive accumulation of pathogenic Aβs triggers a vicious pathogenic cascade including synaptic deficits, altered neuronal activity, hyperphosphorylation of tau/NFT and finally, neuronal death. In cases of familial AD (FAD), mutations in APP or presenilin 1 (PSEN1) trigger this toxic cascade by increasing generation of pathogenic Aβ species [5]. However, no AD animal models derived from FAD mutations have been able to recapitulate both β-amyloid and NFT together [3, 8–10]. Widely used current AD mouse models are generated by overexpressing human APP (hAPP) and/or PSEN1with single or multiple FAD mutations. Although most of them recapitulated β-amyloid plaques and β-amyloid-induced synaptic/memory deficits, all of them fail to recapitulate robust NFT pathology and neuronal death as observed in AD patients [3, 9] (for a graphical summary, see http://www.alzforum.org/research-models; accessed in June 29, 2015). The recent failures of anti-β-amyloid therapies in humans, which were highly effective in mouse models, might be explained by limitation of AD mouse in comprehensively modeling human AD pathologies [11, 12].
Recently, human neurons were generated from fibroblasts of FAD patients using induced pluripotent stem cell (iPSC) technology [13–22]. These neurons share the same genetic makeup of FAD patients and provide a platform for a new generation of AD models that comprehensively recapitulate the pathogenic cascades of the human brain environment. However, it is still technically challenging to demonstrate neurodegenerative disease conditions with iPSC lines in a petri dish, since they generally occur in the late stages of life. Recently, our laboratory reported a unique 3D culture model of human neural progenitor cells overexpressing APP and PSEN1 with human FAD mutations. Seeded in a novel 3D culture system, these cells demonstrated both robust extracellular aggregates of β-amyloid and hyperphosphorylated/aggregated tau pathologies for the first time in either cell or mouse FAD models [23].
What is the purpose of this article?
In this review, we will discuss the progress and limitations of current in vitro and in vivo AD models, provide insight into the challenges of representing AD pathogenic cascades, particularly in human stem cell models, explore the breakthroughs and trajectories of 3D cell culture, and provide our perspective on existing and future obstacles to modeling AD and other diseases in a dish.
Current AD mouse models do not fully recapitulate AD pathologies
Given the prevalence and poor prognosis of AD, the development of animal models has been a research priority to understand pathogenic mechanisms and test therapeutic strategies. The discovery of FAD genes allowed the development of transgenic models that reproduce several neuropathological, neurodegenerative, and behavioral characteristics of the spectrum of disease in AD patients. To date, ~200 FAD mutations in APP or PSEN1 genes have been discovered (see http://ghr.nlm.nih.gov/gene/APP and http://ghr.nlm.nih.gov/gene/PSEN1; accessed in June 29, 2015). Many of these FAD mutations have been incorporated into transgenic mouse models of AD, the most common of which are summarized in Table 1.
Table 1.
Representative mouse models of AD
| Mouse model | Gene/ isoform |
Mutation | Intraneuronal Aβ |
Parenchymal Aβ plaques |
Tau hyperphos -phorylation |
Neurofibrillary tangles | Neuronal loss | Key references |
|---|---|---|---|---|---|---|---|---|
| hAPP models | ||||||||
| PDAPP | hAPP695< 751,770a | Ind | - | Yes | Yes | No | No | [24, 25] |
| APP23 | hAPP751 | Swe | - | Yes | Yes | No | Little | [26] |
| Tg2576 | hAPP695 | Swe | Yes | Yes | - | - | No | [27] |
| J20 | hAPP695< 751,770a | Swe, Ind | - | Yes | No | No | Yes | [31] |
| TgCRND8 | hAPP695 | Swe, Ind | - | Yes | - | No | No | [72] |
| hAPP/PS1 models | ||||||||
| APP/PS1 (Tg2576 × PS1) | hAPP695 | Swe | - | Yes | - | - | - | [33] |
| PSEN1 | M146L | |||||||
| APPswePS1ΔE9 | m/hAPP695b | Swe | - | Yes | No | No | - | [34] |
| PSEN1 | ΔE9 | |||||||
| APPSL/PS1 | hAPP751 | Swe, Lon | Yes | Yes | - | - | Yes | [73] |
| PSEN1 | M146L | |||||||
| APP/PS1KI | hAPP751 | Swe, Lon | Yes | Yes | - | - | Yes | [36] |
| PSEN1 | M233T, L235P | |||||||
| 5xFAD | hAPP695 | Swe, Lon, Flo | Yes | Yes | - | - | Yes | [37] |
| PSEN1 | M146L, L286V | |||||||
| Models with hTau | ||||||||
| TAPP | hAPP695 | Swe | - | Yes | - | Yes | - | [39] |
| hTau-4R0 N | P301L | |||||||
| 3xTg | hAPP695 | Swe | Yes | Yes | Yes | Yes | No | [40] |
| hTau-4R0 N | P301L | |||||||
| PSEN1 | M146V | |||||||
| Other models | ||||||||
| APPSwDI/NOS2−/− | hAPP770 | Swe, Dutch, Iowa | No | Yes | Yes | Yes | Yes | [41] |
| NOS2 | Knockout | |||||||
| TgF344-AD rats | m/hAPP695b | Swe | Yes | Yes | Yes | Yes | Yes | [42] |
| PSEN1 | ΔE9 | |||||||
APP minigene expressing all three isoforms, mostly KPI-positive APPs.
Humanized mouse APP, in which three amino acids in Aβ are changed to the human sequence.
Ind, Indiana; Swe, Swedish; Lon, London
Dash (−) = not reported.
What are the limitations of mouse APP mutation models?
Games and colleagues reported the first AD transgenic model, termed PDAPP mice, which over-expresses hAPP containing the Indiana (V717F) mutation [24]. These mice developed β-amyloid plaques accompanied by dystrophic neurites and extensive gliosis (reactive astrocytes and activated microglia). Although p-tau were detected in some of the dystrophic neurites after 14 months of age, no PHF or NFT were identified [25]. APP23 line over-expresses the hAPP containing the Swedish (K670N/M671L) mutation [26]. Similar to PDAPP mice, APP23 mice showed some dystrophic neurites containing p-tau, which surround β-amyloid plaques, but still no NFT were detected [26]. The Tg2576 mouse model also expresses hAPP containing the Swedish mutation, and shows an age-dependent parenchymal Aβ deposition, but again, no tau hyperphosphorylation at any of the major phosphorylation sites [27]. Neuronal loss is absent in PDAPP and Tg2576 mice while APP23 mice show less than 30% of neuronal loss in the CA1 region of the hippocampus at the age of 14–18 months [28–30]. In order to achieve more robust β-amyloid accumulation, transgenic mouse models harboring two hAPP FAD mutations were generated: J20 and TgCRND8 mice, which express hAPP with the Swedish mutation together with the Indiana mutation [31, 32]. Although these lines exhibited age-dependent robust increase of Aβ40 and Aβ42 levels and parenchymal β-amyloid deposition, no tau hyperphosphorylation occurs at any of the major phosphorylation sites. Also, no clear neuronal loss is evident in these lines.
Is tau pathology observed in mice expressing both APP and PSEN1 mutations?
To increase the levels of pathogenic Aβ42, mouse models over-expressing both hAPP mutations and PSEN1 mutations were generated. The doubly transgenic APP/ PSEN1 line, which over-expresses hAPP containing the Swedish mutation together with the mutant PSEN1 gene (M146L), shows a selective increase in Aβ42 and develops large numbers of β-amyloid deposits that resembles compact Aβ plaques [33]. Next, APPswe PSEN1ΔE9 mice were made by co-injecting two vectors encoding Swedish APP mutant and ΔE9 PSEN1 mutant (lacking exon 9) [34]. As in the case of the APP/PSEN1 mice, these mice begin to develop Aβ deposits earlier than single transgenic mice only harboring APP mutations. APPSL/PSEN1 mice, which carry hAPP with the Swedish and London mutations and human PSEN1 with the M146L mutation, showed about 30% loss of pyramidal neurons in the hippocampal CA1–3 fields as well as β-amyloid plaques [35]. Another line showing neuronal loss is the APP/PS1KI mouse, which expresses hAPP Swedish and London mutations together with two PSEN1 knock-in (KI) mutations (M233T/L235P) [36]. Recently, 5xFAD mice were generated by over-expressing five FAD mutations: hAPP with the Swedish, London and Florida mutations together with mutant PSEN1 (M146L/L286V) [37]. 5xFAD mice exhibited much higher levels of Aβ42 than those of Aβ40, and rapidly accumulated massive amounts of cerebral Aβ42 at young ages. Large pyramidal neurons in cortical layer 5 and subiculum are visibly reduced in number at 9 months. However, no robust p-tau nor NFT was observed, despite extensive β-amyloid plaques, gliosis and neuronal loss [35, 37, 38].
Mouse models expressing tau pathology
In an effort to generate AD mouse models that exhibit β-amyloid plaques and NFT lesions, transgenic mice harboring both FAD and tau mutations linked with FTD and parkinsonism were produced. Lewis and colleagues first crossed Tg2576 mice with a transgenic line JNPL3, which expresses the FTDP-17 Tau (P301L) mutation, generating a bigenic transgenic mouse model referred to as TAPP [39]. While JNPL3 mice develop NFT-like lesions in the absence of β-amyloid pathology, TAPP mice exhibit both β-amyloid plaques and NFT. 3xTg model, developed by Oddo and colleagues, expresses hAPP Swedish and P301L Tau mutations from exogenous transgenes combined with a PSEN1 M146V mutation from the endogenous mouse gene [40]. These mice show progressive β-amyloid deposition, in which intracellular immunoreactivity is detected in some brain regions. Extracellular β-amyloid plaques appear by six months in the frontal cortex and become more extensive by twelve months. The conformation of tau was altered and hyperphosphorylated at multiple residues in the brain after occurrence of β-amyloid plaques (i.e. 12 to 15 months old). Tau reactive dystrophic neurites are also evident in the brains of older 3xTg mice.
In addition to FTD mutations, a cross between transgenic mice containing three APP mutations (Swedish, Dutch, and Iowa) without tau mutations and a strain deficient in nitric oxide synthase 2 (NOS2), termed APPSwDI/NOS2−/− mice were developed. These mice displayed tau hyperphosphorylation and aggregation of their native mouse tau in regions where β-amyloid deposition is particularly dense. However, it is not clear whether these models show the filamentous assemblies of tau protein observed in human AD patients [41].
FAD mouse models and beyond
In summary, FAD mouse models have failed to fully recapitulate AD pathogenesis in humans. They have exhibited β-amyloid pathology resulting in senile plaques similar to those found in AD patients, but none have successfully shown β-amyloid -driven NFT formation. Old PDAPP and APP23 mice exhibit dystrophic neurites containing p-tau, which may represent pre-tangles, but they do not progress to NFT. The transgenic mice that express mutant forms of tau and APP together with or without PSEN1 (3xTg and TAPP, respectively) developed both plaques and tangle-like pathology in brain tissues. However, these mouse models contain a tau mutation associated with FTD, not AD. It is crucial to note that no mutation has been located in the tau gene in AD: in AD, it is normal human tau that becomes pathological. Fundamental species-specific differences in genome and protein composition between mice and humans may preclude the recapitulation of bona fide AD pathological events in mouse models. One of the differences might lay in the dissimilarity of tau gene structures in humans and mice. In this regard, it is interesting to note that a rat FAD model, termed TgF344-AD, which has six tau isoforms similar to human, and expresses Swedish APP and PSEN1ΔE9 mutations, exhibits some aspects of extensive tauopathy, although clear PHF structures were not evident [42].
Human iPSC-derived AD models
Recent advances in reprograming technology have made it possible to generate human neurons derived from the fibroblasts of AD patients. These cells truly have an AD genetic background, and thus are expected precisely to recapitulate AD pathogenesis in vitro when differentiated into mature neurons. Recently, several groups have successfully generated human neurons from AD patients [13–16, 18, 20, 21, 43].
β-amyloid pathologies in human iPSC-derived neurons harboring FAD mutations
Yagi and colleagues reported the first FAD neurons derived from human iPSCs carrying FAD mutations in PSEN1 and PSEN2 [21]. As evidence for pathogenic changes, they showed that the Aβ42/Aβ40 ratio was significantly increased in FAD neurons as compared to neurons from non-AD controls. Human iPSC-derived neurons with different PSEN FAD mutations also showed significant increases in Aβ42/40 levels, confirming that PSEN FAD mutations impair PS/γ-secretase activity [2, 13, 15, 17, 19]. Similar to PSEN FAD mutations, APP FAD mutations (V717I, London) significantly increased the Aβ42/40 ratio in human forebrain neurons [13, 14].
APP duplication mutations robustly increased total Aβ levels, mostly Aβ40, in iPSC-derived human neurons, possibly by increasing the levels of APP, a precursor protein for Aβ generation [13, 20]. Although it does not carry any FAD mutations, Trisomy 21/Down syndrome neurons also showed robust increases in total Aβ levels due to the APP gene duplication located on chromosome 21 [44]. In those Down syndrome neurons, Shi and colleagues showed both intra and extra cellular deposits of Aβ42 species [44]. APP E693Δ, a rare autosomal FAD mutation associated with early-onset AD symptoms without β-amyloid plaques, leads to a reduction in extracellular Aβ levels while inducing the accumulation of intracellular Aβ oligomers in a human iPSC-derived neuronal model [18]. Interestingly, APP E693Δ neurons showed elevated endoplasmic reticulum (ER) and oxidative stress, both of which could be blocked by Docosahexaenoic acid (DHA) treatments [18]. These data may suggest that the accumulation of intracellular Aβ oligomers plays a role in the cognitive decline of AD patients with APP-E693Δ [45].
What can we learn about tau pathology in human iPSC-derived models?
Regarding tau pathologies, Israel and colleagues detected an increase in tau phosphorylation (pThr231) in neurons carrying an APP duplication mutation [20]. Blocking Aβ generation with β-secretase inhibitors significantly decreased tau phosphorylation in the same model, but γ-secretase inhibitor, another Aβ blocker, did not affect tau phosphorylation. APP V717I neurons also showed an increase in levels of total and phosphorylated tau [14]. More importantly, treatment with Aβ-specific antibodies early in culture reverses the phenotype of increased total and phospho tau (pThr231) levels, suggesting that altered Aβ species are responsible for total and phospho tau levels in APP V717I neurons [14]. Recently, Moore and colleagues also reported that cortical neurons derived from iPSCs harboring APP V717I or APP duplication mutation showed increases in both total and phospho tau (pS202/T205 and pS396/pS404) levels [13]. The altered tau metabolism was not observed in neurons carrying PSEN1 FAD mutations (PSEN1 Y111C, M146I and Intron 4). The elevated total and phospho tau levels in these cells were normalized by β-secretase inhibitor treatments but interestingly, not by γ-secretase inhibitor. The altered tau metabolism in APP V717I and duplication neurons may represent a very early stage of tauopathy in AD. Validation in other models will strengthen this intriguing hypothesis.
Together, these reports demonstrate that human iPSC-derived FAD neurons can successfully recapitulate the hallmarks of early stage of AD pathogenesis, including accumulation of pathogenic Aβ species, increases in ER and oxidative stress and possibly very early stage tauopathy. However, these FAD neurons did not show robust extracellular β-amyloid aggregation, NFT pathologies such as aggregated, or hyperphosphorylated tau with PHFs; neither did they display any signs of neurodegeneration, as observed in AD patients. The low levels of Aβ species in the current iPSC-derived human AD neurons, poses a limitation in reconstituting robust β-amyloid accumulation within 6–10 weeks, as we have shown in our 3D culture models.
Modeling Alzheimer’s disease in a 3D human neural cell culture system
Lack of robust AD pathologies in FAD iPSC-derived neurons might be due to the relatively low levels of pathogenic Aβ species in those models. As summarized in Table 2, FAD iPSC neurons generally show moderate to low levels of pathogenic Aβ42 while Aβ levels in AD patient brains are robustly elevated as compared to age-matched healthy controls. For example, the average levels of insoluble Aβ40 and Aβ42/43 were 159 and 1659 pmol/g wet tissue in AD brains and Aβ40 and Aβ42/43 levels were elevated by 330 and 558-fold in AD patients as compared with the age-matched controls, which is much higher than the Aβ levels achieved by FAD iPSC models [46] (see table 2 for summary of Aβ levels in select FAD iPSC neurons).
Table 2.
Summary of human AD-iPSC models and FAD ReN cells
| Features | Aβ40 | Aβ42 | Aβ42/40 ratio |
p-Tau | Extracellular β-amyloid deposits /NFT |
|
|---|---|---|---|---|---|---|
| Yagi et al. [20] |
|
A246E: ~75 pmol/L N141I: ~60 pmol/L |
A246E: ~15 pmol/L N141I: ~10 pmol/L |
~2-fold * increase |
- | - |
| Israel et al. [19] |
|
sAD: ~70 fmol/mg FAD: ~60–80 fmol/mg |
- | - | ~2 fold* increase in p-tau/tau ratio |
- |
| Koch et al. [18] |
|
~70 fmol/mg | ~4 fmol/mg | ~2-fold * Increase |
- | - |
| Kondo et al. [17] |
|
V717L: ~100 pmol/L E693Δ: ~20 pmol/L |
V717L: ~20 pmol/L E693Δ: ~2 pmol/L* |
V717L: ~1.5 fold* |
- | - |
| Muratore et al. [13] |
|
~230 fmol/mg | ~44 fmol/mg* | ~1.6 fold* | ~2 fold* increase in total and p-tau levels |
- |
| Choi et al. [21] |
APPK670N/M671L, V717I, PS1ΔE9; immortalized hNPCs |
mAP: ~10,000 mol/mg mGAP: ~5,200 fmol/mg HReN: ~18,700 fmol/mg |
mAP: ~1,700 fmol/mg mGAP: ~3,400 fmol/mg HReN: ~4,000 fmol/mg |
mAP: ~1.4 fold mGAP: ~5.4 fold HReN: ~1.7 fold increase |
~5–10 fold increase in p-tau levels |
Extracellular β-amyloid deposits
|
Dash (−) = not reported
How do we achieve pathogenic Aβ levels in human neural progenitor cells?
To overcome the limitation of current human iPSC-derived cellular models, we generated human stem cell lines producing high levels of pathogenic Aβ species by overexpressing human APP together with PSEN1 carrying multiple FAD mutations [23]. Similar strategies have previously been applied in AD mouse models to accelerate the severity and onset of β-amyloid deposition in brain tissue [3, 8, 9]. For example, 5XFAD mouse model with five different FAD mutations showed β-amyloid deposits and cognitive deficits in 2–4 months as compared to 9–12 months of Tg2576 mice harboring a single FAD APP mutation [27, 27, 37]. APP K670N/M671L (Swedish), V717I (London) mutations and PSEN1 ΔE9 mutations were chosen to increase pathogenic Aβ42 levels in ReNcell VM (ReN) human neural progenitor cells [23].
Using FACS enrichment protocols, we were able to generate FAD ReN cell lines highly expressing Aβs, especially Αβ42, as compared to previously reported cell models (see table 2 for Αβ levels in FAD cell lines [23]). Next, FAD ReN cells were differentiated into neurons and glial cells by growth factor deprivation in a standard two-dimensional (2D) culture system, Since FAD ReN cells produce high levels of Αβ42, we expected to detect Αβ aggregation after 4–6 weeks of differentiation. However, we could not detect any extracellular β-amyloid aggregates or hyperphosphoylated tau/NFT pathologies (personal observation).
Using Matrigel-based 3D systems to model AD
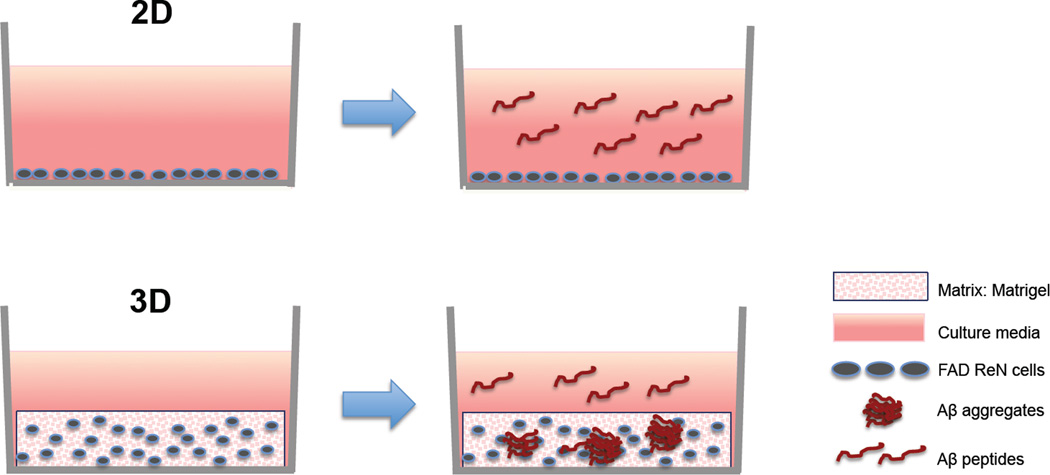
In 2D cell cultures, the secreted Aβ species from FAD ReN cells immediately diffuse into the relatively large volume of cell culture media, and is subsequently removed during regular media changes (commonly performed every two to three days). This likely explains why we could not detect Aβ aggregation in a 2D cell culture system (Fig. 1). We hypothesized that 3D hydrogels would provide a brain tissue-like closed environment, which would accelerate Aβ deposition by limiting the diffusion of secreted Aβ into the cell culture medium and thus provide local niches capable of achieving high enough Aβ concentrations for initiation of Aβ aggregation (Fig. 1) [47–50]. We chose Matrigel specifically as our 3D hydrogel because it can be easily solidified with ReN cells through moderate thermal change, and it provides a brain-like environment rich in structural proteins such as laminin, entactin, collagen, and heparin sulfate proteoglycans [51].
Fig. 1. Impact of 2D vs 3D cell culture systems on β-amyloid aggregation.
Matrigel-based 3D culture conditions promote aggregation of secreted Aβ species from FAD ReN cells.
After switching to the 3D Matrigel culture system, we were able to observe robust extracellular β-amyloid deposits in 6 week-differentiated FAD ReN cells, as detected by Aβ immunostaining and AmyloGlo, a β-amyloid dye [23, 52]. Biochemical analysis also confirmed the presence of β-amyloid aggregates (dimer, trimer, tetramer) in Tris-buffered saline (TBS)-insoluble/Sodium dodecyl sulfate (SDS)-soluble and SDS-insoluble/Formic acid soluble fractions [23]. More importantly, 3D-differentiated FAD cells showed dramatic increases in phospho tau (pSer199/Ser202/Thr205, pSer396/Ser404) levels in detergent-insoluble fractions from FAD ReN cells without significantly affecting total tau levels. In addition, the hyperphosphorylated tau proteins were abnormally accumulated in somatodendritic compartments, silver-stained with a modified Gallyas protocol, and formed Sarkosyl-insoluble filamentous structures detected by immunoelectron microscopy [23] (see table 2 and Figure 2 for summary and timeframe of β-amyloid and tau pathology in our 3D FAD ReN cell model). These analyses clearly demonstrate the recapitulation of β-amyloid and NFT pathologies in our 3D FAD models. We also found that inhibition of Aβ generation with β- or γ-secretase inhibitors not only decreased β-amyloid, but also attenuated phospho tau pathologies, which suggests that excess accumulation of β-amyloid directly causes the hyperphosphorylated tau pathology in this model.
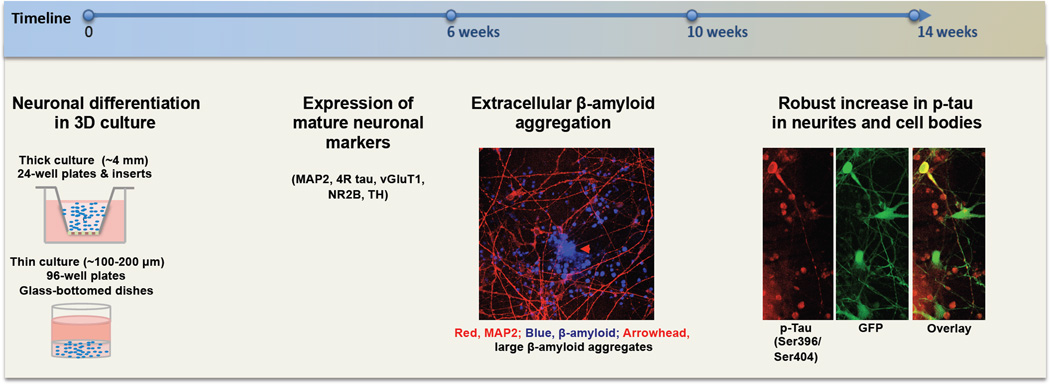
Fig. 2. Timeframe of AD pathologies in 3D FAD ReN cells.
Extracellular Aβ aggregates develop ~6 weeks after differentiation and tauopathy is evident from ~10–14 weeks after differentiation. Day 0 indicates the day on which cells are seeded with Matrigel.
Are there other cellular 3D culture models of AD?
In addition to our human 3D culture model, other 3D systems have recently reported recapitulation of AD pathogenesis. 3D culture technique meets human stem cell technology in a Zhang et al. study, in which human neuroepithelial-like stem cells, It-NES, were cultured in a PuraMatrix hydrogel made by laminin and a self-assembling peptide matrix [53]. Those 3D It-NES cultures were then exposed to a soluble Αβ oligomer preparation from synthetic/monomeric Aβ42. The 24-hr Αβ oligomer treatment largely decreased the activated form of p21-activated kinase (pPAK) and redistributed the pPAK to submembranous regions, which resembles the altered pPAK distribution in both AD patients and mouse models. Interestingly, the same Αβ oligomer preparation showed no impact on pPAK levels or distribution in 2D-cultured It-NES cells, which again clearly demonstrates the advantage of 3D hydrogel systems in studying β-amyloid-induced pathogenesis. Choi and colleagues established a 3D neurospheroid model useful for testing Aβ toxicity in a microfluidic platform [54, 55]. This model consists of prenatal rat, not human, cortical neurons and synthetic Aβ42 and would be more interesting if the system could be extended to human FAD neurons [54, 55].
Why is 3D better than 2D for modeling AD?
We have demonstrated that a powerful advantage of 3D culture for modeling AD is that it provides a local environment that promotes aggregation of β-amyloid, which can trigger pathogenic cascades, including NFT [23]. In addition, other studies have shown that 3D conditions more closely mimic in vivo environments and can accelerate neuronal differentiation and neural network formation [47, 48, 50, 56–59]. Neurons grown in a 2D environment are able to spread freely across the surface of the glass or plastic surface, but they have no support for vertical growth, which can lead to unintended apical-basal polarity rather than the more accurate neuronal stellar morphology [60]. Additionally, the space between cells is not as accurate: 2D models tend to have reduced linkages between neurons, and synaptic distances are usually too large [61]. In addition to morphology, neurons grown in a 3D environment are able to express genes such as neuronal markers more accurately, as compared to neurons grown in a monolayer [62]. Indeed, we also confirmed that 3D differentiation dramatically elevated mature neuronal marker expression as compared with 2D-differentiated ReN cells [23]. More importantly, we found that 3D culture condition greatly elevated 4-repeat adult tau (4R tau) isoforms, which is essential for recapitulating tau pathology [23]. The elevation of 4R tau isoform by 3D culture conditions were also observed in SH-SY5Y neuroblastoma cells [63] and human neural stem cells derived from iPSC although the impact was not as high as ReN cells (personal observation). High levels of 4R tau isoform would also contribute to the robust tauopathy observed in our 3D FAD ReN cell models as well as β-amyloid accumulation. These findings clearly suggest that 3D culture conditions, as compared with 2D, would have additional advantages in recapitulating neurodegenerative disease conditions including those associated with tauopathy.
Do 3D cultures make superior models for diseases other than AD?
Another advantage of 3D culture is that it can simulate mechanical strain, which can be converted to biochemical signals through opening of stress sensitive ion channels, leading to signaling cascades: this is impossible in 2D cultures. Tang-Schomer and colleagues engineered a brain-like cortical tissue comprising an ECM gel strengthened by a scaffold derived from silk proteins. The tissue model, constructed from concentric donut-like layers of differing concentration and mechanical stiffness allowed rat cortical neurons to develop intricate networks that recapitulated traumatic brain injury (TBI) conditions [59]. The 3D cerebral organoid model is another example that highlights the advantage of 3D culture models: developing distinct brain-like regions for up to ten months when cultured in a spinning bioreactor [64]. Using this model, Lancaster and colleagues were able to reconstitute a model of microcephaly, which cannot be achieved using conventional 2D culture systems. These examples demonstrate the enormous potential of 3D culture models for reconstituting neurological diseases.
What are the applications of a 3D model of AD?
The most valuable application of our 3D human neural cell culture model of AD is large-scale high-throughput screening for novel therapeutic targets, and validation of targets during the initial stages of drug discovery, which are not feasible in the current AD mouse models [23, 43, 65, 66]. A major strength of 3D human neural cell culture models is their flexible scalability. A spectrum ranging from 6-well thick-layer to 96-well thin-layer cultures enables a range of purposes from validation of targets to large-scale drug screening. The immortalized and single-clonal ReN cells in our 3D cultures are particularly well suited for large-scale, high throughput studies due to their quick proliferation and stability over repeated passages. Human stem cell-derived models provide a valid system to test the efficacy and potential toxicity of candidate AD drugs. Cross-checking a candidate drug target in both human and mouse based models would minimize chance of failure in the final stages of clinical trials [11].
Another key application of our AD model is investigation into the molecular mechanisms underlying AD pathology. This will provide novel druggable targets and may lead to the discovery of new diagnostic and prognostic biomarkers of AD [67]. While human iPSC-derived AD models allow study of pathogenic mechanisms under physiological conditions, our 3D model provides insight after pathological accumulation of β-amyloid and NFT formation, which are present in moderate to advanced AD. Our neural cell culture model system might also be an ideal model to test the impact of AD-risk genetic variants and environmental factors on AD pathogenesis.
What are the challenges and limitations of modeling AD in 3D cultures?
Although human neural cell culture models of AD provide promise and support for the next generation of AD models, major challenges still exist. One of the most poignant is the difficulty of reconstituting neurodegenerative disease conditions in vitro over a short time-course while the pathogenic changes of AD progress slowly and commence in the later stages of life [68]. Differentiation and maturation of human neural stem cells occurs over a span of months, which may not be enough to establish the aged brain conditions under which patients develop robust neurodegenerative pathologies [68]. In our study, we have shown that β-amyloid pathology can be accelerated by introducing multiple FAD mutations in human neural progenitor cells, together with the 3D culture conditions [23]. However, comprehensive recapitulation of AD-associated neurodegenerative changes is still proving to be an elusive goal using hiPSC-derived neurons from AD patients, which only harbor a single FAD mutation. Supplementation through gene manipulation or by aging-associated stresses, such as DNA damaging agents or proteasome inhibitors, may promote degenerative phenotypes in human iPSC-derived cellular AD models [68–70].
Another limitation in the current human stem cell-based models is the technical difficulty of reconstituting the brain regions most affected in AD, most notably the hippocampus and specific cortical layers. Recent studies demonstrated that forebrain/cortical neurons derived from FAD patient iPSCs showed significant increase of pathogenic Aβ levels [13, 14].
What are the latest innovations in 3D culture technology?
The creation of active, organized, brain-like structures in vitro has proven to be a formidable challenge. Advancements in 3D culture technology are beginning to address the issue. Moore et al. showed that both control and FAD cortical neurons formed a spontaneously active neural network [13]. Still, these neural networks could not completely reflect the organized, layered networks observed in human brains. Tang-Schomer and colleagues recently showed the reconstitution of 3D brain-like cortical tissue with silk protein scaffolds and collagen gel [59]. A “3D cerebral organoids” model recapitulated cerebral cortex-like structures using ES and iPSCs. This innovative system contained progenitor populations with characteristic features of human cortical development [64].
Can we overcome the imaging issue?
One of the major challenges for 3D culture systems is the low optical transparency during high-resolution imaging due to the thick nature of the culture. To analyze the β-amyloid and tau pathologies, we developed both thin layer cultures (~100–300 µm) for high magnification imaging and thick layer cultures (~4 mm) for biochemical analysis (Fig. 2) [23, 52]. Tang-Schomer and colleagues designed a system with a collagen-filled central surrounded by 3D silk-scaffold structures. This model allows live observation of neurite networks [59]. New advances like the “CLARITY” technology may also pave the way for comprehensive imaging of thick-layered 3D cultures [13, 71].
Conclusion and outlook
Human stem cell technology has provided a platform for a new generation of AD models useful both for studying disease pathogenic cascades and drug discovery in a human brain-like environment. In this manuscript, we have reviewed the current progress and explored the limitations of modeling AD in a dish. We have also presented major breakthroughs and current trajectories of 3D cell culture technology. Additionally, we shared our perspective on the future obstacles facing recapitulation of neurodegenerative diseases, including AD, in vitro. Still, many hurdles remain: introduction of neuro inflammatory systems including microglial cells, reconstitution of sporadic AD and recapitulation of neuronal death stemming from β-amyloid and tau pathologies. However, the combination of mature neurons derived from patient fibroblasts partnered with new 3D culture technology will propel our understanding of pathogenic cascades and drug discovery.
ACKNOWLEGEMENTS
This work is supported by the grants from the Cure Alzheimer’s fund to D. Y. K., S. H. C. and R. E. T., the Bio & Medical Technology Development Program of the National Research Foundation (funded by the Korean government, MSIP (2015M3A9C7030151, Y.H.K), and National Institute of Health (1RF1AG048080-01, D.Y.K. and R.E.T.; 5P01AG15379, D.Y.K. and R.E.T.;2R01AG014713, D.Y.K.; 5R37MH060009, R.E.T.). We appreciate Drs. Sang Su Kwak and Raja Bhattacharyya for the critical reading and helpful suggestions.
Footnotes
DECLARATION OF INTEREST
The authors report no competing financial interest.
AUTHOR CONTRIBUTIONS
C.D. and J.A. were equally responsible for designing, writing and preparing the manuscript. S.H.C. Y.H.K. and D.Y.K. contributed to the writing and R.E.T. and D.Y.K supervised and finalized the manuscript.
References
- 1.Alzheimer's Association. 2014 Alzheimer's disease facts and figures. Alzheimers Dement. 2014;10:e47–e92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Götz J, Ittner LM. Animal models of Alzheimer's disease and frontotemporal dementia. Nat Rev Neurosci. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- 4.Glenner GG. The pathobiology of Alzheimer's disease. Annu Rev Med. 1989;40:45–51. doi: 10.1146/annurev.me.40.020189.000401. [DOI] [PubMed] [Google Scholar]
- 5.Hardy J, Selkoe D. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 6.Selkoe D. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 7.Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. 2011:1–15. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 8.Duff K, Rao MV. Progress in the modeling of neurodegenerative diseases in transgenic mice. Curr Opin Neurol. 2001;14:441–447. doi: 10.1097/00019052-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Chin J. Selecting a mouse model of Alzheimer's disease. Methods Mol Biol. 2011;670:169–189. doi: 10.1007/978-1-60761-744-0_13. [DOI] [PubMed] [Google Scholar]
- 10.Li C, Ebrahimi A, Schluesener H. Drug pipeline in neurodegeneration based on transgenic mice models of Alzheimer's disease. Ageing Res Rev. 2013;12:116–140. doi: 10.1016/j.arr.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 11.De Strooper B. Lessons from a failed γ-secretase Alzheimer trial. Cell. 2014;159:721–726. doi: 10.1016/j.cell.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Henley DB, Sundell KL, Sethuraman G, Dowsett SA, et al. Safety profile of semagacestat, a gamma-secretase inhibitor: IDENTITY trial findings. Curr Med Res Opin. 2014:1–12. doi: 10.1185/03007995.2014.939167. [DOI] [PubMed] [Google Scholar]
- 13.Moore S, Evans LDB, Andersson T, Portelius E, et al. APP Metabolism Regulates Tau Proteostasis in Human Cerebral Cortex Neurons. Cell Reports. 2015;11:689–696. doi: 10.1016/j.celrep.2015.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muratore CR, Rice HC, Srikanth P, Callahan DG, et al. The familial Alzheimer's disease APPV717I mutation alters APP processing and Tau expression in iPSC-derived neurons. Hum Mol Genet. 2014;23:3523–3536. doi: 10.1093/hmg/ddu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sproul AA, Jacob S, Pre D, Kim SH, et al. Characterization and molecular profiling of PSEN1 familial Alzheimer's disease iPSC-derived neural progenitors. PLoS ONE. 2014;9:e84547. doi: 10.1371/journal.pone.0084547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan L, Bhattacharyya BJ, Belmadani A, Pan L, et al. Stem cell derived basal forebrain cholinergic neurons from Alzheimer's disease patients are more susceptible to cell death. Mol Neurodegener. 2014;9:3. doi: 10.1186/1750-1326-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodruff G, Young JE, Martinez FJ, Buen F, et al. The Presenilin-1 ΔE9 Mutation Results in Reduced γ-Secretase Activity, but Not Total Loss of PS1 Function, in Isogenic Human Stem Cells. Cell Reports. 2013 doi: 10.1016/j.celrep.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondo T, Asai M, Tsukita K, Kutoku Y, et al. Modeling Alzheimer's disease with iPSCs reveals stress phenotypes associated with intracellular Abeta and differential drug responsiveness. Cell Stem Cell. 2013;12:487–496. doi: 10.1016/j.stem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Koch P, Tamboli IY, Mertens J, Wunderlich P, et al. Presenilin-1 L166P mutant human pluripotent stem cell-derived neurons exhibit partial loss of γ-secretase activity in endogenous amyloid-β generation. Am J Pathol. 2012;180:2404–2416. doi: 10.1016/j.ajpath.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Israel MA, Yuan SH, Bardy C, Reyna SM, et al. Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells. Nature. 2012;482:216–220. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yagi T, Ito D, Okada Y, Akamatsu W, et al. Modeling familial Alzheimer's disease with induced pluripotent stem cells. Hum Mol Genet. 2011;20:4530–4539. doi: 10.1093/hmg/ddr394. [DOI] [PubMed] [Google Scholar]
- 22.Choi SH, Tanzi RE. iPSCs to the rescue in Alzheimer's research. Cell Stem Cell. 2012;10:235–236. doi: 10.1016/j.stem.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Choi SH, Kim YH, Hebisch M, Sliwinski C, et al. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature. 2014;515:274–278. doi: 10.1038/nature13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Games D, Adams D, Alessandrini R, Barbour R, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 25.Masliah E, Sisk A, Mallory M, Games D. Neurofibrillary pathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. J Neuropathol Exp Neurol. 2001;60:357–368. doi: 10.1093/jnen/60.4.357. [DOI] [PubMed] [Google Scholar]
- 26.Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold K-H, et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsiao K, Chapman P, Nilsen S, Eckman C, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 28.Calhoun ME, Wiederhold K-H, Abramowski D, Phinney AL, et al. Neuron loss in APP transgenic mice. Nature. 1998;395:755–756. doi: 10.1038/27351. [DOI] [PubMed] [Google Scholar]
- 29.Chen G, Chen KS, Knox J, Inglis J, et al. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer's disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- 30.Van Dam D, D'Hooge R, Staufenbiel M, Van Ginneken C, et al. Age-dependent cognitive decline in the APP23 model precedes amyloid deposition. Eur J Neurosci. 2003;17:388–396. doi: 10.1046/j.1460-9568.2003.02444.x. [DOI] [PubMed] [Google Scholar]
- 31.Chishti MA, Yang DS, Janus C, Phinney AL, et al. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276:21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- 32.Masliah E, Rockenstein E, Veinbergs I, Sagara Y, et al. beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer“s disease and Parkinson”s disease. Proc Natl Acad Sci U S A. 2001;98:12245–12250. doi: 10.1073/pnas.211412398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holcomb L, Gordon MN, McGowan E, Yu X, et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 34.Jankowsky JL, Slunt HH, Ratovitski T, Jenkins NA, et al. Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomolecular engineering. 2001;17:157–165. doi: 10.1016/s1389-0344(01)00067-3. [DOI] [PubMed] [Google Scholar]
- 35.Casas C, Sergeant N, Itier J-M, Blanchard V, et al. Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Abeta42 accumulation in a novel Alzheimer transgenic model. Am J Pathol. 2004;165:1289–1300. doi: 10.1016/s0002-9440(10)63388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flood DG, Reaume AG, Dorfman KS, Lin YG, et al. FAD mutant PS-1 gene-targeted mice: increased A beta 42 and A beta deposition without APP overproduction. Neurobiol Aging. 2002;23:335–348. doi: 10.1016/s0197-4580(01)00330-x. [DOI] [PubMed] [Google Scholar]
- 37.Oakley H, Cole SL, Logan S, Maus E, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wirths O, Multhaup G, Czech C, Feldmann N, et al. Intraneuronal APP/A beta trafficking and plaque formation in beta-amyloid precursor protein and presenilin-1 transgenic mice. Brain Pathol. 2002;12:275–286. doi: 10.1111/j.1750-3639.2002.tb00442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis J, Dickson DW, Lin WL, Chisholm L, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 40.Oddo S, Caccamo A, Shepherd JD, Murphy MP, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 41.Wilcock DM, Lewis MR, Van Nostrand WE, Davis J, et al. Progression of amyloid pathology to Alzheimer's disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. J Neurosci. 2008;28:1537–1545. doi: 10.1523/JNEUROSCI.5066-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen RM, Rezai-Zadeh K, Weitz TM, Rentsendorj A, et al. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric abeta, and frank neuronal loss. J Neurosci. 2013;33:6245–6256. doi: 10.1523/JNEUROSCI.3672-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohamet L, Miazga NJ, Ward CM. Familial Alzheimer's disease modelling using induced pluripotent stem cell technology. World J Stem Cells. 2014;6:239–247. doi: 10.4252/wjsc.v6.i2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi Y, Kirwan P, Smith J, MacLean G, et al. A human stem cell model of early Alzheimer's disease pathology in Down syndrome. Sci Transl Med. 2012;4:124ra29. doi: 10.1126/scitranslmed.3003771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomiyama T, Nagata T, Shimada H, Teraoka R, et al. A new amyloid beta variant favoring oligomerization in Alzheimer's-type dementia. Ann Neurol. 2008;63:377–387. doi: 10.1002/ana.21321. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Dickson DW, Trojanowski JQ, Lee VM. The levels of soluble versus insoluble brain Abeta distinguish Alzheimer's disease from normal and pathologic aging. Exp Neurol. 1999;158:328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- 47.Liedmann A, Rolfs A, Frech MJ. Cultivation of human neural progenitor cells in a 3-dimensional self-assembling peptide hydrogel. Journal of visualized experiments : JoVE. 2012:e3830. doi: 10.3791/3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LaPlaca MC, Vernekar VN, Shoemaker JT, Cullen DK. Three-Dimensional Neuronal Cultures. In: Morgan JR, Berthiaume F, editors. Methods in Bioengineering: 3D Tissue Engineering. 2010. pp. 187–204. Methods in Bioengineering: 3D Tissue Engineering. [Google Scholar]
- 49.Haycock JW. 3D cell culture: a review of current approaches and techniques. Methods Mol Biol. 2011;695:1–15. doi: 10.1007/978-1-60761-984-0_1. [DOI] [PubMed] [Google Scholar]
- 50.Li H, Wijekoon A, Leipzig ND. 3D differentiation of neural stem cells in macroporous photopolymerizable hydrogel scaffolds. PLoS ONE. 2012;7:e48824. doi: 10.1371/journal.pone.0048824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–1890. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 52.Kim YH, Choi SH, D'Avanzo C, Hebisch M, et al. A 3D human neural cell culture system for modeling Alzheimer’s disease. 2015 doi: 10.1038/nprot.2015.065. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang D, Pekkanen-Mattila M, Shahsavani M, Falk A, et al. A 3D Alzheimer's disease culture model and the induction of P21-activated kinase mediated sensing in iPSC derived neurons. Biomaterials. 2014;35:1420–1428. doi: 10.1016/j.biomaterials.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 54.Choi YJ, Park J, Lee S-H. Size-controllable networked neurospheres as a 3D neuronal tissue model for Alzheimer's disease studies. Biomaterials. 2013;34:2938–2946. doi: 10.1016/j.biomaterials.2013.01.038. [DOI] [PubMed] [Google Scholar]
- 55.Park J, Lee BK, Jeong GS, Hyun JK, et al. Three-dimensional brain-on-a-chip with an interstitial level of flow and its application as an in vitro model of Alzheimer's disease. Lab on a Chip. 2015;15:141–150. doi: 10.1039/c4lc00962b. [DOI] [PubMed] [Google Scholar]
- 56.Ortinau S, Schmich J, Block S, Liedmann A, et al. Effect of 3D-scaffold formation on differentiation and survival in human neural progenitor cells. Biomed Eng Online. 2010;9:70. doi: 10.1186/1475-925X-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suga H, Kadoshima T, Minaguchi M, Ohgushi M, et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature. 2011;480:57–62. doi: 10.1038/nature10637. [DOI] [PubMed] [Google Scholar]
- 58.Liedmann A, Frech S, Morgan PJ, Rolfs A, et al. Differentiation of human neural progenitor cells in functionalized hydrogel matrices. Biores Open Access. 2012;1:16–24. doi: 10.1089/biores.2012.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang-Schomer MD, White JD, Tien LW, Schmitt LI, et al. Bioengineered functional brain-like cortical tissue. Proc Natl Acad Sci USA. 2014;111:13811–13816. doi: 10.1073/pnas.1324214111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cullen DK, Wolf JA, Vernekar VN, Vukasinovic J, et al. Neural tissue engineering and biohybridized microsystems for neurobiological investigation in vitro (Part 1) Crit Rev Biomed Eng. 2011;39:201–240. doi: 10.1615/critrevbiomedeng.v39.i3.30. [DOI] [PubMed] [Google Scholar]
- 62.Seidel D, Krinke D, Jahnke H-G, Hirche A, et al. Induced Tauopathy in a Novel 3D-Culture Model Mediates Neurodegenerative Processes: A Real-Time Study on Biochips. PLoS ONE. 2012;7:e49150. doi: 10.1371/journal.pone.0049150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agholme L, Lindström T, Kågedal K, Marcusson J, et al. An in vitro model for neuroscience: differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J Alzheimers Dis. 2010;20:1069–1082. doi: 10.3233/JAD-2010-091363. [DOI] [PubMed] [Google Scholar]
- 64.Lancaster MA, Renner M, Martin C-A, Wenzel D, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Young JE, Goldstein LS. Alzheimer's disease in a dish: promises and challenges of human stem cell models. Hum Mol Genet. 2012;21:R82–R89. doi: 10.1093/hmg/dds319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young W. Patient-specific Induced Pluripotent Stem Cells as a Platform for Disease Modeling, Drug Discovery and Precision Personalized Medicine. J Stem Cell Res Ther. 2012;01 [Google Scholar]
- 67.Livesey FJ. Human stem cell models of dementia. Hum Mol Genet. 2014;23:R35–R39. doi: 10.1093/hmg/ddu302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu G-H, Ding Z, Izpisua Belmonte JC. iPSC technology to study human aging and aging-related disorders. Curr Opin Cell Biol. 2012;24:765–774. doi: 10.1016/j.ceb.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 69.Nguyen HN, Byers B, Cord B, Shcheglovitov A, et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011;8:267–280. doi: 10.1016/j.stem.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller JD, Ganat YM, Kishinevsky S, Bowman RL, et al. Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell. 2013;13:691–705. doi: 10.1016/j.stem.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chung K, Wallace J, Kim S-Y, Kalyanasundaram S, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332–337. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mucke L, Masliah E, Yu GQ, Mallory M, et al. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmitz C, Rutten BPF, Pielen A, Schäfer S, et al. Hippocampal neuron loss exceeds amyloid plaque load in a transgenic mouse model of Alzheimer's disease. Am J Pathol. 2004;164:1495–1502. doi: 10.1016/S0002-9440(10)63235-X. [DOI] [PMC free article] [PubMed] [Google Scholar]